Abstract
Background:
Bariatric surgery is a popular and effective therapeutic intervention for obesity, which is an abnormal health condition that is prevalent worldwide. Metabolic improvements that precede weight loss after bariatric surgery may be mediated, in part, through the fibroblast growth factor (FGF) 15/19 and FGF21 signaling pathways. Both FGF15/19 and FGF21 are hormone-like members of the FGF family and exert their metabolic effects in an endocrine manner. Enhanced bile acid recycling after bariatric surgery leads to increased circulating levels of FGF15/19 in the distal small intestine. Synthesis of FGF21 is upregulated predominately in the fasting state through peroxisome proliferator-activated receptor pathways and to a lesser extent by FGF15/19.
Key Messages:
The biological functions of FGF15/19 and FGF21 are diverse and complicated. The tissue targeted effects of FGF15/19 and FGF21 of importance after bariatric surgery include the regulation of hepatic bile acid biosynthesis and ketogenesis as well as thermogenesis in adipose tissue, respectively. Furthermore, FGF15/19 and FGF21 function to regulate carbohydrate and lipid metabolism.
Conclusion:
The long-term effects of bariatric surgery on weight loss are undisputable. However, the mechanism for improvements in glucose and lipid homeostasis observed shortly after bariatric surgery is less understood. This review article attempts to describe the known metabolic effects of FGF15/19 and FGF21 that may potentiate these improvements after bariatric surgery.
Keywords: Bile acid, Sleeve gastrectomy, Roux-en-Y gastric bypass, Obesity
Introduction
The prevalence of obesity and its comorbidities are unfortunately now ubiquitous. It is associated with dyslipidemia, and type 2 diabetes mellitus, and nonalcoholic fatty liver disease, all of which have reached endemic proportions [1, 2]. Thus, it warrants great concern for preventing the associated health risks and resulting chronic diseases. Great emphasis and resources have been placed on therapeutic efforts to prevent or delay obesity-related disease by advocating for lifestyle modifications to reverse weight gain. However, when weight loss is refractory to diet or exercise alone, bariatric surgery has become a necessary therapeutic intervention.
Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed bariatric procedures and has been shown to have lasting effects on weight loss in adolescent and adult patients. In addition to reductions in weight gain, RYGB has profound effects on lowering blood glucose, circulating triglycerides, and remitting type 2 diabetes [3]. Mice that underwent RYGB had reduced food intake and increased insulin sensitivity [4]. Patients with uncontrolled type 2 diabetes and moderate obesity who underwent intensive medical therapy and RYGB had improved glycemic control and improved beta cell function after measurements of oral tolerance testing when compared to patients receiving intensive medical therapy alone [3]. After controlling for weight loss, remission of type 2 diabetes was greater after RYGB than laparoscopic gastric banding [5]. The most striking observations after RYGB, however, are the metabolic improvements that occur days after surgery preceding the dramatic weight loss. Rerouting the digestive pathway in RYGB and a second commonly performed bariatric procedure, the vertical sleeve gastrectomy (VSG) both alter the flow of undigested food from the stomach to the distal small intestine. The metabolic improvements after VSG are indeed very similar to that of RYBG, while VSG results in less nutrient deficiency complications.
The long-term benefits of weight loss after bariatric surgery are undisputed; however, the mechanisms underlying the acute improvements in metabolism including glucose and lipid homeostasis are not well elucidated [6]. Among the many candidate molecules considered important to these acute post-surgical benefits include incretin hormones such as glucagon like peptide 1, Peptide YY and more recently circulating levels of bile acids, fibroblast growth factor 19 (FGF19), and FGF21. Both human and murine studies have observed elevations in circulating bile acids, FGF19, and FGF21 after bariatric surgical procedures and this trend may provide invaluable insight into the observed acute physiological responses after surgery [7, 8]. Specifically, a twofold increase in serum FGF15/19 and FGF21 levels was measured in human participants after VSG and it was found that elevated serum FGF15/19 and bile acid levels remained elevated for a 24-month duration. Taken together, this suggests further investigation into the beneficial effects of bile acids, FGF15/19, and FGF21 to reduce the risk of obesity-related diseases after bariatric surgery. Thus, the questions remain whether bariatric surgery alters bile acid mechanics, which influence FGF15/19 activity and, in turn, regulate the physiological effects of FGF21. This article reviews the known literature on bariatric surgery’s impact on FGF15/19 and FGF21 and whether the alterations in these endocrine hormone levels impact the early improvement of obesity-related diseases post-bariatric surgery.
Regulation of FGF19 and FGF21
The FGF family is a vast and diverse group consisting of 22 proteins. In humans the hormone-like members of the FGF family include FGF15/19 and FGF21 [9]. FGF15 is the murine ortholog of FGF19. This selective group of the FGF family has little affinity for heparin/heparin sulfate residues and therefore, are able to exert their metabolic effects in an endocrine manner. Both FGF15/19 and FGF21 exert their physiological effects in vast distal tissues augmenting metabolic processes and there is growing interest to determine their protective roles in metabolic diseases [10].
Fibroblast Growth Factor 19
FGF19 is the founding member of the hormone-like FGF proteins and is synthesized in the distal small intestine or ileum, gall bladder, and brain. In the ileum, transcription of FGF15/19 is regulated by bile acids through the interaction with the nuclear receptor, farnesoid X receptor (FXR) [11]. The expression of FGF15/19 increases with postprandial elevation in bile acids uptake in the distal small intestine. Elevations in circulating FGF19 after bariatric surgery, specifically VSG, occur by the direct stimulation of FXR by bile acids indicating a molecular mechanism by which bariatric surgery initiates metabolic regulation [12, 13].
Fibroblast Growth Factor 21
FGF21, first discovered by Nishimura et al. [23], encodes a 210-amino acid endocrine hormone basally expressed in the liver and to a lesser degree, in the adipose tissue, pancreas in islet β-cells, and thymus. FGF21 can be detected at basal levels in serum. In rodents, hepatic expression and secretion of FGF21 are controlled by a circadian rhythm and fasting [14]. Postprandial elevation of FGF21 occurs 2–3 h after feeding. Circadian rhythm was also noted to coordinate with FGF21 secretion after a 72-hour fast in healthy females [14].
Transcriptional regulation of FGF21 in the fasting state is controlled by several different mechanisms. The most well-known mechanism by which FGF21 expression is upregulated is by the direct action of peroxisome proliferator-activated receptor pathways including peroxisome proliferator-activated receptor-α (PPARα) and peroxisome proliferator-activated receptor-γ (PPARγ). The induction of FGF21 expression by PPARα and PPARγ has been shown by both in vitro and in vivo studies. Hepatic expression of FGF21 was increased by administration of a PPARα agonist in both primary human hepatocytes and ob/ob mice, which have elevated PPARα expression compared to wild-type mice. In contrast, induction of FGF21 expression by a PPARα agonist was unsuccessful in PPARα-deficient mice identifying the role of PPARα to regulate FGF21 gene expression.
Additionally, in a fasting state or exercise when there is a demand for hepatic gluconeogenesis, glucagon may upregulate the expression of FGF21 by a glucagon receptor–mediated increase in PPARα transcriptional activity [15–18]. Sirtuin 1 induction of FGF21 expression in the fasting state is dependent on hydroxymethylglutaryl CoA synthase 2 (HMGCS2), a key regulator for ketone body biosynthesis [19].
FGF19 and FGF21 Signaling: Modulators of Metabolism
FGF15/19 and FGF21 both bind to FGF receptors (FGFRs). Binding of FGF15/19 and FGF21 to FGFRs is dependent on the cofactors αKlotho or βKlotho. FGFR signaling by FGF19 is dependent on αKlotho or βKlotho cofactors, while FGF21 signaling through FGFR is dependent on βKlotho [20]. In the liver, white adipose tissue (WAT) and brown adipose tissue (BAT), FGF21, directly interacts with FGFR1, FGFR2 and FGFR3 in the liver [21]. Activation of hepatic FXR by bile acids (cholic acid and chenodeoxycholic acid) and intestinal FGF15/19 may also upregulate gene expression and secretion of FGF21 in the liver [22]. The metabolic effects of FGF19 and FGF21 include the regulation of bile acid biosynthesis, glucose metabolism, and lipid metabolism in the beta cells of pancreatic islets, liver and adipose tissue. Both FGF15/19 and FGF21 exert their physiologic effects in an endocrine manner on distal tissues. Tissue targets of FGF15/19 include the liver, gallbladder, and brain [21]. Metabolic regulation by FGF21 occurs in insulin target tissues including liver, adipose, pancreas, skeletal muscle, and brain [23]. The metabolic improvements observed after bariatric surgery may be explained in part by understanding the tissue-specific activity of FGF15/19 and FGF21.
Metabolic Effects of FGF15/FGF19 (Fig. 1)
Fig. 1.
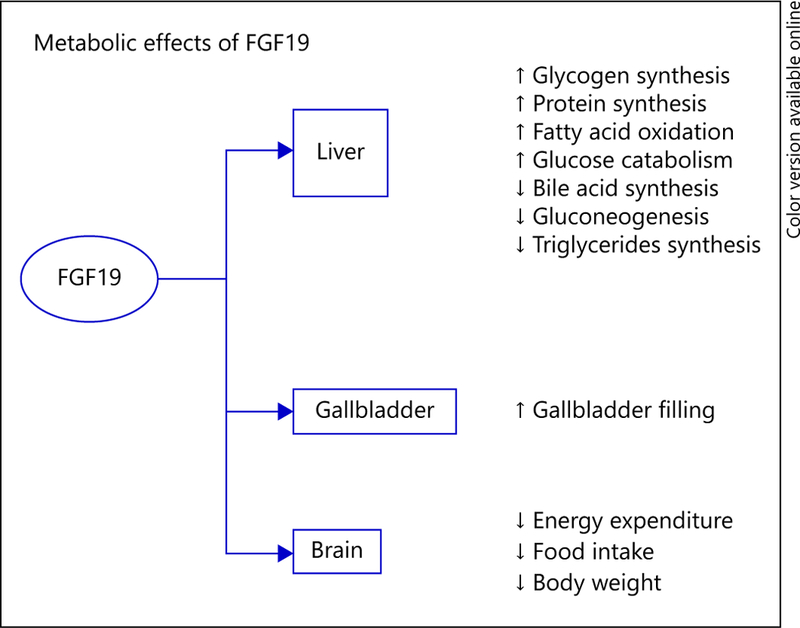
Tissue target functions of FGF19. FGF19 is a hormone produced in the intestine that exerts endocrine-like effects primarily in the liver, gallbladder, and brain. In the liver, FGF19 is involved in regulation of carbohydrate and lipid metabolism, in addition to protein synthesis. FGF19 regulates bile metabolism by increasing bile acid synthesis in the liver and subsequent storage of bile in the gallbladder. The effects of FGF19 in the brain include reducing food intake, in part reducing body weight.
Bile Acid Synthesis
FGF15/19 is released from the distal small intestine and enters the portal venous circulation to act on the liver and regulate hepatic bile acid synthesis. FGF15/19 inhibits the expression of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting step of bile acid biosynthesis through the activation of the transmembrane protein FGFR4, for which FGF15/19 has a high affinity [9, 11, 24]. Preoperative levels of FGF15/19 were lower in diabetic patients who had high CYP7A1 expression. Furthermore, FGF15/19 promotes the refilling of bile and relaxation of the gallbladder [25].
Hepatic Glycogen and Protein Synthesis, Gluconeogenesis
FGF15/19 has been proposed to regulate hepatic protein and glycogen metabolism in an insulin-independent manner [26, 27]. The peak in circulating FGF15/19 levels occurs much longer after insulin corresponds to elevations in bile acids [28]. The increase in FGF15/19 blocks hepatic gluconeogenesis by inhibiting the transcriptional activity of cAMP regulatory element-binding protein (CREB), a primary regulator of peroxisome proliferator-activated receptor γ coactivator protein-1α (PGC-1α) [28]. Alternatively, FGF15/19 demonstrated glucose-lowering effects and reduced gluconeogenesis by the downregulation of glucose 6-phosphatase gene expression after intracerebroventricular injection of FGF15/19 in ob/ob mice [27].
Fatty Acid Oxidation and Triglyceride Synthesis
The metabolic activity of FGF15/19 is similar to insulin; however, FGF15/19 does not induce lipogenesis through the suppression of sterol regulatory element– binding protein 1c (SREBP-1c) [29, 30]. SREBP-1c is the predominant transcription factor for key mediators of triglyceride synthesis and de novo lipogenesis including the rate-limiting enzyme for lipogenesis, acetyl-CoA carboxylase (Acc1 ), fatty acid synthase (Fas), and stearoyl-CoA desaturase 1 (Scd-1) [31]. In the liver, Acc1 dimerizes to form active polymers that convert citrate to malonyl-CoA. Fas then converts malonyl-CoA to palmitate, a saturated fatty acid (C16). The fatty acid elongase complex converts palmitate to stearate, another saturated fatty acid (C18), which is converted to oleate, a monounsaturated fatty acid, by Scd-1. Finally, the remaining product is stored as triglyceride. Additionally, FGF15/19 mediates its physiological effects in the liver and has been shown to increase β-oxidation by reducing the synthesis of malonyl-CoA, an inhibitor of carnitine palmitoyl-transferase 1a (CPT1a), the rate-limiting step of fatty acid oxidation [28].
Metabolic Effects of FGF21 (Fig. 2)
Fig. 2.
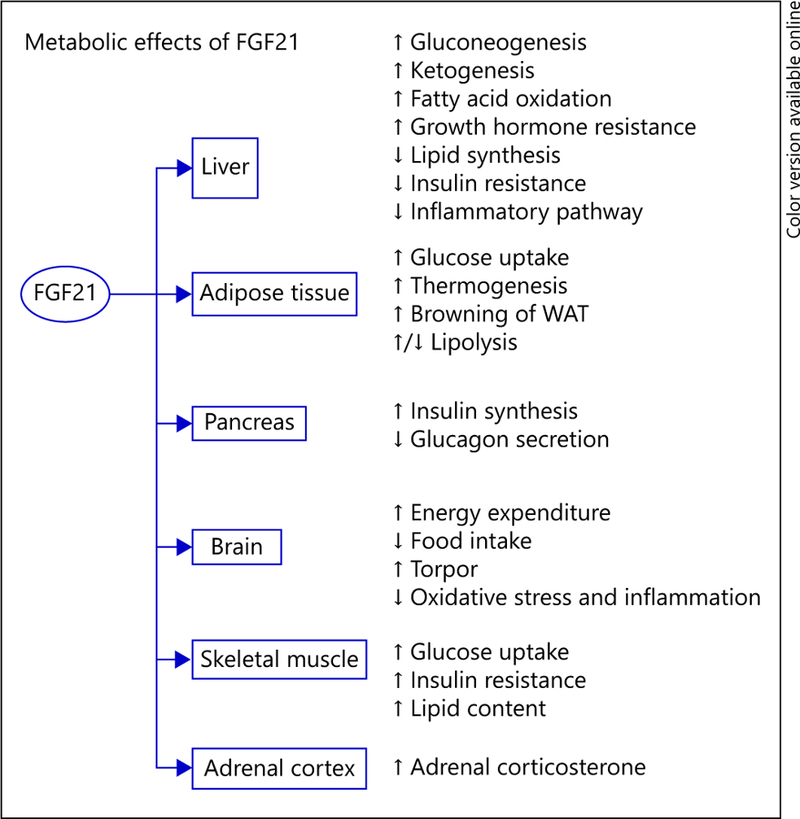
Tissue target functions of FGF21. FGF21, like FGF19, is a hormone that acts to alter a vast array of metabolic processes in an endocrine and autocrine manner. In the liver, FGF21 regulates carbohydrate and lipid metabolism. In adipose tissue, FGF21 induces thermogenesis and browning of WAT. FGF21 indirectly increases insulin synthesis in the pancreas. The hormone effects of FGF21 improve metabolic function, not only by promoting insulin sensitivity, but also reducing body weight by acting on the brain to decrease food intake and increase energy expenditure.
Fatty Acid Oxidation, Triglyceride Synthesis, and Gluconeogenesis
The regulation of FGF21 expression by FGF15/19 may also contribute to increase in fatty acid oxidation through the regulation of SREBP-1c, Acc1, Fas, and Scd-1 [21, 32]. Knockdown of FGF21 expression was associated with reduced capacity for β odixation as measured by a decrease in CPT1a and very long-chain acetyl-CoA dehydrogenase as well as long and medium acetyl-CoA dehydrogenase expression [21, 32]. Mice lacking FGF21 developed hepatic steatosis and had in creased expression of SREBP-1c, indicating increased triglyceride synthesis when fed with a ketogenic diet [33]. Global FGF21 knockout mice also have elevated circulating lipid levels, which indicate that FGF21 may play a role in triglyceride clearance as well as fatty acid oxidation and triglyceride synthesis [32]. FGF21 stimulates hepatic gluconeogenesis by direct effects on PGC-1α; however, the exact molecular mechanism is still uncertain [21].
Ketogenesis
An essential role of the liver during the adaptive fasting response is mediated, in part, by FGF21 signaling [32– 34]. During fasting, energy sources are shifted from carbohydrates to ketone bodies as lipids are mobilized from WAT to the liver. In the hepatocyte, fatty acids enter the mitochondrial through the carnitine shuttle regulated by CPT1a and are rapidly oxidized to produce acetyl-CoA, which can be converted to ketone bodies including aceto-acetate, β-hydroxybutyrate, and acetone by HMGCS2. Murine studies have described the stimulation of FGF21 secretion after low-carbohydrate ketogenic diet feeding. Mice deficient in both FGF21 and PPARα expression have impaired ketogenesis [33, 35]. Transgenic mice overexpressing FGF21 had markedly elevated levels of β-hydroxybutyrate [35]. Similarly, β-hydroxybutyrate was elevated after the administration of exogenous FGF21 to fed PPARα-deficient mice expressing low levels of FGF21 suggesting that FGF21 plays a central role in hepatic ketogenesis [35]. Protein levels of CPT1a and HMGCS2, both transcriptionally regulated by PPARα, were increased in FGF21 transgenic mice suggesting a posttranslational effect by which FGF21 regulates ketogenesis [35]. Mobilization of lipids from adipocytes occurs through the lipolysis of triglycerides, the storage form of fatty acids. Although the role of FGF21 in lipolysis is not well understood, FGF21 has been shown to attenuate lipolysis in human adipocytes and 3T3-L1 adipocytes by downregulating gene expression of lipid droplet-associated phosphoprotein perilipin [36].
Glucose Uptake
The role of FGF21 in maintaining glucose homeostasis has been well elucidated by both in vivo and in vitro studies. Serum FGF21 levels have been observed in obese individuals [37, 38]. However, recent insights may elude to the mitigating effects of FGF21 on insulin resistance [39]. Circulating levels FGF21 from the liver peak after fasting remain elevated during the early refeeding period [39]. Pharmacologic doses of exogenous FGF21 elevated glucose uptake in diabetic high-fat diet fed ob/ob mice and obese diabetic Zucker rats [40]. Transgenic FGF21 mice overexpressing the human FGF21 protein had less body weight and lower blood-glucose levels when compared to fed, wild type mice [40]. The mechanism by which FGF21 stimulates glucose uptake is by the upregulation gene expression of GLUT1 in adipocytes [40, 41].
Thermogenesis in Adipose Tissue
FGF21 directs metabolic effects in BAT through FGFR1 signaling. Alternatively, FGF21 is thought to be a key mediator in browning or beiging of WAT [42]. Increased energy expenditure through thermogenesis has been observed in FGF21 transgenic mice. Overexpression of FGF21 promoted an increase in brown adipocytes [40]. FGF21 regulates PGC-1α promoting browning of adipose tissue and increases gene expression of uncoupling protein 1 in WAT by directly activating CREB [21]. In both human and animal studies, the role of FGF21 in browning of WAT was demonstrated by cold-induced, non-shivering thermogenesis [42]. Additionally, FGF21 concentrations positively correlated with BAT activity upon cold-exposure [42].
Metabolic Regulation via the Brain-Liver Axis
During fasting, FGF21 is released from the liver and enters the brain through the blood-brain barrier to moderate its endocrine activity on neural pathways [43]. As previously stated, global FGF21 knockout mice exhibit impaired gluconeogenesis when compared to wild type mice establishing FGF21 as a key mediator in the adaptive fasting response [43]. One mechanism by which FGF21 regulates gluconeogenesis is through the hypothalamic-pituitary-adrenal axis by acting on hypothalamic neurons to activate the ERK1/2 pathway resulting in increased corticosterone levels in the brain as well as in the adrenal gland in response to adrenocorticotropic hormone [43]. Lastly, continuous intracerebroventricular infusion of FGF15/19 or FGF21 increases energy expenditure as indicated by increased metabolic rate and insulin sensitivity in diet-induced obese rats [12, 44, 45]
Conclusion
The long-term effects of bariatric surgery include weight loss, which decreases the risk for obesity-associated disease. However, the acute metabolic improvements including glycemic and lipid homeostasis observed after bariatric surgery are compelling and may be directed through the bile acid-FGF15/19-FGF21 pathways. Bariatric surgery induces FGF15/19 expression in the distal small intestine by the direct stimulation of FXR by bile acids. FGF15/19 acts in an endocrine-like manner to improve metabolic function on insulin target tissues. Additionally, FGF15/19 may induce hepatic FGF21 expression, which potentiates the beneficial effects on metabolic activity. Future directions to delineate the direct effects of FGF15/19 and FGF21 in reversing insulin resistance and reducing circulating triglyceride and glucose levels include therapeutic targeting of the FGF15/19 and FGF21 molecules.
Footnotes
Disclosure Statement
The co-authors, A.P. and F.H.K. have no information to disclose. Co-author R.K. is supported by a research grant from NGM Biopharmaceuticals, Inc., South San Francisco, Calif., USA.
References
- 1.Ogden CL, et al. : Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016; 315: 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellentani S, et al. : Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010; 28: 155–161. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap SR, et al. : Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013; 36: 2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troy S, et al. : Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008; 8: 201–211. [DOI] [PubMed] [Google Scholar]
- 5.Purnell JQ, et al. : Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016; 39: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumithran P, et al. : Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 7.Steinert RE, et al. : Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013; 21:E660–E668. [DOI] [PubMed] [Google Scholar]
- 8.Haluzíková D, et al. : Laparoscopic sleeve gas-trectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring) 2013; 21: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 9.Itoh N: Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res 2010; 342: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson E, et al. : Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002; 143: 1741–1747. [DOI] [PubMed] [Google Scholar]
- 11.Holt JA, et al. : Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 2003; 17: 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan KK, et al. : FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuipers F, Groen AK: FXR: the key to benefits in bariatric surgery? Nat Med 2014; 20: 337–338. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Infantes D, et al. : Circulating FGF19 and FGF21 surge in early infancy from infra-to supra-adult concentrations. Int J Obes (Lond) 2015; 39: 742–746. [DOI] [PubMed] [Google Scholar]
- 15.Berglund ED, et al. : Hepatic energy state is regulated by glucagon receptor signaling in mice. J Clin Invest 2009; 119: 2412–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglund ED, et al. : Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab 2010; 299:E607–E614. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Cuevas-Ramos D, et al. : Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 2012; 7:e38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cyphert HA, et al. : Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 2014; 9:e94996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilà-Brau A, et al. : Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line. J Biol Chem 2011; 286: 20423–20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, et al. : C-terminal tail of FGF19 determines its specificity toward Klotho co-receptors. J Biol Chem 2008; 283: 33304–33309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher FM, et al. : Integrated regulation of he-patic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 2011; 152: 2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Jadhav K, Zhang Y: Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol 2013; 86: 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, et al. : Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000; 1492: 203–206. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, et al. : Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005; 2: 217–225. [DOI] [PubMed] [Google Scholar]
- 25.Choi M, et al. : Identification of a hormonal basis for gallbladder filling. Nat Med 2006; 12: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 26.Kir S, et al. : FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011; 331: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton GJ, et al. : FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013; 123: 4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potthoff MJ, et al. : Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol 2013; 304:G371–G380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatnagar S, Damron HA, Hillgartner FB: Fi-broblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem 2009; 284: 10023–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. : The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Mol Cell Endocrinol 2011; 342: 41–47. [DOI] [PubMed] [Google Scholar]
- 31.Rui L: Energy metabolism in the liver. Compr Physiol 2014; 4: 177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badman MK, et al. : Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426–437. [DOI] [PubMed] [Google Scholar]
- 33.Badman MK, et al. : Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009; 150: 4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gälman C, et al. : The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–174. [DOI] [PubMed] [Google Scholar]
- 35.Inagaki T, et al. : Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415–425. [DOI] [PubMed] [Google Scholar]
- 36.Arner P, et al. : FGF21 attenuates lipolysis in human adipocytes – a possible link to improved insulin sensitivity. FEBS Lett 2008; 582: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 37.Gallego-Escuredo JM, et al. : Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond) 2015; 39: 121–129. [DOI] [PubMed] [Google Scholar]
- 38.Giannini C, et al. : Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab 2013; 98: 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markan KR, et al. : Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014; 63: 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kharitonenkov A, et al. : FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, et al. : Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009; 58: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanssen MJ, et al. : Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci Rep 2015; 5: 10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Q, et al. : FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014; 63: 4064–4075. [DOI] [PubMed] [Google Scholar]
- 44.Sarruf DA, et al. : Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 2010; 59: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen BM, Mangelsdorf DJ, Kliewer SA: Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab 2015; 26: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


