Abstract
All vertebrates possess mechanisms to restore damaged tissues with outcomes ranging from regeneration to scarring. Unfortunately, the mammalian response to tissue injury most often culminates in scar formation. Accounting for nearly 45% of deaths in the developed world, fibrosis is a process that stands diametrically opposed to functional tissue repair and regeneration. Strategies to improve wound healing outcomes therefore require methods to limit fibrosis. Wound healing is guided by precise spatiotemporal deposition and remodeling of the extracellular matrix (ECM). The ECM, comprising the non-cellular component of tissues, is a signaling depot that is differentially regulated in scarring and regenerative healing. This Review focuses on the role of key matrix components during mammalian wound healing alongside a comparison to scar-free repair and regeneration followed by an overview of matrix-based strategies that attempt to exploit the role of the ECM to facilitate functional tissue remodeling.
Keywords: extracellular matrix (ECM), wound healing, fibrosis, tissue engineering
2. Introduction
The extracellular matrix (ECM) is the complex three-dimensional acellular environment that is present in all tissues and is essential for life. Controlled remodelling of the ECM is required for development, wound healing, and homeostasis; however, uncontrolled remodelling contributes to a variety of disease states. The ECM is crucial for tissue repair, acting as a dynamic scaffold to protect and support healing wounds and as an instructive platform for reciprocal signaling with cells. Precisely regulated spatiotemporal biochemical and biomechanical cell-matrix interactions are key to the efficacy and quality of repair processes. Understanding matrix signaling events during wound healing provides insights for the rational design of matrix mimics to control the outcome of tissue repair.
All vertebrates have evolved mechanisms to replace or restore damaged tissue. The spectrum of wound healing outcomes ranges from complete regeneration in zebrafish and salamanders to scar formation in adult mammals. The mammalian wound healing response is classically characterized by three stages (i.e., inflammation, proliferation and remodeling) with each stage inextricably linked to the ECM. Tissue repair commences immediately following tissue injury and, in post-natal mammals, most often culminates in the replacement of the damaged tissue with an acellular fibrotic matrix (i.e., scar tissue). Fibrosis is a major impediment to functional tissue repair. While scarring of minor skin wounds will have little or no functional effect on the organism, fibrosis in other tissues (e.g., heart, esophagus, spinal cord) contributes to the manifestation and progression of a myriad of diseases. The present review focuses on some of the common regulatory features of ECM during wound healing and tissue regeneration. An overview of the deposited and remodeled matrix components during mammalian wound healing is provided alongside a comparison to natural examples of scar-free repair and regeneration. Finally, we review pre-clinical and clinical matrix-based strategies that attempt to exploit the role of the ECM to facilitate functional tissue remodeling.
3. Matrix deposition and dynamics in the wound healing cascade
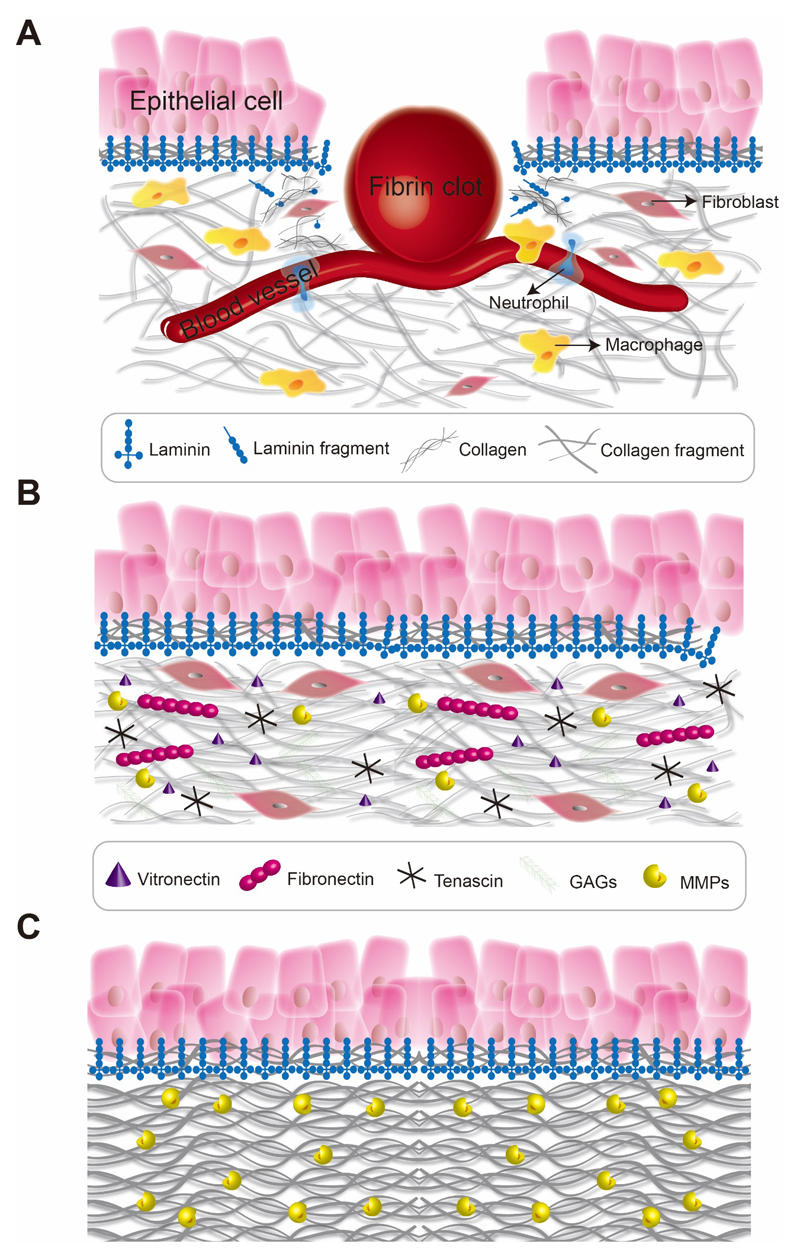
Faithful wound healing requires finely tuned ECM deposition and remodeling. Disruption of matrix remodeling events and excessive matrix deposition can lead to impaired wound healing (i.e., fibrosis). The deposition of ECM proteins and matrix-associated proteins during the wound healing cascade is well-orchestrated, and key to successful wound healing (Table 1). Fibrin and fibronectin are released from vasculature immediately after capillary damage to prevent extensive blood loss. Platelets release chemotactic growth factors to re-establish the epithelium, while neutrophils and macrophages begin clearing excess matrix and cell debris. Fibroblasts activate to myofibroblasts and deposit collagen, glycosaminoglycans (GAGs) and fibronectin (i.e., granulation tissue). Myofibroblasts, originating from epithelial-to-mesenchymal transition of various cells, produce collagen I enriched scar tissue to provide strength and protection to the healing wound. Myofibroblasts contract along matrix fibers to support wound closure and subsequently undergo apoptosis. Epithelial cells deposit a basement membrane to establish tissue integrity and cell polarity. Angiogenesis along the newly deposited basement membrane leads to the formation of blood vessels to restore circulation. Collagen fibrils are then arranged and cross-linked over a period of months, and excess collagen is cleared (Figure 1).
Table 1. Role and consequence of dysregulation of key matrix proteins during tissue repair.
| Matrix protein | Role during tissue repair | Consequences of dysregulation |
|---|---|---|
| Collagen type I |
|
|
| Collagen type III |
|
|
| Collagen type IV |
|
|
| Collagen type VI |
|
|
| Collagen type VII |
|
|
| Collagen type XIV |
|
|
| Plasma fibronectin |
|
|
| Cellular fibronectin |
|
|
| Laminin |
|
|
| Tenascin |
|
|
| Vitronectin |
|
|
| Glycosaminoglycans |
|
|
| SPARC |
|
|
| CCN proteins |
|
|
| Thrombospondin |
|
|
| Osteopontin |
|
|
| Fibulin |
|
|
Figure 1. Extracellular matrix deposition and remodelling during tissue repair.
(A) Tissue injury triggers the accumulation of fibrin and plasma fibronectin that are released from the vasculature to immediately close the wound. Resident and recruited immune cells release growth factors and matrix proteins to initiate the wound healing cascade. Fibroblasts are activated and deposit collagen and fibronectin. (B) The well-orchestrated release and dynamic remodelling of a variety of different matrix proteins enables cell migration, cell differentiation, matrix remodelling and wound closure. (C) Finally, collagen fibers are remodelled by proteolytic enzymes to protect the healing tissue and to provide strength and support.
3.1. Major ECM components in mammalian tissue repair
3.1.1. Collagens
Collagens are the most abundant and diverse ECM proteins with 28 members (type I - XXVIII) of the collagen family identified to date [1]. Collagens are trimers assembled from three polypeptide chains (α-chains) that form a triple helix with repeating amino acid motif Gly-X-Y and are classified as fibrillar, fibril-associated collagens with interrupted triple-helices (FACIT), basement membrane network-forming, hexagonal network-forming, and filamentous. The collagens contribute to tissue repair via cell interaction sites for adhesion and migration, by connecting the basement membrane and the interstitial matrix, and providing mechanical strength to the tissue.
Fibrillar collagens III and I are sequentially deposited during mammalian tissue repair. Collagen III regulates fibroblast phenotype in early stages of tissue repair, and is replaced by collagen I after 2-3 weeks [2]. Individuals with collagen III mutations have impaired wound healing. Collagen III deficiency in murine models triggers increased myofibroblast differentiation and accelerated wound closure alongside increased scar formation. Collagen I, the most abundant collagen type, is expressed by fibroblasts. Collagen I fibers offer strength to protect the healing wound, and are involved in mechano-signaling events guiding tissue repair. The scar matrix is predominantly composed of collagen I and is effectively a barrier to tissue regeneration once formed.
Collagen IV provides strength and integrity to the basement membrane – the sheet-like network underlying all epithelium and endothelium. Collagen IV interacts with cells via integrins, and collagen IV fragments exert a variety of biological activity. During tissue remodeling, the basement membrane, and therefore collagen IV, aids in establishing tissue integrity, epithelial cell polarity, and neo-vascularisation. Basement membrane remodeling by matrix metalloproteinases (MMPs) during angiogenesis releases growth factors that are tethered to the dense membrane network, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [3].
The role of FACIT collagens in tissue repair is not yet well understood. Type XIV collagen appears to regulate early stages of collagen fibrillogenesis, based on studies on Col14a1(-/-) mice [4]. Collagens XII, XIV and VII connect the basement membrane to the interstitial matrix and are crucial to matrix integrity [5, 6]. Collagen VI is expressed by fibroblasts and endothelial cells of newly formed blood vessels in clinical specimens and regulates fibroblast apoptosis in vitro [7]. Collagen VI deficiency weakens the basement membrane and alters collagen fibril architecture in Col6a1(-/-) mice [8], and similar defects are present in the dermis of individuals with collagen VI myopathy [9]. Collagen VI deficient matrices are thinner and contain less total collagen than matrices derived from healthy individuals. Collagen VI also affects cell spreading and migration [10]. Interestingly, macrophages secrete collagen type VI to modify cell-matrix interactions during wound healing [11]. FACIT collagen type VII ensures wound closure through the organisation of laminin in the basement membrane and by supporting fibroblast migration [12].
3.1.2. Fibronectin
Fibronectin is a multidomain glycoprotein comprising 12 FN type I repeats (FN1), two FN type II repeats (FNII), fifteen FN type III repeats (FNIII) and one variable (V) region. Fibronectin exists in circulating plasma form and the structurally unique cellular form, which is expressed and assembled during the wound healing cascade. A variety of cell- and tissue-specific isoforms arise from alternative splicing of FNIII domains. This domain structure allows for flexibility in the protein backbone and mechanical dynamics to modulate fibronectin-cell and fibronectin-matrix interactions [13].
Fibronectin acts as a scaffolding protein during tissue repair and a regulator of cell function. Plasma fibronectin, synthesized by hepatocytes, stabilizes the fibrin clot and is a substrate for platelet adhesion, spreading, and aggregation in early stages of wound healing. Cellular fibronectin, secreted by endothelial cells and fibroblasts, is assembled into a dense fibrillar network that enables fibroblast polarization and provides migration highways for cells within the wound bed. The domain architecture and mechanical flexibility of fibronectin confers cell signaling and matrix interactions as different domains comprise different cell-binding and matrix protein-binding sites. Fibronectin a critical regulator of ECM organization and stability and a pre-formed fibronectin matrix is requisite for collagen network formation [14]. The fibronectin matrix is a signaling depot where growth factors are sequestered and activated upon matrix alterations (e.g., transforming growth factor beta 1 (TGFβ1) and VEGF, amongst others). Interestingly, wound gap closure is primarily dependent on fibronectin deposition rather than the availability of a collagen matrix [15].
3.1.3. Laminin
Laminins are glycosylated, 400 kDa multidomain proteins assembled from an α-, β-, and γ- chain to a cross-like tertiary structure. Twelve different mammalian laminin chains have been identified that can form 16 different isoforms with varying chain length and domain architecture (39). Genetic deletion of laminin causes early lethality [16] in contrast to collagen IV deletion that diminishes the stability of the basement membrane and is lethal at a later developmental stage [17]. Remodeling of the basement membrane during wound healing is critical to re-epithelialisation and the formation of blood vessels.
The formation of a basement membrane network is initiated by laminin. Basement membranes are sheet-like matrices, present in all epithelial and endothelial tissues, providing tissue integrity, instructing cell polarity, and acting as a selective barrier for cell transmigration (37, 38). Vascular basement membrane components are required for the initiation of vascularization, and blood vessel growth and branching requires continuous remodeling of the basement membrane. Endothelial cells are quiescent while bound to the capillary basement membrane, but proteolytic degradation of the basement membrane by MMPs leads to the release of pro-angiogenic growth factors (i.e., VEGF and FGF, and cryptic basement membrane fragments) cell migration and blood vessel formation activity [18]. Growth factor tethering within the basement membrane is maintained by agrin, perlecan, and type XVIII collagen. Basement membrane fragments have been suggested as a tool to assess impaired tissue repair and fibrosis [19]. Isoforms of laminin are involved in endothelial and pericyte cell signalling [20], indicating a regulatory role of basement membrane remodeling in angiogenesis. In mice lacking nidogen-1, a protein that connects laminin chains and stabilizes the basement membrane, epidermis remodeling and resolution of granulation tissue resolution is impaired following skin injury [21].
3.1.4. Tenascin
The tenascin family of matrix proteins comprises four different isoforms arising from alternative splicing and protein modifications. Tenascin-C, a well-studied isoform, is a hexameric, multidomain protein around 200kDa comprising FNIII and EGF-like domains which provide a variety of binding sites for cross-talk with cells and the matrix [22]. Tenascin-C is a highly flexible isoform due to its tandem FN-III modules that can be revealed by force-induced unfolding [23]. Tenascin-C is a developmentally associated protein that is effectively absent in the adult organism except during wound repair. Interestingly, de novo tenascin-C synthesis is characteristic at the wound’s edge during tissue repair and tenascin-C deficiency elicits abnormalities in wound healing [24]. Tenascin-C depletion reduces the deposition of fibronectin and collagen in the wound healing process where fibronectin-tenascin-C interactions alter cell migration and focal adhesions pivotal to wound closure [25]. A recent study found that supplementation of tenascin-C (in conjunction with another developmentally associated protein Fibrillin-2) on acellular lung scaffolds enhances epithelial proliferation, decreases senescence, aids cell attachment and migration, and improves the morphology and structure of regenerated lung tissue in-vitro and ex-vivo [26]. Tenascin-C accumulation is also detected in fibrotic tissue, suggesting spatiotemporal control of tenascin-C is important to tissue repair [27].
3.1.5. Vitronectin
Vitronectin is a 75kDa blood plasma protein that represents 0.2–0.5% of total plasma proteins with binding domains for integrins and matrix proteins (e.g, collagen and heparin) and non-matrix proteins (e.g., plasminogen activator inhibitor-1) [28]. Vitronectin regulates plasminogen activation during wound healing [29]. Plasminogen activation is an extracellular proteolytic cascade generating plasmin—a protease that cleaves matrix, mediates clearance of fibrin clots, and activates MMPs critical for matrix remodeling and angiogenesis [30]. Vitronectin also binds activated platelets and initiates platelet aggregation [31] and vitronectin depletion results in delayed coagulation [32]. The addition of exogenous vitronectin enhances the wound closure rate of corneal epithelial cells in vitro [33]. Vitronectin regulates growth factor activity and has been suggested as a growth facto delivery vehicle for growth factors [34] which has shown promise in early clinical study for treatment of chronic wounds [35].
3.1.6. Glycosaminoglycans and proteoglycans
Glycosaminoglycans (GAGs) such as hyaluronic acid (HA), chondroitin sulfate (CS), dermatan sulfate (DS), and heparan sulfate (HS) are synthesized by fibroblasts and contribute to the hydrophilic granulation matrix [36, 37]. Proteoglycans consist of core proteins or glycoproteins with glycosamino side-chains that interact with granulation tissue, altering matrix biomechanical properties and hydrophobicity, and modifying cell migration and fate. Proteoglycans are a reservoir for growth factors (e.g., TGFβ, VEGF and FGF) critical to tissue repair. For example, the activity of FGF-2 (which stimulates fibroblast growth) is mediated by HS-proteoglycans that facilitates binding and stabilization of FGF-receptors [38].
HA interacting with HA-binding proteoglycans (e.g., aggrecan, versican, neurocan, and brevican) is ubiquitous in the human body and involved in many different tissue repair events, including the regulation of fibroblast phenotypes [39]. Syndecans are transmembrane HS-proteoglycans that control cell migration and wound closure [40], and act as a regulator of heart fibrosis [41]. HA-binding proteoglycans bind growth factors and have been exploited as growth factor delivery vehicles for diabetic wound healing [42]. The HS-proteoglycan perlecan is part of the basement membrane and involved in epithelial cell plasticity and angiogenesis. Recent work has demonstrated the potential to induce angiogenesis and healing of cutaneous wounds with scaffolds functionalised with perlecan and VEGF [43].
3.1.7. SPARC
Secreted protein acidic and rich in cysteine (SPARC) is a 32kDa glycoprotein expressed at sites of injury and matrix remodeling [44]. SPARC is a modulator of matrix organization and cell-matrix interactions through binding of matrix proteins and modulating integrin-linked kinase activity [45]. SPARC-deficient mice exhibit tissue repair defects manifesting as delayed formation of granulation tissue and diminished ECM production [46]. SPARC is also involved in idiopathic pulmonary fibrosis through activation by TGFβ [47]. Inhibition of SPARC attenuates the pro-fibrotic effect of TGFβ [48].
3.1.8. CCN proteins
CCNs are a family of six secreted proteins with key roles in tissue repair and matrix remodeling. CCNs interact with cells and granulation matrix via binding sites for integrins and HS-proteoglycans [49]. Platelets release CCN2, leading to expression of other CCN isoforms at the site of tissue injury. CCN2, alongside TGFβ, promotes expression of matrix proteins and is overexpressed in fibrosis, and is suggested as a potential diagnostic marker of fibrosis [50]. CCN5 has an opposing role to CCN2 in the progression of fibrosis, where CCN5 inhibits cardiac hypertrophy and fibrosis [51]. Interaction with TGFβ-signalling is a common functional motif of CCN proteins. Similarly, CCN4 is up-regulated in idiopathic pulmonary fibrosis. CCN1 is also considered anti-fibrotic as it induces myofibroblasts senescence [52]. CCN1 structurally bridging macrophages to neutrophils through binding of phosphatidylserine on neutrophils and integrins on macrophages and plays a key role in neutrophil clearance, and CCN1 has been suggested as a possible treatment for non-healing chronic wounds [53]. CCN3 promotes fibroblast adhesion and wound healing in skin wounds via integrin-binding and has been demonstrated to be pro-angiogenic [54].
3.1.9. Thrombospondin
Thrombospondins (TSPs) are multidomain oligomeric glycoproteins with distinct domain structure and binding sites for cells, growth factors, GAGs and other matrix proteins [55]. A multivalent structure allows TSPs to link matrix proteins and facilitate matrix organisation. TSP1 is expressed by macrophages and facilitates re-epithelialisation through organising matrix structure and fibrils [56], while also acting as a regulator of latent TGFβ [57]. Depletion of TSP and TSP1/TSP2 results in a prolonged inflammatory response and delayed healing [58]. TSP1 is a mediator of renal fibrosis in the pathophysiology of ischemic kidney failure [59]. TSP2 is necessary for collagen fibrillogenesis [60] and TSP2 is required for platelet aggregation in the early response to tissue injury [61]. TSP2 also acts as an angiogenesis inhibitor and is regulated by protein kinase B (Akt) in fibroblasts, which mediates cell migration, adhesion and differentiation [62].
3.1.10. Osteopontin
Osteopontin is a 44kDa phosphoprotein that has been extensively studied in the context of bone mineralisation and repair. Osteopontin is also a participant in skin and cornea wound healing. Fibroblast phenotype, matrix organisation and collagen fibrillogenesis are all altered when osteopontin is depleted [63, 64].
3.1.11. Fibulin
Fibulins are multidomain proteins with binding sites for several matrix proteins, with strong affinity for basement membrane proteins laminin and nidogen, and consensus sequences for calcium binding [65]. Fibulin is deposited by fibroblasts as extracellular fibers or secreted into the blood, and five different isoforms have been identified. Fibulin fibers regulate TGFβ and fibroblast migration in cardiac tissue [66, 67]. Fibulin-5 accumulation is associated with abnormal tissue repair in chronic obstructive pulmonary disease [68] while the addition of exogenous fibulin-5 promotes skin wound healing [69]. These elastic fibers of fibulin-5 may not directly impact short-term wound healing, but rather are important for long-term function and integrity of the tissue [70]. While the structure-function relationship of fibulin fibers in repair processes is not well understood, fibulin has a role in long-term tissue repair.
3.2. Matrix mechanics – how stiffness and force regulate tissue repair
Mechano-chemical feedback mechanisms between cells and the matrix regulate the wound healing cascade. The dynamic composition and architecture of the matrix during tissue repair alter the viscoelasticity of the wound bed, and in turn growth factor activation, cell adhesion, migration, and differentiation. Further, cell-generated traction leads to mechanical unfolding of proteins that reveals neo-interaction sites as an additional regulatory feature of ECM-mediated wound healing.
Fibronectin and collagens are the first matrix proteins deposited after tissue injury. Fibronectin fibrils can be mechanically unfolded by cells to regulate protein structure/function. Cytoskeletal tension leads to the reversible extension of fibronectin, exposing FNIII motifs and altering integrin-binding affinities and cell signaling [71]. The elasticity of fibronectin fibers assists collagen assembly, as collagen I fibers preferentially co-localize with more-relaxed fibronectin fibrils [13]. Similarly, tenascin-C and tenascin-X, which differ only in number of FNIII domains, can be mechanically unfolded to generate functional diversity and the ability to sustain cell-ECM or ECM-ECM interactions over long extensions [23].
Collagen morphology determines the physicochemical properties of the collagen matrix and affects collagen degradation, fibrogenesis, and integrin-mediated mechanocoupling. Cell motility and the ability of cells to translocate collagen fibers is modulated by collagen stiffness and porosity [72], which together can induce a change in mode of migration [73]. Collagen I, the major constituent of scar tissue, has a fractal microarchitecture that confers stiff matrix properties that alter cellular responses, such as inducing epithelial-to-mesenchymal transition (EMT) and mesenchymal transcription responses through integrin-mediated mechanosensing [74]. Accordingly, in fibrotic processes, excess collagen I deposition triggers an increase in myofibroblasts (originating via EMT) that in turn deposit matrix and propagate tissue fibrosis. The mechanical properties of collagen are also associated with TGFβ activation via myofibroblast’s integrin-mediated growth factor activation [75].
3.3. Proteolytic matrix remodelling
During tissue repair, spatiotemporal expression of enzymes leads to cleavage of matrix proteins that modulates matrix morphology (i.e., mechanical properties) and biochemical signaling by releasing or unravelling bioactive matrix fragments known as cryptic fragments or matricryptins. Among the matrix degrading enzymes important for tissue repair are MMPs, ADAMs (a disintegrin and metalloproteinase), ADAMTSs (a disintegrin and metalloproteinase with thrombospondin-like motifs), and neutrophil elastase. Proteolysis guides cell migration by local matrix degradation and modulate cell signaling. Moreover, matrix-degrading enzymes release (and activate) growth factors from the ECM.
3.3.1. Matrix metalloproteinases (MMPs)
MMPs belong to the zinc proteinase superfamily and are expressed by cells during tissue injury and repair. MMP activity is regulated by the activation of pro-enzyme or inhibition through endogenous tissue inhibitors of metalloproteinases (TIMPs). Twenty-two different human isoforms of MMP with distinct substrates and tissue distribution have been identified. MMPs are either secreted or membrane-bound (MT-MMPs) and are generally classified by their substrate and mechanism of action. However, redundancy of substrates and identification of new substrates may soon render the historical classification of MMPs obsolete. The MMPs involved in tissue repair processes cleave matrix proteins including collagens, gelatin, laminin, fibronectin, proteoglycans, tenascin, vitronectin, and osteopontin in local matrix remodeling events.
MMPs process matrix proteins during tissue injury and repair and also act on cellular junction and adhesion proteins. MMPs have been shown to cleave E-cadherin, an adherens junction protein responsible for epithelial cell sheet formation, to regulate epithelial cell extrusion and cell migration events [76]. MMPs interact with integrins to modulate cell adhesion and migration [77]. MMPs also regulate vascular remodelling by liberating sequestered angiogenic factors and by generating cryptic anti- and pro-angiogenic molecules [78]. Given the key roles of MMPs in tissue repair, increased MMP expression has been correlated with impaired wound healing and fibrosis [79]. Elevated levels of certain MMPs have been suggested as diagnostic markers of disease-state and therefore MMP-inhibition represents a potential therapeutic strategy for improving tissue repair outcomes [80, 81]. However, pharmaceutical targeting of MMPs is problematic, as poor selectivity and off-target effects of exogenous MMP inhibitors has led to multiple failed clinical trials [82], highlighting a need for unique approaches to MMP targeting.
3.3.2. ADAMs and ADAMTSs metalloproteinases
ADAMs and ADAMTSs are members of the zinc proteinase family and are involved in tissue repair, vascular remodeling and activation of growth factors. ADAMs have been found to be important in skin and human lens epithelium wound healing [83–85]. ADAMTSs degrade proteoglycans [86], process pro-collagen to collagen [87], regulate blood coagulation, and facilitate vascularization [88–90].
3.3.3. Neutrophil Elastase
Neutrophil elastase is secreted by neutrophils and macrophages during the early wound healing response. Neutrophil elastase is responsible for extracellular killing of microorganisms [91], and has been shown to process laminin in regulating neutrophil extravasation [92–95].
4. Scar-free healing: Unique matrix environments in the embryo and regenerating organisms
The ECM provides a pro-regenerative microenvironment in fetal tissue repair and in regenerating vertebrates that may provide therapeutic opportunities for improving wound healing in humans (Table 2). In stark contrast to adult wounds, fetal tissue repairs rapidly and scar-free. Differences exist between the ECM of adult and embryonic tissue, highlighting the role of matrix composition and architecture in scar-free healing [96]. The ratio of type I to type III collagen is higher in adult tissue repair processes compared to embryonic repair [97]. Murine models of collagen III deficiency show increased scar formation, indicating an important role of collagen III in the regulation of scar tissue formation. Collagen I provides strength to the closing wound, but is the major constituent of the scar-forming matrix and a physical barrier to regeneration. Tenascin expression occurs earlier in the fetal wound compared to the adult [98]. Elevated levels of HA, a granulation tissue component and regulator of fibroblast phenotype and cell motility, is elevated in the fetal wound matrix which likely provides a more permissive matrix for cell movement and proliferation [99]. It has been further shown that there are differences in MMP and TIMP ratios between fetal and adult wounds, favoring matrix turnover and cell migration [100]. Changes in matrix composition, architecture and MMP expression further impact the release and activation of growth factors tethered to the matrix, such as TGFβ, to accelerate scar-free tissue repair in fetal tissue.
Table 2. Key differences in ECM content in scarring vs. regenerative healing.
| Scarring | Regeneration | Function in regeneration | |
|---|---|---|---|
| Collagen I | High expression, disorganized, and high crosslinking | Low expression, organized, and low crosslinking | Col I maintains tissue structural integrity but excessive levels and high crosslinking are physical barriers to regeneration |
| Collagen III | Decreased expression | Increased expression | Increased levels of col III offers a looser matrix network to allow cell migration, proliferation, and differentiation. Col III is required for col I fibrillogenesis. |
| HA | Low levels | High levels early and sustained | Increased levels of HA in matrix facilitates cell movement, mitigates fibroblast proliferation, and augments inflammation |
| Tenacin C | Late expression | Early expression | Early expression of tenascins enables cell migration for rapid epithelialization and wound closure |
| Fibronectin | Later deposition | Early deposition | Early deposition of fibronectin provides cell anchorage points for rapid wound closure |
| MMP:TIMP ratio | Low | High | High MMP:TIMP ratio favors matrix remodelling and turnover |
Insights into scar-free tissue repair can be gained by understanding how some non-mammalian vertebrates undergo tissue regeneration. Not surprisingly, dynamic breakdown and remodeling of the ECM is pivotal to tissue regeneration. Zebrafish, salamanders, and lizards are known for their regenerative capacity. Biphasic expression of MMPs is critical for faithful limb and tail regeneration in lizards and salamanders. The first wave of MMP expression is thought to promote epithelial cell migration while inhibiting basement membrane formation and the second phase of MMP expression is responsible for ECM remodeling and turnover [101]. The delayed basement membrane formation is an important distinction in regeneration vs. mammalian tissue repair. In most mammals, the basement membrane is restored during early stages of wound healing and is associated with a switch from collagen III to collagen I which is progressively cross-linked to an acellular fibrous scar. In salamanders and fish however, expression of basement membrane constituents (e.g., collagen IV and laminins) is delayed until late stages of tissue repair/regeneration [102, 103]. Synthesis and cross-linking of collagen I is also delayed until final stages of scar free repair [103]. Increased expression of hyaluronic acid, tenascin, collagen III, fibronectin, and developmentally-associated collagens (e.g., collagen XII) may also be important to the regenerative ECM landscape [101].
5. Strategies to reduce scarring
Fibrosis is the major contributor to the manifestation and progression of a myriad diseases and represents one of the most compelling health issues in modern medicine. Scarring causes substantial morbidity; for example there is high incidence of fibrotic adhesions of internal organs in response to trauma, infection, radiation, or surgery that often leads to pain, bowel obstruction, and infertility. In diseases such as idiopathic pulmonary fibrosis, liver cirrhosis, cardiac fibrosis, systemic sclerosis and nephritis, fibrosis can lead to organ failure and death. Despite the fact that fibrotic diseases account for nearly 45% of deaths in the developed world, there is currently no accepted therapy to target fibrosis. However, insights gained over the past 2 decades into the molecular pathways driving fibrosis has informed the development of potential therapies.
Dozens of fibrosis therapies have been developed over the past decade and can be classified as pharmaceuticals, biomaterials, and cell therapies (Table 3). Common therapeutic targets include TGFβ signaling, inflammation, and fibroblasts (i.e., honing, activation, and proliferation). Strategies to target fibrotic disease have had limited clinical success to date, and is reviewed in detail elsewhere [104–106]. Given that fibrosis is often characterized by several simultaneous pro-fibrotic pathways, a multifaceted approach is likely key to success. In the following section, we focus on matrix inspired approaches to limit fibrosis and achieve functional tissue repair.
Table 3. Selected interventions that may limit fibrosis.
| Category | Name | Class | Potential Target/mechanism | Phase |
|---|---|---|---|---|
| Pharmaceuticals | Enalpril | Angiotensin converting enzyme inhibitors | Inhibit fibroblast activation | Clinic |
| Pirfenidone | Small molecule | TGFβ, inflammation | Clinic | |
| Nintedanib | Small molecule | Receptor tyrosine kinsases | Clinic | |
| Anakinra | Monoclonal antibody | Inflammation (IL-1RA) | Clinic | |
| Fresolimumab | Monoclonal antibody | Inflammation (IL-13) | Phase II | |
| Endostatin | Recombinant fragment of col XVIII | TGFβ | Pre-clinical | |
| Lumikine | Peptide fragment of lumican | TGFβ | Pre-clinical | |
| Biomaterials | Integra™ | Silicone and bovine col I-chondroitin sulfate mesh | Collagen and GAG mesh provide environment to facilitate repair | Clinic |
| Surgisis® Oasis® Biodesign® CorMatrix® |
Porcine SIS ECM scaffold |
Multi-functional molecules liberated during matrix degradation mediate inflammation and repair | Clinic | |
| XenMatrix® | Porcine dermis ECM scaffold |
See above | Clinic | |
| Alloderm® Allomax® |
Human dermis ECM scaffold |
See above | Clinic | |
| MatriStem® | Porcine UBM ECM scaffold |
See above | Clinic | |
| VentriGel™ | Porcine myocardial ECM hydrogel |
See above | Phase I | |
| Cell-based therapies | Dermagraft® | Allogeneic neonatal cells on vicryl scaffold | Neonatal cells and scaffold provide environment to facilitate repair | Clinic |
| Apligraf® | Allogeneic neonatal cells on bovine col I matrix | Neonatal cells and scaffold provide environment to facilitate repair | Clinic | |
| Mesenchymal stem cells | Allogeneic stem cells | Paracrine signalling alters inflammation | Phase I |
6. Matrix-based materials for modulating tissue repair
The intimate role of the ECM in post-inflammatory tissue repair, wound healing, and fibrosis dispels the historic view of ECM as simply an inert support structure for cells. The ECM microenvironment in which cells reside is comprised of structural fibers, functional molecules, and a signalling repository which is highly heterogeneous and varies from tissue to tissue. ECM-cell interactions activate cellular responses (e.g., proliferation, differentiation, and migration) and tissue-specific cells are constantly secreting, assembling, and remodelling the ECM. Accordingly, the design and fabrication of ECM mimicking materials has been pursued for decades and represents a core strategy in tissue engineering. Due to the complexity of composition, structural organization, biomechanics, and dynamic roles of the ECM, synthesis of a high fidelity ECM mimic is not yet feasible. For this reason, typical biomaterial approaches focus on a few of the mechanisms by which ECM influences cells and attempts to effectively present these cues for a given tissue. Thus, essentially every material studied in tissue engineering can be classified as an ECM-like material but this review will focus on biologically derived ECM-like materials.
6.1. Components and cryptic fragments of the ECM
In mammals, the ECM consists of over 300 proteins including collagens, proteoglycans, and glycoproteins. Individual ECM components have been isolated from tissues to study constituent effects on cell behaviour and to manufacture scaffolds for various applications. Matrix materials have been generated from purified collagens, fibronectin, fibrin, laminin, HA, and CS, among others. Purified Type I collagen derived scaffolds are among the most common ECM-derived protein and are FDA allowed for several clinical applications, mostly as hemostatics and soft tissue fillers. Enzymatic cleavage of many ECM proteins can release or reveal bioactive fragments that exert biological activity which is distinct from the parent molecule. ECM proteins and their subdomains bind integrins and other cell surface receptors to regulate gene expression, cell differentiation, mitogenesis, and migration. Through the role of ECM fragments in directing cell activity and cell fate, matrix components regulate inflammation, angiogenesis, and scarring outcomes during wound healing. Given the interaction of the ECM with the wound healing process, there has been considerable interest in the cryptic fragments and degradation products of ECM for tuning wound healing.
Cryptic fragments have been described for a variety of matrix proteins and contribute to tissue repair events ranging from cell migration and epithelial-to-mesenchymal transition to the formation of blood vessels. Selective ECM degradation generates microenvironments with unique biochemical and morphological ECM properties to instruct cell behaviour [107]. Past studies have shown relative success in use of matrikines as inhibitors of angiogenesis and inflammation, and angiogenesis inhibitors have been proposed in clinical studies for cancer therapy [108, 109]. Individual ECM fragments can also alter wound healing outcomes. A peptide fragment of lumican, a small leucine rich proteoglycan, has been shown to promote corneal wound healing following epithelial debridement, in part by mitigating TGFβ signalling [110]. Collagen IV contains hidden signaling molecules, such as arresten and canstatin that become active through proteolytic processing by MMPs and regulate angiogenesis after tissue injury by coordinating basement membrane remodelling and vascular sprouting [111–113]. Similarly, cryptic laminin fragments impact epithelial and endothelial cell migration and adhesion, angiogenesis, MMP activation, and EMT-related events involved in fibroblast differentiation and wound closure [18, 114, 115]. The laminin β1-chain-derived fragment, liberated by MMP processing, interacts with cells via α3β1-integrins and triggers down-regulation of active MMP2 in mouse and human ESCs [116], and was recently shown in a pre-clinical study to prevent peritoneal fibrosis [117].
Individual fragments and cryptic sites of ECM provide interesting insights to the complex process of ECM remodelling during wound repair. Bioactive ECM fragments are potential drugs and have been tested in experimental models of cancer, wound healing, fibrosis and infectious diseases either as full-length fragments, modified fragments or peptides derived from their sequences, but their translation into the clinics is challenging. Clinical trials with recombinant collagen XVIII fragment (i.e., endostatin) as a cancer therapy have been largely unsuccessful in the US despite approved clinical use of endostatin in China [118]. However, there is still potential for endostatin as an anti-fibrotic [119]. Translation to date has been limited largely due to low potency of individual ECM fragments relative to the cognate ligands for the receptors known to trigger cell activation; for example, IL-8 binds and activates neutrophils at nanomolar concentrations [120], while micromolar concentrations of chemotactic matrix-derived peptides are needed to activate neutrophils [121].
The combination of several ECM bioactive fragments as an alternative approach to increase their biological effect has been studied by examining the products released from ECM materials as they degrade. The degradation products of decellularized tissues releases numerous protein fragments and peptides with reported biologic function. Early study in this area showed that peptides generated during solubilisation of SIS are chemoattractants for endothelial cells [122]. The fragments generated by enzymatic degradation of a decellularized urinary bladder matrix (UBM) have shown an increased proliferative and migration response in perivascular stem cells [123], especially in hypoxic conditions similar to oxygen levels after injury [124]. Host-mediated degradation of UBM has also been shown to be chemotactic for progenitor cells, which established a link between chemotactic properties that result from in vitro methods of ECM scaffold degradation and the in vivo process of scaffold remodeling [125]. Degradation products of UBM injected at the site of digit amputation caused an accumulation of multipotent progenitors expressing Sox2, Sca1, and Rex1 [126] and these effects were later attributed in part to a cryptic peptide from the C-terminal region of collagen III [127]. Bioactive components of degraded fetal cardiac ECM [128] and partially degraded adult cardiac ECM [129] have also been shown to promote the expansion of cardiomyocytes. Most frequently, these studies were conducted using a complex mix of degraded ECM materials which makes it difficult identifying contributions and interpreting mechanism of action. However, it’s unquestionable that degradation products of ECM have measureable bioactivity that may be a therapeutic strategy to enhance tissue repair. One approach to deliver several bioactive fragments as a therapeutic that has gained attention over the past decade uses scaffolds and hydrogels derived from decellularized tissue.
6.2. Decellularized matrices
The concept of tissue decellularization is not new. The earliest reports of tissue decellularization date back nearly 7 decades, where in 1948 pulverization and freeze-thaw cycles were used to render muscle tissue acellular [130]. In 1991, an early tissue engineering study used allogeneic acellular dermal matrices as a biomaterial to facilitate reconstruction of skin with the use of autologous keratinocytes and fibroblasts [131]. The first use of xenogeneic tissue was described in 1989, where small intestinal submucosa (SIS) was used as a scaffold for vascular applications [132]. Since then, methods for preparing ECM scaffolds been developed and refined for nearly every tissue in the body [133]. All tissues are comprised predominantly of the same base biopolymers (i.e., collagens, glycans, laminin, elastin) with collagen I representing the majority of the matrix by weight. This unique properties of different decellularized tissues are therefore attributed to differences in biopolymer compositions and structures attributed to the cellular composition of the tissue. Numerous ECM scaffolds are now FDA-allowed and commercially available from a range of source tissues and species (Table 3).
The objective of decellularization is to remove cell and antigenic components from a tissue while limiting damage to the underlying native ECM. The methods required to extract cells is a tissue-dependent procedure due to differences in cell density, matrix density, tissue thickness and shape amongst different tissues. Disruption of and removal of cells from tissues is typically achieved with physical treatments such as freeze-thawing, agitation, sonicaton, or mechanical pressure combined with detergents, ionic solutions, and/or enzymatic treatment which invariably causes some disruption of matrix. Methods of tissue decellularization has been reviewed in detail elsewhere [133]. Optimal decellularization processes require a balance between adequate cell removal and preservation of the matrix. Decellularization processes that are too harsh or too gentle can elicit adverse outcomes and pro-inflammatory responses arising from ineffective removal of antigens (e.g., α-gal or MHC-I epitopes), residual detergents, endotoxin contamination, or changes in the ECM structure [134, 135]. Complete removal of all cell organelles, lipid membranes, cell-derived nucleic acids is likely not possible and there is measureable cellular debris in every ECM scaffold including those in clinical use [136, 137], but given that such scaffolds generally not associated with adverse reactions by patients it is suggested that levels of cellular debris below some threshold is acceptable. Despite potentially dire consequences of incomplete decellularization [138], quantitative metrics for decellularization still do not exist.
When prepared properly, ECM scaffolds can act as an inductive template for tissue repair. Numerous materials derived from decellularized tissues are used clinically to repair tissue in diverse applications including plastic and reconstructive, dental, orthopaedics, and cardiovascular procedures among others. The most extensive clinical use of ECM scaffolds is for hernia repair, where xenogeneic and allogeneic decellularized tissues like Surgisis®, Allomax®, and AlloDerm®, are considered to have improved performance characteristics over synthetic mesh materials [139–142]. Decellularized tissues have also aided in the repair of critically sized skin defects. Alloderm® has been shown to increase skin flexibility and improve scar quality in full-thickness wounds [143] while Oasis® has facilitated complete healing in 60% of patients with chronic leg ulcers compared to 35% with the standard treatment [144]. Various decellularized dermis mesh materials are increasingly used in breast reconstructive procedures [145, 146], and Alloderm® has also been used to facilitate frozen-banked ovarian tissue transplantation in the first reported pregnancies and live birth using this technique [147]. Decellularized tissues are commercially available in cardiac applications for the repair of heart valves (e.g., SynerGraft®) and pericardium (e.g., CorMatrix® ECM).
Along with the widespread use of ECM-based products, many preclinical and clinical studies continue to use decellularized matrices to improve repair of tissues including muscle, oesophageal, and cardiac. In a clinical report of 13 patients from an ongoing trial (ClinicalTrials.gov Identifier: NCT01292876) with volumetric muscle loss, decellularized tissue was implanted and combined with aggressive physical therapy improved strength by 37% and range-of-motion tasks by 27% [148]. While more study is necessary, particularly to control for the effect of physical therapy and differences amongst 3 different ECM scaffolds used, this and another study [149] suggest ECM can enhance the repair of volumetric muscle lesions to improve strength and function, albeit increased strength may not be attributable to appreciable de novo muscle formation [150].
Decellularized tissues have also supported the reconstruction of tissues in the gastrointestinal tract [151]. In patients with superficial adenocarcinoma, restoration of the normal epithelial lining of the esophagus was achieved by implantation of a tubular ECM scaffold following en bloc circumferential resection of the diseased mucosa and submucosa [152]. A recent follow-up study showed this same strategy of mucosal resection with ECM scaffold placement also facilitates repair of tissue at the opposite end of the gastrointestinal tract in the colon/rectum [153]. The technique, informed by preclinical studies showing the ECM scaffold was necessary to support regrowth of mucosal tissue and prevent recalcitrant stricture [154, 155] is currently being studied in patients with esophageal high grade dysplasia (ClinicalTrials.gov Identifier: NCT02396745).
While an enhanced tissue healing response has been demonstrated with ECM materials, the ultimate goal to achieve scarless wound healing remains largely unmet. Studies in highly regenerative species like the salamander and zebrafish have highlighted the importance of the ECM in regenerative healing [101]. Building upon this concept, a recent study has shown that cardiac injection of particulate ECM derived from a healing zebrafish heart induced a regenerative effect in the murine heart following myocardial infarct. The authors showed proliferation of cardiomyocytes and cardiac precursor cells was induced by the injection of decellularized zebrafish cardiac tissue. The effect was associated with the higher level of neuregulin-1 in ECM derived from regenerating zebrafish myocardium compared to adult mouse cardiac ECM, and ErbB2 signaling was involved in inducing the regenerative outcome [156].
Despite many successes of decellularized tissues in preclinical and clinical settings, the field is still developing. It should be noted that outcomes associated with the use of ECM scaffolds have ranged from success to failure, and the reasons for this disparity are now mostly understood. Stated simply, not all commercially available decellularized tissues are created equal—differences exist amongst the source tissue, sizing, storage, proprietary processing, and sterilization. A recognized drawback on decellularized tissue matrices is the inherent heterogeneity amongst source tissues. For example, the composition of decellularized myocardial matrix from different cadaveric donors has measurable donor-to-donor variation [157]. Quantitative proteomics provides a tool that will likely improve the characterization and quality control of tissue derived scaffolds. A recent study described the use of proteomic methods to enable refinement of processing methods to attain high fidelity ECM constructs from lung tissue [158]. Still, heterogeneity between donor tissues can result in heterogeneity in decellularization. Implantation of the first decellularized porcine valve introduced in Europe, SynerGraft®, resulted in the death of 3 of 4 of the pediatric patients undergoing valve repair. Analysis of the SynerGraft® valves showed that decellularization was incomplete and resulted in severe inflammation and valve failure [138]. This sobering outcome together with limitations due to tissue heterogeneity highlight the need for robust quality control measures for decellularized tissues.
6.3. Tissue-derived injectable hydrogels
The generation of tissue derived hydrogels from SIS in 1998 prompted the expansion in use of decellularized materials beyond the sheet or mesh materials described previously [159]. Tissue-derived hydrogels, or ECM hydrogels, represent a growing subset of injectable biomaterials in tissue engineering. Hydrogels are defined as highly hydrated polymeric materials with polymer chains and crosslinks to provide structural integrity. Natural ECM mimicking hydrogels contain natural polymers (e.g., collagen, aliginate, chitosan, or gelatin) and have been extensively studied and reviewed in detail elsewhere. Such hydrogels are composed of one or more purified ECM proteins and have promising applications. Single protein or collection of purified ECM components, although well-defined and relatively simple materials, is not able to recapitulate the complexity of the ECM microenvironment. Matrix-derived hydrogels with increasing complexity, although less defined, can be produced from decellularized tissues and have shown promise in mediating tissue repair outcomes.
Tissue derived hydrogels are formed through solubilisation of decellularized tissues. These gels are typically digested at low pH in a pepsin solution. Once the decellularized tissue is soluble, salt concentration and pH are balanced to physiologic levels and a gel forms spontaneously when warmed to 37°C. The soluble mixture is a viscous liquid below 22°C, and displays intermediate solid-like and fluid-like properties at 37°C [160]. Gel formation occurs through collagen self-assembly and is augmented by the presence glycosaminoglycans and other ECM proteins [161]. The source tissue from which the hydrogel is derived will impact the biochemical makeup and in turn the mechanical properties of the gel. Hydrogels have been formed and characterized from a range of decellularized tissues including adipose, gastrointestinal, heart, lung, and skin among others. A recent review nicely summarizes the structural and functional characteristics of such gels [162]. Tissue derived hydrogels have mechanical properties that are tunable by varying the concentration of matrix, providing some application-specific control of material properties. In some instances the hydrogels retain cues from the source tissue that can enhance cellular responses of tissue-specific progenitors. ECM hydrogels are cell-friendly and commonly used as a cell culture substrate. Several studies have shown improved or equivalent responses of cells grown on/in ECM hydrogels when compared to reconstituted basement membrane extract from Englebreth-Holm-Swarm tumor (i.e., Matrigel®) or purified collagen. Promising results from in-vitro studies with ECM hydrogels has spurred increased study of such hydrogels in pre-clinical in-vivo studies. The delivery of solubilized ECM via catheter or syringe to irregularly-shaped anatomic sites provides increased utility of ECM-derived materials.
ECM hydrogels are injectable materials that allow for minimally invasive delivery to target tissues. The viscous soluble pre-gel ECM material is shear thinning, exhibiting a decrease in viscosity under shear, which enables injection of the ECM. Low viscosity of the pre-gel solution and gelation kinetics can be optimised to achieve minimally invasive injection with sufficient time required for delivery of the pre-gel solution to the anatomic site before gelation begins. The injectability through catheters or 18-27 gauge syringes has been demonstrated demonstrated for ECM hydrogels derived from adipose, cartilage, dermis, intestine, liver, lung, muscle, and urinary bladder. The in-vivo application of ECM hydrogels has shown promise in a number of applications, particularly where there is an existing unmet need for minimally invasive therapies.
Tissue derived hydrogels have been tested pre-clinically as a therapeutic for skeletal muscle injury, defects in the central nervous system (CNS), myocardial infarction, and ulcerative colitis. In-situ polymerizing hydrogels composed of ECM enhance skeletal muscle remodelling in injury models. Hydrogels derived from both UBM and dermal ECM skeletal promote muscle remodelling in partial thickness abdominal wall defects with UBM treatment resulting in relatively higher myogenesis [163]. ECM hydrogel has also shown therapeutic benefits in treating skeletal muscle ischemia reperfusion injuries. In a rat hindlimb ischemia model, treatment with injectable ECM derived from skeletal muscle and umbilical cord increased tissue perfusion with an enhanced regenerative response elicited by skeletal muscle derived ECM hydrogel [164]. A number of studies have investigated the therapeutic potential of ECM hydrogels in treating tissue defects in the CNS. A recently described technique was used to deliver ECM hydrogels intracerebrally to a stroke cavity [165], where UBM hydrogel at 8 mg/mL was shown to induce a cell infiltrate consisting of 60/30 mix of brain-derived/peripheral macrophage phenotypes that may potentially be exploited for treating stroke lesions [166]. ECM hydrogels have also been applied to traumatic injuries of the brain and spinal cord. Traumatic brain injuries treated with brain-derived ECM had reduced lesions volumes and improved neurobehavioral function [167], while UBM hydrogels reduced lesion size and improved motor function despite no measureable improvement in cognitive recovery [168]. Decellularized spinal cord and UBM hydrogels were used as a substrate to bridge lesions following spinal cord injury [169]. Catheter delivery of porcine myocardial matrix hydrogel has also been demonstrated as safe and effective in improving cardiac function following myocardial infarction in preclinical studies [170] and is currently the subject of clinical study (ClinicalTrials.gov Identifier:NCT02305602). A recent preclinical study in in rats with ulcerative colitis showed functional improvement in epithelial barrier function following treatment with SIS hydrogel [171]. The ECM was delivered as an enema and following in-situ gelation adhered to the colon wall more than 24 hours, potentially expanding the applications for tissue derived hydrogels beyond treating only compartments or lesions of tissues.
6.4. Method of repair: modulating the inflammatory wound healing process
While many studies have demonstrated the therapeutic effect of decellularized tissues, either as a scaffold or hydrogel, the exact mechanism of repair remains to be fully understood. The implantation or injection ECM materials is inextricably linked to acute tissue injury at the site of application, and it’s not surprising that the success of ECM materials has largely been attributed to the ability of ECM to modulate the host response that commences upon implantation or injection. ECM materials are rapidly, within hours of implantation or injection, infiltrated by mononuclear cells. The ECM material then undergoes host-mediated biodegradation which is followed by an orchestrated spatiotemporal cellular response characterized by immune modulation and stem/progenitor cell mobilization, proliferation, and differentiation.
ECM degradation is a requirement for constructive tissue remodelling. The ECM material is infiltrated by host cells, especially macrophages, and the release of matrix metalloproteinases (MMPs) and other proteases contribute to the degradation of ECM. As a depot of biological signals, the degradable ECM materials act as vehicles for delivery of a variety of functional molecules. Decellularized tissues are rich in growth factors, bifunctional molecules such as fibronectin and various types of collagen, among other structural and functional molecules. Bioactive fragments of parent ECM molecules such as collagen, fibronectin, and laminin, are also generated by proteolytic degradation of the ECM [116, 117, 122, 172, 173]. The degradation rate of ECM scaffolds varies with the source tissue and the site of application. Besides a few studies with SIS ECM scaffolds, there is limited data on the degradation rate of ECM scaffolds. When used as a scaffold for Achilles tendon repair 60% of the SIS ECM mass was lost by one month after surgery, and the graft was completely resorbed by three months [174]. When placed in the urinary bladder, less than 10% of the original SIS ECM remained after 3 months [175]. In a model of abdominal wall repair, only 20% of the original the SIS-ECM remained after 4 weeks and the SIS ECM was resorbed by 6 months after implantation [176]. The degradation of ECM hydrogels is relatively fast compared to ECM scaffolds and, again, the rate of degradation/ resorption is dependent on the application. For instance, ECM hydrogels injected into a tissue compartment degrade over weeks [170], while enema preparations of ECM hydrogels delivered to the colon are cleared over days [171]. In summary, depending on the application, ECM degradation is necessary for constructive outcomes and ECM scaffolds are degraded over weeks to months while ECM hydrogels are degraded over days to weeks. Processing methods that affect the degradation of ECM materials, such as chemical cross-linking, will alter the release profile of degradation products and impair tissue remodelling.
Immune modulation is a necessary feature of ECM mediated tissue remodelling. Despite being an underlying contributor to many degenerative and chronic diseases, inflammation plays an essential role in mitigating infection and mediating tissue repair. An intact immune system is necessary for faithful regeneration of the salamander limb [177], zebrafish fin [178, 179] and zebrafish heart [180]. The regenerative capacity of mammalian tissues also relies upon an intact and tuned immune response [181]. While tissue repair and regeneration are initiated by inflammation, excessive or prolonged inflammation often promotes fibrosis. Macrophages, which have garnered the majority of attention in relation to tissue regeneration, act not only through their immune functions, but also control cell function and ECM remodelling. Macrophages are phenotypically diverse, and are key to both initiation/maintenance of inflammation as well as the inflammation resolution/regeneration processes. Research over the past 10 years with ECM-derived materials has correlated specific macrophage phenotypes with regenerative outcomes [182]. Decellularized tissues skew macrophages towards a predominantly M2 phenotype, which is regulatory and anti-inflammatory, and away from the pro-inflammatory M1 phenotype. This M2 majority seems to arise from a reduction in M1-like macrophages rather than an increase in M2-like macrophages. For example, ECM glycoproteins derived from various matrices diminished M1 function [183], and delivering a porcine SIS gel to the colon in chemically-induced colitis abated the inflammatory response resulting in increased proportions of M2 macrophages [171]. Relatively less studied in the context of regeneration is the adaptive immune response but recent work has begun to elucidate the role of T cells in the immune microenvironment and, similar to macrophages, Th-2 cells have been shown to accelerate bone healing [184] and regeneration of injured muscle [185, 186]. There is still much to be learned about the mechanisms of matrix-based materials in generating a therapeutic immune response, but it’s clear that a regulated scaffold immune microenvironment is a critical component of the regenerative niche [106, 187, 188].
The formation of functional tissue requires that stem/progenitor cells are recruited to the site, proliferate, and site-appropriately differentiate. Chemotactic properties of matrix components for a variety of cells (e.g., skeletal muscle cells, esophageal progenitors, perivascular stem cells, among others) have been demonstrated in-vitro. Matrix degradation products have also been shown to affect differentiation and function of stem/progenitor cells. These effects have also been shown in pre-clinical and clinical application of matrix-based materials, where a variety of endogenous stem/progenitors are recruited and accumulate at the site of tissue remodelling.
7. Conclusions
The essential role of the matrix influencing cell function and guiding wound healing provides a model for therapies to improve tissue repair outcomes. Scar-free healing in the embryo and regenerative organisms is guided by the ECM, and these examples provide opportunities for enhancing mammalian tissue reconstruction and regeneration. Matrix based scaffolds derived from decellularized tissue have been used clinically in reconstructive surgeries for decades. The success of these scaffolds in facilitating functional tissue repair is dependent largely upon a scaffold immune microenvironment and scaffold degradation, which has led to increased study of matrix degradation products. Components and cryptic fragments of ECM are revealed during matrix remodelling and elicit a wide range of signalling events and individual components have been used to tune wound healing outcomes. Hydrogels derived from decellularized tissues, which represents a complex mixture of ECM bioactive fragments, has emerged in recent years as a strategy to modulate the repair of various tissues. Improved understanding of the dynamic matrix properties during regeneration, scar-free healing, and fibrotic outcomes will provide intervention checkpoints that may be exploited to guide clinical methodologies for improved tissue repair.
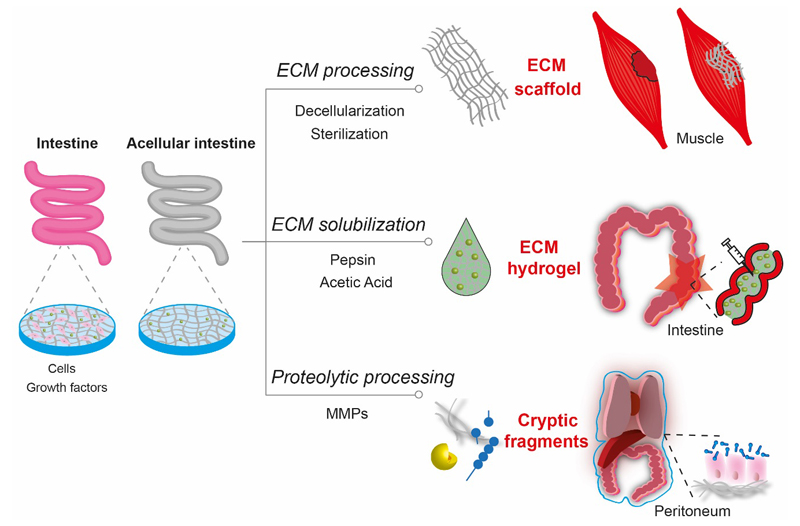
Figure 2. Overview of matrix-based strategies to regulate tissue repair outcomes.
The extracellular matrix (ECM) represents the non-cellular component of every tissue and organ. The matrix can be utilized in various ways based on application of interest. Appropriate tissue decellularization and sterilization processes can be used to prepare a mesh material suitable for filling/bridging tissue defects. Acellular tissue can also be solubilized (e.g., with pepsin or acetic acid) to produce an ECM hydrogel that is suitable for injection or catheter delivery in minimally invasive procedures. Alternatively, specific bioactivity of defined components or cryptic fragments of ECM components can be exploited to mediate tissue repair processes.
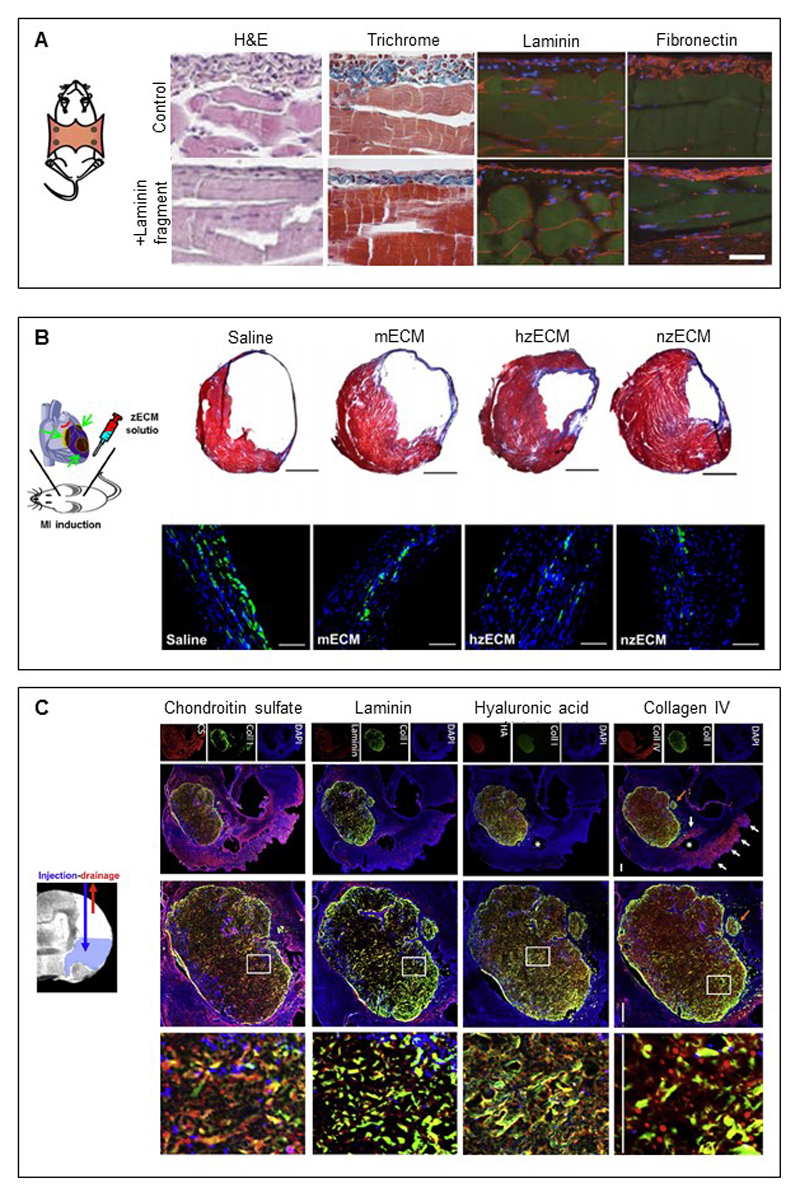
Figure 3. Matrix-based therapies modulate tissue repair.
(A) A cryptic laminin fragment functionalized biomaterial was prevents fibrosis in an established model of peritoneal fibrosis. Compared to controls, mice treated with the cryptic fragment showed a reduced fibrotic response demonstrated by H&E, Trichrome, and fibronectin stains alongside minimal disruption to the basement membrane evidenced by laminin staining (scale bars = 40 μm, Adapted from [117]). (B) Zebrafish extracellular matrix induces mammalian heart regeneration. Extracellular matrix from normal (nzECM) and healing (hzECM) cardiac tissue was micronized and injected in an adult mouse acute myocardial infarct model. Compared to adult mouse cardiac ECM (mECM), the zebrafish ECM, especially the hzECM mitigated scarring as seen in Masson’s trichrome stained sections (collagen in blue, cardiac muscle in red, scale bars = 1mm, Adapted from [156]). Heart sections labelled with CD68 showed a reduction in chronic inflammation at 6 weeks post-infarct when mice were treated with hzECM (CD68 in green, scale bars = 50 μm). (C) Extracellular matrix hydrogels implanted in a stroke cavity using a newly developed injection-drainage strategy promotes an acute endogenous repair response that could potentially be exploited to treat stroke. The hydrogel is infiltrated by host cells, typically of microglia, macrophage, or neural and oligodendrocyte progenitor phenotype, forms to the shape of the lesion, and the ECM components including GAGs (chondroitin sulphate and hyaluronic acid) and basement membrane proteins (laminin and collagen IV) can be detected over time (scale bars = 500 μm, Adapted from [165]).
Acknowledgments
TJK was supported by the Whitaker International Program, Institute of International Education, United States of America. CMH was supported by the Swedish Research Council (VR 4-478/2016). MMS. acknowledges the support of the UK Regenerative Medicine Platform Hub “Acellular Approaches for Therapeutic Delivery” (MR/K026682/1) and the grant “State of the Art Biomaterials Development and Characterization of the Cell-Biomaterial Interface” (MR/L012677/1) from the MRC.
Abbreviations
- ECM
extracellular matrix
- ADAM
a disintegrin and metalloproteinase
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin-like motifs
- CS
chondroitin sulfate
- DS
dermatan sulfate
- EMT
epithelial-to-mesenchymal transition
- FACIT
fibril-associated collagens with interrupted triple-helices
- FGF
fibroblast growth factor
- GAGs
glycosaminoglycans
- HA
hylaluronic acid
- HS
heparin sulfate
- MMPs
matrix metalloproteinases
- SIS
small intestinal submucosa
- SPARC
Secreted protein acidic and rich in cysteine
- TGFβ
transforming growth factor beta
- TIMPs
tissue inhibitors of metalloproteinases
- TSP
Thrombospondin
- UBM
urinary bladder matrix
- VEGF
vascular endothelial growth factor
References
- [1].Ricard-Blum S. The Collagen Family. Cold Spring Harbor Perspectives in Biology. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished Type III Collagen Promotes Myofibroblast Differentiation and Increases Scar Deposition in Cutaneous Wound Healing. Cells, Tissues, Organs. 2011;194:25–37. doi: 10.1159/000322399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- [4].Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE. Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. The Journal of Biological Chemistry. 2009;284:8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Betz P, Nerlich A, Wilske J, Tübel J, Wiest I, Penning R, Eisenmenger W. The time-dependent rearrangement of the epithelial basement membrane in human skin wounds —immunohistochemical localization of Collagen IV and VII. International Journal of Legal Medicine. 1992;105:93–97. doi: 10.1007/BF02340831. [DOI] [PubMed] [Google Scholar]
- [6].Thierry L, Geiser AS, Hansen A, Tesche F, Herken R, Miosge N. Collagen types XII and XIV are present in basement membrane zones during human embryonic development. Journal of Molecular Histology. 2004;35:803–810. doi: 10.1007/s10735-004-1132-y. [DOI] [PubMed] [Google Scholar]
- [7].Rühl M, Sahin E, Johannsen M, Somasundaram R, Manski D, Riecken EO, Schuppan D. Soluble Collagen VI Drives Serum-starved Fibroblasts through S Phase and Prevents Apoptosis via Down-regulation of Bax. Journal of Biological Chemistry. 1999;274:34361–34368. doi: 10.1074/jbc.274.48.34361. [DOI] [PubMed] [Google Scholar]
- [8].Lettmann S, Bloch W, Maaß T, Niehoff A, Schulz J-N, Eckes B, Eming SA, Bonaldo P, Paulsson M, Wagener R. Col6a1 Null Mice as a Model to Study Skin Phenotypes in Patients with Collagen VI Related Myopathies: Expression of Classical and Novel Collagen VI Variants during Wound Healing. PLOS ONE. 2014;9:e105686. doi: 10.1371/journal.pone.0105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jimenez-Mallebrera C, Maioli MA, Kim J, Brown SC, Feng L, Lampe AK, Bushby K, Hicks D, Flanigan KM, Bonnemann C, Sewry CA, et al. A comparative analysis of collagen VI production in muscle, skin and fibroblasts from 14 Ullrich congenital muscular dystrophy patients with dominant and recessive COL6A mutations. Neuromuscular Disorders. 2006;16:571–582. doi: 10.1016/j.nmd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- [10].Theocharidis G, Drymoussi Z, Kao AP, Barber AH, Lee DA, Braun KM, Connelly JT. Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. Journal of Investigative Dermatology. 2016;136:74–83. doi: 10.1038/JID.2015.352. [DOI] [PubMed] [Google Scholar]
- [11].Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, Rauterberg J, Lorkowski S. Production of Type VI Collagen by Human Macrophages: A New Dimension in Macrophage Functional Heterogeneity. The Journal of Immunology. 2008;180:5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- [12].Nystr xFmA, Velati D, Mittapalli VR, Fritsch A, Kern JS, Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. The Journal of Clinical Investigation. 2013;123:3498–3509. doi: 10.1172/JCI68127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nature Communications. 2015;6 doi: 10.1038/ncomms9026. 8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of Type I and III Collagens Is Dependent On Fibronectin and Enhanced By Integrins α11β1and α2β1. Journal of Biological Chemistry. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- [15].Sakar MS, Eyckmans J, Pieters R, Eberli D, Nelson BJ, Chen CS. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat Commun. 2016;7 doi: 10.1038/ncomms11036. 11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miner JH, Cunningham J, Sanes JR. Roles for Laminin in Embryogenesis: Exencephaly, Syndactyly, and Placentopathy in Mice Lacking the Laminin α5 Chain. The Journal of Cell Biology. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- [18].Horejs C-M. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. European Journal of Cell Biology. 2016 doi: 10.1016/j.ejcb.2016.06.002. in press. [DOI] [PubMed] [Google Scholar]
- [19].Murawaki Y, Koda M, Okamoto K, Mimura K, Kawasaki H. Diagnostic value of serum type IV collagen test in comparison with platelet count for predicting the fibrotic stage in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2001;16 doi: 10.1046/j.1440-1746.2001.02515.x. [DOI] [PubMed] [Google Scholar]
- [20].Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Seminars in Cancer Biology. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- [21].Baranowsky A, Mokkapati S, Bechtel M, Krügel J, Miosge N, Wickenhauser C, Smyth N, Nischt R. Impaired wound healing in mice lacking the basement membrane protein nidogen 1. Matrix Biology. 2010;29:15–21. doi: 10.1016/j.matbio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [22].Tucker RP, Chiquet-Ehrismann R. Tenascin-C: Its functions as an integrin ligand. The International Journal of Biochemistry & Cell Biology. 2015;65:165–168. doi: 10.1016/j.biocel.2015.06.003. [DOI] [PubMed] [Google Scholar]
- [23].Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 1998;393:181–185. doi: 10.1038/30270. [DOI] [PubMed] [Google Scholar]
- [24].Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. The Journal of Cell Biology. 1988;107:2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;53:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- [26].Gilpin SE, Li Q, Evangelista-Leite D, Ren X, Reinhardt DP, Frey BL, Ott HC. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials. 2017;140:212–219. doi: 10.1016/j.biomaterials.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, Lafyatis R, Lee J, Hinchcliff M, Feghali-Bostwick C, Lakota K, et al. Tenascin-C drives persistence of organ fibrosis. Nature Communications. 2016;7 doi: 10.1038/ncomms11703. 11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Preissner KT, Seiffert D. Role of Vitronectin and Its Receptors in Haemostasis and Vascular Remodeling. Thrombosis Research. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- [29].Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- [30].Deryugina EI, Quigley JP. Cell Surface Remodeling by Plasmin: A New Function for an Old Enzyme. Journal of Biomedicine and Biotechnology. 2012;2012:21. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Asch E, Podack E. Vitronectin binds to activated human platelets and plays a role in platelet aggregation. The Journal of Clinical Investigation. 1990;85:1372–1378. doi: 10.1172/JCI114581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jang Y-C, Tsou R, Gibran NS, Isik FF. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery. 2000;127:696–704. doi: 10.1067/msy.2000.105858. [DOI] [PubMed] [Google Scholar]
- [33].Chow S, Di Girolamo N. Vitronectin: A Migration and Wound Healing Factor for Human Corneal Epithelial CellsCorneal Wound Healing With Vitronectin. Investigative Ophthalmology & Visual Science. 2014;55:6590–6600. doi: 10.1167/iovs.14-15054. [DOI] [PubMed] [Google Scholar]
- [34].Upton Z, Cuttle L, Noble A, Kempf M, Topping G, Malda J, Xie Y, Mill J, Harkin DG, Kravchuk O, Leavesley DI, et al. Vitronectin: Growth Factor Complexes Hold Potential as a Wound Therapy Approach. Journal of Investigative Dermatology. 2008;128:1535–1544. doi: 10.1038/sj.jid.5701148. [DOI] [PubMed] [Google Scholar]
- [35].Upton Z, Wallace HJ, Shooter GK, van Lonkhuyzen DR, Yeoh-Ellerton S, Rayment EA, Fleming JM, Broszczak D, Queen D, Sibbald RG, Leavesley DI, et al. Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. International Wound Journal. 2011;8:522–532. doi: 10.1111/j.1742-481X.2011.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kosir MA, Quinn CCV, Wang W, Tromp G. Matrix Glycosaminoglycans in the Growth Phase of Fibroblasts: More of the Story in Wound Healing. Journal of Surgical Research. 2000;92:45–52. doi: 10.1006/jsre.2000.5840. [DOI] [PubMed] [Google Scholar]
- [37].Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Misra S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. International Journal of Cell Biology. 2015;2015:20. doi: 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Walker A, Turnbull JE, Gallagher JT. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. Journal of Biological Chemistry. 1994;269:931–935. [PubMed] [Google Scholar]
- [39].Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of Hyaluronan in Regulation of Fibroblast Phenotype. Journal of Biological Chemistry. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- [40].Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biology. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [41].Lunde IG, Herum KM, Carlson CC, Christensen G. Syndecans in heart fibrosis. Cell and Tissue Research. 2016;365:539–552. doi: 10.1007/s00441-016-2454-2. [DOI] [PubMed] [Google Scholar]
- [42].Das S, Singh G, Majid M, Sherman MB, Mukhopadhyay S, Wright CS, Martin PE, Dunn AK, Baker AB. Syndesome Therapeutics for Enhancing Diabetic Wound Healing. Advanced Healthcare Materials. 2016;5:2248–2260. doi: 10.1002/adhm.201600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lord MS, Ellis AL, Farrugia BL, Whitelock JM, Grenett H, Li C, O'Grady RL, DeCarlo AA. Perlecan and vascular endothelial growth factor-encoding DNA-loaded chitosan scaffolds promote angiogenesis and wound healing. Journal of Controlled Release. 2017;250:48–61. doi: 10.1016/j.jconrel.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell–matrix. Matrix Biology. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- [45].Barker TH, Baneyx G, Cardó-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, et al. SPARC Regulates Extracellular Matrix Organization through Its Modulation of Integrin-linked Kinase Activity. Journal of Biological Chemistry. 2005;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- [46].Basu A, Kligman LH, Samulewicz SJ, Howe CC. Impaired wound healing in mice deficient in a matricellular protein SPARC (osteonectin, BM-40) BMC Cell Biology. 2001;2:15–15. doi: 10.1186/1471-2121-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shibata S, Ishiyama J. Secreted protein acidic and rich in cysteine (SPARC) is upregulated by transforming growth factor (TGF)-β and is required for TGF-β-induced hydrogen peroxide production in fibroblasts. Fibrogenesis & Tissue Repair. 2013;6:6. doi: 10.1186/1755-1536-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhou X, Tan FK, Guo X, Wallis D, Milewicz DM, Xue S, Arnett FC. Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor β1 in cultured normal human fibroblasts. Arthritis & Rheumatism. 2005;52:257–261. doi: 10.1002/art.20785. [DOI] [PubMed] [Google Scholar]
- [49].Perbal B. CCN proteins: multifunctional signalling regulators. The Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- [50].Leask A, Parapuram SK, Shi-wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? Journal of Cell Communication and Signaling. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yoon PO, Lee M-A, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. Journal of Molecular and Cellular Cardiology. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- [52].Jun J-I, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jun J-I, Kim K-H, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nature Communications. 2015;6 doi: 10.1038/ncomms8386. 7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lin CG, Chen C-C, Leu S-J, Grzeszkiewicz TM, Lau LF. Integrin-dependent Functions of the Angiogenic Inducer NOV (CCN3) IMPLICATION IN WOUND HEALING. Journal of Biological Chemistry. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- [55].Adams JC, Lawler J. The Thrombospondins. Cold Spring Harbor Perspectives in Biology. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. The American Journal of Pathology. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- [57].Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biology. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Agah A, Kyriakides TR, Lawler J, Bornstein P. The Lack of Thrombospondin-1 (TSP1) Dictates the Course of Wound Healing in Double-TSP1/TSP2-Null Mice. The American Journal of Pathology. 161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. Journal of Clinical Investigation. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kyriakides TR, Zhu Y-H, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, et al. Mice That Lack Thrombospondin 2 Display Connective Tissue Abnormalities That Are Associated with Disordered Collagen Fibrillogenesis, an Increased Vascular Density, and a Bleeding Diathesis. The Journal of Cell Biology. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kristofik N, Calabro NE, Tian W, Meng A, MacLauchlan S, Wang Y, Breuer CK, Tellides G, Niklason LE, Kyriakides TR. Impaired von Willebrand factor adhesion and platelet response in thrombospondin-2 knockout mice. Blood. 2016;128:1642–1650. doi: 10.1182/blood-2016-03-702845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].MacLauchlan S, Yu J, Parrish M, Asoulin TA, Schleicher M, Krady MM, Zeng J, Huang PL, Sessa WC, Kyriakides TR. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1137–E1145. doi: 10.1073/pnas.1104357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) The Journal of Clinical Investigation. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu J, Wang Z. Osteopontin improves adhesion and migration of human primary renal cortical epithelial cells during wound healing. Oncology Letters. 2016;12:4556–4560. doi: 10.3892/ol.2016.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Timpl R, Sasaki T, Kostka G, Chu M-L. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–489. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- [66].Chowdhury A, Hasselbach L, Echtermeyer F, Jyotsana N, Theilmeier G, Herzog C. Fibulin-6 regulates pro-fibrotic TGF-β responses in neonatal mouse ventricular cardiac fibroblasts. Scientific Reports. 2017;7:42725. doi: 10.1038/srep42725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, et al. Latent TGF-β binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brandsma C-A, van den Berge M, Postma DS, Jonker MR, Brouwer S, Paré PD, Sin DD, Bossé Y, Laviolette M, Karjalainen J, Fehrmann RSN, et al. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax. 2015;70:21–32. doi: 10.1136/thoraxjnl-2014-205091. [DOI] [PubMed] [Google Scholar]
- [69].Lee MJ, Roy NK, Mogford JE, Schiemann WP, Mustoe TA. Fibulin-5 promotes wound healing in vivo1. Journal of the American College of Surgeons. 2004;199:403–410. doi: 10.1016/j.jamcollsurg.2004.04.021. [DOI] [PubMed] [Google Scholar]
- [70].Zheng Q, Choi J, Rouleau L, Leask RL, Richardson JA, Davis EC, Yanagisawa H. Normal Wound Healing in Mice Deficient for Fibulin-5, an Elastin Binding Protein Essential for Dermal Elastic Fiber Assembly. Journal of Investigative Dermatology. 2006;126:2707–2714. doi: 10.1038/sj.jid.5700501. [DOI] [PubMed] [Google Scholar]
- [71].Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proceedings of the National Academy of Sciences. 1999;96:1351–1356. doi: 10.1073/pnas.96.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Miron-Mendoza M, Seemann J, Grinnell F. THE DIFFERENTIAL REGULATION OF CELL MOTILE ACTIVITY THROUGH MATRIX STIFFNESS AND POROSITY IN THREE DIMENSIONAL COLLAGEN MATRICES. Biomaterials. 2010;31:6425–6435. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. The Journal of Cell Biology. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dingal PCDP, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wipff P-J, Rifkin DB, Meister J-J, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. The Journal of Cell Biology. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. Journal of Cell Science. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- [77].Kryczka J, Stasiak M, Dziki L, Mik M, Dziki A, Cierniewski CS. Matrix Metalloproteinase-2 Cleavage of the β1 Integrin Ectodomain Facilitates Colon Cancer Cell Motility. Journal of Biological Chemistry. 2012;287:36556–36566. doi: 10.1074/jbc.M112.384909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix Metalloproteinases Regulate Neovascularization by Acting as Pericellular Fibrinolysins. Cell. 95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- [79].Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biology. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- [80].Caley MP, Martins VLC, O'Toole EA. Metalloproteinases and Wound Healing. Advances in Wound Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lazaro JL, Izzo V, Meaume S, Davies AH, Lobmann R, Uccioli L. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. Journal of Wound Care. 2016;25:277–287. doi: 10.12968/jowc.2016.25.5.277. [DOI] [PubMed] [Google Scholar]
- [82].Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol. 2014;109:424. doi: 10.1007/s00395-014-0424-y. [DOI] [PubMed] [Google Scholar]
- [83].Kawaguchi M, Hearing VJ. The Roles of ADAMs Family Proteinases in Skin Diseases. Enzyme Research. 2011;2011 doi: 10.4061/2011/482498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- [85].Hodgkinson LM, Wang L, Duncan G, Edwards DR, Wormstone IM. ADAM and ADAMTS gene expression in native and wound healing human lens epithelial cells. Molecular Vision. 2010;16:2765–2776. [PMC free article] [PubMed] [Google Scholar]
- [86].Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812:1616–1629. doi: 10.1016/j.bbadis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [87].Wang W-M, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS. Transforming Growth Factor-β Induces Secretion of Activated ADAMTS-2: A PROCOLLAGEN III N-PROTEINASE. Journal of Biological Chemistry. 2003;278:19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- [88].Rodríguez-Manzaneque JC, Fernández-Rodríguez R, Rodríguez-Baena FJ, Iruela-Arispe ML. ADAMTS proteases in vascular biology. Matrix Biology. 2015;44–46:38–45. doi: 10.1016/j.matbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Reinecke IR, Weber CF, Budde U, Seifried E, Miesbach WA. Prospective evaluation of ADAMTS-13 and von Willebrand factor multimers in cardiac surgery. Blood Coagulation & Fibrinolysis. 2016;27:886–891. doi: 10.1097/MBC.0000000000000510. [DOI] [PubMed] [Google Scholar]
- [90].Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- [91].Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- [92].Heck LW, Blackburn WD, Irwin MH. Abrahamson DR, Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. The American Journal of Pathology. 1990;136:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- [93].Steadman R, Irwin MH, John PL, St, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. Journal of Leukocyte Biology. 1993;53:354–365. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- [94].Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, Senior RM. Neutrophil Elastase Cleaves Laminin-332 (Laminin-5) Generating Peptides That Are Chemotactic for Neutrophils. Journal of Biological Chemistry. 2008;283:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang S, Voisin M-B, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. The Journal of Experimental Medicine. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Larson BJ, Longaker MT, Lorenz HP. Scarless Fetal Wound Healing: A Basic Science Review. Plastic and reconstructive surgery. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187:493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- [98].Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MW. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. Journal of Cell Science. 1991;99:583–586. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]
- [99].Longaker MT, Chiu ES, Harrison MR, Crombleholme TM, Langer JC, Duncan BW, Adzick NS, Verrier ED, Stern R. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Annals of Surgery. 1989;210:667–672. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless Fetal Wounds Are Associated with an Increased Matrix Metalloproteinase–to–Tissue-Derived Inhibitor of Metalloproteinase Ratio. Plastic and Reconstructive Surgery. 2003;111:2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- [101].Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar-free repair and regeneration: insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol. 2014;56:47–55. doi: 10.1016/j.biocel.2014.10.011. [DOI] [PubMed] [Google Scholar]
- [102].Levesque M, Villiard E, Roy S. Skin wound healing in axolotls: a scarless process. J Exp Zool B Mol Dev Evol. 2010;314:684–697. doi: 10.1002/jez.b.21371. [DOI] [PubMed] [Google Scholar]
- [103].Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7:e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- [106].Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- [107].Solomonov I, Zehorai E, Talmi-Frank D, Wolf SG, Shainskaya A, Zhuravlev A, Kartvelishvily E, Visse R, Levin Y, Kampf N, Jaitin DA, et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:10884–10889. doi: 10.1073/pnas.1519676113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bellon G, Martiny L, Robinet A. Matrix metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol Hemat. 2004;49:203–220. doi: 10.1016/j.critrevonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [109].Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. Journal of Clinical Investigation. 2016;126:3176–3184. doi: 10.1172/JCI83147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gesteira TF, Coulson-Thomas VJ, Yuan Y, Zhang J, Nader HB, Kao WW. Lumican Peptides: Rational Design Targeting ALK5/TGFBRI. Sci Rep. 2017;7 doi: 10.1038/srep42057. 42057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, et al. Anti-angiogenic Cues from Vascular Basement Membrane Collagen. Cancer Research. 2000;60:2520–2526. [PubMed] [Google Scholar]
- [112].Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Hudson BG, Brooks PC. New Functions for Non-collagenous Domains of Human Collagen Type IV: NOVEL INTEGRIN LIGANDS INHIBITING ANGIOGENESIS AND TUMOR GROWTHIN VIVO. Journal of Biological Chemistry. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- [113].Ricard-Blum S, Ballut L. Matricryptins derived from collagens and proteoglycans. Front Biosci (Landmark Ed) 2011;16:674–697. doi: 10.2741/3712. [DOI] [PubMed] [Google Scholar]
- [114].Horejs C-M, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proceedings of the National Academy of Sciences. 2014;111:5908–5913. doi: 10.1073/pnas.1403139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Horejs C-M, St-Pierre J-P, Ojala JRM, Steele JAM, da Silva PB, Rynne-Vidal A, Maynard SA, Hansel CS, Rodríguez-Fernández C, Mazo MM, You AYF, et al. Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information. 2017;8 doi: 10.1038/ncomms15509. 15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Horejs CM, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:5908–5913. doi: 10.1073/pnas.1403139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Horejs CM, St-Pierre JP, Ojala JRM, Steele JAM, da Silva PB, Rynne-Vidal A, Maynard SA, Hansel CS, Rodriguez-Fernandez C, Mazo MM, You AYF, et al. Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information. Nat Commun. 2017;8 doi: 10.1038/ncomms15509. 15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin's emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015;1850:2422–2438. doi: 10.1016/j.bbagen.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yamaguchi Y, Takihara T, Chambers RA, Veraldi KL, Larregina AT, Feghali-Bostwick CA. A peptide derived from endostatin ameliorates organ fibrosis. Sci Transl Med. 2012;4:136ra171. doi: 10.1126/scitranslmed.3003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lee J, Horuk R, Rice GC, Bennett GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- [121].Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36:1306–1316. [PubMed] [Google Scholar]
- [122].Li F, Li W, Johnson SA, Ingram DA, Yoder MC, Badylak SF. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium-J Endoth. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- [123].Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation Products of Extracellular Matrix Affect Cell Migration and Proliferation. Tissue Eng Pt A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- [124].Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular Matrix Degradation Products and Low-Oxygen Conditions Enhance the Regenerative Potential of Perivascular Stem Cells. Tissue Eng Pt A. 2011;17:37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of Progenitor Cells by Remodeling Extracellular Matrix Scaffolds. Tissue Eng Pt A. 2009;15:1119–1125. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, Hirschi KK, Braunhut S, Gudas LJ, Badylak SF. Epimorphic regeneration approach to tissue replacement in adult mammals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3351–3355. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of Progenitor Cells by an Extracellular Matrix Cryptic Peptide in a Mouse Model of Digit Amputation. Tissue Eng Pt A. 2011;17:2435–2443. doi: 10.1089/ten.tea.2011.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Williams C, Quinn KP, Georgakoudi I, Black LD., 3rd Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 2014;10:194–204. doi: 10.1016/j.actbio.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Williams C, Sullivan K, Black LD., 3rd Partially Digested Adult Cardiac Extracellular Matrix Promotes Cardiomyocyte Proliferation In Vitro. Adv Healthc Mater. 2015;4:1545–1554. doi: 10.1002/adhm.201500035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Poel WE. Preparation of Acellular Homogenates From Muscle Samples. Science. 1948;108:390–391. doi: 10.1126/science.108.2806.390-a. [DOI] [PubMed] [Google Scholar]
- [131].Krejci NC, Cuono CB, Langdon RC, McGuire J. In vitro reconstitution of skin: fibroblasts facilitate keratinocyte growth and differentiation on acellular reticular dermis. J Invest Dermatol. 1991;97:843–848. doi: 10.1111/1523-1747.ep12491522. [DOI] [PubMed] [Google Scholar]
- [132].Badylak SF, Lantz GC, Coffey A, Geddes LA. Small Intestinal Submucosa as a Large Diameter Vascular Graft in the Dog. Journal of Surgical Research. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- [133].Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- [134].Keane TJ, Badylak SF. The host response to allogeneic and xenogeneic biological scaffold materials. J Tissue Eng Regen Med. 2015;9:504–511. doi: 10.1002/term.1874. [DOI] [PubMed] [Google Scholar]
- [135].Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- [136].Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in Biologic Scaffold Materials. Journal of Surgical Research. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB, Badylak SF. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–1006. doi: 10.1016/s1010-7940(03)00094-0. discussion 1006. [DOI] [PubMed] [Google Scholar]
- [139].Nie X, Xiao D, Wang W, Song Z, Yang Z, Chen Y, Gu Y. Comparison of Porcine Small Intestinal Submucosa versus Polypropylene in Open Inguinal Hernia Repair: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0135073. doi: 10.1371/journal.pone.0135073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Golla D, Russo CC. Outcomes following placement of non-cross-linked porcine-derived acellular dermal matrix in complex ventral hernia repair. Int Surg. 2014;99:235–240. doi: 10.9738/INTSURG-D-13-00170.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ferzoco SJ. A systematic review of outcomes following repair of complex ventral incisional hernias with biologic mesh. Int Surg. 2013;98:399–408. doi: 10.9738/INTSURG-D-12-00002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Roth JS, Brathwaite C, Hacker K, Fisher K, King J. Complex ventral hernia repair with a human acellular dermal matrix. Hernia. 2015;19:247–252. doi: 10.1007/s10029-014-1245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Juhasz I, Kiss B, Lukacs L, Erdei I, Peter Z, Remenyik E. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010 doi: 10.1155/2010/210150. 210150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, O.V.U.S. Group Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41:837–843. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- [145].Salibian AA, Frey JD, Choi M, Karp NS. Subcutaneous Implant-based Breast Reconstruction with Acellular Dermal Matrix/Mesh: A Systematic Review. Plast Reconstr Surg Glob Open. 2016;4:e1139. doi: 10.1097/GOX.0000000000001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Valdatta L, Cattaneo AG, Pellegatta I, Scamoni S, Minuti A, Cherubino M. Acellular dermal matrices and radiotherapy in breast reconstruction: a systematic review and meta-analysis of the literature. Plast Surg Int. 2014;2014 doi: 10.1155/2014/472604. 472604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214 doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Dziki JL, Sicari BM, Wolf MT, Cramer MC, Badylak SF. Immunomodulation and Mobilization of Progenitor Cells by Extracellular Matrix Bioscaffolds for Volumetric Muscle Loss Treatment. Tissue Eng Part A. 2016;22:1129–1139. doi: 10.1089/ten.TEA.2016.0340. [DOI] [PubMed] [Google Scholar]
- [149].Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra258. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Corona BT, Greising SM. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials. 2016;104:238–246. doi: 10.1016/j.biomaterials.2016.07.020. [DOI] [PubMed] [Google Scholar]
- [151].(!!! INVALID CITATION !!! {Hussey, 2017 #310;Hussey, 2017 #10})
- [152].Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Dziki JL, Keane TJ, Shaffiey S, Cognetti D, Turner N, Nagle D, Hackam D, Badylak S. Bioscaffold-mediated mucosal remodeling following short-segment colonic mucosal resection. Journal of Surgical Research. 2017;218:353–360. doi: 10.1016/j.jss.2017.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [155].Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, Badylak SF. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–296. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- [156].Chen WC, Wang Z, Missinato MA, Park DW, Long DW, Liu HJ, Zeng X, Yates NA, Kim K, Wang Y. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci Adv. 2016;2:e1600844. doi: 10.1126/sciadv.1600844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Johnson TD, Hill RC, Dzieciatkowska M, Nigam V, Behfar A, Christman KL, Hansen KC. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl. 2016;10:75–83. doi: 10.1002/prca.201500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Calle EA, Hill RC, Leiby KL, Le AV, Gard AL, Madri JA, Hansen KC, Niklason LE. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomaterialia. 2016;46:91–100. doi: 10.1016/j.actbio.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Voytik-Harbin SL, Brightman AO, Waisner BZ, Robinson JP, Lamar CH. Small intestinal submucosa: A tissue-derived extracellular matrix that promotes tissue-specific growth and differentiation of cells in vitro. Tissue Eng. 1998;4:157–174. [Google Scholar]
- [160].Johnson TD, Lin SY, Christman KL. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology. 2011;22:494015. doi: 10.1088/0957-4484/22/49/494015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222–234. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [162].Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D'Amore A, Nagarkar SP, Velankar SS, Badylak SF. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ungerleider JL, Johnson TD, Hernandez MJ, Elhag DI, Braden RL, Dzieciatkowska M, Osborn KG, Hansen KC, Mahmud E, Christman KL. Extracellular Matrix Hydrogel Promotes Tissue Remodeling Arteriogenesis, and Perfusion in a Rat Hindlimb Ischemia Model. JACC Basic Transl Sci. 2016;1:32–44. doi: 10.1016/j.jacbts.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Massensini AR, Ghuman H, Saldin LT, Medberry CJ, Keane TJ, Nicholls FJ, Velankar SS, Badylak SF, Modo M. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015;27:116–130. doi: 10.1016/j.actbio.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, Modo M. ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate. Biomaterials. 2016;91:166–181. doi: 10.1016/j.biomaterials.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu Y, Wang J, Shi Y, Pu H, Leak RK, Liou AK, Badylak SF, Liu Z, Zhang J, Chen J, Chen L. Implantation of Brain-derived Extracellular Matrix Enhances Neurological Recovery after Traumatic Brain Injury. Cell Transplant. 2016 doi: 10.1177/0963689717714090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhang L, Zhang F, Weng Z, Brown BN, Yan H, Ma XM, Vosler PS, Badylak SF, Dixon CE, Cui XT, Chen J. Effect of an inductive hydrogel composed of urinary bladder matrix upon functional recovery following traumatic brain injury. Tissue Eng Part A. 2013;19:1909–1918. doi: 10.1089/ten.tea.2012.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Tukmachev D, Forostyak S, Koci Z, Zaviskova K, Vackova I, Vyborny K, Sandvig I, Sandvig A, Medberry CJ, Badylak SF, Sykova E, et al. Injectable Extracellular Matrix Hydrogels as Scaffolds for Spinal Cord Injury Repair. Tissue Eng Pt A. 2016;22:306–317. doi: 10.1089/ten.tea.2015.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Science Translational Medicine. 2013;5 doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Keane TJ, Dziki J, Sobieski E, Smoulder A, Castleton A, Turner N, White LJ, Badylak SF. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J Crohns Colitis. 2017;11:360–368. doi: 10.1093/ecco-jcc/jjw149. [DOI] [PubMed] [Google Scholar]
- [172].Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annual Review of Cell and Developmental Biology. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sarikaya A, Record R, Wu CC, Tullius B, Badylak S, Ladisch M. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 2002;8:63–71. doi: 10.1089/107632702753503063. [DOI] [PubMed] [Google Scholar]
- [174].Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- [175].Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, Badylak SF. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653–2659. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- [176].Carey LE, Dearth CL, Johnson SA, Londono R, Medberry CJ, Daly KA, Badylak SF. In vivo degradation of 14C-labeled porcine dermis biologic scaffold. Biomaterials. 2014;35:8297–8304. doi: 10.1016/j.biomaterials.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Pei W, Tanaka K, Huang SC, Xu L, Liu B, Sinclair J, Idol J, Varshney GK, Huang H, Lin S, Nussenblatt RB, et al. Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation. 2016;1 doi: 10.1038/npjregenmed.2016.13. 16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141:2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].de Preux Charles AS, Bise T, Baier F, Marro J, Jazwinska A. Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart. Open Biol. 2016;6 doi: 10.1098/rsob.160102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Han C, Nie Y, Lian H, Liu R, He F, Huang H, Hu S. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 2015;25:1137–1151. doi: 10.1038/cr.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Morris AH, Stamer DK, Kyriakides TR. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol. 2017 doi: 10.1016/j.smim.2017.01.002. [DOI] [PubMed] [Google Scholar]
- [183].Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat Commun. 2016;7 doi: 10.1038/ncomms10928. 10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Fishman JM, Lowdell MW, Urbani L, Ansari T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ, Birchall MA, et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci U S A. 2013;110:14360–14365. doi: 10.1073/pnas.1213228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352:366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Badylak SF. TISSUE REGENERATION. A scaffold immune microenvironment. Science. 2016;352:298. doi: 10.1126/science.aaf7587. [DOI] [PubMed] [Google Scholar]
- [188].Chung L, Maestas DR, Jr, Housseau F, Elisseeff JH. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]