Abstract
Lifetime reproductive success (LRS) is what counts in terms of evolution, but investments in reproduction entail costs for an organism. The idea that telomere dynamics may be shaped in response to such costs is already established; however, we still lack information on whether this relation translates to overall fitness. Here, we quantified LRS (number of fledged young) and longitudinal telomere dynamics of small passerine birds—the blue tits (Cyanistes caeruleus). We found that individual telomere erosion rate was positively associated with lifetime fledgling number. Birds with more fledged young experienced increased telomere attrition. We show that telomere attrition rate, but not telomere length, is related to individual fitness and suggest that telomere dynamics may underlie reproductive costs experienced by animals as a consequence of prioritizing their lifetime fitness. This is the first study, to our knowledge, to provide evidence that more pronounced telomere erosion is associated with higher fitness gain.
Keywords: cost of reproduction, telomere shortening, ageing, life-history, regression to the mean
1. Introduction
Telomeres are non-coding repeats of DNA found at the ends of eukaryotic chromosomes that protect the integrity of the functional genome. Because of an imperfect replication process on the lagging DNA strand, telomeres shorten with each cellular division [1]. Their shortening beyond a critical length invokes cellular senescence and subsequent arrest in the cell cycle [2]. There is a large body of literature demonstrating that telomere erosion rate is not simply an effect of the end-replication problem, but can reflect the cumulative action of various extrinsic factors [3–6]. Many studies consider telomere length (TL) as a proxy for individual quality because short telomeres are associated with high mortality risk [7]. Evidence that reproduction is yet another factor contributing to telomere loss, in both correlative [8,9] and experimental studies [10,11], is also accumulating. To the best of our knowledge, all of this research is based on single breeding events. In expectation of further breeding events, iteroparous animals have to tailor their current reproductive effort to their actual capacity in order to maximize overall fitness. An approach that accounts for cumulative breeding decisions across many breeding events could increase our understanding of the observed relationships between telomere dynamics and breeding success for several reasons. First, it has been shown that the effect of reproduction on TL did not persist beyond the duration of a reproductive treatment [8]. Next, if a relation between reproductive effort and telomere dynamics exists, the investment made into subsequent reproduction will not be independent of telomere loss experienced in the current breeding. Finally, both reproductive investment and telomere dynamics may be age-dependent, therefore studying early and late reproductive events in isolation can further alter the observed patterns. These issues can be avoided by investigating lifetime reproductive success (LRS), which is the number of raised young during an individual's lifespan. It is considered to be a reliable proxy for fitness and therefore constitutes a crucial trait in understanding life-history evolution [12].
Here, we used a dataset collected in a long-term study of a wild passerine—the blue tit (Cyanistes caeruleus). We aimed to investigate the relationship between observed LRS in terms of an overall number of fledged young and telomere dynamics measured within each individual parent throughout its recorded lifetime. We predicted that birds showing high lifetime reproductive output will be subjected to pronounced telomere shortening. Since telomere dynamics include two components: length and erosion rate, we also investigated if TL at first reproduction could predict LRS. Since long initial telomeres correlate positively with attained lifespan [8], we hypothesized the existence of a positive association between initial TL and LRS.
2. Material and methods
The study constitutes a part of the long-term monitoring of a nest-box breeding blue tit population inhabiting the southern part of Gotland, Sweden (see [13] for detailed study site description) and for the present analysis we considered data from breeding seasons 2009–2015 (see the electronic supplementary material for details on study species, LRS assessment and sampling).
To quantify TL change in a longitudinal manner, in the current analysis, we considered a subset of birds caught at least twice, and for which the first capture took place in their first breeding season (i.e. 1-year-olds) to standardize age-related effects across all individuals. We excluded birds that underwent experimental manipulations altering the number of fledged young (brood size manipulations, hormonal egg injections). For some individuals, a blood sample was not available; therefore, our final dataset comprises 105 samples belonging to 48 individuals (up to four samples per individual collected at yearly intervals in 23 males and 25 females).
TL was assessed using real-time quantitative PCR assay adapted for birds [14] from whole blood stored in ethanol. We used relative TL, expressed as the ratio (T/S) of a telomere copy number (T) and a single control gene copy number (S, which was GAPDH [15]). For details on qPCR, please refer to the electronic supplementary material.
(a). Statistics
We calculated D, which is a measure of temporal telomere attrition rate that adjusts for regression to the mean (RTM) [16,17]. To calculate within-individual D (see Box 1), we used initial TL (measured for all individuals at the age of 1 year) and the last TL available (at the last capture) for an individual (initial and final TL was log-transformed for normality in all analyses). We then fitted D as a response variable in a linear model and tested if this attrition rate was influenced by LRS. Apart from the focal LRS (the number of young fledged during an individual's life, defined as a fixed continuous variable), we included sex as a categorical fixed factor and TL at first sampling (invariably at age 1—to test if the relationship between D and LRS was influenced by initial TL) and maximum attained age (to check if the relation is not simply caused by older individuals having more fledglings) as fixed continuous covariates. Initially, nest ID (for birds sharing breeding pairs) and year of sampling were included as random effects in a mixed model approach, but variance for both terms was null, thus the terms were deemed redundant, allowing to simplify the main model (for details, see the electronic supplementary material). We began with a full model, and then we culled non-significant (p > 0.05) interaction terms (see the electronic supplementary material: R code). We removed one data point from the final analysis, i.e. a female with very high D (0.511); after performing model diagnostics, this point turned out to be a highly influential outlier (Cook's distance ∼ 1). This led to a final sample size of 23 males and 24 females. To test if TL influenced LRS, we fitted LRS as a response variable and we included TL at first sampling, maximum attained age and sex as fixed explanatory variables. Analyses were performed in R (v. 3.3.1) [18].
Box 1. How to calculate telomere attrition rate D, after [16].
To adjust each value for the regression to the mean, we applied an equation:
where
X1: initial TL (measured for all individuals at the age of 1 year); mean value of X1,
X2: last TL available (at the last capture); mean value of X2,
r: correlation between X1 and X2,
s: standard deviation,
s2: variance.
3. Results and discussion
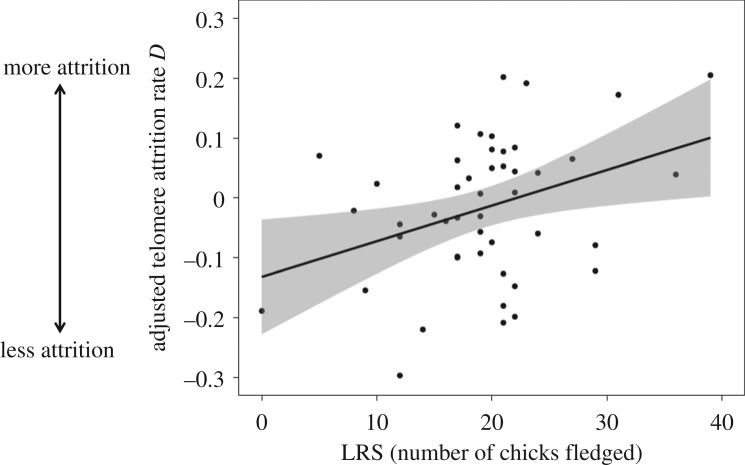
Here, we provide the first demonstration, to our knowledge, of a relationship between telomere dynamics and LRS. In accordance with our predictions, birds that produced more fledglings in their life experienced faster telomere loss (table 1 and figure 1). TL at first sampling was not related to LRS (F1,42 = 0.134, p = 0.717, see electronic supplementary material, table S1), suggesting that telomere erosion rather than TL may serve as a better proxy for overall fitness, at least in our study system. Maximum attained age was highly correlated with LRS (F1,20 = 42.376, p < 0.001, see electronic supplementary material, table S1) and did not influence telomere loss (F1,42 = 0.003, p = 0.957, electronic supplementary material, table S2). To avoid collinearity of the two variables, we then excluded age from the final model (table 1). Excluding age improved the fit of our model (AIC of a model including age = 121.82, and of the model without age = 119.82). We interpret the correlation between telomere shortening rate and LRS as yet more support for the hypothesis that reproduction may have consequences in terms of telomere erosion. As demonstrated in experimental studies [10,11], telomere shortening possibly reflects cumulative DNA damage caused by high reproductive effort. Elevated reproduction may cause telomere shortening owing to faster cellular turnover and overproduction of telomere-damaging free radicals by increasing metabolic rate ([19,20], but see [21,22]), but also divert resources from telomere maintenance, i.e. telomerase activity or antioxidant protection [1,23]. Both mechanisms could underlie the observed positive association of investment in LRS and telomere loss in the blue tit. This relation can be observed similarly in both sexes, as indicated by the non-significant interaction of sex by LRS (F1,42 = 1.270, p = 0.266). In blue tits, both parents perform parental care such as chick provisioning and nest sanitation [24], and there are no apparent sex-specific differences in workload. Even though larger reproductive investment could be expected in females, as they produce and incubate eggs, potential negative effects of this additional energetic burden on telomeres could be counteracted by better protection and reparation mechanisms enhanced by the action of oestrogen [25,26].
Table 1.
Final linear model explaining the effects of lifetime reproductive success (LRS), sex and telomere length at first sampling (TL1, at age 1) on RTM-corrected telomere attrition rate D. Significant fixed effects (p < 0.05) marked in bold. Estimates for continuous variables are shown after Z-score scaling.
| fixed effects | estimates ± s.e. | d.f. | F | p (>F) |
|---|---|---|---|---|
| intercept | −0.159 ± 0.170 | 1, 43.0 | 0.874 | 0.355 |
| LRS | 0.293 ± 0.132 | 1, 43.0 | 4.941 | 0.032 |
| sex | 0.136 ± 0.251 | 1, 43.0 | 0.292 | 0.592 |
| TL1 | −0.022 ± 0.121 | 1, 43.0 | 0.034 | 0.854 |
Figure 1.
Within-individual telomere attrition rate D [change in the relative telomere length: the ratio (T/S), telomere copy number (T) to single control gene copy (S)] adjusted for regression to the mean in relation to lifetime reproductive success (LRS, number of fledged young within an individual's lifespan). Raw data shown. The line is the best-fit linear regression through the data ± 95%. CI in grey.
To summarize, we observed that high LRS is associated with pronounced telomere loss. It is important to note that we did not measure reproductive effort in this study, to which costs of reproduction are usually referred. Given the correlative nature of our study, we cannot infer causality, but we demonstrate that more fecund parents can possibly bear consequences in terms of faster telomere attrition. Pronounced telomere attrition, as a measure of somatic damage rate, can potentially relate to impaired survival prospects, which could help to reveal where the long-studied cost of reproduction actually lies. However, in the studied blue tit population, we do not observe any effects of the pronounced shortening on subsequent survival [11]. It is possible that in short-lived birds with high extrinsic mortality rates, such as the blue tits, telomere attrition does not entail shortened lifespan. In seasonal breeders, investment in reproduction, rather than in self-maintenance, appears to be an optimal strategy. In the light of our results, investigating the relationship between LRS and telomere attrition and its consequences in long-lived animals and/or opportunistic breeders could be extremely stimulating for life-history studies.
Supplementary Material
Acknowledgements
We thank Anna Dubiec and Edyta Podmokła for helping with sample collection.
Ethics
All applicable institutional and/or national guidelines for the care and use of animals were followed. Permit number: dnr 37-15 issued by Jordbruksverket, Sweden.
Data accessibility
Data, including R codes, are available in the online electronic supplementary material, and via the Dryad Digital Repository: https://doi.org/10.5061/dryad.m073h5q [27].
Authors' contributions
J.S. conceived the idea; A.A., S.M.D., M.C. and J.S. collected the samples; J.S. performed laboratory analyses and analysed the data; L.G. provided logistical support in the field; J.S. led the writing of the manuscript. All authors contributed critically to the drafts, agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This research was financed by the grant of the Polish National Science Centre no. DEC-2013/09/N/NZ8/03211 awarded to J.S.
References
- 1.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 2.Blackburn EH. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. 579, 859–862. ( 10.1016/j.febslet.2004.11.036) [DOI] [PubMed] [Google Scholar]
- 3.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F.. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 4.Boonekamp JJ, Mulder G, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol. Lett. 11, 20140938 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 7.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ, Nussey DH. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 ( 10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichert S, Stier A, Zahn S, Arrivé M, Bize P, Massemin S, Criscuolo F. 2014. Increased brood size leads to persistent eroded telomeres. Front. Ecol. Evol. 2, 1–11. ( 10.3389/fevo.2014.00009) [DOI] [Google Scholar]
- 11.Sudyka J, Arct A, Drobniak SM, Dubiec A, Gustafsson L, Cichoń M. 2014. Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J. Evol. Biol. 27, 2258–2264. ( 10.1111/jeb.12479) [DOI] [PubMed] [Google Scholar]
- 12.Charnov EL, Warne R, Moses M. 2007. Lifetime reproductive effort. Am. Nat. 170, E129–E142. ( 10.1086/522840) [DOI] [PubMed] [Google Scholar]
- 13.Przybylo R, Sheldon BC, Merilä J. 2000. Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J. Anim. Ecol. 69, 395–403. ( 10.1046/j.1365-2656.2000.00401.x) [DOI] [Google Scholar]
- 14.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 15.Cawthon RM. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 ( 10.1093/nar/30.10.e47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly C, Price TD. 2005. Correcting for regression to the mean in behavior and ecology. Am. Nat. 166, 700–707. ( 10.1086/497402) [DOI] [PubMed] [Google Scholar]
- 17.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur. J. Epidemiol. 28, 859–866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org/. [Google Scholar]
- 19.Deerenberg C, Pen I, Dijkstra C, Arkies BJ, Visser GH, Daan S. 1995. Parental energy expenditure in relation to manipulated brood size in the European kestrel Falco tinnunculus. Zool. Jena 99, 39–48. [Google Scholar]
- 20.Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. 2004. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 7, 363–368. ( 10.1111/j.1461-0248.2004.00594.x) [DOI] [Google Scholar]
- 21.Boonekamp JJ, Bauch C, Mulder E, Verhulst S. 2017. Does oxidative stress shorten telomeres? Biol. Lett. 13, 20170164 ( 10.1098/rsbl.2017.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudyka J, Casasole G, Rutkowska J, Cichoń M. 2016. Elevated reproduction does not affect telomere dynamics and oxidative stress. Behav. Ecol. Sociobiol. 70, 2223–2233. ( 10.1007/s00265-016-2226-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiersma P, Selman C, Speakman JR, Verhulst S. 2004. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. B 271, S360–S363. ( 10.1098/rsbl.2004.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bańbura J, Perreti P, Blondeli J, Sauvages A, Galan M, Lambrechts MM. 2001. Sex differences in parental care in a Corsican Blue Tit Parus caeruleus population. Ardea 89, 517–526. [Google Scholar]
- 25.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. 1999. Estrogen activates telomerase. Cancer Res. 59, 5917–5921. [PubMed] [Google Scholar]
- 26.Sengupta S, Wasylyk B. 2004. Physiological and pathological consequences of the interactions of the p53 tumor suppressor with the glucocorticoid, androgen, and estrogen receptors. Ann. N. Y. Acad. Sci. 1024, 54–71. ( 10.1196/annals.1321.005) [DOI] [PubMed] [Google Scholar]
- 27.Sudyka J, Arct A, Drobniak SM, Gustafsson L, Cichoń M. 2019. Data from: Birds with high lifetime reproductive success experience increased telomere loss Dryad Digital Repository. ( 10.5061/dryad.m073h5q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sudyka J, Arct A, Drobniak SM, Gustafsson L, Cichoń M. 2019. Data from: Birds with high lifetime reproductive success experience increased telomere loss Dryad Digital Repository. ( 10.5061/dryad.m073h5q) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data, including R codes, are available in the online electronic supplementary material, and via the Dryad Digital Repository: https://doi.org/10.5061/dryad.m073h5q [27].