Abstract
The Chronic Kidney Disease (CKD) Mineral and Bone Disorder (MBD) encompasses changes in mineral ion and vitamin D metabolism that are widespread in the setting of CKD and end-stage renal disease (ESRD). MBD components associate with cardiovascular disease in many epidemiologic studies. Through impacts on hypertension, activation of the renin-angiotensin-aldosterone system, vascular calcification, endothelial function, and cardiac remodeling and conduction, MBD may be a direct and targetable cause of cardiovascular disease. However, assessment and treatment of MBD is rife with challenges due to biological tensions between its many components, such as: calcium and phosphorus with their regulatory hormones fibroblast growth factor 23 and parathyroid hormone; fibroblast growth factor 23 with its co-receptor klotho; and vitamin D with control of calcium and phosphorus. These complex interactions between MBD components hinder the simple translation to clinical trials, which are ultimately needed to prove the benefits of treating MBD. Deeper investigation using precision medicine tools and principles, including genomics and individualized risk assessment and therapy may help move the field closer towards clinical applications. This review will provide a high level overview of conventional and ‘precision’ epidemiology in MBD, potential mechanisms of cardiovascular disease pathogenesis, and guiding therapeutic principles for established and emerging treatments.
Keywords: cardiovascular disease, phosphorus, fibroblast growth factor 23, klotho, vitamin D, mineral metabolism
Introduction
Abnormal bone and mineral ion homeostasis frequently complicates chronic kidney disease (CKD) and is a prime suspect in the accelerated development of cardiovascular disease. By definition, the syndrome of CKD mineral and bone disorder (MBD) involves changes in mineral ion homeostasis, bone quality and turnover, and ectopic extraosseous calcification.1 The current prevailing paradigm roots the pathogenesis of the syndrome in the abnormal handling of phosphorus, calcium and vitamin D, which will be the focus of this review.2
Although the full complexity of MBD physiology is beyond our scope, a simplified paradigm is critical to understanding the multiple components in MBD and their inherent collusion in the pathogenesis of cardiovascular disease. Under this simplified paradigm, the ability to filter and excrete a phosphorus load is progressively compromised as kidney disease progresses, requiring higher fractional excretion of phosphorus to maintain balance. The fractional excretion of phosphorus is controlled by fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH) each of which reduce expression of sodium-phosphate co-transporters in the kidney’s proximal tubule. As FGF23 and then PTH rise in CKD,3 they exert opposing effects on vitamin D metabolism creating a physiologic “tug of war” culminating in CKD-MBD. For instance, whereas PTH stimulates transcription of 1-α hydroxylase that converts 25-hydroxyvitamin D (25-D to 1,25-dihydroxyvitamin D (1,25-D), FGF23 inhibits it. Rising FGF23 creates a state of 1,25-D deficiency that drives down serum calcium and further elevates PTH.4 In the distal tubule of the kidney, both PTH and FGF23 appear to increase calcium reabsorption (Figure 1).5,6 In the case of FGF23 this action may compensate for low gastrointestinal calcium absorption induced by 1,25-D deficiency. In the case of PTH this action helps raise serum calcium, the dominant function of PTH. In both cases calcium reabsorption prevents urine calcium phosphorus supersaturation which may otherwise result from enhanced phosphaturia.5,6
Figure 1. Simplified Schematic of Mineral and Bone Disorder (MBD) Physiology in Chronic Kidney Disease.
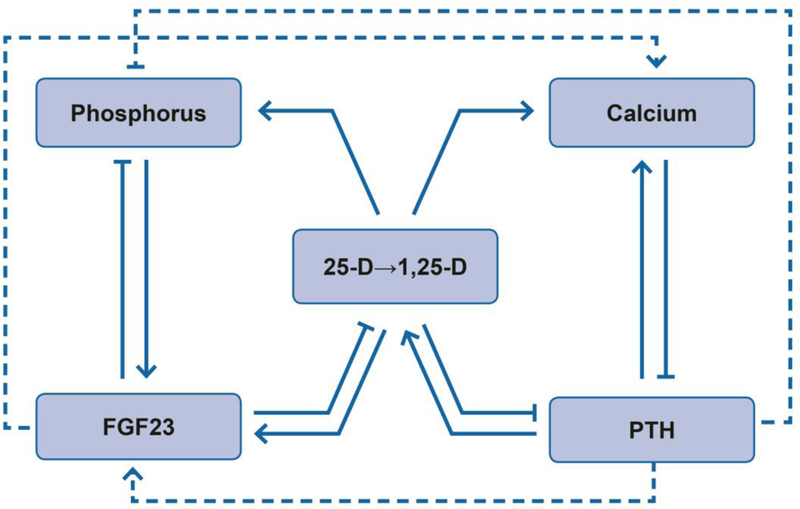
Solid lines and arrows depict dominant physiologic effects on circulating MBD factors including: (1) effects of fibroblast growth factor 23 (FGF23) to lower serum phosphorus by inhibiting 1α-hydroxylase that converts 25-hydroxyvitamin D (25-D) to 1,25-dihydroxyvitamin D (1,25-D) and stimulating urinary phosphorus excretion; (2) effects of parathyroid hormone (PTH) to raise serum calcium by stimulating 1α-hydroxylase and increasing urinary calcium reabsorption and bone remodeling; (3) effects of vitamin D to promote gastrointestinal absorption of calcium and phosphorus and feedback on FGF23 (stimulation) and PTH (inhibition). Dashed lines and arrows depict additional non-dominant effects of FGF23 to increase urinary calcium reabsorption and PTH to increase urinary phosphorus excretion and FGF23 transcription. In each case, arrows (→) represent actions that raise the associated MBD factor whereas capped lines (┤) represent actions that lower the associated MBD factor. Altogether, the physiology encompasses major effects of FGF23 to reduce phosphorus and vitamin D and major effects of PTH to increase calcium and vitamin D, along with substantial redundancy, feedback, and crosstalk.
Exacerbating this physiologic spiral in CKD is a simultaneous loss of expression of α-klotho, the co-receptor for FGF23.7 Klotho is a membrane-bound protein that interacts with fibroblast growth factor (FGF) receptors to increase their specificity for FGF23 and to drive signaling pathways that are responsible for its effects on mineral ion balance and vitamin D metabolism.8 In animals, klotho transcription can be suppressed by FGF23, phosphorus loading and vitamin D deficiency, suggesting additional physiologic feedback.9 Additionally, klotho can be cleaved or alternatively spliced to result in soluble forms of unclear physiologic significance.10–12 Ultimately, the additional loss of klotho activity creates resistance to FGF23, further driving levels to concentrations up to 100–1000 times normal in end stage renal disease (ESRD).13,14
In this review we provide a high-level overview of the current knowledge linking the above described changes in mineral ion and vitamin D homeostasis with cardiovascular disease in CKD. With many high quality and comprehensive reviews in the field, here we focus on integrating across epidemiology, basic biology, precision medicine, and therapeutic trials to derive ‘big picture’ lessons on the road ahead. We highlight the many promising and biologically intertwined targets in MBD and argue that its inherent complexity and feedback will require a holistic, multifaceted approach aimed at the full mineral and vitamin D axis to provide the highest likelihood of success in identifying and combating its role in cardiovascular disease.
Epidemiologic Associations of MBD and Clinical Cardiovascular Disease
The recognition of MBD as a risk factor for premature mortality and cardiovascular disease largely began with observations about risk related to higher levels of serum phosphorus.15 Reports linked higher serum phosphorus to heightened risk of all-cause and cardiovascular mortality, cardiovascular events, and heart failure in many populations including patients with kidney disease and the general population.16–21 Meanwhile, as this literature proliferated, FGF23 was discovered as a novel hormone regulating both phosphorus and vitamin D homeostasis.22,23
Since its discovery, higher FGF23 has been strongly associated with numerous adverse outcomes including all-cause and cardiovascular mortality, cardiovascular events, atrial fibrillation, and heart failure with a generally larger magnitude of association than observed in studies of phosphorus.14,24–33 Like phosphorus, increased cardiovascular risk associated with higher FGF23 was reported in a wide variety of groups including those with ESRD, CKD, and the general population with the strongest risks in patients with CKD and ESRD.34 Although FGF23 has been associated with many non-cardiovascular outcomes as well,29,33,35–38 some of the most commonly reported and robust associations are with heart failure.34
FGF23 has a feedback relationship with its co-receptor, klotho, such that klotho deficiency can raise FGF23 and high FGF23 can exacerbate klotho deficiency via low 1,25-D.9 Using epidemiology to disentangle the exact role of klotho versus FGF23 in cardiovascular disease is challenging because klotho functions primarily as a membrane-bound receptor in tissues which are not regularly sampled in human studies. Cleaved and alternatively spliced forms of klotho circulate, but their role is not firmly established.10 Current measurement methods to detect circulating klotho have significant limitations due to assays and sample stability.7,39 For instance, although animal models show decline in membrane-bound klotho with worsening kidney function, many of the existing studies of circulating klotho in humans show only a modest or no decline in klotho expression with worse CKD.40–42 In light of these challenges, most epidemiologic studies of klotho are small with limited power and show mixed results.39,40,43–46 Only a few studies report that reduced circulating klotho is associated with increased mortality or worse cardiovascular outcomes including atrial fibrillation.39,46
Vitamin D deficiency is a central link between FGF23, klotho, phosphorus and other MBD components such as PTH and calcium. A large observational literature reports associations between low levels of vitamin D with incidence of several cardiovascular phenotypes, including cardiovascular mortality and major cardiovascular events, with most studies focused on 25-D.47–50Despite many studies, relationships between vitamin D and cardiovascular disease have not been robust across different populations,51,52 in meta-analyses of cohort studies, or in clinical trials primarily of surrogate outcomes.53,54 However, not all of these studies have targeted CKD populations in which vitamin D metabolism is deranged resulting in low levels of 1,25-D specifically. Observational studies within the ESRD population in particular demonstrate that treatment with 1,25-D in the form of calcitriol or vitamin D analogs is associated with improved mortality.55,56 Placebo-controlled clinical trials have not been performed to confirm these benefits in ESRD. In pre-dialysis CKD, trials of 1,25-D with surrogate cardiovascular outcomes have been performed with mixed results.57–59
One of the challenges with treating and studying vitamin D in the CKD and ESRD populations is that 1,25-D is often not measured clinically or in cohorts and may not be reliably assessed in the circulation due to its shorter half-life and significant local production and paracrine action. High PTH and lower serum calcium both are common clinical ‘read-outs’ of 1,25-D deficiency that are associated with mortality in some observational studies in CKD, but not consistently.17 Magnesium is an additional factor that may interact with vitamin D and calcium homeostasis promoting deficiency of these MBD components.60 Serum and dietary magnesium have received increasing attention as risk factors for cardiovascular disease in the general population and populations with CKD.21,61,62 In ESRD patients on hemodialysis, low magnesium dialysate which depletes total body magnesium also associates with higher cardiovascular risk.63
MBD and Mechanisms of Cardiovascular Disease
The well-established epidemiologic links of the MBD components with clinical cardiovascular disease have stimulated a large body of work evaluating pathways that could mediate these effects. Discovery of biologically plausible pathways of cardiovascular disease that can be demonstrated experimentally in animals or observed consistently in studies of patients can help bolster the conclusion that the epidemiologic associations are potentially causal and not merely the result of confounding or other biases. In this section we briefly review some of the candidate mechanisms with support in the literature.
Vascular and Endothelial Function
Vascular calcification is common and premature in patients with kidney disease leading to poor vascular compliance and end organ damage.64–66 Procalcific stimuli in kidney disease include increased serum phosphorus, excess use of vitamin D, calcium loading from calcium-based phosphorus binders, supplements and dialysate, and inflammation. These effects can be exacerbated by insufficient activity of calcification inhibitors, including magnesium, vitamin K, or reduced klotho expression.67,68 For instance, phosphorus loading and klotho depletion consistently induce calcification and reduce vascular compliance in vitro and in vivo in animals.69–71 Similarly, restoration of normal klotho expression in animals with vitamin D agonists, or reduction in phosphorus exposure through diet can reverse or prevent the calcific phenotype.72,73 In patients, simultaneously reducing procalcific stimuli, such as phosphorus, while bolstering calcification inhibitors through use of vitamin D, magnesium or vitamin K may be a strong rational for combination therapy of MBD, but trials are needed.
Apart from calcification per se, vascular and endothelial dysfunction are common sequelae of CKD, with several studies implicating features of MBD in this altered physiology. High phosphorus impairs endothelial function in vitro and in patients,74–76 whereas soluble klotho appears to have beneficial effects on the vasculature by promoting the survival of vascular smooth muscle cells and stimulating nitric oxide production.76,77 Due to their effects on klotho or directly via the vitamin D receptor, vitamin D and vitamin D receptor agonists improve endothelial function and vascular stiffness in multiple pilot studies in CKD.58,78–81 The role of FGF23 in the vasculature is less clear, however indirect effects are plausible via its interaction with the renin-angiotensin-aldosterone system (RAAS), promotion of inflammation, and antagonism of active vitamin D.82–84 To better understand the landscape of ongoing, but unpublished, studies in MBD and cardiovascular disease we performed a review of registered ongoing studies in ClinicalTrials.gov using keyword terms for MBD components and focused on interventional studies in active, ongoing or recruiting status. Based on our review, the role of 1,25-D, magnesium, and vitamin K in reducing vascular calcification in CKD and the role of dietary phosphorus in vascular stiffness and endothelial function are the subject of ongoing studies (See Selected Examples in Table 1)85,88.
Table 1.
Selected Registered Clinical Trials* Addressing Mineral and Bone Disorder (MBD) and Cardiovascular Disease (CVD) or Personalized MBD Therapy
|
Example Ongoing
Studies of MBD and CVD Registered on Clinicaltrials.gov | ||||||
|---|---|---|---|---|---|---|
| NCT # | MBD/CVD | Population |
ClinicalTrials.gov Status |
Intervention | Duration | CVD Outcome |
| 03234361 | Inorganic phosphorus and BP |
Healthy individuals |
Ongoing | Sodium phosphate vs. sodium chloride |
4 weeks | 24 hour ABPM |
| 02837328 | Magnesium and arrhythmia |
Healthy individuals |
Recruitment completed |
Magnesium citrate vs. placebo |
4 weeks | Premature atrial contractions |
| 02620449 | Dietary phosphorus and vascular function |
Healthy individuals | Ongoing | Diet low in additive- based phosphorus |
10 weeks | Pulse wave velocity; Flow- mediated dilatation- |
| 02545426 | Dialysate calcium and myocardial stunning |
Patients with ESRD on hemodialysis |
Recruitment completed |
Dialysate calcium 2.5 vs. 3.5 mEq/L |
1 week | Echocardiographic strain |
| 02224144 | 1,25-D and vascular calcification |
Kidney transplant recipients |
Active, not recruiting |
Calcitriol vs. placebo added to vitamin D3 |
12 months |
Lower
extremity vascular calcifications |
| 02542319 | Magnesium supplementation and vascular calcification |
Patients with CKD | Recruiting | Oral magnesium supplementation |
12 months |
Coronary artery calcification score |
| 02087683 | Vitamin D and cardiac remodeling |
Patients with heart failure and vitamin D deficiency |
Recruiting | Vitamin D supplementation |
12 months |
Change in myocardial function and structure |
|
01528800 02870829 01742273 |
Vitamin K and vascular calcification |
Patients with ESRD on hemodialysis |
Active,
not recruiting/ Recruiting |
Vitamin K | 12– 18month s |
Coronary artery calcification |
| 02258074 | Phosphorus lowering and cardiac remodeling |
Patients with CKD | Active, not recruiting |
Factorial: Lanthanum carbonate vs. placebo, nicotinamide vs. placebo |
12 months |
Left ventricular mass and geometry |
|
Example Ongoing
Studies of Personalized MBD Therapy Registered on Clinicaltrials.gov | ||||||
| NCT # | MBD Theme | Population |
ClinicalTrials.gov Status |
Intervention/Question | Duration | Personalized Outcome |
| 02925195 | Vitamin D response | General population | Ongoing | Cholecalciferol; Effect modification by clinical traits and genes |
16 weeks | Vitamin
D concentrations; PTH |
| 02802449 | Vitamin D in African Americans |
African Americans | Active, not recruiting |
Vitamin
D supplementation; Interaction with genetic and biologic factors |
8 weeks | PTH; inflammatory markers |
| 03134417 | Vitamin D and magnesium |
Individuals with obesity |
Recruiting | Vitamin D vs. Vitamin D plus magnesium vs. placebo; Magnesium as critical co-factor |
12 weeks | PTH; lipids; BP |
| 02572960 | Vitamin D, PTH and RAAS interaction |
Vitamin D deficiency and secondary hyperparathyroidism |
Recruiting | Factorial: Valsartan vs. placebo, Vitamin D3 vs. placebo; Interaction of Vitamin D and RAAS |
12 weeks | Aldosterone; PTH; vascular measures |
NCT , ClinicTrials.gov identifier; BP, blood pressure; ABPM, ambulatory blood pressure monitoring; ESRD, end-stage renal disease; 1,25-D, 1,25-dihydroxyvitamin D; CKD, chronic kidney disease; PTH, parathyroid hormone; RAAS, renin angiotensin aldosterone system
Studies of MBD were identified by systematically searching ClinicalTrials.gov using keywords including “phosphorus”, “calcium”, “parathyroid”, “vitamin D”, “fibroblast growth factor 23”, “magnesium”, and “mineral and bone disorder”. Subsequently, example interventional studies were selected from among those in active, ongoing or recruiting status.
Hypertension and RAAS Activation
Elevated blood pressure is a major risk factor for clinical cardiovascular disease, including coronary artery disease, stroke and heart failure, with a growing burden globally.89 Vitamin D deficiency has long been linked to hypertension observationally.53 High levels of vitamin D inhibit renin thereby downregulating RAAS and potentially blood pressure.90 Clinical trials have not confirmed benefits of vitamin D on brachial blood pressure,53 however a recent study suggests a potential impact on central blood pressure which was not measured in many prior studies.91
In animal models high dietary phosphorus also raises blood pressure.92,93 Investigators have suggested potential mechanisms including changes in sympathetic activity,92 activation of the RAAS,93 or effects of phosphorus-regulatory hormones on sodium excretion.94 Observational human studies have largely reported a neutral or favorable association between higher phosphorus intake and lower blood pressure;95–97 However these studies cannot easily distinguish inorganic phosphorus common in dietary additives from healthy sources of phosphorus such as whole grains, legumes, dairy, and animal-based protein that are believed to have blood pressure lowering effects.98 Other minerals including calcium and magnesium may also be important in blood pressure regulation and often correlate with dietary phosphorus intake.99–101 Ongoing interventional studies may help reconcile the effects of phosphorus additives on blood pressure in healthy individuals (Table 1).88
The role of FGF23 and klotho in blood pressure is less well studied. Recent reports suggest an association of FGF23 with hypertension, potentially due to increasing expression of the sodium chloride co-transporter in the distal tubule.94,102 FGF23 may also increase blood pressure indirectly through its inhibition of 1,25-D or by activating RAAS directly.103 Some support for this hypothesis comes from studies demonstrating an impact of FGF23 to reduce angiotensin converting enzyme 2, an enzyme which degrades angiotensin.82,103 Although this area of investigation is immature, remarkably high risk of blood pressure dependent outcomes such as heart failure and stroke,34 and interactions between circulating FGF23, phosphorus and efficacy of RAAS blockade and diuretics suggests that this is a fruitful area for additional investigation.104–109
Abnormal Cardiac Geometry and Function
In many human studies, abnormalities of MBD components including phosphorus, vitamin D, PTH and FGF23 associate with adverse changes in cardiac structure or function that are independent of static blood pressure.16,18,53,110 Relationships of higher FGF23 with left ventricular hypertrophy, concentric and eccentric remodeling and diastolic dysfunction are some of the most widely reported, with independent associations found in adults and children with and without kidney disease. 31,111–117.Experiments in preclinical models suggest that these associations may be the result of direct effects of FGF23 on the myocardium,113,118,119 due to biased signaling via fibroblast growth factor receptor 4 (FGFR4) without klotho as a co-receptor.120,121 Perhaps by promoting this klotho-independent signaling pathway, cardiac hypertrophy is present in heterozygous klotho knockout mice who also have higher FGF23.113,122,123 Additionally, experimental manipulations that increase klotho can partially abrogate hypertrophy related to these and other stimuli.122,124 Given their inherent feedback in many studies it is difficult to disentangle direct klotho effects from compensatory changes in FGF23. Nonetheless, the current framework in which adverse cardiac effects of FGF23 rely on klotho-independent signaling,120 suggests a unifying paradigm in which high FGF23 coupled with low klotho expression increasingly directs FGF23 signaling towards its klotho-independent adverse effects.121 With this in mind interventions to reduce FGF23, raise klotho, or both need to be tested in patients with CKD to improve cardiac remodeling and function. Reduction in dietary or serum phosphorus could be one strategy supported by some observations, as could interventions to increase klotho expression via 1,25-D.18,110 Initial trials of phosphorus reduction are underway (Table 1).88,125 Trials of active vitamin D sterols in patients with CKD have not demonstrated the expected benefits on cardiac remodeling to date.57,59
Abnormal Cardiac Rhythm and Sudden Death
Managing MBD can be particularly difficult because of the critical role of calcium and magnesium in cardiac conduction. Uncontrolled MBD is often characterized by hypocalcemia related to severe 1,25-D deficiency. Furthermore, core treatments for MBD including nutritional and active vitamin D, calcimimetics and phosphorus binders can affect these divalent cations as major adverse effects. Use of low dialysate calcium (<2.5 mEq/L) and magnesium (<1.0 mEq/L) have each been associated with increased risk of sudden death, presumably of an arrhythmic cause.63,126 Other mineral metabolites including high levels of FGF23 and low klotho have been associated with arrhythmias such as atrial fibrillation in some studies, potentially due to effects on cardiac remodeling.25,28,46
Confounding and Limitations
Despite compelling and consistent associations of many components of MBD with cardiovascular disease and plausible disease pathways, residual confounding of these relationships is hard to rule out without interventional trials. For example, high levels of FGF23 may be driven by other adverse risk factors including iron deficiency, inflammation, obesity and hyperaldosteronism.83,127–129 Additionally, inadequate nutrition and chronic disease such as gastrointestinal and liver disease may impact levels of 25-D, calcium, and magnesium resulting in confounding.60,130 25-D may be impacted by reduced outdoor activity, obesity or an unhealthful diet without normal dietary sources such as fatty fish and fortified foods,54,130 and multiple MBD factors including 25-D, phosphorus, FGF23 and PTH are affected by kidney function (Figure 2).3 Each of these factors and others may confound the associations seen in many observational studies.
Figure 2. Examples of Major Factors that Influence Mineral and Bone Disorder (MBD).
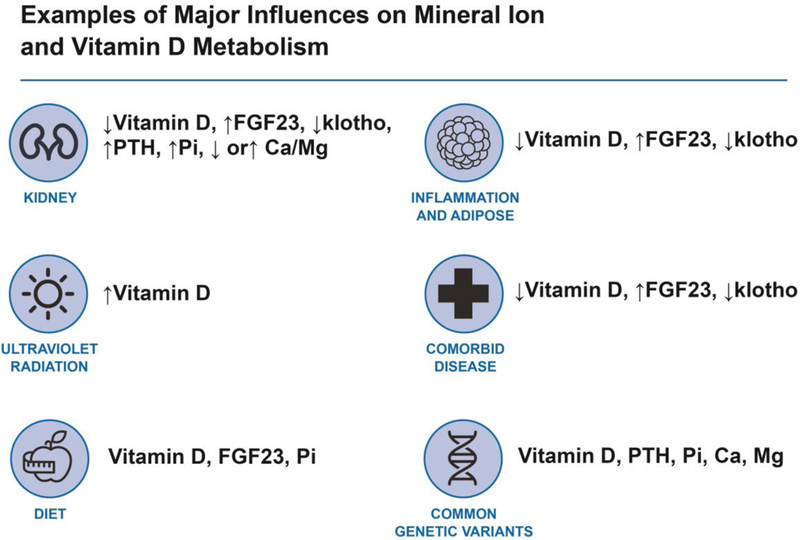
These factors may confound associations or interact to modify associations leading to heterogeneity across studies. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; Pi, inorganic phosphorus; Ca, calcium; Mg, magnesium.
In animal models, FGF23 transcription is induced by adverse physiologic events such as myocardial ischemia, myocardial pressure overload and acute kidney injury.131–133 Expression of FGF23 has also been reported in coronary plaques and cardiac tissue from CKD patients.134 These studies raise the possibility that strong associations between FGF23, cardiovascular outcomes and mortality could be driven by reverse causality where FGF23 is a result, not a cause, of pathophysiologic changes. In nearly all cases, the complex web of MBD changes induced by interventions in animal models makes it difficult to isolate a single factor as the cause of the phenotype. With this in mind interpreting the full body of evidence including observational studies, animal studies, trials and other robust designs is needed to evaluate the most promising targets.
“Precision” Epidemiology of MBD and Cardiovascular Disease
In light of the numerous risks reported in human and animal studies, targeting MBD risk factors for cardiovascular disease prevention appears obvious and deceivingly simple at first glance. However, initial biologic agents aimed at reducing FGF23 demonstrated increased mortality in preclinical rat models,135 and phosphorus lowering therapies paradoxically associated with increased vascular calcification in initial small clinical trials.136 Small trials of dietary phosphorus reduction and vitamin D supplementation show mixed effectiveness on surrogate outcomes such as MBD risk factors and cardiac geometry.16,53,125 Fully-powered placebo-controlled trials of phosphorus binders and vitamin D sterols have not been conducted, but an initial large trial of cinacalcet to lower PTH failed to demonstrate a reduction in cardiovascular disease.137 These observations could suggest that the epidemiologic associations described above are not truly causal, but are instead the result of confounding and other biases. Alternatively, heterogeneous risks and treatment effects across groups, or the substantial biological tensions in MBD physiology (Box) may cause discrepant, and sometimes unexpected, results across studies. The precision medicine paradigm provides a deeper lens to bolster causal inference and to explore heterogeneity and interactions among different MBD factors. For instance, if multiple disparate factors that converge to raise 25-D show expected associations with a disease outcome, then the evidence for a central role of 25-D in the disease process is strengthened.
In the next section we review the emerging epidemiologic literature with a precision focus, evaluating known genetic, environmental and other contextual factors that can help strengthen causal inference around MBD and cardiovascular disease and calibrate MBD biomarkers and health interventions to specific populations. Although not exhaustive, the examples described demonstrate the role these studies may play in understanding the potentially causal role of MBD in cardiovascular disease and in resolving across-study heterogeneity.
Genomics
A robust body of literature describes differences in MBD physiology by race and ethnicity, suggesting potentially important genetic contributions.138 A search of the Database of Genotypes and Phenotypes identifies over 50 unique single nucleotide polymorphisms (SNPs) in or near >40 genes that have been linked to circulating MBD factors, including phosphorus, calcium, magnesium, vitamin D, vitamin D binding protein and PTH.139–149 A selected list of the most relevant genes and their functions related to MBD are provided in Table 2. Some of these candidate MBD genes have at least suggestive associations with cardiovascular phenotypes including coronary heart disease (GALNT2; and ATP2B1)150,151 and inflammation (GCKR)152–155 in genome wide studies, and hypertension in genome wide and confirmatory studies (CASR; and ATP2B1).9,156–166 Variants in the gene encoding the calcium-sensing receptor (CASR) have been marginally associated with cardiac valve calcification in small studies.167 These common associations could suggest that mineral metabolites are in fact on the causal pathway between these genes and cardiovascular disease ( Figure 3a); however other possibilities include pleiotropic gene effects that affect both pathways independently (Figure 3b), such as effects of GALNT2 on serum lipids,168,169 or GCKR on inflammation.152–155
Table 2.
Selected Examples of Proposed Genes Related to Mineral and Bone Disorder (MBD) Identified on Database of Genotypes and Phenotypes (dbGaP)139 and Published Links to Cardiovascular Disease (CVD)
| MBD Phenotype | Example Proposed Gene | MBD-Related Function | Example of Described Relationship to CVD |
|---|---|---|---|
| Vitamin D | |||
| Circulating DBP147 | GC | Encodes DBP | |
| ST6GALNAC3 | Glycosylates DBP | ||
| GALNT2 | Glycosylates DBP | Lipids168,169; Coronary Artery Disease151 | |
| 25-D143,145 | DHCR7 | Biosynthesis of vitamin D3 | |
| CYP2R1 | Biosynthesis of 25-D | Coronary Artery Disease183 | |
| GC | Encodes DBP | ||
| Calcium (Ca) 141,142 | |||
| CASR | Calcium-sensing receptor | Myocardial Infarction175, Hypertension166 | |
| CYP24A1 | Degrades 25-D and 1,25-D | Coronary Artery Calcification184 | |
| GATA3† | Differentiation of parathyroid gland |
||
| CARS | Linked to Mendelian calcemic disorders |
||
| GCKR | Unknown; May decrease serum albumin |
Inflammation152–155 | |
| Phosphorus146 | |||
| CASR† | Calcium -sensing receptor | Myocardial Infarction175, Hypertension166 | |
| FGF23† | Phosphaturic hormone | ||
| SLC34A1† | Sodium-phosphate co- transporter |
||
| ALPL† | Encodes alkaline phosphatase |
||
| Magnesium (Mg) 148,149 | |||
| TRMP6 | Magnesium transport in kidney and gut |
||
| ATP2B1† | Ca-ATPase; may control Mg cellular efflux |
Hypertension9,156–165; Coronary Artery Disease150 |
|
| Parathyroid Hormone144 | |||
| CYP24A1 | Degrades 25-and 1,25-D | ||
| CASR† | Calcium-sensing receptor | Myocardial Infarction175, Hypertension166 | |
| CLDN14† | Paracellular transport of calcium and magnesium |
Note: Single nucleotide polymorphism (SNP) associated with MBD phenotype is within the proposed gene unless noted otherwise.
Proposed gene is located near or in strong linkage disequilibrium with a SNP that has been associated with the MBD phenotype MBD, mineral and bone disorder; CVD, cardiovascular disease; DBP, vitamin D binding protein; 25-D, 25-hydroxyvitamin D; 1,25-D, 1,25-dihydroxyvitamin D
Figure 3. Theoretical Framework for Incorporating Genetic Data in Studies of Mineral and Bone Disorder (MBD) and Cardiovascular Disease.
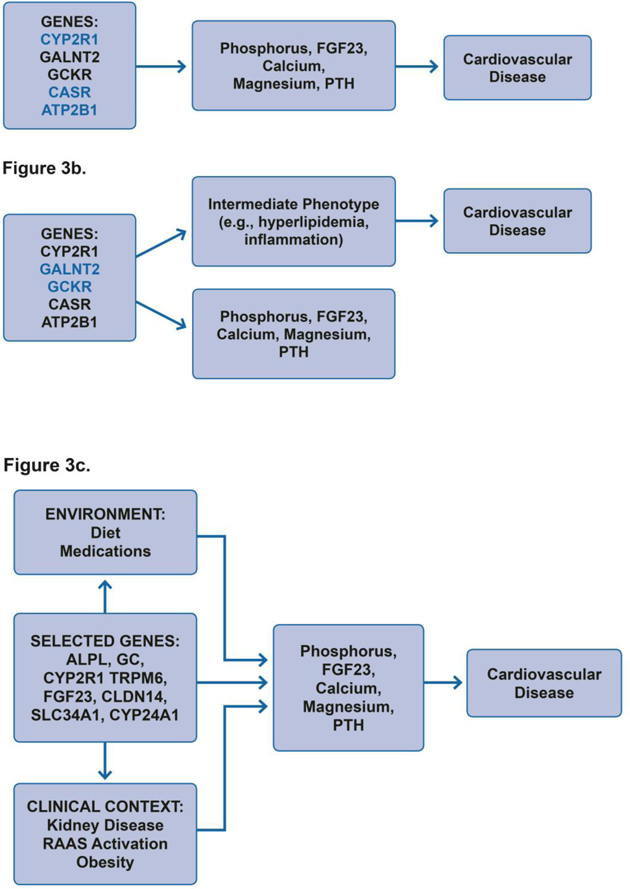
In panel A genetic variants impact cardiovascular disease via effects on MBD intermediates without any other influences on cardiovascular disease pathways supporting causal inference. In panel B genetic variants may influence both MBD and other unrelated intermediate pathways leading to cardiovascular disease, demonstrating pleiotropy. Candidate genes with known primary biological effects on MBD and without other known unrelated biological effects may provide stronger causal inference (blue 3a) compared to genes with known pleiotropic effects (blue 3b). In panel C MBD related variants may interact with other patient and environmental factors to more strongly influence MBD and cardiovascular disease. Studies elucidating these effects may help identify subtle effects on common environmental exposures, explain across study heterogeneity, and allow personalized therapy. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; RAAS, renin angiotensin aldosterone system.
However a large number of MBD genes, including some of the candidates that are most likely to directly affect mineral metabolite levels based on our current biological understanding, have not emerged in genome wide screens for cardiovascular disease.170–174 On the one hand the lack of associations could suggest that the MBD-disease associations are not causal, but these conclusions are challenging because some MBD traits may not be strongly influenced by common genetic variants. In addition, known MBD genes may have small effects on the biological risk factors and disease that are difficult to rule out in large scale genetic studies that employ stringent statistical criteria for significance due to the large number of tests performed. Candidate gene and Mendelian Randomization studies can evaluate the effect of specific genes that are known to be important in MBD and determine purportedly causal associations with cardiovascular risk. Only a few studies to date have evaluated cardiovascular outcomes related to SNPs influencing vitamin D, phosphorus, calcium, magnesium, PTH, klotho and FGF23 as candidates. In some, variants in CASR associate with high PTH as well as coronary artery disease.175 In others, no clear associations have emerged.176 Some, but not all,177–182 candidate gene and Mendelian Randomization studies have linked genetic differences in vitamin D metabolism to cardiovascular disease including hypertension, heart failure, coronary artery calcification, and atherosclerotic coronary artery disease.183–186 Variants in the klotho gene have been linked to mortality in ESRD, particularly in the absence of active vitamin D sterols which augment endogenous klotho.187 In some small studies klotho variants associate with vascular disease or risk of kidney disease.188,189 However, associations of klotho variants with vascular calcification was not observed in a larger, healthy population.190
Inconsistent and absent genetic associations may also reflect substantial complexity in the role of MBD factors in cardiovascular disease pathogenesis. For instance, effects of MBD genes on cardiovascular disease could depend upon other factors including environmental and host factors that interact to cause disease. An emerging body of literature supports interaction between vitamin D-related genes and circulating vitamin D for some cardiovascular event types, such as heart failure and possibly stroke, as well as other adverse event composites.51,191–193 Others demonstrate interactions between the genotype of vitamin D pathway genes and the efficacy of vitamin D supplementation.194 To continue to deepen our understanding of how MBD may contribute to cardiovascular disease pathogenesis we need additional studies that account for the confluence of multiple biological factors, including genetic factors, and exacerbating factors in our environment and patients (Figure 3c).
Environmental influences and biologic processing
Environmental factors can interact with MBD components to alter their pharmacokinetics. These environmental influences need to be considered not only confounders but also as potential biological interactions that may affect the impact of genes, treatments or other exposures related to MBD. For instance, cutaneous synthesis of vitamin D, which is a primary source of circulating 25-D in humans, is increased by environmental factors that expose individuals to greater intensity and duration of sunlight. Factors that have been described in the literature include latitude closer to the equator, outdoor occupations and recreational activities, and seasonal changes, all of which affect intensity and cumulative exposure to sunlight-derived ultraviolet radiation.130,195,196 Similarly, dietary composition and use of mineral/vitamin supplements influence circulating levels of calcium, phosphorus, magnesium, and 25-D. A recent trial demonstrated increased efficacy of vitamin D supplementation in the setting of common variants in vitamin D pathway genes including the vitamin D receptor, CYP2R1 encoding the 25-hydroxylase, and CYP24A1 encoding the 24-hydroxylase.194 Additional studies in this area are ongoing (Table 1). Studies of gene-environment interaction in other MBD components may identify additional complex determinants of both circulating levels and, ultimately, treatment efficacy.
Certain medications and comorbid illnesses may also influence pharmacokinetics of MBD components and modify the effectiveness of supplementation or restriction. Examples include intake of prebiotics, such as non-digestible oligosaccharides, that increase intestinal absorption of calcium, magnesium, and other minerals,197,198 as well as oral contraceptives that may increase 25-D in women by increasing circulating levels of vitamin D binding protein.199,200 Obesity also may interact with 25-D levels by providing a storage depot for the fat-soluble 25-D.130 Thus, deeper study of the complex interaction of genomic, dietary and other biologic factors could help resolve inconsistency across studies and personalize therapy. Our review of ClinicalTrials.gov identified several examples of ongoing studies leveraging interaction between MBD components and genes, obesity and other biologic factors (Table 1).
Treatment of MBD and Cardiovascular Disease
Although few well-powered clinical trials are currently available to guide management of MBD, initial proof-of-concept studies in the general population, CKD and ESRD continue to proliferate to help solidify the role of MBD in cardiovascular disease and refine future approaches. Several key principles that could guide management of MBD include the following:
1. Reduce exposure to highly absorbable phosphorus.
High phosphorus exposure can induce adverse biochemical changes including rise in serum phosphorus, FGF23, PTH, and fall in klotho and 1,25-D.16 Whether phosphorus binders or other pharmacologic agents, such as niacin derivatives and tenapanor that block gastrointestinal phosphorus absorption, can reverse these changes in CKD or ESRD remains controversial and is an area of active investigation.125,201–206 In observational comparative effectiveness studies, use of phosphorus binders associates with improved survival in ESRD and CKD.207–209 In ESRD, studies of alternative phosphorus binders have been conducted,210–213 but there are not adequately powered studies of phosphorus binders versus placebo or alternative serum phosphorus targets.214 In CKD, initial studies have provided some warnings including a paradoxically higher risk of vascular calcification with phosphorus binders and higher mortality in trials of niacin.136,215 Before advocating expanded indications for these treatments adequately powered and rigorous studies are needed.
At a population level, reducing exposure to food additives containing highly absorbable phosphorus salts is an attractive strategy for preventive health. Although only a few observational studies have clearly demonstrated risks of high dietary phosphorus intake, interventional studies have shown that increased intake of highly absorbable phosphorus salts can drive higher FGF23, lower 1,25-D and, to a lesser extent, improve serum phosphorus, with reduced intake mitigating some of these changes.16,216,217 Ongoing studies continue to evaluate this critical public health question (Table 1).88
2. Pharmacologic replacement of vitamin D.
Although observational studies suggest benefits of 1,25-D and activated vitamin D analogs in patients with ESRD,55,56 no clinical trials have demonstrated a reduction in cardiovascular events or mortality with these therapies. Their widespread use in ESRD to control secondary hyperparathyroidism will make placebo controlled trials unlikely. Effects of 1,25-D to increase calcium and phosphorus also need to be weighed. Theoretically, additional effects of 1,25-D to raise FGF23 may be harmful or offset by stimulation of endogenous klotho expression. Studies evaluating dosing and monitoring of vitamin D agents in CKD are needed to provide optimal replacement without adverse MBD-related effects. Trials of 1,25-D or active vitamin D analogs in pre-dialysis CKD are currently sparse, with some studies demonstrating modest benefits on surrogate outcomes,58,78–81,218 but several studies demonstrating no benefit.57,59 Currently there are no adequately powered, placebo-controlled trials of hard clinical events.219
Treatment of 25-D deficiency with high dose cholecalciferol may lower PTH in CKD,220 however definitive effects on cardiovascular disease have not been demonstrated.219 Ongoing trials may shed some light in these areas including a large-scale study in the general population focused on the effects of 25-D supplementation on cardiovascular events. Nested ancillary studies will evaluate mechanisms including hypertension and atrial fibrillation.88,221,222
3. Calcimimetics to lower PTH and FGF23.
Whereas vitamin D receptor agonists raise FGF23 through a positive feedback loop, treatment with calcimimetics lowers FGF23, perhaps as an indirect effect of PTH reduction.4,223–225 As expected based on the integrated physiology of MBD (Figure 1), calcimimetics also reduce 1,25-D. Thus, in ESRD when PTH and FGF23 are no longer effective at promoting urinary phosphorus excretion, they have the additional biochemical benefit of lowering phosphorus. On the other hand, lowered 1,25-D and impaired PTH response results in calcium lowering as well, a potentially dangerous side effect.137 Despite favorable effects of the calcimimetic cinacalcet on phosphorus and FGF23, a reduction in cardiovascular events was not seen among patient with ESRD on dialysis randomized to cinacalcet compared with placebo in the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial.137 An observational post-hoc analysis of EVOLVE demonstrated a reduction in cardiovascular events among participants randomized to cinacalcet that achieved a ≥30% reduction in FGF23 compared to those who did not.223 This nonrandomized result should be viewed cautiously due to null results in the overall trial137 and because reduction in FGF23 could be more simply a marker of adherence to therapy. More adherent participants regularly experience better outcomes compared to less adherent participants in trials even with placebo or ineffective interventions.226
With multiple examples of biological interactions and tensions between MBD components, approaches may need to consider strategies that achieve balance in MBD control (Box). For instance therapies that lower FGF23 directly, such as cinacalcet, may be most effective in ESRD on dialysis, but have risks in CKD where FGF23 promotes urinary phosphorus excretion and maintains phosphorus control. Optimal dosing of vitamin D, cinacalcet and phosphorus binders must achieve the potential benefits without excessive effects on calcium and phosphorus that could be harmful. The interaction of many MBD components suggests potential value of multifactorial therapy for MBD. In clinical trials of patients with ESRD on dialysis, combination therapy with vitamin D agonists and calcimimetics yields the most favorable changes across all MBD components due to their opposing effects on phosphorus, calcium and FGF23.227,228 Studies of clinical outcomes using combination treatment strategies are needed. In the future, our growing understanding of FGF23 and klotho signaling may also suggest novel biologic agents that could interrupt adverse signaling pathways while maintaining function of critical homeostatic functions.
Challenges and Future Directions in MBD and Cardiovascular Disease
Translating the complex biology of MBD to ultimately benefit patients will require observational and interventional studies to capture and consider a wide variety of influences on MBD, including genes, environment, diet, and disease status, to identify target groups and best approaches (Figure 2). Recent pharmacogenomics studies provide a few examples that may point to the future of MBD-cardiovascular disease studies, including identification of common variants in CASR that predict adverse hypocalcemic side effects of cinacalcet; variants in vitamin D binding protein and the vitamin D receptor that modify associations between 25-D and risk; and variants in vitamin D metabolic pathways that predict the response to vitamin D supplementation.51,194,229 To understand these important disease and therapeutic modifiers, future studies will need to enroll and study diverse populations with adequate power for subgroup and interaction testing. Expansion of big data through electronic health records and a growing commitment to data sharing should make such studies more feasible and promote advances.
Additionally, widespread improvements in measurement of the MBD will also transform our ability to study and treat its potential effects on cardiovascular disease. Critical measurement challenges include diurnal, seasonal and random variation in many MBD components that limit reproducibility and comparability over time and across individuals.230,231 Poor assay calibration and standardization continues to hinder studies of novel MBD risk factors, such as soluble klotho and FGF23, and limit the utility of established clinical measurements for PTH and vitamin D.7,232,233 Ongoing efforts to improve research assays, and standardize performance and calibration for clinical assays will enable progress in the field.234,235
While daunting, ‘next generation’ epidemiologic studies and targeted trials may help support causal inference and resolve heterogeneity in MBD-cardiovascular disease studies to risk stratify and optimally treat patients for MBD. The tremendous biological insights of the last two decades in MBD broadly have set the stage for rational therapies approaching the complex and intertwined physiology, ultimately leading to real success for future patients.
Text Box Key Biological and Therapeutic Tensions in CKD-MBD.
Balance between FGF23 and klotho
Potential tradeoff between FGF23 and phosphorus control (CKD)
Tradeoff between PTH and calcium control
Therapeutic window of Vitamin D and calcium-based binders
Acknowledgements
This study was supported in part by R01DK111952 (JS) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Duke O’Brien Center for Kidney Research (P30DK096493). The perspectives presented here are the opinions of the authors and do not necessarily reflect the position of the NIDDK.
Financial Support: JJS is supported by R01DK111952 and P30DK096493
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relevant Disclosures: none
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Mineral and Bone Disorder Working Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter CS, Slatopolsky E. Phosphate Toxicity in CKD: The Killer among Us. Clin J Am Soc Nephrol 2016;11(6):1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naveh-Many T, Silver J. The Pas de Trois of Vitamin D, FGF23, and PTH. J Am Soc Nephrol 2017;28(2):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Yang J, Li L, Huang J, King G, Quarles LD. Conditional Deletion of Fgfr1 in the Proximal and Distal Tubule Identifies Distinct Roles in Phosphate and Calcium Transport. PLoS One 2016;11(2):e0147845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 2014;33(3):229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 9.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol 2013;180:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrukhova O, Bayer J, Schuler C, Zeitz U, Murali SK, Ada S, Alvarez-Pez JM, Smorodchenko A, Erben RG. Klotho Lacks an FGF23-Independent Role in Mineral Homeostasis. J Bone Miner Res 2017;32(10):2049–2061. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 2014;25(10):2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW. Renal Production, Uptake, and Handling of Circulating alphaKlotho. J Am Soc Nephrol 2016;27(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, Shafi T, Coresh J, Powe NR, Melamed ML. Race, Mineral Homeostasis and Mortality in Patients with End-Stage Renal Disease on Dialysis. Am J Nephrol 2015;42(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359(6):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 1998;31(4):607–617. [DOI] [PubMed] [Google Scholar]
- 16.Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014;10(5):268–278. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011;305(11):1119–1127. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra R, Gona P, Benjamin E, Wang T, Aragam J, D’Agostino R, Kannel W, Vasan R. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail 2010;12(8):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhingra R, Sullivan L, Fox C, Wang T, D’Agostino RS, Gaziano J, Vasan R. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007;167(9):879–885. [DOI] [PubMed] [Google Scholar]
- 20.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112(17):2627–2633. [DOI] [PubMed] [Google Scholar]
- 21.Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, Folsom AR. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2014;100(3):756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consortium A Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet 2000;26(3):345–348. [DOI] [PubMed] [Google Scholar]
- 23.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 2005;280(4):2543–2549. [DOI] [PubMed] [Google Scholar]
- 24.Scialla J, Xie H, Rahman M, Anderson A, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald J, Raj D, Yang W, He J, Lash J, Go A, Kusek J, Feldman H, Wolf M. Fibroblast Growth Factor 23 and Cardiovascular Events in Chronic Kidney Disease. J Am Soc Nephrol 2014;25(2):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M, Chronic Renal Insufficiency Cohort Study I. Association of Fibroblast Growth Factor 23 With Atrial Fibrillation in Chronic Kidney Disease, From the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol 2016;1(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011;22(10):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, Alonso A, Chonchol M, Deo R, Ix JH, Siscovick DS, Kestenbaum B, de Boer IH. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation 2014;130(4):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. J Am Soc Nephrol 2016;27(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH, Coresh J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc 2014;3(3):e000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, de Boer IH. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail 2014;7(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panwar B, Jenny NS, Howard VJ, Wadley VG, Muntner P, Kissela BM, Judd SE, Gutierrez OM. Fibroblast growth factor 23 and risk of incident stroke in community-living adults. Stroke 2015;46(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MS, DeRosa JT, Silverberg SJ, Mendez AJ, Dong C, Wright CB, Wolf M. Fibroblast Growth Factor 23 and Cause-Specific Mortality in the General Population: The Northern Manhattan Study. J Clin Endocrinol Metab 2016;101(10):3779–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scialla JJ. Epidemiologic insights on the role of fibroblast growth factor 23 in cardiovascular disease. Curr Opin Nephrol Hypertens 2015;24(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scialla J, Astor B, Isakova T, Xie H, Appel L, Wolf M. Mineral metabolites and chronic kidney disease progression in African Americans. J Am Soc Nephrol 2012;24(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.e KL, Bartz TM, Dalrymple L, de Boer IH, Kestenbaum B, Shlipak MG, Garimella PS, Ix JH, Chonchol M. Fibroblast Growth Factor 23 and the Risk of Infection-Related Hospitalization in Older Adults. J Am Soc Nephrol 2017;28(4):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishigami J, Jaar BG, Rebholz CM, Grams ME, Michos ED, Wolf M, Kovesdy CP, Uchida S, Coresh J, Lutsey PL, Matsushita K. Biomarkers of Mineral and Bone Metabolism and 20-Year Risk of Hospitalization With Infection: The Atherosclerosis Risk in Communities Study. J Clin Endocrinol Metab 2017;102(12):4648–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, Genet L, Kuentz F, Lataillade D, Legrand E, Moreau -Gaudry X, Jean G, Fouque D. Circulating Klotho Associates With Cardiovascular Morbidity and Mortality During Hemodialysis. J Clin Endocrinol Metab 2017;102(9):3154–3161. [DOI] [PubMed] [Google Scholar]
- 40.Seiler S, Wen M, Roth HJ, Fehrenz M, Flugge F, Herath E, Weihrauch A, Fliser D, Heine GH. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 2013;83(1):121–128. [DOI] [PubMed] [Google Scholar]
- 41.Brandenburg VM, Kleber ME, Vervloet MG, Larsson TE, Tomaschitz A, Pilz S, Stojakovic T, Delgado G, Grammer TB, Marx N, Marz W, Scharnagl H. Soluble klotho and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2015;242(2):483–489. [DOI] [PubMed] [Google Scholar]
- 42.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011;22(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, Floege J, Fliser D, Heine GH. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin J Am Soc Nephrol 2014;9(6):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo TH, Kang SW, Han DS, Han SH. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis 2013;61(6):899–909. [DOI] [PubMed] [Google Scholar]
- 45.Pavik I, Jaeger P, Ebner L, Wagner C, Petzold K, Spichtig D, Poster D, Wüthrich R, Russmann S, Serra A. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant 2013;28(2):352–359. [DOI] [PubMed] [Google Scholar]
- 46.Nowak A, Friedrich B, Artunc F, Serra AL, Breidthardt T, Twerenbold R, Peter M, Mueller C. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One 2014;9(7):e100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117(4):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melamed M, Michos E, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168(15):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168(12):1340–1349. [DOI] [PubMed] [Google Scholar]
- 50.de Boer IH, Levin G, Robinson-Cohen C, Biggs ML, Hoofnagle AN, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med 2012;156(9):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, Kritchevsky SB, Cauley JA, Tanaka T, Ferrucci L, Bandinelli S, Patel KV, Hagstrom E, Michaelsson K, Melhus H, Wang T, Wolf M, Psaty BM, Siscovick D, Kestenbaum B. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012;308(18):1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA 2013;310(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al Mheid I, Quyyumi AA. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J Am Coll Cardiol 2017;70(1):89–100. [DOI] [PubMed] [Google Scholar]
- 55.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 2003;349(5):446–456. [DOI] [PubMed] [Google Scholar]
- 56.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr., Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol 2005;16(4):1115–1125. [DOI] [PubMed] [Google Scholar]
- 57.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012;307(7):674–684. [DOI] [PubMed] [Google Scholar]
- 58.Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, Bolignano D, Cutrupi S, Politi R, Tripepi G, Ghiadoni L, Thadhani R, Mallamaci F. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 2014;64(5):1005–1011. [DOI] [PubMed] [Google Scholar]
- 59.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, Lo G, Lai KN, Lo WK, Lam CW, Yu CM. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol 2014;25(1):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosanoff A, Dai Q, Shapses SA. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv Nutr 2016;7(1):25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L, Streja E, Rhee CM, Mehrotra R, Soohoo M, Brunelli SM, Kovesdy CP, Kalantar-Zadeh K. Hypomagnesemia and Mortality in Incident Hemodialysis Patients. Am J Kidney Dis 2015;66(6):1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reffelmann T, Ittermann T, Dorr M, Volzke H, Reinthaler M, Petersmann A, Felix SB. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 2011;219(1):280–284. [DOI] [PubMed] [Google Scholar]
- 63.Pun PH, Middleton JP. Dialysate Potassium, Dialysate Magnesium, and Hemodialysis Risk. J Am Soc Nephrol 2017;28(12):3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodman W, London GM, Amann K, Block GA, Giachelli CM, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43(3):572–579. [DOI] [PubMed] [Google Scholar]
- 65.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001;38(3):434–438. [DOI] [PubMed] [Google Scholar]
- 66.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003;18(9):1731–1740. [DOI] [PubMed] [Google Scholar]
- 67.Gungor O, Kocyigit I, Yilmaz MI, Sezer S. Role of vascular calcification inhibitors in preventing vascular dysfunction and mortality in hemodialysis patients. Semin Dial 2017;31(1):72–81. [DOI] [PubMed] [Google Scholar]
- 68.Chen NX, Moe SM. Pathophysiology of Vascular Calcification. Curr Osteoporos Rep 2015;13(6):372–380. [DOI] [PubMed] [Google Scholar]
- 69.El-Abbadi M, Pai A, Leaf E, Yang H-Y, Bartley B, Quan K, Ingalls C, Liao H, Giachelli C. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 2009;75:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 71.Scialla J, Lau W, Reilly M, Isakova T, Yang H, Crouthamel M, Chavkin N, Rahman M, Wahl P, Amaral A, Hamano T, Master S, Nessel L, Chai B, Xie D, Kallem R, Chen J, Lash J, Kusek J, Budoff M, Giachelli C, Wolf M. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 2013;83(6):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 2012;82(12):1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 2007;18(7):2116–2124. [DOI] [PubMed] [Google Scholar]
- 74.Di Marco G, Konig M, Stock C, Wiesinger A, Hillebrand U, Reiermann S, Reuter S, Amler S, Kohler G, Buck F, Fobker M, Kumpers P, Oberleithner H, Hausberg M, Lang D, Pavenstadt H, Brand M. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int 2013;83:213–222. [DOI] [PubMed] [Google Scholar]
- 75.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 2009;20:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, Drueke TB, Massy ZA. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One 2014;9(4):e93423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, Siow RC. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med 2017;21(3):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lundwall K, Jörneskog G, Jacobson SH, Spaak J. Paricalcitol, Microvascular and Endothelial Function in Non-Diabetic Chronic Kidney Disease: A Randomized Trial. Am J Nephrol 2015;42(4):265–273. [DOI] [PubMed] [Google Scholar]
- 79.Chitalia N, Ismail T, Tooth L, Boa F, Hampson G, Goldsmith D, Kaski JC, Banerjee D. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One 2014;9(3):e91363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levin A, Tang M, Perry T, Zalunardo N, Beaulieu M, Dubland JA, Zerr K, Djurdjev O. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin J Am Soc Nephrol 2017;12(9):1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar V, Yadav AK, Lal A, Kumar V, Singhal M, Billot L, Gupta KL, Banerjee D, Jha V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J Am Soc Nephrol 2017;28(10):3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles L. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One 2012;7(9):e44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016;90(5):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M, Chronic Renal Insufficiency C. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 2012;7(7):1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bressendorff I, Hansen D, Schou M, Kragelund C, Brandi L. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): essential study design and rationale. BMJ Open 2017;7(6):e016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, Pedersen L, Rasmussen LM, Brandi L. Oral Magnesium Supplementation in Chronic Kidney Disease Stages 3 and 4: Efficacy, Safety, and Effect on Serum Calcification Propensity-A Prospective Randomized Double-Blinded Placebo-Controlled Clinical Trial. Kidney Int Rep 2017;2(3):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holden RM, Booth SL, Day AG, Clase CM, Zimmerman D, Moist L, Shea MK, McCabe KM, Jamal SA, Tobe S, Weinstein J, Madhumathi R, Adams MA, Heyland DK. Inhibiting the progression of arterial calcification with vitamin K in HemoDialysis patients (iPACK-HD) trial: rationale and study design for a randomized trial of vitamin K in patients with end stage kidney disease. Can J Kidney Health Dis 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.ClinicalTrials.gov. 2017; http://clinicaltrials.gov. Accessed December 6, 2017.
- 89.Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, Heidenreich PA, Huffman MD, Mayosi BM, Mendis S, Murray CJ, Perel P, Pineiro DJ, Smith SC Jr., Taubert KA, Wood DA, Zhao D, Zoghbi WA. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Modeling Study From the American Heart Association and World Heart Federation. Circulation 2016;133(23):e674–690. [DOI] [PubMed] [Google Scholar]
- 90.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010;55(5):1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sluyter JD, Camargo CA Jr., Stewart AW, Waayer D, Lawes CMM, Toop L, Khaw KT, Thom SAM, Hametner B, Wassertheurer S, Parker KH, Hughes AD, Scragg R. Effect of Monthly, High-Dose, Long-Term Vitamin D Supplementation on Central Blood Pressure Parameters: A Randomized Controlled Trial Substudy. J Am Heart Assoc 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mizuno M, Mitchell JH, Crawford S, Huang CL, Maalouf N, Hu MC, Moe OW, Smith SA, Vongpatanasin W. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 2016;311(1):R39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bozic M, Panizo S, Sevilla MA, Riera M, Soler MJ, Pascual J, Lopez I, Freixenet M, Fernandez E, Valdivielso JM. High phosphate diet increases arterial blood pressure via a parathyroid hormone mediated increase of renin. J Hypertens 2014;32(9):1822–1832. [DOI] [PubMed] [Google Scholar]
- 94.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 2014;6(6):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J, Group ICR. Dietary phosphorus and blood pressure: international study of macro-and micro-nutrients and blood pressure. Hypertension 2008;51(3):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR Jr. Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension 2010;55(3):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van Horn L, Daviglus ML, Stamler J, Butte AJ, Ioannidis JP, Elliott P. A nutrient-wide association study on blood pressure. Circulation 2012;126(21):2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336(16):1117–1124. [DOI] [PubMed] [Google Scholar]
- 99.Jimenez ZNC, Silva BC, dos Reis L, Castro MCM, Ramos CD, Costa-Hong V, Bortolotto LA, Consolim-Colombo F, Dominguez WV, Oliveira IB, Moyses RMA, Elias RM. High Dialysate Calcium Concentration May Cause More Sympathetic Stimulus During Hemodialysis. Kidney Blood Press Res 2016;41(6):978–985. [DOI] [PubMed] [Google Scholar]
- 100.Sabanayagam C, Shankar A. Serum calcium levels and hypertension among U.S. adults. J Clin Hypertens 2011;13(10):716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016;68(2):324–333. [DOI] [PubMed] [Google Scholar]
- 102.Fyfe-Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, Lutsey PL. Serum fibroblast growth factor-23 and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. J Hyperten 2016;34(7):1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011;22(9):1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wohlfahrt P, Melenovsky V, Kotrc M, Benes J, Jabor A, Franekova J, Lemaire S, Kautzner J, Jarolim P. Association of Fibroblast Growth Factor-23 Levels and Angiotensin-Converting Enzyme Inhibition in Chronic Systolic Heart Failure. JACC Heart Fail 2015;3(10):829–839. [DOI] [PubMed] [Google Scholar]
- 105.de Jong MA, Mirkovic K, Mencke R, Hoenderop JG, Bindels RJ, Vervloet MG, Hillebrands JL, van den Born J, Navis G, de Borst MH. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant 2017;32(1):73–80. [DOI] [PubMed] [Google Scholar]
- 106.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 2011;22(10):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Humalda JK, Lambers Heerspink HJ, Kwakernaak AJ, Slagman MC, Waanders F, Vervloet MG, Ter Wee PM, Navis G, de Borst MH. Fibroblast growth factor 23 and the antiproteinuric response to dietary sodium restriction during renin-angiotensin-aldosterone system blockade. Am J Kidney Dis 2015;65(2):259–266. [DOI] [PubMed] [Google Scholar]
- 108.Jovanovich AJ, Chonchol MB, Sobhi A, Kendrick JB, Cheung AK, Kaufman JS, Smits G, Jablonski KL. Mineral Metabolites, Angiotensin II Inhibition and Outcomes in Advanced Chronic Kidney Disease. Am J Nephrol 2015;42(5):361–368. [DOI] [PubMed] [Google Scholar]
- 109.Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O’Donnell TF, Vora AN, Omland T, Solomon SD, Pfeffer MA, Braunwald E, Sabatine MS. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol 2014;63(22):2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamamoto K, Robinson-Cohen C, de Oliveira M, Kostina A, Nettleton J, Ix J, Nguyen H, Eng J, Lima J, Siscovick D, Weiss N, Kestenbaum B. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int 2013;83(4):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tranaeus Lindblad Y, Olauson H, Vavilis G, Hammar U, Herthelius M, Axelsson J, Barany P. The FGF23-Klotho axis and cardiac tissue Doppler imaging in pediatric chronic kidney disease-a prospective cohort study. Pediatr Nephrol 2018;33(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanaka S, Fujita S, Kizawa S, Morita H, Ishizaka N. Association between FGF23, alpha-Klotho, and Cardiac Abnormalities among Patients with Various Chronic Kidney Disease Stages. PLoS One 2016;11(7):e0156860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, John Sutton M St, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009;119(19):2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saab G, Whooley M, Schiller N, Ix J. Association of serum phosphorus with left ventricular mass in men and women with stable cardiovascular disease: Data from the heart and soul study. Am J Kidney Dis 2010;56(3):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 2009;207(2):546–551. [DOI] [PubMed] [Google Scholar]
- 117.Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Ped Nephrol 2012;27(11):2129–2136. [DOI] [PubMed] [Google Scholar]
- 118.Han X, Ross J, Kolumam G, Pi M, Sonoda J, King G, Quarles LD. Cardiovascular Effects of Renal Distal Tubule Deletion of the FGF Receptor 1 Gene. J Am Soc Nephrol 2018;29(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Touchberry C, Green T, Tchikrizov V, Mannix J, Mao T, Carney B, Girgis M, Vincent R, Wetmore L, Dawn B, Bonewald L, Stubbs J, Wacker M. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab 2013;304:E863–E873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstadt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab 2015;22(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grabner A, Faul C. The role of fibroblast growth factor 23 and Klotho in uremic cardiomyopathy. Curr Opin Nephrol Hypertens 2016;25(4):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J Am Soc Nephrol 2015;26(5):1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015;26(6):1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J. Klotho Protects Against Indoxyl Sulphate-Induced Myocardial Hypertrophy. J Am Soc Nephrol 2015;26(10):2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. J Am Soc Nephrol 2015;26(10):2328–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol 2013;8(5):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zaheer S, de Boer IH, Allison M, Brown JM, Psaty BM, Robinson-Cohen C, Michos ED, Ix JH, Kestenbaum B, Siscovick D, Vaidya A. Fibroblast Growth Factor 23, Mineral Metabolism, and Adiposity in Normal Kidney Function. J Clin Endocrinol Metab 2017;102(4):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang B, Umbach AT, Chen H, Yan J, Fakhri H, Fajol A, Salker MS, Spichtig D, Daryadel A, Wagner CA, Foller M, Lang F. Up-regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun 2016;470(2):384–390. [DOI] [PubMed] [Google Scholar]
- 129.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 2013;28(8):1793–1803. [DOI] [PubMed] [Google Scholar]
- 130.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 131.Slavic S, Ford K, Modert M, Becirovic A, Handschuh S, Baierl A, Katica N, Zeitz U, Erben RG, Andrukhova O. Genetic Ablation of Fgf23 or Klotho Does not Modulate Experimental Heart Hypertrophy Induced by Pressure Overload. Sci Rep 2017;7(1):11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andrukhova O, Slavic S, Odorfer KI, Erben RG. Experimental Myocardial Infarction Upregulates Circulating Fibroblast Growth Factor-23. J Bone Miner Res 2015;30(10):1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christov M, Waikar S, Pereira R, Havasi A, Leaf D, Goltzman D, Pajevic P, Wolf M, Juppner H. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int 2013;84(4):776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leifheit-Nestler M, Grosse Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, Klintschar M, Becker JU, Erbersdobler A, Aufricht C, Seeman T, Fischer DC, Faul C, Haffner D. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant 2016;31(7):1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shalhoub V, Shatzen E, Ward S, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai M, Cattley R, Wronski T, Xia X, Li X, Henley C, Eschenberg M, Richards W. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 2012;122(7):2543–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Block G, Wheeler D, Persky M, Kestenbaum B, Ketteler M, Spiegel D, Allison M, Asplin J, Smits G, Hoofnagle A, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow G. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012;23(8):1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367(26):2482–2494. [DOI] [PubMed] [Google Scholar]
- 138.Shieh A, Aloia JF. Assessing Vitamin D Status in African Americans and the Influence of Vitamin D on Skeletal Health Parameters. Endocrinol Metab Clin North Am 2017;46(1):135–152. [DOI] [PubMed] [Google Scholar]
- 139.National Center for Biotechnology Information. Database of Genotypes and Phenotypes https://www.ncbi.nlm.nih.gov/gap. Accessed November 28, 2017.
- 140.Chang X, Li J, Guo Y, Wei Z, Mentch FD, Hou C, Zhao Y, Qiu H, Kim C, Sleiman PM, Hakonarson H. Genome-wide association study of serum minerals levels in children of different ethnic background. PloS one 2015;10(4):e0123499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Seaghdha CM, Wu H, Yang Q, Kapur K, Guessous I, Zuber AM, Kottgen A, Stoudmann C, Teumer A, Kutalik Z, Mangino M, Dehghan A, Zhang W, Eiriksdottir G, Li G, Tanaka T, Portas L, Lopez LM, Hayward C, Lohman K, Matsuda K, Padmanabhan S, Firsov D, Sorice R, Ulivi S, Brockhaus AC, Kleber ME, Mahajan A, Ernst FD, Gudnason V, Launer LJ, Mace A, Boerwinckle E, Arking DE, Tanikawa C, Nakamura Y, Brown MJ, Gaspoz JM, Theler JM, Siscovick DS, Psaty BM, Bergmann S, Vollenweider P, Vitart V, Wright AF, Zemunik T, Boban M, Kolcic I, Navarro P, Brown EM, Estrada K, Ding J, Harris TB, Bandinelli S, Hernandez D, Singleton AB, Girotto G, Ruggiero D, d’Adamo AP, Robino A, Meitinger T, Meisinger C, Davies G, Starr JM, Chambers JC, Boehm BO, Winkelmann BR, Huang J, Murgia F, Wild SH, Campbell H, Morris AP, Franco OH, Hofman A, Uitterlinden AG, Rivadeneira F, Volker U, Hannemann A, Biffar R, Hoffmann W, Shin SY, Lescuyer P, Henry H, Schurmann C, Consortium S, Consortium G, Munroe PB, Gasparini P, Pirastu N, Ciullo M, Gieger C, Marz W, Lind L, Spector TD, Smith AV, Rudan I, Wilson JF, Polasek O, Deary IJ, Pirastu M, Ferrucci L, Liu Y, Kestenbaum B, Kooner JS, Witteman JC, Nauck M, Kao WH, Wallaschofski H, Bonny O, Fox CS, Bochud M. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet 2013;9(9):e1003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vinayagamoorthy N, Yim SH, Jung SH, Park SW, Kim YJ, Kim BJ, Chung YJ. Association of common variants in the calcium-sensing receptor gene with serum calcium levels in East Asians. J Hum Genet 2015;60(8):407–412. [DOI] [PubMed] [Google Scholar]
- 143.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376(9736):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robinson-Cohen C, Lutsey PL, Kleber ME, Nielson CM, Mitchell BD, Bis JC, Eny KM, Portas L, Eriksson J, Lorentzon M, Koller DL, Milaneschi Y, Teumer A, Pilz S, Nethander M, Selvin E, Tang W, Weng LC, Wong HS, Lai D, Peacock M, Hannemann A, Volker U, Homuth G, Nauk M, Murgia F, Pattee JW, Orwoll E, Zmuda JM, Riancho JA, Wolf M, Williams F, Penninx B, Econs MJ, Ryan KA, Ohlsson C, Paterson AD, Psaty BM, Siscovick DS, Rotter JI, Pirastu M, Streeten E, Marz W, Fox C, Coresh J, Wallaschofski H, Pankow JS, de Boer IH, Kestenbaum B. Genetic Variants Associated with Circulating Parathyroid Hormone. J Am Soc Nephrol 2017;28(5):1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19(13):2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kestenbaum B, Glazer NL, Kottgen A, Felix JF, Hwang SJ, Liu Y, Lohman K, Kritchevsky SB, Hausman DB, Petersen AK, Gieger C, Ried JS, Meitinger T, Strom TM, Wichmann HE, Campbell H, Hayward C, Rudan I, de Boer IH, Psaty BM, Rice KM, Chen YD, Li M, Arking DE, Boerwinkle E, Coresh J, Yang Q, Levy D, van Rooij FJ, Dehghan A, Rivadeneira F, Uitterlinden AG, Hofman A, van Duijn CM, Shlipak MG, Kao WH, Witteman JC, Siscovick DS, Fox CS. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol 2010;21(7):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moy KA, Mondul AM, Zhang H, Weinstein SJ, Wheeler W, Chung CC, Mannisto S, Yu K, Chanock SJ, Albanes D. Genome-wide association study of circulating vitamin D-binding protein. Am J Clin Nutr 2014;99(6):1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A, Genetic Factors for Osteoporosis C, Meta Analysis of G, Insulin Related Traits C. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet 2010;6(8):e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tin A, Kottgen A, Folsom AR, Maruthur NM, Tajuddin SM, Nalls MA, Evans MK, Zonderman AB, Friedrich CA, Boerwinkle E, Coresh J, Kao WH. Genetic loci for serum magnesium among African-Americans and gene-environment interaction at MUC1 and TRPM6 in European-Americans: the Atherosclerosis Risk in Communities (ARIC) study. BMC Genet 2015;16(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferguson JF, Matthews GJ, Townsend RR, Raj DS, Kanetsky PA, Budoff M, Fischer MJ, Rosas SE, Kanthety R, Rahman M, Master SR, Qasim A, Li M, Mehta NN, Shen H, Mitchell BD, O’Connell JR, Shuldiner AR, Ho WK, Young R, Rasheed A, Danesh J, He J, Kusek JW, Ojo AO, Flack J, Go AS, Gadegbeku CA, Wright JT Jr., Saleheen D, Feldman HI, Rader DJ, Foulkes AS, Reilly MP. Candidate gene association study of coronary artery calcification in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). J Am Coll Cardiol 2013;62(9):789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie G, Myint PK, Voora D, Laskowitz DT, Shi P, Ren F, Wang H, Yang Y, Huo Y, Gao W, Wu Y. Genome-wide association study on progression of carotid artery intima media thickness over 10 years in a Chinese cohort. Atherosclerosis 2015;243(1):30–37. [DOI] [PubMed] [Google Scholar]
- 152.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, Pellikka N, Wallaschofski H, Kettunen J, Henneman P, Baumert J, Strachan DP, Fuchsberger C, Vitart V, Wilson JF, Pare G, Naitza S, Rudock ME, Surakka I, de Geus EJ, Alizadeh BZ, Guralnik J, Shuldiner A, Tanaka T, Zee RY, Schnabel RB, Nambi V, Kavousi M, Ripatti S, Nauck M, Smith NL, Smith AV, Sundvall J, Scheet P, Liu Y, Ruokonen A, Rose LM, Larson MG, Hoogeveen RC, Freimer NB, Teumer A, Tracy RP, Launer LJ, Buring JE, Yamamoto JF, Folsom AR, Sijbrands EJ, Pankow J, Elliott P, Keaney JF, Sun W, Sarin AP, Fontes JD, Badola S, Astor BC, Hofman A, Pouta A, Werdan K, Greiser KH, Kuss O, Meyer zu Schwabedissen HE, Thiery J, Jamshidi Y, Nolte IM, Soranzo N, Spector TD, Volzke H, Parker AN, Aspelund T, Bates D, Young L, Tsui K, Siscovick DS, Guo X, Rotter JI, Uda M, Schlessinger D, Rudan I, Hicks AA, Penninx BW, Thorand B, Gieger C, Coresh J, Willemsen G, Harris TB, Uitterlinden AG, Jarvelin MR, Rice K, Radke D, Salomaa V, Willems van Dijk K, Boerwinkle E, Vasan RS, Ferrucci L, Gibson QD, Bandinelli S, Snieder H, Boomsma DI, Xiao X, Campbell H, Hayward C, Pramstaller PP, van Duijn CM, Peltonen L, Psaty BM, Gudnason V, Ridker PM, Homuth G, Koenig W, Ballantyne CM, Witteman JC, Benjamin EJ, Perola M, Chasman DI. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 2011;123(7):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellis J, Lange EM, Li J, Dupuis J, Baumert J, Walston JD, Keating BJ, Durda P, Fox ER, Palmer CD, Meng YA, Young T, Farlow DN, Schnabel RB, Marzi CS, Larkin E, Martin LW, Bis JC, Auer P, Ramachandran VS, Gabriel SB, Willis MS, Pankow JS, Papanicolaou GJ, Rotter JI, Ballantyne CM, Gross MD, Lettre G, Wilson JG, Peters U, Koenig W, Tracy RP, Redline S, Reiner AP, Benjamin EJ, Lange LA. Large multiethnic Candidate Gene Study for C-reactive protein levels: identification of a novel association at CD36 in African Americans. Hum Genet 2014;133(8):985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hong EP, Kim DH, Suh JG, Park JW. Genetic risk assessment for cardiovascular disease with seven genes associated with plasma C-reactive protein concentrations in Asian populations. Hyperten Res 2014;37(7):692–698. [DOI] [PubMed] [Google Scholar]
- 155.Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, Kwiatkowski D, Cook NR, Miletich JP, Chasman DI. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet 2008;82(5):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fontana V, McDonough CW, Gong Y, El Rouby NM, Sa AC, Taylor KD, Chen YD, Gums JG, Chapman AB, Turner ST, Pepine CJ, Johnson JA, Cooper-DeHoff RM. Large-scale gene-centric analysis identifies polymorphisms for resistant hypertension. J Am Heart Assoc 2014;3(6):e001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hong KW, Go MJ, Jin HS, Lim JE, Lee JY, Han BG, Hwang SY, Lee SH, Park HK, Cho YS, Oh B. Genetic variations in ATP2B1, CSK, ARSG and CSMD1 loci are related to blood pressure and/or hypertension in two Korean cohorts. J Hum Hyperten 2010;24(6):367–372. [DOI] [PubMed] [Google Scholar]
- 158.Hong KW, Jin HS, Lim JE, Kim S, Go MJ, Oh B. Recapitulation of two genomewide association studies on blood pressure and essential hypertension in the Korean population. J Hum Genet 2010;55(6):336–341. [DOI] [PubMed] [Google Scholar]
- 159.Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, Morris RW, Tzoulaki I, O’Brien ET, Poulter NR, Sever P, Shields DC, Thom S, Wannamethee SG, Whincup PH, Brown MJ, Connell JM, Dobson RJ, Howard PJ, Mein CA, Onipinla A, Shaw-Hawkins S, Zhang Y, Davey Smith G, Day IN, Lawlor DA, Goodall AH, Fowkes FG, Abecasis GR, Elliott P, Gateva V, Braund PS, Burton PR, Nelson CP, Tobin MD, van der Harst P, Glorioso N, Neuvrith H, Salvi E, Staessen JA, Stucchi A, Devos N, Jeunemaitre X, Plouin PF, Tichet J, Juhanson P, Org E, Putku M, Sober S, Veldre G, Viigimaa M, Levinsson A, Rosengren A, Thelle DS, Hastie CE, Hedner T, Lee WK, Melander O, Wahlstrand B, Hardy R, Wong A, Cooper JA, Palmen J, Chen L, Stewart AF, Wells GA, Westra HJ, Wolfs MG, Clarke R, Franzosi MG, Goel A, Hamsten A, Lathrop M, Peden JF, Seedorf U, Watkins H, Ouwehand WH, Sambrook J, Stephens J, Casas JP, Drenos F, Holmes MV, Kivimaki M, Shah S, Shah T, Talmud PJ, Whittaker J, Wallace C, Delles C, Laan M, Kuh D, Humphries SE, Nyberg F, Cusi D, Roberts R, Newton-Cheh C, Franke L, Stanton AV, Dominiczak AF, Farrall M, Hingorani AD, Samani NJ, Caulfield MJ, Munroe PB. Blood pressure loci identified with a gene-centric array. Am J Hum Genet 2011;89(6):688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kelly TN, Takeuchi F, Tabara Y, Edwards TL, Kim YJ, Chen P, Li H, Wu Y, Yang CF, Zhang Y, Gu D, Katsuya T, Ohkubo T, Gao YT, Go MJ, Teo YY, Lu L, Lee NR, Chang LC, Peng H, Zhao Q, Nakashima E, Kita Y, Shu XO, Kim NH, Tai ES, Wang Y, Adair LS, Chen CH, Zhang S, Li C, Nabika T, Umemura S, Cai Q, Cho YS, Wong TY, Zhu J, Wu JY, Gao X, Hixson JE, Cai H, Lee J, Cheng CY, Rao DC, Xiang YB, Cho MC, Han BG, Wang A, Tsai FJ, Mohlke K, Lin X, Ikram MK, Lee JY, Zheng W, Tetsuro M, Kato N, He J. Genome-wide association study meta-analysis reveals transethnic replication of mean arterial and pulse pressure loci. Hypertension 2013;62(5):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009;41(6):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, Shen H, He J, Zhu J, Li H, Hixson JE, Wu T, Dai J, Lu L, Shen C, Chen S, He L, Mo Z, Hao Y, Mo X, Yang X, Li J, Cao J, Chen J, Fan Z, Li Y, Zhao L, Li H, Lu F, Yao C, Yu L, Xu L, Mu J, Wu X, Deng Y, Hu D, Zhang W, Ji X, Guo D, Guo Z, Zhou Z, Yang Z, Wang R, Yang J, Zhou X, Yan W, Sun N, Gao P, Gu D. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 2015;24(3):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miyaki K, Htun NC, Song Y, Ikeda S, Muramatsu M, Shimbo T. The combined impact of 12 common variants on hypertension in Japanese men, considering GWAS results. J Hum Hyperten 2012;26(7):430–436. [DOI] [PubMed] [Google Scholar]
- 164.Tabara Y, Kohara K, Kita Y, Hirawa N, Katsuya T, Ohkubo T, Hiura Y, Tajima A, Morisaki T, Miyata T, Nakayama T, Takashima N, Nakura J, Kawamoto R, Takahashi N, Hata A, Soma M, Imai Y, Kokubo Y, Okamura T, Tomoike H, Iwai N, Ogihara T, Inoue I, Tokunaga K, Johnson T, Caulfield M, Munroe P, Umemura S, Ueshima H, Miki T. Common variants in the ATP2B1 gene are associated with susceptibility to hypertension: the Japanese Millennium Genome Project. Hypertension 2010;56(5):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 2010;121(21):2302–2309. [DOI] [PubMed] [Google Scholar]
- 166.Tobin MD, Tomaszewski M, Braund PS, Hajat C, Raleigh SM, Palmer TM, Caulfield M, Burton PR, Samani NJ. Common variants in genes underlying monogenic hypertension and hypotension and blood pressure in the general population. Hypertension 2008;51(6):1658–1664. [DOI] [PubMed] [Google Scholar]
- 167.Kutikhin AG, Yuzhalin AE, Brusina EB, Ponasenko AV, Golovkin AS, Barbarash OL. Genetic predisposition to calcific aortic stenosis and mitral annular calcification. Mol Biol Rep 2014;41(9):5645–5663. [DOI] [PubMed] [Google Scholar]
- 168.Guo T, Yin RX, Huang F, Yao LM, Lin WX, Pan SL. Association between the DOCK7, PCSK9 and GALNT2 Gene Polymorphisms and Serum Lipid levels. Sci Rep 2016;6:19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008;40(2):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fox ER, Musani SK, Barbalic M, Lin H, Yu B, Ogunyankin KO, Smith NL, Kutlar A, Glazer NL, Post WS, Paltoo DN, Dries DL, Farlow DN, Duarte CW, Kardia SL, Meyers KJ, Sun YV, Arnett DK, Patki AA, Sha J, Cui X, Samdarshi TE, Penman AD, Bibbins-Domingo K, Buzkova P, Benjamin EJ, Bluemke DA, Morrison AC, Heiss G, Carr JJ, Tracy RP, Mosley TH, Taylor HA, Psaty BM, Heckbert SR, Cappola TP, Vasan RS. Genome-wide association study of cardiac structure and systolic function in African Americans: the Candidate Gene Association Resource (CARe) study. Circ Cardiovasc Genet 2013;6(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qi L, Qi Q, Prudente S, Mendonca C, Andreozzi F, di Pietro N, Sturma M, Novelli V, Mannino GC, Formoso G, Gervino EV, Hauser TH, Muehlschlegel JD, Niewczas MA, Krolewski AS, Biolo G, Pandolfi A, Rimm E, Sesti G, Trischitta V, Hu F, Doria A. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 2013;310(8):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith NL, Felix JF, Morrison AC, Demissie S, Glazer NL, Loehr LR, Cupples LA, Dehghan A, Lumley T, Rosamond WD, Lieb W, Rivadeneira F, Bis JC, Folsom AR, Benjamin E, Aulchenko YS, Haritunians T, Couper D, Murabito J, Wang YA, Stricker BH, Gottdiener JS, Chang PP, Wang TJ, Rice KM, Hofman A, Heckbert SR, Fox ER, O’Donnell CJ, Uitterlinden AG, Rotter JI, Willerson JT, Levy D, van Duijn CM, Psaty BM, Witteman JC, Boerwinkle E, Vasan RS. Association of genome-wide variation with the risk of incident heart failure in adults of European and African ancestry: a prospective meta-analysis from the cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ Cardiovasc Genet 2010;3(3):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Swerdlow DI, Hingorani AD, Humphries SE. Genetic risk factors and Mendelian randomization in cardiovascular disease. Curr Cardiol Rep 2015;17(5):33. [DOI] [PubMed] [Google Scholar]
- 174.Wild PS, Felix JF, Schillert A, Teumer A, Chen MH, Leening MJG, Volker U, Grossmann V, Brody JA, Irvin MR, Shah SJ, Pramana S, Lieb W, Schmidt R, Stanton AV, Malzahn D, Smith AV, Sundstrom J, Minelli C, Ruggiero D, Lyytikainen LP, Tiller D, Smith JG, Monnereau C, Di Tullio MR, Musani SK, Morrison AC, Pers TH, Morley M, Kleber ME, Aragam J, Benjamin EJ, Bis JC, Bisping E, Broeckel U, Cheng S, Deckers JW, Del Greco MF, Edelmann F, Fornage M, Franke L, Friedrich N, Harris TB, Hofer E, Hofman A, Huang J, Hughes AD, Kahonen M, Investigators K, Kruppa J, Lackner KJ, Lannfelt L, Laskowski R, Launer LJ, Leosdottir M, Lin H, Lindgren CM, Loley C, MacRae CA, Mascalzoni D, Mayet J, Medenwald D, Morris AP, Muller C, Muller-Nurasyid M, Nappo S, Nilsson PM, Nuding S, Nutile T, Peters A, Pfeufer A, Pietzner D, Pramstaller PP, Raitakari OT, Rice KM, Rivadeneira F, Rotter JI, Ruohonen ST, Sacco RL, Samdarshi TE, Schmidt H, Sharp ASP, Shields DC, Sorice R, Sotoodehnia N, Stricker BH, Surendran P, Thom S, Toglhofer AM, Uitterlinden AG, Wachter R, Volzke H, Ziegler A, Munzel T, Marz W, Cappola TP, Hirschhorn JN, Mitchell GF, Smith NL, Fox ER, Dueker ND, Jaddoe VWV, Melander O, Russ M, Lehtimaki T, Ciullo M, Hicks AA, Lind L, Gudnason V, Pieske B, Barron AJ, Zweiker R, Schunkert H, Ingelsson E, Liu K, Arnett DK, Psaty BM, Blankenberg S, Larson MG, Felix SB, Franco OH, Zeller T, Vasan RS, Dorr M. Large-scale genome-wide analysis identifies genetic variants associated with cardiac structure and function. J Clin Invest 2017;127(5):1798–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marz W, Seelhorst U, Wellnitz B, Tiran B, Obermayer-Pietsch B, Renner W, Boehm BO, Ritz E, Hoffmann MM. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab 2007;92(6):2363–2369. [DOI] [PubMed] [Google Scholar]
- 176.Babinsky VN, Hannan FM, Youhanna SC, Marechal C, Jadoul M, Devuyst O, Thakker RV. Association studies of calcium-sensing receptor (CaSR) polymorphisms with serum concentrations of glucose and phosphate, and vascular calcification in renal transplant recipients. PloS one 2015;10(3):e0119459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brondum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol . 2015;44(2):651–661. [DOI] [PubMed] [Google Scholar]
- 178.Daffara V, Verdoia M, Rolla R, Nardin M, Marino P, Bellomo G, Carriero A, De Luca G. Impact of polymorphism rs7041 and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr Metab Cardiovasc Dis 2017;27(9):775–783. [DOI] [PubMed] [Google Scholar]
- 179.Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njolstad I, Lochen ML, Figenschau Y, Berg JP, Svartberg J, Grimnes G. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromso Study. PloS one 2012;7(5):e37295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W, Pieber TR, Obermayer-Pietsch B, Renner W. Vitamin D and mortality: a Mendelian randomization study. Clin Chem 2013;59(5):793–797. [DOI] [PubMed] [Google Scholar]
- 181.Wang L, Chu A, Buring JE, Ridker PM, Chasman DI, Sesso HD. Common genetic variations in the vitamin D pathway in relation to blood pressure. Am J Hyperten 2014;27(11):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hassanein SI, Abu El Maaty MA, Sleem HM, Gad MZ. Triangular relationship between single nucleotide polymorphisms in the CYP2R1 gene (rs10741657 and rs12794714), 25-hydroxyvitamin d levels, and coronary artery disease incidence. Biomarkers 2014;19(6):488–492. [DOI] [PubMed] [Google Scholar]
- 184.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O’Connell JR, Hixson JE, Kardia SL, Sun YV, Jhun MA, Wang X, Mehta NN, Li M, Koller DL, Hakonarson H, Keating BJ, Rader DJ, Shuldiner AR, Peyser PA, Reilly MP, Mitchell BD. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterio Thromb Vasc Biol 2010;30(12):2648–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M, Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schottker B, Saum KU, McCarthy MI, Dupuis J, Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G, Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP, Hernandez DG, Byberg L, Hagstrom E, Melhus H, Ingelsson E, Mellstrom D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O, Wilson JF, Rudan I, Salomaa V, Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaelsson K, Bandinelli S, Frayling TM, Hartman CA, Sorensen TI, Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimaki T, Kuh D, Humphries SE, Cooper C, Ohlsson C, Marz W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C, Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD, Pilz S, Whittaker JC, Jarvelin MR, Hypponen E. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diab Endocrinol 2014;2(9):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wilke RA, Simpson RU, Mukesh BN, Bhupathi SV, Dart RA, Ghebranious NR, McCarty CA. Genetic variation in CYP27B1 is associated with congestive heart failure in patients with hypertension. Pharmacogenomics 2009;10(11):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Friedman DJ, Afkarian M, Tamez H, Bhan I, Isakova T, Wolf M, Ankers E, Ye J, Tonelli M, Zoccali C, Kuro-o M, Moe O, Karumanchi SA, Thadhani R. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res 2009;24(11):1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Donate-Correa J, Martin-Nunez E, Martinez-Sanz R, Muros-de-Fuentes M, Mora-Fernandez C, Perez-Delgado N, Navarro-Gonzalez JF. Influence of Klotho gene polymorphisms on vascular gene expression and its relationship to cardiovascular disease. J Cell Mol Med 2016;20(1):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Elghoroury EA, Fadel FI, Elshamaa MF, Kandil D, Salah DM, El-Sonbaty MM, Farouk H, Raafat M, Nasr S. Klotho G-395A gene polymorphism: impact on progression of end-stage renal disease and development of cardiovascular complications in children on dialysis. Pediatr Nephrol 2018. [DOI] [PubMed] [Google Scholar]
- 190.Tangri N, Alam A, Wooten EC, Huggins GS. Lack of association of Klotho gene variants with valvular and vascular calcification in Caucasians: a candidate gene study of the Framingham Offspring Cohort. Nephrol Dial Transplant 2011;26(12):3998–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, Reis JP, Gross M, Eckfeldt JH, Folsom AR. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Failure 2015;3(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schneider AL, Lutsey PL, Selvin E, Mosley TH, Sharrett AR, Carson KA, Post WS, Pankow JS, Folsom AR, Gottesman RF, Michos ED. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol 2015;22(8):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Verdoia M, Daffara V, Pergolini P, Rolla R, Marino P, Bellomo G, Carriero A, De Luca G. Vitamin D Binding Protein rs7041 polymorphism and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Vasc Pharmacol 2017;93–95:42–47. [DOI] [PubMed] [Google Scholar]
- 194.Barry EL, Rees JR, Peacock JL, Mott LA, Amos CI, Bostick RM, Figueiredo JC, Ahnen DJ, Bresalier RS, Burke CA, Baron JA. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab 2014;99(10):E2133–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Durazo-Arvizu RA, Camacho P, Bovet P, Forrester T, Lambert EV, Plange-Rhule J, Hoofnagle AN, Aloia J, Tayo B, Dugas LR, Cooper RS, Luke A. 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr 2014;100(3):908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 2010;171(8):903–908. [DOI] [PubMed] [Google Scholar]
- 197.Whisner CM, Castillo LF. Prebiotics, Bone and Mineral Metabolism. Calcif Tissue Int 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Acil Y, Gluer CC, Schrezenmeir J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr 2007;137(3 Suppl 2):838S–846S. [DOI] [PubMed] [Google Scholar]
- 199.Harmon QE, Umbach DM, Baird DD. Use of Estrogen-Containing Contraception Is Associated With Increased Concentrations of 25-Hydroxy Vitamin D. J Clin Endocrinol Metab 2016;101(9):3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Harris SS, Dawson-Hughes B. The association of oral contraceptive use with plasma 25-hydroxyvitamin D levels. J Am Coll Nutr 1998;17(3):282–284. [DOI] [PubMed] [Google Scholar]
- 201.Malhotra R, Katz R, Hoofnagle A, Bostom A, Rifkin DE, McBride R, Probstfield J, Block G, Ix JH. The Effect of Extended Release Niacin on Markers of Mineral Metabolism in CKD. Clin J Am Soc Nephrol 2018; 13(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Astrand M, Johansson S, Knutsson M, Langkilde AM, Chertow GM. Effect of Tenapanor on Serum Phosphate in Patients Receiving Hemodialysis. J Am Soc Nephrol 2017;28(6):1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012;23(8):1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, Ross W, Windus D, Dávila-Román VG, Hruska KA. Effects of Phosphate Binder Therapy on Vascular Stiffness in Early-Stage Chronic Kidney Disease. Am J Nephrol 2013;38(2):158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liabeuf S, Ryckelynck JP, El Esper N, Urena P, Combe C, Dussol B, Fouque D, Vanhille P, Frimat L, Thervet E, Mentaverri R, Prie D, Choukroun G. Randomized Clinical Trial of Sevelamer Carbonate on Serum Klotho and Fibroblast Growth Factor 23 in CKD. Clin J Am Soc Nephrol 2017;12(12):1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Urena-Torres P, Prie D, Keddad K, Preston P, Wilde P, Wan H, Copley JB. Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol 2014;15(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Isakova T, Gutierrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 2009;20(2):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K. Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis 2010;56(5):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lopes AA, Tong L, Thumma J, Li Y, Fuller DS, Morgenstern H, Bommer J, Kerr PG, Tentori F, Akiba T, Gillespie BW, Robinson BM, Port FK, Pisoni RL. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis 2012;60(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 2007;71(5):438–441. [DOI] [PubMed] [Google Scholar]
- 211.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005;68(4):1815–1824. [DOI] [PubMed] [Google Scholar]
- 212.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62(1):245–252. [DOI] [PubMed] [Google Scholar]
- 213.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 2007;72(9):1130–1137. [DOI] [PubMed] [Google Scholar]
- 214.Olivo RE, Scialla JJ. Getting Out of the Phosphate Bind: Trials to Guide Treatment Targets. Clin J Am Soc Nephrol 2017;12(6):868–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kalil RS, Wang JH, de Boer IH, Mathew RO, Ix JH, Asif A, Shi X, Boden WE. Effect of extended-release niacin on cardiovascular events and kidney function in chronic kidney disease: a post hoc analysis of the AIM-HIGH trial. Kidney Int 2015;87(6):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gutierrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR Jr., Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism. J Clin Endocrinol Metab 2015;100(11):4264–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 2006;91(8):3144–3149. [DOI] [PubMed] [Google Scholar]
- 218.Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Singh B, Zehnder D, Pachika A, Manning WJ, Shah A, Solomon SD, Thadhani R. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 2012;164(6):902–909 e902. [DOI] [PubMed] [Google Scholar]
- 219.Lu RJ, Zhu SM, Tang FL, Zhu XS, Fan ZD, Wang GL, Jiang YF, Zhang Y. Effects of vitamin D or its analogues on the mortality of patients with chronic kidney disease: an updated systematic review and meta-analysis. Eur J Clin Nutr 2017;71(6):683–693. [DOI] [PubMed] [Google Scholar]
- 220.Westerberg PA, Sterner G, Ljunggren O, Isaksson E, Elvarson F, Dezfoolian H, Linde T. High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD Stages 3–4: results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 2017:gfx059–gfx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bassuk SS, Manson JE, Lee IM, Cook NR, Christen WG, Bubes VY, Gordon DS, Copeland T, Friedenberg G, D’Agostino DM, Ridge CY, MacFadyen JG, Kalan K, Buring JE. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 2016;47:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drueke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation 2015;132(1):27–39. [DOI] [PubMed] [Google Scholar]
- 224.Sprague SM, Wetmore JB, Gurevich K, Da Roza G, Buerkert J, Reiner M, Goodman W, Cooper K. Effect of Cinacalcet and Vitamin D Analogs on Fibroblast Growth Factor-23 during the Treatment of Secondary Hyperparathyroidism. Clin J Am Soc Nephrol 2015;10(6):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 2010;5(1):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.May GS, DeMets DL, Friedman LM, Furberg C, Passamani E. The randomized clinical trial: bias in analysis. Circulation 1981;64(4):669–673. [DOI] [PubMed] [Google Scholar]
- 227.Fishbane S, Shapiro W, Corry D, Vicks S, Roppolo M, Rappaport K, Ling X, Goodman W, Turner S, Charytan C. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, Bushinsky DA. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant 2008;23(7):2311–2318. [DOI] [PubMed] [Google Scholar]
- 229.Moe SM, Wetherill L, Decker BS, Lai D, Abdalla S, Long J, Vatta M, Foroud TM, Chertow GM. Calcium-Sensing Receptor Genotype and Response to Cinacalcet in Patients Undergoing Hemodialysis. Clin J Am Soc Nephrol 2017;12(7):1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ix JH, Anderson CA, Smits G, Persky MS, Block GA. Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: a crossover study. Am J Clin Nutr 2014;100(5):1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jukic AMZ, Hoofnagle AN, Lutsey PL. Measurement of Vitamin D for Epidemiologic and Clinical Research: Shining Light on a Complex Decision. Am J Epidemiol 2017:kwx297–kwx297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Smith ER, McMahon LP, Holt SG. Method-specific differences in plasma fibroblast growth factor 23 measurement using four commercial ELISAs. Clin Chem Lab Med 2013;51(10):1971–1981. [DOI] [PubMed] [Google Scholar]
- 233.Cantor T, Yang Z, Caraiani N, Ilamathi E. Lack of comparability of intact parathyroid hormone measurements among commercial assays for end-stage renal disease patients: implication for treatment decisions. Clin Chem 2006;52(9):1771–1776. [DOI] [PubMed] [Google Scholar]
- 234.Binkley N, Sempos CT, Program VDS. Standardizing vitamin D assays: the way forward. J Bone Miner Res 2014;29(8):1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sturgeon CM, Sprague S, Almond A, Cavalier E, Fraser WD, Algeciras-Schimnich A, Singh R, Souberbielle JC, Vesper HW. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement -A view from the IFCC Working Group for PTH. Clin Chim Acta 2017;467(Supplement C):42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


