Abstract
Visualizing and quantifying molecular motion and interactions inside living cells provides crucial insight into the mechanisms underlying cell function. This has been achieved by super-resolution localization microscopy and single-molecule tracking in conjunction with photoactivatable fluorescent proteins (PA-FPs). An alternative labelling approach relies on genetically-encoded protein tags with cell-permeable fluorescent ligands which are brighter and less prone to photobleaching than fluorescent proteins but require a laborious labelling process. Either labelling method is associated with significant advantages and disadvantages that should be taken into consideration depending on the microscopy experiment planned. Here, we describe an optimised procedure for labelling Halo-tagged proteins in live Escherichia coli cells. We provide a side-by-side comparison of Halo tag with different fluorescent ligands against the popular photoactivatable fluorescent protein PAmCherry. Using test proteins with different intracellular dynamics, we evaluated fluorescence intensity, background, photostability, and results from single-molecule localization and tracking experiments. Capitalising on the brightness and extended spectral range of fluorescent Halo ligands, we also demonstrate high-speed and dual-colour single-molecule tracking.
Keywords: single-molecule tracking, super-resolution microscopy, Halo tag, photoactivatable fluorescent protein, DNA-binding proteins, Escherichia coli, fluorophores
Introduction
The combination of super-resolution localization microscopy with single-particle tracking has proven very powerful as it allows direct visualization of the activity of thousands of proteins inside individual living cells. The localizations, mobility, and movement patterns of each molecule report on the molecular interactions and reactions in real-time. These data provide important quantitative information about those interactions, such as the diffusion coefficients of the reactants, dissociation constants, and the spatial distribution of interaction sites relative to other landmarks in the cell. Various implementations of this approach have provided crucial insights into many fundamental molecular processes in prokaryotic and eukaryotic cells [1–7].
Super-resolution localization microscopy is based on the sequential detection and localization of individual fluorescent molecules over the course of a movie [8]. This demands fluorescent labels with certain properties [9–11], most notably (i) means for specific labelling of target proteins ideally in a genetically-encoded manner, (ii) sufficient brightness for detection and accurate localization of single molecules, and (iii) the ability to switch fluorophores between fluorescent and non-fluorescent states so that only a fraction of molecules is visible at any time. For single-molecule tracking, photostability is also an important requirement as bleaching limits the observation time per molecule.
Live-cell localization microscopy was enabled by the development of photoactivatable fluorescent proteins (PA-FPs) such as PA-GFP, PAmCherry, mEOS, and other variants [10–12]. In fixed and permeabilized cells, antibody staining permits the use of synthetic dyes which are generally brighter and more photostable than fluorescent proteins. Fixed samples can be imaged in special buffers that induce reversible photoswitching of many types of synthetic fluorophores [13, 14]. The photophysical properties, cell permeability, and conjugation chemistries of different classes of synthetic dyes have been engineered for microscopy applications. Especially rhodamine, cyanine, and oxazine dye derivatives are commonly used in super-resolution microscopy and single-molecule fluorescence studies [9]. Genetically encoded protein tags have been developed for labelling with synthetic dyes that permeate live cells [15]. Especially Halo tag [16], SNAP tag [17], and CLIP tag [18] are becoming increasingly popular in fluorescence microscopy. In principle, these tags combine the advantages of genetically encoded FPs with the superior brightness and photostability of synthetic dyes. Because of this, they have found many successful applications in super-resolution microscopy [19, 20], single-molecule imaging [21, 22], and single-molecule tracking techniques [5, 7, 23], both in eukaryotic and in prokaryotic cells.
Halo tag is a monomeric 33 kDa protein that was engineered from a bacterial hydrolase enzyme to form a covalent bond with a chloroalkane linker of a ligand. This reaction is rapid and irreversible under physiological intracellular conditions [16]. Promega have commercialised the system and supply a range of fluorescent ligands. These include tetramethylrhodamine (TMR), as well as Oregon Green, and diAcFAM dyes which are less bright than TMR [24]. Alexa fluors are very popular in super-resolution microscopy with fixed cells; however, the Promega Alexa Halo tag ligands do not permeate into live cells. A new palette of bright cell-permeable fluorescent Halo tag ligands has been developed based on the TMR scaffold and termed Janelia Fluors [25]. There are also significant disadvantages and considerations concerning protein tags compared to fluorescent proteins, as the extra labelling step introduces additional experimental complexity and labour, and labelling specificity is not guaranteed. Nevertheless, the separation of protein expression and fluorescence labelling provides another means of experimental control and opportunities for new microscopy-based assays.
Many factors need to be considered when choosing the optimal labelling strategy for any microscopy experiment, and the additional temporal dimension in single-molecule tracking presents further challenges. To facilitate the decision, we evaluated the Halo tag with different fluorescent ligands in a detailed side-by-side comparison against PAmCherry, a popular PA-FP [12]. PAmCherry has excitation/emission maxima of 564/595 nm, quantum yield of 0.46, and extinction coefficient of 1.8 × 104 M−1 cm−1 [12]. Based on previous successful applications (e.g. [5, 20, 21]), we first evaluated the Halo ligand TMR, which has excitation/emission maxima of 548/572 nm, quantum yield of 0.41, and extinction coefficient of 7.8 × 104 M−1 cm−1 [25]. We also tested the dyes JF549 and PA-JF549 which were derived from TMR and have similar spectral characteristics [25, 26]. JF549 has excitation/emission maxima of 549/571 nm, quantum yield of 0.88, and extinction coefficient of 1.0 × 105 M−1 cm−1 [26]. We also utilized the red-shifted silicon-rhodamine dye JF646 [26, 27] in combination with JF549 for dual-colour imaging. JF646 has excitation/emission maxima of 646/664 nm, quantum yield of 0.54, and extinction coefficient of 1.5 × 105 M−1 cm−1 [26]. After optimising a protocol for Halo tag labelling, we tested labelling specificity and background, quantified the brightness and photostability of the labels, and performed tracking experiments on several different fusion proteins. We compile these assessments to provide a practical guide for choosing the most suitable label for single-molecule tracking applications in live E. coli cells.
Fluorescent labelling of intracellular Halo tag proteins
To evaluate the performance of Halo tag and PAmCherry we chose test proteins that had previously been characterised. MukB is a structural maintenance of chromosomes (SMC) protein that acts in chromosome segregation together with accessory proteins MukE and MukF at a copy number of ~200 MukB dimers per cell [28]. Tracking MukB-PAmCherry revealed the presence of mobile and immobile SMC complexes that accumulated in distinct foci inside cells [29]. These foci appear to position the chromosome replication origin and subsequently aid in the segregation of the replicated origins into the two daughter cells [30]. As a second test protein with different intracellular mobility and localization, we chose DNA polymerase I (PolI), a monomeric protein that is much smaller than the SMC complex and present at ~400 copies per cell [31]. PolI performs DNA synthesis during the processing of Okazaki fragments and in DNA excision repair pathways [32]. Following DNA damage treatment, PolI-PAmCherry molecules become transiently immobilised at DNA repair sites [31]. To complement the existing PAmCherry fusions of MukB and PolI [29, 31], we generated endogenous C-terminal translational Halo tag fusions using the Lambda Red recombination technique [33]. We detected no signs of any growth defects that would indicate impairment of the function of MukB or PolI by the Halo fusions. Single-molecule imaging and tracking was performed on a custom-built total internal reflection fluorescence microscope equipped with an electron-multiplying CCD camera [34]. To image cytoplasmic proteins within ~1 µm thick E. coli cells, laser excitation was adjusted to highly inclined illumination mode [35].
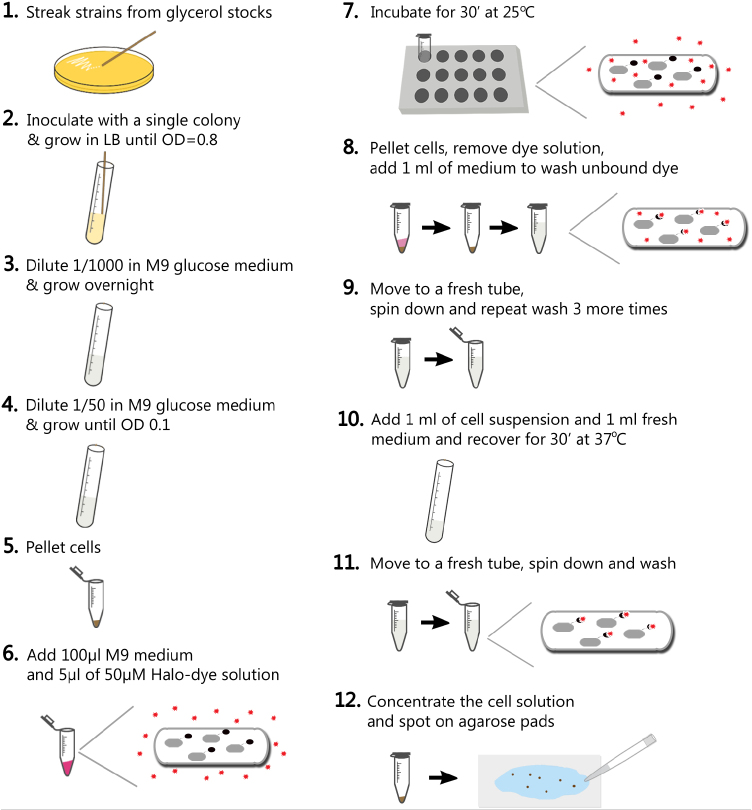
We optimized a procedure for labelling Halo tag in live E. coli with the commercially available dye TMR (figure 1). Briefly, we grew cell cultures expressing PolI-Halo to early exponential phase at 37 °C in M9 minimal growth medium containing glucose and amino acids. 1 ml of culture was concentrated by centrifugation, resuspended in 100 µl of growth medium and incubated with 2.5 µM TMR dye (5 µl of 50 µM TMR stock solution) at 25 °C for 30 min. Removal of unbound dye was crucial to minimize fluorescent background for robust detection of single molecules inside cells. This is especially important for imaging bacteria directly on the surface of the coverslip where fluorescent particles adhere. We optimized the washing procedure by measuring the background over multiple centrifugation and resuspension cycles (figure 2(a)). After each wash, a small amount of cell suspension was immobilized on an agarose gel pad sandwiched between two microscope coverslips and imaged using 561 nm laser excitation. Intracellular fluorescence became visible above the background noise after 2–3 washes, but substantial background remained both on the coverslip surface and as diffuse background within the agarose pad. Therefore, to facilitate release of unbound dye and to recover the cells from the prolonged centrifugation, we performed a 4th wash and incubated the diluted labelled culture at 37 °C for 30 min in a shaking incubator, followed by a final 5th wash. This removed the fluorescent background almost entirely. The entire labelling, washing, and recovery procedure takes approximately 90 min. Note that cell growth and division during the recovery dilutes the labelled Halo proteins exponentially while new unlabelled Halo is synthesised.
Figure 1.
Procedure for labelling Halo tag with a fluorescent ligand in live E. coli cells.
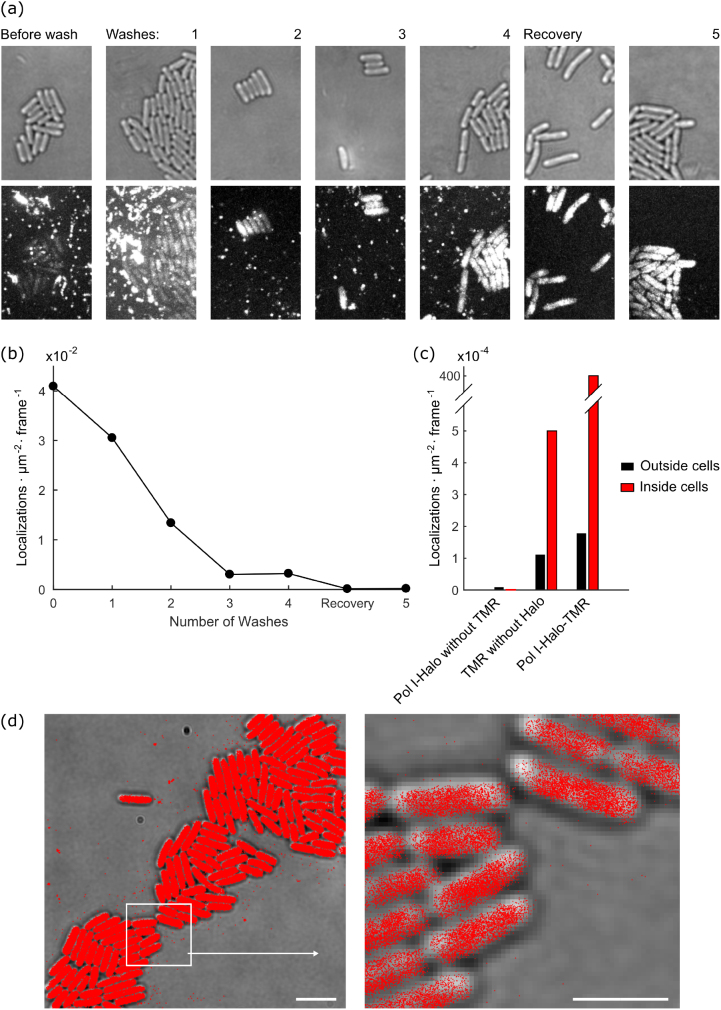
Figure 2.
Removal of fluorescent background after Halo tag labelling. (a) E. coli expressing PolI-Halo-TMR were labelled and a super-resolution movie of 15 000 frames (15.48 ms frame−1) was recorded after each washing step to remove unbound dye. Transmitted light images show cell outlines (top). Fluorescence images show maximum intensity projections from the super-resolution movie (bottom, identical grey scaling across images for all washes). (b) Frequency of background localizations outside cells during the washing procedure. The frequency represents the average number of localizations detected per area per frame. (c) Frequency of localizations outside cells and inside cells: (i) for cells without TMR labelling, (ii) for cells that do not express Halo tag but were labelled with TMR (5 washes), (iii) for cells expressing PolI-Halo that were labelled with TMR (5 washes). (d) Map of PolI-Halo-TMR localizations (5 washes) plotted on transmitted light snapshot (scale bar 4 µm). The boxed area is shown magnified (scale bar 2 µm).
To quantify how the background affects single-molecule localization microscopy, we acquired movies and performed localization analysis as described below and in our previous work [36]. The frequency of background localizations outside cells decreased with each washing cycle from an average of 4.1 · 10−2 localizations · µm−2 · frame−1 without washing to 1.7 · 10−4 localizations · µm−2 · frame−1 after 5 washes (figure 2(b)). The remaining background after the washing procedure was much lower than the desired signal from PolI-Halo-TMR inside cells (average frequency of 4 · 10−2 localizations · µm−2 · frame−1, figures 2(c) and (d)). To assess the level of non-specific labelling, we measured the localization frequency of TMR inside cells that do not express Halo tag. The localization frequency was approximately 5-fold above the background outside cells, but more than 2 orders of magnitude lower than the specific localizations of PolI-Halo-TMR (figure 2(c)). Furthermore, the localization frequency inside cells without any TMR labelling was as low as 2 · 10−6 localizations · µm−2 · frame−1 (figure 2(c)). Therefore, the optimised labelling and washing procedure, produces reliable single-molecule localization data for Halo-TMR in live E. coli cells with little non-specific background (figure 2(d)).
Single-molecule localization microscopy of Halo tag and PAmCherry fusions
To test the utility of the Halo tag for imaging intracellular protein localization, we recorded snapshots of MukB-Halo-TMR using a standard Nikon epifluorescence microscope. Comparison with a fusion of MukB to the conventional fluorescent protein mCherry shows that both labelling strategies reproduce the characteristic foci of MukB [29] with similar image quality (figure 3(a)). The procedure for recording single-molecule localization or tracking data with Halo-TMR differs from PAmCherry. PAmCherry is initially in a non-fluorescent state and becomes converted by 405 nm illumination to the fluorescent state which can be excited at 561 nm [10, 12]. Consequently, the sample appears dark at the beginning of the experiment apart from a small number of spontaneously activated molecules (figure 3(b)). On the one hand, this can be a technical inconvenience, as the absence of any signal makes it harder to find regions of interest and to adjust the focus. On the other hand, illumination at 561 nm without 405 nm photoactivation allows bleaching any background fluorescence before data acquisition. Simultaneous 405 nm and 561 nm illumination during the acquisition causes continuous activation and bleaching of PAmCherry for sequential localization of individual molecules (figure 3(b)). Adjusting the 405 nm intensity provides accurate control over the density of fluorescent dyes at any time to facilitate single-molecule detection. Because most PAmCherry molecules bleach irreversibly after one photoactivation cycle, the decay in the density of fluorescent molecules can be counteracted by gradually increasing the 405 nm intensity during an acquisition.
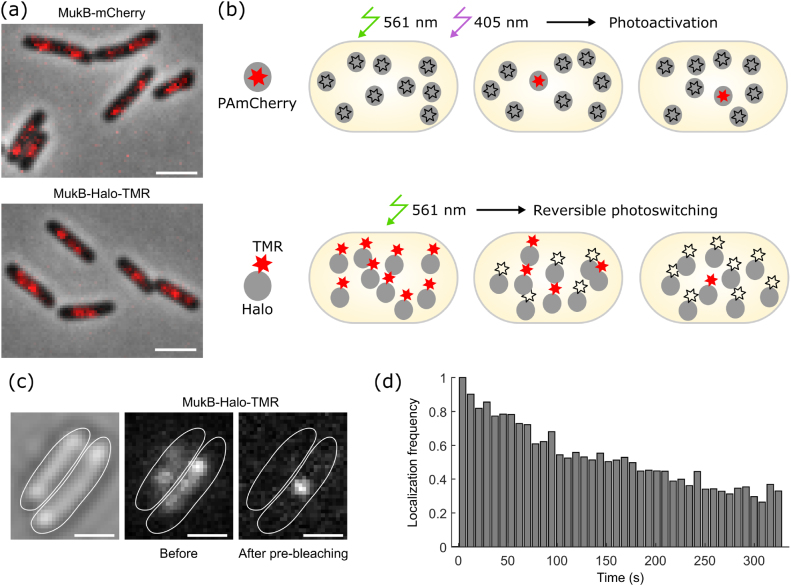
Figure 3.
Microscopy data acquisition using Halo tag. (a) Snapshots of MukB-Halo-TMR and MukB-mCherry recorded with identical settings on an epifluorescence microscope (red: fluorescence with 150 ms exposure, grey: phase contrast). Scale bars 2 µm. (b) Principle of data acquisition for single-molecule localization microscopy using PAmCherry or Halo-TMR labels. (c) Snapshots showing MukB-Halo-TMR intensity before and after pre-bleaching (15 ms exposure). Scale bars 1 µm. (d) Frequency of MukB-Halo-TMR localizations over the course of a movie at constant 561 nm excitation (0.2 kW cm−2), normalised to the initial frequency.
Contrary to PAmCherry, TMR-labelled Halo fusions are initially in the fluorescent state (figures 3(b) and (c)). Therefore, pre-bleaching is required before single-molecule imaging, but this can be useful for capturing a conventional epifluorescence image as a low-resolution reference (figure 3(a)). Under continuous illumination at 561 nm, TMR showed both photobleaching and reversible photoswitching within live cells. Initially, the number of visible fluorophores decayed rapidly due to reversible deactivation of the dye (figure 3(c)), followed by a long period of spontaneous photoswitching (figure 3(d)). The frequency of localisations decreased slowly over the course of a movie because of photobleaching (figure 3(d)). Importantly, imaging was performed in M9 minimal medium containing only supplements for cell growth (glucose, amino acids, and thiamine). There was no requirement for special buffer additives to induce photoswitching (e.g. oxygen scavenging and reducing agents) because the reducing environment inside cells promotes the photoinduced formation of a dark radical anion state that is recovered by molecular oxygen [37]. The lifetime of the reversible dark state of TMR was sufficiently long such that only a sparse subset of molecules appeared fluorescent at any time, which is an important requirement for accurate single-molecule localization and tracking. Compared to PAmCherry, the density of fluorescent TMR molecules cannot be precisely controlled at this stage in the experiment. However, if the density is too high, the initial photobleaching can be prolonged before data acquisition or substoichiometric labelling can be achieved by using a lower concentration of the dye.
We generally detected more localizations from Halo-TMR compared to PAmCherry under the same acquisition settings. This was mostly due to the repeated photoswitching cycles of TMR as opposed to irreversible activation and bleaching of PAmCherry. Although repeated photoswitching of TMR provides more localization data from the same number of molecules per cell, this may complicate quantitative analysis of the localization data. In particular, blinking of single molecules produces apparent clusters of localizations, which are difficult to distinguish from genuine molecular assemblies [38]. This issue also affects PA-FPs, albeit to a lesser extent than photoswitchable dyes [39]. Several data analysis strategies have been reported to correct for blinking effects in molecule counting and clustering analysis [38–42].
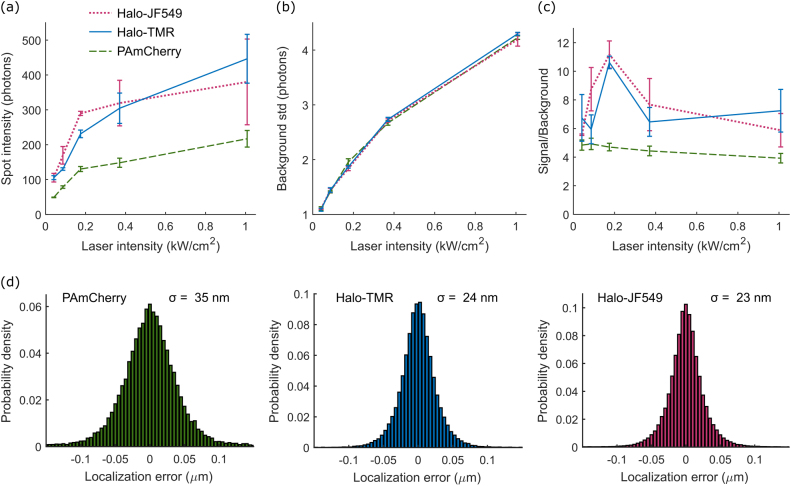
The average intensity of single MukB-Halo-TMR molecules was approximately 2-fold higher than MukB-PAmCherry under the same imaging conditions (figure 4(a)). Notably, fluorescence brightness saturated with increasing 561 nm excitation intensity for both dyes, while the background shot noise continues to increase (figure 4(b)). This creates a peak in the signal to noise ratio at ~0.2 kW cm−2 561 nm intensity (figure 4(c)). We also tested the Halo ligand JF549, which is a derivative of TMR with improved brightness and photostability [25]. We achieved excellent labelling of MukB-Halo-JF549 using the same protocol as for TMR, and found that it showed similar photoswitching behaviour. JF549 was significantly brighter than TMR at excitation intensities up to 0.2 kW cm−2 but there was little difference for higher intensities (figures 4(a)–(c)). To quantify the localization precision for the different labels, we imaged MukB fusions in cells that were chemically fixed with paraformaldehyde (figure 4(d)). At the optimal excitation intensity of 0.2 kW cm−2 we measured localization errors (standard deviation) of σ = 35 nm (PAmCherry), 24 nm (TMR), and 23 nm (JF549). Photoactivatable Halo tag ligands have also been developed [26]. We tested one such dye, PA-JF549, but found that it did not label MukB-Halo efficiently using our protocol. Only ~1% of cells showed intracellular fluorescence, likely because the dye does not pass the E. coli cell wall.
Figure 4.
Fluorescence intensity and localization error comparison of PAmCherry, Halo-TMR, and Halo-JF549. (a) Fluorescence spot intensity of single MukB fusion proteins tagged with the indicated fluorophores as a function of 561 nm excitation intensity with 15 ms exposure time. Error bars are ±SEM from 3 repeats. (b) Standard deviation of the background noise per pixel as a function of 561 nm excitation intensity with 15 ms exposure time. Error bars are ±SEM from 3 repeats. (c) Signal to background ratio for the data in a-b, calculated from the peak pixel intensity in spots divided by the background std per pixel. Error bars are ±SEM from 3 repeats. (d) Localization error was determined as the standard deviation of repeated localizations of the same immobile molecules in fixed cells at 15 ms exposure time.
Single-molecule tracking of Halo tag and PAmCherry fusions
The use of photoactivatable or photoswitchable fluorophores generalizes single-particle tracking, such that a very large number of trajectories can be recorded per cell, irrespective of the protein expression level or labelling density [43]. A major appeal of single-molecule tracking experiments is the ability to directly observe and quantify molecular interactions. PA-FPs have been used successfully for this purpose [2, 4, 6, 44]. However, low brightness and photostability of these fluorophores have been major limitations. Fluorophore brightness not only determines the spatial resolution, but also sets a limit on the temporal resolution at which molecules can be tracked. Measuring protein motion in the bacterial cytoplasm requires imaging at high frame rates of typically 10–1000 frames s−1. Because photobleaching restricts the observation time per molecule, it has been challenging to record long-lived, rare, or transient molecular events. We thus tested whether the enhanced photophysical characteristics of fluorescent Halo tag ligands enable faster and longer observations, which would open avenues for new biological applications.
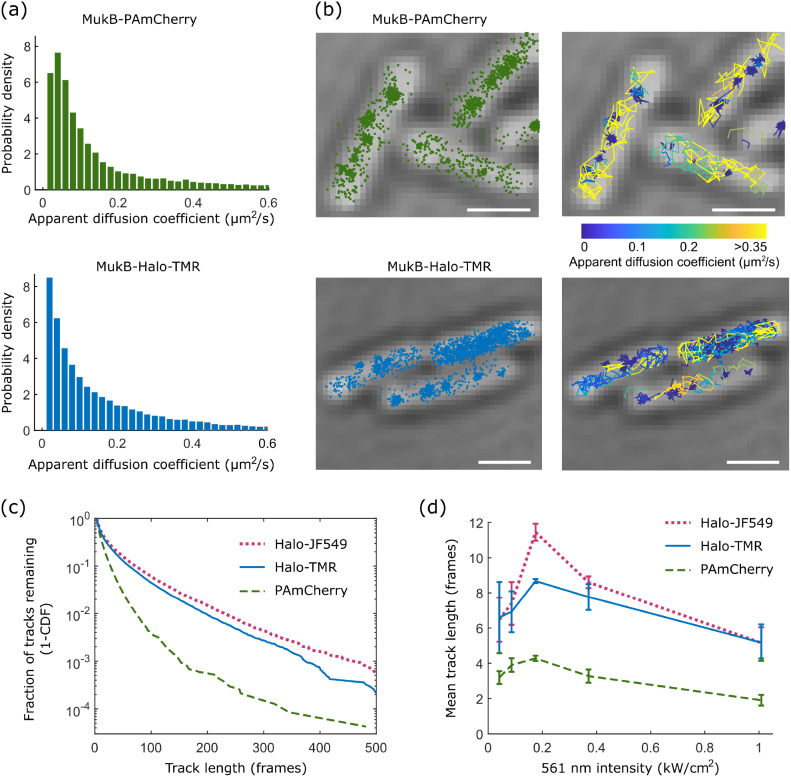
Using a tracking algorithm, 2D localizations were linked to tracks if they appeared within a radius of 768 nm (8 pixels) in consecutive frames [36]. To reconnect tracks with single missed localizations, we used a memory parameter of 1 frame. An apparent diffusion coefficient D* = MSD/4Δt was calculated from the mean squared displacement (MSD) between consecutive localizations of individual tracks with Δt = 15.48 ms. For our purposes, D* serves as a relative measure of mobility but does not represent the accurate diffusion coefficient of a molecule because of several systematic biases, such as motion confinement, motion blurring, and localization error. If required, these biases can be estimated and corrected for according to previously described procedures [31, 36]. For example, the localization error σ adds an offset of σ2/Δt to the apparent diffusion coefficient. The distribution of D* values for MukB-Halo-TMR showed a mixture of immobile and diffusing molecules (figure 5(a)). Stationary MukB molecules were localized in clusters, whereas diffusing molecules moved randomly within the nucleoid (figure 5(b)). These results match our observations for MukB-PAmCherry (figures 5(a) and (b)), and reproduce previous findings [29].
Figure 5.
Application of PAmCherry and Halo tag labels for single-molecule tracking of MukB. All data recorded at 15.48 ms frame−1. (a) Distributions of the apparent diffusion coefficient for MukB-PAmCherry and MukB-Halo-TMR. (b) Localisations and tracks of MukB-PAmCherry and MukB-Halo-TMR. Track colours correspond to the apparent diffusion coefficient. Scale bars 1 µm. (c) Track length distributions at 0.2 kW cm–2 561 nm excitation intensity for MukB labelled with PAmCherry, Halo-TMR, and Halo-JF549. (d) Mean track length as a function of 561 nm excitation intensity. Error bars are ±SEM from 3 repeats.
Next, we analysed the duration of single-molecule tracks for MukB-PAmCherry, MukB-Halo-TMR, and MukB-Halo-JF549. For PAmCherry, trajectories are terminated mainly by photobleaching, whereas both reversible photoswitching and photobleaching occur for TMR and JF549. Track durations were significantly longer for the Halo dyes compared to PAmCherry (figure 5(c)). At optimal excitation intensity (0.2 kW cm−2 561 nm, figure 5(d)), the average track durations were 4.3, 8.7, and 11.5 frames for PAmCherry, TMR, and JF549, respectively. The distribution of track durations shows a long tail with 4.5% and 6.1% of TMR and JF549 molecules lasting for more than 100 frames, compared to 0.4% for PAmCherry. This enables direct observations of protein function within living cells over much longer time scales than previously possible with PA-FPs.
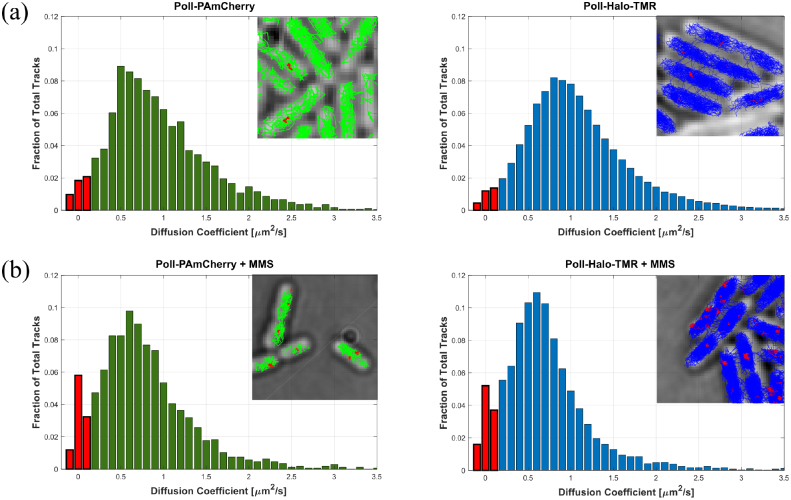
We used the PolI fusions to test if the Halo tag is also suitable for the measurement of transient molecular interactions. PolI is an essential factor for the repair of DNA base damage, filling DNA gaps that are generated by the base excision repair machinery [32]. The majority of PolI-Halo-TMR molecules were diffusing with D* ~ 0.7 µm2 s−1 in cells before DNA damage treatment (figure 6(a)). To monitor the DNA damage response, we first labelled PolI-Halo with TMR, and subsequently treated cells on agarose pads containing the DNA damaging agent methyl methanesulfonate (MMS). We observed immobilization of individual PolI molecules after MMS exposure for PAmCherry and Halo-TMR fusions (figure 6(b)), as previously shown [31].
Figure 6.
Tracking DNA repair activity of PolI. Cells with PolI-PAmCherry (left) or PolI-Halo-TMR (right) were imaged before (a) and after treatment with 100 mM MMS for 15 min. Immobile molecules with D* < 0.15 µm2 s−1 are shown in red. All data recorded at 15.48 ms frame−1.
Halo labelling enables high-speed and dual-colour tracking
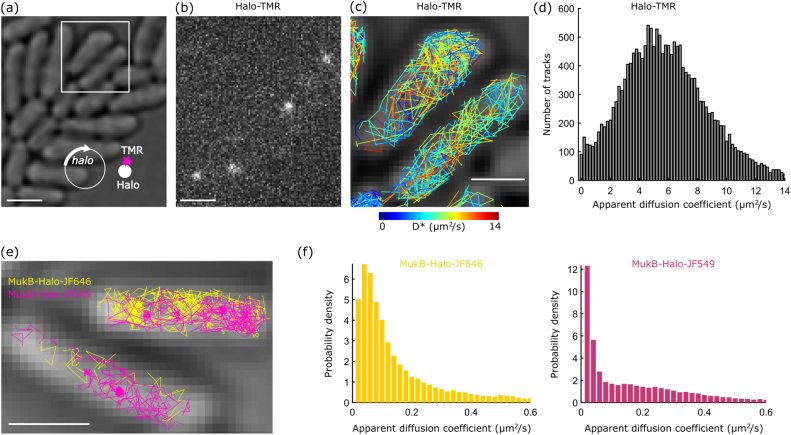
To track rapidly diffusing proteins, previous studies relied on stroboscopic illumination patterns in order to reduce blurring of the fluorescent spots due to movement of molecules during the exposure [45, 46]. The superior brightness of fluorescent Halo ligands may enable tracking fast molecular motion with simple continuous illumination. Besides the technical convenience, this also avoids the use of very high excitation intensities required for stroboscopic illumination pulses (i.e. hundreds of kW cm−2 [46]). To test the limits of high-speed single-molecule tracking, we measured unconjugated Halo molecules that were expressed from a plasmid and labelled with TMR (figure 7(a)). With continuous 561 nm illumination (0.4 kW cm−2) and a frame rate of 134 frames s−1 (7.48 ms frame−1), fluorescent spots were sufficiently bright for robust localization and tracking despite residual motion blurring (figures 7(b)–(d)). This was in stark contrast to unconjugated PAmCherry molecules, which were almost indistinguishable from the background noise at such short exposure times. Because any protein fused to Halo should have a lower mobility than the Halo tag alone, this result demonstrates the technical feasibility of tracking even the most rapidly diffusing fusion proteins with simple continuous illumination. Stable non-specific binding of unconjugated Halo molecules was reported in eukaryotic nuclei [47]. This complicates the interpretation of the tracking data and may perturb the function of fusion proteins. Here, we observed only a small fraction of apparently immobile Halo-TMR molecules in E. coli (1% of all tracks, figure 7(d)), which was likely due to residual fluorescent background particles (as quantified in figure 2).
Figure 7.
High-speed and dual-colour single-molecule tracking using Halo tag. (a) Cells expressing unconjugated Halo tag from a plasmid. Scale bar 2 µm. (b) Snapshot from a super-resolution movie showing Halo-TMR molecules at 7.48 ms exposure time. Scale bar 2 µm. (c) Halo-TMR tracks from the boxed area in panel a, shown in colours corresponding to the apparent diffusion coefficient. Scale bar 1 µm. (d) Histogram of apparent diffusion coefficients for unconjugated Halo-TMR. (e) Dual-colour tracking of MukB-Halo-JF646 (yellow) and MukB-Halo-JF549 (pink), recorded at 15.48 ms frame−1. Scale bar 1 µm. (f) Histogram of apparent diffusion coefficients from dual-colour tracking of MukB-Halo-JF646 and MukB-Halo-JF549.
To choose appropriate imaging conditions for high-speed tracking experiments, first the exposure time per frame should be sufficiently short such that motion blurring does not prevent single-molecule localization. This may require reducing the size of the field of view to increase the readout speed of the camera. On the other hand, molecular motion should yield significant displacements beyond the localization error between successive frames to accurately measure mobility. After determining the exposure time and frame rate, the excitation intensity should be adjusted to optimise localization precision and track duration as shown (figures 4 and 5). Especially for rapidly diffusing proteins, it is crucial to maintain a low density of fluorescent spots during data acquisition to avoid tracking errors.
The ability to use Halo tag with a range of different ligands is a further advantage of this labelling approach. It facilitates multi-colour imaging and enables the use of fluorophores in the far-red spectrum where cellular auto-fluorescence and phototoxicity are less pronounced. We tested dual-colour tracking using Halo labelling with JF549 and the red-shifted dye JF646 [25]. As a proof-of-principle, we first labelled MukB-Halo with JF646 according to the protocol in figure 1. Following 2 h of cell recovery, we labelled the newly synthesised MukB-Halo with JF549 using the same protocol. We imaged the two labels sequentially, recording 21 000 frames under 640 nm excitation (0.24 kW cm−2) followed by 21 000 frames under 561 nm excitation (0.2 kW cm−2). The dual-colour tracking data shows the expected diffusing and immobile MukB molecules for both labels (figures 7(e) and (f)). Under our imaging conditions, JF646 had a lower intensity (116 photons/frame) compared to JF549 (290 photons/frame). As a result, the mean apparent diffusion coefficient was marginally higher for MukB-JF646 (0.190 ± 0.005 µm2 s−1, SEM) compared to MukB-JF549 (0.182 ± 0.007 µm2 s−1, SEM) due to the increased localization error of JF646.
Choosing the right label for single-molecule tracking experiments
Applications that require maximum fluorophore brightness for fast tracking or high localization precision benefit from the use of protein tags with synthetic dyes. The high excitation intensities required for super-resolution microscopy and single-molecule tracking cause significant phototoxicity in cells [48, 49]. Especially near-UV light required for photoactivation of PA-FPs is problematic [48]. Ongoing development of synthetic fluorophores will further improve their photophysical characteristics, cell permeability, and availability. However, several important limitations need to be taken into account. The requirement for labelling and extensive washing of the fluorescent ligand may complicate applications that require precisely controlled growth conditions or cell perturbations. This may be overcome using certain fluorophores such as JF646 whose fluorescence increases upon conjugation to a protein tag, thereby reducing the need for removal of unconjugated dye [25]. Prolonged time-lapse imaging benefits from the continuous synthesis and replenishment of fluorescent proteins. Improved fluorescent proteins are also reported, with extended spectral range [50], beneficial photoactivation/conversion/switching properties, reduced maturation time [51]. We close with a table showing what we consider the most important practical considerations in favour and against PA-FPs and synthetic dyes for protein tags (table 1).
Table 1.
Practical considerations regarding labelling approaches for single-molecule localization and tracking experiments in live bacterial cells.
| Advantages | Disadvantages | |
|---|---|---|
| Photoactivatable fluorescent protein (e.g. PAmCherry) |
|
|
| Protein tag labelled with synthetic fluorophore (e.g. Halo-TMR) |
|
|
Acknowledgments
We thank Luke Lavis for providing the Halo ligand dyes JF549, PA-JF549, and JF646, and David J Sherratt for discussions. Imaging in the Micron Advanced Microscopy Facility was funded by Wellcome Trust Strategic Award 107457/Z/15/Z. This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant No. 206159/Z/17/Z) and a Wellcome-Beit Prize (206159/Z/17/B) to SU. SU holds a Hugh Price Fellowship at Jesus College Oxford.
Author contributions
NB and JM contributed equally to the work. SU initiated the study. NB, JM, SU performed experiments and analysed the data. NB, JM, SU prepared manuscript figures. SU wrote the manuscript with input from all authors.
References
- 1.Saxton M J, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997;26:373–99. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- 2.Gahlmann A, Moerner W E. Exploring bacterial cell biology with single-molecule tracking and super-resolution imaging. Nat. Rev. Microbiol. 2013;12:9–22. doi: 10.1038/nrmicro3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapanidis A N, Uphoff S, Stracy M. Understanding protein mobility in bacteria by tracking single molecules. J. Mol. Biol. 2018;430:4443–55. doi: 10.1016/j.jmb.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uphoff S, Sherratt D J. Single-molecule analysis of bacterial DNA repair and mutagenesis. Annu. Rev. Biophys. 2017;46:411–32. doi: 10.1146/annurev-biophys-070816-034106. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell. 2014;156:1274–85. doi: 10.1016/j.cell.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Schroeder J W, Simmons L A, Biteen J S. Visualizing bacterial DNA replication and repair with molecular resolution. Curr. Opin. Microbiol. 2018;43:38–45. doi: 10.1016/j.mib.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight S C, et al. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–6. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 8.Huang B, Bates M, Zhuang X. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 2009;78:993–1016. doi: 10.1146/annurev.biochem.77.061906.092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Linde S, Heilemann M, Sauer M. Live-cell super-resolution imaging with synthetic fluorophores. Annu. Rev. Phys. Chem. 2012;63:519–40. doi: 10.1146/annurev-physchem-032811-112012. [DOI] [PubMed] [Google Scholar]
- 10.Lippincott-Schwartz J, Patterson G H. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol. 2009;19:555–65. doi: 10.1016/j.tcb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Moffitt J R, Dempsey G T, Xie X S, Zhuang X. Characterization and development of photoactivatable fluorescent proteins for single-molecule–based superresolution imaging. Proc. Natl Acad. Sci. 2014;111:8452–7. doi: 10.1073/pnas.1406593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subach F V, Patterson G H, Manley S, Gillette J M, Lippincott-Schwartz J, Verkhusha V V. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat. Methods. 2009;6:153–9. doi: 10.1038/nmeth.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilemann M, Dedecker P, Hofkens J, Sauer M. Photoswitches: key molecules for subdiffraction-resolution fluorescence imaging and molecular quantification. Laser Photonics Rev. 2009;3:180–202. doi: 10.1002/lpor.200810043. [DOI] [Google Scholar]
- 14.Vogelsang J, Steinhauer C, Forthmann C, Stein I H, Person-Skegro B, Cordes T, Tinnefeld P. Make them blink: probes for super-resolution microscopy. ChemPhysChem. 2010;11:2475–90. doi: 10.1002/cphc.201000189. [DOI] [PubMed] [Google Scholar]
- 15.Crivat G, Taraska J W. Imaging proteins inside cells with fluorescent tags. Trends Biotechnol. 2012;30:8–16. doi: 10.1016/j.tibtech.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Los G V, et al. Halo tag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008;3:373–82. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 17.Juillerat A, Gronemeyer T, Keppler A, Gendreizig S, Pick H, Vogel H, Johnsson K. Directed evolution of O6-alkylguanine-DNA alkyltransferase for efficient labeling of fusion proteins with small molecules in vivo. Chem. Biol. 2003;10:313–7. doi: 10.1016/S1074-5521(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 18.Gautier A, Juillerat A, Heinis C, Corrêa I R, Jr, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 2008;15:128–36. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Hein B, Willig K I, Wurm C A, Westphal V, Jakobs S, Hell S W. Stimulated emission depletion nanoscopy of living cells using SNAP-tag fusion proteins. Biophys. J. 2010;98:158–63. doi: 10.1016/j.bpj.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlag B, et al. Single molecule super-resolution imaging of proteins in living Salmonella enterica using self-labelling enzymes. Sci. Rep. 2016;6:31601. doi: 10.1038/srep31601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke N, Landgraf D, Paulsson J, Berkmen M. Visualization of periplasmic and cytoplasmic proteins with a self-labeling protein tag. J. Bacteriol. 2016;198:1035–43. doi: 10.1128/JB.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talyor H, Lepore A, Landgraf D, Okumus B, Jaramillo-Riveri S, McLaren L, Paulsson J, Karoui M E. Quantification of very low-abundant proteins in bacteria using the HaloTag and epi-fluorescence microscopy. 2017:237248. doi: 10.1101/237248. (bioRxiv) ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes J D, Mazza D, Nasmyth K A, Uphoff S. Scc2/Nipbl hops between chromosomal cohesin rings after loading. eLife. 2017;6:e30000. doi: 10.7554/eLife.30000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris S R, Núñez M F, Verhey K J. Influence of fluorescent tag on the motility properties of kinesin-1 in single-molecule assays. Biophys. J. 2015;108:1133–43. doi: 10.1016/j.bpj.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm J B, et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods. 2015;12:244–50. doi: 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm J B, et al. Bright photoactivatable fluorophores for single-molecule imaging. Nat. Methods. 2016;13:985–8. doi: 10.1038/nmeth.4034. [DOI] [PubMed] [Google Scholar]
- 27.Lukinavičius G, et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013;5:132–9. doi: 10.1038/nchem.1546. [DOI] [PubMed] [Google Scholar]
- 28.Rybenkov V V, Herrera V, Petrushenko Z M, Zhao H. MukBEF, a chromosomal organizer. J. Mol. Microbiol. Biotechnol. 2014;24:371–83. doi: 10.1159/000369099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badrinarayanan A, Reyes-Lamothe R, Uphoff S, Leake M C, Sherratt D J. In vivo architecture and action of bacterial structural maintenance of chromosome proteins. Science. 2012;338:528–31. doi: 10.1126/science.1227126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes-Lamothe R, Nicolas E, Sherratt D J. Chromosome replication and segregation in bacteria. Annu. Rev. Genet. 2012;46:121–43. doi: 10.1146/annurev-genet-110711-155421. [DOI] [PubMed] [Google Scholar]
- 31.Uphoff S, Reyes-Lamothe R, de Leon F G, Sherratt D J, Kapanidis A N. Single-molecule DNA repair in live bacteria. Proc. Natl Acad. Sci. USA. 2013;110:8063–8. doi: 10.1073/pnas.1301804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedberg E C. DNA Repair and Mutagenesis. 2nd edn. Washington, DC: American Society for Microbiology; 2006. [Google Scholar]
- 33.Datsenko K A, Wanner B L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wegel E, Göhler A, Lagerholm B C, Wainman A, Uphoff S, Kaufmann R, Dobbie I M. Imaging cellular structures in super-resolution with SIM, STED and localisation microscopy: a practical comparison. Sci. Rep. 2016;6:27290. doi: 10.1038/srep27290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokunaga M, Imamoto N, Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods. 2008;5:159–61. doi: 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- 36.Uphoff S, Sherratt D J, Kapanidis A N. Visualizing protein-DNA interactions in live bacterial cells using photoactivated single-molecule tracking. J. Vis. Exp. 2014;85:e51177. doi: 10.3791/51177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Linde S, Krstić I, Prisner T, Doose S, Heilemann M, Sauer M. Photoinduced formation of reversible dye radicals and their impact on super-resolution imaging. Photochem. Photobiol. Sci. 2011;10:499–506. doi: 10.1039/C0PP00317D. [DOI] [PubMed] [Google Scholar]
- 38.Annibale P, Vanni S, Scarselli M, Rothlisberger U, Radenovic A. Quantitative photo activated localization microscopy: unraveling the effects of photoblinking. PLoS One. 2011;6:e22678. doi: 10.1371/journal.pone.0022678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S-H, Shin J Y, Lee A, Bustamante C. Counting single photoactivatable fluorescent molecules by photoactivated localization microscopy (PALM) Proc. Natl Acad. Sci. 2012;109:17436–41. doi: 10.1073/pnas.1215175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coltharp C, Kessler R P, Xiao J. Accurate construction of photoactivated localization microscopy (PALM) images for quantitative measurements. PLoS One. 2012;7:e51725. doi: 10.1371/journal.pone.0051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta P, Jovanovic-Talisman T, Lippincott-Schwartz J. Quantifying spatial organization in point-localization superresolution images using pair correlation analysis. Nat. Protocols. 2013;8:345–54. doi: 10.1038/nprot.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fricke F, Beaudouin J, Eils R, Heilemann M. One, two or three? Probing the stoichiometry of membrane proteins by single-molecule localization microscopy. Sci. Rep. 2015;5:14072. doi: 10.1038/srep14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manley S, Gillette J M, Patterson G H, Shroff H, Hess H F, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods. 2008;5:155–7. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 44.Dersch S, Graumann P L. The ultimate picture-the combination of live cell superresolution microscopy and single molecule tracking yields highest spatio-temporal resolution. Curr. Opin. Microbiol. 2018;43:55–61. doi: 10.1016/j.mib.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Elf J, Li G-W, Xie X S. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007;316:1191–4. doi: 10.1126/science.1141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.English B P, Hauryliuk V, Sanamrad A, Tankov S, Dekker N H, Elf J. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc. Natl Acad. Sci. 2011;108:E365–73. doi: 10.1073/pnas.1102255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebhardt J C M, Suter D M, Roy R, Zhao Z W, Chapman A R, Basu S, Maniatis T, Xie X S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods. 2013;10:421–6. doi: 10.1038/nmeth.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wäldchen S, Lehmann J, Klein T, van de Linde S, Sauer M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015;5:15348. doi: 10.1038/srep15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Icha J, Weber M, Waters J C, Norden C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays. 2017;39:1700003. doi: 10.1002/bies.201700003. [DOI] [PubMed] [Google Scholar]
- 50.Shcherbakova D M, Baloban M, Emelyanov A V, Brenowitz M, Guo P, Verkhusha V V. Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging. Nat. Commun. 2016;7:12405. doi: 10.1038/ncomms12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balleza E, Kim J M, Cluzel P. Systematic characterization of maturation time of fluorescent proteins in living cells. Nat. Methods. 2018;15:47–51. doi: 10.1038/nmeth.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]