As health care costs continue to rise, greater is being placed on the assessment of appropriateness of cardiovascular (CV) diagnostic studies and on reserving the use of testing to patients who may derive additional benefits from it (1). At the same time, the role of CV testing for many patients with stable symptoms has been questioned (2) due to lack of data that such testing, or coronary revascularization in this setting, can improve outcomes, and the fact that most tests do no lead to any significant changes in patient management (3). These concerns, together with the expansion of testing to lower risk patients, has led to a recognition by payers, health care providers, and medical societies that better methods are needed to identify patients in whom testing can be safely deferred.
WHAT IS THE GOAL OF TESTING STABLE PATIENTS?
As detailed in the 2012 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the diagnosis and management of patients with stable ischemic heart disease, the main goal of testing is to initiate guideline-directed medical therapy and to consider coronary revascularization if it can improve symptoms or survival (4). An equally important goal of testing is to identify patients who are at low risk for coronary artery disease (CAD) and would therefore be unlikely to benefit from additional testing or procedures.
WHO ARE LOW-RISK PATIENTS?
Ideally, low-risk patients are ones whose risk of future events is low enough that testing would not reduce their risk further. However, there is no widely agreed upon definition of what constitutes “low risk” and no data on how to reliably identify such a group across different health care settings.
Most risk scores used to evaluate patients with stable chest pain were developed with the goal of estimating the likelihood of obstructive CAD. The first such score was developed by Diamond and Forrester (5) over 3 decades ago when the prevalence of CAD was significantly higher and is still recommended by current guidelines (4). Nevertheless, this score and others have been shown to overestimate the risk of obstructive CAD in contemporary cohorts (6,7). To address these limitations, a recalibrated score using the same variables (namely age, sex, and type of chest pain) has been derived from contemporary patients undergoing invasive angiography or coronary computed tomography angiography: the CAD consortium basic score (8). This score has been found to result in a greater proportion of patients who are classified as low risk and to offer improved discrimination for both obstructive CAD and for major adverse cardiac events (7,9). Consequently, the CAD consortium basic score is currently recommended by the European Society of Cardiology (ESC) in its stable CAD guidelines (10).
More recently, the new National Institute for Health and Care Excellence (NICE) guidelines adopted a symptom-based strategy that does not rely on calculating a pre-test probability to identify low-risk patients who should not undergo further testing (11,12). According to these guidelines, no testing is indicated for patients who have nonanginal symptoms and a normal electrocardiogram.
Before considering the utility of these risk assessment tools, it is important to emphasize that the use of these scores is only needed when there is uncertainty regarding the role of testing. Testing should not be performed when there is a clear alternative explanation for a patient’s symptoms or when testing is unlikely to result in any change to therapy. An additional option to consider when the role of testing is unclear is coronary artery calcium scoring. When negative (coronary artery calcium score = 0), it can identify low-risk patients better than any risk score that has been identified to date (13) and may represent an effective “gate-keeper” for further testing among low- to intermediate-risk patients.
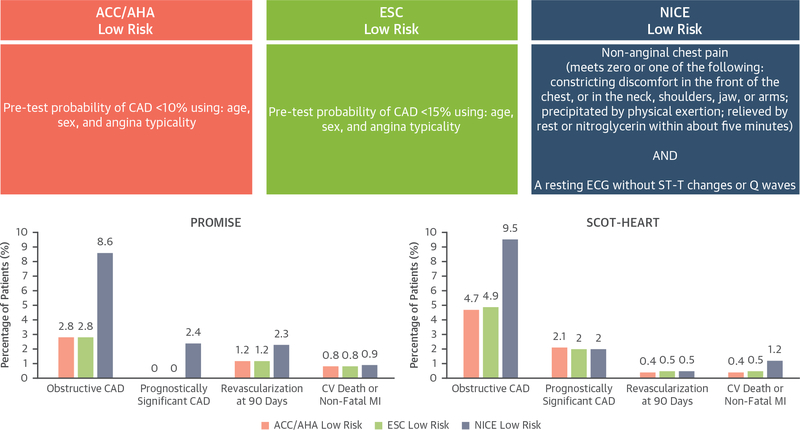
In this issue of iJACC, Adamson et al. (14) compared the efficiency and safety of the preceding 3 guidelines-based definitions of low risk in the 2 largest prospective trials of coronary computed tomography angiography for stable symptoms performed to date: the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) and the SCOT-HEART (Scottish Computed Tomography of the Heart) studies. A summary of how low risk was defined by each strategy, and the ensuing outcomes of these groups, is shown in Figure 1.
FIGURE 1. Definitions of Low-Risk Categorization and Patient Outcomes.
Definitions of low-risk categorization according to the American College of Cardiology/American Heart Association (ACC/AHA), European Society of Cardiology (ESC), and National Institute of Health and Care Excellence (NICE) guidelines and patient outcomes. CAD = coronary artery disease; CV = cardiovascular; ECG = electrocardiogram; MI = myocardial infarction; PROMISE = Prospective Multicenter Imaging Study for Evaluation of Chest Pain; SCOT-HEART = Scottish Computed Tomography of the Heart.
This study has several notable findings. First, regardless of the guideline used to identify low-risk patients, the prevalence of obstructive CAD—defined as ≥70% area stenosis in any major epicardial vessel, or ≥50% in the left main by coronary computed tomography angiography—was <10% in all groups, with a prevalence of prognostically significant CAD (defined as 3-vessel disease, 2-vessel disease including the proximal left anterior descending artery, or obstructive disease involving the left main) or the need for subsequent coronary revascularization at 90 days occurring far less frequently. Second, the rate of CV death or nonfatal myocardial infarction in the subsequent 1 (PROMISE) to 3 (SCOT-HEART) years was extremely low: <1% in most cases, but 1.2% for the low-risk group identified by the NICE guidelines in the SCOT-HEART group. Third, the symptom-focused NICE strategy identified a larger group of low-risk patients in both trials than the probability-based strategies endorsed by the ACC/AHA and ESC guidelines. Specifically, the proportions of patients identified as low risk by the ACC/AHA, ESC, and NICE guidelines, respectively, were 2.5%, 2.5%, and 10% within PROMISE, and 14.0%, 19.8%, and 38.4% within SCOT-HEART.
In both trial cohorts, low-risk identification was associated with a negative predictive value for coronary revascularization of >0.97 regardless of the strategy used. The negative predictive value for CV death/nonfatal myocardial infarction in a combined analysis of both trial cohorts was lower when applying the NICE strategy than either the ACC/AHA or ESC strategies, but it remained >0.98 for each of the 3 strategies.
Due to the low event rate in the various low-risk groups compared, the study by Adamson et al. (14) lacks the power to show whether any particular strategy may be safer. However, it appears that the rate of CV death or nonfatal myocardial infarction were numerically higher in the low-risk group identified by the NICE guidelines; perhaps not an unexpected finding given the substantially larger size of that group. This finding serves as a reminder that identifying a larger low-risk group (i.e., “better efficiency”) will ultimately result in a trade-off with respect to identifying the safest low-risk group (i.e., “better safety”).
Whereas the results of this study reaffirm the importance of history-taking, there are certainly questions regarding how symptoms were interpreted in PROMISE and SCOT-HEART and whether age and sex have a role in pre-test risk assessment once nonanginal symptoms are identified. For instance, nonanginal symptoms were less frequent in PROMISE than in SCOT-HEART (11% vs. 41%), and thus fewer patients were identified as low risk in this study. Yet the rate of obstructive CAD and the event rates were higher in the SCOT-HEART study, which Adamson et al. (14) suggest may be due to a higher rate of patients with non-chest pain symptoms (e.g., dyspnea, fatigue) in the PROMISE study. In clinical practice, such nonspecific symptoms can represent a true anginal equivalent, and thus over-relying on the actual presence of chest pain may not be appropriate in all cases. Similarly, patients’ chest pain symptoms may not always be consistently brought on with activities of daily living, and the performance of an exercise treadmill test for the majority of patients in the SCOT-HEART study may have improved the detection of anginal symptoms.
Another important observation from this study in iJACC that the investigators did not highlight is that 100% of the patients identified as low risk by the ACC/AHA and ESC strategies in the PROMISE trial cohort were women. In the SCOT-HEART trial cohort, 88.1% and 97.2% of the low-risk patients were women by the ACC/AHA and ESC criteria, respectively. The NICE strategy, which does not consider age or sex, had a much more even sex distribution: 56.3% and 46.2% of the low-risk patients were women in the PROMISE and SCOT-HEART trial cohorts, respectively. The results of the ACC/AHA and ESC categorization strategies would suggest that almost all men presenting with chest pain, regardless of typicality, should be considered as candidates for noninvasive testing. However, a study from 2013 that followed patients with suspected CAD and/or angina and at least 1 prior abnormal cardiac test result who underwent their first angiogram found that symptoms were very similar among men and women with obstructive CAD (15). By removing sex from risk stratification, the NICE strategy may help to diminish the notions that women’s symptoms should be handled differently and that atypical symptoms in women, but not men, should not warrant further testing.
An important limitation of this study is the relatively short follow-up time in each trial. Patients were only followed up to 1 year in PROMISE and up to 3 years in SCOT-HEART for fatal and nonfatal CV events. Another is the focus on identifying obstructive CAD. It is now well-known that the identification of extensive nonobstructive atherosclerotic plaque can be prognostically important (16), may occur more commonly in women (17), and is actionable if patients are not on medical therapy (18). Thus, future studies should also assess how these risk assessment techniques compare in identifying patients who have no plaque and no stenoses.
Although additional prospective studies are needed regarding the clinical implications of these risk scores, how they compare to other approaches such as coronary artery calcium testing, and how often patients return with persistent symptoms when testing is deferred, Adamson et al. (14) should be congratulated on providing helpful data on how to identify low-risk patients in whom further testing can be deferred. Their work highlights the potential benefits, challenges, and trade-offs of performing risk assessments in stable patients with suspected CAD, and as such, they should also be commended for encouraging us all to place more emphasis on the history and characterization of chest pain and perhaps less emphasis on probabilistic risk equations.
Acknowledgments
Dr. Divakaran is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Editorials published in JACC: Cardiovascular Imaging reflect the views of the authors and do not necessarily represent the views of JACC: Cardiovascular Imaging or the American College of Cardiology.
REFERENCES
- 1.Shaw LJ, Blankstein R, Jacobs JE, et al. Defining quality in cardiovascular imaging: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging 2017;10:e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad V, Cheung M, Cifu A. Chest pain in the emergency department: the case against our current practice of routine noninvasive testing. Arch Intern Med 2012;172:1506–9. [DOI] [PubMed] [Google Scholar]
- 3.Hachamovitch R, Nutter B, Hlatky MA, et al. , for the SPARC Investigators. Patient management after noninvasive cardiac imaging results from SPARC (Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease). J Am Coll Cardiol 2012;59: 462–74. [DOI] [PubMed] [Google Scholar]
- 4.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–164. [DOI] [PubMed] [Google Scholar]
- 5.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–8. [DOI] [PubMed] [Google Scholar]
- 6.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry (CONFIRM). Circulation 2011; 124:2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond and Forrester score: the Partners Registry. Circulation 2016;134:201–11. [DOI] [PubMed] [Google Scholar]
- 8.Genders TS, Steyerberg EW, Alkadhi H, et al. , for the CAD Consortium. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J 2011; 32:1316–30. [DOI] [PubMed] [Google Scholar]
- 9.Baskaran L, Danad I, Gransar H, et al. A comparison of the updated Diamond-Forrester, CAD Consortium, and CONFIRM history-based risk scores for predicting obstructive coronary artery disease in patients with stable chest pain: the SCOT-HEART coronary CTA cohort. J Am Coll Cardiol Img 2018. April 13 [DOI] [PubMed] [Google Scholar]
- 10.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence. Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin Clinical Guideline 95 London, UK: NICE; 2016. [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin Clinical Guideline 95 London, UK: NICE; 2010. [Google Scholar]
- 13.Budoff MJ, Mayrhofer T, Ferencik M, et al. , for the PROMISE Investigators. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136:1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamson PD, Newby DE, Hill CL, Coles A, Douglas PS, Fordyce CB. Comparison of international guidelines for assessment of suspected stable angina: Insights from the PROMISE and SCOT-HEART. J Am Coll Cardiol Img 2018;11:1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreatsoulas C, Shannon HS, Giacomini M, Velianou JL, Anand SS. Reconstructing angina: cardiac symptoms are the same in women and men. JAMA Intern Med 2013;173:829–31. [DOI] [PubMed] [Google Scholar]
- 16.Chow BJ, Small G, Yam Y, et al. , for the CONFIRM Investigators. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) registry. Arterioscler Thromb Vasc Biol 2015;35:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulten E, Bittencourt MS, Singh A, et al. Coronary artery disease detected by coronary computed tomographic angiography is associated with intensification of preventive medical therapy and lower low-density lipoprotein cholesterol. Circ Cardiovasc Imaging 2014;7:629–38. [DOI] [PubMed] [Google Scholar]