Abstract
Background
Trimetazidine (TMZ) is an anti-ischemic drug. In spite of its protective effects on cardiovascular system, there is no scientific study on the usefulness of TMZ treatment for prolonged QT interval and cardiac hypertrophy induced by diabetes.
Objectives
To evaluate the effects of TMZ on QT interval prolongation and cardiac hypertrophy in the diabetic rats.
Methods
Twenty-four male Sprague-Dawley rats (200-250 g) were randomly assigned into three groups (n = 8) by simple random sampling method. Control (C), diabetic (D), and diabetic administrated with TMZ at 10 mg/kg (T10). TMZ was administrated for 8 weeks. The echocardiogram was recorded before isolating the hearts and transfer to a Langendorff apparatus. Hemodynamic parameters, QT and corrected QT interval (QTc) intervals, heart rate and antioxidant enzymes were measured. The hypertrophy index was calculated. The results were evaluated by one-way ANOVA and paired t-test using SPSS (version 16) and p < 0.05 was regarded as significant.
Results
The diabetic rats significantly indicated increased hypertrophy, QT and QTc intervals and decreased Left ventricular systolic pressure (LVSP), Left ventricular developed pressure (LVDP), rate pressure product (RPP), Max dp/dt, and min dp/dt (±dp/dt max), heart rate, superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase in the heart. Treatment with TMZ in the diabetic animals was significantly improved these parameters in comparison to the untreated diabetic group.
Conclusions
TMZ improves QTc interval prolongation and cardiac hypertrophy in diabetes.
Keywords: Diabetes Mellitus, Trimetadizine, Cardiomegaly, Electrocardiology, Oxidative Stress, Rats
Introduction
Diabetes is associated with cardiovascular disorders and increased mortality rate in diabetic patients.1 The statistic reveals that 30 million people were suffered from diabetes worldwide in 1985 and recently, it is predicted by WHO, there will be 300 million by the year 2025.2
Diabetic cardiomyopathy is known as the structural and functional alterations in the heart induced by diabetes that are associated with cardiac hypertrophy, diastolic and/or systolic dysfunction in the absence of hypertension, valvular and ischemic heart diseases and other cardiac disorders.3,4
QT and QTc intervals are electrocardiographic parameters that regarded as critical predictors of mortality and stroke in diabetic patients.5,6 The pathological QT prolongation is known as a risk factor that increases ventricular arrhythmias and other heart diseases. Moreover, ventricular hypertrophy plays an important role in developing prolonged QT interval-related diabetes.7 A previous study has confirmed the negative effects of hypertrophy and QT interval prolongation on the function of heart in diabetes.8 The homeostasis of energy is effective in decreasing the hypertrophy in the heart.9
Trimetazidine (TMZ) is an anti-angina agent that is known to improve metabolism of energy in the heart subjected to ischemia.10,11 Previous studies have indicated reduced fatty acid oxidation via reducing mitochondrial 3-ketoacyl CoA thiolase (3-KAT) activity in beta-oxidation by TMZ treatment.12 Others also indicated that TMZ has protective effects on cardiac fibrosis resulted from pressure overload.13 In addition, there are some other investigations showing that the treatment with TMZ has positive effects on cardiac function in diabetic individuals with cardiovascular disorders.14 Taken together, these results from related studies make evidence that TMZ has beneficial effects on cardiovascular system. However, the role of TMZ in QT interval prolongation and cardiac hypertrophy improvement in diabetes was still unknown. Therefore, the present study was undertaken to evaluate the effects of TMZ on QT interval prolongation and cardiac hypertrophy in the diabetic animals.
Methods
Chemical
Trimetazidine (TMZ), heparin and alloxan were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and Ketamine and xylazine purchased from Alfasan Co (Woderen- Holland).
Animal
Twenty-four adult male Sprague-Dawley rats (250 ± 20 g) were housed under standard conditions (20 ± 5°C, 12-hour light/dark cycle, and free available to water and food) during the study period. All the experimental protocols followed the Consensus Author Guidelines on Animal Ethics and Welfare and the national guidelines for conducting animal studies (Ethics Committee permission No. APRC-94-25 Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran).15
The sample size of each group was computed to be eight by the formula:16
where S12 and S22 are means.
The animals were randomly divided into three groups (n = 8) by simple random sampling method. Control (C), diabetic (D) and diabetic administrated with TMZ at 10 mg/kg (T10).17 TMZ was treated orally by gavage once daily for 8 weeks.
Diabetic model
Diabetes was induced by intraperitoneal administration of alloxan at 120 mg/kg. After 6 h, the animals were orally treated with 10% glucose solution (10 mL). They were further kept for 24 h on 5% glucose solution to reduce fatal hypoglycemic resulted from alloxan. The rats, indicating fasting blood glucose ≥ 250 mg/dL, reduced body weight, dyslipidemia, increased hepatic enzymes and clear signs of polyuria, polyphagia and polydipsia after 4 days were regarded as diabetic animals and used for the experiment.18
Electrocardiography
The animals were anesthetized by heparin, ketamine and xylazine (1000 U/kg, 50, and 5 mg/kg, respectively), lead II was recorded by Bio Amp and controlled using a Power Lab system (AD Instruments, Australia). QT interval and heart rate were measured. Corrected QT interval (QTc) was calculated by Bazett formula normalized as QTc = QT/(RR/f)1/2, where RR is R-R interval and f = 150 ms.19,20
Isolation of hearts
After echocardiogram (ECG) recording, the cannulation and ventilation of trachea were performed using an animal ventilator (UGO BASILE, model: 7025). The cannulation of aorta was carried out by a central incision in the aorta. The hearts were conveyed to the Langendorff system. The perfusion of heart was carried out by Krebs-Henseleit solution (5% carbon dioxide and 95% oxygen, 37°C, pH = 7.4, 8 ml/min). A latex balloon was inserted in the left ventricle for the measurement of left ventricular pressure (LVP) by Power Lab system (AD Instruments, Australia). Left ventricular end diastolic pressure (LVEDP) was approximately regulated 5-10 mmHg by the alteration of balloon volume. Left ventricular systolic pressure (LVSP), Max dp/dt, and min dp/dt (±dp/dt max) were measure.21 Left ventricular developed pressure (LVDP) and rate pressure product (RPP) were calculated by following formula:
Measurement of hypertrophy
After assessment of hemodynamic parameters using the Langendorff system, the hearts were removed and put in saline, then on a paper for assessment of the heart weight. Cardiac hypertrophy index (mg/g) was calculated from the total heart weight (mg) relative to total body weight (g) of the rat.22
Measurement of antioxidant enzymes
After measurement of hypertrophy, 100 mg of heart tissue was frozen in liquid nitrogen and stored at -70°C. The tissue samples were homogenized in phosphate buffered saline (PBS; 50 mM at pH of 7.4) using a Homogenizer (Heidolph Silenterosher M, Germany), and centrifuged at 14000 g for 15 minutes. The assessment of enzyme levels including glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) was performed on supernatant. GPx and SOD were measured using Randox kits (Randox Lab, UK) and CAT activity was evaluated using Zellbio kit (Zellbio Lab, Ulm, Germany).
Statistical analysis
The results were indicated as mean and standard deviation (SD). In the present study, the normal distribution of the results was carried out by Kolmogorov-Smirnov analysis. One-way ANOVA and Least Significant Difference (LSD) test were used for comparison between the various groups. The comparison of pre and post metabolic in each group was performed by paired t-test using SPSS (version 16). A p < 0.05 was regarded statistically significant.
Results
Electrocardiographic parameters
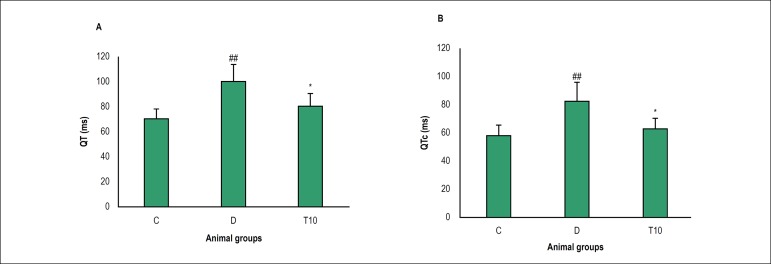
The QT and QTc intervals significantly increased in the diabetic animals in comparison with the control group (100 ± 13.80 vs. 70 ± 8.34, 82.52 ± 13.03 vs. 58.4 ± 7.33, p = 0.007 and p=0.009, respectively). TMZ treatment was associated with a significant reduction in the QT and QTc intervals in comparison with the untreated diabetic rats (80 ± 10.69 vs. 100 ± 13.80, 63.11 ± 7.05 vs. 82.52 ± 13.03, p = 0.043 and p = 0.040, respectively, Figure 1). As shown in Table 1, the diabetic rats indicated a decrease in the heart rate compared to the control rats (198 ± 41.21 vs. 268 ± 27.99, p = 0.002). Obviously, the administration of diabetic group with TMZ significantly increased the heart rate compared to the untreated diabetic rats (263 ± 35.02 vs. 198 ± 41.21, p = 0.006).
Figure 1.
QT interval (a), QTc interval (b) values in control (C), diabetic (D) and diabetic treated with TMZ (10 mg/kg, T10) groups eight weeks after treatment in the rats. The results were presented as mean ± SD. ## p < 0.01 compared to the control group, * p < 0.05 compared to the diabetic group.
Table 1.
Hemodynamic parameters in the heart
| Groups | C | D | T10 | P valueD VS. C | P valueT10 VS. D |
|---|---|---|---|---|---|
| Heart rate (beats/min) | 268 ± 27.99 | 198 ± 41.21 | 263 ± 35.02 | 0.002## | 0.006** |
| LVSP (mmHg) | 75 ± 20.91 | 60.78 ± 16.76 | 79.75 ± 10.16 | 0.041# | 0.028* |
| LVDP (mmHg) | 74.37 ± 18.76 | 56 ± 18.37 | 74.25 ± 9.93 | 0.030# | 0.031* |
| RPP (mmHg) | 14965 ± 5582 | 10184 ± 4589 | 14099 ± 3859 | 0.041# | 0.049* |
| Max +dp/dt(mmHg) | 2294 ± 255.27 | 1035 ± 370.33 | 1727 ± 410.60 | < 0.001### | 0.001** |
| Min -dp/dt (mmHg) | -1220 ± 229.09 | -594.77 ± 210 | -962 ± 194 | < 0.001### | 0.002** |
(Mean ± SD, n = 8) in control (C), diabetic (D) and diabetic treated with TMZ (T10), (one-way ANOVA followed by LSD post hoc test). LVSP: left ventricular systolic pressure; LVDP: left ventricular developed pressure; RPP: rate pressure product.
Markers of cardiac function
At the end of the experiment, LVSP, LVDP, ±dp/dt max and RPP were observed significantly lower in the diabetic group than control group. However, TMZ administration for 8 weeks was associated with a significant increase in these parameters in comparison with the untreated diabetic rats (Table 1).
Effect of TMZ on myocardial hypertrophy
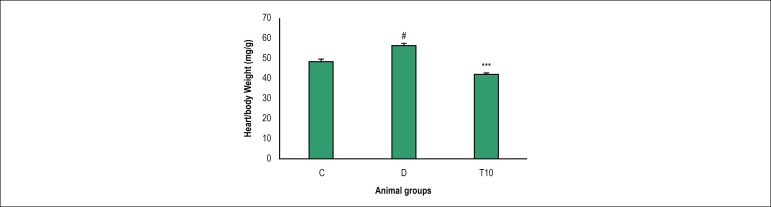
As indicated, the hypertrophy index increased significantly in the diabetic rats on 8 weeks compared to the control group (56.62 ± 6.50 vs. 48.62 ± 7.90, p = 0.039). According to our findings, in the diabetic rats, administration with TMZ remarkably decreased the hypertrophy index when compared to the diabetic rats (41.87 ± 7.50 vs. 56.62 ± 6.50, p < 0.001, Figure 2).
Figure 2.
Hypertrophy value in control (C), diabetic (D) and diabetic treated with TMZ (T10) groups eight weeks after treatment in the rats. The results were presented as mean ± SD. # p < 0.05 compared with control group, ***p < 0.001 compared to the untreated diabetic group.
Effect of TMZ on antioxidant enzymes
As indicated in Table 2, antioxidant enzymes, GPx, CAT and SOD significantly decreased in the heart of diabetic animals as compared to the control group (p < 0.001, p = 0.002, respectively). However, oral administration with TMZ was significantly improved GPx, CAT and SOD (p < 0.001, p < 0.049, respectively).
Table 2.
Antioxidant enzymes activities
| Groups | C | D | T10 | P value D vs. C | P valueT10 vs. D |
|---|---|---|---|---|---|
| SOD (U/dl) | 8.46 ± 1.51 | 5.86 ± 0.69 | 7 ± 1.54 | 0.002## | 0.049* |
| CAT (U/ dl) | 10.52 ± 0.60 | 1.90 ± 4.08 | 10.71 ± 0.50 | 0.002## | < 0.001*** |
| GPx (U/ dl) | 28.50 ± 2.67 | 13.22 ± 0.95 | 24.03 ± 1.73 | < 0.001### | < 0.001*** |
(Mean ± SD, n = 8) in control (C), diabetic (D) and diabetic treated with TMZ (T10), (one-way ANOVA followed by LSD post hoc test). SOD: superoxide dismutase. CAT: catalase; GPx: glutathione peroxidase.
Discussion
Our results indicated that alloxan injection significantly increased QT and QTc intervals and decreased heart rate, LVSP, LVDP, RPP, ±dp/dt max, and cardiac hypertrophy, SOD, GPx and CAT in the heart of the diabetic rats when compared with control group. However, treatment with TMZ was able to improve QT and QTc intervals, heart rate, hemodynamic parameters, SOD, CAT and hypertrophy significantly. Previous studies have demonstrated that diabetes is associated with the alterations of electromechanical and prolonged QTc interval in the heart.23
Diastolic and systolic dysfunctions are the earliest manifestations in the development of diabetic cardiomyopathy.24 The ±dp/dt max, LVSP, LVDP, RPP, cardiac diastolic and systolic indexes, are widely used to evaluate cardiac function. The alloxan-induced diabetic rats progressed cardiac dysfunction as demonstrated by a significant decrease in ±dp/dt LVSP, LVDP, RPP. TMZ treatment in turn improved each of these parameters.
In our model of type 1 diabetes, ECG indicated prolonged QTc, a finding that is consistent with previous studies. Treatment with TMZ significantly decreased these QT and QTc dispersions. This result is in agreement with previous reports which indicated that TMZ treatment improved QT prolongation in individuals with kidney disorders.25,26
In the present study, we also observed that diabetes led to bradycardia in the diabetic animals. It is revealed that in the diabetic rats heart rate tends to decrease after eight weeks.27 On the other hand, diabetes increases vagal tone and decreases sympathetic tone in diabetic rats.28 In addition, treatment with TMZ improves autonomic tone in individuals with acute coronary syndrome.29 Improved sympathetic and parasympathetic tone can partly explain the increased heart rate in the diabetic rats treated with TMZ.
Diabetic cardiomyopathy is associated with cardiac hypertrophy and dysfunction. High blood glucose and oxidative stress maybe considered to be critical factors that involved in hypertrophy and dysfunction of the heart.30 In the present study, the diabetic rats showed cardiac hypertrophy demonstrated by the increased heart wieght/body wieght ratio. Similar results have been indicated in previous studies.31 It is well established that, increased VLDL-c and decreased HDL-c levels can result in reduction in anti-oxidant defense system.27 In a previous study, it was indicated that the impairment of lipid profile levels in diabetic animals could be attributed to increased lipid breakdown and release of a large amount of free fatty acids.17 The released free fatty acids are susceptible to oxidation which result in decreased anti-oxidant level and anti-oxidant defense system.32 Increased level of fatty acid oxidation in the diabetic heart leads to lipid accumulation and cardiac hypertrophy.33 Reduction in fatty acid oxidation and oxidative stress by TMZ treatment can partly attribute to improvement of cardiac hypertrophy.
Previous studies have indicated that SOD level reduced in type 1 diabetes and it is mostly demonstrated that increased reactive oxygen species (ROS) negatively associated with the enzyme antioxidant values such as SOD and GPx.34 SOD quickly alters O2 to H2O2, which is further destroyed via GPx and CAT. The antioxidant enzyme levels are sensitive to the oxidative stress, and enhanced or reduced values have been indicated in various pathologies in which an increase of ROS is a cause or result of the disorder such as diabetes.35,36 In addition, superoxide anions and ROS have also been indicated to be contributed to cardiac hypertrophy resulted from various stimuli; therefore, SOD is a primary defense against oxidative stress that involves in the hypertrophy of the heart.37 Our findings indicated that SOD and CAT levels in hearts from TMZ treated diabetic rats was significantly higher than that in the untreated diabetic animals. GPx values was slightly but not significantly more in the hearts from TMZ treated diabetic animals compared to the diabetic rats.
Taken together, these findings indicated that the diabetic rats showed hypertrophy and dysfunction in the heart as well as increased cardiac oxidative damage in comparison with the control animals, showing that these undesirable factors are connected. TMZ probably improved these factors by antioxidant effects. Based on the results of present study, more studies require to be carried out to assessment mechanisms involved in the improvement of hypertrophy and cardiovascular disorders resulted from diabetes using TMZ treatment.
Conclusions
All these observations show that TMZ treatment contributes to the improvement of impaired function and electrical activity as well as hypertrophy of the heart in diabetic cardiomyopathy in rats. Improvements observed in TMZ treatment is associated with decrease oxidative stress.
Funding Statement
This study was funded by Ahvaz Jundishapur University of Medical Sciences (grant No. APRC-94-25).
Footnotes
Sources of Funding
This study was funded by Ahvaz Jundishapur University of Medical Sciences (grant No. APRC-94-25).
Study Association
This article is part of the thesis of Doctoral submitted by Fatemeh Ramezani-Aliakbari, from Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences.
Ethics approval and consent to participate
This study was approved by the Ethics Committee on Animal Experiments of the Ahvaz Jundishapur University of Medical Sciences under the protocol number APRC-94-25.
Author contributions
Design and conception of the study: Ramezani-Aliakbari F, Badavi M; Acquisition of data: Dianat M, Mard SA, Ahangarpour A; Analysis and interpretation of the data: Dianat M, Mard SA, Ahangarpour A; Statistical analysis: Ramezani-Aliakbari F, Badavi M; Obtaining financing: Badavi M; Writing of the manuscript: Ramezani-Aliakbari F, Badavi M; Critical revision of the manuscript for intellectual content: Badavi M.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115(25):3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 2.Gohari A, Noorafshan A, Akmali M, Zamani-Garmsiri, Seghatoleslam A. Urtica Dioica Distillate (Aragh Gazaneh) regenerates pancreatic beta cells in streptozotocin-induced diabetic rat. Iran J Med Sci. 2018;43(2):174–183. [PMC free article] [PubMed] [Google Scholar]
- 3.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121(9):748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta Diabetol. 2011;48(3):173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso CR, Salles GF, Deccache W. QTc interval prolongation is a predictor of future strokes in patients with type 2 diabetes mellitus. Stroke. 2003;34(9):2187–2194. doi: 10.1161/01.STR.0000085084.15144.66. [DOI] [PubMed] [Google Scholar]
- 6.Christensen PK, Gall MA, Major-Pedersen A, Sato A, Rossing P, Breum L, et al. QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand J Clin Lab Invest. 2000;60(4):323–332. doi: 10.1080/003655100750046486. [DOI] [PubMed] [Google Scholar]
- 7.Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Wachtell K, Papademetriou V, et al. Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hypertens. 2001;19(10):1883–1891. doi: 10.1097/00004872-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp Cell Res. 2017;360(1):12–18. doi: 10.1016/j.yexcr.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V. Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol. 2009;46(6):952–959. doi: 10.1016/j.yjmcc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y, et al. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol. 2012;59(10):913–922. doi: 10.1016/j.jacc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Tsioufis K, Andrikopoulos G, Manolis A. Trimetazidine and cardioprotection: facts and perspectives. Angiology. 2015;66(3):204–210. doi: 10.1177/0003319714530040. [DOI] [PubMed] [Google Scholar]
- 12.Lopatin YM, Rosano GM, Fragasso G, Lopaschuk GD, Seferovic PM, Gowdak LH, et al. Rationale and benefits of trimetazidine by acting on cardiac metabolism in heart failure. Int J Cardiol. 2016 Jan 15;203:909–915. doi: 10.1016/j.ijcard.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Gai Y, Liu F, Gao W, Zhang Y, Xu M, et al. Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase-ROS-CTGF pathway. Cardiovasc Res. 2010;88(1):150–158. doi: 10.1093/cvr/cvq181. [DOI] [PubMed] [Google Scholar]
- 14.Rosano GM, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003 Nov 28;2:16–16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olfert CB, McWilliam AA. Guide to the care nd use of experimental animals. 2nd ed. Canada: McWilliam; 1998. [Google Scholar]
- 16.Rosner B. Fundamentals of biostatistics. 6th ed. Boston: Brooks/Cole; 2005. [Google Scholar]
- 17.Xiang YL, He L, Xiao J, Xia S, Deng SB, Xiu Y, et al. Effect of trimetazidine treatment on the transient outward potassium current of the left ventricular myocytes of rats with streptozotocin-induced type 1 diabetes mellitus. Braz J Med Biol Res. 2012;45(3):205–211. doi: 10.1590/S0100-879X2012007500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargouri M, Magne C, El Feki A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr Res. 2016;36(11):1255–1268. doi: 10.1016/j.nutres.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Joukar S, Ghasemipour-Afshar E, Sheibani M, Naghsh N, Bashiri A. Protective effects of saffron (Crocus sativus) against lethal ventricular arrhythmias induced by heart reperfusion in rat: a potential anti-arrhythmic agent. Pharm Biol. 2013;51(7):836–843. doi: 10.3109/13880209.2013.767362. [DOI] [PubMed] [Google Scholar]
- 20.Joukar S, Zarisfi Z, Sepehri G, Bashiri A. Efficacy of Melissa officinalis in suppressing ventricular arrhythmias following ischemia-reperfusion of the heart: a comparison with amiodarone. Med Princ Pract. 2014;23(4):340–345. doi: 10.1159/000363452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radmanesh E, Dianat M, Badavi M, Goudarzi G, Mard SA. The cardioprotective effect of vanillic acid on hemodynamic parameters, malondialdehyde, and infarct size in ischemia-reperfusion isolated rat heart exposed to PM10. Iran J Basic Med Sci. 2017;20(7):760–768. doi: 10.22038/IJBMS.2017.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorri Mashhadi F, Zavvar Reza J, Jamhiri M, Hafizi Z, Zare Mehrjardi F, Safari F. The effect of resveratrol on angiotensin II levels and the rate of transcription of its receptors in the rat cardiac hypertrophy model. J Physiol Sci. 2017;67(2):303–309. doi: 10.1007/s12576-016-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casis O, Echevarria E. Diabetic cardiomyopathy: electromechanical cellular alterations. Curr Vasc Pharmacol. 2004;2(3):237–248. doi: 10.2174/1570161043385655. [DOI] [PubMed] [Google Scholar]
- 24.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98(1-2):33–39. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 25.Suner A, Cetin M. The effect of trimetazidine on ventricular repolarization indexes and left ventricular diastolic function in patients with coronary slow flow. Coron Artery Dis. 2016;27(5):398–404. doi: 10.1097/MCA.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 26.Balenovic D, Prkacin I, Cavric G, Horvat I, Pocanic D, Baotic I. The effects of trimetazidine on QT-interval prolongation in patients with chronic kidney disease stage III-IV (predialysis CRD) Acta Med Croatica. 2012;66(3):153–156. [PubMed] [Google Scholar]
- 27.Badavi M, Abedi HA, Dianat M, Sarkaki AR. Exercise Training and Grape Seed Extract Co-Administration Improves Lipid Profile, Weight Loss, Bradycardia, and Hypotension of STZ-Induced Diabetic Rats. Int Cardiovasc Res J. 2013;7(4):111–117. [PMC free article] [PubMed] [Google Scholar]
- 28.Vinik AI, Erbas T. Diabetic autonomic neuropathy. Handb Clin Neurol. 2013;117:279–294. doi: 10.1016/B978-0-444-53491-0.00022-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, He S, Wang X, Wang D. Effect of trimetazidine on heart rate variability in elderly patients with acute coronary syndrome. Pak J Med Sci. 2016;32(1):75–78. doi: 10.12669/pjms.321.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adebiyi AO, Adebiyi OO, Owira PM. Naringin Mitigates Cardiac Hypertrophy by Reducing Oxidative Stress and Inactivating c-Jun Nuclear Kinase-1 Protein in Type I Diabetes. J Cardiovasc Pharmacol. 2016;67(2):136–144. doi: 10.1097/FJC.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 31.Al-Rasheed NM, Al-Rasheed NM, Hasan IH, Al-Amin MA, Al-Ajmi HN, Mahmoud AM. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des Devel Ther. 2016 Jun 28;10:2095–2107. doi: 10.2147/DDDT.S109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prisacaru AE. Effect of antioxidants on polyunsaturated fatty acids - review. Acta Sci Pol Technol Aliment. 2016;15(2):121–129. doi: 10.17306/J.AFS.2016.2.12. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Carillon J, Rugale C, Rouanet JM, Cristol JP, Lacan D, Jover B. Endogenous antioxidant defense induction by melon superoxide dismutase reduces cardiac hypertrophy in spontaneously hypertensive rats. Int J Food Sci Nutr. 2014;65(5):602–609. doi: 10.3109/09637486.2014.893286. [DOI] [PubMed] [Google Scholar]
- 35.Navarro-Arevalo A, Canavate C, Sanchez-del-Pino MJ. Myocardial and skeletal muscle aging and changes in oxidative stress in relationship to rigorous exercise training. Mech Ageing Dev. 1999;108(3):207–217. doi: 10.1016/s0047-6374(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 36.Ulker S, McMaster D, McKeown PP, Bayraktutan U. Impaired activities of antioxidant enzymes elicit endothelial dysfunction in spontaneous hypertensive rats despite enhanced vascular nitric oxide generation. Cardiovasc Res. 2003;59(2):488–500. doi: 10.1016/s0008-6363(03)00424-3. [DOI] [PubMed] [Google Scholar]
- 37.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol. 2009;29(4):309–318. doi: 10.1159/000163767. [DOI] [PubMed] [Google Scholar]