Abstract
Background: Adiposity traits have been associated with risk of many cancers in observational studies, but whether these associations are causal is unclear. Mendelian randomization (MR) uses genetic predictors of risk factors as instrumental variables to eliminate reverse causation and reduce confounding bias. We performed MR analyses to assess the possible causal relationship of birthweight, childhood and adult body mass index (BMI), and waist-hip ratio (WHR) on the risks of breast, ovarian, prostate, colorectal and lung cancers.
Methods: We tested the association between genetic risk scores and each trait using summary statistics from published genome-wide association studies (GWAS) and from 51 537 cancer cases and 61 600 controls in the Genetic Associations and Mechanisms in Oncology (GAME-ON) Consortium.
Results: We found an inverse association between the genetic score for childhood BMI and risk of breast cancer [odds ratio (OR) = 0.71 per standard deviation (s.d.) increase in childhood BMI; 95% confidence interval (CI): 0.60, 0.80; P = 6.5 × 10 -5 ). We also found the genetic score for adult BMI to be inversely associated with breast cancer risk (OR = 0.66 per s.d. increase in BMI; 95% CI: 0.57, 0.77; P = 2.5 × 10 -7 ), and positively associated with ovarian cancer (OR = 1.35; 95% CI: 1.05, 1.72; P = 0.017), lung cancer (OR = 1.27; 95% CI: 1.09, 1.49; P = 2.9 × 10 -3 ) and colorectal cancer (OR = 1.39; 95% CI: 1.06, 1.82, P = 0.016). The inverse association between genetically predicted adult BMI and breast cancer risk remained even after adjusting for directional pleiotropy via MR-Egger regression.
Conclusions: Findings from this study provide additional understandings of the complex relationship between adiposity and cancer risks. Our results for breast and lung cancer are particularly interesting, given previous reports of effect heterogeneity by menopausal status and smoking status.
Keywords: Cancer risk, body mass index, waist-to-hip ratio, Mendelian randomization, post-GWAS study
Key Messages
Adiposity traits have been associated with risk of many cancers in observational studies, but whether these associations are causal is unclear.
We performed Mendelian randomization analyses to assess the possible causal relationship of birthweight, childhood and adult body mass index (BMI), and waist-hip ratio on the risks of breast, ovarian, prostate, colorectal and lung cancers.
We found that genetic score for higher adult BMI is associated with decreased risk of breast cancer and increased risk of ovarian, lung and colorectal cancer. We also observed an inverse association between genetically predicted childhood BMI and risk of breast cancer.
These results provide additional understanding of the complex relationship between adiposity and cancer risks.
Introduction
Obesity influences risk for many chronic diseases such as cancer, cardiovascular disease and diabetes. 1,2 Observational studies have found associations between body mass index (BMI) and various cancer types including increasing risk of postmenopausal breast, 3 colorectal, 4 endometrial 5 and pancreatic cancer, 6,7 and decreasing risk of lung cancer and premenopausal breast cancer. 8 However, the mechanisms underlying the contribution of obesity to cancer risk remains poorly understood. It is also unclear whether these associations between obesity and cancer in observational studies are causal. For instance, the observed increased risk of lung cancer among individuals with low BMI may be due to residual confounding by smoking or weight loss resulting from chronic lung disease. 9
Recent studies have also found time-dependent associations between assessment of adiposity and subsequent cancer risk. Higher adiposity at young ages is inversely associated with both pre- and postmenopausal breast cancer. 10 In contrast, higher adult BMI is positively associated with postmenopausal breast cancer risk. 3,11,12 Evidence also suggests that childhood obesity may be associated with ovarian cancer independent of adult BMI. 13 These findings demonstrate a dynamic relationship between adiposity and cancer development during different time frames of life, which requires a deeper investigation.
Elevated waist-to-hip ratio (WHR), representing a higher abdominal fat distribution, is associated with multiple hormonal and metabolic changes including insulin resistance and hyperinsulinaemia which may increase risk of chronic disease such as cancer. 14–16 Previous studies examining WHR and breast cancer risk indicated a positive association which remained positive after adjusting for BMI. 12,17 Some studies also suggest that measures of abdominal adiposity are more predictive of colorectal cancer than BMI. 18,19 Thus, further investigations on the contribution of WHR to cancer risk may improve our understanding of the relationship between body fat distribution, obesity and cancerogenesis.
Mendelian randomization (MR) is a technique that uses genetic predictors of risk factors as instrumental variables to assess the possible causal associations between risk factors and diseases. 20 As genetic variants are fixed at conception and are generally independent of confounders, such an approach seeks to eliminate potential reverse causality and reduce confounding bias. 21,22 To our knowledge, there has not been any large-scale MR study assessing the potential causal relationship between obesity across different life stages and risk of multiple cancers.
In this study, we performed MR analysis to estimate the causal relationship between adiposity at different life stages (birthweight, childhood BMI, adult BMI and WHR) and risk of breast, ovarian, prostate, colorectal and lung cancers. We leveraged the results of recently published large-scale genome-wide association studies (GWAS) of adiposity-related traits to define a genetic score for each trait. We then assessed the associations between these scores and risks of five cancers from the Genetic Associations and Mechanisms in Oncology (GAME-ON) Consortium, which include 51 537 cancer cases and 61 600 controls from 32 participating studies.
Materials and Methods
The GAME-ON post-GWAS initiative
The Genetic Associations and Mechanisms in Oncology (GAME-ON) Initiative is a network of cancer-specific consortia engaged in GWAS and post-GWAS research. It includes five cancer-specific consortia: DRIVE (breast), CORECT (colorectal), ELLIPSE (prostate), FOCI (ovarian) and TRICL (lung) ( Table 1 ). GWAS data from 32 studies (all European ancestry) contributing to the GAME-ON consortium were imputed using the 1000 Genomes reference panel (phase I version 3). Studies contributed summary statistics only to cancer-specific meta-analyses. Further information regarding imputation and analyses can be found in Fehringer et al.23 and Zhang et al.24
Table 1.
Participants and studies included in the Genetic Associations and Mechanisms in Oncology (GAME-ON) consortium by cancer site and subtype
| Cancer type | Cancer subtype | Cases | Controls | GWAS studies |
|---|---|---|---|---|
| Breast | All | 15748 | 18084 | 11 |
| ER-negative | 4939 | 13128 | 8 | |
| Colorectal | All | 5100 | 4831 | 6 |
| Lung | All | 12160 | 16838 | 6 |
| Adenocarcinoma | 3718 | 15871 | 6 | |
| Squamous | 3422 | 16015 | 6 | |
| Ovarian | All | 4369 | 9123 | 3 |
| Clear-cell | 356 | 9123 | 3 | |
| Endometrioid | 715 | 9123 | 3 | |
| Serous | 2556 | 9123 | 3 | |
| Prostate | All | 14160 | 12724 | 6 |
| Aggressive | 4450 | 12724 | 6 | |
| Total | All | 51537 | 61600 | 32 |
Identification of single nucleotide polymorphisms associated with birthweight, childhood obesity and adult BMI and WHR
To calculate the genetic scores, we considered single nucleotide polymorphisms (SNPs) that were genome-wide significant ( P < 5 × 10 -8 ) in the largest GWAS to date for each trait as follows: (i) 7 SNPs of birthweight from Horikoshi et al. ; 25 (ii) 15 SNPs of childhood BMI from Felix et al. ; 26 (iii) 77 SNPs of adult BMI from Locke et al. (SNPs from primary meta-analysis of European-descents only); 27 and (iv) 14 SNPs of adult WHR from Heid et al.28 All GWAS were restricted to individuals of European ancestry. For all identified SNPs, we obtained the chromosome and position, the nearest gene, the risk allele and trait-specific association estimates and standard errors reported in the papers above. For each SNP, we also extracted cancer-specific effect estimates and P -values from the GAME-ON consortium ( Supplementary Table 1 , available as Supplementary data at IJE online).
Several SNPs associated with birthweight, childhood BMI, adult BMI and WHR were not found in GAME-ON data for ovarian endometrioid cancer subtype, lung cancer or colorectal cancer. For these SNPs, proxy SNPs (r 2 > 0.9, 1000 Genomes Northern and Western European population) were used in the analysis instead ( Supplementary Table 2 , available as Supplementary data at IJE online) There were no overlaps (lead SNPs within 250 kb) among the GWAS-identified loci for different adiposity-related traits except childhood BMI and adult BMI, for which we found 10 overlap regions: SEC16B, TNNI3K, FTO, MC4R, TMEM18, TFAP2B, OLFM4, ADCY3, GPR61/GNAT2 and GNPDA2 ( Supplementary Figure 1 , available as Supplementary data at IJE online).
Statistical analysis
We conducted MR analyses to estimate the association between adiposity-related traits and cancer using summary genetic association statistics, as described in Burgess et al.29 Specifically, the ratio estimate ( ) of the effect of a risk factor (X) on disease outcome (Y) using genetic variants k = 1,…, K can be calculated as:
where x k is the per-allele effect of SNP k with the risk factor, Y k is the per-allele change in the log odds ratio for the cancer being tested and σ Yk2 is the standard error for Y k . The summary statistics X k , Y k and σ Yk2 are taken from the GWAS for the risk factor and for cancer, respectively. The standard error of is given by: 16, 21 Under certain assumptions, 30 the ratio estimate can be interpreted as the causal log odds ratio of cancer risk associated with one unit change in the adiposity-related traits (birthweight, childhood BMI, adult BMI and WHR).
Since some cancers demonstrate aetiological heterogeneity by histological subtype or clinical characteristics, we also conducted the following cancer-specific subgroup analyses: estrogen receptor-negative (ER-) breast cancer; clear cell, endometrioid and serous ovarian cancer; adenocarcinoma and squamous lung cancer; and aggressive prostate cancer (defined as a Gleason score of ≥ 8, a disease stage of ‘distant’, a prostate-specific antigen level of > 100 ng/ml or death from prostate cancer. 31 In addition, sensitivity analyses were performed excluding the overlap loci between childhood BMI and adult BMI. One key assumption for MR analysis is no pleiotropic effect. Thus, Egger regression was performed to evaluate directional pleotropic effect for adult and childhood BMI 32 to provide effect estimates after adjusting for potential pleiotropic effects. The intercept from Egger regression provides a test for directional pleiotropy (the average direct effects of adiposity-increasing variants increase [or decrease] cancer risk). Under the assumption that the SNPs’ direct effects on cancer risk are independent of their association with body mass index, Egger regression provides an unbiased estimate of the causal effect of genetically predicted BMI on cancer. Unless otherwise noted, all P -values are unadjusted for multiple testing.
Results
We estimated the associations between adiposity-related genetic scores and risk of five cancers ( Table 1 ). Figures comparing results across cancers are shown in Supplementary Figure 2 (available as Supplementary data at IJE online).
Breast cancer
The risk of breast cancer decreased with increasing genetic score for childhood BMI [odds ratio (OR) = 0.71 per standard deviation (s.d.) increase in childhood BMI; 95% confidence interval (CI): 0.60, 0.80; P = 6.5 × 10 -5 ] and also with increasing genetic score for adult BMI (OR = 0.66 per s.d. increase in adult BMI; 95% CI: 0.57, 0.77; P = 2.5 × 10 -7 ) ( Table 2 ). Similar associations were found for ER-negative breast cancer (OR = 0.69; 95% CI: 0.53, 0.98, P = 5.8 × 10 -3 for childhood BMI; OR = 0.59; 95% CI: 0.46, 0.75; P = 2.0 × 10 -5 for adult BMI). We did not observe an association between the genetic score for birthweight and breast cancer and observed an inverse association between the genetic score for WHR and breast cancer risk (OR = 0.73; 95% CI: 0.54, 1.00; P = 0.05).
Table 2.
Mendelian randomization odds ratios of birthweight, childhood BMI, adult BMI, and waist-hip ratio across five different cancer types obtained using summary data from GAME-ON consortium
|
|
Birthweight
|
Childhood BMI
|
Adult BMI
|
Waist-hip ratio
|
|||||
|---|---|---|---|---|---|---|---|---|---|
|
Cancer type
|
OR | P -value | OR | P -value | OR | P -value | OR | P -value | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||
| Breast cancer | All | 1.22 | 0.15 | 0.71 | 6.5 × 10 -5 | 0.66 | 2.5 × 10 -7* | 0.73 | 0.051 |
| (0.93, 1.60) | (0.60, 0.80) | (0.57, 0.77) | (0.53,1.00) | ||||||
| ER-negative | 1.01 | 0.98 | 0.69 | 0.0058 | 0.59 | 2.0 × 10 -5* | 0.74 | 0.23 | |
| (0.66, 1.53) | (0.53, 0.98) | (0.46, 0.75) | (0.45, 1.21) | ||||||
| Ovarian cancer | All | 1.07 | 0.75 | 1.07 | 0.62 | 1.35 | 0.017 | 1.19 | 0.50 |
| (0.69, 1.65) | (0.82, 1.39) | (1.05,1.72) | (0.73, 1.94) | ||||||
| Clear-cell | 2.75 | 0.10 | 1.45 | 0.34 | 1.68 | 0.14 | 1.31 | 0.71 | |
| (0.82, 9.30) | (0.68, 3.09) | (0.84, 3.36) | (0.32, 5.30) | ||||||
| Endometrioid | 0.79 | 0.60 | 1.47 | 0.16 | 1.34 | 0.26 | 1.03 | 0.95 | |
| (0.33, 1.92) | (0.86, 2.52) | (0.80, 2.26) | (0.38, 2.84) | ||||||
| Serous | 0.85 | 0.56 | 0.91 | 0.56 | 1.30 | 0.089 | 1.34 | 0.34 | |
| (0.50, 1.45) | (0.65, 1.26) | (0.97, 1.76) | (0.73, 2.46) | ||||||
| Prostate cancer | All | 1.33 | 0.082 | 1.01 | 0.91 | 1.01 | 0.97 | 1.02 | 0.90 |
| (0.96, 1.82) | (0.83, 1.22) | (0.84, 1.21) | (0.72, 1.46) | ||||||
| Aggressive | 1.63 | 0.037 | 1.10 | 0.49 | 1.11 | 0.44 | 1.19 | 0.51 | |
| (1.03, 2.57) | (0.83, 1.45) | (0.85, 1.44) | (0.71, 1.98) | ||||||
| Lung cancer | All | 0.93 | 0.64 | 1.01 | 0.90 | 1.27 | 2.9 × 10 -3 | 1.15 | 0.46 |
| (0.70, 1.23) | (0.85, 1.2) | (1.09, 1.49) | (0.80, 1.66) | ||||||
| Adenocarcinoma | 0.95 | 0.83 | 0.90 | 0.47 | 0.93 | 0.59 | 0.90 | 0.71 | |
| (0.62, 1.46) | (0.69, 1.19) | (0.73, 1.19) | (0.51, 1.58) | ||||||
| Squamous | 0.99 | 0.94 | 1.08 | 0.57 | 1.54 | 6.6 × 10 -4* | 1.33 | 0.33 | |
| (0.64, 1.52) | (0.82, 1.43) | (1.20, 1.96) | (0.75, 2.36) | ||||||
| Colorectal cancer | All | 0.69 | 0.12 | 1.20 | 0.21 | 1.39 | 0.016 | 1.29 | 0.35 |
| (0.44, 1.10) | (0.90, 1.59) | (1.06, 1.82) | (0.75, 2.22) | ||||||
BMI SNP rs12016871 has been merged into rs9581854 and thus rs9581854 was used for analysis instead.
*Denotes analyses that have P < 0.001 after Bonferroni correction for 48 tests.
Ovarian cancer
The estimated association between the genetic scores for higher adult BMI is associated with increased risk of overall ovarian cancer. One standard deviation increase in genetically predicted adult BMI was associated with 35% increased risk of ovarian cancer (OR = 1.35; 95% CI: 1.05,1.72; P = 0.017). We did not find strong evidence of associations between genetically predicted birthweight, childhood BMI or WHR and ovarian cancer risk.
Lung cancer
We observed a positive association between genetically predicted adult BMI and overall lung cancer (OR = 1.27; 95% CI: 1.09, 1.49; P -value = 2.9 × 10 -3 ) ( Table 2 ). This association appeared restricted to squamous cell lung cancer (OR = 1.54; 95% CI: 1.20, 1.96; P = 6.6 × 10 -4 ), as we found no strong evidence for association with lung adenocarcinoma (OR = 0.93; 95% CI:0.73, 1.19, P = 0.59). We also did not find strong evidence for association between either genetically predicted birthweight or childhood BMI and lung cancer risk.
Prostate cancer
We found a positive association between the genetic score for birthweight and aggressive prostate cancer (OR = 1.63 per s.d. unit increase in birthweight; 95% CI: 1.03, 2.57; P = 0.037). No strong evidence was found for associations between prostate cancer and any other adiposity measures.
Colorectal cancer
We found an increase in risk of colorectal cancer per s.d. increase of genetically predicted adult BMI (OR = 1.39; 95% CI: 1.06, 1.82; P = 0.016). No associations were found between birthweight, childhood BMI or waist-hip-ratio and colorectal cancer risk.
Overlap in adiposity SNP scores
None of the pairs of adiposity-trait SNP scores overlap (within 250 kb) except childhood BMI and adult BMI, which overlap at 10 loci: SEC16B, TNNI3K, FTO, MC4R, TMEM18, TFAP2B, OLFM4, ADCY3, GPR61/GNAT2 and GNPDA2. To assess the specificity of the observed associations between childhood and adult BMI and cancer risk, we repeated the analyses after removing the SNPs from the overlapping loci. The associations remained between adult BMI and breast and lung cancer, whereas the associations between childhood BMI and breast were attenuated after removing the overlapping loci ( Table 3 ).
Table 3.
Mendelian randomization odds ratios of childhood BMI and adult BMI across five different cancer types obtained using summary data from GAME-ON consortium, excluding overlap loci ( SEC16B, TNNI3K, FTO, MC4R, TMEM18, TFAP2B, GNAT2, OLFM4, ADCY3, GNPDA2 )
|
Childhood BMI
|
Adult BMI
|
||||
|---|---|---|---|---|---|
| OR | P -value | OR | P -value | ||
| (95% CI) | (95% CI) | ||||
| Breast cancer | All | 1.05 | 0.80 | 0.75 | 4.7 × 10 -3* |
| (0.74, 1.48) | (0.62, 0.92) | ||||
| ER-negative | 1.17 | 0.57 | 0.66 | 0.011 | |
| (0.68, 2.03) | (0.49, 0.91) | ||||
| Ovarian cancer | All | 0.58 | 0.053 | 1.26 | 0.14 |
| (0.34, 1.01) | (0.93, 1.72) | ||||
| Clear-cell | 0.70 | 0.69 | 1.44 | 0.42 | |
| (0.15, 3.25) | (0.60, 3, 43) | ||||
| Endometrioid | 0.67 | 0.47 | 0.84 | 0.61 | |
| (0.22, 2.03) | (0.43, 1.64) | ||||
| Serous | 0.54 | 0.07 | 1.43 | 0.062 | |
| (0.27, 1.06) | (0.98, 2.10) | ||||
| Prostate cancer | All | 1.29 | 0.19 | 1.09 | 0.48 |
| (0.88, 1.87) | (0.86, 1.37) | ||||
| Aggressive | 1.32 | 0.32 | 1.24 | 0.20 | |
| (0.77, 2.29) | (0.89, 1.73) | ||||
| Lung cancer | All | 0.90 | 0.55 | 1.41 | 6.8 × 10 -4* |
| (0.63, 1.28) | (1.16, 1.73) | ||||
| Adenocarcinoma | 1.06 | 0.83 | 1.00 | 0.99 | |
| (0.62, 1.83) | (0.74, 1.36) | ||||
| Squamous | 0.66 | 0.13 | 1.73 | 5.3 × 10 -4* | |
| (0.38, 1.14) | (1.27, 2.38) | ||||
| Colorectal cancer | All | 0.85 | 0.57 | 1.36 | 0.08 |
| (0.48, 1.50) | (0.96, 1.92 | ||||
*Denotes analyses that have P < 0.001 after Bonferroni correction for 48 tests.
Egger regression
With the possible exception of genetically predicted childhood BMI and breast cancer risk, the Egger regression did not reveal any strong directional pleiotropic effect on the risk estimation of genetically predicted adult BMI/childhood BMI/WHR/birthweight on various cancers ( Table 4 ). All estimated intercepts from the Egger regression are near zero. The effect estimates from the Egger regression are generally in the same direction as the estimates from the MR analysis and larger in magnitude, except for lung cancer. We detect no strong pleiotropic effect on the risk estimation of genetically predicted adult BMI and lung cancer (intercept = 0.011; P = 0.057) but found no positive association between the BMI score on lung cancer in the Egger regression analysis (OR = 0.90; 95% CI: 0.51, 1.29; P = 0.59).
Table 4.
Effect estimates from Egger regression for adult BMI, childhood BMI, birthweight and WHR
| Adult BMI |
Egger regression
|
||||||
|---|---|---|---|---|---|---|---|
| MR OR | Intercept | Standard error | P | OR Egger | Standard error | P | |
| Breast cancer | 0.66 | 0.0035 | 0.0056 | 0.53 | 0.59 | 0.20 | 0.0076 |
| (0.57, 0.77) | |||||||
| Ovarian cancer | 1.35 | −0.0093 | 0.0088 | 0.29 | 1.80 | 0.31 | 0.054 |
| (1.05, 1.72) | |||||||
| Prostate cancer | 1.01 | 0.0096 | 0.0066 | 0.15 | 0.74 | 0.23 | 0.19 |
| (0.84, 1.21) | |||||||
| Lung cancer | 1.27 | 0.011 | 0.0057 | 0.057 | 0.90 | 0.20 | 0.59 |
| (1.09, 1.49) | |||||||
| Colorectal cancer | 1.39 | 0.0082 | 0.0098 | 0.40 | 1.08 | 0.33 | 0.82 |
| (1.06, 1.82) | |||||||
|
|
Egger regression
|
||||||
|---|---|---|---|---|---|---|---|
| Childhood BMI | MR OR | Intercept | Standard error | P | OR Egger | Standard error | P |
| Breast cancer | 0.71 | 0.048 | 0.027 | 0.026 | 0.34 | 0.21 | 0.0017 |
| (0.60, 0.80) | |||||||
| Ovarian cancer | 1.07 | −0.053 | 0.044 | 0.12 | 2.44 | 0.33 | 0.10 |
| (0.82, 1.39) | |||||||
| Prostate cancer | 1.01 | −0.020 | 0.033 | 0.42 | 1.38 | 0.25 | 0.42 |
| (0.83, 1.22) | |||||||
| Lung cancer | 1.01 | −0.0015 | 0.088 | 0.95 | 1.04 | 0.21 | 0.92 |
| (0.85, 1.2) | |||||||
| Colorectal cancer | 1.20 | −0.020 | 0.15 | 0.41 | 1.63 | 0.35 | 0.22 |
| (0.90, 1.59) | |||||||
|
|
Egger regression
|
||||||
|---|---|---|---|---|---|---|---|
| WHR | MR OR | Intercept | Standard error | P | OR Egger | Standard error | P |
| Breast cancer | 0.73 | 0.0048 | 0.026 | 0.85 | 0.63 | 0.83 | 0.58 |
| (0.53,1.00) | |||||||
| Ovarian cancer | 1.19 | −0.037 | 0.042 | 0.38 | 3.67 | 1.32 | 0.32 |
| (0.73, 1.94) | |||||||
| Prostate cancer | 1.02 | 0.046 | 0.031 | 0.14 | 0.25 | 0.97 | 0.15 |
| (0.72, 1.46) | |||||||
| Lung cancer | 1.15 | −0.017 | 0.032 | 0.60 | 1.97 | 1.04 | 0.52 |
| (0.80, 1.66) | |||||||
| Colorectal cancer | 1.29 | −0.068 | 0.046 | 0.14 | 10.38 | 1.43 | 0.10 |
| (0.75, 2.22) | |||||||
|
Egger regression |
|||||||
|---|---|---|---|---|---|---|---|
| Birthweight | MR OR | Intercept | Standard error | P | OR Egger | Standard error | P |
| Breast cancer | 1.22 | 0.040 | 0.030 | 0.18 | 1.75 | 0.59 | 0.34 |
| (0.93, 1.60) | |||||||
| Ovarian cancer | 1.07 | 0.069 | 0.048 | 0.15 | 3.46 | 0.93 | 0.18 |
| (0.69, 1.65) | |||||||
| Prostate cancer | 1.33 | 0.0043 | 0.035 | 0.90 | 0.82 | 0.69 | 0.77 |
| (0.96, 1.82) | |||||||
| Lung cancer | 0.93 | 0.0011 | 0.031 | 0.97 | 1.10 | 0.60 | 0.88 |
| (0.70, 1.23) | |||||||
| Colorectal cancer | 0.69 | −0.026 | 0.051 | 0.96 | 1.38 | 0.100 | 0.75 |
| (0.44, 1.10) | |||||||
|
| |||||||
Associations between individual adiposity-related SNPs and cancer risk
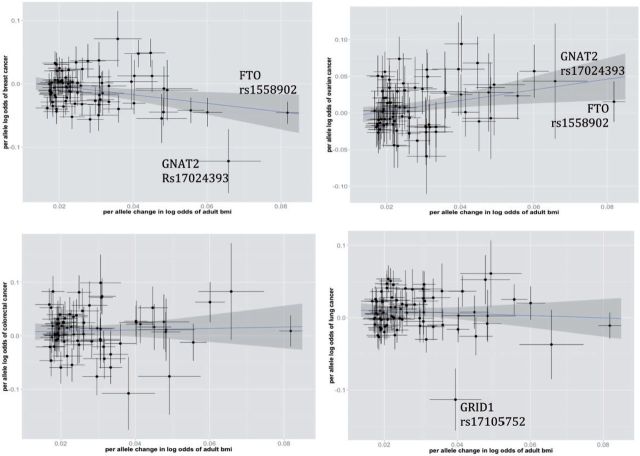
Figure 1 illustrates SNP-specific associations with risk of breast (top left), ovarian (top right), colorectal (bottom left) and lung cancer (bottom right) versus the documented associations between each SNP and adult BMI. After excluding potential outliers (rs1558902 and rs17024393 for breast and ovarian cancer; rs17105752 for lung cancer), the MR analysis still shows strong evidence for association between predicted adult BMI and cancer (for breast cancer, OR = 0.69 per s.d. increase in BMI; 95% CI: 0.58, 0.82; P = 3.0 × 10 -5 ; for ovarian cancer, OR = 1.32 per s.d. increase in BMI; 95% CI: 1.01, 1.74; P = 0.041; for lung cancer, OR = 1.30 per s.d. increase in BMI; 95% CI: 1.10, 1.52; P = 1.5 × 10 -3 ).
Figure 1.
Scatterplot of SNP-specific effects for the associations with adult BMI and a) breast cancer(top left), b) ovarian cancer risk(top right), c) colorectal cancer(bottom left), d) lung cancer(bottom right) for all 77 BMI-associated SNPs. SNP-specific vertical and horizontal bars correspond to standard errors for the breast/ovarian/colorectal/lung cancer association and BMI association respectively. The shaded region corresponds to 95%CI of the association between BMI and cancer risk.
Discussion
In this study, we found an inverse association between the genetic scores for childhood BMI and adult BMI and risk of both overall and ER-negative breast cancer. Further, the genetic score for adult BMI was associated with increased risk of ovarian, lung, squamous lung and colorectal cancer.
Consistent with our results, observational studies have shown an inverse association between higher childhood BMI and both premenopausal and postmenopausal breast cancer. 10,33,34 In contrast to our findings, observational studies have found that higher adult BMI was positively associated with postmenopausal breast cancer; 35,36 this includes a recent instrumental variables analysis using offspring BMI as an instrument for parental BMI. 37 However, we found decreased risk of breast cancer with higher adult BMI genetic score, even though the majority of women who contributed to our analysis were postmenopausal (62%). We did not have access to summary statistics stratified by menopausal status, but findings from a recent MR analysis of a large data set from the Collaborative Oncological Gene-Environment Study (COGS) are consistent with our study. The MR estimate from that study for 5kg/m 2 increase in BMI was 0.65 (95% CI: 0.56, -0.75; P = 3.3 2 × 10 -10 ) for overall breast cancer. This inverse association was consistent across both pre- and postmenopausal women: OR = 0.44; 95% CI: 0.31, 0.62; P = 9.91 × 10 -8 for premenopausal women, and OR = 0.57; 95% CI: 0.46, 0.71; P = 1.88 × 10 -6 for postmenopausal women. 38
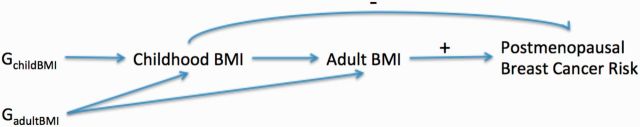
Thus, at first sight, our results might suggest that increasing adult BMI is associated with reduced postmenopausal breast cancer risk, contradicting the epidemiological evidence. There are several possible explanations for this discrepancy. One hypothesis to explain this is illustrated in the causal graph in Figure 2 . The positive association between observed adult BMI and postmenopausal breast cancer in observational studies may be driven by adult weight gain, which has been linked to increased postmenopausal breast cancer risk. 39 This weight gain could be due to environmental factors that are not captured by genetic risk scores. 40 . The effects of the BMI-associated SNPs on breast cancer risk may be mediated through their effects on BMI in childhood and young adulthood, which have been shown to be inversely associated with postmenopausal breast cancer risk (as shown in Figure 2 by a negative sign). 10,33,34 It is also possible that the adult BMI genetic score is a stronger instrumental variable for early life BMI as compared with later life BMI that is largely determined by environment, and that the inverse association of early life BMI with breast cancer may counterbalance the association with BMI later in life.
Figure 2.
DAG demonstrating one potential explanation of how genetic variants influence postmenopausal breast cancer risk.
Consistent with our hypothesis, an observational study examining the association between weight change across the life course and breast cancer risk in the Nurses Health Study (77 232 women from 1980 to 2012) found that weight at age 18 was inversely associated with both pre-and postmenopausal incidence of breast cancer. In contrast, adult weight gain was positively associated with both pre and postmenopausal breast cancer risks (B. Rosner, personal communication).
Three of the four strongest (largest effect size) adult BMI SNPs are also associated with childhood BMI. In sensitivity analyses excluding overlapping loci from the adult and childhood BMI scores, we still observed an inverse association with breast cancer for the genetic score for adult BMI (OR = 0.75, 95% CI: 0.62, 0.92; P = 4.7 × 10 -3 ); but the association between childhood BMI score and breast cancer was attenuated ( Table 3 ). However, we found the genetic instrument for adult BMI was associated with childhood BMI (and vice versa, Supplementary Table 5 , available as Supplementary data at IJE online) even after removing the overlapping loci. This suggests that care is required when interpreting these results. The association between predicted adult BMI and breast cancer risk may reflect effects on a pathway distinct from childhood BMI, or it may simply reflect the shared genetics of early- and later-life BMI.
We found that a genetic risk score predicting higher BMI was associated with increased risk of lung cancer overall and lung squamous carcinoma in particular. Studies have found obesity to be associated with high insulin resistance 41 which is positively associated with lung cancer risk, 42 suggesting that the observed positive associations may be mediated by insulin resistance. Multiple studies have reported an inverse relationship between BMI and lung cancer among smokers but no or a weakened association among never smokers. 8,43–45 These results may be due to residual confounding, reverse causation or effect modification by smoking. 9,44,45 We did not have access to individual-level genetic and smoking data for this study, so our Mendelian randomization estimate of the effect of body mass index on cancer risk should be interpreted with care: it represents an average of the effects across smoking status (83% of the participants in the lung cancer GWAS were ever smokers). Future work in the large OncoArray Network will be able to perform stratified analysis by smoking status. 46
Another concern with our MR analyses on adult BMI and lung cancer risk is that some BMI-associated SNPs are associated with neurological response and stress-related behaviour that affect smoking. 27,47,48 To assess whether our results were driven by pleiotropic effects, we performed additional analysis excluding SNPs that are associated with smoking initiation or schizophrenia (rs1191560, rs11030104 27 ). We still observe a positive association between genetically predicted adult BMI and lung cancer (OR = 1.25; 95% CI: 1.07,1.47; P = 6.0 × 10 -3 ). It is also worth noting that, although we detect limited directional pleiotropy for the association between predicted adult BMI and lung cancer risk, we found inverse association between the genetically predicted adult BMI and lung cancer risk in the MR Egger regression analysis ( P = 0.59). This could be due to bias caused by other type of pleiotropy or lack of statistical power.
Our MR results showed an increased risk in ovarian cancer with increasing adiposity measures across different life stages; this is consistent with previous observational studies. 49,50 Obesity in adolescence is associated with increased risk of ovulatory infertility that may increase risk of ovarian cancer. 51 In addition, obesity is also associated with an increased level of insulin-like growth factor 1 (IGF-1) which increases cell proliferation and modulates synthesis and bioavailability of sex steroids hormones that are involved in ovarian cancer aetiology. 52,53 The opposite risk profiles between breast and ovarian cancer also suggest that adiposity determined by genetic variants has different underlying mechanisms in relation to breast versus ovarian cancer carcinogenesis.
Our analyses suggest that adult BMI is associated with increased risk of colorectal cancer, consistent with the published epidemiological literature. Keimling et al. found a 14% increase in colorectal cancer risk per s.d. increase in BMI. 54 A recently published MR study also found that genetically influenced BMI was associated with higher risk of colorectal cancer (OR = 1.50 per 5 kg/m 2 increase; 95% CI: 1.13, 2.01). 55 The mechanisms linking adiposity and colorectal cancer are not yet fully understood. One possible explanation is that obese individuals have higher leptin secretion from the white adipose tissue, and the binding of leptin to its receptor in the colon epithelium activates biological pathways implicated with colorectal cancer. 56
Although there is evidence that genetically predicted BMI is associated with breast and lung cancer, the underlying mechanisms remain unknown. There are many factors that can influence both adiposity and cancer risks such as physical activity, mental stress, insulin resistance and exposure to hormones secreted by adipose tissue. Further studies incorporating these factors might provide a better understanding of the mechanism underlying the relationship between adiposity and cancer risk. As data on SNP-specific function emerge, future studies can also carefully categorize SNPs by their functionality, and perform MR analysis for different groups of SNPs. This will allow us to parse out specific sets of SNPs and further evaluate which pathway(s) are of importance in the adiposity-cancer association. In addition, gene-environmental interaction can also provide additional insights in understanding the mechanism underlying adiposity and cancer risk. Although not feasible in the GAME-ON data, in the newly completed OncoArray data, where we have individual data on menopausal status, hormone therapy, reproductive factors for breast cancer and smoking status for lung cancer, we will be able to perform gene-environment interaction analysis in the near future. 46
Our study has several limitations. The summary-level statistics approach does not allow us to perform analyses stratified by covariates such as menopausal or smoking status. The summary statistics also did not permit us to explore the non-linearity of the association between obesity and cancer risk, which has been observed in a previous study. 8 We note that non-linearity does not invalidate the test of association, although it may complicate the interpretation of the effect estimate. 57 Finally, the statistical power is limited by both the proportion of the adiposity risk factors explained by the genetic instruments, and the sample size in the cancer genetic association studies, 58 and this is particularly an issue for analyses of rare cancer subtypes.
MR analyses are only valid under a few strong assumptions: 30,59 (i) valid association between SNPs and risk factors; (ii) SNPs are not associated with other confounders of the risk factors and outcome; and (iii) SNPs only affect the outcome through their effect on the risk factors (no pleiotropic effects). The second and third assumptions are the most concerning and require careful interpretation. For (ii), population stratification may be a source of confounding but the original studies saw little evidence for such bias and all have appropriately controlled for it. Assumption (iii) raises the most concern, especially for relationship between genetically predicted adult BMI and breast cancer risk. As noted before, the association between the genetic instrument for adult BMI and childhood BMI (and vice versa) makes the associations between these instruments and breast cancer difficult to distinguish. This is a situation where the InSIDE (Instrument Strength Independent of Direct Effects) assumption—the direct effect of an SNP on cancer risk is uncorrelated with its association with trait of interest—does not hold. 32 There are other reasons why assumption (iii) might not hold. For example, two SNPs known to be associated with breast cancer are near the FTO gene, raising the possibility that obesity-related variants may affect cancer risk through other pathways. 60 To test for and correct for bias due to pleiotropy where the InSIDE assumption holds, we performed Egger regression for all traits investigated ( Table 4 and Supplementary Table 4 , available as Supplementary data at IJE online). Egger regression shows limited evidence for any directional pleiotropic effects influencing associations between genetically predicted adiposity traits and the cancer studied here.
Despite these issues, our study also has several important strengths. Many studies examining BMI and cancer risk in the past were susceptible for recall bias, confounding and reverse causation, 61 none of which are concerns of MR studies. In addition, we used summary statistics from the largest meta-analyses of primary GWAS of these cancer types to date, which improves our power of detecting real causal effects. Moreover, by comparing results across cancer types, we are able to demonstrate specificity of the association between genetic markers of adiposity and particular cancers.
In summary, we found associations between genetic scores for higher adult BMI and increased risk of lung, colorectal and ovarian cancers. Additionally, we observed an inverse association of both genetically predicted childhood BMI and adult BMI with breast cancer. Given the strength of the epidemiological and biological studies linking obesity after menopause with increased risk of breast cancer, this highlights the need for caution when interpreting the results of MR analyses. Our study supports the hypothesis of dynamic relationships between genetic variation underlying obesity and different cancer risks throughout life. To better interpret the complexity of the relationship between adiposity and breast cancer, future investigations that effectively distinguish childhood versus adulthood obesity need to be undertaken. In addition, MR studies stratifying by menopausal status or smoking status can add additional insight in understanding the relationship between adiposity and breast or lung cancer risk.
Supplementary Material
Acknowledgements
DRIVE acknowledges the following genome-wide association studies and investigators from the Breast Cancer Association Consortium (BCAC) that shared genome-wide summary data as part of the breast-cancer GWAS meta-analysis: the Australian Breast Cancer Family Study (ABCFS) (John L. Hopper, Melissa C. Southey, Enes Makalic, Daniel F. Schmidt), the British Breast Cancer Study (BBCS) (Olivia Fletcher, Julian Peto, Lorna Gibson, Isabel dos Santos Silva), the Dutch Familial Bilateral Breast Cancer Study (DFBBCS) (Quinten Waisfisz, Hanne Meijers-Heijboer, Muriel Adank, Rob B van der Luijt, Andre G Uitterlinden, Albert Hofman), German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) (Alfons Meindl, Rita K. Schmutzler, Bertram Müller-Myhsok, Peter Lichtner), the Helsinki breast cancer family Study (HEBCS) (Heli Nevanlinna, Taru A Muranen, Kristiina Aittomäki, Carl Blomqvist), the Mammary Carcinoma Risk factor Investigation (MARIE) (Jenny Chang-Claude, Rebecca Hein, Norbert Dahmen, Lars Beckman), the Singapore and Sweden Breast Cancer Study (SASBAC) (Per Hall, Kamila Czene, Astrid Irwanto, Jianjun Liu) and UK2 (Douglas F Easton, Clare Turnbull, Nazneen Rahman).
FOCI acknowledges the following genome-wide association studies and investigators from the Ovarian Cancer Association Consortium (OCAC) that shared genome-wide summary data as part of the ovarian-cancer GWAS meta-analysis: the Mayo Clinic Ovarian Cancer Case Control Study (Ellen Goode), the North Carolina Ovarian Cancer Study (NCOCS) (Joellen Schildkraut), the New England Case-Control Study (NECC) (Daniel Cramer, Kathryn Terry), the Tampa Bay Ovarian Cancer Study (TBOCS) (Rebecca Sutphen), the Familial Ovarian Tumor Study (FOTS) (Steven Narod, Catherine Phelan, Harvey Risch), the Royal Marsden Hospital Ovarian Cancer Study, the UK Studies of Epidemiology and Risk Factors in Cancer Heredity Ovarian Cancer Study (SEARCH), the UK Familial Ovarian Cancer Registry (UKFOCR) (Paul Pharoah), the UK Ovarian Cancer Population Study (UKOPS) (Simon Gayther, Usha Menon) and the Polish Ovarian cancer Case Control Study (POCS) (Nicolas Wentzensen).
Conflict of interest : None declared.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the Genetic Associations and Mechanisms in Oncology Network, GAME-ON [ http://epi.grants.cancer.gov/gameon/], which includes the following consortia: Colorectal Transdisciplinary Study, CORECT, PI: S. Gruber (grant number U19 CA148107); Discovery, Biology, and Risk of Inherited Variants in Breast Cancer, DRIVE, PI: D. Hunter (U19 CA148065); Elucidating Loci Involved in Prostate Cancer Susceptibility, ELLIPSE, PI: B. Henderson (grant number U19 CA148537); Follow-up of Ovarian Cancer genetic association and Interaction studies, FOCI, PI: T. Sellers (U19 CA148112); Transdisciplinary Research in Cancer of the Lung, TRICL, PI: C. Amos (U19 CA148127). This work was also supported by Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation (NIEHS K99ES023504, NIEHS R21 ES025052); Cancer Research UK (C1287/A16563); and National Institutes of Health (CA173785 and CA165131).
We also acknowledge the following funding sources for breast cancer genome-wide association studies: BBCS: this work was funded by Cancer Research UK (C150/A5660, C1178/A3947); Breakthrough Breast Cancer; and the Institut National de Cancer.
We acknowledge National Health Service funding to the NIHR Biomedical Research Centre and the National Cancer Research Network (NCRN). Funding for the project was provided by the Wellcome Trust (076113, 085475). Controls from the Dutch Familial Bilateral Breast Cancer Study (DFBBCS) are drawn from the Rotterdam Study: Rotterdam Study is supported by: the Netherlands Organisation of Scientific Research NWO Investments (no. 175.010.2005.011, 911‐03‐012); the Genetic Laboratory of the Department of Internal Medicine; Erasmus MC; the Research Institute for Diseases in the Elderly (014‐93‐015; RIDE2); the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO); Netherlands Consortium for Healthy Aging (NCHA)(no. 050‐060‐810); Erasmus Medical Center and Erasmus University, Rotterdam; Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DGx II); and the Municipality of Rotterdam.
We also acknowledge the following funding sources for the Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI): the National Cancer Institute’s Cancer Post-GWAS Initiative; Genetic Associations and Mechanisms in Oncology (GAME-ON) (U19-CA148112), (R01-CA114343, R01-CA114343-S1); National Cancer Institute (P30-CA15083); Cancer Research UK (C490/A8339); and the Wellcome Trust (076113).
References
- 1. McCallum V, Schneider-Levinson W . Obesity: A Provocative Question and the Search for Answers . Bethesda, MD: : Division of Cancer Epidemiology & Genetics, National Cancer Institute; , 2015. . [Google Scholar]
- 2. Noto H, Goto A, Tsujimoto T, Osame K, Noda M . Latest insights into the risk of cancer in diabetes . J Diabetes Invest 2013. ; 4:225 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L . Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women . Int J Cancer 2003. ; 106:96102 . [DOI] [PubMed] [Google Scholar]
- 4. Song X, Pukkala E, Dyba T, et al. . Body mass index and cancer incidence: the FINRISK study . Eur J Epidemiol 2014. ; 29:477 – 87 . [DOI] [PubMed] [Google Scholar]
- 5. Lu L, Risch H, Irwin ML, et al. . Long-term overweight and weight gain in early adulthood in association with risk of endometrial cancer . Int J Cancer 2011. ; 129:1237 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arslan AA, Helzlsouer KJ, Kooperberg C, et al. . Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) . Arch Intern Med 2010. ; 170:791 – 802 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs ÇS . Physical activity, obesity, height, and the risk of pancreatic cancer . JAMA 2001. ; 286:921 – 29 . [DOI] [PubMed] [Google Scholar]
- 8. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L . Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults . Lancet 2014. ; 384 : 755 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith L, Brinton LA, Spitz MR, et al. . Body mass index and risk of lung cancer among never, former, and current smokers . J Natl Cancer Inst 2012. ; 104:778 – 89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baer HJ, Tworoger SS, Hankinson SE, Willett WC . Body fatness at young ages and risk of breast cancer throughout life . Am J Epidemiol 2010. ; 171:1183 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morimoto LM, White E, Chen Z, et al. . Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) . Cancer Causes Control. 2002 Oct. ; 13 ( 8 ) :741 – 51 . [DOI] [PubMed] [Google Scholar]
- 12. White AJ, Nichols HB, Bradshaw PT, Sandler DP . Overall and central adiposity and breast cancer risk in the sister study . Cancer 2015. ; 121 ( 20 ) :3700 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lubin F . Body mass index at age 18 years and during adult life and ovarian cancer risk . Am J Epidemiol 2003. ; 157:113 – 20 . [DOI] [PubMed] [Google Scholar]
- 14. Stoll BA . Obesity and breast cancer . Int J Obes Relat Metab Disord 1996. ; 20:389 – 92 . [PubMed] [Google Scholar]
- 15. Hollmann M, Runnebaum B, Gerhard I . Impact of waist-hip-ratio and body-mass-index on hormonal and metabolic parameters in young, obese women . Int J Obes Relat Metab Disord 1997. ; 21:476 – 83 . [DOI] [PubMed] [Google Scholar]
- 16. Borugian MJ . Waist-to-hip ratio and breast cancer mortality . Am J Epidemiol 2003. ; 158:963 – 68 . [DOI] [PubMed] [Google Scholar]
- 17. Huang Z, Willett WC, Colditz GA, et al. . Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study . Am J Epidemiol 1999 Dec 15. ; 150 ( 12 ) :1316 – 24 . [DOI] [PubMed] [Google Scholar]
- 18. Moore LL, Bradlee ML, Singer MR, et al. . BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults . Int J Obes Relat Metab Disord 2004. ; 28:559 – 67 . [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Jacobs EJ, Patel AV, et al. . A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence . Cancer Causes Control 2008. ; 19:783 – 92 . [DOI] [PubMed] [Google Scholar]
- 20. Davey Smith G, Hemani G . Mendelian randomization: genetic anchors for causal inference in epidemiological studies . Hum Mol Genet 2014. ; 23:R89 – 98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmer TM, Sterne JA, Harbord RM, et al. . Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses . Am J Epidemiol 2011. ; 173:1392 – 403 . [DOI] [PubMed] [Google Scholar]
- 22. Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW . Mendelian randomization: use of genetics to enable causal inference in observational studies . Nephrol Dial Transplant 2010. ; 25:1394 – 98 . [DOI] [PubMed] [Google Scholar]
- 23. Fehringer G, Kraft P, Pharoah PD, et al. . Cross-cancer genome-wide analysis of lung, ovary, breast, prostate and colorectal cancer reveals novel pleiotropic associations. Cancer Res. 2016 Apr 20. pii: canres.2980.2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Doherty JA, Burgess S, et al. . Genetic determinants of telomere length and risk of common cancers: a Mendelian randomization study . Hum Mol Genet . 2015 Sep 15. ; 24 ( 18 ) :5356 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. . New loci associated with birthweight identify genetic links between intrauterine growth and adult height and metabolism . Nat Genet 2013. ; 45:76 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felix JF, Bradfield JP, Monnereau C, et al. . Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index . Hum Mol Genet . 2016 Jan 15. ; 25 ( 2 ) :389 – 403 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locke AE, Kahali B, Berndt SI, et al. . Genetic studies of body mass index yield new insights for obesity biology . Nature 2015. ; 518:197 – 206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heid IM, Jackson AU, Randall JC, et al. . Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution . Nat Genet 2010. ; 42:949 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burgess S, Butterworth A, Thompson SG . Mendelian randomization analysis with multiple genetic variants using summarized data . Genet Epidemiol 2013. ; 37:658 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P . Methodological challenges in mendelian randomization . Epidemiology 2014. ; 25:427 – 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al Olama AA, Kote-Jarai Z, Berndt SI, et al. . A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer . Nat Genet 2014. ; 46:1103 – 09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowden J, Davey Smith G, Burgess S . Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression . Int J Epidemiol 2015. ; 44:512 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baer HJ, Colditz GA, Willett WC, Dorgan JF . Adiposity and sex hormones in girls . Cancer Epidemiol Biomarkers Prev 2007. ; 16:1880 – 08 . [DOI] [PubMed] [Google Scholar]
- 34. Baer HJ, Colditz GA, Rosner B, et al. . Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study . Breast Cancer Res 2005. ; 7:R314 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magnusson C, Baron J, Persson I, et al. . Body size in different periods of life and breast cancer risk in post-menopausal women . Int J Cancer 1998 Mar 30. ; 76 ( 1 ) :29 – 34 . [DOI] [PubMed] [Google Scholar]
- 36. van den Brandt PA, Spiegelman D, Yaun SS, et al. . Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk . Am J Epidemiol 2000 Sep 15. ; 152 ( 6 ) :514 – 27 . [DOI] [PubMed] [Google Scholar]
- 37. Davey Smith G, Sterne JA, Fraser A, Tynelius P, Lawlor DA, Rasmussen F . The association between BMI and mortality using offspring BMI as an indicator of own BMI: large intergenerational mortality study . BMJ 2009. ; 339:b5043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo Y, Andersen SW, Shu X-O, Michailidou K, et al. . Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 women of European descent [Accepted] . PLOS Medicine 2016. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emaus MJ, van Gils CH, Bakker MF, et al. . Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study . Int J Cancer 2014. ; 135:2887 – 99 . [DOI] [PubMed] [Google Scholar]
- 40. Sandholt CH, Allin KH, Toft U, et al. . The effect of GWAS identified BMI loci on changes in body weight among middle-aged Danes during a five-year period . Obesity (Silver Spring) 2014. ; 22:901 – 08 . [DOI] [PubMed] [Google Scholar]
- 41. Bardou M, Barkun AN, Martel M . Obesity and colorectal cancer . Gut 2013. ; 62:933 – 47 . [DOI] [PubMed] [Google Scholar]
- 42. Petridou ET, Sergentanis TN, Antonopoulos CN, et al. . Insulin resistance: an independent risk factor for lung cancer? Metabolism 2011. ; 60:1100 – 06 . [DOI] [PubMed] [Google Scholar]
- 43. Kabat GC, Miller AB, Rohan TE . Body mass index and lung cancer risk in women . Epidemiology 2007. ; 18:607 – 12 . [DOI] [PubMed] [Google Scholar]
- 44. Tarnaud C, Guida F, Papadopoulos A, et al. . Body mass index and lung cancer risk: results from the ICARE study, a large, population-based case-control study . Cancer Causes Control 2012. ; 23:1113 – 26 . [DOI] [PubMed] [Google Scholar]
- 45. El-Zein M, Parent ME, Nicolau B, Koushik A, Siemiatycki J, Rousseau MC . Body mass index, lifetime smoking intensity and lung cancer risk . Int J Cancer 2013. ; 133:1721 – 31 . [DOI] [PubMed] [Google Scholar]
- 46. OncoArray Network . http://epi.grants.cancer.gov/oncoarray/ .
- 47. McKee SA, Sinha R, Weinberger A, et al. . Stress decreases the ability to resist smoking and potentiates smoking intensity and reward . J Psychopharmacol 2011. ; 25:490 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Childs E, de Wit H . Effects of acute psychosocial stress on cigarette craving and smoking . Nicotine Tob Res 2010. ; 12:449 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aune D, Navarro Rosenblatt DA, Chan DS, et al. . Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies . Int J Cancer 2015. ; 136:1888 – 98 . [DOI] [PubMed] [Google Scholar]
- 50. Baer HJ, Hankinson SE, Tworoger SS . Body size in early life and risk of epithelial ovarian cancer: results from the Nurses’ Health Studies . Br J Cancer 2008. ; 99:1916 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE . Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk . Am J Epidemiol 2007. ; 166:894 – 901 . [DOI] [PubMed] [Google Scholar]
- 52. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM . Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis . Eur J Cancer 2007. ; 43:690 – 709 . [DOI] [PubMed] [Google Scholar]
- 53. Risch HA . Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone . J Natl Cancer Inst. 1998 Dec 2. ; 90 ( 23 ) :1774 – 86 . [DOI] [PubMed] [Google Scholar]
- 54. Keimling M, Renehan AG, Behrens G, et al. . Comparison of associations of body mass index, abdominal adiposity, and risk of colorectal cancer in a large prospective cohort study . Cancer Epidemiol Biomarkers Prev 2013. ; 22:1383 – 94 . [DOI] [PubMed] [Google Scholar]
- 55. Thrift AP, Gong J, Peters U, et al. . Mendelian randomization study of body mass index and colorectal cancer risk . Cancer Epidemiol Biomarkers Prev 2015. ; 24:1024 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harriss DJ, Atkinson G, George K, et al. . Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index . Colorectal Dis 2009. ; 11:547 – 63 . [DOI] [PubMed] [Google Scholar]
- 57. Burgess S, Davies NM, Thompson SG ; Consortium EP-I . Instrumental variable analysis with a nonlinear exposure-outcome relationship . Epidemiology 2014. ; 25:877 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burgess S . Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome . Int J Epidemiol 2014. ; 43:922 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomas DC, Conti DV . Commentary: The concept of ‘Mendelian randomization’ . Int J Epidemiol 2004. ; 33:21 – 25 . [DOI] [PubMed] [Google Scholar]
- 60. Michailidou K, Beesley J, Lindstrom S, et al. . Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer . Nat Genet 2015. ; 47:373 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spencer EA, Appleby PN, Davey GK, Key TJ . Validity of self-reported height and weight in 4808 EPIC-Oxford participants . Public Health Nutr 2002. ; 5:561 – 65 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.