Abstract
Background:
Sensorineural hearing loss due to ototoxic cancer therapy is well established; effects on the vestibular system are unknown. We examined the feasibility of implementing vestibular screens for pediatric cancer survivors exposed to ototoxic agents. The prevalence of screening failures is reported.
Methods:
Cancer survivors who were 6 – 17 years, at least one-month post-treatment, and received ototoxic therapy (radiation to the head/neck, cisplatin, carboplatin) were eligible. Screening measures included 1.) Pediatric Vestibular Symptom Questionnaire (PVSQ), 2.) Modified Clinical Test of Sensory Interaction on Balance (MCTSIB), 3.) Dynamic Visual Acuity (DVA).
Results:
Vestibular screening failures were observed in 30 participants (60%). Patients with a brain tumor diagnosis were at increased risk for failures compared to non-brain tumor patients, (74.2% vs. 36.8%, p = 0.009). Patients who underwent brain surgery were at increased risk for failures compared to patients without brain surgery, (71% vs. 42%, p = 0.043). Patients with a longer duration between end of treatment and vestibular screening had a reduced risk of failures, with an almost 20% decrease for each year between the time points, (OR = 0.812 CI: 0.683–0.964, p = 0.018). Receiving carboplatin correlated with a decreased risk of failure (p = 0.016), due to a negative correlation with other clinical risk factors (diagnosis of a brain tumor, major brain surgery) shown to be associated with vestibular screening failure.
Conclusion:
Vestibular screening failures are highly prevalent in childhood cancer survivors who received ototoxic therapy. Broad screening of this population and further characterization of these patients is warranted.
Keywords: pediatric, survivors, ototoxic, vestibular, screening, brain tumor
Introduction
Cancer survival rates have increased for the pediatric population in recent years, with the five-year survival rate in excess of 80%.1 Though the goal is for childhood cancer survivors to advance into adulthood and lead healthy lives – including physical, emotional, and educational aspects – some of these survivors experience late effects from their therapies. 2, 3 In order for medical providers to identify affected patients, reliable and efficient screening tools that can be administered in a clinical setting are needed to allow affected patients to be identified and referred for appropriate rehabilitative services. It is well-established that treatment, such as radiation to the head and neck and platin-based therapies, like cisplatin and carboplatin, are ototoxic.4–10 Previous studies in both pediatric and adult oncology populations have shown significant relationships between these agents and hearing loss during therapy, even years after therapy completion.11–14 However, there have been little to no previous efforts in investigating how these therapies affect the vestibular system, the other component of the inner ear.
This study examined the ability to administer a battery of screening tools to assess vestibular function in our ambulatory pediatric oncology program. The aims of this study were to assess the feasibility of implementing vestibular screening in ambulatory oncology clinics and to estimate the prevalence of vestibular screening failures in pediatric cancer survivors exposed to ototoxic therapies. We hypothesized that patients who experienced toxicity to their hearing would also demonstrate vestibular difficulties.
Patients and Methods
This pilot study was a non-randomized cross-sectional study design and included 50 participants. The Protocol Review and Monitoring Committee (PRMC) and Institutional Review Board (IRB) at Washington University School of Medicine reviewed the design, materials, and various aspects of the study. The study was approved on July 19, 2016 and August 12, 2016, by PRMC and IRB respectively. Participants for this study were consented and enrolled between September 1, 2016 and January 26, 2017.
Participants
This study population included oncology patients from St. Louis Children’s Hospital, who received at least one of the following ototoxic therapies: radiation to head/neck, cisplatin, or carboplatin. Patients were 6 – 17 years old, as the questionnaire included in the methods was validated in this aged population. Inclusion criteria stated that participants had to be at least one-month from completion of their cancer therapy, English speaking, ambulatory and physically able to complete the screens. Participants were excluded if they were non-English speaking, non-ambulatory, had prosthetic lower limbs, had insufficient cognitive abilities to answer the questionnaire and/or follow directions for the screens, or had a neurologic deficit that was known to impair balance. Participants could be excluded after consent and enrollment, if they were unable to maintain the first condition of the MCTSIB (standing on the floor with eyes open for 30 seconds). Participants were also excluded if they were unable to read the letters on the Snellen eye chart. A total of 50 participants consented, enrolled, and completed the vestibular screen. A team of seven examiners were trained to properly administer each of the screens. Only trained team members were allowed to approach, enroll, consent, and administer the screens to the research subjects. Every team member completed a 20-minute training led by a vestibular audiologist on how to properly administer each of the screens.
Pediatric Vestibular Symptom Questionnaire
Subjective measures, like questionnaires, can be helpful in determining a person’s perception of difficulties. Though there are many vestibular questionnaires, most focus on adult populations, like the Dizziness Handicap Inventory (DHI); the few questionnaires that are available for children, such as the Dizziness Handicap Inventory for Patient Caregivers (DHI-PC), are created to assess children with known vestibular issues and therefore, do not screen for potential vestibular dysfunction or abnormalities.15, 16 The Pediatric Vestibular Symptom Questionnaire (PVSQ) was subsequently developed to quantify vestibular symptom severity and validated the measure in children 6 – 17 years of age.17
The 11-item PVSQ was completed by the participant if he/she was 11 years of age or older; the guardian completed the questionnaire on behalf of the participant if he/she was 10 years old or younger (Supplementary Table S1). Each item was to be rated on a scale from 0 to 3, with an optional “Don’t know” answer. The total score was an average numeric value for the answer to each question, with higher scores indicating greater symptom severity. A score was significant if it was greater than 0.68.
Modified Clinical Test of Sensory Interaction on Balance
Assessment of the vestibular system often requires the use of large and expensive equipment that is not portable. Among these, the Sensory Organization Test (SOT) is a functional test that assesses a person’s integration of visual, proprioception, and vestibular cues through a series of six conditions. These include standing on a firm platform with eyes open, closed, and with sway-referenced visual field and then with a sway reference platform with eyes open, closed, and with sway-referenced visual field. Screening tools have been created to allow for ease of administration in various settings. The SOT has been adapted into the Modified Clinical Test of Sensory Interaction on Balance (MCTSIB), and is comprised of four test conditions. Studies testing the reliability and accuracy of the MCTSIB in pediatric participants have found that MCTSIB scores correlated with SOT scores.18
The MCTSIB was administered after the questionnaire and required the participant to maintain four different conditions for 30 seconds, with a maximum of 3 trials per condition. Conditions were as follows: Condition 1 – standing on the ground, eyes opened; Condition 2 – standing on the ground, eyes closed; Condition 3 – standing on foam pad, eyes opened; Condition 4 – standing on foam pad, eyes closed. During the “eyes closed” conditions (Conditions 2 and 4), the participant wore a blindfold to ensure compliance of the denied vision condition; during the foam pad conditions (Conditions 3 and 4), an Airex Balance Pad (19.7” × 16.1” × 2.4”) was used. The participant completed this screen without wearing shoes in order to increase their proprioception of the support surface, however, socks were permissible. The distance between the feet was no wider than 12 inches. If the participant took a step, touched the wall, an object, the clinician, or asked to stop the trial, the trial was terminated and the time of termination was recorded. The participant had up to 3 trials per condition to maintain balance for 30 seconds. After successfully maintaining balance for 30 seconds or attempting all 3 trials for the condition, the participant proceeded to the next condition, until all four conditions were obtained. The average time for each condition was calculated, and each of the average scores were added together for a total score, for a maximum of 120 seconds. Based on previous published studies, a score of less than 110 seconds was significant, and for the purposes of this study, this score represented a vestibular screening failure.18
Dynamic Visual Acuity
The Dynamic Visual Acuity (DVA) test assesses another aspect of the vestibular system called the vestibulo-ocular reflex (VOR). The VOR is responsible for stabilizing a person’s gaze; a dysfunctional VOR would result in blurry vision due to retinal image instability and thus affect a person’s visual acuity during head movement. Investigators have assessed the utility of the DVA in children and determined its specificity and sensitivity to be 100% for their pediatric test population.19
The DVA screen consisted of a test where the participant was seated in 20 feet from a Snellen eye chart, which was placed at eye level for the participant. The participant was allowed to wear eye glasses during this test. To determine the participant’s static visual acuity, the participant was asked to read the smallest line, while reading all of the letters correctly. After establishing and recording the line of static visual acuity, the examiner stood behind the participant and rotated his/her head side to side, at a speed of 2 Hertz (Hz) or 240 beats per minute (BPM); a metronome was used to ensure the appropriate speed was maintained throughout. To determine the dynamic visual acuity, the participant was again asked to read the smallest line possible in which all of the letters were read correctly, while his/her head was moving. The screen constituted a “pass” if the difference between the static visual acuity and dynamic visual acuity was 2 lines or less. A change of 3 or more lines was considered a screen “fail.”
For the purposes of this study, a “vestibular screening failure” is defined as any participant who had at least one significant score or screen fail. In addition to screening failures, data collection from patient charts included gender, age, race, cancer diagnosis, date of diagnosis, number of and nature of prior brain surgeries, ototoxic chemotherapy exposures, prior vancomycin and aminoglycoside administrations, and hearing thresholds from the most recent audiogram. Referring to the most recent audiograms, a participant’s hearing loss was defined in this protocol utilizing the SIOP (International Society of Pediatric Oncology), an ototoxicity grading scale, to assess cancer therapy induced hearing loss 12. Hearing loss for this study was defined as a SIOP grade 1 or greater, using the worse grade between the two ears, in the event there was an asymmetric hearing loss.
Statistical Methods
Patient demographic and clinical characteristics were presented as mean, medians, standard deviations and ranges for continuous variables and frequencies (percentage of group) for the categorical variables. For the purposes of this study, a vestibular screening failure is defined as any participant who had at least one significant score or screening failure. The prevalence of vestibular screening failures and its 95% confidence interval were calculated. Logistic regression analysis was performed to identify significant risk factors (age, race, gender, diagnosis, radiation therapy, surgery, aminoglycoside exposure or hearing loss) that may be associated with the development of vestibular screening failure. All analyses were conducted with a 2-sided test at a significance level of 0.05 using SAS 9.3 (SAS Institute, Cary, NC).
Results
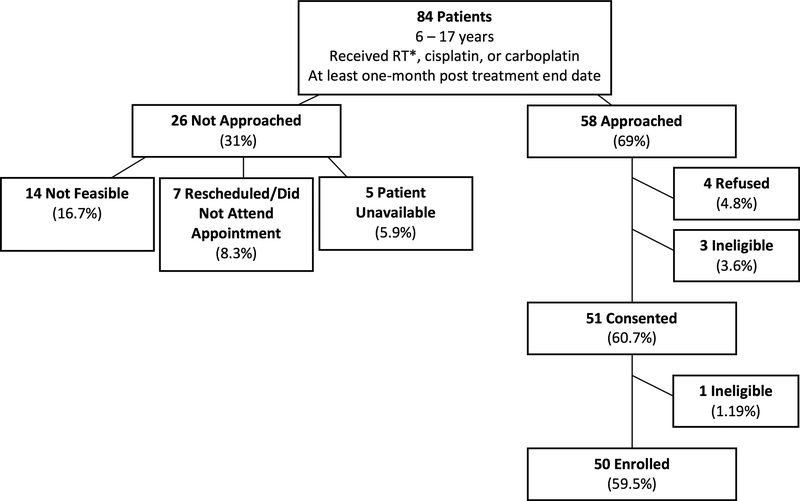
Figure 1 describes the patient eligibility and accrual of the study. There were 84 eligible participants. A total of 26 (30.9%) were not approached due to either alteration in their scheduled appointments, no team member was available for screening, or the patient was unable to be approached during the appointment. The remaining 58 patients (69%) were approached. There were 3 patients who were ineligible according to the exclusion criteria and were never enrolled. Only 4 patients refused to participate. The remaining 51 were consented and enrolled. However, one participant was unable to complete the MCTSIB Condition 1 screen, and therefore became ineligible to participate in the remainder of the study. Thus, 50 participants were enrolled and completed the screening process; this number represents 59.5% of all eligible patients and 86.2% of all patients approached to participate.
Fig. 1.
Patient Eligibility and Accrual: Accrual of participants from 84 consecutive patients who were identified as eligible for enrollment. Factors accounting for patients not approached for enrollment are cited.
*RT refers to radiation to the head/neck
The characteristics of the participants are displayed in Table 1. There were 28 males (56%) and 22 females (44%). Participants were divided into the following diagnostic categories: 1.) brain tumor (medulloblastoma, astrocytoma, pineoblastoma, ependymoma, etc.) or, 2.) non-brain tumor (osteosarcoma, neuroblastoma, ALL, retinoblastoma, etc.) representing 31 (62%) and 19 (38%) participants, respectively. Brain surgery was performed on 31 of the participants (63%). The mean age of diagnosis was 7.39 years, and the mean age at screening was 13.2 years; the average duration between end of treatment and vestibular screening was 4.77 years.
TABLE 1.
Participant characteristics
| N = 50 | |
|---|---|
| Gender | |
| Male | 28 (56%) |
| Female | 22 (44%) |
| Race | |
| African-American | 7 (14%) |
| Caucasian | 43 (86%) |
| Diagnosis | |
| Brain tumor | 31 (62%) |
| Non-brain tumor | 19 (38%) |
| Age at diagnosis (yrs) | |
| Mean | 7.39 |
| Median (Range) | 6.90 (0.40 – 16.20) |
| Age at screening (yrs) | |
| Mean | 13.2 |
| Median (Range) | 14.2 (6.1 – 17.99) |
| Brain Surgery | |
| Yes | 31 (62%) |
| No | 19 (38%) |
| Primary tumor in brain | |
| None/biopsy of brain | 5 (10%) |
| Partial/total resection | 27 (54%) |
| No primary tumor in brain | |
| None/biopsy of brain | 18 (36%) |
| Radiation to head/neck | |
| Yes | 35 (70%) |
| No | 15 (30%) |
| Cisplatin | |
| Yes | 19 (38%) |
| No | 31 (62%) |
| Carboplatin | |
| Yes | 14 (28%) |
| No | 36 (72%) |
| Vancomycin | |
| Yes | 9 (19.6%) |
| No | 37 (80.4%) |
| Missing | 4 |
| Aminoglycosides | |
| Yes | 3 (6.5%) |
| No | 43 (93.5%) |
| Missing | 4 |
| Hearing loss | |
| Yes | 17 (40.5%) |
| No | 25 (59.5%) |
| Missing | 8 |
| End of treatment to screening (yrs) | |
| Mean | 4.77 |
| Median (Range) | 4.25 (0.17 – 13.88) |
Vestibular failures were observed in 30 participants (60%, 95% CI: 45–75%). Of the 30 failures, 22 participants failed one screen, and 8 participants failed two of the screens; none of the participants failed all three screens. Table 2 describes the results from each of the vestibular screens. The PVSQ scores ranged from 0 to 2.10, with an average score of 0.67 (SD of 0.59). The MCTSIB scores ranged from 120 to 90 seconds, with an average score of 118 seconds (SD of 5.45).
TABLE 2.
Vestibular screening results
| Measure | N = 50 |
|---|---|
| PVSQ | |
| Significant (> 0.68)* | 21 (42%) |
| Non-significant (≤ 0.68) | 29 (58%) |
| MCTSIB | |
| Significant (< 110 sec)* | 3 (6%) |
| Non-significant (≥ 110 sec) | 47 (94%) |
| DVA | |
| Fail ( ≥ 3 line change)* | 14 (28%) |
| Pass ( ≤ 2 line change) | 36 (72%) |
Score represents vestibular screening failure.
Table 3 describes the associations between participant characteristics and vestibular failures. Gender, radiation, cisplatin administration, vancomycin, aminoglycosides, and hearing loss were not significantly associated with vestibular failure. Participants with brain tumors were at increased risk for failure, with 74% failing at least one screen compared to only 37% of non-brain tumor patients (OR = 4.929, p = 0.009). Participants undergoing major surgical procedures of the brain (partial or gross total resections) were compared to those who did not undergo brain surgery or who only underwent a biopsy procedure, (non-brain surgery). Those with a history of undergoing a major surgical resection procedure to the brain were also at increased risk for failure compared to the non-brain surgery group (71% vs. 42%, OR = 3.448, p = 0.043). Surprisingly those participants receiving carboplatin demonstrated a decreased risk of vestibular failure (36% vs. 69%, OR = 0.244, p = 0.016). Given carboplatin’s relatively low ototoxic properties, alternative explanations for this association were sought.
TABLE 3.
Factor associations with vestibular screening failures
| Vestibular Fail |
|||||
|---|---|---|---|---|---|
| Covariate | Level | No (N=20) | Yes (N=30) | Odds Ratio | Parametric P-value* |
| Gender | Male | 12 (42.86) | 16 (57.14) | 0.762 | 0.642 |
| Female | 8 (36.36) | 14 (63.64) | |||
| Diagnosis | Brain tumor | 8 (25.81) | 23 (74.19) | 4.929 | 0.009 |
| No brain tumor | 12 (63.16) | 7 (36.84) | |||
| Age at Diagnosis | Median (range) | 5.5 (0.7–15.4) | 7.0 (0.4–16.2) | 1.121 | 0.096 |
| Brain surgery | No | 11 (57.89) | 8 (42.11) | 0.298 | 0.043 |
| Yes | 9 (29.03) | 22 (70.97) | |||
| Radiation to head/neck | No | 7 (46.67) | 8 (53.33) | 0.675 | 0.529 |
| Yes | 13 (37.14) | 22 (62.86) | |||
| Cisplatin | No | 15 (48.39) | 16 (51.61) | 0.381 | 0.122 |
| Yes | 5 (26.32) | 14 (73.68) | |||
| Carboplatin | No | 11 (30.56) | 25 (69.44) | 4.090 | 0.029 |
| Yes | 9 (64.29) | 5 (35.71) | |||
| Vancomycin | No | 16 (43.24) | 21 (56.76) | 0.375 | 0.247 |
| Yes | 2 (22.22) | 7 (77.78) | |||
| Aminoglycosides | No | 18 (41.86) | 25 (58.14) | 0.000 | 0.151 |
| Yes | 0 (0) | 3 (100) | |||
| Hearing loss | No | 12 (48.00) | 13 (52.00) | 0.451 | 0.228 |
| Yes | 5 (29.41) | 12 (70.59) | |||
| Years after total treatment | Median (range) | 5.9 (0.2–13.9) | 2.8 (0.2–10.6) | 0.812 | 0.018 |
The parametric p-value was calculated by univariate logistic regression (for Age at diagnosis and Years after total treatment) or chi-square test (for all others).
Table 4 details an analysis of the use of carboplatin in our participant population and its relationship with a brain tumor diagnosis. Comparing the administration of carboplatin and diagnosis, the analysis revealed that receiving carboplatin was negatively associated with brain tumor diagnosis and a decreased risk of vestibular failure (p = 0.016). Thus, carboplatin exposure was non-randomly associated with not having a brain tumor diagnosis, a risk factor which we had demonstrated to be strongly associated with a vestibular screening failure. Thus, carboplatin’s negative association with a vestibular screening failure may have been attributed to its corresponding negative correlation to a brain tumor diagnosis, rather than having a protective effect.
TABLE 4.
Relationship of a brain tumor diagnosis and carboplatin exposure
| Vestibular Fail |
||||
|---|---|---|---|---|
| Covariate | Level | No (N=20) | Yes (N=30) | Parametric P-value* |
| Diagnosis_Carboplatin | No brain tumor & no Carbo | 6 (50) | 6 (50) | 0.016 |
| No brain tumor & Carbo | 6 (85.71) | 1 (14.29) | ||
| Brain tumor & no Carbo | 5 (20.83) | 19 (79.17) | ||
| Brain tumor & Carbo | 3 (42.86) | 4 (57.14) | ||
The parametric p-value was calculated by chi-square test.
Conversely, in contrast to carboplatin, patients who received cisplatin failed to demonstrate a significant increase in vestibular failure (Table 3). However, when chemotherapy was categorized and analyzed as “neither cisplatin nor carboplatin” (N = 21), “cisplatin only” (N = 15), “carboplatin only” (N = 10), and “combined cisplatin and carboplatin” (N = 4), the vestibular failure rates were 57%, 86%, 40%, and 25%, respectively. The findings suggested that participants who received “cisplatin only” have a higher rate of vestibular screening failures (86%) than those who received “carboplatin only” (40%) (p = 0.028) (data not shown). Further analysis was completed to examine if dosages of cisplatin and/or carboplatin affected outcomes. We categorized the cumulative dose of cisplatin and carboplatin respectively by its medians, and the vestibular failure rates across subgroups (below median vs. above median) were very close for both cisplatin (78% vs. 70%, p > 0.99) and carboplatin (33% vs. 40%, p > 0.99). Thus, although there is some indication that cisplatin may influence the prevalence of vestibular screen failures, further analyses failed to clearly implicate it as a risk factor, perhaps due to the small sample size of this study population.
Initial statistical analysis revealed no significant associations for vestibular screening failures and the presence of hearing loss. Though not statistically significant, there is a considerable difference between vestibular failures rates of 71% versus 52% for those with and without hearing loss, respectively. We subsequently refined our analysis to determine if there was a relationship with SIOP grade and vestibular failure, in contrast to our initial effort examining the presence or absence of hearing loss. Our findings show that participants with a higher SIOP grade (more severe hearing loss) tend to have a higher rate of vestibular failure, though the difference is not significant (70% vs. 52%, p=0.339) (data not shown).
In examining the relationship of the amount of time from completion of treatment to the vestibular screening, we observed that participants with a longer duration between end of treatment and the vestibular screening had a reduced risk of failures, with an almost 20% reduction in the risk with each year between the end of treatment and the vestibular screening (OR = 0.81, 95% CI: 0.68–0.96, p = 0.018). This suggests that the longer interval from treatment to evaluation potentially gives patients the opportunity to adjust to their vestibular abnormality, resulting in improved performance during the vestibular screening procedure.
Discussion
With the high accrual acceptance rate, and the low rate of ineligibility (6.9%), we demonstrated that this vestibular screen process is a feasible tool to implement within an ambulatory clinical setting. These measures were easily completed during a typical clinical appointment, and the entire screen was completed in fifteen minutes. The costs for implementing these screens was relatively low, with supplies that are modest in cost (Snellen eye charts, high density foam pads, blindfolds). Furthermore, the screening measures were administered by multiple providers (7 team members) in various clinics (3 different sites), indicating that this screening battery can be adapted to a variety of clinic settings. A 20-miunte training session enabled each member to appropriately administer the screens. Thus, this vestibular screening is relatively low in cost in regard to both training and time.
Vestibular screening failures were highly prevalent in this population of pediatric cancer survivors. Failures were particularly common in patients with a brain tumor diagnosis, and in individuals who underwent extensive brain surgery. Initial logistic regression analysis suggested that exposure to ototoxic agents, which we had hypothesized as a risk factor for vestibular toxicity, was not associated with failing this battery of vestibular screens. Thus, our inability to demonstrate this correlation suggest that an even broader population may be affected.
Our restriction of enrollment to patients receiving ototoxic therapy may have generated an underestimate of the prevalence of vestibular screening failures in pediatric cancer patients. Further investigation will provide a better estimate of the true prevalence of this clinical condition. However, given the high prevalence in the population tested, (60% of the whole population, and 70% of patients with a brain tumor diagnosis), vestibular difficulties may indeed impact a substantial fraction of the pediatric cancer survivors. Of note, all patients undergo complete neurologic examinations in the Neuro-onc and Late Effects Clinics. Given the lack of appreciation of this clinical entity, our findings reinforce the likely inability of routine exams to reliably identify these patients. Therefore, this simple battery should be considered as a routine screening in order to identify patients at risk. Once these patients are identified, further diagnostic vestibular testing can then fully characterize the nature of their deficits.
Examination of the prevalence of vestibular screening failures in the pediatric oncology population is warranted due to the negative impact of vestibular dysfunction on gaze stabilization and/or poor postural control. These deficits translate into difficulties in academic areas such as reading, motor development and control, and overall balance. 20 The inability to maintain a stable gaze may significantly impact the patient’s capabilities in walking and driving. 21 Thus, vestibular dysfunction could adversely affect education and quality of life for this population.
Participants with a shortened duration between end of treatment and vestibular screening were at increased risk for vestibular failure. The compensatory nature of the vestibular system highlights the potential of rehabilitative services to improve the outcome of this population.
There were clear limitations of this study. Eligible participants were recruited based on their upcoming appointments and availability, and therefore resulted in a non-heterogeneous population. However, by recruiting and screening consecutive patients visiting the institution, we believe this participant recruitment method reduced potential biases. An additional limitation was the sample size, as a larger cohort may potentially have enrolled a more diverse population. Furthermore, the age of the cohort was influenced by the screens utilized to assess vestibular function, as there is a limited number of validated, age appropriate tools available. Another limitation to this study was missing data, specifically, variables for medication administrations and hearing tests, with a total of 8 participants without a documented hearing test. Thus, although we could not demonstrate that the presence of hearing loss was linked to vestibular difficulties, the analysis was complicated by incomplete hearing evaluations on all the patients. For the DVA screen, poor static visual acuity may have masked vestibular screening fails for as many as three brain tumor participants. These participants had poor visual acuity in that they could only read the third line from the top of the eye chart. Since this screen requires a 3-line difference for a failure, these participants all passed the screen. Lastly, there was a large team of professionals for this study. While 40 participants were screened by the same team member, the remaining 10 participants were screened by three other team members. However, no obvious variations were noted by the investigators.
Conclusions
Survivors of childhood brain tumors and those individuals who received brain surgery are more likely to fail vestibular screening testing than other childhood cancer survivors. Future research should focus on identifying additional risk factors associated with vestibular screening failures and further characterization of the deficits to better define the nature of their difficulties. Given the high prevalence observed, implementation of screening programs like that described in this report may substantially improve the care of these patients.
Supplementary Material
Supplemental Fig. S1. Pediatric Vestibular Symptom Questionnaire (PVSQ)
Abbreviations Key:
- PVSQ
Pediatric Vestibular Symptom Questionnaire
- MCTSIB
Modified Clinical Test of Sensory Interaction on Balance
- DVA
Dynamic Visual Acuity
- PRMC
Protocol Review and Monitoring Committee
- IRB
Institutional Review Board
- DHI
Dizziness Handicap Inventory
- DHI-PC
Dizziness Handicap Inventory for Patient Caregivers
- SOT
Sensory Organization Test
- VOR
Vestibulo-ocular Reflex
- SIOP
International Society of Pediatric Oncology
Footnotes
Conflicts of Interest
Robert Hayashi and Susan Hayashi were consultants for Otonomy Inc., and Belinda Sinks was a consultant for Barron Associates Inc.
Contributor Information
Miranda L. Camet, Program in Audiology and Communication Sciences Washington University School of Medicine.
Susan S. Hayashi, Department of Audiology, St. Louis Children’s Hospital.
Belinda C. Sinks, Dizziness and Balance Center, Department of Otolaryngology, Washington University School of Medicine.
Jennifer Henry, Department of Pediatrics, Washington University School of Medicine.
Katie Gettinger, Division of Pediatric Hematology/Oncology, St. Louis Children’s Hospital.
Ashley Hite, Department of Pediatrics, Washington University School of Medicine.
Juliann Kiefer, Pediatric Hematology/Oncology, Riley Hospital for Children.
Caroline Mohrmann, Department of Pediatrics, Washington University School of Medicine.
Taryn Sandheinrich, Division of Pediatric Hematology/Oncology St. Louis Children’s Hospital.
Feng Gao, Department of Surgery, Public Health Sciences, Siteman Cancer Center, Washington University School of Medicine.
Robert J. Hayashi, Division of Pediatric Hematology/Oncology, Department of Pediatrics Washington University School of Medicine.
REFERENCES
- 1.Ward E, DeSantis C, Robbins A, Kohler B, & Jemal A Childhood and adolescent cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64: 83–103. [DOI] [PubMed] [Google Scholar]
- 2.Robinson LL & Hudson MM Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nature. 2014; 14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClellan W, Klemp JR, Krebill H, et al. Understanding the functional late effects and informational needs for adult survivors of childhood cancer. Oncology Nursing Forum. 2013;40:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen WC, Liao CT, Tsai HC, et al. Radiation-induced hearing impairment in patients treated for malignant parotid tumor. The Annals of Otology, Rhinology, and Laryngology. 1999;108:1159–1164. [DOI] [PubMed] [Google Scholar]
- 5.Anteunis LJ, Wanders SL, Hendriks JJ, Langendijk JA, Manni JJ, & de Jong JM A prospective longitudinal study on radiation-induced hearing loss. American Journal of Surgery. 1994;168:408–411. [DOI] [PubMed] [Google Scholar]
- 6.Mujica-Mota M, Waissbluth S, & Daniel SJ Characteristics of radiation-induced sensorineural hearing loss in head and neck cancer: A systematic review. Head & Neck. 2013;35:1662–1668. [DOI] [PubMed] [Google Scholar]
- 7.McHaney VA, Thibadoux G, Hayes FA, & Green AA Hearing loss in children receiving cisplatin chemotherapy. The Journal of Pediatrics. 1983;102:314–317. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney DH, Weaver T, Steuber CP, & Starling KA Ototoxicity with cisplatin therapy. The Journal of Pediatrics. 1983;103:1006–1007. [DOI] [PubMed] [Google Scholar]
- 9.Brock P, Pritchard J, Bellman S, & Pinkerton CR Ototoxicity of high-dose cis-platinum in children. Medical and Pediatric Oncology. 1988;16:368–369. [DOI] [PubMed] [Google Scholar]
- 10.Skinner R, Pearson ADJ, Amineddine HA, Mathias DB & Craft AW Ototoxicity of cisplatinum in children and adolescents. British Journal of Cancer. 1990;61:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Khatib T, Cohen N, Carret AS, & Daniel S Cisplatinum ototoxicity in children, long-term follow up. International Journal of Pediatric Otorhinolaryngology. 2010;74:913–919. [DOI] [PubMed] [Google Scholar]
- 12.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology boston ototoxicity scale. Journal of Clinical Oncology. 2012;30:2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, & Hayashi RJ Hearing loss in pediatric oncology patients receiving carboplatin-containing regimens. Journal of Pediatric Hematology Oncology. 2008;30:130–134. [DOI] [PubMed] [Google Scholar]
- 14.Kolinsky DC, Hayashi SS, Karzon R, Mao J, & Hayashi RJ Late onset hearing loss: A significant complication of cancer survivors treated with cisplatin containing chemotherapy regimens. Journal of Pediatric Hematology Oncology. 2010;32:119–123. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson GP, & Newman CW The development of the dizziness handicap inventory. Archives of Otolaryngology - Head and Neck Surgery. 1990;116:424–427. [DOI] [PubMed] [Google Scholar]
- 16.McCaslin DL, Jacobson GP, Lambert W, English LN, & Kemph AJ The development of the vanderbilt pediatric dizziness handicap inventory for patient caregivers (DHI-PC). International Journal of Pediatric Otorhinolaryngology. 2015;79: 1662–1666. [DOI] [PubMed] [Google Scholar]
- 17.Pavlou M, Whitney S, Alkathiry AA, et al. The pediatric vestibular symptom questionnaire: A validation study. The Journal of Pediatrics. 2016;168:171–177. [DOI] [PubMed] [Google Scholar]
- 18.Christy JB, Payne J, Azuero A, & Formby C Reliability and diagnostic accuracy of clinical tests of vestibular function for children. Pediatric Physical Therapy. 2014;26:180–189. [DOI] [PubMed] [Google Scholar]
- 19.Rine RM & Braswell J A clinical test of dynamic visual acuity for children. International Journal of Pediatric Otorhinolaryngology. 2003;67:1195–1201. [DOI] [PubMed] [Google Scholar]
- 20.Rine RM Growing evidence for balance and vestibular problems in children. Audiological Medicine. 2009; 7:138–142. [Google Scholar]
- 21.Rine RM & Wiener-Vacher S Evaluation and treatment of vestibular dysfunction in children. NeuroRehabilitation. 2013; 32:507–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Pediatric Vestibular Symptom Questionnaire (PVSQ)