Abstract
Wetland soils are globally important carbon stores, and natural wetlands provide a sink for atmospheric carbon dioxide (CO2) through ongoing carbon accumulation. Recognition of coastal wetlands as a significant contributor to carbon storage (blue carbon) has generated interest into the climate change mitigation benefits of restoring or recreating saltmarsh habitat. However, the length of time a re-created marsh will take to become functionally equivalent to a natural (reference) system, or indeed, whether reference conditions are attainable, is largely unknown. Here, we describe a combined field chronosequence and modelling study of saltmarsh carbon accumulation and provide empirically based predictions of changes in the carbon sequestration rate over time following saltmarsh restoration. Carbon accumulation was initially rapid (average 1.04 t C ha−1 yr−1 during the first 20 years), slowing to a steady rate of around 0.65 t C ha−1 yr−1 thereafter. The resulting increase in C stock gave an estimated total C accumulation of 74 t C ha−1 in the century following restoration. This is approximately the same as our observations of natural marsh C content (69 t C ha−1), suggesting that it takes approximately 100 years for restored saltmarsh to obtain the same carbon stock as natural sites.
Keywords: carbon sequestration, managed realignment, blue carbon, coastal wetlands, climate change mitigation
1. Introduction
Wetland soils provide a major global store of carbon, and natural wetlands provide a sink for atmospheric carbon dioxide (CO2) through ongoing carbon accumulation. There is increasing interest in both protecting existing wetland carbon stores from degradation (avoided CO2 emissions) and in either enhancing or re-establishing the carbon sink function of wetlands (so-called negative emissions) via altered land management and wetland restoration strategies [1]. While much of the focus to date has been on inland organic soils (peatlands), increasing attention is now being paid to other wetland types, notably coastal wetlands.
To date, the study of coastal wetlands as carbon stores has addressed their sustainability and resilience to continued exploitation and modification [2]. Threats to coastal wetland ecosystems include reclamation of saltmarsh and mangrove forest for agriculture, dredging and pollution of seagrass beds, and loss of coastal habitat to port development and sea defences. Approximately 50% of saltmarsh has been degraded or even lost worldwide [3] with a further 30–40% loss expected over the next 100 years [4]. The recognition of rates of habitat and carbon loss, and of potential ‘blue’ carbon sequestration (and sea defence) benefits from reversing this process, have generated interest in the restoration and/or recreation of coastal wetlands. For saltmarsh, this primarily occurs via ‘managed realignment’: the landward retreat of coastal defences and subsequent tidal inundation of previously reclaimed agricultural land [5].
The restoration of biological and physical characteristics can be a slow process [6], but restored sites in the UK are at most 24 years old, insufficient to determine how long a re-created marsh will take to become functionally equivalent to a natural (reference) system, or indeed whether reference conditions are attainable. However, several formerly reclaimed saltmarshes in the UK have been accidentally breached during storms, providing long-term analogues for saltmarsh restoration. A previous study [7] estimated that restored sites would attain equivalent soil carbon pools to natural saltmarshes approximately 100 years after creation; however, this estimate was based on a single location, and required extrapolation of carbon stock increases from a site that had only been restored 15 years previously. Consequently, both the spatial consistency and the long-term trajectory of carbon stock change in restored saltmarshes remain uncertain.
Here, we describe a combined field chronosequence and modelling study of saltmarsh carbon accumulation, in order to determine the trajectory and timescale of habitat recovery, and provide empirically based predictions of changes in the rate of carbon sequestration over time following saltmarsh restoration.
2. Material and methods
In October 2011, we sampled saltmarshes in Eastern England, representing a chronosequence from 16 to 114 years since restoration of tidal flow. The experimental design consisted of nine sites, 3 × 16–20, 3 × 58–66 and 3 × 114 years since restoration. Natural saltmarsh at all nine sites was also sampled, along with adjacent unrestored agricultural fields where access permitted (four sites). There is no active management or livestock grazing on any of the saltmarsh study sites. The restored sites were used as agricultural fields prior to restoration of tidal flow. Further site information is available in [8] and the electronic supplementary material. At each saltmarsh site, soil cores (4 cm diameter, 30 cm depth) were taken from four locations in permanently vegetated marsh above 1.5 m OD. The methods used for soil property measurements are given in the electronic supplementary material.
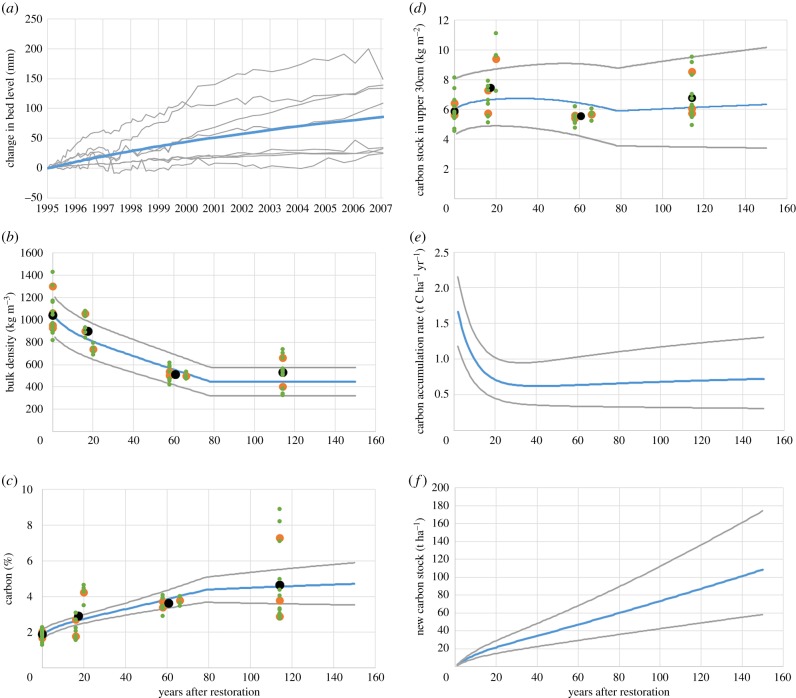
Carbon accumulation in restored saltmarsh was modelled by first fitting a standard curve to the mean of eight sets of elevation change measurements (using sediment erosion bars) made over a 12-year period at the Tollesbury managed realignment site. The fitted curve (figure 1a) comprised an exponential component (representing a gradually declining growth rate following sea-defence breaching) and a linear component (representing ongoing growth of natural saltmarsh due to isostatic changes in relative sea level for the study region). This growth rate was extrapolated to 150 years after restoration. We defined initial soil %C and bulk density (BD) from agricultural field samples, and ‘grew’ the saltmarsh soil above the field surface according to the growth curve. We assumed that new saltmarsh soil had the same BD as our natural saltmarsh sampling sites, and that saltmarsh %C would converge exponentially towards the natural saltmarsh value. The modelling accounts for the combined field and saltmarsh sediment in the 30 cm cores within restored sites, estimating total stock within the core, rather than differentiating between carbon sources (figure 1d). The model then goes a step further by estimating the depth of newly deposited sediment per year and therefore estimates the amount of ‘new’ carbon by using the data for field and natural saltmarsh proportionately. Figure 1f shows the accumulation of this ‘new’ carbon only (without any of the underlying relic land surface) over time.
Figure 1.
(a) Transect data (over 1.5 m OD) for Tollesbury managed realignment site, including the fitted curve (blue line). Outputs of C accumulation model (b–f). Blue lines are model predictions. Grey lines provide an indication of uncertainty in predictions. Black dots are age group means, orange dots are site means and green dots are individual sample results. (f) ‘New’ carbon = carbon accumulated in the sediment above the original land surface.
3. Results
We observed changes in all soil properties consistent with the age of site (table 1). Mean soil conductivity, moisture content, below ground biomass and %C all increased from field, to restored sites of increasing age, to natural saltmarsh, while BD decreased with age. Natural marshes had approximately six times more below ground biomass than the youngest managed realignment sites, and double the %C.
Table 1.
Soil properties. Means ± standard deviation.
| years since restoration |
|||||
|---|---|---|---|---|---|
| field | 16–20 | 58–66 | 114 | natural | |
| soil conductivity (mS cm−1) | 0.1 ± 0.1a | 12.1 ± 2.8 | 25.8 ± 3.4 | 29.3 ± 9.4 | 33.9 ± 15.7 |
| moisture content (%) | 18.0 ± 0.7 | 31.4 ± 5.9 | 49.9 ± 3.4 | 50.2 ± 7.9 | 56.0 ± 8.3 |
| bulk density (g cm−3) | 1.0 ± 0.2 | 0.90 ± 0.14 | 0.51 ± 0.06 | 0.53 ± 0.13 | 0.45 ± 0.13 |
| below ground biomass (kg m−2 to 30 cm depth) | 0.1 ± 0.2a | 0.61 ± 0.5 | 1.3 ± 0.8 | 2.4 ± 2.0 | 3.8 ± 2.7 |
| C (%) | 1.9 ± 0.3 | 2.9 ± 1.1 | 3.6 ± 0.4 | 4.7 ± 2.2 | 5.8 ± 2.7 |
| carbon content, 0–30 cm (kg m−2) | 5.9 ± 1.0 | 7.5 ± 1.8 | 5.6 ± 0.5 | 6.8 ± 1.5 | 6.9 ± 1.4 |
aTollesbury site data only.
There was no clear relationship between age and 0–30 cm (topsoil) carbon stock, with the youngest restored sites having the highest stocks and the intermediate (58–66 years) sites having the lowest. Topsoil C stock was similar to a natural marsh after 114 years of saltmarsh development. Because the younger restored sites still have relic field soil within 30 cm of the surface, topsoil C stock is not a particularly meaningful indicator of true C accumulation rates; however, we were able to estimate this using the saltmarsh growth curve within the C accumulation model. The fitted model (figure 1b–d) was able to capture much of the observed variation in topsoil BD, %C and C stock across the chronosequence. Topsoil BD declines steadily to around 80 years, at which point the entire upper 30 cm of soil comprises new saltmarsh material (according to the saltmarsh growth curve) and no further change occurs. The trajectory of %C change is similar, but the fitted model predicts an ongoing slow increase beyond 80 years because %C in the 114-year sites was found to still be below that of the natural saltmarsh sites. The model was also able to capture some of the uneven variation in topsoil C stock as new (high %C, low BD) saltmarsh soil accumulates over (low %C, high BD) field soil.
While the observational data incorporate the (variable) contribution of relic field soils to upper 30 cm C stocks, the model can also be used to estimate ‘new’ C accumulation above this reference level (figure 1e,f). Modelled C accumulation was initially very rapid (average 1.04 t C ha−1 yr−1 during the first 20 years) after restoration, slowing to a fairly constant rate of around 0.65 t C ha−1 yr−1 thereafter (table 2). The resulting steady increase in C stock gave an estimated total ‘new’ C accumulation of around 73 t C ha−1 in the century following restoration.
Table 2.
Estimated C benefit of saltmarsh restoration over short (0–20 years), medium (20–50 years) and long term (50–100 years). Lower and upper estimates of uncertainty in brackets.
| timescale (years) | average C accumulation rate (t C ha−1 yr−1) | total new carbon stock (t C ha−1) |
||
|---|---|---|---|---|
| 0–20 | 1.04 | (0.70–1.41) | 21.5 | (14.6–29.0) |
| 20–50 | 0.64 | (0.38–0.97) | 40.7 | (25.9–58.0) |
| 50–100 | 0.65 | (0.33–1.08) | 73.4 | (42.5–112.3) |
4. Discussion
The estimated new carbon accumulation of around 74 t C ha−1 in the century following restoration is approximately the same as our observations of natural marsh C content (69 t C ha−1), further strengthening the 100-year time frame for restored sites to reach equivalency. Previous studies have estimated various timescales from approximately 65 years [9] to 100 years [7]. From the results of the soil variables measured here, there is a clear transition gradient from field, to restored sites of increasing age, to natural saltmarsh on an approximate 100 year timescale.
The global average carbon accumulation rate in saltmarsh sediments is 2.42 (±0.26) t C ha−1 yr−1, with a slightly higher estimate for Northern Europe of 3.15 (±0.63) t C ha−1 yr−1 [10], although there is no equivalent carbon loss rate calculated at a global scale. High plant productivity is the main contributor to these high rates of carbon sequestration with below ground production contributing directly to soil carbon stocks. Roots can be deeper than 1 m in saltmarshes and are not easily broken down in the waterlogged and anoxic conditions, contributing to long-term stores [11]. Plant diversity increases soil organic carbon storage [12] across a range of ecosystems in combination with climate, soil type and direct management. In natural saltmarshes, carbon stocks vary considerably with marsh maturity, geomorphology and environmental setting [13]. In restored marshes, it can take many decades for plant communities in restored marshes to resemble those of natural marshes, if at all [6,8]. This discrepancy is likely to be a constraint in restored marshes reaching functional equivalency with natural systems.
Models that link carbon sequestration to wetland management are needed to improve decision-making when considering options for shoreline management planning [14] and climate change mitigation. Current IPCC guidelines [15] for reporting carbon emissions associated with tidal marsh rewetting offer a ‘Tier 1’ default emission factor of −0.91 t C ha−1 yr−1 and suggest that country-specific ‘Tier 2’ factors be developed using empirical data where possible. A more sophisticated ‘Tier 3’ approach requires the development of process models that take account of changes over time and responses to different environmental factors. The simple model presented here could thus represent a first step towards a more sophisticated emission reporting methodology.
Although considerable recent attention and research have been devoted to the potential role of ‘blue carbon’ in climate mitigation, quantitative empirical data on rates of C accumulation following saltmarsh restoration remain scarce. Habitat creation and restoration of tidal flows will not necessarily result in ecosystems that behave or respond in the same way as natural systems, or in the reinstatement of pre-disturbance carbon stocks. However, the data presented here suggest that reconnecting former saltmarsh to the tide, either through managed realignment or through natural storm breaches, will eventually allow the land to return to conditions typical of a natural saltmarsh. Our data indicate that saltmarsh restoration can lead to an initially rapid and subsequently sustained accumulation of carbon, in large part due to CO2 uptake and carbon accumulation by saltmarsh plants growing on saturated soils. This supports the view that saltmarsh restoration can contribute to climate change mitigation, and this potential should be considered alongside other valuable ecosystem services, to enhance and inform future land management decisions.
Supplementary Material
Acknowledgements
The authors thank Kate Batchelor for below ground biomass measurements, James Tempest for bulk density analysis and Simon Oakley for %C analysis.
Data accessibility
Datasets are available from the Environmental Information Data Centre repository (www.eidc.ceh.ac.uk). The saltmarsh soil properties data [16]: https://doi.org/10.5285/0b1faab4-3539-457f-9169-b0b1fbd59bc2. The elevational change data [17]: https://doi.org/10.5285/f9513ece-a913-4774-8808-273dcf7ed0be.
Authors' contributions
A.B. carried out the fieldwork, participated in study design and data analysis, and drafted the manuscript; A.G. carried out the fieldwork, participated in study design and revised the manuscript. C.D.E. developed the saltmarsh growth model and contributed to drafting the manuscript. All authors agree to be held accountable for the content and approved the final version of the manuscript.
Competing interests
None of the authors of this manuscript has any competing interests relating to this article.
Funding
This work was funded as part of the NERC Centre for Ecology & Hydrology's Multi-functional land-use options project (C03463), with funding support from NERC (NE/R010846/1). This publication is a contribution to the C-SIDE (Carbon Storage in Intertidal Environments) project.
References
- 1.Paustian K, Lehmann J, Ogle S, Reay D, Robertson GP, Smith P. 2016. Climate-smart soils. Nature 532, 49–57. ( 10.1038/nature17174) [DOI] [PubMed] [Google Scholar]
- 2.Beaumont NJ, Jones L, Garbutt A, Hansom JD, Toberman M. 2014. The value of carbon sequestration and storage in coastal habitats. Estuar. Coast. Shelf Sci. 137, 32–40 ( 10.1016/j.ecss.2013.11.022) [DOI] [Google Scholar]
- 3.Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. 2011. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. ( 10.1890/10-1510.1) [DOI] [Google Scholar]
- 4.Pendleton L, et al. 2012. Estimating global ‘blue carbon’ emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7, e43542 ( 10.1371/journal.pone.0043542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbutt RA, Reading CJ, Wolters M, Gray AJ, Rothery P. 2006. Monitoring the development of intertidal habitats on former agricultural land after the managed realignment of coastal defences at Tollesbury, Essex, UK. Mar. Pollut. Bull. 53, 155–164. ( 10.1016/j.marpolbul.2005.09.015) [DOI] [PubMed] [Google Scholar]
- 6.Mossman HL, Davy AJ, Grant A. 2012. Does managed coastal realignment create saltmarshes with ‘equivalent biological characteristics' to natural reference sites? J. Appl. Ecol. 49, 1446–1456. ( 10.1111/j.1365-2664.2012.02198.x) [DOI] [Google Scholar]
- 7.Burden A, Garbutt RA, Evans CD, Jones DL, Cooper DM. 2013. Carbon sequestration and biogeochemical cycling in a saltmarsh subject to coastal managed realignment. Estuar. Coast. Shelf Sci. 120, 12–20. ( 10.1016/j.ecss.2013.01.014) [DOI] [Google Scholar]
- 8.Garbutt A, Wolters M. 2008. The natural regeneration of saltmarsh on formerly reclaimed land. Appl. Veget. Sci. 11, 335–344. ( 10.3170/2008-7-18451) [DOI] [Google Scholar]
- 9.Craft C, Megonigal P, Broome S, Stevenson J, Freese R, Cornell J, Zheng L, Sacco J. 2003. The pace of ecosystem development of constructed Spartina alterniflora marshes. Ecol. Appl. 13, 1417–1432. ( 10.1890/02-5086) [DOI] [Google Scholar]
- 10.Ouyang X, Lee SY. 2014. Updates estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11, 5057–5071. ( 10.5194/bg-11-5057-2014) [DOI] [Google Scholar]
- 11.Yu OT, Chmura GL. 2009. Soil carbon may be maintained under grazing in a St Lawrence Estuary tidal marsh. Environ. Conserv. 36, 312–320. ( 10.1017/S0376892910000184) [DOI] [Google Scholar]
- 12.Chen S, et al. 2018. Plant diversity enhances productivity and soil carbon storage. Proc. Natl Acad. Sci. USA 115, 4027–4032. ( 10.1073/pnas.1700298114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa AI, Lillebø AI, Pardal MA, Caçador I. 2010. The influence of Spartina maritima on carbon retention capacity in saltmarshes from warm-temperate estuaries. Mar. Pollut. Bull. 61, 215–223. ( 10.1016/j.marpolbul.2010.02.018) [DOI] [PubMed] [Google Scholar]
- 14.Hansen VD, Nestlerode JA. 2014. Carbon sequestration in wetland soils of the northern Gulf of Mexico coastal region. Wetl. Ecol. Manage. 22, 289–303. ( 10.1007/s11273-013-9330-6) [DOI] [Google Scholar]
- 15.Intergovernmental Panel on Climate Change (IPCC). 2014. 2013 Supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: wetlands (eds Hiraishi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG). Switzerland: IPCC. [Google Scholar]
- 16.Burden A, Garbutt A, Hughes S, Oakley S, Tempest JA. 2018. Soil biochemical measurements from saltmarshes of different ages on the Essex coast, UK (2011) NERC Environmental Information Data Centre; ( 10.5285/0b1faab4-3539-457f-9169-b0b1fbd59bc2) [DOI] [Google Scholar]
- 17.Garbutt A. 2018. Bed level change within the Tollesbury managed realignment site, Blackwater estuary, Essex, UK between 1995 and 2007 NERC Environmental Information Data Centre; ( 10.5285/f9513ece-a913-4774-8808-273dcf7ed0be) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets are available from the Environmental Information Data Centre repository (www.eidc.ceh.ac.uk). The saltmarsh soil properties data [16]: https://doi.org/10.5285/0b1faab4-3539-457f-9169-b0b1fbd59bc2. The elevational change data [17]: https://doi.org/10.5285/f9513ece-a913-4774-8808-273dcf7ed0be.