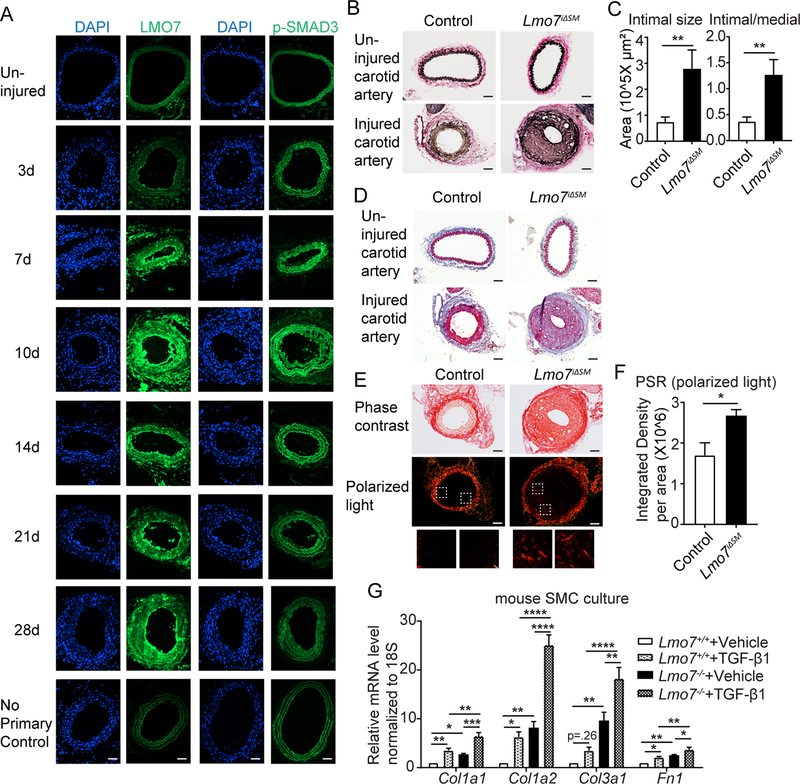
Figure 1. Loss of LMO7 in SMC exacerbates intimal hyperplasia and ECM deposition.
(A) WT mice were subjected to carotid artery ligation and injured vessels were harvested at time points ranging from 3 to 28 days. Cryosections were immunostained for LMO7 (green) (left panel). Adjacent sections were immunostained for p-SMAD3 (green) (right panel). DAPI was used to visualize nuclei. Representative images from two mice at each time point are shown. 20 sections were stained for LMO7 or pSMAD3 per individual per time point. (B) EVG staining of tissue sections from control or Lmo7iΔSM mice 28 days after carotid artery ligation. (C) Quantification of intimal size and intimal/medial ratio of injured carotid arteries (n=10). (D) Trichrome staining of cross-sections of contralateral uninjured and injured arteries of control or Lmo7iΔSM mice after carotid ligation. (E) Picro-Sirius Red staining of cross-sections of injured arteries of control or Lmo7iΔSM mice at 28 days post carotid ligation by phase contrast (upper panel) or polarized light (lower panel) microscopy. (F) Quantification of relative integrated intensity of the signal in medial and intimal layers shown in polarized light images in (E) (n=5). (G) Mouse aortic SMCs were treated with 0.5ng/ml TGF-β1 for 24hrs and mRNA was harvested for qPCR analysis of ECM genes (n=4 independent experiments). Two-way ANOVA revealed a significant effect of LMO7 depletion and TGF-β1 treatment on mRNA expression. For Col1a2 mRNA only, LMO7 depletion significantly enhanced the TGF-β1 effect on expression (p=0.0009). Scale bar=50 μm. Data are expressed as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001