Abstract
Purpose:
Sepsis damages the endothelial glycocalyx, contributing to fluid extravasation, organ injury, and death. Our goal was to determine if syndecan-1 level is associated with the risk of intubation and modifying effect of intravenous fluids in these patients.
Methods:
Syndecan-1 was measured at enrollment in patients underdoing protocolized resuscitation for severe sepsis or septic shock. The primary outcome was difference in syndecan-1 based on subsequent intubation status, with in-hospital mortality and acute kidney injury serving as secondary outcomes. Logistic regression was performed to evaluate the effect of intravenous fluid volume on each outcome.
Results:
Syndecan-1 was measured in 175 patients. 22% met the primary outcome, 21% died, and 57% developed kidney injury. Syndecan-1 was non-significantly higher in intubated patients, and significantly higher in non-survivors and those with kidney injury. High syndecan-1 was defined as >240 ng/mL. Intravenous fluids did not differ significantly between high and low syndecan-1 groups. Fluid volume was not associated with intubation in patients with a low syndecan-1 level, but was associated with intubation in those with high syndecan-1 levels.
Conclusions:
Syndecan-1 is elevated in ED sepsis non-survivors. Patients with high syndecan-1 may represent a cohort at particular risk for intubation following large volume fluid administration.
Keywords: Sepsis, intravenous fluids, glycocalyx, endothelium, syndecan-1, risk stratification
Purpose
Despite recent data suggesting some improvement in severe sepsis mortality, the risk of death in severe sepsis still exceeds 20%.(1) One of the cornerstones of early sepsis resuscitation is the administration of intravenous crystalloid,.(2) with the goals of restoring intravascular fluid volume loss, increasing venous return to the heart, and ultimately increasing cardiac output. Numerous studies have investigated the value of physiologic measurements to predict fluid responsiveness. Static measurements such as central venous pressure(3) and pulmonary capillary wedge pressure(4) carry significant limitations and are of unclear value. Dynamic measurements such as stroke volume variation(5) and straight leg raise(6) testing demonstrate better test characteristics, but have limitations that prevent widespread adoption. Investigations related to these measurements are often limited by short-term hemodynamic outcomes, and rarely address patient centered outcomes. While fluid boluses can lead to temporary improvements in hemodynamic measurements, a proportion of crystalloid fluid diffuses into the extravascular space, a process exacerbated in sepsis secondary to endothelial damage.(7) This extravascular fluid may promote the development of acute respiratory distress syndrome and acute kidney injury. Thus, defining the relative risks and benefits of fluid resuscitation remains a critically important area of investigation.
Clinical data regarding the relative risks and benefits of intravenous fluids in sepsis remains mixed. Implementation of resuscitation protocols have been associated with both decreased mortality and increased volume of crystalloid administration,(8;9) though it is unclear whether changes in fluid management are responsible for improved outcomes. Data from three recent large randomized control trials of early quantitative resuscitation strategies suggest that the volume of crystalloids administered varies significantly internationally, with median volumes ranging from approximately 2.5 L in Australia(10) to 4 L in the United Kingdom(11) to 5 L in the United States.(12) Despite this variation, the three cohorts exhibited comparable mortality rates. In other settings, the FEAST trial found patients in low-resource environments with a high predominance of anemia have an increased risk of death following fluid bolus therapy, demonstrating the importance of clinical environment and specific patient factors when assessing this therapy.(13) Finally, clinical trial data suggest patients with acute lung injury benefit from a restrictive fluid strategy.(14) Taken together, the ideal volume and timing of crystalloid resuscitation in severe sepsis remains unclear.
The endothelium plays several physiologic functions; one of the most critical is the separation between the intravascular and extravascular spaces. Severe sepsis damages the endothelium.(15) In the intraluminal space, the endothelium is covered with glycocalyx composed of a number of proteins including syndecan-1.(16) The glycocalyx is hypothesized to be damaged prior to the endothelium itself, and may be an earlier and more sensitive indicator of injury.(15) Syndecan-1 levels are elevated in patients with sepsis, particularly non-survivors.(15;17;18) In this study, we wished to extend these studies by investigating the possibility that glycocalyx damage plays a direct pathophysiologic role in sepsis by predisposing patients to organ failure. We hypothesized that glycocalyx damage may contribute to increased extravascular fluid extravasation, observed clinically as an increase in the risk of intubation, that would be especially pronounced among patients receiving larger volumes of intravenous crystalloids.
Materials and Methods
Patient selection:
Patients from a single emergency department participating in a previously published multicenter clinical trial comparing two early resuscitation strategies for severe sepsis were enrolled. The study took place from 2007–2009.(19) The study was IRB approved and all patients or their surrogates gave informed consent. Inclusion criteria included age > 17 years, 2 or more systemic inflammatory response criteria, and either hypotension following a fluid challenge or lactate > 4 mmol/L. Patients were excluded if they or a surrogate could not provide informed consent, they had an absolute contraindication to central venous line placement, or they required emergent surgical intervention. All patients underwent a standardized resuscitation based on serial iterative steps of IV crystalloid to achieve central venous pressure, mean arterial pressure, and either Scvo2 ≥ 70% or lactate clearance goals. The protocol was continued until all end points were achieved or a maximum of 6 hours. The published results of this study showed a 6% (95% CI, −3% to 14%) in-hospital mortality difference favoring the lactate clearance group, confirming the primary hypothesis of noninferiority between the two strategies.(19)
Syndecan-1 measurements:
Blood was drawn at enrollment into EDTA tubes and processed immediately by centrifugation at 3000 g at 4 °C for 10 minutes. Aliquots were stored at −80 °C without any freeze-thaw cycles until the time of measurement. Investigators blinded to the clinical status of the patient measured syndecan-1 using ELISA (Abcam, Cambridge MA) in duplicate, and results were averaged. The lower limit of the detection for the test was 4.94 ng/mL.
Clinical outcomes:
All clinical data including demographics and therapies administrated, including volume of intravenous fluid (IVF) administered in the emergency department, were recorded prospectively. The primary outcome of this study was the difference in sydencan-1 levels among patients who did and did not require endotracheal intubation. Secondary outcomes included differences in syndecan-1 among in-hospital survivors and non-survivors, and those who did and did not develop acute kidney injury. Acute kidney injury (AKI) was defined as a creatinine increase of > 50% at any point during the hospitalization, consistent with the “Risk” component of the RIFLE criteria.(20) Timing of intubation and development of AKI was not collected prospectively, and the relationship of these outcomes to the time of blood draw was not available for this analysis.
Data analysis:
Simple descriptive statistics, chi-square and Fisher exact tests, and Wilcoxon rank sum were used to analyze the data as appropriate. A receiver operator characteristic (ROC) curve using intubation as the dependent variable was created and the area under the curve (AUC) was calculated. An optimum cutoff was chosen and patients were grouped into high and low syndecan-1 groups accordingly. In order to assess the potential of high syndecan-1 levels as a predictor variable, a multivariable logistic regression model was constructed using intubation as the dependent variable. Candidate variables for the model were chosen based on the results of the univariate analysis comparing patients who did and did not meet the primary outcome. Initial variables included those variables with a p-value < 0.1, and other variables logically expected to increase the risk of intubation (such as a history of chronic obstruction pulmonary disease). SOFA (sequential organ failure assessment) score(21) was not placed in the model as a whole due to the potential for colinarity, as the respiratory component of the SOFA score is highly reflective of the dependent variable of interest (intubation) and the central nervous system component is based on the Glasgow Coma Score, which may be artificially inaccurate in intubated patients secondary to sedative medications and has been shown to have low inter-rater reliability.(22) We forced the variable of intravenous fluid volume administered into the final logistic regression model. The model was refined using backwards stepwise elimination, maintaining variables with a p < 0.1. Model diagnostics included jackknife and bootstrap replications, which demonstrated similar results. The final model was checked for overfitting using the method of Hosmer and Lomeshow.
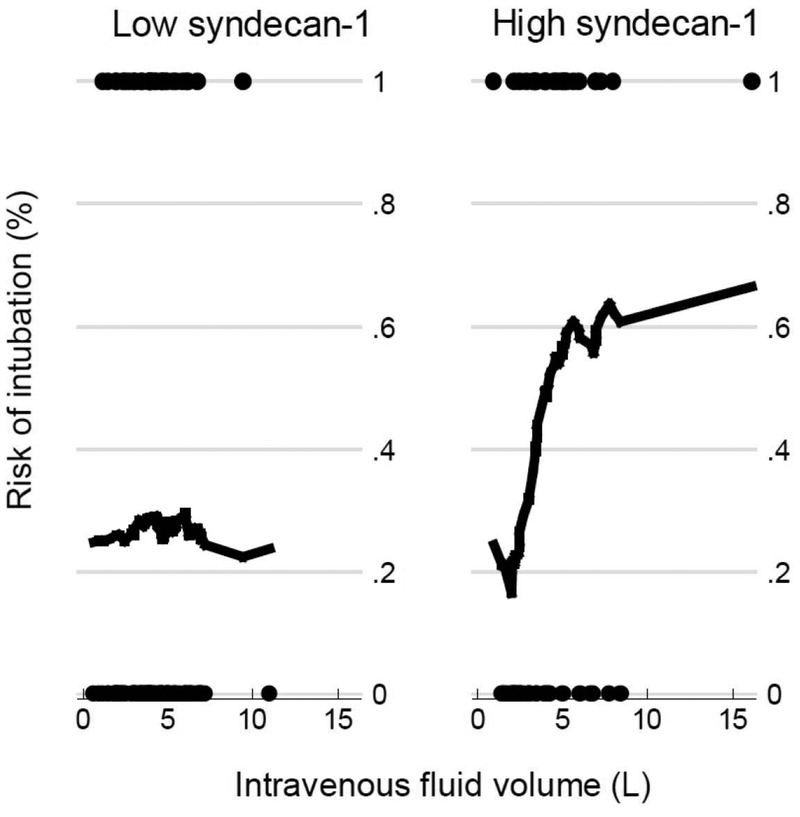
Finally, logistic regression of patients with high and low syndecan-1 levels was performed in order to evaluate the risk of intubation associated with the administration of IV fluid in each of these subgroups of patients. These data were visualized using the Lowess smoothing function and are presented in Figure 2. All analyses were conducted using STATA 10.0 (College Station, TX). Tests were 2-sided, and p-values ≤ 0.05 were considered significant.
Figures 2a and 2b:
Lowess smoothing plots demonstrating the relationship between intravenous fluid volume administered in the ED among patients with low and high syndecan-1 levels. There was no relationship between IVF volume and risk of intubation in the group with low syndencan-1 (p = 0.99) but a significantly higher risk of intubation in patients with a high syndecan-1 level (p < 0.05).
Results
One hundred ninety three patients were enrolled, and 175 had plasma available for analysis from the time of enrollment. Fifty two patients (22%) met the primary outcome of intubation and 34 (21%) died. One hundred (57%) met the study definition of AKI. Baseline demographics and clinical characteristics of the entire cohort and those meeting primary outcome are summarized in Table 1. Intubated patients tended to be older, were more likely to have dementia and require dialysis. They also had a higher severity of illness, had longer lengths of stay, and a higher mortality rate.
Table 1 -.
Baseline demographics and clinical characteristics of the entire cohort, and those meeting (n = 57) or not meeting (n = 118) the primary outcome of endotracheal intubation.
| All patients (n = 175) | Not intubated (n = 118) | Intubated (n = 57) | p-value | |
|---|---|---|---|---|
| Age; years (IQR) | 61 (48, 71) | 58 (46, 70) | 66 (54, 74) | 0.05 |
| Sex; n, (%) | ||||
| Male | 93 (53) | 64 (54) | 29 (51) | 0.68 |
| Female | 82 (47) | 54 (46) | 28 (49) | |
| Race n, (%) | ||||
| White | 94 (54) | 60 (49) | 34 (44) | 0.86 |
| African American | 78 (45) | 55 (47) | 23 (40) | |
| Asian | 2 (1) | 2 (2) | 0 (0) | |
| Other | 1 (1) | 1 (1) | 0 (0) | |
| Ethnicity; n, (%) | ||||
| Hispanic | 9 (5) | 8 (7) | 1 (2) | 0.16 |
| Non-hispanic | 166 (95) | 110 (93) | 56 (98) | |
| Past medical history; n, (%) | ||||
| Hypertension | 102 (58) | 63 (53) | 39 (68) | 0.06 |
| Congestive heart failure | 24 (14) | 17 (14) | 7 (12) | 0.70 |
| Dementia | 16 (9) | 4 (3) | 12 (21) | <0.01 |
| End-stage renal disease | 14 (8) | 13 (11) | 1 (2) | 0.03 |
| Chronic obstructive pulmonary disease | 32 (19) | 18 (15) | 14 (25) | 0.13 |
| Source; n (%) | ||||
| Pneumonia | 57 (33) | 35 (30) | 22 (39) | 0.24 |
| Urinary | 47 (27) | 33 (28) | 14 (26) | 0.63 |
| Intraabdominal | 30 (17) | 20 (17) | 10 (18) | 0.92 |
| Skin/Soft tissue | 15 (8) | 8 (7) | 7 (12) | 0.22 |
| Other | 26 (29) | 22 (19) | 4 (7)23 | 0.05 |
| Labs (enrollment) | ||||
| White blood count; cells/mm3 (IQR) | 12.6 (8.1, 17.7) | 12.4 (8.2, 17.3) | 12.8 (6.8, 20.0) | 0.57 |
| Platelets; cells/mm3 (IQR) | 199 (133, 280) | 199 (136, 284) | 197 (106, 271) | 0.25 |
| Hemoglobin; mg/dL (IQR) | 11.6 (10, 13.4) | 11.5 (10.2, 13.2) | 11.9 (9.8, 13.6) | 0.92 |
| Creatinine; mg/dL (IQR) | 1.8 (1.2, 2.9) | 1.5 (1.0, 2.8) | 2.1 (1.5, 3.4) | 0.03 |
| Total Bilirubin; mg/dL (IQR) | 0.9 (0.6, 1.6) | 0.9 (0.6, 1.8) | 1.0 (0.5, 1.5) | 0.97 |
| SOFA score; n (IQR) | 7 (4, 9) | 6 (4, 8) | 10 (8, 12) | <0.01 |
| Therapies | ||||
| Total intravenous fluids; L (IQR) | 4.0 (2.6, 5.1) | 4.0 (2.5, 5.0) | 4.1 (3.3, 5.3) | 0.14 |
| Outcomes | ||||
| ICU length of stay; days (IQR) | 3 (2, 7) | 2 (1, 4) | 8 (3, 12) | <0.01 |
| Hospital length of stay; days (IQR) | 8 (5, 13) | 7 (5, 11) | 11 (5, 19) | 0.03 |
| In-hospital mortality; n (%) | 36 (21) | 13 (11) | 23 (40) | <0.01 |
IQR - Interquartile range; SOFA – Sequential organ failure assessment
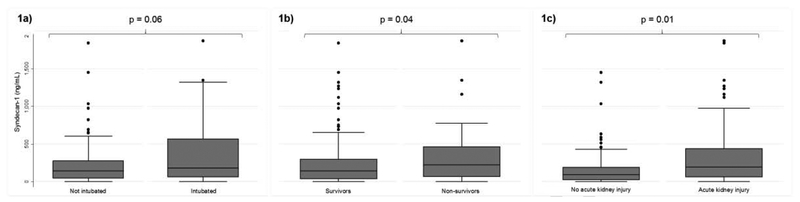
Median syndecan-1 levels were 152 ng/mL (IQR 49, 345), with a range from undetectable (<4.94 ng/mL; 14 patients) to 1870 ng/mL. Levels of syndecan-1 were non-significantly higher in intubated patients [181 ng/mL (IQR 61, 568) vs 141 ng/mL (IQR 46, 275); p = 0.06] and significantly higher in non-survivors [223 ng/mL (IQR 67, 464) vs 142 ng/mL (IQR 38, 294); p = 0.04] (Figures 1a and 1b). Syndecan-1 levels were significantly higher in patients with evidence of AKI [193 ng/mL (IQR 63, 441) vs 93 ng/mL (IQR 23, 187); p <0.001] (Figure 1c).
Figures 1a, 1b, and 1c:
Boxplots of syndecan-1 levels stratified by 1a) intubation status, 1b) survival status, and 1c) acute kidney injury. Syndecan-1 levels were non-significantly higher in patients who were later intubated (p = 0.06), significantly higher among non-survivors (p = 0.04), and significantly higher among patients with acute kidney injury (p = 0.01).
High syndecan-1 was significantly associated with intubation (OR 2.3, 95% CI 1.1–4.6) which remained a significant independent predictor in our final multivariable model (OR 2.7, 95% CI 1.3–5.6; p < 0.01). Other variables in the final model included history of hypertension (OR 2.4, 95% CI 1.1–5.0), dementia (10.5, 95% CI 3.0–36.1), and total IVF volume administered in the emergency department (OR 1.2 per L of fluid, 95% CI 1.0–1.5). These data are summarized in Table 3.
Table 3 -.
Summary of variables included in logistic regression model to predict intubation, with odds ratios and 95% confidence intervals.
| Low syndecan-1 (n = 117) | High syndecan-1 (n = 58) | P-value | |
|---|---|---|---|
| Age; years (IQR) | 61 (48, 71) | 62 (52, 71) | 0.74 |
| Sex; n, (%) | |||
| Male | 60 (51) | 33 (57) | 0.48 |
| Female | 57 (49) | 25 (43) | |
| Race n, (%) | |||
| White | 64 (55) | 30 (52) | 0.78 |
| African American | 50 (43) | 28 (48) | |
| Asian | 2 (2) | 0 (0) | |
| Other | 1 (1) | 0 (0) | |
| Ethnicity; n, (%) | |||
| Hispanic | 5 (4) | 4 (7) | 0.45 |
| Non-Hispanic | 113 (96) | 54 (93) | |
| Past medical history; n, (%) | |||
| Hypertension | 67 (57) | 35 (60) | 0.70 |
| Congestive heart failure | 18 (15) | 6 (10) | 0.36 |
| Dementia | 12 (10) | 4 (7) | 0.47 |
| End-stage renal disease | 9 (8) | 5 (9) | 0.83 |
| Chronic obstructive pulmonary disease | 21 (18) | 11 (19) | 0.87 |
| Source; n (%) | |||
| Pneumonia | 43 (37) | 14 (24) | 0.09 |
| Urinary | 32 (27) | 15 (26) | 0.83 |
| Intraabdominal | 16 (14) | 14 (24) | 0.08 |
| Skin / Soft tissue | 9 (8) | 6 (10) | 0.56 |
| Other | 17 (15) | 9 (16) | 0.86 |
| Labs (enrollment) | |||
| White blood count; cells/mm3 (IQR) | 12.7 (8.5, 17.0) | 12.8 (6.7, 20.6) | 0.59 |
| Platelets; cells/mm3 (IQR) | 213 (145, 294) | 167 (106, 245) | 0.02 |
| Hemoglobin; mg/dL (IQR) | 11.8 (10.4, 13.2) | 10.7 (9.6, 13.6) | 0.25 |
| Creatinine; mg/dL (IQR) | 1.7 (0.7, 2.8) | 2.0 (1.2, 3.0) | 0.20 |
| Total Bilirubin; mg/dL (IQR) | 0.8 (0.5, 1.4) | 1.1 (0.7, 2.7) | <0.01 |
| SOFA score; n (IQR) | 6 (4, 8) | 8.5 (6, 12) | <0.01 |
| Therapies | |||
| Total intravenous fluids; L (IQR) | 4.0 (3.0, 5.1) | 3.5 (2.4, 5.0) | 0.36 |
| Endotracheal intubation; n (%) | 31 (26) | 26 (45) | 0.01 |
| Outcomes | |||
| ICU length of stay; days (IQR) | 3 (1, 6) | 4 (2, 10) | 0.07 |
| Hospital length of stay; days (IQR) | 8 (5, 11) | 10 (5, 25) | 0.03 |
| In-hospital mortality; n (%) | 18 (15) | 18 (31) | 0.02 |
IQR - Interquartile range; SOFA – Sequential organ failure assessment
The area under the curve (AUC) to predict the primary outcome of intubation was 0.58 (95% CI 0.49, 0.68), indicating that syndecan-1 level alone is a poor predictor of the need for intubation. Based on the ROC analysis, high syndecan-1 levels were defined as >240 ng/mL, representing 58 (33%) patients. The associated sensitivity and specificity to predict the primary outcome of intubation at the cutoff of 240 ng/mL were 46% and 73%, respectively, demonstrating a fair predictive value at best in the cohort as a whole. Baseline demographics and clinical characteristics of patients with high and low syndecan-1 levels are summarized in Table 2. Patients with high levels tended to have a lower platelet count, higher bilirubin, and a higher SOFA score, and high levels were associated with an increased risk of intubation, a longer length of stay, and higher mortality.
Table 2-.
Demographics and clinical characteristics comparing patients with a low versus high syndecan-1 level.
| Variable | Odds ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Syndecan-1 level (high or low) | 2.71 | 1.33 | 5.55 | <0.01 |
| Intravenous fluid volume (per 1 L) | 1.24 | 1.02 | 1.48 | 0.02 |
| Past history of hypertension | 2.35 | 1.11 | 5.00 | 0.03 |
| Past history of dementia | 10.50 | 3.03 | 36.10 | <0.01 |
Median IVF volume administered in the emergency department was similar to contemporaneous cohorts of patients enrolled in clinical trials in the United States: 4.0 L (IQR 2.0, 5.3). Administered crystalloid volume did not differ significantly differ between intubated and non-intubated patients [4.1 L (IQR 3.3, 5.3) vs 4.0 L (IQR 2.5, 5.0 L); p = 0.14] nor did it differ between high and low syndecan-1 groups [4.0 L (IQR 3.3, 5.3) vs 3.5. L (IQR 2.4, 5.0); p = 0.36]. Crystalloids, predominantly normal saline, represented almost all (> 97%) of the volume administered. IVF volume was not associated with intubation in patients with a low syndecan-1 level (p = 0.99), but demonstrated a significant linear relationship with the risk of intubation in patients with high syndecan-1 levels (p = 0.045; Figure 2). Among patients with a high syndecan-1 level (n = 58, 33% of the cohort), those receiving > 4 L of intravenous fluid (n = 26) demonstrated an especially high risk of intubation versus those who received <4 L intravenous fluid. (61 vs 30%, p = 0.02). This is compared to an intubation rate of 26% of those patients with a low level. Similar stratified analyses using in-hospital mortality and acute kidney injury as the dependent variables did not demonstrate any significant relationship between intravenous fluid volume and the secondary outcomes of interest.
Discussion
In this study, we externally validated the finding that syndecan-1 levels are elevated in sepsis non-survivors as compared to survivors, supporting the hypothesis that glycocalyx damage is associated with adverse clinical outcomes. Whether glycocalyx damage plays a role in the pathophysiology of sepsis or is simply a marker of illness severity remains unclear. To further investigate this possibility, we evaluated the relationship between syndecan-1 and intubation. We found syndecan-1 levels are non-significantly elevated in patients requiring intubation in the cohort as a whole. However, when stratified by an empiric derived cutoff, we found a “high” syndecan-1 levels were significantly associated with an increased risk of intubation even after controlling for other confounding variables.
Several studies have previously investigated the prognostic and potential pathophysiologic roles of syndecan-1 measurements in human patients with sepsis. Ostrowski et al examined several cohorts of 321 total patients (no infection, local infection, sepsis, severe sepsis, and septic shock), and found increasing syndecan-1 levels across the cohorts as well as significant associations with the Sequential Organ Failure Assessment (SOFA) score. Furthermore, survival curves demonstrated significant differences between those patients with high and low levels at enrollment, consistent with our results. Taking these data further, two related studies by the same group in another cohort of patients demonstrated consistent findings with regard to higher levels of sydencan-1 in non-survivors, but interestingly an inverse correlation with fibrinolysis, a hypothesis that was not investigated further in our study. It is worth noting that levels of soluble thrombomodulin,(sTM) a deeper component of the glycocalyx, demonstrated similar relationships. Johansen et al directly compared both of these markers, syndecan-1 and sTM, in a cohort of over 1,100 critically ill patients (with a majority exhibiting sepsis). While both markers were significant predictors of mortality in their univariate analysis, only sTM remained a significant predictor in the adjusted model. Interestingly, the cutoff for the highest syndecan-1 quartile in the Johansen study was 240 ng/mL, identical to the cutoff utilized in our study, providing some external validity for our finding.
The most striking finding, and the novel aspect contributed by our study, was the interaction between intravenous fluid volume and the risk of intubation among patients with a high sydencan-1 level at enrollment. In this group, those who received a significant (> 4 L) volume of intravenous fluid twice as likely to require intubation (>60%) compared to patients receiving <4 L of fluids (30%). Taken together, these data raise the possibility that syndecan-1 may represent a prognostic biomarker to risk stratify patients at an increased risk of intubation following large volume fluid resuscitation, perhaps due to increased vascular permeability and extravascular fluid extravasation, though that hypothesis was not specifically tested in this study.
Despite its primacy in the earliest stages of sepsis resuscitation, the ideal role and volume of fluid resuscitation in patients with sepsis remains frustratingly unclear. While the adverse consequences of fluid resuscitation are becoming increasingly recognized,(23) they provide an immediate, widely available therapy that leads to, at a minimum, short-term improvement in physiologic indicators of hemodynamic function. What remains unclear is the ideal volume of fluid to administer to patients. Given the heterogeneity of the sepsis syndrome, it is unlikely a “one size fits all” approach will be efficacious, and any clinical trial directly comparing two different volumes of fluid resuscitation will likely lead to a null result. A targeted or ‘precision’ approach to identify patients particularly likely to derive benefit or harm from intravenous fluids may assist in development of personalized resuscitation strategies.
On one side of this clinical risk-benefit assessment, dynamic hemodynamic indices may help identify “fluid-responsive” patients more likely to benefit from fluid resuscitation. On the other hand, while clinicians may use past medical history such as renal or heart failure to hone the volume of administered fluid, objective measures to identify patients at the highest risk of harm remain scarce. Our data suggest that syndecan-1 may be a useful biomarker to identify a particularly high risk cohort of patients that might derive harm from large volume fluid resuscitation. Should these data be validated and rapid tests be developed, we could envision a clinical scenario where patients at low risk of intubation (as indicated by a low syndecan-1 level) are first resuscitated with fluids and transitioned to vasopressors only if they do not respond to fluid loading, while patients at high risk of intubation receive limited fluid resuscitation and undergo rapid central venous line placement and begin early vasopressor therapy. Such an approach could minimize the risk of unnecessary central venous line placement in patients more tolerant of intravenous fluids, while minimizing the harm of excessive fluid resuscitation in patients at higher risk of their adverse effects. Given the heterogeneity of the sepsis syndrome, we believe such risk-stratification may provide a path forward towards more personalized resuscitation strategies.
There are several limitations to this study that must be considered. First, this was a hypothesis generating study. We did not specifically test the hypothesis that patients with high sydecan-1 levels have increased levels of extravascular fluid, but rather relied on clinical surrogate outcomes such as intubation. Whether or not extravascular fluid extravasation proves to be the predominant pathophysiologic mechanism, the potential value of syndecan-1 as a risk-stratification tool remains. Second, the empiric cutoff derived in this study will bias the study results towards more dramatic findings. Therefore, external validation in a prospective cohort of patients is necessary. Similarly, there was a significant range of values on both groups, and while the differences between groups were statistically significant, the clinical utility of this test has yet to be established. Third, while all patients underwent a standardized resuscitation, it is possible that clinicians altered their strategy based on unmeasured variables, such as radiograph findings or albumin levels. However, given the very high rates of adherence in the parent study (with ~90% attainment of all of the targeted physiologic goals)(19), these results are likely still valid and generalizable. Fourth, while our definition of AKI is consistent with the risk stage of the RIFLE criteria, another definition may have yielded different results. Closely related to this issue, the timing of development of AKI and intubation times were unavailable, and we were unable to assess with temporal relationship between the exposure variable (syndecan-1 level) and the outcomes, which remains a topic for further investigation. Similarly, we cannot rule-out the possibility that other clinical factors besides extravascular lung water contributed to the need for intubation, such as sepsis-induced altered mental status or cardiovascular deterioration. Finally, the volumes of fluids administered in this study, while consistent with contemporaneous geographic cohorts, are higher than those administered for the same condition in the trials conducted in the United Kingdom and Australia, which could limit applicability of our results depending on local clinical practice. However, this threshold type effect may be important to consider in future studies comparing fluid usage between different practice environments.
Conclusion
Syndecan-1 levels are significantly elevated in ED sepsis non-survivors, those with acute kidney injury, and non-significantly higher among those requiring intubation. Patients with high syndecan-1 levels may represent a high-risk cohort at particular risk for intubation following large volume fluid administration. External validation of these findings in a prospective cohort is indicated.
Acknowledgements:
Dr. Puskarich has received salary support through K23GM113041–01 from the National Institute of General Medical Sciences/National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA, ePub ahead of print. 2014. [DOI] [PubMed] [Google Scholar]
- (2).Dellinger R, Levy M, Rhodes A, Annane D, Gerlach H, Opal SM et al. Surviving Sepsis Campaign: International Guidelines for the Management of Severe Sepsis and Septic Shock 2012. Crit Care Med 41[2], 580–637. 2013. [DOI] [PubMed] [Google Scholar]
- (3).Eskesen TG, Wetterslev M, Perner A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2015. [DOI] [PubMed] [Google Scholar]
- (4).Harvey S, Harrison DA, Singer M et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomized controlled trial. Lancet. 2005;366(9484):472–477. [DOI] [PubMed] [Google Scholar]
- (5).Zhang Z, Lu B, Sheng X et al. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904–916. [DOI] [PubMed] [Google Scholar]
- (6).Duus N, Shogilev DJ, Skibsted S et al. The reliability and validity of passive leg raise and fluid bolus to assess fluid responsiveness in spontaneously breathing emergency department patients. J Crit Care. 2015;30(1):217–5. [DOI] [PubMed] [Google Scholar]
- (7).Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101(10):3765–3777. [DOI] [PubMed] [Google Scholar]
- (8).Rivers E, Nguyen B, Havstad S et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. [DOI] [PubMed] [Google Scholar]
- (9).Jones AE, Focht A, Horton JM et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Peake SL, Delaney A, Bailey M et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. [DOI] [PubMed] [Google Scholar]
- (11).Mouncey PR, Osborn TM, Power GS et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N Engl J Med. 2015. [DOI] [PubMed] [Google Scholar]
- (12).The ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med, ePub ahead of print. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Maitland K, Kiguli S, Opoka RO et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. [DOI] [PubMed] [Google Scholar]
- (14).Wiedemann HP, Wheeler AP, Bernard GR et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- (15).Johansen ME, Johansson PI, Ostrowski SR et al. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost. 2015;41(1):16–25. [DOI] [PubMed] [Google Scholar]
- (16).Henrich M, Gruss M, Weigand MA. Sepsis-induced degradation of endothelial glycocalix. Scientific World Journal. 2010;10:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ostrowski SR, Gaini S, Pedersen C et al. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015;30(1):90–96. [DOI] [PubMed] [Google Scholar]
- (18).Ostrowski SR, Haase N, Muller RB et al. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care. 2015;19:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jones A, Shapiro N, Trzeciak S, Arnold R, Kline J. Multi-center randomized controlled trial of lactate clearance versus central venus oxygen saturation as the endpoint of early sepsis resuscitation. Acad Emerg Med 16[4 Suppl 1], S143 2009. [Google Scholar]
- (20).Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33(3):409–413. [DOI] [PubMed] [Google Scholar]
- (21).Vincent JL, Moreno R, Takala J et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- (22).Rowley G, Fielding K. Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet. 1991;337(8740):535–538. [DOI] [PubMed] [Google Scholar]
- (23).Yunos NM, Bellomo R, Hegarty C et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. [DOI] [PubMed] [Google Scholar]