Abstract
Oligodendrocyte development and myelination are processes in the central nervous system that are regulated by cell intrinsic and extrinsic mechanisms. Organotypic slice cultures provide a simple method for studying factors that affect oligodendrocyte proliferation, differentiation, and myelination in the context of the local cellular environment. Here we show that major glial cell types and neurons are preserved in slice cultures from postnatal mouse forebrain, and their morphological characteristics are retained. We further demonstrate that cellular processes requiring interactions with neighboring cells such as myelination can proceed in slice culture.
Keywords: Oligodendrocyte, Myelin, Oligodendrocyte precursor, NG2, PDGF, Pdgfrα, Proliferation, Organotypic slice culture
1. Introduction
NG2 cells, also known as oligodendrocyte precursor cells (OPCs) or polydendrocytes, constitute a major glial cell type in the developing and mature central nervous system (CNS). While NG2 cells exhibit some lineage plasticity, those in the postnatal CNS are mostly restricted to the oligodendrocyte lineage. They undergo either self-renewal or differentiation into oligodendrocytes, which myelinate axons [1–4]. NG2 cells remain in the cell cycle and continue to generate new oligodendrocytes in the adult. NG2 cells in different CNS regions proliferate and differentiate into oligodendrocytes at different rates [5–8]. While dissociated cultures of NG2 cells from different CNS regions exhibit different proliferative and differentiation behaviors, it has been difficult to assess whether the observed behaviors are due to inherent properties of the cells or caused by taking the cells out of their natural context [2, 3, 9]. Homo- and heterotopic transplantation of NG2 cells from different CNS regions have revealed both clearly intrinsic [10] and somewhat more equivocal properties of their fate [11].
Organotypic slice culture is an isolated and three-dimensional ex vivo system, which preserves the architecture and microenvironment of the brain tissue and provides an effective approach to studying the mechanism underlying cellular behavior in the multicellular context of the tissue. Ross Harrison first succeeded in culturing explants from frog embryos in ymph clots using the hanging drop method [12]. Half a century later, Murray and colleagues grew explants from chick dorsal root ganglia on rat tail collagen-coated coverslips in Maximov chamber slides and demonstrated peripheral myelination in vitro [13]. Shortly thereafter, Hild [14] reported myelination of CNS tissue using roller tube cultures of newborn cat cerebellum, but the analysis was limited due to the difficulty of sequential observation and significant loss of the original tissue architecture. This was improved by culturing newborn cat and rat cerebellum in Maximov chamber slides, which provided more definitive demonstration of myelinogenesis in CNS explant culture [15] and further confirmation of myelin by electron microscopy [16].
The development of an air-liquid interphase culture method [17] significantly improved experimental consistency and facilitated its use in a standard laboratory by eliminating the need for plasma clots or a roller tube drum. In organotypic slice cultures, slices of CNS tissues are placed on a porous membrane and cultured at the interface between culture medium and air. By capillary action the medium crosses the membrane and forms a thin film over the slices, ensuring sufficient oxygenation of the tissue. Compared to dissociated cell cultures, organotypic slice cultures allow investigation of cellular interactions, and in certain CNS regions such as the hippocampus, a simple neuronal circuitry is retained [17, 18]. Compared to in vivo approaches, organotypic slice cultures are more accessible to pharmacological manipulations and time-lapse imaging [19–21].
We have previously used slice cultures from postnatal forebrain and cerebellum to demonstrate differential proliferative response of NG2 cells in gray and white matter to platelet-derived growth factor (PDGF) AA [22] and used time-lapse imaging to show that acute demyelination accelerates oligodendrocyte differentiation from divided NG2 cells during the critical temporal window after their division [23]. Here we describe a simple method of organotypic forebrain slice cultures with a slight modification to the previously published method [24].
2. Materials
2.1. Animals
Slices are obtained from postnatal day 8 (P8) mice and can be taken from various genetic mutants to label NG2 cells, e.g., NG2cre:zeg mice [22, 25] (see Note 1), or other cell types (see Note 2).
2.2. Reagents
Dissection buffer: 124 mM NaCl, 3.004 mM KCl, 1.25 mM KH2PO4,4.004mM MgSO4 (anhydrous), 2.0mM CaCl2∙2H2O, 26 mM NaHCO3, 10 mM D-(+)-glucose, 2 mM ascorbic acid, 0.075 mM adenosine in MilliQ Water (see Note 3).
Slice medium: 50% Minimum Essential Medium, 25% Hank’s Balanced Salt Solution, 25% horse serum (see Note 4), 25 mM HEPES, 1 mM L-glutamine, 20 ng/mL insulin, 0.4 mM ascorbic acid (see Note 5).
5-Ethynyl-2′-deoxyuridine (EDU).
Fixative solution: 4% Paraformaldehyde (PFA), dissolved in 0.1 M sodium phosphate buffer, pH 7.4.
Blocking solution: 1% bovine serum albumin (BSA) and 0.1% Triton-×100 in PBS.
Primary antibodies: Anti-GFP antibody (to increase the signal of the GFP or EYFP reporter), anti-platelet-derived growth factor receptor alpha (PDGFRα) antibody (to label NG2 cells), anti-Iba1 antibody (to detect microglia), anti-GFAP antibody (to label astrocytes), anti-NeuN antibody (to label neurons), and anti-myelin basic protein antibody (to label myelin).
Secondary antibodies: Alexa 488-conjugated antibodies, Cy3-conjugated antibodies, and Alexa 647-conjugated antibodies.
Fluorescent mounting medium.
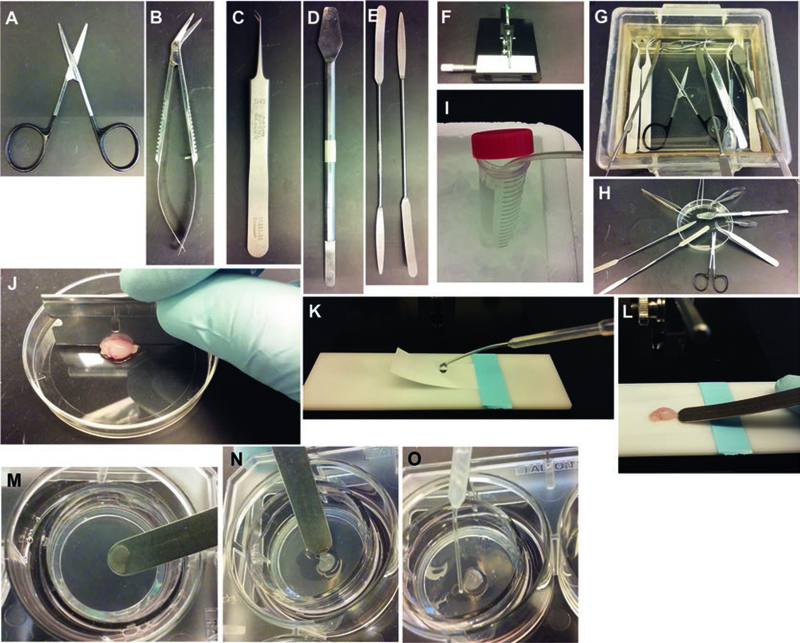
2.3. Supplies and Tools (Fig. 1)
Fig. 1.
Setup for making organotypic slice cultures from P8 mouse brain. (a–e) Tools. (f) Tissue chopper. (g, h) Treatment of tools with 70% ethanol. (i) Bubbling dissection buffer with 90% O2/5% CO2 gas mixture and chilling it on ice. (j) Cutting the dissected brain into two hemispheres. (k) Preparation of the stage for slicing using Whatman paper to hold the brain in place. (l) Picking up sliced hemisphere with a spatula. (m, n) Sliding a slice onto the membrane of a Millicell insert. O: Removing extra dissection buffer from around the slice using a transfer pipet
Millicell culture membrane inserts, 30 mm diameter.
Sterile six-well tissue culture plates.
Small dissection scissors for cutting the skin.
Small scissors with rounded tips for cutting the skull (e.g., Fine Science Tools Bone Strabismus-scissors (Fig. 1a) or iridectomy scissors (Fig. 1b)) (see Note 6).
-
Forceps.
Serrated forceps work best for holding the skin. Fine forceps with angled tips work well for separating the slices (Fig. 1c).
Hippocampal spatulas (Fig. 1d) or small weighing spatulas (Fig. 1e) (see Note 7).
Single- and doubled-edged razor blades.
Whatman filter paper (no. 1) cut into ~5 × 2 cm rectangular pieces to be placed on the chopper to secure the brain in place.
70% Ethanol.
Disposable transfer pipettes.
35 and 60 mm sterile Petri dishes.
2.4. Equipment
Manual tissue chopper (Fig. 1f).
95% O2/5% CO2 tank and tubing.
Laminar flow hood and tissue culture incubator.
Stereomicroscope.
Inverted microscope.
Fluorescence microscope.
3. Methods
3.1. Preparation of Dissection Setup
In a laminar flow hood, gently place tissue culture inserts in each well of a six-well plate filled with 1 mL of slice medium. Avoid getting air trapped between the medium and insert.
Transfer the plates to the cell culture incubator set at 37 °C with 5% CO2 and let equilibrate for 1 h before dissection (at least 2 h before placing the slices).
Sterilize the tools by soaking them in 70% ethanol for >5 min (Fig. 1g). Then take them out of the ethanol vat and let them completely dry on a clean surface (Fig. 1h) (see Note 8).
Sterilize the fully assembled tissue chopper mounted with a double-edged razor blade by spraying it with 70% ethanol.
Spray ethanol on the pieces of Whatman filter paper and let them dry, propped up against the wall of a Petri dish.
Put 20–30 mL of dissection buffer into a sterile conical 50 mL tube and bubble it with 95% O2/5% CO2 on ice for at least 15 min prior to slicing (Fig. 1i) (see Note 9).
3.2. Preparation of Slices
Anesthetize mouse pups on ice for 5–10 min according to the approved animal protocol and confirm the lack of response by pinching the toes and tail (see Note 10). All animal procedures should be approved by the Institutional Animal Care and Use Committee (IACUC).
Once the depth of anesthesia is ascertained, decapitate using sharp scissors.
Spray 70% ethanol over the skin to sterilize. Remove the skin and skull quickly from over the forebrain.
Dissect out the brain by severing the cranial nerves from the ventral surface of the brain.
Place the brain in a prechilled 35 mm Petri dish containing a drop of ice-cold oxygenated dissection buffer (see Note 9) for 1 min.
Quickly remove the cerebellum from forebrain using a razor blade and cut the forebrain sagittally into two hemispheres (Fig. 1j).
Tape a piece of a sterile Whatman filter paper on the stage of the tissue chopper and moisten it with ice-cold dissection buf fer (Fig. 1k). Wick off excess buffer with sterile kimwipes. Place one hemisphere on the filter paper on the tissue chopper and cut 300 μm coronal slices (see Note 11).
Transfer the sliced brain en bloc using a spatula (Fig. 1l) to a prechilled 35 mm Petri dish containing approximately 0.5 mL of bubbled ice-cold dissection buffer and place the Petri dish on ice under the stereomicroscope.
Use a spatula to separate individual slices, gently supporting the brain with forceps held in the other hand (see Note 12).
Take out the preincubated slice culture inserts from the incubator and slide each slice onto the membrane of the culture insert (Fig. 1m, n).
Remove any excess dissection buffer around the slice on the membrane using a P-200 pipette tip or a transfer pipette (Fig. 1o).
Immediately return the plate to the incubator (see Note 13).
On the following day, replace the slice medium with fresh warmed medium.
Then, change the medium every other day.
Look at the slices under the microscope every day. Use slices that have become transparent after 5–7 days in vitro (DIV). Do not use slices with uneven and white opaque regions. The vasculature should be visible on good slices (see Note 14).
For proliferation assays, EDU can be added to the medium at a final concentration of 10 μM during the last 4 h of culture before fixation.
Slices can also be used to examine the effects of pharmacological agents (see Note 15) or genetic manipulation (see Note 16) on specific processes such as myelination.
3.3. Fixation and Immunohisto chemistry
Remove medium from the wells.
Gently add 1 mL of fixative solution to the top and bottom of inserts in each well and let sit at RT for 30 min.
Wash slices with PBS 3 times for 15 min each.
Cut the membrane around each slice using scissors and tweezers.
Transfer the slices to individual wells of a 24 well plate containing PBS (see Note 17).
Incubate slices with blocking solution for 1 h.
Incubate the slices in primary antibodies diluted with 1% BSA or 5% normal goat serum in PBS at 4 °C overnight.
On the second day, wash slices in PBS 3 times for 15 min each, then add secondary antibodies diluted with 1% BSA or 5% normal goat serum in PBS for 1 h at RT.
Wash slices in PBS 3 times for 15 min each and mount on slides using fluorescent mounting medium containing DAPI. The slices should be mounted with the membrane side down and the free slice side up adjacent to the coverslip.
For the detection of EDU, we use Alexa 555- or Alexa 647-conjugated Click-IT system (InVitrogen) according to the manufacturer’s protocol (see Note 18).
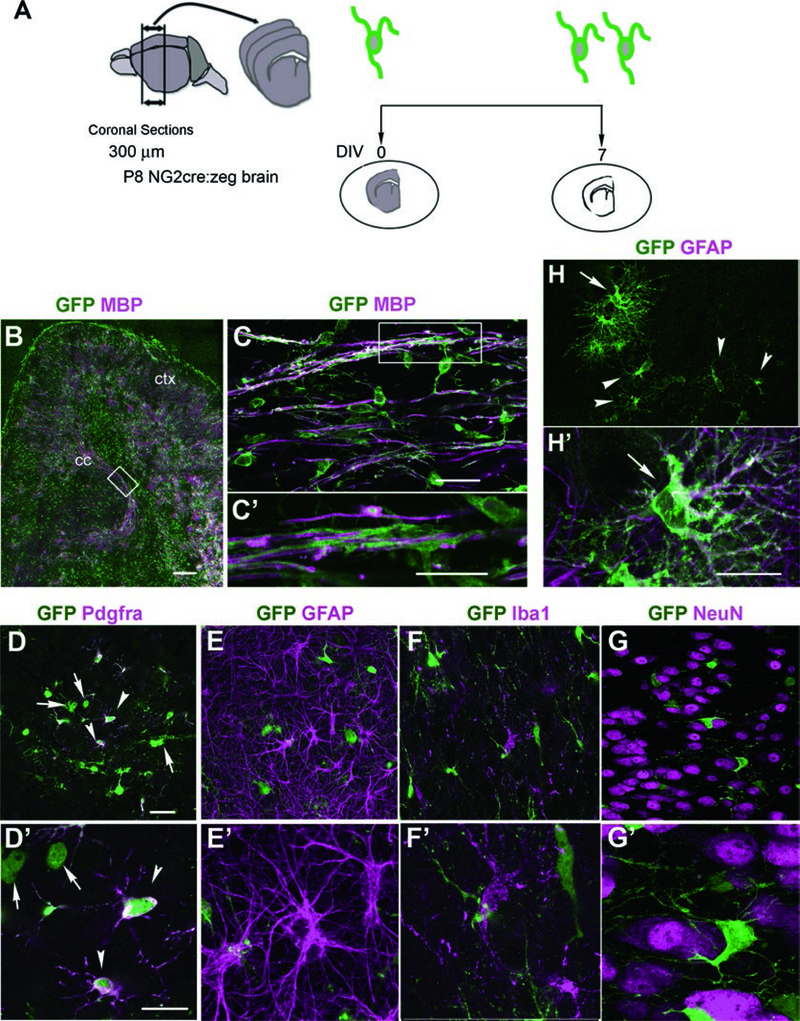
Analyze labeled sections by fluorescent microscopy (see Note 19) (Fig. 2).
Fig. 2.
CNS structures and cell types identified in slice cultures from P8 NG2cre:zeg mice after 7 DIV. (a) A schematic showing the culture strategy. (b) Low-magnification image of a coronal anterior forebrain slice depicting GFP+ cells and MBP immunostaining in both cortex (ctx; gray matter) and corpus callosum (cc; white matter) in forebrain cultures by 7 DIV. Scale bar, 100 μm. (c, c′) High-magnification images taken from the boxed region of the white matter in (b) showing GFP+ cells and their processes extending into parallel MBP+ myelin sheaths. (c′) represents area shown in box in (c). Since myelination in the mouse corpus callosum does not begin until P11 [26], this likely represents myelination that occurred during slice culture. Scale bars, 20 μm. (d, d′) GFP+ PDGFRα+ NG2 cells (arrowheads) and GFP+ PDGFRα-negative cells, which are presumably oligodendrocytes (arrows). Scale bars, 20 μm. (e, e′) GFP+ oligodendrocyte lineage cells in the cortex are distinct from GFAP+ astrocytes that exhibit the typical stellate morphology. (f, f′) GFP+ oligodendrocyte lineage cells are distinct from Iba1+ microglial cells but the two cell types are closely associated with each other. (g, g′) NeuN+ neurons in the cortex. GFP+ oligodendrocyte lineage cells are often found in satellite position to neuronal cell bodies. (h, h′) Slice cultures from Olig2flfl:NG2cre:zeg mice showing GFAP+ bushy protoplasmic astrocytes (arrows) that are GFP+ and hence are the progeny of NG2 cells. Arrowheads in (h) and (h′) denote cells with polydendrocyte morphology that are likely to be NG2 cells. Scale, 20 μm
4. Notes
Reporter expression is highly preserved in slice cultures and provide a reliable way to follow cells in real time (Fig. 2). In addition to DsRed expression in NG2DsRed mice and GFP expression in NG2cre:zeg mice [25, 27], we have used inducible NG2creER:YFP mice [8, 28] in which YFP expression can be induced by tamoxifen either in vivo prior to slice preparation or in slices. The GFP or YFP reporter can also be used to study the fate of NG2 cells under various conditions, for example, after transcription factor manipulation [29, 30]. To induce Cre in slice cultures, 4-hydroxytamoxifen, dissolved in ethanol or DMSO, must be used instead of tamoxifen because tamoxifen cannot be converted into the active form in the absence of intact liver.
One can also use mouse lines that express the reporter in other cell types such as the Cx3CR1-creER-ires-EYFP mice [31] that expresses EYFP in microglia in a Credependent manner, to examine the role of non-oligodendrocyte lineage cells in the process of oligodendrocyte maturation and myelination. To study the dynamics of oligodendrocytes and myelin, slices from PLP-EGFP mice can be used [32]. We have used PLP- DsRed mice [33] to examine the dynamics of oligodendrocyte differentiation from divided NG2 cells following acute demyelination induced in slice culture [23]. Murai and colleagues used slice cultures transduced with Semliki forest virus encoding membrane-anchored enhanced green fluorescent protein (EGFP) to perform high resolution time-lapse imaging of single oligodendrocyte lineage cells and myelin and demonstrate the extent of their growth cone activity and myelin remodeling at different differentiation stages [34].
Filter the final dissection buffer using a Nalgene bottle-top filter with 0.45 μm pore size and store at 4 °C for short term, up to 2 weeks. Aliquots of 40 mL can be stored in sterile 50 mL polypropylene conical tubes in −20 °C for a few months.
Heat-inactivate horse serum at 56 °C for 30 min and freeze in 45 mL aliquots.
After all reagents are mixed, adjust the pH to 7.2. Sterilize the medium using a bottle-top filter (Nalgene, 0.45 μm) and store at 4 °C. Do not use antibiotics.
The rounded tips allow the blade to be inserted between the skull and the brain without damaging the underlying brain.
We have obtained them from Ted Pella in the past. If they are no longer available, weighing spatulas (Fig. 1e) will do, although they are slightly thicker.
We put the handles on the side of Petri dishes so that the tips do not come into contact with the bench or other non-sterile surfaces while drying (Fig. 1h).
The buffer should be equilibrated with 95% O2/5% CO2 for at least 15 min before use. It is critical that the buffer be kept chilled at all times for the best preservation of tissue.
The healthiest slice cultures are prepared from P4 to P8 mouse brains. Slices from mice older than P12 do not recover well and often remain opaque with large areas of tissue death.
Smaller explant cultures of 300 μm3 pieces can also be used [22]. To dissect out subregions from a slice, use two sterile 30-gauge needles attached to 1.0 mL syringes, and use a spatula to slide each piece onto the membrane.
This step should be done as quickly as possible to minimize damage.
Slice viability significantly decreases with delay in putting the slices into the incubator. It is best to do one hemisphere at a time. Up to 3 slices can be placed on each membrane insert. Take 3–4 slices from each hemisphere. The slices hold up best when taken from the level of anterior striatum to the septum.
Since most dead cells and tissue debris created by the slicing procedure must be cleared from the surface of the slices, the slices cannot be used immediately after culturing. It is best to wait for 5–7 days before analyzing or manipulating the slice cultures.
Pharmacological agents such as small molecule compounds, growth factors, and larger proteins such as antibodies penetrate the slices well [22]. This makes the slice cultures ideally suited for addressing mechanistic questions that require the retention of tissue architecture without having to infuse compounds into living animals. For example, cerebellar slice cultures were used to examine the effects of testosterone on remyelination [35]. It may be important to wait for 7 days to allow the slices to stabilize after the initial tissue reorganization before adding the pharmacological reagents. It is likely that the slices become more penetrable as they flatten and become translucent. A slightly higher concentration of the agents may be needed for the optimum response.
It is very inefficient to transfect cells in slice cultures with plasmids. Cells in slices can be transduced with viral constructs such as AAV (adeno-associated virus). While viral transduction is a viable approach, generating constructs in AAV or lentiviral vectors is much more time-consuming than generating plasmid constructs. Biolistic transfection is effective for expressing reporter genes in a small number of isolated target cells for detailed morphological analyses [34]. Some of these shortcomings can be overcome by transplanting genetically modified cells into host slices. For example, induced oligodendrocyte progenitor cells were transplanted into slices from the myelin deficient shiverer mice lacking the MBP gene and shown to produce myelin [36].
Use the membrane to pick up slices and avoid touching the tissue during the transfer.
When mounting slices after EDU detection kit, 100 μg/mL Hoechst 33342 should be used instead of DAPI.
Organotypic slice cultures from P8 mouse forebrain and cerebellum preserve the morphology and intercellular spatial relationship of neurons, astrocytes, microglia, and oligodendrocyte lineage cells including myelin. NG2 cells in gray and white matter retain their regionally distinct proliferative properties, as well as their specific fate when transcription factors are manipulated. The slices undergo considerable reorganization during the first few days in culture and flatten from the original 300 μm to less than 150 μm. The top and bottom surfaces of the slices undergo changes that are reminiscent of reactive changes after injury, and amoeboid microglia and astrocytes with reactive morphology are seen, while the cells in the middle portion of the slices resemble those in the intact CNS in vivo. Therefore, care should be taken to restrict the analysis to the middle layer of the slices and avoid the top and bottom of the slices that have altered tissue structure. Barateiro and Fernandes [37] provide a comparison of different culture models and also demonstrate the detection of myelin, NG2 cells, astrocytes, and microglia in cerebellar slice cultures.
Acknowledgments
This work was supported by grants from NIH (R01 NS073425 and R01 NS074870) to AN. We thank Youfen Sun for maintaining the transgenic mouse colony.
References
- 1.Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24(2):476–488 [DOI] [PubMed] [Google Scholar]
- 2.Hill RA, Nishiyama A (2014) NG2 cells (poly-dendrocytes): listeners to the neural network with diverse properties. Glia 62(8):1195–1210. 10.1002/glia.22664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiyama A (2007) Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13(1):62–76. 10.1177/1073858406295586 [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama A, Komitova M, Suzuki R, Zhu X (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10(1):9–22 [DOI] [PubMed] [Google Scholar]
- 5.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M (2008) Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28(41):10434–10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE (2010) NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68(4):668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77(5):873–885. 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A (2011) Age-dependent fate and lineage restriction of single NG2 cells. Development 138(4):745–753. 10.1242/dev.047951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power J, Mayer-Proschel M, Smith J, Noble M (2002) Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev Biol 245(2):362–375. 10.1006/dbio.2002.0610 [DOI] [PubMed] [Google Scholar]
- 10.Fanarraga ML, Griffiths IR, Zhao M, Duncan ID (1998) Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J Comp Neurol 399(1):94–100 [PubMed] [Google Scholar]
- 11.Vigano F, Mobius W, Gotz M, Dimou L (2013) Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16(10):1370–1372. 10.1038/nn.3503 [DOI] [PubMed] [Google Scholar]
- 12.Harrison RG (1907) Observations on the living developing nerve fiber. Proc Soc. Biol Med 4:140–143 [Google Scholar]
- 13.Peterson ER, Murray MR (1955) Myelin sheath formation in cultures of avian spinal ganglia. Am J Anat 96(3):319–355. 10.1002/aja.1000960302 [DOI] [PubMed] [Google Scholar]
- 14.Hild W (1957) Myelogenesis in cultures of mammalian central nervous tissue. Z Zellforsch Mikrosk Anat 46(1):71–95 [DOI] [PubMed] [Google Scholar]
- 15.Bornstein MB, Murray MR (1958) Serial observations on patterns of growth, myelin formation, maintenance and degeneration in cultures of new-born rat and kitten cerebellum. J Biophys Biochem Cytol 4(5):499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross LL, Bornstein MB, Lehrer GM (1962) Electron microscopic observations of rat and mouse cerebellum in tissue culture. J Cell Biol 14:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37(2):173–182 [DOI] [PubMed] [Google Scholar]
- 18.Bahr BA, Kessler M, Rivera S, Vanderklish PW, Hall RA, Mutneja MS, Gall C, Hoffman KB (1995) Stable maintenance of glutamate receptors and other synaptic components in longterm hippocampal slices. Hippocampus 5(5):425–439. 10.1002/hipo.450050505 [DOI] [PubMed] [Google Scholar]
- 19.Cho S, Liu D, Fairman D, Li P, Jenkins L, McGonigle P, Wood A (2004) Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochem Int 45(1):117–127. 10.1016/j.neuint.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Pringle AK, Sundstrom LE, Wilde GJ, Williams LR, Iannotti F (1996) Brain-derived neurotrophic factor, but not neurotrophin-3, prevents ischaemia-induced neuronal cell death in organotypic rat hippocampal slice cultures. Neurosci Lett 211(3):203–206 [DOI] [PubMed] [Google Scholar]
- 21.Ray AM, Owen DE, Evans ML, Davis JB, Benham CD (2000) Caspase inhibitors are functionally neuroprotective against oxygen glucose deprivation induced CA1 death in rat organotypic hippocampal slices. Brain Res 867(1–2):62–69 [DOI] [PubMed] [Google Scholar]
- 22.Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A (2013) NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci 33(36):14558–14566. 10.1523/JNEUROSCI.2001-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A (2014) Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci 17(11):1518–1527. 10.1038/nn.3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill RA, Medved J, Patel KD, Nishiyama A (2014) Organotypic slice cultures to study oligodendrocyte dynamics and myelination. J Vis Exp 90:e51835 10.3791/51835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Bergles DE, Nishiyama A (2008) NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135(1): 145–157 [DOI] [PubMed] [Google Scholar]
- 26.Sturrock RR (1980) Myelination in the mouse corpus callosum. Neuropathol Appl Neurobiol 6(6):415–420. [DOI] [PubMed] [Google Scholar]
- 27.Novak A, Guo C, Yang W, Nagy A, Lobe CG (2000) Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28(3–4):147–155 [PubMed] [Google Scholar]
- 28.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A (2012) Olig2-dependent developmental fate switch of NG2 cells. Development 139(13):2299–2307. 10.1242/dev.078873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo H, Hill RA, Sherafat AM, Lu QR, Nishiyama A (2018) Age-dependent decline in fate switch from NG2 cells to astrocytes after Olig2 deletion. J Neurosci 38(9):2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155(7):1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallon BS, Shick HE, Kidd GJ, Macklin WB (2002) Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci 22(3):876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirrlinger PG, Scheller A, Braun C, Quintela-Schneider M, Fuss B, Hirrlinger J, Kirchhoff F (2005) Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci 30(3):291–303. 10.1016/j.mcn.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Haber M, Vautrin S, Fry EJ, Murai KK (2009) Subtype-specific oligodendrocyte dynamics in organotypic culture. Glia 57(9):1000–1013. 10.1002/glia.20824 [DOI] [PubMed] [Google Scholar]
- 35.Hussain R, Ghoumari AM, Bielecki B, Steibel J, Boehm N, Liere P, Macklin WB, Kumar N, Habert R, Mhaouty-Kodja S, Tronche F, Sitruk-Ware R, Schumacher M, Ghandour MS (2013) The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain 136(Pt 1):132–146. 10.1093/brain/aws284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ (2013) Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol 31(5):426–433. 10.1038/nbt.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barateiro A, Fernandes A (2014) Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta 1843(9):1917–1929. 10.1016/j.bbamcr.2014.04.018 [DOI] [PubMed] [Google Scholar]