Abstract
Impulsive personality traits refer to a group of self-reported dispositions about self-regulatory capacity, several of which have been linked to diverse forms of psychopathology. One of these is negative urgency (NUR), the propensity to act out when experiencing negative emotions, which has been linked to substance use disorders and eating disorders. However, few laboratory studies have investigated the extent to which self-reported NUR relates to an individual’s in vivo emotional and behavioral responses. Harmonizing two archival datasets on alcohol and high energy dense (HED) food motivation, the current study investigated NUR as a moderator of reactivity to stressful situations elicited by two commonly-used stress manipulations, the Trier Social Stress Test and a stress imagery induction. To do so, 148 adults were assessed for NUR, severity of alcohol misuse or binge eating, and measures of negative affect and psychophysiological arousal (i.e., heart rate and blood pressure) prior to and following one of the two manipulations. In addition, a behavioral multiple-choice procedure assessing the relative reinforcing value of alcohol or HED foods followed the manipulations. As predicted, NUR positively moderated the effects of stress induction on self-reported negative affect and relative reinforcing value, although not arousal. Individuals exhibiting elevated NUR also exhibited greater alcohol misuse, although not greater binge eating severity. These findings provide in vivo validation of the construct of NUR and its measurement using the UPPS-P. More broadly, these findings inform the understanding of deficits that are characteristic of self-regulatory disorders.
Keywords: Negative Urgency, Impulsivity, Stress, Relative Reinforcing Value
While “impulsivity” has consistently been linked with a variety of externalizing behaviors and clinical disorders, it is increasingly recognized to be a complex and multi-dimensional construct (De Wit, 2009; Dick et al., 2010; Whiteside & Lynam, 2001). According to a data-driven model of personality originally proposed by Whiteside and Lynam (Whiteside & Lynam, 2001) and then expanded by Cyders and Smith (Cyders & Smith, 2007, 2008b), one important facet of impulsivity in understanding its role in maladaptive behavior is the propensity to engage in rash action when experiencing intense negative emotion, which has been termed negative urgency (NUR). A recent meta-analysis found NUR to be the personality index of impulsivity most strongly linked with alcohol dependence and problematic alcohol consumption (Coskunpinar, Dir, & Cyders, 2013) and it has been associated with smoking, drug use, gambling, binge eating, risky sex, and conduct disorder (Cyders & Smith, 2008a; Doran et al., 2013; Kaiser, Bonsu, Charnigo, Milich, & Lynam, 2016; Pearson, Combs, Zapolski, & Smith, 2012; Settles et al., 2012; Settles, Zapolski, & Smith, 2014; Zapolski, Cyders, & Smith, 2009).
Despite its repeated association with problematic real-world behaviors, little laboratory research has evaluated the extent to which levels of NUR modulate changes to psychological state caused by unpleasant experiences. Since the UPPS-P asks individuals to report on their general behaviors retrospectively, it is possible that it is susceptible to biases in memory or responding. Therefore, empirical evidence is needed to affirm that trait NUR is related to negative emotional response and rash decisions in specific situations that provoke negative affect. The only study to address this question in relation to alcohol use found that NUR was associated with reductions in mood and greater alcohol seeking behavior following a sad mood induction (VanderVeen et al., 2016). Two studies have been conducted addressing this question in HED food consumption, one which used a frustration based negative mood induction to demonstrate a moderating effect of NUR on consumption of a variety of HED foods (Emery, King, & Levine, 2014) and another which used an anger inducing manipulation based on autobiographical recall to demonstrate NUR had a moderating effect on HED food consumption (Becker, Fischer, Smith, & Miller, 2016). However, these results may not extend to other negative emotions, such as stress, and different antecedents that elicit them. Stress is typically defined as a subjectively negative emotional response to challenging life events that is characterized by increased autonomic arousal. Individuals experiencing high levels of stress are more prone to developing substance use disorders (Koob, Kreek, Ph, & Kreek, 2007; Kreek & Koob, 1998) and stressful events following cessation are often triggers of relapse to substance use in individuals in recovery (Sinha, 2013). High levels of stress are also associated with binge eating episodes and loss of control of eating behavior in individuals with binge eating disorder (Freeman & Gil, 2004; Telch & Agras, 1996). This makes stress particularly pertinent to NUR, which is strongly associated with substance use disorders (Coskunpinar et al., 2013) and dysregulated eating (Fischer, Peterson, & McCarthy, 2012; Racine et al., 2013; Racine & Martin, 2016).
In a harmonized dataset from two archival studies, the current investigation sought to determine whether high NUR is associated with greater emotional and behavioral responsiveness to stressful situations. The study’s overarching hypothesis was that NUR would be a positive moderator of stress reactivity. This was tested by having participants undergo one of two stress inductions, the Trier Social Stress Test (Kirschbaum, Pirke, & Hellhammer, 1993; Kudielka, Wüst, Kirschbaum, & Hellhammer, 2007) or the Guided Stress Imagery Procedure (Sinha, 2013). These manipulations were used in the archival studies, one on motivation for alcohol and the other on motivation for high-energy density (HED) food, respectively. The datasets were harmonized on theoretical and statistical grounds. There is an increasing recognition that overconsumption of psychoactive drugs and food share important features (Davis et al., 2011; Ifland et al., 2009; Volkow & Wise, 2005). Indeed, alcohol and HED foods can be highly reinforcing and when an individual’s valuation of those reinforcers becomes problematic there is a similar series of biological and psychological changes that take place, including alterations of the dopaminergic system and development of classically conditioned response to cues relevant to that reinforcer (Benton & Young, 2016; Carr, Daniel, Lin, & Epstein, 2011; Gearhardt et al., 2011; Johnson & Kenny, 2010; Smith & Robbins, 2013; Volkow, Wang, & Baler, 2011). Additionally, trait impulsiveness is associated with both alcohol use disorder (Courtney et al., 2012; Kaiser et al., 2016) and dysregulated eating behavior (Murphy, Stojek, & MacKillop, 2014; Raymond & Lovell, 2015; Sutin et al., 2013; VanderBroek-Stice, Stojek, Beach, vanDellen, & MacKillop, 2017), suggesting that the construct of NUR may help explain pathological consumption of alcohol and food in response to stressful situations. The last theoretical basis for aggregating these two studies was that it would permit evaluation of the generality of NUR as a moderator of stress reactivity, as absence of study-specific effects would obliquely support the consistency of the relationship.
The specific aim of the current study was to test the hypothesis that high levels of NUR would be associated with greater negative emotional response following a stressful situation (i.e., positive moderation). Whether or not NUR is associated with greater emotional reactivity is a critical question and stress has not been addressed in previous laboratory studies. The current study also tested the hypothesis that NUR would moderate stress effects on consumable (alcohol or HED food) reinforcing value via a multiple-choice procedure (Griffiths, Roland, Rush, Craig, & Puhala, 1996; Griffiths, Troisi, Silverman, & Mumford, 1993). This paradigm allows participants to forgo a monetary reward to obtain in vivo access to a preferred reinforcer, in this case, alcohol or HED food, with the amount of money representing their motivation for alcohol or HED food in monetary terms. Support for these hypotheses would further validate the construct of NUR and its measurement using the UPPS-P. Additionally, the relationship between NUR and alcohol and binge eating severity was examined to contextualize the findings in relation to psychopathology and speak to the extent NUR is pertinent to overconsumption of alcohol or HED foods. In doing so, the study sought to inform the understanding of the self-regulatory deficits that are characteristic of alcohol misuse or binge eating.
Methods
Participants
The current study was executed in a harmonized dataset from two previous laboratory studies, one on motivation for alcohol that recruited heavy drinkers (Amlung & MacKillop, 2014) and the other on motivation for food that recruited individuals who reported liking HED foods (Stojek, Fischer, & MacKillop, 2015). The purpose of the original study recruiting heavy drinkers was to explore the effects of a stress manipulation on several behavioral economic measures used frequently to study substance use disorders (delay discounting, an alcohol purchase task, the multiple choice procedure described below) (Amlung & MacKillop, 2014). The purpose of the original study recruiting HED food-liking individuals was to investigate whether the same behavioral economic paradigms typically used to investigate substance use disorders could be applied to food motivation in a similar way (Stojek et al., 2015). The two studies were executed in the same laboratory and recruited participants from the same catchment area (Athens, GA). Inclusion/exclusion criteria were also similar between the studies, with the exception of one study enrolling healthy individuals who were heavy drinkers (i.e., >14/7 drinks/week for men/women, above U.S. federal guidelines (National Institute on Alcohol Abuse and Alcoholism, 2010) and the other enrolling healthy individuals who reported enjoying HED food. Details of each study’s eligibility criteria are reported in manuscripts reporting primary findings from the respective studies (Amlung & MacKillop, 2014; Stojek et al., 2015). The current study is an analysis of a harmonized dataset comprising 148 participants who underwent a baseline assessment immediately followed by one of the two stress inductions with n = 83 receiving the TSST (Amlung & MacKillop, 2014) and n = 65 receiving personalized imagery (Stojek et al., 2015). Participant characteristics overall and by study are in Table 1. There were significant differences between the two studies in participant age and level of education. However, these variables were not significantly associated with NUR (r = −.03, p = .68 for age, r = −.11, p = .20 for years of education). They were also not associated with relative reinforcing value or negative affect before or after the stress induction, nor were they associated with the difference in either from pre- to –post induction (all p-values < .05). No significant differences were present for income, sex, race, Hispanic ethnicity, or NUR. Both studies were approved by the University of Georgia Institutional Review Board (protocols 2012-10408 and 2012-10794).
Table 1.
Participant demographics, overall and by manipulation.
| Variable | Overall N = 148 |
Heavy Alcohol Use Study Mean (SD)/Count n = 83 |
HED Food Liking Study Mean (SD)/Count n = 65 |
t/χ2 | p |
|---|---|---|---|---|---|
| Age | 21.52 (2.30) | 22.25 (2.25) | 20.58 (2.02) | 4.68 | <.001 |
| Years Education | 14.76 (1.54) | 15.26 (1.24) | 14.12 (1.67) | 4.80 | <.001 |
| Negative Urgency | 2.16 (0.49) | 2.12 (0.54) | 2.19 (0.41) | 0.89 | 38 |
| Sex | 0.00 | 98 | |||
| Male | 49.3% | 49.4% | 49.2% | ||
| Female | 50.7% | 50.6% | 50.8 | ||
| Income | 2.00 | 0.58 | |||
| $0-$30,000 | 25% | 27.7% | 21.5% | ||
| $30,001-$60,000 | 18.2% | 20.5% | 15.4% | ||
| $60,001-$90,000 | 18.9% | 18.1% | 20.0% | ||
| $90,000-$120,000 | 37.8% | 33.7% | 43.1% | ||
| Race | 1.80 | 0.77 | |||
| Caucasian | 62.2% | 63.9% | 60.0% | ||
| African American | 23.6% | 20.5% | 27.7% | ||
| American Indian | 7% | 1.2% | 0.0% | ||
| Asian | 10.1% | 10.8% | 9.2% | ||
| Mixed | 3.4% | 3.6% | 3.1% | ||
| Hispanic | .01 | 0.92 | |||
| Yes | 7.4% | 7.2% | 7.7% | ||
| No | 92.6% | 92.8% | 92.3% |
Note: Participants in the study for heavy drinkers received Trier Social Stress Test, while participants in the study of high energy dense (HED) foods received the guided imagery paradigm.
Assessments
UPPS-P Impulsive Behavior Scale (UPPS-P)
The UPPS-P (Lynam, Smith, Cyders, Fischer, & Whiteside, 2007) was administered at baseline, prior to the stress induction procedures. The UPPS-P assesses five different facets of impulsivity: sensation seeking, lack of premeditation, lack of planning, positive urgency, and negative urgency (NUR). NUR was the focus of the current study. Cronabach’s alpha for NUR was .79. A tertile split was conducted on NUR to create 3 groups: Low NUR (≤ 1.92, n = 51), Mid NUR (> 1.92 and < 2.42, n = 45), High NUR (≥ 2.42, n = 52). The mean score overall for NUR was 2.16 (SD = .49, Range = 1.00 – 3.25) and NUR was normally distributed (skewness = −.02, kurtosis = −.42). NUR group membership was not significantly different between the two studies (F = .08, p = .77, = .001).
Multiple Choice Procedure (MCP)
An analogous MCP was employed by both studies that assessed participants’ choice between a series of escalating monetary rewards and immediate access to alcohol or HED foods (Benson, Little, Henslee, & Correia, 2009; Griffiths et al., 1996, 1993). In the study recruiting heavy drinkers (Amlung & MacKillop, 2014) participants chose between monetary rewards escalating from $0 to $15 and one drink of their preferred alcoholic beverage to be consumed immediately on site. In the study recruiting participants who liked HED foods (Stojek et al., 2015), participants chose between monetary rewards escalating from $0 to $15 and access to a HED food buffet for 30 minutes. For each participant, an index of the relative reinforcing value of food or alcohol (RRV) was calculated as the mean of the last prices at which participants selected alcohol or HED food and the first price at which they selected money, which has been termed the crossover point.
Visual Analog Scales (VAS)
A VAS used to measure self-reported negative affect before and after the stress induction (Folstein & Luria, 1973). This measure used an 11-point scale in which 0 indicated “Not at all” and 10 indicated “Extremely”. Participants used this scale to indicate the extent to which they felt each of three negative mood states: nervous, stressed, and sad. These three scores were averaged to create a negative affect composite score for each participant. The Cronbach’s α for this composite was .79 pre-induction and .75 post-induction.
Psychophysiological Arousal
To assess the effect of each induction on autonomic arousal, blood pressure and heart rate (HR) were collected before and after the stress inductions. This was measured using an electronic wrist blood pressure cuff (Welch Allyn, Inc.; Skaneateles Falls, NY). Blood pressure was converted to mean arterial pressure (MAP), a metric that incorporates systolic and diastolic pressure to create an index of the average pressure in arteries during one cardiac cycle. Heart rate was measured in beats per minute over the course of the blood pressure reading (approximately 30 to 60 seconds). Both were recorded at baseline following 10 minutes of sitting in a neutral room listening to soothing music. They were recorded again immediately following the stress induction procedure.
Alcohol Use Disorder Identification Test (AUDIT)
The AUDIT (Reinert & Allen, 2007) was collected at baseline in the sample reporting heavy alcohol use and is a self-report measure of alcohol misuse. A score of 8 has been validated as a clinically significant cut-off for hazardous alcohol use (Conigrave, Hall, & Saunders, 1995). The average score of the heavy drinking sample was 10.37 (SD = 4.61) with 28% meeting AUDIT criteria for hazardous alcohol use.
Binge Eating Scale (BES)
The BES (Gormally, Black, Daston, & Rardin, 1982) was collected at baseline in the HED food sample and is a self-report measure of binge eating severity. A score of 17 has been validated as a clinically significant cut-off for binge eating (Gormally et al., 1982). The average score on the BES of the HED food liking sample was 8.34 (SD = 5.95) with 11% meeting the criteria for clinically significant binge eating.
Procedures
Trier Social Stress Test
The stress inductions administered to the heavy alcohol using participants was the Trier Social Stress Test as described by Kirschbaum and colleagues (1993). In order to standardize baseline levels of mood and arousal, participants first completed a relaxation period before completing baseline assessments. Then participants were told they would complete a mock job interview for their “dream job” and were given 5 minutes to prepare a brief speech on their qualifications. They were then escorted to a room with three seated confederates wearing lab coats at the far end of a conference table and were asked to speak for five minutes on their qualifications while receiving no verbal or non-verbal feedback. If participants stopped before 5 minutes had elapsed, they were prompted to continue using standard interview-type questions. They were then asked to complete a serial subtraction task in which they were instructed to count aloud from 1,022 to 0 in units of 13 for five minutes. After incorrect responses participants were required to start from the beginning until the 5 minutes had elapsed. Both interviewing for a job and completing challenging math problems while being evaluated are considered to be highly stressful and prior literature shows this procedure is effective in eliciting a stress response (Birkett, 2011; Hellhammer & Schubert, 2012; Kirschbaum et al., 1993).
Guided Stress Imagery Procedure
The stress induction administered to the HED food liking participants was a guided stress imagery procedure following a manualized protocol for guided imagery (Sinha, 2013). In this procedure, participants were asked to identify stressful experience that had occurred within the past year and rate their distress on a 10-point Likert scale (1 = not at all stressful, 10 = most stressful). In order to ensure all situations produced significant stress, only situations rated eight and above were used. Subsequently, participants described the event in detail to the experimenter so that a detailed script could be written about this description. A research assistant who had not interacted with the participant then audio recorded the script resulting in clips approximately five minutes in length. Before the experiment began, participants underwent imagery training to ensure they imagined the scene they had described vividly and to reduce variability in mood among participants. Then participants completed a relaxation period to standardize mood and arousal before completing baseline measures. During the stress induction itself, participants listened to the audio tape of the script describing their stressful event and were instructed to imagine the event using skills from the imagery training they had previously completed. Prior literature suggests that participants experience a stress response while completing the imagery procedure (Sinha, 2009; Sinha, Catapano, & O’Malley, 1999; Sinha, Fuse, Aubin, & O’Malley, 2000; Sinha & O’Malley, 1999).
Data Analysis
To investigate differences in NUR as a moderator of response to stress induction, a series of four 2 (time: Pre/Post) × 3 (NUR: High/Mid/Low) ANCOVAs were conducted on RRV, the negative affect composite, and the two psychophysiological measures collected (MAP and HR). Main effects of time (before and after induction) on RRV, mood, and psychophysiological response were inspected to ensure the efficacy of the stress induction in producing a stress response. However, the primary focus was the time × NUR interaction as a test of moderation. A categorical approach to NUR was employed for clear interpretation of the interaction and for compatibility with previous laboratory studies. To ensure results were not due to differences between the two studies being collapsed, follow-up 2 (Time: Pre/Post) × 3 (NUR: High/Mid/Low) × 2 (Study: Heavy Drinkers/HED Food Liking) ANCOVAs were completed to test for interactions with Study. Subsequently, to put the NUR moderator in the context of psychopathology, severity of alcohol misuse or binge eating were tested for relationship with NUR in each study sample separately. To do this, one-way between subject ANCOVAs were completed testing for differences in alcohol use disorder severity based on the AUDIT (in the heavy drinking study) or binge eating severity based on the BES (in the HED food liking study) between the three levels of NUR. Age and education were used as covariates in these analyses. Additionally, differences in between the two studies were examined regarding the level of psychopathology present by comparing the percentage of participants with clinically significant levels of hazardous alcohol use and binge eating symptoms based on established cut-off values for the AUDIT and BES using a χ2 test.
Results
Negative Urgency as Moderator of Stress Reactivity
In mixed ANCOVA models, an interaction reflecting evidence of moderation was found between time and NUR group for negative affect (F = 3.38, p = .03, = .05), but not for HR (F = 2.46, p = .07, = .04) or MAP (F = .15, p = .86, = .002) (Figure 1A). Additionally, an interaction reflecting evidence of moderation was found between time and NUR group for RRV (F = 3.61, p = .02, = .05) (Figure 1B). Three-way interactions with Study were not present for negative affect (F = .49, p = .62, = .01) or RRV (F = 1.33, p = .27, = .02), suggesting that moderation of stress reactivity by NUR did not differ between the two studies.1
Figure 1.
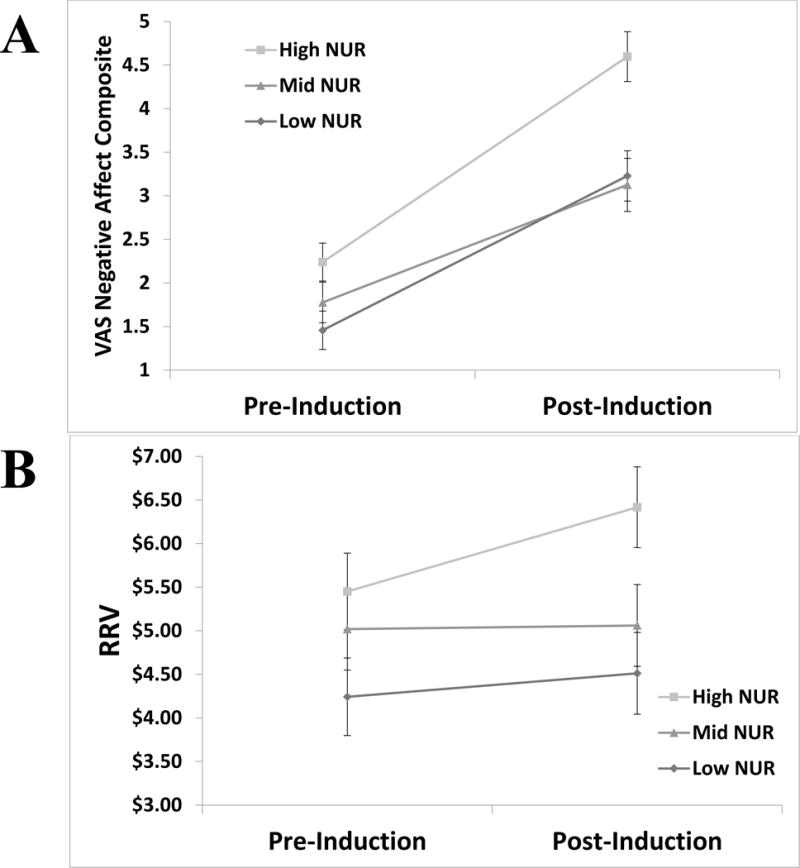
Visualization of significant interactions on pre- to post- induction measures with level of negative urgency levels. All interactions are significant at p < .05. Panel A depicts changes in the relative reinforcing value of alcohol or high energy dense food indicated across high, mid, and low negative urgency groups. Panel B depicts changes in negative affect across high, mid, and low negative urgency groups. Note: NUR= Negative Urgency; VAS = Visual Analog Scale; RRV = relative reinforcing value.
When analyses were conducted separately for each study sample, for the heavy drinking study, an interaction reflecting evidence of moderation was found between time and NUR group for RRV (F = 3.44, p = .04, = .08), but not for negative affect (F = 1.40, p = .25, = .04), HR (F = 1.19, p = .31, = .03), or MAP (F = .29, p = .75, = .01). For the HED food-liking study, an interaction reflecting evidence of moderation was found between time and NUR group for negative affect (F = 3.07, p = .05, = .09), but not for RRV (F = .57, p = .57, = .02), HR (F = 1.65, p = .20, = .05), or MAP (F = .02, p = .98, = .001).
Relationship to Psychopathology
In the heavy drinking study, a univariate ANCOVA of the NUR moderator variable indicated significant differences in AUDIT score between NUR groups (F = 6.85, p = .002, = .15), with post-hoc pairwise comparisons showing significant differences between low NUR and High NUR and between mid NUR and high NUR (Figure 2). In the HED food-liking study, a univariate ANCOVA of the NUR moderator variable did not indicate significant differences between NUR groups (F = 1.24, p = .30, = .04). Notably, there was a significant difference between the two study samples in percent of participants meeting the criteria of clinical severity indicated on the AUDIT/BES (χ2 = 55.68, p < .001), suggesting that, although both cohorts had meaningful variability, greater psychopathology was present in the heavy drinking sample.
Figure 2.
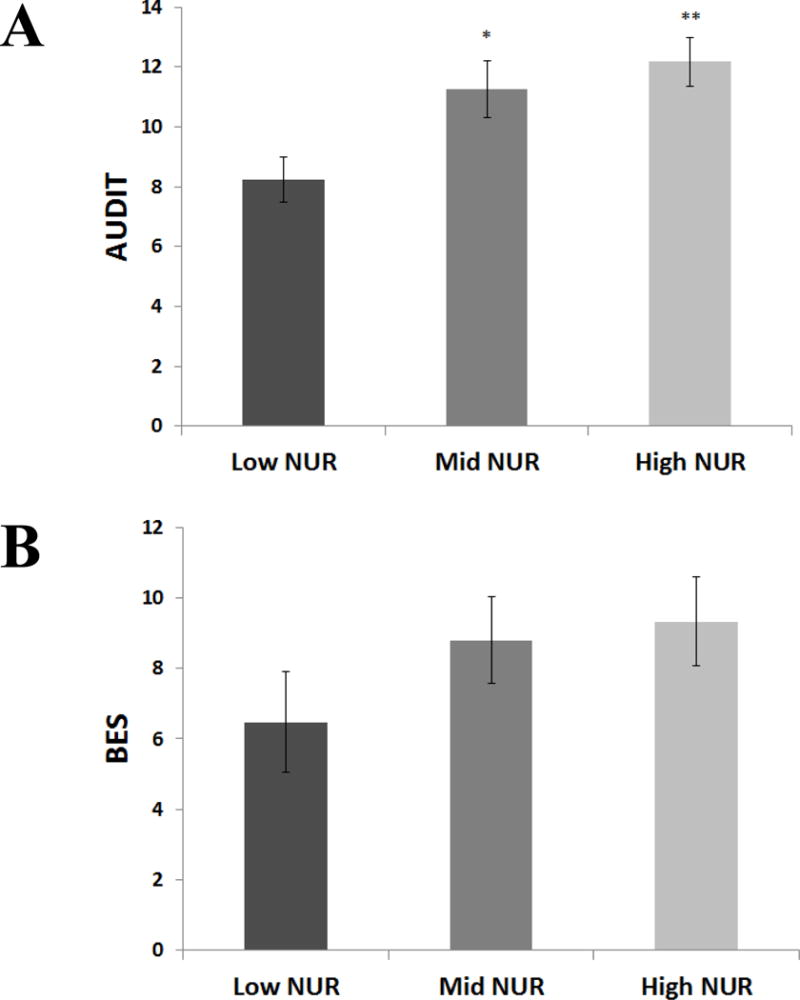
Relationship between negative urgency and alcohol/binge eating severity. Panel A depicts differences in Alcohol Use Disorder Identification Test (AUDIT) score according to the trichotomous negative urgency moderator variable in the heavy drinking study; asterisks reflect follow-up contrasts with Low. Panel B depicts differences in Binge Eating Scale Score (BES) according to the trichotomous negative urgency moderator variable in the HED food liking study; asterisks reflect follow-up contrasts with Low. Note: *p< .05, **p<.005.
Discussion
Results of the current study suggest that NUR moderates the effects of stress induction on both negative affect and the reinforcing value of alcohol, both with medium effect sizes. Across all participants, those high in NUR showed a differentially greater increase in negative affect and RRV in response to one of two stress inductions. When these analyses were conducted on each study sample separately, in the heavy drinking study, those high in NUR showed a differentially greater increase in RRV of alcohol in response stress induction whereas, in the HED food liking study, those high in NUR showed greater increases in self-reported negative affect. The finding of the moderation of negative emotional response by NUR is consistent with our hypothesis that those high in NUR would show greater emotional responding to stress manipulations. This finding suggests that greater emotional reactivity to stressful events may be part of the reason why individuals high in NUR act rashly in these situations. That NUR moderated the effects of stress induction on RRV with a medium effect size confirms our hypothesis that high NUR individuals do indeed behave rashly when experiencing negative emotionality, in this case by showing increased willingness to defer monetary rewards for the opportunity to immediately engage in a behavior with the potential to alleviate negative affect. This is entirely consistent with theoretical accounts of NUR, which state that rash behavior in response to negative affect is the core construct being measured by the NUR subscale of the UPPS-P (Lynam, Smith, Whiteside, & Cyders, 2006; Lynam et al., 2007).
The results of the current study are consistent with the existing literature on the laboratory validity of the construct of NUR, with previous studies having focused on sadness, frustration, and anger (Becker et al., 2016; Emery et al., 2014; VanderVeen et al., 2016). All of those studies found that those high in NUR demonstrated greater willingness to engage in behaviors which would putatively reduce their emotional distress. However, the current study is the first to show that higher NUR is associated with increased reactivity to stressful situations. In addition, this was the first study to test the relationship of NUR to physiological stress response, showing that NUR did not moderate the effects of a stressful experience on HR or blood pressure. It is not particularly surprising that NUR is more related to self-reported negative emotion and the behavioral response than to other elements of the stress response, such as physiological responses. While stressors have been shown to increase heart rate and blood pressure (Birkett, 2011; Hjortskov et al., 2004; Shapiro, Jamner, Goldstein, & Delfino, 2001; Vrijkotte, van Doornen, & de Geus, 2000), NUR has been conceptualized as pertaining primarily to the subjective experience of distress (Cyders & Smith, 2007, 2008b) and consequently there has been little effort to investigate its relationship to psychophysiological responsiveness. The current results provide some preliminary supporting evidence of this conceptualization. Taken together, these findings support the existing conceptualization of NUR and suggest that individuals high in NUR do, in fact, experience greater negative affect and augmentation of the value of consumables in the face of stress. Interestingly, however, the current study suggests that these effects are specific to these domains and do not extend to psychophysiological arousal.
The current study’s results may also provide helpful information in understanding overconsumption of alcohol and food more broadly. The study found that greater NUR was associated with higher levels of alcohol misuse, highlighting the relevance of NUR to alcohol use disorder. This is consistent with prior findings that high levels of NUR are found in those with clinically significant levels of alcohol use (Coskunpinar et al., 2013; Lynam et al., 2007; Settles et al., 2012). However, statistically significant differences were not present for binge eating severity, likely due to the smaller number of participants (and thus lower power) and lower general severity in terms of binge eating symptomology. Previously, binge eating has been associated with higher levels of NUR in several prior studies (Fischer et al., 2012; Racine et al., 2013; Racine & Martin, 2016). Thus, the current findings robustly implicate NUR in problematic alcohol use, but are more equivocal for problematic food consumption. Further investigation of the link between NUR and binge eating is warranted to more definitively ascertain the relationship.
A strength of the current study is its comparatively large sample for a laboratory investigation, which was achieved by the combining of two studies. However, the current analysis is limited in its ability to parse the differences found between the two study samples when analyzed separately. These differences may indicate a true contrast in stress response between the two studies or may be the result of limited power when the two studies are considered individually. Given that higher levels of psychopathology were present in the heavy drinkers, the difference between results might indicate that, in those with higher levels of psychopathology, NUR is more strongly associated with impulsive behavior, while in samples with less psychopathology it is associated with more negative affect than impulsive behavior. Alternately, it might indicate that snack food was a less effective means of managing negative affect in that study sample, whereas the heavy drinking sample showed less negative affect because of the effectiveness of alcohol as means of managing negative affect. However, it is equally plausible that these differences represent statistical noise that would dissipate if each study’s sample were larger. In addition, a further consideration is that the purely continuous analysis of NUR revealed robust main effects and only directionally consistent moderation. This suggests that the moderating relationship is not purely continuous and is compatible with the primary analyses finding the high NUR group showed marked increases in negative affect and RRV, while the low and intermediate groups showed modest increase in negative affect and almost no increase in RRV. Addressing this conjecture definitively is not possible the current study, but certainly warrants investigation in future studies. Another limitation that is worth noting is that although biological assessments were included in the form of HR and MAP, stress hormones were not. As such, the study cannot speak to whether NUR is a moderator of endocrine response to stress.
This study contributes to the literature by validating the construct of NUR, a self-reported trait that is associated with multiple forms of externalizing problems and psychological disorders (Coskunpinar et al., 2013; Doran et al., 2013; Kaiser et al., 2016; VanderVeen et al., 2016), and its assessment using the UPPS-P. Specifically, it suggests that trait NUR is, in fact, associated more rash behavior following a stressful experience and these findings suggest that studies using the UPPS-P to measure NUR may proceed with greater certainty that it is successfully measuring the intended trait. Further, these results replicate prior work linking NUR with alcohol misuse, and thus contribute to the understanding of self-regulatory deficits in these forms of dysregulated consumption.
General Scientific Summary.
Negative urgency, the tendency to act out when experiencing negative emotion, is associated with several psychiatric disorders, but has received little laboratory investigation. This study provides laboratory support for the validity of negative urgency by demonstrating it is positively associated with greater emotional and behavioral reactivity in response to acutely stressful events. In addition, negative urgency was systematically related to severity of alcohol misuse. Collectively, these findings validate the construct of negative urgency in relation to stress reactivity and inform the understanding of self-control deficits in relation to disorders associated with excessive consumption.
Acknowledgments
This work was partially supported by the Peter Boris Chair in Addictions Research, the Peter Boris Centre for Addictions Research, NIH grant P30 DA027827, and NIH grant K23 AA016936. Drs. Amlung and Stojek equally contributed to this work and are listed alphabetically. Preliminary version of these findings was presented at the 2017 Research Society on Alcoholism Annual Convention in Denver, DO.
Footnotes
The authors have no conflicts of interest to declare.
While our planned analyses used a trichotomized measure of NUR for consistency with previous studies and interpretability, we also report analyses using a dimensional measure of NUR for completeness. This analysis revealed main effects of NUR for negative affect (F = 13.18, p < .001, = .08) and RRV (F = 7.99, p = .005, = .05) but non-significant interactions for negative affect (F = 1.06, p = .31, = .01) and RRV (F = 1.46, p = .23, = .01), suggesting the relationship is not fully continuous (see Discussion).
References
- Amlung M, MacKillop J. Understanding the effects of stress and alcohol cues on motivation for alcohol via behavioral economics. Alcoholism, Clinical and Experimental Research. 2014;38(6):1780–9. doi: 10.1111/acer.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KD, Fischer S, Smith GT, Miller JD. The influence of negative urgency, attentional bias, and emotional dimensions on palatable food consumption. Appetite. 2016;100:236–243. doi: 10.1016/j.appet.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Benson TA, Little CS, Henslee AM, Correia CJ. Effects of reinforcer magnitude and alternative reinforcer delay on preference for alcohol during a multiple-choice procedure. Drug and Alcohol Dependence. 2009;100(1–2):161–163. doi: 10.1016/j.drugalcdep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Benton D, Young HA. A meta-analysis of the relationship between brain dopamine receptors and obesity: a matter of changes in behavior rather than food addiction[quest] Int J Obes. 2016;40(S1):S12–S21. doi: 10.1038/ijo.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett MA. The Trier Social Stress Test protocol for inducing psychological stress. Journal of Visualized Experiments : JoVE. 2011;(56):1–6. doi: 10.3791/3238. [DOI] [PMC free article] [PubMed]
- Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement pathology and obesity. Current Drug Abuse Reviews. 2011;4(3):190–6. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90(10):1349–1356. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: A meta-analysis using the UPPS model of impulsivity. Alcoholism: Clinical and Experimental Research. 2013;37(9):1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Gálvan A, Poldrack RA, Mackillop J, Ray LA. The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach. Alcoholism, Clinical and Experimental Research. 2012;36(6):923–31. doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Mood-based rash action and its components: Positive and negative urgency. Personality and Individual Differences. 2007;43(4):839–850. doi: 10.1016/j.paid.2007.02.008. [DOI] [Google Scholar]
- Cyders MA, Smith GT. Clarifying the role of personality dispositions in risk for increased gambling behavior. Personality and Individual Differences. 2008a;45(6):503–508. doi: 10.1016/j.paid.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychological Bulletin. 2008b;134(6):807–28. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that “food addiction” is a valid phenotype of obesity. Appetite. 2011;57(3):711–717. doi: 10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009 doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010 doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed]
- Doran N, Khoddam R, Sanders PE, Schweizer CA, Trim RS, Myers MG. A prospective study of the Acquired Preparedness Model: The effects of impulsivity and expectancies on smoking initiation in college students. Psychology of Addictive Behaviors. 2013;27(3):714–722. doi: 10.1037/a0028988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RL, King KM, Levine MD. The moderating role of negative urgency on the associations between affect, dietary restraint, and calorie intake: An experimental study. Personality and Individual Differences. 2014;59:38–43. doi: 10.1016/j.paid.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Peterson CM, McCarthy D. A Prospective Test of the Influence of Negative Urgency and Expectancies on Binge Eating and Purging. Psychology of Addictive Behaviors. 2012;27(1):294–300. doi: 10.1037/a0029323. [DOI] [PubMed] [Google Scholar]
- Folstein M, Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychological Medicine. 1973 doi: 10.1017/S0033291700054283. [DOI] [PubMed]
- Freeman LMY, Gil KM. Daily stress, coping, and dietary restraint in binge eating. International Journal of Eating Disorders. 2004;36(2):204–212. doi: 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of General Psychiatry. 2011;68(8):808–16. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Park DK. Splitting a predictor at the upper quarter or third and the lower quarter or third. American Statistician. 2009;63(1):1–8. doi: 10.1198/tast.2009.0001. [DOI] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Roland R, Rush CR, Craig R, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Experimental and Clinical Psychopharmacology. 1996;4(1):97–106. doi: 10.1037/1064-1297.4.1.97. [DOI] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behavioural Pharmacology. 1993;4(1):3–13. doi: 10.1097/00008877-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Schubert M. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology. 2012;37(1):119–124. doi: 10.1016/j.psyneuen.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hjortskov N, Rissén D, Blangsted AK, Fallentin N, Lundberg U, Søgaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. European Journal of Applied Physiology. 2004;92(1–2):84–89. doi: 10.1007/s00421-004-1055-z. [DOI] [PubMed] [Google Scholar]
- Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, Manso G. Refined food addiction: A classic substance use disorder. Medical Hypotheses. 2009;72(5):518–526. doi: 10.1016/j.mehy.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Bonsu JA, Charnigo RJ, Milich R, Lynam DR. Impulsive Personality and Alcohol Use: Bidirectional Relations Over One Year. Journal of Studies on Alcohol and Drugs. 2016;77(3):473–82. doi: 10.15288/jsad.2016.77.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ, Ph D, Kreek MJ. Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence. American Journal of Psychiatry. 2007;164(4):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug and Alcohol Dependence. 1998 doi: 10.1016/S0376-8716(98)00064-7. [DOI] [PubMed]
- Kudielka BM, Wüst S, Kirschbaum C, Hellhammer DH. Trier Social Stress Test. Encyclopedia of Stress. 2007;3:776–781. doi: 10.1016/B978-012373947-6.00681-4. [DOI] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SAP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette: 2006. [Google Scholar]
- Lynam D, Smith GT, Cyders MA, Fischer S, Whiteside SA. The UPPS-P: A multidimensional measure of risk for impulsive behavior 2007 [Google Scholar]
- Murphy CM, Stojek MK, MacKillop J. Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite. 2014;73:45–50. doi: 10.1016/j.appet.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, N. I. of H. U. S. D. of H. and H. S. Rethinking drinking: Alcohol and your health 2010 [Google Scholar]
- Pearson CM, Combs JL, Zapolski TCB, Smith GT. A longitudinal transactional risk model for early eating disorder onset. Journal of Abnormal Psychology. 2012;121(3):707–718. doi: 10.1037/a0027567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Exploring the relationship between negative urgency and dysregulated eating: etiologic associations and the role of negative affect. Journal of Abnormal Psychology. 2013;122(2):433–44. doi: 10.1037/a0031250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Martin SJ. Exploring divergent trajectories: Disorder-specific moderators of the association between negative urgency and dysregulated eating. Appetite. 2016;103:45–53. doi: 10.1016/j.appet.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Raymond KL, Lovell GP. Food addiction symptomology, impulsivity, mood, and body mass index in people with type two diabetes. Appetite. 2015;95:383–389. doi: 10.1016/j.appet.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcoholism, Clinical and Experimental Research. 2007;31(2):185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT. Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of Abnormal Psychology. 2012;121(1):160–72. doi: 10.1037/a0024948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles RE, Zapolski TCB, Smith GT. Longitudinal test of a developmental model of the transition to early drinking. Journal of Abnormal Psychology. 2014;123(1):141–51. doi: 10.1037/a0035670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Goldstein IB, Delfino RJ. Striking a chord: moods, blood pressure, and heart rate in everyday life. Psychophysiology. 2001;38(2):197–204. doi: 10.1017/S004857720199225X. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling relapse situations in the human laboratory. Behavioral Neurobiology of Alcohol Addiction. 2013 doi: 10.1007/7854. [DOI] [PMC free article] [PubMed]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, O’Malley S. Craving for alcohol: findings from the clinic and the laboratory. Alcohol and Alcoholism. 1999;34(2):223–230. doi: 10.1093/alcalc/34.2.223. Retrieved from http://alcalc.oxfordjournals.org/content/34/2/223.short. [DOI] [PubMed] [Google Scholar]
- Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed]
- Snijders T, Bosker R. Multilevel Analysis: An introduction to basic and advanced multilevel modeling. London: Sage Publications; 1999. [Google Scholar]
- Stojek MK, Fischer S, MacKillop J. Stress, cues, and eating behavior. Using drug addiction paradigms to understand motivation for food. Appetite. 2015;92:252–260. doi: 10.1016/j.appet.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Costa PT, Chan W, Milaneschi Y, Eaton WW, Zonderman AB, Terracciano A. I know not to, but i can’t help it: weight gain and changes in impulsivity-related personality traits. Psychological Science. 2013;24(7):1323–8. doi: 10.1177/0956797612469212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch CF, Agras WS. Do emotional states influence binge eating in the obese? International Journal of Eating Disorders. 1996;20(3):271–279. doi: 10.1002/(SICI)1098-108X(199611)20:3<271::AID-EAT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- VanderBroek-Stice L, Stojek MK, Beach SRH, vanDellen MR, MacKillop J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite. 2017;112:59–68. doi: 10.1016/j.appet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken DA, O’Connor S, Cyders MA. Negative urgency, mood induction, and alcohol seeking behaviors. Drug and Alcohol Dependence. 2016;165:151–158. doi: 10.1016/j.drugalcdep.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Vrijkotte TG, van Doornen LJ, de Geus EJ. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35(4):880–886. doi: 10.1161/01.HYP.35.4.880. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Zapolski TCB, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors. 2009;23(2):348–54. doi: 10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]


