Abstract
Primary ciliary dyskinesia (PCD) is a genetic disorder in which impaired ciliary function leads to chronic airway disease. Exome sequencing of a PCD subject identified an apparent homozygous frameshift variant, c.887_890delTAAG (p.Val296Glyfs∗13), in exon 5; this frameshift introduces a stop codon in amino acid 308 of the growth arrest-specific protein 2-like 2 (GAS2L2). Further genetic screening of unrelated PCD subjects identified a second proband with a compound heterozygous variant carrying the identical frameshift variant and a large deletion (c.867_∗343+1207del; p.?) starting in exon 5. Both individuals had clinical features of PCD but normal ciliary axoneme structure. In this research, using human nasal cells, mouse models, and X.laevis embryos, we show that GAS2L2 is abundant at the apical surface of ciliated cells, where it localizes with basal bodies, basal feet, rootlets, and actin filaments. Cultured GAS2L2-deficient nasal epithelial cells from one of the affected individuals showed defects in ciliary orientation and had an asynchronous and hyperkinetic (GAS2L2-deficient = 19.8 Hz versus control = 15.8 Hz) ciliary-beat pattern. These results were recapitulated in Gas2l2−/− mouse tracheal epithelial cell (mTEC) cultures and in X. laevis embryos treated with Gas2l2 morpholinos. In mice, the absence of Gas2l2 caused neonatal death, and the conditional deletion of Gas2l2 impaired mucociliary clearance (MCC) and led to mucus accumulation. These results show that a pathogenic variant in GAS2L2 causes a genetic defect in ciliary orientation and impairs MCC and results in PCD.
Keywords: primary ciliary dyskinesia, PCD, GAS2L2, ciliary orientation, mucociliary clearance, MCC
Introduction
Primary ciliary dyskinesia (PCD) is a rare genetic disorder (MIM: 244400) in which dysfunction of motile cilia1 results in defective mucociliary clearance (MCC) and chronic airway disease. The clinical features of PCD include neonatal respiratory distress, bronchiectasis, chronic rhinosinusitis, low nasal nitric oxide (nNO) production, male infertility, and a spectrum of laterality defects.1, 2 Genetic variants in >40 genes have been identified in individuals with PCD,3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and ultrastructural cilia defects are observed in ∼70% of the cases;3 however, in many other cases of PCD, the genetic lesions and ciliary defect are still unknown.
The coordinated ciliary beat, with a specific frequency and waveform, directs fluid and foreign materials toward the mouth to be swallowed or expectorated.13 This coordination results from proper orientation and stabilization of cilia along the tissue axis.14, 15 Stabilization of basal bodies at the surface of ciliated cells provides the foundation for ciliary axoneme extension and the directionality of the ciliary beat.16 The direction of the ciliary beat is evidenced by the alignment of the basal-body basal foot16 or basal-body rootlet17 within individual cells. Mouse models with disrupted rootlets18 or basal feet19 suggest that the stabilization of basal bodies is essential for long-term maintenance of ciliary function. In PCD, the ciliary beat and waveform vary from absent to a range of uncoordinated motions,20, 21 impairing MCC and leading to the chronic airway disease that characterizes PCD.22, 23
We identified a PCD-causing apparent homozygous variant and a compound heterozygous variant in the growth-arrest-specific 2-like 2 (GAS2L2; [MIM: 611398], [GenBank: NM_139285.3, 17q12]) in two individuals with symptoms of PCD but with normal axoneme structure. GAS2L2 is a member of the GAS2 family, which includes GAS2, GAS2L1, GAS2L2, and GAS2L3.24, 25 The GAS2 family mediates crosstalk between actin filaments and microtubules (MT). They all contain a calponin homology (CH) domain (a putative actin binding site) and a GAS2-related (GAR) domain (a putative MT binding domain), but only the GAS2-like proteins contain a larger unstructured C terminus that contains the conserved microtubule-tip localization sequences Ser/Thr-Xaa-Ile/Leu-Pro (or SxIP motifs) necessary for interaction with microtubule plus-end-binding (EB) proteins.25, 26 The GAS2 [MIM: 602835] is expressed in many human tissues27 and is involved in the regulation of microfilament dynamics during both the cell cycle and apoptosis.28, 29 The overexpression of GAS2 is a hallmark in myeloid leukemia,30 and its absence has been related to infertility due to follicle growth impairment in mice.31 GAS2L1 [MIM: 602128] is also expressed in multiple human tissues.24 It localizes to the proximal end of mature centrioles and participates in centriole dynamics and centrosome disjunction,32 inhibits the growth of red blood cells downstream of thyroid receptor signaling,33 and is downregulated in myeloid leukemia.34 GAS2L3 [MIM: 617224] is expressed in many cell types.35 It is essential for brain morphogenesis and development36 and might play a role in tumorigenesis.37 GAS2L2 has six exons, encodes a 97 kDa protein, and is the least characterized member of the family. Previous studies showed that GAS2L2 localized with both actin stress fibers and microtubules and thereby contributed to different levels of actin-microtubule co-alignment.25 A separate study showed that the transfection of GAS2L2 into HEK293 cells stabilized the interaction of the A2A adenosine receptor with the Gsα subunit, increasing the cellular cAMP content.38 However, little is known about the localization and function of GAS2L2 in native tissues. We sought to determine the expression and localization of GAS2L2 specifically in airway cells, and its role in PCD development. In normal airway ciliated cells, GAS2L2 localizes throughout the cytoplasm but is abundant near basal bodies. In human and mouse airway cell cultures, the absence of GAS2L2 impaired ciliary orientation, and the ciliary beat was hyperactive and uncoordinated. Similarly, in X. laevis the absence of Gas2l2 disrupted cilia rotational polarity. Knockout of Gas2l2 in mice resulted in neonatal death. Adult Gas2l2-conditional-KO mice developed a PCD phenotype with impaired nasal MCC. These results demonstrate that defective ciliary orientation, resulting from a genetic variant in GAS2L2, causes PCD.
Material and Methods
Subjects
Individuals included in the study had a clinical diagnosis of PCD confirmed by standard clinical diagnostic criteria. For studies of affected individuals and their families, all individuals gave their signed and informed consent. All protocols involving human studies were approved by the University of North Carolina Medical School Institutional Review Board and the Ethics Review Board of the Comité de Protection des Personnes CPP Ile-de-France III (France) (approvals no. CPP07729 and CPP02748).
Genetic Analysis
Identification of GAS2L2 variants was performed either by whole-exome sequencing as previously described39 or by parallel sequencing with a custom targeted-capture panel encompassing 38 genes implicated in PCD (Table S1) and 250 candidate genes (SeqCap EZ Choice, Roche Diagnostics; details available on request). Copy-number-variation analysis was performed with a depth-ratio comparison between the individuals sequenced in the same run. Performing Sanger sequencing (Life Technologies) validated the genetic GAS2L2 variants found in the affected individuals. Segregation analysis was performed on the available DNA from family members (UNC-362). The primers used are listed in Table S2. More than 455 unrelated individuals suspected of having PCD were screened for biallelic genetic variants in GAS2L2 by various methodologies, including whole-exome sequencing, panel testing, and Sanger sequencing.
Airway Epithelial Cell Cultures
Human bronchial epithelial (HBE) cells were obtained from male and female, non-smoking donors lacking respiratory pathologies. The HBE cells were provided by the Cystic Fibrosis Center Tissue Procurement and Cell Culture Core under protocols approved by the University of North Carolina (UNC) Medical School Institutional Review Board. HBE cells were cultured at the air/liquid interphase (ALI) as previously described.40
Human nasal epithelial (HNE) cells from proband PCD-1367 and controls were obtained as described.41 The nasal cells were expanded as conditionally reprogrammed cells (CRCs).42 In brief, irradiated 3T3J2 fibroblasts were seeded on collagen-coated culture dishes 12 hr before coculture with HNE cells in CRC media supplemented with 5 μM of the Rho-associated kinase (ROCK) inhibitor (Y-27632, Sigma SCM075). To induce cell differentiation, we seeded cells in collagen-coated Millicell inserts (Millipore) and cultured at the air-liquid interface.
Mouse trachea epithelial cells (mTECs) were isolated according to established protocols.43 Tracheas were dissected from adult mice or 18.5 days post-coito (dpc) embryos. The cells were expanded and cultured as described above.
Reverse-Transcription Polymerase Chain Reaction
Semiquantitative reverse-transcription polymerase chain reaction (RT-PCR) for detection of GAS2L2 was performed via standard protocols. In brief, total RNA from human tissues was obtained from Takara Bio (Takara Bio). Total RNA from wild-type mouse tissues and from airway epithelial cell cultures (human and mouse) were isolated with an RNeasy kit (QIAGEN) according to the manufacturer’s protocols. First-strand cDNA was synthesized with SuperScript II Reverse Transcriptase (Thermo Fisher cat.# 18064014). PCR was performed with PrimeSTAR HS DNA Polymerase (Takara Bio) according to the manufacturer’s protocols. For the normalization of RT-PCR analysis of human tissues, PPIA (cyclophilin A) was used as a reference44 and DNAI1 as a control of ciliogenesis progression. RT-PCR analysis of mouse tissues was normalized with TATA box protein (Tbp) and β-actin (Actb).45 The primers used are listed in Table S3.
Immunofluorescence and Co-localization Analysis
Immunofluorescence was performed as described previously.46 For whole-mount staining, ALI cultures were washed with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized with 0.2% Triton X-100 in Tris-buffered saline for 30 min, and then incubated for 1 hr at room temperature in blocking solution (1% BSA, 1% fish gelatin, 0.1% Triton X-100, and 5% fetal bovine serum). After primary antibody incubation, antibody binding was detected with secondary antibodies conjugated with Alexa Fluor-488, Alexa Fluor-647 (Life Technologies), indocarbocyanine- (CY3), or Rhodamine Red-X (RRX) (Jackson ImmunoResearch Laboratories). Actin filaments were stained with phalloidin conjugated with Alexa Fluor 488 (Life Technologies A12379). For DNA staining, Hoechst 33342 trihydrochloride, trihydrate-FluoroPure (Life Technologies) was used. No detectable staining was observed for isotype-matched control antibodies. Membranes were mounted on slides with ProLong Diamond antifade mountant (Thermo Fisher). The antibodies used are listed in Table S4. Whole-mount ALI cultures were imaged with a Leica SP8 inverted confocal or a Nikon N-SIM microscope. Single cells were imaged with a Nikon N-SIM microscopy system. Images were processed and analyzed with FIJI.47 For co-localization analysis, single cells were embedded in 100 mM β-mercaptoethylamine (Sigma) for ground-state depletion (GSD) super-resolution microscopy (Leica). So that Pearson coefficients (r: values between −1 to 1; where −1 = inverse association and 1 = full association) and Manders coefficients for magenta (M1) and green (M2) channels (M1 and M2; values vary from 0–1, where 0 = no overlapping and 1 = 100% co-localization)48 could be obtained, images were analyzed with the Coloc2 plugin of ImageJ49.
Immunoblots and Targeted Proteomics Analysis
Human nasal epithelial cells were lysed in RIPA buffer (Thermofisher) supplemented with protease inhibitor cocktail (Sigma). Protein concentration was determined via the BCA method (Pierce BCA kit, ThermoFisher Scientific). The samples (20 μg) were electrophoresed on NuPage 4–12% Bis-Tris gels in MES-SDS running buffer (ThermoFisher Scientific) and transferred to 0.45 μm nitro-cellulose membrane for immunoblotting. Targeted proteomics is described in the Supplemental Data. The antibodies used are listed in Table S4.
Analysis of Ciliary Function
Ciliary beat frequency was measured as previously described.50 In brief, three to four cultures were individually visualized with a Nikon Eclipse TE2000 inverted microscope with phase optics and a 20× objective (NA = 0.45). The temperature was maintained at 37°C via a Tokai HIT controller (model INU-TIZ-F1) and a microscope stage-heater block. High-speed videos (60 frames/s) were recorded with a Basler acA1300-200um camera controlled by SAVA software (Ammons Engineering) and were analyzed via SAVA whole-field analysis. For evaluation of the waveform and beat direction, high-resolution videos of ciliated cells were recorded at 200 fps with a 60× Plan-Apo oil objective lens (NA = 1.4), DIC optics, and ambient temperature. Analysis of videos was conducted by investigators blinded to the cells’ genotype. Videos were replayed in slow motion (1/7 of the true speed). For waveform analysis, three cilia were traced manually at the end recovery and end effective positions in four ciliated cells from each genotype. To evaluate the direction of the ciliary beat, we manually traced the tip positions of four to five individual cilia, and the orientation of the ciliary beat was represented in a two-dimensional plot.
Transmission Electron Microscopy and Scoring of Ciliary Orientation
Fixed cultures were sectioned (1 μm) and stained.51 From three biological replicates, 10 to 15 images of individual cells were taken with a Zeiss 900 TEM at 10,000×. So that ciliary orientation could be scored, the angle (0°–360°) between the center of the basal body to the tip of the basal foot was determined with ImageJ. The length of the mean vector (r)52 and the average of the length of the mean vector (R) for each genotype were calculated. The mean vector lengths were plotted with Oriana 4. The R values were plotted with GraphPad Prism 7.1 and compared via a Student’s t test.
Transgenic Gas2l2 Mice
All protocols were approved by the University of North Carolina at Chapel Hill (UNC) Institutional Animal Care and Use Committee (IACUC). A knockout-first Gas2l2tm1a(KOMP)Wts/i/+ (Gas2l2+/−) mouse in a C57BL/6N; C57BL/6N-Atm1Brd/a background was acquired from the Wellcome Trust Sanger Institute knockout mouse project (KOMP).53 After several unsuccessful attempts at breeding, the mouse was euthanized, and in vitro fertilization was used for generation of the Gas2l2+/− line in the same genetic background. Gas2l2fl/fl mice were generated from a cross of Gas2l2+/− mice to a Rosa26::FLPe mouse (Jackson Laboratory stock no.: 003946) so that the lacZ and neo cassettes were removed. The Gas2l2fl/fl mice therefore contain loxP sites flanking exon 2 of Gas2l2. Tamoxifen inducible Gas2l2 conditional-knockout (Gas2l2 conditional-KO) mice were obtained by crossing Gas2l2fl/fl mice to RosaCreER mice (Jackson Laboratory stock no.: 004847) or to Foxj1CreERT2::GFP/+ mice (Jackson Laboratory stock no.: 027012).54 Treatment with tamoxifen activates Cre and causes the deletion of exon 2. Primers used for genotyping are listed in Table S3.
Tamoxifen Treatment and CT-Scan Analysis
For all studies, Gas2l2fl/fl:Foxj1CreERT2::GFP/+ (Gas2l2fl/fl:Foxj1CreERT2) and control animals were treated with tamoxifen55 (dissolved in pure corn oil at 20 mg/mL; Sigma-Aldrich). Animals received three intraperitoneal injections of tamoxifen (75 mg/kg body weight) every other day so that the initial deletion of Gas2l2 would be induced, and thereafter they were maintained on a tamoxifen-supplemented diet (Envigo TD.130860). In most experiments, Gas2l2 conditional-KO animals were compared to litter mate control mice of genotypes Gas2l2fl/fl or Gas2l2fl/+:Foxj1CreERT2. For evaluation of the development and progression of the PCD phenotype, the nasal cavity of the mice was scanned by micro-computed tomography (micro-CT) four times, starting at the end of the last tamoxifen injection (baseline) and repeating every 30 days. Micro-CT scans were analyzed with Mimics Research 18.0, and the volume of the nasal air space (V) was calculated. The change in nasal air space (ΔV) was calculated by subtracting the baseline nasal air space (vbaseline) from the volume measured at 90 days (V90).
Measurement of MCC
For measurement of MCC in the nasal cavity and in the anterior nasopharynx, the clearance of radiolabeled 99mtechnetium colloid particles56 or dry green-fluorescent beads,57 respectively, was recorded. Analyses were performed using ImageJ48 and Mosaic plugin.58
Histology of the Nasal Cavity
After measurement of MCC, the heads of the mice were fixed and processed as previously described.59 Coronal sections of the nasal cavity were stained with hematoxylin and eosin (H&E) and with alcian blue and periodic acid Schiff (AB-PAS) stain so that mucus accumulation could be visualized. For comparisons between genotypes, the samples were screened for the presence of mucus and for degeneration of the epithelium. The presence of mucus was scored from one to four as previously described.59 Images were taken with an Olympus VS120 virtual slide scanner microscope.
Xenopus laevis Embryo Manipulation and Analysis
Xenopus embryos were obtained by in vitro fertilization according to standard procedures.60 All protocols were approved by the UNC IACUC committee. The X. laevis genome contains two largely identical Gas2l2 copies that have different start codons. Two separate morpholinos (Table S3) targeting the start codon of each isoform were designed on the basis of the sequences from the NIBB database and obtained from Gene Tools. To deplete both isoforms simultaneously, Gas2l2 morpholinos (Gas2l2-MO) were injected together at 20-60 ng/blastomere into the two ventral cells at the four-cell stage so the ciliated epidermis was targeted. Coinjection of Centrin 4-RFP mRNA (Unigene ID: Xl.50473) and Clamp-GFP mRNA (Unigene ID: Xl.26316) labeled basal bodies and ciliary rootlets, respectively. For immunostaining and confocal imaging analysis, X. laevis embryos at stage 28 were processed as previously described.61 Antibodies anti-γ-tubulin and anti-Gas2l2 were the same as described above. All imaging of X. laevis ciliated cells was performed on a Nikon A1R laser scanning confocal microscope with a 60× oil plan-Apo objective lens (1.4 NA). Cilia orientation was scored as previously described.62 In brief, individual rootlet orientation was scored manually by measurement of the angle of orientation of the rootlets relative to the anterior–posterior axis of the embryo. Mean vector-length calculations and graphical representation of cilia orientation via circular plots were performed with Oriana 4.02 software.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD) of n experiments. In these studies, we used the following statistical methods: two-tailed Student’s t test with Welch’s correction, Paired t test, one-way ANOVA with Tukey’s multiple comparisons, chi-square test, and Pearson’s correlation test. Figure legends specify the statistical method used for each experiment. P values less than 0.05 were considered significant.
Results
Discovery of Genetic Variants in GAS2L2
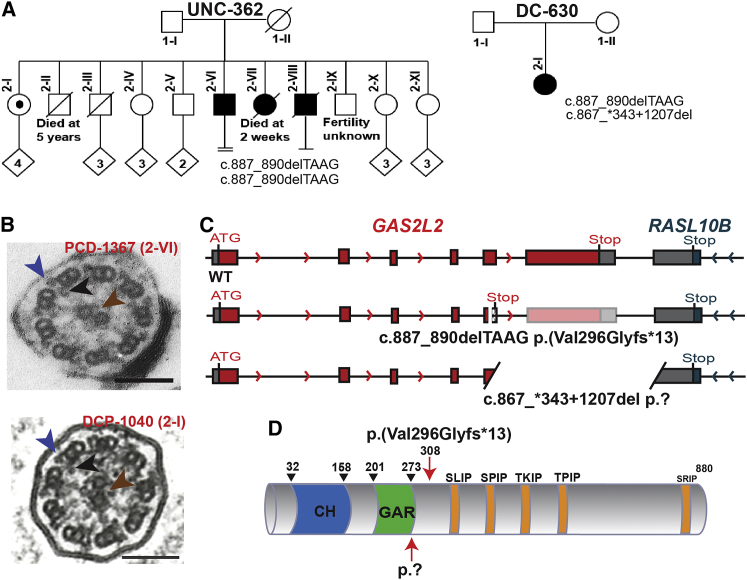
Although genetic variants in more than 40 genes have been identified as causes of PCD,1, 4, 5, 6, 7, 8 in many cases of PCD the genetic lesion has not yet been identified. To uncover additional variants that cause PCD, we performed whole-exome sequencing of a subject with clinical features consistent with PCD (Table S5). We identified an apparent homozygous frameshift variant, c.887_890delTAAG (p.Val296Glyfs∗13) (dbSNP: rs587633197) in exon 5 of GAS2L2 (GenBank: NM_139285.3) (Figures 1A and S1A). Proband PCD-1367 from family UNC-362 (2-VI) was diagnosed at the age of 56 years. DNA from the parents was not available; however, subject 2-I is a heterozygous carrier, and the family history suggested that two other siblings were affected by PCD (Figure 1A); these included a sister who had neonatal respiratory distress and severe hypoxemia and died at 2 weeks and a brother who had a daily cough and bronchiectasis and underwent a lobectomy. During the course of this research, a second individual with a compound heterozygote genetic variant in GAS2L2 was identified (Figure 1A). Proband DCP-1040 (2-I) from family DC-630 carries the same c.887_890delTAAG (p. Val296Glyfs∗13) frameshift variant and a large deletion (c.867_∗343+1207del p.?) (LOVD: 0000439768] starting in exon 5 (Figure S1B). DNA from the parents was not available, and they had no report of upper or lower chronic respiratory disease. The location of the two deletions that proband DCP-1040 carries indicates that she is an obligatory compound heterozygote. Indeed, within GAS2L2 exon 5, the 5′ breakpoint of the large deletion c.867_∗343+1207del (with a 3′ breakpoint in the adjacent gene RASL10B and, therefore, encompassing GAS2L2 exon 6) is upstream of the small four-nucleotide deletion c.887_890del, also located in exon 5. Next-generation sequencing (NGS) data (Figure S2A) highlights the absence of a normal allele for exon 5 because reads either include c.867_∗343+1207del or c.887_890del. Additionally, copy-number analysis based on NGS read depth (Figure S2B) confirmed the heterozygous deletion of exon 6 and the partial heterozygous deletion of exon 5. Both individuals have similar clinical phenotypes, including bronchiectasis, otitis media, and rhinosinusitis (Table S5). However, the ultrastructure of the ciliary axoneme was normal (Figure 1B), and one of the individuals had normal levels of nasal NO (nNO = 342.6 nL/min, cutoff 77 nL/min)63 (Table S5). The GAS2L2 genetic variants that we identified in the PCD-affected families (Figure 1C) are both predicted to result in the absence of protein production as a result of immature full-length transcripts and/or nonsense-mediated mRNA decay. GAS2L2 possesses a GAS2-related (GAR) domain for interacting with microtubules and a calponin homology (CH) domain for interacting with actin filaments.24, 25 Interestingly, GAS2L2 has a long C terminus with five putative SxIP sites for interaction with microtubule-end-binding (EB) proteins (Figure 1D). The frameshift variant induces a stop codon in amino acid 308, after the microtubule-binding domain (Figure 1D). The large deletion (3,931 nucleotides) might disrupt the putative protein after the GAR domain (Figure 1D). Because of these findings, we sought to determine the expression and localization of GAS2L2 specifically in airway cells and its possible role in PCD development.
Figure 1.
GAS2L2 Pathogenic Variants in Individuals with PCD
(A) Segregation analysis of GAS2L2 genetic variants found in family UNC-362 (c.887_890delTAAG [p. Val296Glyfs∗13]) and in family DC-630 (c.887_890delTAAG;867_∗343+1207del [p.Val296Glyfs∗13;?]). Filled symbols indicate PCD-affected individuals. In family UNC-362, individual 2-I is a heterozygous carrier, individual 2-II died at age 5 years as a result of a ruptured appendicitis. Individual 2-III was a lifelong smoker and died of lung cancer at ∼55 years. Individual 2-VI is proband PCD-1367. Individual 2-VII was a term neonate suspected to have had PCD as a result of neonatal respiratory distress and severe hypoxemia. She died at 2 weeks. Individual 2-VIII is suspected to have had PCD as a result of phenotypic features including daily cough and bronchiectasis at age 10 years with subsequent lobectomy. He passed away from lymphoma at 50+ years.
(B) A transmission electron micrograph of axonemal cross sections of nasal epithelium from both probands shows the central pair (brown arrowhead) surrounded by nine microtubule doublets. Outer dynein arms (blue arrowhead) and inner dynein arms (black arrowhead) project from each doublet normally.
(C) Schematic representation of the GAS2L2 variants found in the PCD-affected individuals. The top panel shows normal exon-intron structure, the middle panel shows the small four-nucleotide deletion (c.887_890del) that introduces an early stop codon, and the bottom panel shows the large deletion (c.867_∗343+1207del) that is also located in exon 5. Red boxes indicate the coding exons, patterned boxes indicate out-of-frame sequence, and gray boxes indicate the 5′ and 3′ UTRs.
(D) Schematic representation of GAS2L2 structure. It contains a calponin homology (CH, blue) domain and a GAS2-related domain (GAR, green), which might mediate binding to the actin cytoskeleton and MT, respectively. Conserved SxIP motifs for interaction with EB proteins are indicated in orange. The location of the mutations is indicated by the red arrows. The small deletion induces a frameshift variant that introduces a stop codon in amino acid 308. The large deletion disrupts the protein after the GAR domain.
GAS2L2 Is a Ciliated-Cell-Specific Protein
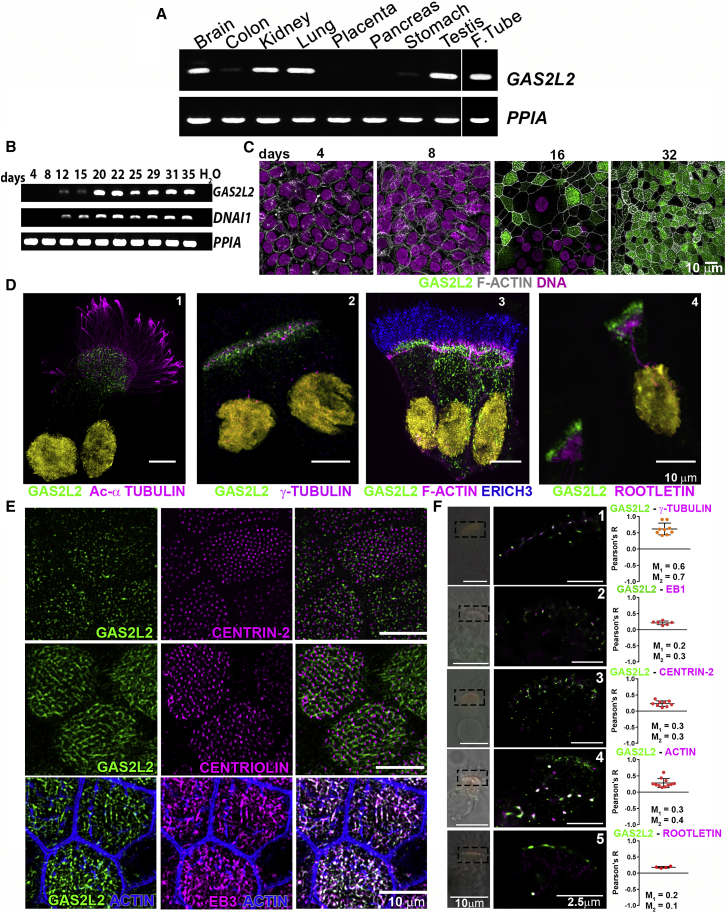
In control human tissues, GAS2L2 transcript was abundant in tissues with motile cilia; including brain, lung, testis, and fallopian tube. GAS2L2 expression was also detected in kidney tissue, and less abundantly in stomach and colon tissue (Figure 2A). To determine the expression and localization of GAS2L2 during HBE cell differentiation, we used RT-PCR and immunofluorescence, respectively (Figures 2B and 2C). The expression of GAS2L2 was not detected in undifferentiated cells. By day 12 of ALI culture (Figure 2B), we observed a weak GAS2L2 signal correlating with the expression of DNAI1 [MIM: 604366]. At later time points, GAS2L2 was strongly expressed in a similar pattern to that of DNAI1 (Figure 2B), and the protein was abundant in the cytoplasm of HBE cells (Figure 2C). In single-cell immunostaining, GAS2L2 strongly localized at the base of the cilia (Figure 2D-1), close to γ-tubulin (a basal body marker, Figure 2D-2). Also, GAS2L2 was detected in the cytoplasm of ciliated cells, and it partially localized with actin filaments (Figure 2D-3) and rootletin (a rootlet marker; Figure 2D-4). In whole-mount cultures (Figure 2E), we confirmed the cytoplasmic localization of GAS2L2 with centrin-2 (Figure 2E, top panel) and centriolin (basal feet marker, Figure 2E, middle panel) at the base of the cilia. GAS2L2 also localized with EB3 (Figure 2E, bottom panel), an interaction previously described.25 GAS2L2 was not detected in secretory cells labeled with CC10 and MUC5B (Figures S3A and S3B). Co-localization analysis showed that GAS2L2 co-localizes with γ-tubulin (Pearson’s coefficient, r = 0.62 ± 0.18; Manders coefficients, M1 = 0.6, M2 = 0.7; Figure 2F-1) and partially co-localizes with EB1 (r = 0.21 ± 0.06; M1 = 0.2, M2 = 0.3, Figure 2F-2), centrin-2 (r = 0.23 ± 0.08; M1 = 0.3, M2 = 0.3; Figure 2F-3), and actin (r = 0.28 ± 0.14; M1 = 0.3, M2 = 0.4; Figure 2F-4) but not with rootletin (r = 0.18 ± 0.02; M1 = 0.2 and M2 = 0.1; Figure 2F-5). These results suggest that GAS2L2 interacts closely with proteins present at the basal body.
Figure 2.
Expression and Localization of GAS2L2
(A) Total RNA from different human tissues was used for determining the expression of GAS2L2. PPIA was used as a reference.
(B) Expression of GAS2L2 in normal HBE cells. Total RNA was extracted at the different indicated days of ALI culture. The expression of GAS2L2 correlated with the expression of DNAI1, a ciliated-cell-specific gene. PPIA was used as a reference.
(C) Whole-mount immunofluorescence. HBE cultures were fixed at different days during the differentiation of airway cells and stained for actin filaments (gray) and GAS2L2 (green), and nuclei (magenta) were labeled with Hoechst 33342. GAS2L2 was not detected in undifferentiated cells (days four through eight). It was observed in the cytoplasm of HBE cells (day 16), and it was strongly present in fully differentiated cultures (day 32).
(D) Isolated cell immunofluorescence. Cells were stained for GAS2L2 in green and in magenta: (1) the ciliary axoneme marker Acetylated-αtubulin; (2) basal-body marker γ-tubulin; (3) actin filaments and ERICH3 (blue), a ciliary axoneme marker; and (4) the rootlet marker rootletin. Nuclei (yellow) were labeled with Hoechst 33342.
(E) Whole-mount culture immunofluorescence. GAS2L2 is closely associated with centrin-2 (top panel) and centriolin (middle panel). GAS2L2 strongly localizes with EB3 (bottom panel).
(F) Single-molecule detection using GSD super-resolution microscopy showing representative images of cells stained with GAS2L2 (green) and in magenta: (1) γ-tubulin, (2) EB1, (3) centrin-2, (4) actin, and (5) rootletin. The boxed area in the bright-field image was scanned in GSD mode. Pearson coefficients for each analyzed cell are represented in the graph (n ≥ 4). The average Manders coefficient for GAS2L2 (M1) and the partner protein (M2) are indicated.
The Loss of GAS2L2 Disrupts Cilia Beat Frequency and Orientation
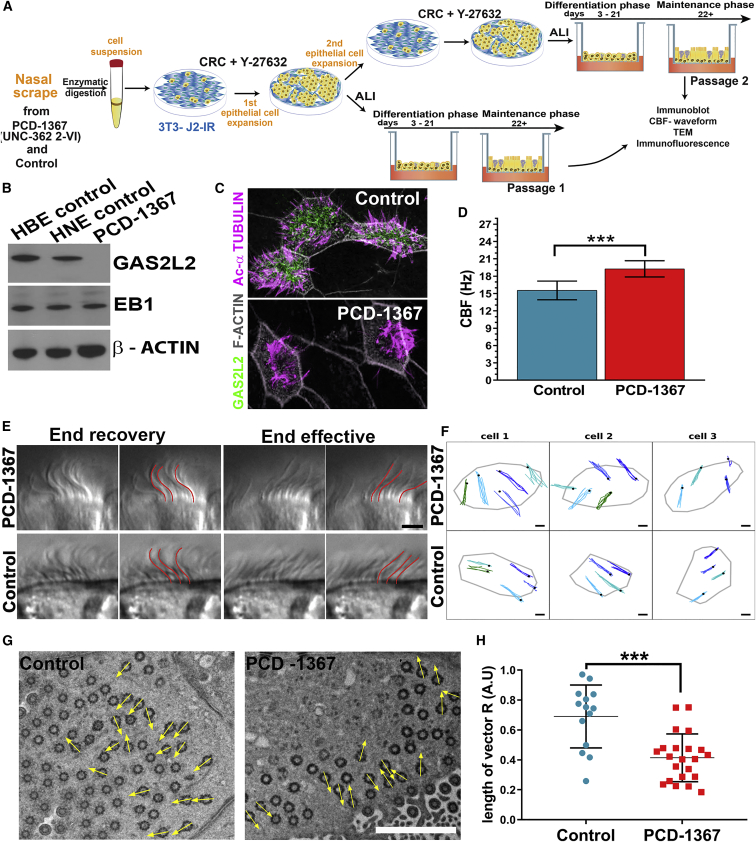
To investigate the functional role of GAS2L2 in ciliated cells, we cultured HNE cells from the proband PCD-1367 (GAS2L2-deficient) and control cells and expanded them as CRCs.42 (Figure 3A). The screening of GAS2L2-deficient cells by immunoblot and immunofluorescence showed complete absence of GAS2L2 (Figures 3B and 3C). Also, a truncated form of the protein was not detected by targeted proteomics (Figure S4A and Table S6; see also Supplemental Methods). In PCD-1367 cells, the transcript of GAS2L2 was reduced (Figure S4B), possibly as a result of nonsense-mediated decay of the mRNA. The ciliary beat frequency (CBF) was significantly faster in GAS2L2-deficient (19.8 ± 0.2 Hz) cells than in control cells (15.8 ± 0.1 Hz; p = 0.0002; Figure 3D). Although the waveform appeared normal in ciliated cells from proband PCD-1367 (Figure 3E, additional traces are shown in Figure S5 and Videos S1 and S2), the direction of the ciliary beat appeared to be randomized (Figure 3F, top panel; also Video S3) in comparison to control cells (Figure 3F, bottom panel; also Video S4). To further analyze the coordination of the ciliary beat, we measured the orientation of the basal feet in individual ciliated cells. In cell culture, the direction of the ciliary beat varies between cells, but it is well coordinated within individual cells; however, in GAS2L2-deficient cells the alignment of the basal feet was significantly randomized (Figure 3G). The length of the mean vector (r) was shorter in the GAS2L2-deficient cells (r = 0.05) than in control cells (r = 0.469). This was further reflected by a low average length of the mean vector (R) for GAS2L2-deficient cells compared to control cells (0.41 + 0.034 versus 0.65 + 0.065; p < 0.001, Figure 3H). These results demonstrate that the absence of GAS2L2 in human airway ciliated cells causes a ciliary orientation defect and affects the performance of the cilia.
Figure 3.
The Absence of GAS2L2 Causes a Defective Ciliary Beat
(A) Nasal epithelial cells from individual PCD-1367 (family UNC-362, 2-VI) and a control were dissociated into single cells. The HNE cells were conditionally reprogramed and expanded by co-culture with irradiated 3T3J2 fibroblasts for one to two passages in the presence of Y-27632, a selective inhibitor of Rho-associated protein kinase (ROCK). After expansion, we cultured the HNE cells at the ALI to conduct multiple assays.
(B and C) The absence of GAS2L2 in PCD-1367 was confirmed by immunoblot and immunofluorescence.
(D) The CBF in GAS2L2-deficient cells was hyperkinetic in comparison to controls (n = 6; data are represented as the mean + SD; Student’s t test, ∗∗∗p = 0.0002).
(E) High-resolution videos of ciliated cells were observed and showed a normal waveform pattern of ciliary beat in control and GAS2L2-deficient cells. The images represent a time point of the cilia seen in profile at end recovery (left panels) and end effective positions (right panels). For each position, a panel showing the manual tracing of the cilia (highlighted in red) is shown. (n = 4; the scale bar represents 4 μm).
(F) Direction of ciliary beat. Four to five cilia were tracked manually in three individual cells. The black dot represents the position at the end recovery phase. The direction of the ciliary beat was plotted on an X-Y graph (the scale bar represents 2 μm).
(G) Representative electron micrograph showing the analysis of alignment of the basal body and basal foot in control cells (n = 14) and GAS2L2-deficient cells (n = 22). The arrows in each micrograph are pointing in the direction of the basal foot tip (the scale bar represents 2 μm).
(H) The length of the vector (0 < R < 1) for each cell is represented in the graph. The average length of the vector was significantly shorter in GAS2L2-deficient nasal ciliated cells than in control cells (data are expressed as the mean + SD; Student’s t test, ∗∗∗p = 0.0004).
The video was recorded at 200 fps with a 60× objective lens with DIC optics. Playback speed is 15% of normal speed. The scale bar represents 4 μm.
the video was recorded at 200 fps with a 60× objective lens with DIC optics. Playback speed is 15% of normal speed. The scale bar represents 4 μm.
The video matches exactly cell 2 shown in Figure 3F (top panel, middle) of the main document. Light gray areas in the video are areas that extended beyond the original frames. The playback speed is 15% of the original 200 frames per second. The scale bar represents 2 μm.
The video matches exactly cell 2 (arrow) shown in Figure 3F (bottom panel, middle) of the main document. Light gray areas in the video are areas that extended beyond the original frames. The playback speed is 15% of the original 200 frames per second. The scale bar represents 2 μm.
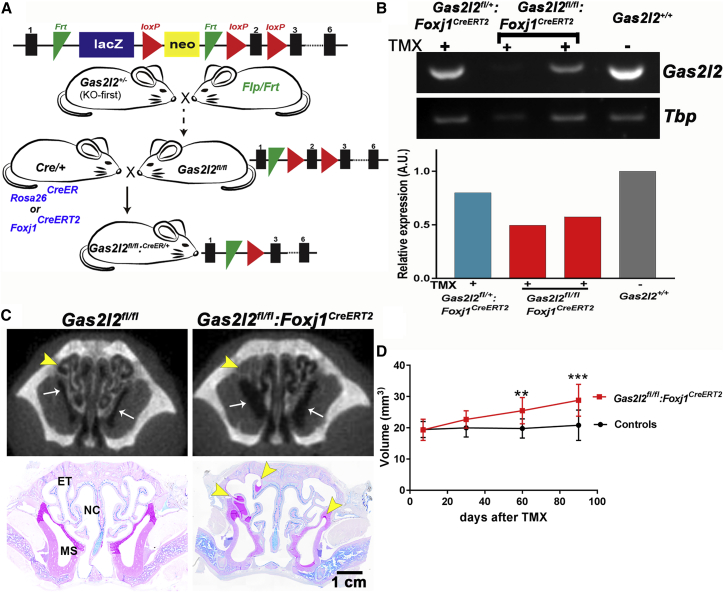
Deletion of Gas2l2 Causes Neonatal Lethality in a Mouse Model
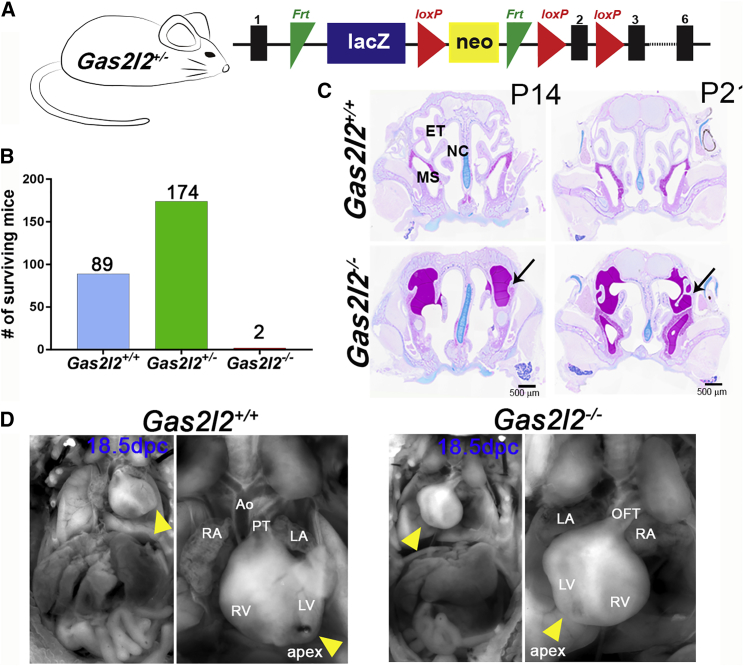
To analyze the function of GAS2L2 in vivo, we used a Gas2l2-knockout-first mouse (Gas2l2tm1a(KOMP)Wtsi or Gas2l2+/−) (Figure 4A). More than 90% of the Gas2l2+/− mice survived, and they had no signs of a PCD phenotype. Because in mouse, as in humans, the localization of Gas2l2 expression is largely unknown, we took advantage of the presence of the lacZ reporter in Gas2l2+/− mice and detected β-galactosidase activity in tissues with motile cilia; such tissues included those of the brain, nasal septum, trachea, lungs, and testis (Figure S6A). RT-PCR corroborated these results (Figure S6B). Matings between heterozygous Gas2l2+/− mice resulted in a high incidence of neonatal death (∼29%). Of the 265 mice that survived, only two were Gas2l2−/− (Figure 4B). These mice showed signs of hydrocephalus at 14 and 21 days of age. Histology of the head (Figure 4C) showed mucus accumulation in multiple sinuses and remodeling of the nasal cavity, a typical phenotype of murine PCD models (e.g., Dnaic1−/− mice).59 To evaluate inheritance ratios, we genotyped 18.5 dpc embryos; ∼13% (6/48) were homozygous (x2obs = 6.00, with 2 degrees of freedom; p = 0.05; Figure S6C), consistent with Mendelian distribution. Of the six Gas2l2−/− embryos collected, three showed dextrocardia (Figure 4D), suggesting that Gas2l2 is expressed in ciliated cells at the node and might play a role in situs determination.
Figure 4.
Deletion of Gas2l2 In Vivo Causes a Lethal Trait in Mice
(A) The knockout-first allele contains an IRES:lacZ and a promoter-driven neo cassette inserted into an intron of Gas2l2, disrupting Gas2l2 expression.53 See Material and Methods for details.
(B) From the Gas2l2+/− × Gas2l2+/− crosses, 265 mice survived; of these, two were homozygous.
(C) Histological head sections of the two Gas2l2−/− and control mice at P14 and P21. The heads were stained with AB-PAS, which revealed severe mucus accumulation in the sinuses of both Gas2l2−/− mice (arrows). Abbreviations are as follows: ET, ethmoturbinates; MS, maxillary sinus; and NC, nasal cavity.
(D) Situs ambiguous was observed in three out of six Gas2l2−/− mouse embryos dissected at 18.5 dpc. Dextrocardia was clearly observed as the apex of the heart (yellow arrow head) was oriented to the right side of the body axis. Abbreviations are as follows: RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium; Ao, Aorta; PT, pulmonary trunk; and OFT, out-flow tract.
Deletion of Gas2l2 in mTEC Cultures and in X. laevis Embryos Recapitulates the Ciliary Phenotype Observed in Humans
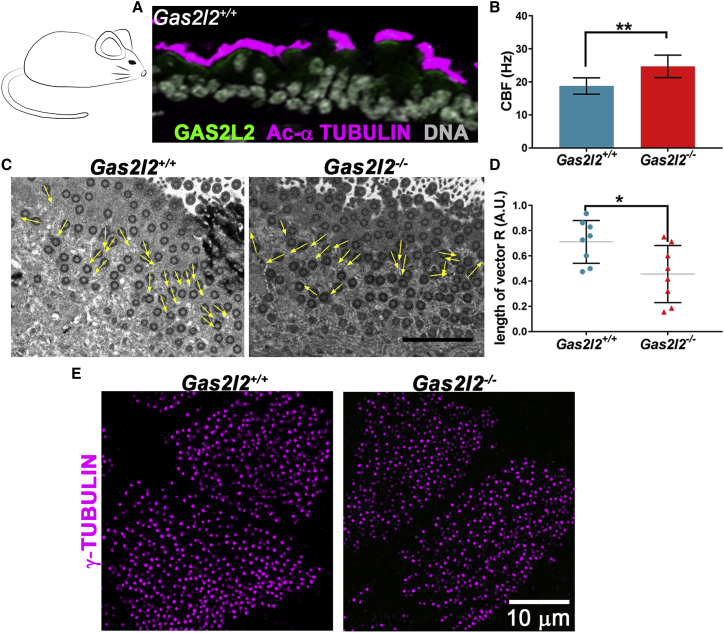
To further investigate the role of GAS2L2 in ciliary function, we used two vertebrate models: the knockout mouse and X. laevis embryos. In wild-type cells from both species, GAS2L2 localized at the base of the cilia, near the basal bodies (Figures 5A and 6A). To investigate the effects of Gas2l2 deletion, we cultured mTEC isolated from embryonic tracheas. We pooled tracheas of the same genotype, digested them to obtain a single cell suspension, expanded them as CRC cells, and then cultured them at an ALI until they were differentiated. The absence of Gas2l2 was confirmed by RT-PCR (Figure S7A). The CBF of Gas2l2−/− mTEC was hyperkinetic (24.71 ± 1.28 Hz) in comparison to controls (Gas2l2+/+ = 18.77 ± 0.94 Hz, Figure 5B and Gas2l2+/− = 20.57 ± 0.80 Hz; data not shown). We observed a lack of alignment of the basal feet within individual Gas2l2−/− cells (r = 0.40; R = 0.46 ± 0.08) compared to Gas2l2+/+ cells (r = 0.60; R = 0.71 ± 0.06, Figures 5C and 5D) and Gas2l2+/− cells (r = 0.51; R = 0.76 ± 0.036; data not shown). The distribution and spacing of the basal bodies were similar between the Gas2l2−/− and the wild-type cells (Figure 5E).
Figure 5.
Absence of GAS2L2 in Mouse Tracheal Ciliated Cells Affects CBF and Ciliary Orientation
(A) Detection of GAS2L2 in tracheal ciliated cells from a wild-type mouse by immunofluorescence. Tracheal sections were stained for GAS2L2 (green) and Acetylated-α tubulin (magenta). Nuclei (gray) were labeled with Hoechst 33342.
(B) The CBF in Gas2l2−/− mTEC cells was hyperkinetic in comparison to control (n = 9; data are represented as the mean + SD; Student’s t test, ∗∗p = 0.0033).
(C) Representative electron micrographs showing the analysis of alignment of the basal body and basal foot in cells from Gas2l2+/+ (n = 8) and Gas2l2−/− (n = 8) cultures. The scale bar represents 2 μm.
(D) The length of the vector (0 < R < 1) for each cell is represented in the graph. The average length of the vector was significantly shorter in Gas2l2−/− ciliated cells than in control cells (data are expressed as the mean + SD; Student’s t test, ∗∗p = 0.0232).
(E) Normal distribution of basal bodies in wild-type and Gas2l2−/− mTEC cells.
Figure 6.
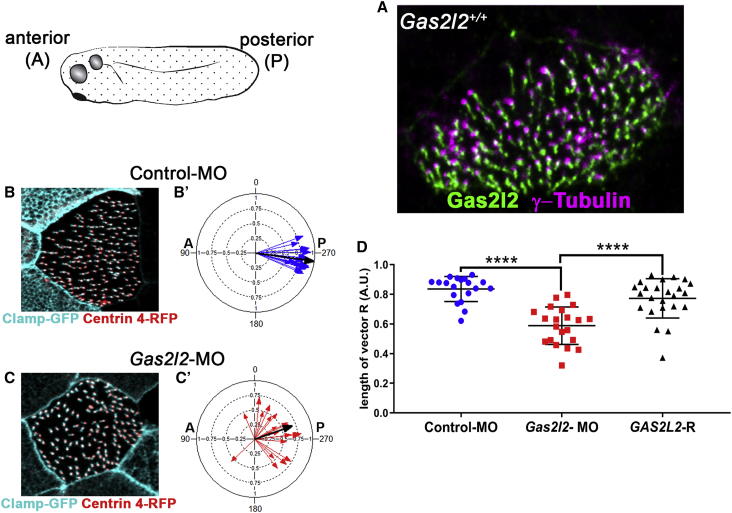
Absence of Gas2l2 in X. laevis Affects Cilia Rotational Polarity
(A) Detection of Gas2l2 in a wild-type skin ciliated cell by immunofluorescence.
(B and C) Representative immunofluorescence images visualizing basal bodies (Clamp-GFP) and rootlet (Centrin 4-RFP) to score basal body-rootlet alignment.
(B′ and C′) Alignment of the basal body and rootlet in control-MO (n = 19, mean vector, black arrow, r = 0.98, CSD = 11.5°) and Gas2l2-MO (n = 21, r = 0.67, CSD = 51.2).
(D) The vector length (R) of each analyzed cell is represented in the graph. The average length of the vector was significantly shorter in Gas2l2-MO (R = 0.5884 ± 0.1259) cells than in controls (R = 0.836 ± 0.0847). The introduction of human GAS2L2 mRNA in morpholino-treated embryos (GAS2L2-R) rescued the phenotype (n = 23, R = 0.7728 ± 0.1322) (ANOVA, multiple comparison, p < 0.0001).
The multiciliated skin cells of X. laevis embryos generate a robust flow oriented from anterior to posterior. X. laevis expresses two isoforms of Gas2l2; therefore, morpholinos (MO) targeting both isoforms were injected into embryos (Gas2l2-MO embryos). To visualize and score the orientation of individual cilia, the embryos were co-injected with Centrin 4-RFP (rootlet marker) and Clamp-GFP (basal-body marker) mRNA.61 The targeting of Gas2l2 resulted in mosaic embryos (Figure S7B). The decreased levels of Gas2l2 affected the number and distribution of basal bodies on the surface of ciliated cells (Figures 6B and 6C). The ciliary orientation was significantly reduced in Gas2l2-MO embryos (r = 0.67, CSD = 51.2°) compared to controls (r = 0.98, CSD = 11.5°; Figures 6B′ and 6C′). This phenotype was rescued when human GAS2L2 mRNA (GAS2L2-R) was co-injected (r = 0.91, CSD = 25.6°; p < 0.0001). In addition, the average of the length of the vectors from ciliated cells in Gas2l2-MO embryos was significantly shorter than in control-MO and GAS2L2-R embryos (Figure 6D).
Gas2l2 Conditional-KO Mouse Develops a PCD-like Phenotype
To understand the role of GAS2L2 in PCD development, we generated conditional-knockout (KO) mice (Figure 7A). The Gas2l2fl/fl mice were first crossed to a ROSA26CreER mouse. However, we detected by PCR a high incidence of spontaneous deletion of Gas2l2 (Figure S8A), and most of the Gas2l2fl/fl:RosaCreER/+ mice died soon after birth. Tracheal cells from surviving Gas2l2fl/fl:RosaCreER/+ and control mice were expanded as CRCs and cultured at ALI. To induce the complete deletion of Gas2l2, we treated the cultures with tamoxifen (TMX; 1 μM)64 and confirmed the deletion by RT-PCR (Figure S8B). The TMX-treated cultures (Figures S8C and S8D) recapitulated the phenotypes observed in GAS2L2-deficient HNE cells (Figure 3) and in mTEC cultures from Gas2l2−/− embryonic trachea (Figure 5).
Figure 7.
Partial Deletion of Gas2l2 in Mice Induces a PCD-like Phenotype
(A) Schematic representation of the generation of Gas2l2-conditional-KO mouse. See Material and Methods for details.
(B) Reduced expression of Gas2l2 was confirmed by RT-PCR with RNA extracted from trachea rings. Tbp was used as reference for normalization.
(C) Representative micro-CT scan and AB-PAS histology of animal heads after 90 days of treatment. Mucus accumulation in the turbinate (arrow head) and increased volume of the sinuses (arrow) was observed in the conditional-KO mice but not in the control. Abbreviations are as follows: ET, ethmoturbinates; MS, maxillary sinus; and NC, nasal cavity.
(D) Measurement of total nasal-cavity air space in all the animals (n = 41) included in these experiments. A significant increase in nasal air space in the conditional-KO mice (red line) compared to controls (black line) was first observed at 60 days after treatment (each data point corresponds to the mean + SD; ∗∗p = 0.0013; ∗∗∗p = 0.0003, Student’s t test, Welch-corrected).
We generated viable conditional-KO (Gas2l2fl/fl:FoxJ1CreERT2) mice by crossing Gas2l2fl/fl mice to Foxj1CreERT2::GFP/+ (knockin/knockout) mice expressing Cre recombinase exclusively in cells with motile cilia. The offspring from this cross had a high rate of neonatal survival (∼98%), but we observed isolated cases of hydrocephalus (∼3%). Conditional-KO and control animals were injected with TMX and maintained on a TMX- supplemented diet. In a cohort of 41 mice included in these experiments, the TMX-supplemented diet did not affect the weight or survival of the mice. However, this treatment did not completely abolish Gas2l2 expression in the trachea (Figures 7B and S9).
Mouse models of PCD develop a phenotype characterized by chronic rhinosinusitis and accumulation of mucus in the nasal cavity.59 To follow the development of rhinosinusitis and mucus accumulation, the nasal cavities of the mice were imaged using micro-CT. The mice were imaged four times at four-week intervals, starting after the third TMX injection (baseline). The CT scans were compared to histological sections (Figure 7C). Of the 21 Gas2l2fl/fl:Foxj1CreERT2 mice treated with TMX, ∼80% developed mucus plugging in multiple nasal cavities and increased nasal air space due to remodeling of the nasal cavity. The nasal air space increased over time and was significant at two and three months after treatment (Figure 7D). These results confirm that the deletion of Gas2l2 caused a PCD phenotype in mice.
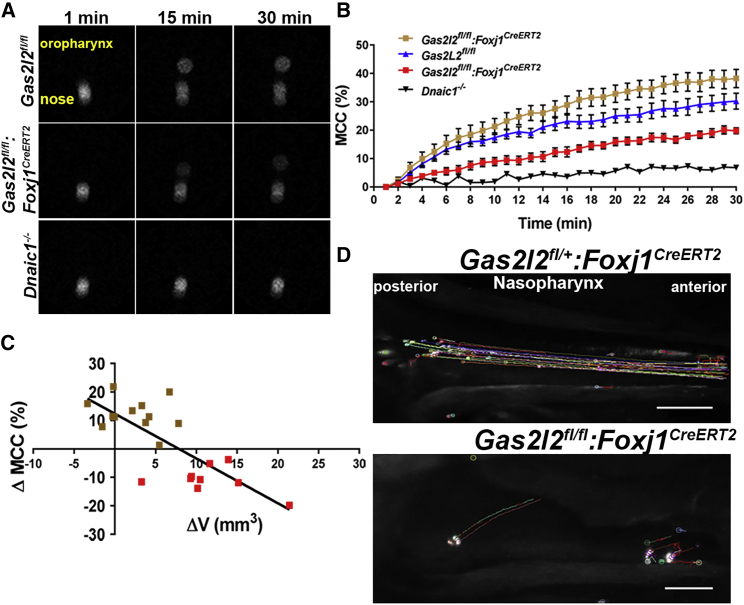
Impaired MCC in Gas2l2 conditional-KO mice
To directly determine the effect of the deletion of Gas2l2 on MCC, we used a noninvasive method to monitor in real time the nasal clearance of Gas2l2fl/fl:Foxj1CreERT2 and control mice.56 After the intranasal administration of the radioactive tracer Technetium-99 (99mTc), radioactive particles were observed at the tip of the nose at time 0 (Figure 8A, left panel). These particles were then cleared toward the oropharynx, where they accumulated over time (Figure 8A, central and right panels). The average clearance in Gas2l2-conditional-KO mice was significant in comparison to that of Dnaic1−/− mice, which show no MCC (Figure 8B). However, in the Gas2l2-conditional-KO mice we observed that the impairment of clearance (with respect to that in control mice) correlated with changes in nasal air space (ΔV = V90 − Vbaseline, Figure 8C, Pearson’s r = −0.7672, p < 0.0001) and the presence of mucus in the nose. Those mice that had normal clearance had no change in ΔV and no mucus accumulation (five mice), or had ΔV < 5 mm3 and a mucus-accumulation score of 1 (seven mice). The mice with impaired clearance (nine mice) had ΔV > 5 mm3 and obvious mucus accumulation (score = 2). Additionally, we measured the clearance of fluorescent microspheres in the nasopharynx at the end of the study. In this technique, which avoids retention of particles in the nasal turbinate, the fluorescent beads were delivered at the ventral wall of the nasopharyngeal (NP) meatus. The effective speed of MCC in Gas2l2fl/fl:Foxj1CreERT2 mice (11.63 ± 2.97 mm/sec; n = 21) was significantly slower than that in control mice (13.31 ± 2.17 mm/sec, p = 0.0168, paired t test; n = 20), and the directionality of the flow was significantly affected in Gas2l2-conditional-KO mice compared to controls (chi-square x2 = 1.71; df = 1, Figure 8D, Videos S5 and S6). The beads were transported in various directions, usually at an angle, and the forward movement was intermittent (Figure 8D and Video S6). This phenotype was observed in the 16 mice that had increased nasal air space and mucus accumulation in the nose. These results show that GAS2L2 is necessary for effective MCC in the mouse upper airways.
Figure 8.
Deletion of Gas2l2 Affects Mucociliary Clearance in Mice
(A) Representative scintigraphy images of real-time measurements of nasal clearance in mice via Technetium-99. Radiolabeled particles were delivered into the nose (left panel). The clearance was recorded over time (middle and right panels) with a gamma camera.
(B) Cumulative nasal clearance in Gas2l2-conditional-KO (n = 21), controls (n = 20), and Dnaic1−/− (n = 3) treated with TMX. In the Gas2l2-conditional-KO two groups were observed: a group with normal clearance (brown squares, n = 12) and a group with impaired clearance (red squares, n = 9).
(C) Correlation between the change in MCC in Gas2l2-conditional-KO mice relative to controls and ΔV in the two groups of Gas2l2-conditional-KO mice (r = −0.7151, p = 0.0018).
(D) Time-lapse images of 600 frames (5 frames/s) representing the nasopharyngeal clearance of fluorescence beads in control and Gas2l2-conditional-KO mice (the scale bar represents 0.5 mm).
The clearance of particles was tracked with Fiji’s plugin Mosaic (Supplemental Material and Methods). The video was created from an image sequence of 200 frames at 5 fps. the scale bar represents 0.5 mm.
The clearance of particles was tracked with Fiji’s plugin Mosaic (Supplemental Material and Methods). The video was created from an image sequence of 200 frames at 5 fps. The scale bar represents 0.5 mm.
Discussion
We have identified two unrelated probands who have clinical symptoms of PCD and harbor pathogenic variants in GAS2L2. Proband PCD-1367 carries an apparent homozygous frameshift variant (c.887_890delTAAG [p. Val296Glyfs∗13]), and proband DCP-1040 harbors compound heterozygous variants (c.887_890delTAAG [p.Val296Glyfs∗13] and c.867_∗343+1207del p.?), consistent with a likely recessive trait. Interestingly, more than 400 unrelated individuals with symptoms of PCD were screened, but none harbored bi-allelic variants in GAS2L2. The pathogenic variants that we describe are rare in the public databases. For example, in gnomAD65 the c.887_890delTAAG variant had an allele frequency of 4.924 × 10−4 (130 of 276,682 alleles); none were homozygous. Thus, on the basis of allele frequency, the chance that this variant would appear in a homozygous state is approximately one in 4.5 million individuals. The GAS2L2 genetic variants were considered pathogenic because (1) GAS2L2 showed coordinated expression with multiple other genes known to be associated with human PCD in UGET,66 (2) both variants are predicted to produce a truncated protein or no protein, and (3) GAS2L2 is not implicated in any other inherited disorder.
Both affected individuals had normal ciliary axonemal ultrastructure by TEM. Several studies have provided examples of individuals who suffer from PCD but have normal ultrastructure of the ciliary axoneme by TEM; for example, such individuals might have genetic variants in DNAH1167 [MIM: 603339] or CCDC6568 [MIM: 611088]. Although in these cases the ciliary axoneme appears normal, the variants result in a stiff and hyperkinetic ciliary beat.67, 68 In contrast, GAS2L2-deficient ciliated cells had a normal waveform but a hyperkinetic ciliary beat. Importantly, the orientation of basal bodies, and therefore the direction of ciliary beating, was disrupted. Previously, the disorientation of cilia was described in several cases of PCD.69, 70, 71 In all these cases, the cilia had normal axonemal ultrastructure and a normal ciliary beat frequency, but they had impaired ciliary orientation and clearance. Because of these reports, it was suggested that ciliary disorientation could be a different variant of PCD. However, ciliary disorientation was also found in individuals with chronic respiratory-tract inflammation, and it was reversed by treatment with antibiotics.72 This suggested that ciliary disorientation was a secondary effect of infection or inflammation and was not the cause of PCD. Independent of the causes leading to disorientation of the cilia, these previous results showed that, although the subjects had normal ciliary structure and waveform, MCC was inefficient. More recently, variants in RPGR [MIM: 300029] have been shown to cause an X-linked PCD variant associated with ciliary disorientation,73 and a genetic variant in STK36 [MIM: 605030] caused disorientation of cilia in an individual with PCD.4 Here we show that ciliary disorientation can occur as a direct result of a genetic lesion in GAS2L2, and the PCD phenotype observed is due to inefficient MCC that results from improper ciliary orientation.
The proband carrying the apparent homozygous variant in GAS2L2 (PCD-1367) has a rate of nNO production within the normal range.63 In contrast, the proband carrying the compound heterozygous variant in GAS2L2 (DCP-1040) has a low rate of nNO production.74 The difference between nNO values could be due to the age of the subjects, their overall health status at the time of measurement, and/or other genetic or environmental factors. In healthy individuals, NO is produced constitutively within the paranasal sinuses and in the nasal mucosa in response to inflammation,75 but individuals with chronic rhinosinusitis have a decreased rate of nNO production.76 The biological significance of NO in the nasal region remains unclear, but it might be essential for local host defense77 and the regulation of MCC in the respiratory tract.78 The mechanisms involved in the reduced production of nNO found in most PCD subjects are unknown; more studies will need to clarify the role of ciliary function in the production of nNO.
Until this study, no evidence of the localization, expression, or role of GAS2L2 in airway cells was available. Here we show that the expression of GAS2L2 follows a similar pattern to that of other cilia-specific genes.79 Although we were able to immunoprecipitate GAS2L2 from cultured airway cells, co-immunoprecipitation studies aimed at identifying interacting proteins were unsuccessful (not shown). However, using a combination of high-resolution SIM and single-molecule GSD microscopy, we detected GAS2L2 localizing closely with markers of basal bodies (γ-tubulin and centrin-2) and basal feet (centriolin), and in the proximity of actin filaments and the rootlet. These results, together with the known ability of GAS2L2 to bind both actin and microtubules,25 suggest that in ciliated cells GAS2L2 is part of the protein network at the base of the ciliary axoneme and could provide mechanical support to the cilia during the constant physical stress of ciliary beating. GAS2L2 could mediate the crosstalk between microtubules, actin filaments, and the proteins that constitute the basal body and its appendages, thus ensuring the proper orientation/stabilization of basal bodies and coordination of the ciliary beating. In GAS2L2-deficient human cells the basal bodies were significantly less aligned than control cells. This phenotype was recapitulated in Gas2l2−/− mTEC and in X. laevis embryos injected with antisense Gas2l2-MO. These results suggest a conserved role for GAS2L2 in the alignment of basal bodies. Previous studies in X. laevis and mice showed that the docking of basal bodies at the membrane and their relative spacing at the apical cortex is driven by the actin cytoskeleton,15 whereas cortical microtubules are important for the alignment of basal bodies.15, 80 In human and mTEC GAS2L2-deficient cells, the basal bodies were normally spaced, but their alignment was impaired. This suggests that the lack of GAS2L2 does not disrupt the actin cytoskeleton but might affect the stabilization of cortical MT and, subsequently, basal body alignment. It will be interesting to look in detail and over time at the organization of the microtubule network at the base of the cilium in GAS2L2-deficient cells. In contrast, in X. laevis embryos injected with antisense Gas2l2-MO targeting both Gas2l2 isoforms, we observed a decrease in both the number and alignment of basal bodies. The severity of the phenotype suggests that the Gas2l2 isoforms play other roles independent of their role in cilia orientation.
Surprisingly, Gas2l2-knockout mice die neonatally. The high lethality in Gas2l2−/− mice could be associated with developmental defects. During early embryogenesis, the motile cilia at the embryonic node generates the right-to-left flow required for establishing the left-right body axis,81 resulting in the normal localization of the organs in the body (situs solitus). We observed situs defects in 50% (3/6) of the Gas2l2−/− mouse embryos dissected at 18.5 dpc, suggesting that GAS2L2 might be necessary for proper ciliary function at the embryonic node. Another common phenotype in mouse models of PCD is hydrocephalus.82 Hydrocephalic PCD mice develop enlarged heads as a result of ventricular dilatation during the neonatal period and die within the first month of life.82, 83 Although we observed hydrocephalus in the two Gas2l2−/− mice that survived, it is unlikely to be the primary cause of neonatal death in the Gas2l2−/− mice. The development of hydrocephalus could be related to other genetic modifiers that play a role in the susceptibility to severe PCD-associated hydrocephalus in the C57BL/6J background.82, 84 Additionally, the majority of the GAS2 family members have been implicated in controlling cell proliferation and apoptosis. For example, GAS2 is required for proper follicular development in mice,31 GAS2L1 might be important for the maintenance of genome stability by controlling centriole motility and ensuring the timing of centrosome disjunction during cell division,32 and GAS2L3 is important for brain morphogenesis and development.36 The fact that human and mouse GAS2L2-deficient airway cells proliferate and differentiate normally in culture suggests that GAS2L2 might not play a role in regulating cell proliferation or differentiation. However, in the Gas2l2−/− mice, GAS2L2 might be important for maintaining homeostasis in the tissues in which it is expressed. These phenotypes of the Gas2l2−/− mice—neonatal death, situs abnormalities, and hydrocephalus—require further investigation.
The conditional deletion of Gas2l2 in vivo caused the typical mouse PCD phenotype: impaired MCC accompanied by mucus accumulation in the nasal cavity and remodeling of the nasal cavity. In Gas2l2-conditional-KO mice we observed significantly impaired clearance directionality in the nasopharynx and a significant correlation between impaired MCC and nasal remodeling. We observed that ∼20% of the conditional-KO mice included in the study had normal MCC, no change in nasal air space, and no mucus accumulation, most likely because the tamoxifen treatment was insufficient to completely delete Gas2l2. This possibility is supported by data showing expression of Gas2l2 in tamoxifen-treated mice. Alternatively, in the unaffected mice the deletion of Gas2l2 could require more time to show its effect. The tamoxifen treatment started when the mice were 4 weeks old; thus, the animals possessed a full complement of properly orientated cilia. Although previous studies have suggested that ciliated cells and ciliary proteins are very stable,64, 85 the turnover of ciliated cells and ciliary proteins in vivo is unknown. Our results suggest that over time all mice lacking Gas2l2 will develop PCD.
In summary, we propose that GAS2L2 plays a critical role in the airways by inter-connecting cytoskeletal elements, basal bodies, and basal feet. Thus, GAS2L2 helps to maintain the correct orientation of basal bodies in ciliated cells. Our results demonstrate that the proper orientation of the cilia is crucial for ensuring effective MCC and that pathogenic variants in GAS2L2 result in poorly aligned cilia, a hyperkinetic ciliary beat, and PCD.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
The authors would like to thank the PCD subjects and family members for their participation and thank the US PCD Foundation and the investigators and coordinators of the Genetic Disorders of Mucociliary Clearance Consortium, part of the Rare Disease Clinical Research Network. We thank Jay Shendure, Deborah Nickerson, and Michael Bamshad from University of Washington School of Medicine (National Institutes of Health [NHI]/ National Human Genome Research Institute [NHGRI] U54HG0006493), Seattle GO Sequencing Project (HL-102926), RS & G at Northwest Genomics Center at the University of Washington (NIH-National Heart, Lung, and Blood Institute [NHLBI] HHSN268201100037); Shrikant Mane, Francesc Lopez-Giraldez, and Weilai Dong from the Yale Center (UM1 HG006504) for whole-exome sequencing and bioinformatics support; Kimberly Burns for histology and electron-microscopy support; Whitney Wolf and Lu Huang for technical assistance; Hong Dang for bioinformatics assistance; Robert Tarran for providing the Leica Sp8 confocal and GSD microscopes; Michael Chua and Tony Perdue for assistance with confocal imaging; Frank Conlon and Panna Tandon for providing Xenopus laevis oocytes; Uma Nagarajan and Amy Kiatthanapaiboon for providing samples of human fallopian-tube total RNA; Julie Kimbell for assistance with Mimics Research 18.0; and Scott H. Randell and the UNC Cell Culture Core for providing human airway cells. The UNC Cell Core Facility is supported by BOUCHE15R0 and P30DK065988 grants. The UNC Proteomics Core Facility is supported in part by P30 CA016086. The Small Animal Imaging Facility at the UNC Biomedical Imaging Research Center is supported in part by a National Cancer Institute cancer core grant, P30-CA016086-40. Funding support for this research was provided to B.J.M. by R01GM089970 from the National Institute of General Medical Sciences at the NIH; to M.R.K and M.A.Z by grant 5U54HL096458 from the Office of Rare Diseases Research and NHLBI at the NIH; to M.R.K., L.E.O., and M.A.Z. by NIH-NHLBI grant R01HL071798; to L.E.O., M.A.Z., and M.R.K by NIH-NHLBI grant R01HL117836; and to the University of North Carolina at Chapel Hill by grant UL1 TR000083 from the National Center for Advancing Translational Sciences at the NIH. Work by S.A., E.E., M.L., and L.T. is supported by the Fondation pour la Recherche Médicale grant DEQ20120323689, by Institut National de la Santé et de la Recherche Médicale (INSERM) grant ANR-10-COHO-003, and the Legs Poix grant from the Chancellerie des Universités of Sorbonne Université.
Published: January 17, 2019
Footnotes
The Supplemental Data include Supplemental Material and Methods, nine figures, six tables, and six videos and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.12.009.
Contributor Information
Maimoona A. Zariwala, Email: maimoona_zariwala@med.unc.edu.
Lawrence E. Ostrowski, Email: ostro@med.unc.edu.
Accession Numbers
c.867_∗343+1207del p.? has been deposited in LOVD: 0000439768.
Web Resources
1000 Genomes, http://www.internationalgenome.org/
OMIM, http://www.omim.org/
Uniprot, https://www.uniprot.org/
Supplemental Data
References
- 1.Knowles M.R., Zariwala M., Leigh M. Primary Ciliary Dyskinesia. Clin. Chest Med. 2016;37:449–461. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro A.J., Zariwala M.A., Ferkol T., Davis S.D., Sagel S.D., Dell S.D., Rosenfeld M., Olivier K.N., Milla C., Daniel S.J. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr. Pulmonol. 2016;51:115–132. doi: 10.1002/ppul.23304. Published online September 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zariwala M.A., Knowles M.R., Leigh M.W. Primary Ciliary Dyskinesia. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Bird T.D., Ledbetter N., Mefford H.C., Smith R.J.H., editors. GeneReviews. University of Washington; 2007 [Updated 2015]. [Google Scholar]
- 4.Edelbusch C., Cindrić S., Dougherty G.W., Loges N.T., Olbrich H., Rivlin J., Wallmeier J., Pennekamp P., Amirav I., Omran H. Mutation of serine/threonine protein kinase 36 (STK36) causes primary ciliary dyskinesia with a central pair defect. Hum. Mutat. 2017;38:964–969. doi: 10.1002/humu.23261. [DOI] [PubMed] [Google Scholar]
- 5.Olcese C., Patel M.P., Shoemark A., Kiviluoto S., Legendre M., Williams H.J., Vaughan C.K., Hayward J., Goldenberg A., Emes R.D. X-linked primary ciliary dyskinesia due to mutations in the cytoplasmic axonemal dynein assembly factor PIH1D3. Nat. Commun. 2017;8:14279. doi: 10.1038/ncomms14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paff T., Loges N.T., Aprea I., Wu K., Bakey Z., Haarman E.G., Daniels J.M.A., Sistermans E.A., Bogunovic N., Dougherty G.W. Mutations in PIH1D3 cause X-linked primary ciliary dyskinesia with outer and inner dynein arm defects. Am. J. Hum. Genet. 2017;100:160–168. doi: 10.1016/j.ajhg.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallmeier J., Shiratori H., Dougherty G.W., Edelbusch C., Hjeij R., Loges N.T., Menchen T., Olbrich H., Pennekamp P., Raidt J. TTC25 deficiency results in defects of the outer dynein arm docking machinery and primary ciliary dyskinesia with left-right body asymmetry randomization. Am. J. Hum. Genet. 2016;99:460–469. doi: 10.1016/j.ajhg.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Khouri E., Thomas L., Jeanson L., Bequignon E., Vallette B., Duquesnoy P., Montantin G., Copin B., Dastot-Le Moal F., Blanchon S. Mutations in DNAJB13, encoding an HSP40 family member, cause primary ciliary dyskinesia and male infertility. Am. J. Hum. Genet. 2016;99:489–500. doi: 10.1016/j.ajhg.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Höben I.M., Hjeij R., Olbrich H., Dougherty G.W., Nöthe-Menchen T., Aprea I., Frank D., Pennekamp P., Dworniczak B., Wallmeier J. Mutations in C11orf70 cause primary ciliary dyskinesia with randomization of left/right body asymmetry due to defects of outer and inner dynein arms. Am. J. Hum. Genet. 2018;102:973–984. doi: 10.1016/j.ajhg.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassad M.R., Shoemark A., le Borgne P., Koll F., Patel M., Dixon M., Hayward J., Richardson C., Frost E., Jenkins L. C11orf70 mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2018;102:956–972. doi: 10.1016/j.ajhg.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olbrich H., Cremers C., Loges N.T., Werner C., Nielsen K.G., Marthin J.K., Philipsen M., Wallmeier J., Pennekamp P., Menchen T. Loss-of-function GAS8 mutations cause primary ciliary dyskinesia and disrupt the nexin-dynein regulatory complex. Am. J. Hum. Genet. 2015;97:546–554. doi: 10.1016/j.ajhg.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnefoy S., Watson C.M., Kernohan K.D., Lemos M., Hutchinson S., Poulter J.A., Crinnion L.A., Berry I., Simmonds J., Vasudevan P., Care4Rare Canada Consortium Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am. J. Hum. Genet. 2018;103:727–739. doi: 10.1016/j.ajhg.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustamante-Marin X.M., Ostrowski L.E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 2017;9:9. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vladar E.K., Bayly R.D., Sangoram A.M., Scott M.P., Axelrod J.D. Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner M.E., Hwang P., Huisman F., Taborek P., Yu C.C., Mitchell B.J. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall W.F. Basal Bodies: Platforms for Building Cilia. In: Paul M., editor. Current Topics in Developmental Biology Wassarman. Academic Press; 2008. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 17.Vertii A., Hung H.-F., Hehnly H., Doxsey S. Human basal body basics. Cilia. 2016;5:13. doi: 10.1186/s13630-016-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Gao J., Adamian M., Wen X.H., Pawlyk B., Zhang L., Sanderson M.J., Zuo J., Makino C.L., Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol. Cell. Biol. 2005;25:4129–4137. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200. doi: 10.1016/j.cell.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 20.Raidt J., Wallmeier J., Hjeij R., Onnebrink J.G., Pennekamp P., Loges N.T., Olbrich H., Häffner K., Dougherty G.W., Omran H., Werner C. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 2014;44:1579–1588. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
- 21.Chilvers M.A., Rutman A., O’Callaghan C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 2003;112:518–524. doi: 10.1016/S0091-6749(03)01799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baum G.L., Zwas S.T., Katz I., Roth Y. Mucociliary clearance from central airways in patients with excessive sputum production with and without primary ciliary dyskinesia. Chest. 1990;98:608–612. doi: 10.1378/chest.98.3.608. [DOI] [PubMed] [Google Scholar]
- 23.Noone P.G., Bennett W.D., Regnis J.A., Zeman K.L., Carson J.L., King M., Boucher R.C., Knowles M.R. Effect of aerosolized uridine-5′-triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 1999;160:144–149. doi: 10.1164/ajrccm.160.1.9806146. [DOI] [PubMed] [Google Scholar]
- 24.Goriounov D., Leung C.L., Liem R.K. Protein products of human Gas2-related genes on chromosomes 17 and 22 (hGAR17 and hGAR22) associate with both microfilaments and microtubules. J. Cell Sci. 2003;116:1045–1058. doi: 10.1242/jcs.00272. [DOI] [PubMed] [Google Scholar]
- 25.Stroud M.J., Nazgiewicz A., McKenzie E.A., Wang Y., Kammerer R.A., Ballestrem C. GAS2-like proteins mediate communication between microtubules and actin through interactions with end-binding proteins. J. Cell Sci. 2014;127:2672–2682. doi: 10.1242/jcs.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang K., Toedt G., Montenegro Gouveia S., Davey N.E., Hua S., van der Vaart B., Grigoriev I., Larsen J., Pedersen L.B., Bezstarosti K. A Proteome-wide screen for mammalian SxIP motif-containing microtubule plus-end tracking proteins. Curr. Biol. 2012;22:1800–1807. doi: 10.1016/j.cub.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 27.Collavin L., Buzzai M., Saccone S., Bernard L., Federico C., DellaValle G., Brancolini C., Schneider C. cDNA characterization and chromosome mapping of the human GAS2 gene. Genomics. 1998;48:265–269. doi: 10.1006/geno.1997.5172. [DOI] [PubMed] [Google Scholar]
- 28.Brancolini C., Benedetti M., Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995;14:5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T., Dayanandan B., Rouiller I., Lawrence E.J., Mandato C.A. Growth-arrest-specific protein 2 inhibits cell division in Xenopus embryos. PLoS ONE. 2011;6:e24698. doi: 10.1371/journal.pone.0024698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H., Ge Y., Sun L., Ma W., Wu J., Zhang X., Hu X., Eaves C.J., Wu D., Zhao Y. Growth arrest specific 2 is up-regulated in chronic myeloid leukemia cells and required for their growth. PLoS ONE. 2014;9:e86195. doi: 10.1371/journal.pone.0086195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.York J.P., Ren Y.A., Zeng J., Bin Zhang, Wang F., Chen R., Liu J., Xia X., Zhang P. Growth arrest specific 2 (GAS2) is a critical mediator of germ cell cyst breakdown and folliculogenesis in mice. Sci. Rep. 2016;6:34956. doi: 10.1038/srep34956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Au F.K.C., Jia Y., Jiang K., Grigoriev I., Hau B.K.T., Shen Y., Du S., Akhmanova A., Qi R.Z. GAS2L1 is a centriole-associated protein required for centrosome dynamics and disjunction. Dev. Cell. 2017;40:81–94. doi: 10.1016/j.devcel.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Gamper I., Koh K.R., Ruau D., Ullrich K., Bartunkova J., Piroth D., Hacker C., Bartunek P., Zenke M. GAR22: A novel target gene of thyroid hormone receptor causes growth inhibition in human erythroid cells. Exp. Hematol. 2009;37:539–548.e4. doi: 10.1016/j.exphem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Desmond J.C., Raynaud S., Tung E., Hofmann W.K., Haferlach T., Koeffler H.P. Discovery of epigenetically silenced genes in acute myeloid leukemias. Leukemia. 2007;21:1026–1034. doi: 10.1038/sj.leu.2404611. [DOI] [PubMed] [Google Scholar]
- 35.Stroud M.J., Kammerer R.A., Ballestrem C. Characterization of G2L3 (GAS2-like 3), a new microtubule- and actin-binding protein related to spectraplakins. J. Biol. Chem. 2011;286:24987–24995. doi: 10.1074/jbc.M111.242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharaby Y., Lahmi R., Amar O., Elbaz I., Lerer-Goldshtein T., Weiss A.M., Appelbaum L., Tzur A. Gas2l3 is essential for brain morphogenesis and development. Dev. Biol. 2014;394:305–313. doi: 10.1016/j.ydbio.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Wolter P., Schmitt K., Fackler M., Kremling H., Probst L., Hauser S., Gruss O.J., Gaubatz S. GAS2L3, a target gene of the DREAM complex, is required for proper cytokinesis and genomic stability. J. Cell Sci. 2012;125:2393–2406. doi: 10.1242/jcs.097253. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y.-C., Lai H.-L., Chang W.-C., Lin J.-T., Liu Y.-J., Chern Y. A novel Gαs-binding protein, Gas-2 like 2, facilitates the signaling of the A2A adenosine receptor. Biochim. Biophys. Acta. 2013;1833:3145–3154. doi: 10.1016/j.bbamcr.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Knowles M.R., Ostrowski L.E., Leigh M.W., Sears P.R., Davis S.D., Wolf W.E., Hazucha M.J., Carson J.L., Olivier K.N., Sagel S.D. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am. J. Respir. Crit. Care Med. 2014;189:707–717. doi: 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulcher M.L., Randell S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 41.Müller L., Brighton L.E., Carson J.L., Fischer W.A., 2nd, Jaspers I. Culturing of human nasal epithelial cells at the air liquid interface. J. Vis. Exp. 2013;(80) doi: 10.3791/50646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gentzsch M., Boyles S.E., Cheluvaraju C., Chaudhry I.G., Quinney N.L., Cho C., Dang H., Liu X., Schlegel R., Randell S.H. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2017;56:568–574. doi: 10.1165/rcmb.2016-0276MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You Y., Richer E.J., Huang T., Brody S.L. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 44.He J.Q., Sandford A.J., Wang I.M., Stepaniants S., Knight D.A., Kicic A., Stick S.M., Paré P.D. Selection of housekeeping genes for real-time PCR in atopic human bronchial epithelial cells. Eur. Respir. J. 2008;32:755–762. doi: 10.1183/09031936.00129107. [DOI] [PubMed] [Google Scholar]
- 45.Yüzbaşioğlu A., Onbaşilar I., Kocaefe C., Ozgüç M. Assessment of housekeeping genes for use in normalization of real time PCR in skeletal muscle with chronic degenerative changes. Exp. Mol. Pathol. 2010;88:326–329. doi: 10.1016/j.yexmp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Blackburn K., Bustamante-Marin X., Yin W., Goshe M.B., Ostrowski L.E. Quantitative proteomic analysis of human airway cilia identifies previously uncharacterized proteins of high abundance. J. Proteome Res. 2017;16:1579–1592. doi: 10.1021/acs.jproteome.6b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn K.W., Kamocka M.M., McDonald J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011;300:C723–C742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sears P.R., Yin W.N., Ostrowski L.E. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309:L99–L108. doi: 10.1152/ajplung.00024.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batschelet E. Circular Statistics in Biology. In: Sibson R., C.J.E., editors. Mathematics in Biology. Academic Press; 1981. p. 371. [Google Scholar]
- 53.Skarnes W.C., Rosen B., West A.P., Koutsourakis M., Bushell W., Iyer V., Mujica A.O., Thomas M., Harrow J., Cox T. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muthusamy N., Vijayakumar A., Cheng G., Ghashghaei H.T. A knock-in Foxj1(CreERT2:GFP) mouse for recombination in epithelial cells with motile cilia. Genesis. 2014;52:350–358. doi: 10.1002/dvg.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi S., McMahon A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 56.Hua X., Zeman K.L., Zhou B., Hua Q., Senior B.A., Tilley S.L., Bennett W.D. Noninvasive real-time measurement of nasal mucociliary clearance in mice by pinhole gamma scintigraphy. J. Appl. Physiol. 2010;108:189–196. doi: 10.1152/japplphysiol.00669.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grubb B.R., Livraghi-Butrico A., Rogers T.D., Yin W., Button B., Ostrowski L.E. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L860–L867. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao X., Geyer V.F., Bowne-Anderson H., Howard J., Sbalzarini I.F. Automatic optimal filament segmentation with sub-pixel accuracy using generalized linear models and B-spline level-sets. Med. Image Anal. 2016;32:157–172. doi: 10.1016/j.media.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ostrowski L.E., Yin W., Rogers T.D., Busalacchi K.B., Chua M., O’Neal W.K., Grubb B.R. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am. J. Respir. Cell Mol. Biol. 2010;43:55–63. doi: 10.1165/rcmb.2009-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sive H.L., Grainger R.M., Harland R.M. Cold Spring Harbor Laboratory Press; 2000. Early development of Xenopus laevis: A laboratory manual. [Google Scholar]
- 61.Werner M.E., Mitchell B.J. Using Xenopus skin to study cilia development and function. Methods Enzymol. 2013;525:191–217. doi: 10.1016/B978-0-12-397944-5.00010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell B., Jacobs R., Li J., Chien S., Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 63.Leigh M.W., Hazucha M.J., Chawla K.K., Baker B.R., Shapiro A.J., Brown D.E., Lavange L.M., Horton B.J., Qaqish B., Carson J.L. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 2013;10:574–581. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostrowski L.E., Yin W., Patel M., Sechelski J., Rogers T., Burns K., Grubb B.R., Olsen J.C. Restoring ciliary function to differentiated primary ciliary dyskinesia cells with a lentiviral vector. Gene Ther. 2014;21:253–261. doi: 10.1038/gt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Day A., Dong J., Funari V.A., Harry B., Strom S.P., Cohn D.H., Nelson S.F. Disease gene characterization through large-scale co-expression analysis. PLoS ONE. 2009;4:e8491. doi: 10.1371/journal.pone.0008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwabe G.C., Hoffmann K., Loges N.T., Birker D., Rossier C., de Santi M.M., Olbrich H., Fliegauf M., Failly M., Liebers U. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008;29:289–298. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 68.Horani A., Brody S.L., Ferkol T.W., Shoseyov D., Wasserman M.G., Ta-shma A., Wilson K.S., Bayly P.V., Amirav I., Cohen-Cymberknoh M. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS ONE. 2013;8:e72299. doi: 10.1371/journal.pone.0072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutland J., de Iongh R.U. Random ciliary orientation. A cause of respiratory tract disease. N. Engl. J. Med. 1990;323:1681–1684. doi: 10.1056/NEJM199012133232406. [DOI] [PubMed] [Google Scholar]
- 70.Rutman A., Cullinan P., Woodhead M., Cole P.J., Wilson R. Ciliary disorientation: a possible variant of primary ciliary dyskinesia. Thorax. 1993;48:770–771. doi: 10.1136/thx.48.7.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rayner C.F., Rutman A., Dewar A., Greenstone M.A., Cole P.J., Wilson R. Ciliary disorientation alone as a cause of primary ciliary dyskinesia syndrome. Am. J. Respir. Crit. Care Med. 1996;153:1123–1129. doi: 10.1164/ajrccm.153.3.8630555. [DOI] [PubMed] [Google Scholar]
- 72.Rayner C.F., Rutman A., Dewar A., Cole P.J., Wilson R. Ciliary disorientation in patients with chronic upper respiratory tract inflammation. Am. J. Respir. Crit. Care Med. 1995;151:800–804. doi: 10.1164/ajrccm.151.3.7881674. [DOI] [PubMed] [Google Scholar]
- 73.Bukowy-Bieryłło Z., Ziętkiewicz E., Loges N.T., Wittmer M., Geremek M., Olbrich H., Fliegauf M., Voelkel K., Rutkiewicz E., Rutland J. RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr. Pulmonol. 2013;48:352–363. doi: 10.1002/ppul.22632. [DOI] [PubMed] [Google Scholar]
- 74.Beydon N., Chambellan A., Alberti C., de Blic J., Clément A., Escudier E., Le Bourgeois M. Technical and practical issues for tidal breathing measurements of nasal nitric oxide in children. Pediatr. Pulmonol. 2015;50:1374–1382. doi: 10.1002/ppul.23167. [DOI] [PubMed] [Google Scholar]
- 75.Lundberg J.O. Nitric oxide and the paranasal sinuses. Anat. Rec. (Hoboken) 2008;291:1479–1484. doi: 10.1002/ar.20782. [DOI] [PubMed] [Google Scholar]
- 76.Dabholkar Y.G., Saberwal A.A., Velankar H.K., Shetty A.K., Chordia N.P., Budhwani S.R. Correlation of nasal nitric oxide measurement with computed tomography findings in chronic rhinosinusitis. Indian J. Otolaryngol. Head Neck Surg. 2014;66:92–96. doi: 10.1007/s12070-013-0689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 78.Runer T., Lindberg S. Effects of nitric oxide on blood flow and mucociliary activity in the human nose. Ann. Otol. Rhinol. Laryngol. 1998;107:40–46. doi: 10.1177/000348949810700108. [DOI] [PubMed] [Google Scholar]
- 79.Knowles M.R., Ostrowski L.E., Loges N.T., Hurd T., Leigh M.W., Huang L., Wolf W.E., Carson J.L., Hazucha M.J., Yin W. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 2013;93:711–720. doi: 10.1016/j.ajhg.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clare D.K., Magescas J., Piolot T., Dumoux M., Vesque C., Pichard E., Dang T., Duvauchelle B., Poirier F., Delacour D. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun. 2014;5:4888. doi: 10.1038/ncomms5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 82.Lee L. Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J. Neurosci. Res. 2013;91:1117–1132. doi: 10.1002/jnr.23238. [DOI] [PubMed] [Google Scholar]
- 83.Ibañez-Tallon I., Gorokhova S., Heintz N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum. Mol. Genet. 2002;11:715–721. doi: 10.1093/hmg/11.6.715. [DOI] [PubMed] [Google Scholar]
- 84.Finn R., Evans C.C., Lee L. Strain-dependent brain defects in mouse models of primary ciliary dyskinesia with mutations in Pcdp1 and Spef2. Neuroscience. 2014;277:552–567. doi: 10.1016/j.neuroscience.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leigh M.W., Carson J.L., Gambling T.M., Boat T.F. Loss of cilia and altered phenotypic expression of ciliated cells after acute sulfur dioxide exposure. Chest. 1992;101(3, Suppl):16S. doi: 10.1378/chest.101.3_supplement.16s-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video was recorded at 200 fps with a 60× objective lens with DIC optics. Playback speed is 15% of normal speed. The scale bar represents 4 μm.
the video was recorded at 200 fps with a 60× objective lens with DIC optics. Playback speed is 15% of normal speed. The scale bar represents 4 μm.
The video matches exactly cell 2 shown in Figure 3F (top panel, middle) of the main document. Light gray areas in the video are areas that extended beyond the original frames. The playback speed is 15% of the original 200 frames per second. The scale bar represents 2 μm.
The video matches exactly cell 2 (arrow) shown in Figure 3F (bottom panel, middle) of the main document. Light gray areas in the video are areas that extended beyond the original frames. The playback speed is 15% of the original 200 frames per second. The scale bar represents 2 μm.
The clearance of particles was tracked with Fiji’s plugin Mosaic (Supplemental Material and Methods). The video was created from an image sequence of 200 frames at 5 fps. the scale bar represents 0.5 mm.
The clearance of particles was tracked with Fiji’s plugin Mosaic (Supplemental Material and Methods). The video was created from an image sequence of 200 frames at 5 fps. The scale bar represents 0.5 mm.