Abstract
Schistosomiasis is an intravascular parasitic infection estimated to affect over 206 million people, the majority of whom live in Africa where the trematode worms Schistosoma mansoni and Schistosoma haematobium are the major causative agents. While a number of drugs have been used to treat schistosomiasis, praziquantel (PZQ) is the only one that is widely available, relatively cheap, and easy to use. The reliance on a single drug for the treatment of such a prevalent disease is a cause for concern due to the potential for resistance to render PZQ ineffective. In this study, we examine the transcriptome of three generations of a laboratory strain of S. mansoni (PR1) whose susceptibility to PZQ has been diminished across 9 passages through exposure to increasing sub-lethal doses of the drug. Miracidial susceptibility was significantly reduced after exposure to 2 × 50 mg/Kg PZQ during the first passage. Susceptibility of worms in vivo was first assessed during passage 5 when mice infected with PZQ-selected schistosomes were dosed with a lethal dose of 3 × 300 mg/kg PZQ resulting in only a 10% reduction in worm number compared to control treatment. The emergence of reduced sensitivity was marked by a shift in sex ratio from a predominantly male to a female population, a reduction in the length of females and ultimately the loss of the PZQ-selected line after passage 9. Analysis of differentially regulated transcripts did not suggest that any particular gene product or pathway was associated with drug resistance suggesting either a loss of function mutation to a single gene or an epistatic interaction of multiple gene products as the underlying cause of reduced susceptibility.
Keywords: Praziquantel, Schistosoma, drug resistance, schistosomiasis, helminth, transcriptome
Summary
Selection of praziquantel resistance reduces the length of female schistosomes and changes the sex ratio of off-spring to favor females before loss of the line.
Graphical abstract’

1. Introduction
Schistosomiasis is a chronic neglected parasitic disease caused by digenetic blood flukes of the genus Schistosoma. As of 2016, the disease was estimated to affect approximately 206 million people worldwide though it is most prevalent in sub-Saharan Africa where Schistosoma mansoni and Schistosoma haematobium are the major causative agents [1]. This is a significant improvement on the previous estimate of approximately 259 million infected in 2014 [2]. One reason for this decline is that the number of individuals receiving treatment for the disease has risen from approximately 62 million in 2014 to 89 million in 2016 [1,2].
Praziquantel (PZQ) is the most widely available drug for the treatment of schistosomiasis and remains effective against all schistosomes that infect humans [3]. While PZQ has been used for over 30 years the exact mechanism of action of the drug remains unknown. One drawback is that sexually immature, juvenile schistosomes are resistant to its action [4,5] and, as the drug is usually administrated in endemic areas as a single dose, complete effectiveness is unlikely with a reservoir of PZQ resistant juvenile parasites likely remaining in the host.
With no other readily available treatment options, and as the number of PZQ tablets dispensed increases each year, there are significant concerns that the drug’s usefulness may be diminished or lost to resistance. A number of reports of schistosomes with reduced PZQ sensitivity have appeared in the literature though not all of these document increasing resistance. For example, Stelma et al. [6] reported a cure rate of only 18% with PZQ in Senegal in 1991 and suggested PZQ resistance as one explanation, however, Fallon et al. [7] reported that isolates from this population had a slower maturation rate rendering the schistosomes less sensitive to the drug at the times tested. Of more concern were the eight isolates derived from Egyptian patients who had received multiple, curative doses of PZQ [8]. These had less sensitivity to the drug compared to controls and retained reduced sensitivity over multiple passages through mice. In a subsequent study, William et al., [9] found 3 of the 6 strains retained reduced PZQ sensitivity in the absence of selective PZQ pressure but this came at the cost of diminished reproductive fitness which may account for the failure to find PZQ resistance in the same area of Egypt 10 years after the initial finding [10]. Melman et al. [11] reported a correlation between the number of PZQ treatments received by Kenyan patients and the sensitivity of miracidia hatched from eggs isolated form patient fecal samples. Similar to the experience reported by William et al. [9], a line of S. mansoni derived from one patient that showed reduced PZQ sensitivity could not be maintained in the lab beyond a few generations.
While it has proven difficult to find and maintain PZQ resistant Schistosoma spp. in field isolates, resistance has been successfully generated in the laboratory by maintaining schistosomes in mice with exposure to increasing sublethal doses of PZQ over several generations [12–15]. The S. mansoni isolates generated by Coeli et al. [13] were also unstable and showed reduced genetic diversity. Remarkably, Couto et al. [16] generated S. mansoni with reduced sensitivity after one exposure to PZQ in infected snails.
The molecular mechanism underpinning resistance in field or laboratory-induced isolates is unknown and it may be that there is more than one route to reduced sensitivity. ATP-binding cassette (ABC) proteins, which are known to be involved in the transport of a wide variety of compounds, including drugs, across membranes have been implicated in the natural resistance of juvenile S. mansoni [17–20]. We have suggested that the aberrant expression of ABC transporters may underlie acquired resistance in adults [21] and, using a laboratory selected strain of PZQ resistant S. mansoni Pinto-Almeida et al. [15] demonstrated the possible involvement of ABC transporter SmMDR2, belonging to the ABCB1 (Pgp-like) subfamily, in male worms displaying the resistance phenotype.
As indicated above, PZQ resistance has been shown to come with a fitness tradeoff [9,11,13,22], and may provide one explanation for the absence of sustained PZQ resistance in the field where there is a large refugium of drug sensitive schistosomes. As the number of individuals treated with PZQ continues to rise, the question of resistance in field isolates remains important for both researchers and government agencies trying to implement policy for the control of schistosomiasis. In the absence of alternative drugs or a vaccine, and as the number of PZQ tablets dispensed continues to rise [1], a better understanding of PZQ resistance is needed to comprehend the risk that it poses. Here we present data that outlines the production of a PZQ-selected line of S. mansoni with reduced susceptibility to the drug and chart the change in the transcriptome of the line over 3 passages in comparison to drug sensitive controls.
2. Materials and Methods
2.1. Ethics statement
All animal procedures in this study were reviewed and approved by the University of New Mexico Institutional Animal Care and Use Committee. This study followed the U.S. National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
2.2. Experimental procedures for selection of reduced PZQ sensitivity in S. mansoni
Passage 1:
Thirty Swiss Webster (SW) 10–12 week old outbred male mice (Charles River, Kingston, NY) were each infected percutaneously with 100–150 S. mansoni Puerto Rican 1 (PR1) cercariae. Mice were randomly divided into 2 groups of 15 and treated by oral gavage at 35 and 37 days post infection (dpi) with either 2% Cremaphore EL (Sigma-Aldrich, St. Louis, MO) in aqueous suspension (control group) or 50 mg/kg PZQ (PZQ-selected group) in aqueous suspension with 2 % Cremaphore EL. Mice were euthanized and perfused with RPMI 1640 media (RPMI; Sigma-Aldrich) at 49 dpi. Adult parasites were collected by perfusion, counted and sexed, then placed in RNeasy lysis buffer with 1% 2-mercaptoethanol (Sigma-Aldrich) and stored at −80 °C for RNA isolation. In addition, 10 parasites of each sex and treatment were chosen randomly and placed in RPMI for measurement of their length and breadth (see below).
After perfusion, mouse livers were harvested for egg collection. The eggs were pulse blended in artificial spring water (ASW) and placed under direct light for 1 h to hatch. Miracidia were collected, pooled and used to infect 24 laboratory reared Biomphalaria glabrata for each treatment group by exposing individual snails to 5 miracidia overnight. Snails were housed in 10-gallon tanks in ASW and after 8 weeks placed in individual wells under direct light to induce cercarial shedding. Cercariae from each treatment were pooled and used to infect mice as described above.
Passage 2–9:
For each successive passage (P), groups of 10 SW mice were infected percutaneously with 100–150 S. mansoni PR1 cercariae per mouse derived from either the PZQ-selected or control line of the previous generation. Animals in the control group received 2% Cremaphore EL in aqueous suspension and those in the selected group received PZQ with the dose and time of treatment varying depending on the passage number.
Mice were perfused and euthanized at 49 dpi and the parasites and livers processed as described for P1. Miracidia derived from eggs obtained from livers of PZQ-selected or control mice were used to found the next generation of PZQ-selected and control schistosomes respectively. A 2-way ANOVA followed by a Sidak’s multiple comparisons test was used to compare the number of recovered parasites from the control and PZQ-selected groups across each passage. All statistical analyses were conducted using GraphPad Prism (GraphPad Software Inc., La Jolla, CA).
2.3. Dimensions of S. mansoni
Parasites (n = 10 parasites/sex/treatment/generation) were photographed 1 h after perfusion using a Zeiss SteREO Discovery V12 microscope and AxioCam HRc camera (Carl Zeiss Microscopy, Thornwood, NY). Parasite length and breadth were measured using AxioVision 4.8.2 software (Carl Zeiss Microscopy) with at least 3 and up to 5 measurements made per worm for length and breadth. The significance of observed differences between control and PZQ-selected parasites across sex and generation was assessed using 2-way ANOVA followed by Sidak’s multiple comparisons test.
2.4. In vivo assay of S. mansoni PZQ resistance
From P5 onwards, to estimate the effectiveness of the PZQ dosing regime in producing drug resistant parasites, mice were infected with approximately 100–150 control or PZQ-selected cercariae (n = 5 per group) derived from the previous generation and subsequently received a single dose of 300 mg/kg PZQ by oral gavage on each of days 28, 35 and 37 post infection (i.e. 3 × 300 mg/kg in total). The mice were perfused and euthanized at 49 dpi and the number of parasites that survived counted and parasite burden reduction calculated using the following modified formula [11]
A 2-way ANOVA followed by Sidak’s multiple comparisons test was used to compare the number of recovered parasites from the control and selected groups across generations.
2.5. PZQ susceptibility assay of S. mansoni miracidia
Miracidia were tested for PZQ sensitivity using a modified version of the protocol developed by Melman et al. [11]. Three to five PZQ-selected or control miracidia were placed in 40 μL ASW in up to 12 wells of a 96-well plate with a final concentration of 0 or 10−5 M PZQ. Dimethyl sulfoxide (DMSO) at 1% was used to prepare 10−4 M PZQ stock and all wells received a final concentration of 0.1% DMSO including those with no drug. A dissecting microscope was used to observe the miracidia prior to administration (t=0) and after 20 min incubation. Miracidia were counted blind as alive or dead i.e. remained immobile. The percentages of surviving miracidia were calculated as described by Mwangi et al. [14]. The surviving miracidia percentages were compared using a multiple t-test with the Holm-Sidak method.
2.6. RNA isolation and sequencing
Isolation of parasite total RNA was performed using an RNeasy Mini Kit (Qiagen, Redwood City, CA) according to the manufacturer’s instructions and included incubation with RNase-free DNase (Qiagen) followed by RNA cleanup and concentration (Zymo Research, Irvine, CA).
Total RNA was quantified and quality assessed using a Qubit Fluorometer (Thermo Fisher Scientific, Waltham, MA) and Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA) respectively. Bioanalyzer traces from each generation and treatment showed no significant RNA degradation (Supplementary Figure 1; Fig S1.). RNA was stored at −80 °C until required.
RNA sequencing (RNA-Seq) was performed on 4 biological replicates of control and PZQ-selected schistosomes from P6, 7, and 8 using an Illumina NextSeq 500 instrument (Illumina, San Diego, CA). Libraries and indexing were prepared using the KAPA mRNA HyperPrep kit (KAPA Biosystems, Wilmington, MA) following the manufactures instructions. A Qubit Fluorometer and KAPA Library Quantification Kit for Illumina Platform were employed to quantify the libraries. The quality and fragment size for each library was examined with the Agilent 2100 Bioanalyzer. Samples passing quality control were pooled and sequenced to generate 150 base paired-end reads at the Molecular Biology Core Facility, University of New Mexico, NM.
2.7. RNA-Seq data processing
Raw sequence reads were trimmed and filtered using Trimmomatic v0.36 [23] with a slide window of 4 nucleotides, average score above 20 and minimum length of 36 nucleotides. Reads passing filters were mapped to the S. mansoni transcriptome annotated from assembly GCA_000237925.2 ASM23792v2 (NCBI) using Bowtie2 v2.2.9 [24]. Gene expression levels were estimated using RSEM v1.2.31 [25] and differential gene expression analysis performed using DESeq2 v 1.18.1 [26] EdgeR v 3.20.8 [27], and EBSeq v1.18.0 [28]. Results were organized using SARTools [29] with cutoffs for all programs set at an adjusted p ≤0.05 with log2 fold change of either <−1 (down-regulated genes) or >1 (up-regulated genes). Differentially expressed gene (DEG) lists for the average (4 replicates per treatment/generation) of each treatment resulting from each program were displayed by Venn diagram and only genes common to at least two bioinformatic approaches were retained for further analysis. All sequencing data sets generated for this study were deposited with Gene Expression Omnibus and can be accessed through accession number GSE120682.
2.8. Functional annotation
DEG lists and annotation file S. mansoni ASM23792v2 were imported into Blast2GO v5.0.13 [30] to classify genes with ontology terms in order to identify functionality. We performed Fisher’s Exact Test (FET) with multiple corrections to identify Gene Ontology (GO) terms significantly (p < 0.05) over-represented in the DEG list compared to the whole gene set [31].
2.9. Quantitative real-time PCR
RNA samples for quantitative real-time PCR (qRT-PCR) originated from the corresponding samples used for RNA-Seq in order to validate Illumina data set expression patterns. Genes were chosen based on qRT-PCR primers passing efficiency tests and their differential expression patterns at P6–8. qRT-PCR was performed on 1 μg total RNA reverse transcribed to cDNA (20 μL reactions) using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. All qRTPCR reactions were performed in biological triplicates with technical duplicates using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad Laboratories). Each 20 μL reaction contained of 100 ng cDNA and 0.5 μM primer. Reactions were performed using a C1000 96 Touch Thermo Cycler (Bio-Rad Laboratories) using S. mansoni gene qRTPCR primers (Table S1) designed with the Integrated DNA Technologies OligoAnalyzer tool (www.idtdna.com).
Cycling conditions for S. mansoni gene expression were as follows; 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s, 60 °C for 1 min and 60 °C for 20 s. CFX Manager™ Software v3.1 (Bio-Rad Laboratories) was used to calculate fold change (2−ΔΔCt method) relative to the reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Expression of this gene did not vary significantly between treatment groups (Table S2).
Statistical analysis was performed using a Student’s t-test for normalized gene expression between treatment groups, and Pearson’s correlation was used to examine the correlation between qRT-PCR and RNA-Seq data.
3. Results and Discussion
3.1. Selection of praziquantel resistance in S. mansoni
The initial goal of this study was to select for a PZQ resistant line of S. mansoni using a modification of the protocol employed by Fallon and Doenhoff [12]. In the original protocol, isolates of S. mansoni from Puerto Rico, Brazil, Kenya and Egypt were used to provide sufficient genetic variation to facilitate selection of resistance after 6 passages with increasing drug pressure. Using a similar protocol, we were able to induce resistance in S. mansoni derived from 7 Kenyan field isolates within 5 generations [14]. In contrast, at least 2 other studies have generated PZQ resistant schistosomes by selecting on a single (LE) strain [13,16]. Due to the success of these latter experiments in selecting for resistance on (probable) limited genetic variation, we chose S. mansoni PR1 as the sole source of genetic material for our experiments. Survival of at least 90% of schistosomes after exposure to 300 mg/kg PZQ on each of 28, 35 and 37 dpi was chosen as the indicator of acquisition of PZQ resistance as we found that this regime would reduce PR1 burden from 69.3 + 2.5 schistosomes in control mice to 1.3 + 2.3 in PZQ treated mice 10 days after the final treatment (P < 0.0001). This was also the drug regimen chosen in 2 previous studies to assess drug resistance [12,13]. The number and gender of parasites recovered across 9 passages from control and PZQ-selected treatment lines are shown in Table 1.
Table 1.
Worms recovered from S. mansoni infected mice after treatment with PZQ over 9 passages
| 1 | - | 36 + 36 | 20 + 19 | 16 + 16 | 1.1 |
| 2 × 100a | 31 + 19 | 17 + 9 | 14 + 10 | 1.2 | |
| 3 | - | 23 + 4 | 14 + 4 | 9 + 2 | 1.6 |
| 2 × 250a | 40 + 17 | 17 + 8** | 23 + 11* | 0.7 | |
| 5 | - | 51 + 11 | 32 + 8 | 19 + 10 | 1.7 |
| 3 × 300b | 36 + 15 | 17 + 7 | 19 + 10 | 0.9 | |
| 7 | - | 35 + 17 | 22 + 9 | 13 + 5 | 1.9 |
| 3 × 300b | 27 + 10*** | 12 + 6** | 15 + 6 | 0.8 | |
| 9 | - | 20 + 4 | 13 + 3 | 7 + 2 | 1.9 |
PZQ in 2% Cremaphore EL or 2% Cremaphore EL alone were administered at 35 and 37 dpi by oral gavage.
PZQ in 2% Cremaphore EL or 2% Cremaphore EL alone were administered at 28, 35 and 37 dpi by oral gavage.
Statistical significance of changes in male and female parasite burden comparing control and PZQ-selected lines at each passage was determined using the Holm-Sidak method with alpha = 0.05. Adjusted p values are shown with * = p<0.05; ** = p<0.01, *** = p<0.001, **** = p<0.0001.
During P1–4, administration of 50–250 mg/kg PZQ administered on each of 35 and 37 dpi did not result in a significant reduction of parasite burden compared with the control group. Through the first 3 passages there was a male bias in both treatment groups which is in accord with the sex distribution associated with bisexual infection [32]. By P4, however, the PZQ-selected group showed a female bias for the first time. Beginning P5, the dose of PZQ was increased to 300 mg/kg on each of days 28, 35 and 37 dpi. Egg laying begins at around 28 dpi and this earlier start to treatment ensured that few eggs were deposited prior to the application of drug pressure. Again, there was no significant difference in the number of worms recovered from PZQ treated and control mice at 49 dpi and, as with P4, drug pressure during P5–9 resulted in a female bias that was not observed with the control treatment. Statistically significant declines in the number of males in the PZQ-selected group compared to the control group at P4, 5, 7, 8 and 9 suggests that this was the underlying cause of the female bias. A significant increase in females was only observed at P4 and 9. Two earlier studies also observed a decrease in male/female ratio following PZQ treatment [22,33]. One explanation could be that female parasites are less susceptible to PZQ in vivo due simply to reduced exposure to the drug as they sit in the male gynecophoric canal. Alternatively, reduced PZQ sensitivity may be an inherent trait and Pica-Mattoccia and Cioli [4] observed that the in vitro ED50 of immature females derived from bisexual infections is three times that of male parasites while Shaw and Erasmus [34] reported that the tegument of male worms is more susceptible to PZQ than that of females. The observation of reduced female sensitivity is not universal. Coeli et al. [13] found more males in the 11th passage of LE strain worms selected for PZQ resistance suggesting sex selection through drug pressure may be a more complex phenomenon than can be explained simply by the sensitivity or degree of exposure of each sex to the drug.
To confirm that the PZQ-selected line had reduced sensitivity to the drug, mice infected with either the PZQ-selected or control lines were treated with 300 mg/kg PZQ on each of days 28, 35 and 37 dpi, euthanized at 49 dpi and the number of surviving worms calculated (Table 2).
Table 2.
Percentage reduction in parasite burden of control and selected parasites in passages 5–9 after treatment with PZQ
| Passage (P)_Line | Treatment PZQ (mg/kg) | Mean ± SD Parasite number | % Reduction | Statistical Significance (p-value) |
|---|---|---|---|---|
| P5_control | - | 51 ± 11 | 46 | <0.001b |
| 3 × 300a | 28 ± 15 | |||
| P5_PZQ-selected | - | 42 ± 21 | 10 | NSc |
| 3 × 300 | 37 ± 12 | |||
| P6_control | - | 22 ± 9 | 83 | <0.01 |
| 3 × 300 | 4 ± 3 | |||
| P6_PZQ-selected | - | 36 ± 15 | 0 | NS |
| 3 × 300 | 37 ± 11 | |||
| P7_control | - | 35 ± 17 | 44 | <0.05 |
| 3 × 300 | 20 ± 5 | |||
| P7_PZQ-selected | - | 26 ± 5 | 0 | NS |
| 3 × 300 | 32 ± 4 | |||
| P8_control | - | 44 ± 13 | 67 | <0.0001 |
| 3 × 300 | 14 ± 4 | |||
| P8_PZQ-selected | - | 27 ± 10 | 20 | NS |
| 3 × 300 | 22 ± 2 | |||
| P9_control | - | 20 ± 4 | 37 | NS |
| 3 × 300 | 13 ± 5 | |||
| P9_PZQ-selected | - | 17 ± 6 | 4 | NS |
| 3 × 300 | 16 ± 6 |
PZQ was administered on 28, 35 and 37 dpi. Animals were euthanized and worm burden counted at 49 dpi.
Statistical significance of parasite burden in control and PZQ-selected lines was determined using the Holm-Sidak method with alpha = 0.05.
NS = not significant.
While there was a 46 (P < 0.001) and 83% (P < 0.01) reduction in worm burden of control line mice, there was only a non-significant 10 and 0% reduction in the PZQ-selected line at P5 and 6 respectively. Similar significant reductions were seen in the control line at P7 and 8 but not in the PZQ line. Although there was a 37% reduction in worm burden in the control line at P9, this failed to reach statistical significance and there was only a 4% reduction in worm burden in the PZQ line at this point. These results confirm that after the 5th passage the PZQ-selected line had become significantly less susceptible to the drug and this status was maintained until P9.
Unfortunately, we were unable to maintain the PZQ-selected line after P9 as the few eggs that were extracted from the liver of infected mice did not hatch. This is similar to the experience of Coeli et al. [13] who lost their line after 11 passages. Loss may be due to a trade-off between PZQ ‘resistance’ and fitness cost [9] and/or a skewed sex ratio leading to a loss of fecundity [13,22] as observed in this study.
In addition to the shift towards females from P4 onwards, we also observed that while the length (Fig 1A) and width (Fig 1B) of PZQ-selected and control male worms did not differ over the 9 passages, females became significantly shorter in the PZQ-selected cohort compared to controls from P6 onwards (Fig 1C). The greatest difference was seen at P8 where the female schistosomes in the control line had a mean length of 5751.8 + 654.2 μm compared to 4150.4 + 440.3 μm (p < 0.001) in the PZQ-selected line. At P9, the mean length of females was 5832.9 + 790.7 μm, while those from the PZQ-selected line were 4336.2 + 599.9 μm (p < 0.001). No differences in the width of female worms were observed between the two groups (Fig 1D). While these data are suggestive of a fitness cost we cannot rule out that the stunted growth was due to the female schistosome’s apparent need for the presence of males to reach maturity [35].
Fig 1. Changes in S. mansoni length and width during PZQ-selection.
The length and width of male and female schistosomes isolated during passages (P) 1–9 are shown. (A) Length of male parasites. (B) Width of male parasites. (C) Length of female parasites. (D) Width of female parasites. Significance of observed differences between control and PZQ-selected schistosomes were assessed with 2-way ANOVA with Sidak’s multiple comparisons test. Error bars represent mean with standard deviation, n = 10 sex/treatment/passage. * = p< 0.05, and *** = p< 0.001.
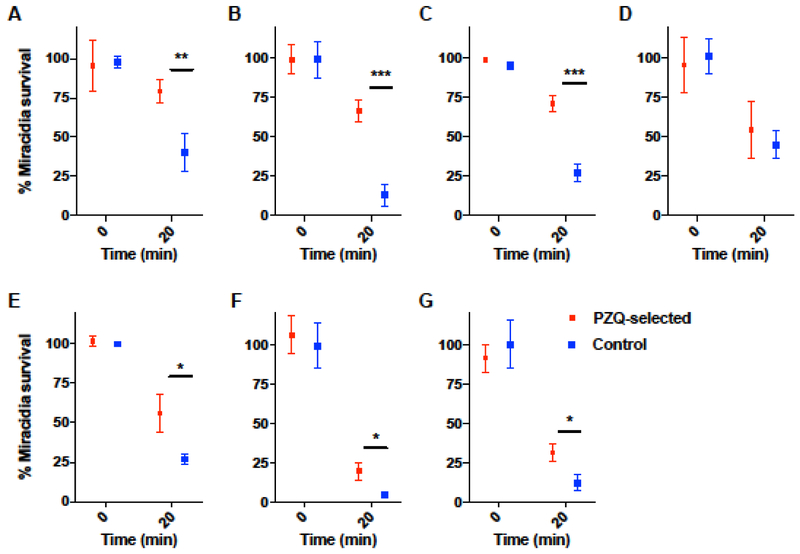
In vitro treatment of miracidia hatched from control and PZQ-selected eggs were assessed for their ability to survive 20 min exposure to 10−5 M PZQ as an indication of PZQ sensitivity. Remarkably, our results suggest the PZQ-selected miracidia were less susceptible to the drug after the first exposure of the adults to 50 mg/kg on each of days 35 and 37 post infection and across most subsequent generations tested (P3, 4, 6, 7 and 8) with the exception of P5 (Fig 2 A–G). The rapid acquisition of reduced miracidial PZQ sensitivity was surprising to us and contrary to the observation of Mwangi et al. [14] who only observed a reduction in sensitivity after exposure of mouse infections to 2 × 250 mg/kg during P4 and 3 × 300 mg/kg during P5. Also surprising was our observation that this reduced sensitivity was greatest during the initial passages (i.e. P1, P3 and P4). In contrast, miracidial sensitivity at P7 and P8, while still statistically significant, was less clear-cut perhaps suggesting an inherent weakness in the PZQ-selected line that foreshadowed its loss.
Fig 2. In vitro assay of S. mansoni miracidia survival after exposure to PZQ.
Miracidia were hatched from control and PZQ-selected groups and exposed to 10−5 M PZQ for 20 min and the number surviving counted. (A) P1, (B) P3, (C) P4, (D) P5, (E) P6, (F) P7, and (G) P8. Passage 2 and P9 data are not included due to low miracidia yield. Statistical significance determined between control and PZQ-selected parasites using the Holm-Sidak multiple comparisons method with alpha = 0.05. Error bars represent mean with standard deviation. * = p< 0.05, ** = p< 0.01, and *** = p< 0.001.
Overall, our results suggest that sexually mature S. mansoni PR1 had acquired significantly reduced sensitivity to PZQ by the completion of P5 and this was maintained through P9. Thus, transcriptomic analysis of adult schistosomes from control and PZQ-selected lines was undertaken to identify genes associated with reduced sensitivity.
3.2. Transcriptome sequencing and analysis
To compare changes in the S. mansoni transcriptome, cDNA libraries were prepared from pooled, mixed sex schistosomes derived from 10 mice infected with PZQ-selected or control lines at P6, 7 and 8. On removing adapter sequences and any ambiguous, low quality reads (Q < 20), a total of 315 million 150 base paired end reads were obtained. In each of the six groups the mean number of reads was between 11.5 (P8, PZQ-selected) and 17.8 million (P6, control), and a mean of 50.0% (P8, PZQ-selected) and 62.5% (P6 & P7, control) paired reads mapped to the S. mansoni genome (Table S3). Each group contained 4 biological replicates/generation and the number of reads provided enough coverage for differential expression analysis [36].
Principle Component Analysis (PCA) resulted in transcriptomes of the PZQ-selected and control groups segregating broadly into two distinct regions with individual transcriptomes generally clustering with others from their treatment/control group. P6 PZQ-selected samples were the most diverse with two of the 4 samples clustering more with the control selected samples than PZQ-selected (Fig S2). All samples were retained for further analyses to maintain an n of 4 across all groups.
Three programs (DESeq, EBSeq, and EdgeR) were used to identify differential transcript expression and PZQ-selected samples were normalized to control samples for each generation. The number of DEGs observed using each program is shown in Fig S3 and transcripts that were identified by at least 2 of the programs were retained for further analyses. Both the number of up- and down-regulated DEGs increased with each successive generation (Table S4) with a total of 197, 652 and 1,003 DEG identified at P6, 7 and 8 respectively. Tables identifying all differentially regulated genes are available through Gene Expression Omnibus with accession number GSE120682.
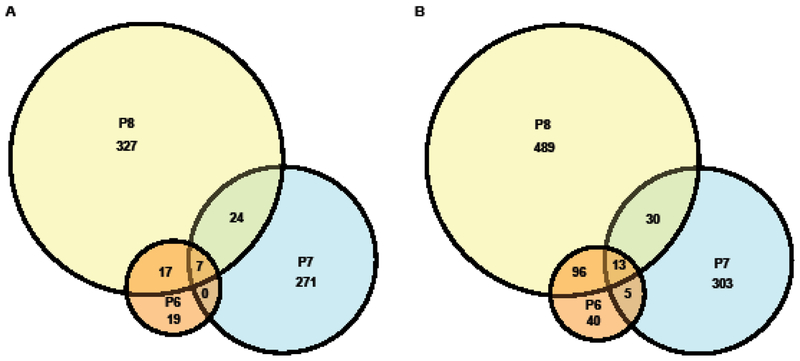
The number of DEGs present across all 3 generations was small in comparison to the total numbers with only 7 up-regulated and 13 down-regulated genes shared across P6–8 (Fig 3).
Fig 3. Venn diagrams of differentially expressed genes.
Indicated in the Venn diagrams are the numbers of (A) up-regulated and (B) down-regulated differentially expressed genes shared between or exclusive to S. mansoni derived from P6, P7 and P8. The diameter of each circle is proportional to the number of transcripts it represents.
Gene Ontology (GO) analysis was employed to provide a statistical assessment of over-represented GO Biological Process terms and S. mansoni reference sequences. The over-represented up-regulated sequences in PZQ-selected schistosomes were weighted towards cell cycling processes at P7 but had no other obvious discernable pattern (Fig S4A, C and E). The over-represented down-regulated sequences were overwhelmingly involved in metabolic processes across P6–8: (Fig S4B, D and F) with 8 transcripts designated as ‘drug metabolic process’ being amongst most overrepresented sequence at P7. On further examination 4 of these were hypothetical proteins while the remaining transcripts encoded putative pyruvate kinase (Smp_065610), adenosine kinase (Smp_008370), phosphatidylcholine-sterol acyltransferase (Smp_082120) and a kunitz-type protease inhibitor (Smp_180810). Overall, however, it was difficult to discern a significant pattern in the GO data related to selection for PZQ resistance.
3.3. Expression analysis of transcripts related to PZQ-selection
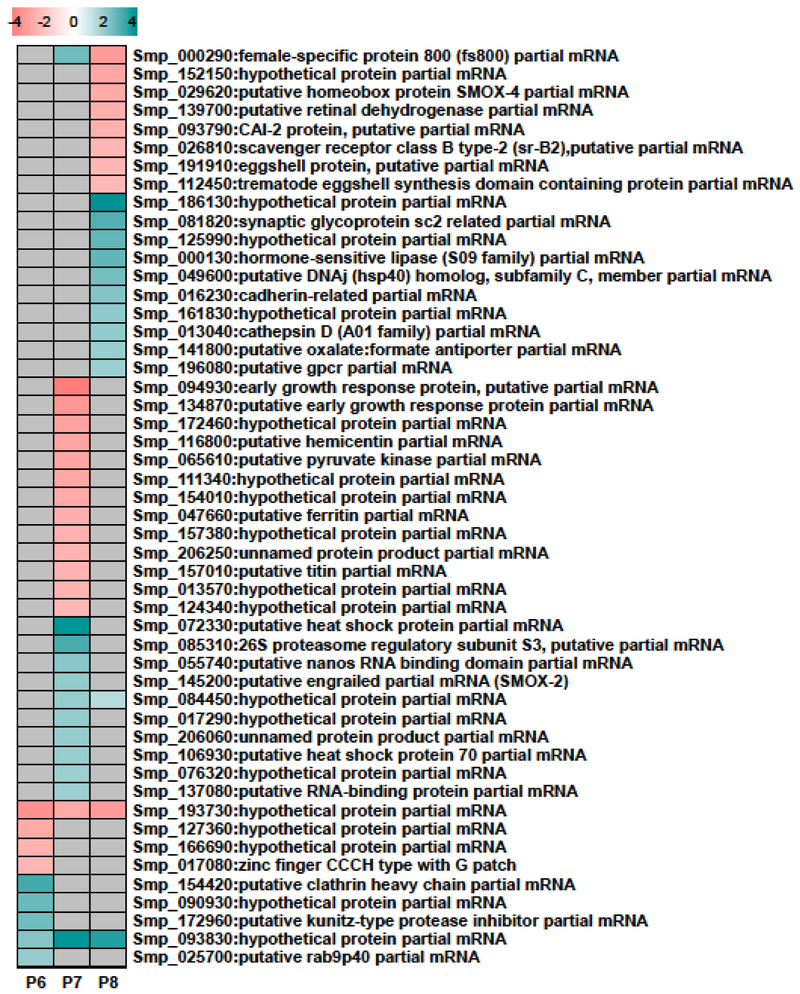
A heat map was constructed to examine those genes that showed the greatest levels of differential regulation in the PZQ-selected versus control lines at P6–8 (Fig 4). The top 25 up- and 25 down-regulated genes were selected based on their expression at any of these 3 passages. Twenty genes encoded ‘hypothetical’ or ‘unnamed’ proteins underlining the relative paucity of original annotation of Schistosoma spp. genes as well as the evolutionary distance between Schistosoma spp. and the classical model organisms. Only 4 genes showed expression in S. mansoni at more than one passage and only two (both hypothetical) showed expression across all three passages. Among the highly differentially regulated genes with functional annotation there were some interesting observations. Two of the putative gene products were similar to transcriptional regulator Homeobox proteins engrailed-like SMOX-2 (Smp_145200) and SMOX-4 (Smp_029620), two other products matched the putative DNA-binding Early Growth Response Protein (EGRP; Smp_094930 and Smp_134870) and two encoded homologs of RNA binding proteins (Smp_055740 and Smp_137080). While SMOX-2 and both RNA binding proteins were up-regulated in P7, both EGRP genes were down-regulated at this time and SMOX-4 was down-regulated at P8. These changes in expression of transcriptional and post-transcriptional regulators presumably reflect the increased transcriptional dynamics associated with the PZQ-selected line and are indirect consequences of drug selection. The down-regulation of EGRP was in contrast to the findings of You et al. who observed that this transcript was up-regulated in both male and female S. japonicum exposed in vivo to a sublethal dose of PZQ 0.5 – 24 h before the mouse host was sacrificed [37]. Other genes of note included the reproduction associated genes encoding female-specific protein 800 (Smp_000290) which is mooted to play a role in vitellogenesis [38], eggshell protein (Smp_191910) and trematode eggshell synthesis domain containing protein (Smp_112450), each of which were down-regulated only at P8, perhaps presaging the loss of the PZQ-selected line due to the lack of viable egg production.
Fig 4. Expression of selected S. mansoni genes during passages (P) 6, 7 and 8.
Heat map of the 50 most differentially expressed genes (25 up- and 25 down-regulated) in PZQ-selected parasites relative to controls. Genes not differentially expressed are colored gray and increased and decreased gene expression are teal and pink respectively. The color scale indicates log2 fold change for the average of four biological replicates.
Although the mechanism of action of PZQ and the molecular basis of juvenile resistance is not yet understood, previous studies have identified gene products that may play a role in both [18–20,39,40]. We have examined the differential regulation of a number of these genes previously and identified stress related genes that are differentially regulated in response to PZQ exposure in vitro [19] and in vivo [20]. One of these, ferritin (Smp_047660), was highly up regulated in juvenile and sexually mature male S. mansoni after exposure to 250 mg/kg/day on each of 4 consecutive days but was among highly down-regulated genes at P7 (Fig 4). In addition to ferritin, one other transcript encoding a previously identified PZQ induced stress protein transcript, thioredoxin peroxidase (TPX-1; Smp_059480), was also down-regulated at P7 (log2 change = −1.16, p<0.05) while a superoxide dismutase (SOD) precursor transcript (Smp_095980) was down-regulated at P6 (−1.80, p<0.05) and P8 (−3.41, p<0.0001) and a second SOD transcript (Smp_174810) was down-regulated at P7 (−1.06, p<0.01). Both thioredoxin peroxidase and SOD were identified by You et al. as up-regulated in female but not male S. japonicum exposed in vivo as described above [37]. No other stress transcripts that have been identified previously as being differentially regulated in response to PZQ treatment were significantly altered though a number of other heat shock proteins that can also be responsive to stress (Smp_106930, Smp_072330 and Smp_049600) were significantly up-regulated at P7 or P8.
Exposure of schistosomes to PZQ results in a muscular paralysis that has been attributed to drug induced influx of Ca2+ [41]. You et al. [37] reported an up-regulation of genes associated with the ‘calcium signaling pathway’ in male S. japonicum exposed to PZQ that were generally down-regulated in females. Here we observed that, with the exception of two instances, all putative Ca2+ binding or regulatory genes were down regulated at P6–8 in our mixed sex samples. These included transcripts encoding Ca2+ binding protein at P6 (Smp_033000; −1.15, p<0.05) and P8 (−1.47, p<0.0001), Ca2+ binding protein 2 at P7 (Smp_025390; −2.85, p<0.0001), a Ca2+ activated K+ channel (Smp_166620; −1.08, p<0.001) as well as calmodulin at P7 (Smp_026560; −2.12, p<0.0001 and Smp_032950; −1.86, p<0.0001) and P8 (Smp_032970; −1.6, p<0.01) and calponin at P7 (Smp_168650; −1.77, p<0.0001). The only transcripts to be up-regulated were that of an I-type Ca2+ channel at P7 (Smp_134050, 1.04, p<0.01) and calponin at P8 (Smp_168650; 1.97, p<0.0001). This data confirms PZQ disturbs Ca2+ homeostasis and lends some support to the observations of You et al. [37] regarding sex-specific regulation as the P6–P8 cohorts were female biased. One caveat, however, is that this sex-specific effect would have had to be relatively long lasting as in this study the transcriptomes were sampled 12 days after the final dose of PZQ was administered to the host.
ABC superfamily members, especially those of the ABCB, ABCC and ABCG families have been shown repeatedly to excrete structurally unrelated drugs, potentially conferring multiple drug resistance on cells in which they are expressed [42]. At least 21 ABC transporter genes have been identified in S. mansoni [43] and we found four of these to be differentially regulated including 3 members of the ABCB and ABCC subfamilies. Pinto-Almeida et al. [15] examined a stable resistance phenotype to PZQ and found the relative expression of the ABCB1 homolog SmMDR2 (Smp_055780) increased in PZQ resistant strain males compared to susceptible strain while resistant strain female SmMDR2 levels were actually lower than the susceptible strains. We identified this ABCB1 homolog previously to be highly up-regulated in drug resistant mixed sex juveniles but not adult males [20]. In this study, Smp_055780 was not differentially regulated but this may be explained by the significantly higher number of females than males contributing to our data set. In addition to identifying an increase in the expression of Smp_055780, Sanchez et al. [20] also identified significant increases of a second ABCB1 homolog (Smp_089200) in mixed sex juveniles but not a third (Smp_170820). Here, a fourth ABCB1 homolog, Smp_137080, was up-regulated at P7 (1.94, p<0.05). S. mansoni subfamily homologs ABCC4 (Smp_167610) and ABCC10 (Smp_147250) were also differentially regulated at P7, however, while ABCC10 expression was decreased at P7 (−1.45, p<0.5), ABCC4 was increased (1.04, p<0.05). Therefore, while drug resistance in juvenile worms may be associated with increased expression of ABCB, ABCC and ABCG family members [20] there is only limited evidence from this data set that reduced susceptibility in P6–8 schistosomes is associated with the overexpression of ABC subfamily members. Each of these differences between previous and current data sets may, however, be due to the sampling of the transcriptome in this study having taken place 12 days after the last PZQ treatment rather that in the period immediately after the last administration of the drug allowing the protective stress and ABC transporter response induced acutely on drug exposure to dissipate as the PZQ is metabolized. If true, this would also infer that reduced susceptibility may be the result of changes in facultative rather than constitutive expression states.
Pinto-Almeida et al. [40] revealed proteins associated with S. mansoni PZQ resistance through comparative proteomics. PZQ resistance was generated in a fully susceptible isogenic S. mansoni strain treated with 0.3 μM PZQ and compared to a fully susceptible strain using high throughput LC-MS/MS identification of protein spots after 2-dimensional gel electrophosesis. Of the eight proteins Pinto-Almeida and colleagues identified in the mixed sex PZQ-resistant strain, none were identified in our data set though one of the three proteins associated with PZQ-resistance exclusive to females, a receptor for activated Protein Kinase C was down-regulated in P8 (Smp_102040; −1.10, p<0.05).
3.4. RNA-Seq validation
Quantitative real-time PCR (qRT-PCR) was employed to validate results obtained by RNA-Seq analysis. Six transcripts encoding homologs of cytochrome oxidase C (Smp_144000), cercarial elastase (Smp_119130), myosin 2 light chain (Smp_062250), SIL 1 (Smp_180180), kinesin K1F6 (Smp_197070) and histone deacetylase (Smp_191310) were validated based on their differential expression across P6, 7 and 8 (Fig S5A). Patterns observed in the qRT-PCR data mirrored those obtained by the RNA-Seq data and showed significant correlation at P6 (r = 0.85, p <0.0312; Fig S5B), P7 (r = 0.98, p <0.0007; Fig S5C) and P8 (r = 0.98, p <0.0005; Fig S5C).
4. Conclusions
The underlying basis for the reduction in sensitivity to PZQ seen in this study could have a number of explanations. For example, there may be one or more mutations in a single gene, such as that encoding the PZQ binding target, that causes a loss of function without a change in expression. Alternatively, a mutation or epigenetic change in a single gene may lead to a change in its expression which can in turn alter the toxicity and/or half-life of a drug. While the transcriptomic analysis carried out here did provide insight into genes that are differentially regulated between PZQ-selected and sensitive lines it did not immediately suggest any single candidate gene or pathway as being responsible for reduced susceptibility. For example, while there were some differences in expression of ABC transporters that might enhance drug efflux, there was not enough consistency in expression for any single ABC gene to suggest that this superfamily alone played a significant role. William et al. [9] concluded the factors leading to selection and maintenance for PZQ resistance varied among isolates and a third drug resistance mechanism may be, as Roquis et al., [44] suggested, ‘due to the epistatic interaction of multiple gene products rather than an (epi)mutation of a single locus’. For example, S. mansoni resistance to the anthelmintic drugs hycanthone and oxamniquine emerged readily in natural populations [45]. A single autosomal recessive locus was found to encode both oxamniquine and hycanthone resistance in two strains [46] and subsequently, non-synonymous coding mutations associated with a sulfotransferase gene were identified, thus establishing the mechanism of resistance for both of these compounds [47]. Subsequently, however, Roquis et al. [44] found laboratory generated hycanthone-resistant parasites that do not carry these mutation but instead carry a high number of epigenetic changes compared to controls. Changes in chromatin structure between resistant and sensitive worms were identified and 5 genes involved in stress responses and one ABC transporter were among 69 genes associated with these modifications. Thus, while we also see changes to stress, ABC transporter, and Receptor for activated PKC gene expression, like Roquis et al. [44] and Pinto-Almeida et al. [40] we also see no obvious single gene or pathway behind the observed PZQ resistance in S. mansoni PR1 and therefore do not rule out the possibility of such an ‘epistatic interaction of multiple gene products’. Identifying the true genetic actors in such interactions may need either more powerful transcriptomic or proteomic analyses or meta-analyses as well as a systems approach to understanding of the role of epigenetics in the generation of resistance. In addition, the mechanism by which resistance is generated may differ depending on whether relatively genetically homogeneous laboratory strains are targeted for selection as opposed to heterogeneous field populations in which the classic monogenic pattern of resistance can more easily arise.
Supplementary Material
Highlights.
A line of schistosomes with reduced sensitivity to praziquantel was generated.
Reduced miracidial sensitivity to praziquantel was evident from the outset.
Reduced sensitivity was marked by a reduction in the length of female schistosomes.
The sex of schistosomes shifted from a predominantly male to a female population.
No single gene or pathway is associated with reduced drug sensitivity.
Acknowledgements
We would like to thank the staff of the UNM Department of Biology Animal Resource Facility for care and husbandry of research animals and the staff of the Center for Evolutionary and Theoretical Immunology (CETI) Molecular and Cell Biology Core Facilities for their help and advice. Support through CETI was provided by the NIH/NIGMS under award P30GM110907. Funding was provided to CC and PMC by the NIH/NIAID award R01 AI 087807. This material is also based on work supported in part by the National Science Foundation. Any opinion, finding, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None
References
- [1].World Health Organization, 2017. Schistosomiasis and soil transmitted helminthiases: number of people treated in 2016. Wkly. Epidemiol. Rec 92, 749–760. [PubMed] [Google Scholar]
- [2].World Health Organization, 2016. Schistosomiasis: number of people treated worldwide in 2014. Wkly. Epidemiol. Rec 91, 53–60. [PubMed] [Google Scholar]
- [3].Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuenté L-A, 2004. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 20, 92–97. 10.1016/j.pt.2003.11.010 [DOI] [PubMed] [Google Scholar]
- [4].Aragon AD, Imani RA, Blackburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C, 2009. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol 164, 57–65. 10.1016/j.molbiopara.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pica-Mattoccia L, Cioli D, 2004. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol 34, 527–533. 10.1016/j.ijpara.2003.12.003 [DOI] [PubMed] [Google Scholar]
- [6].Stelma FF, Talla I, Sow S, Kongs A, Niang M, Polman K, Deedler AM, Gryseels B, 1995. Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am. J. Trop. Med. Hyg 53, 167–170. 10.4269/ajtmh.1995.53.167 [DOI] [PubMed] [Google Scholar]
- [7].Fallon PG, Mubarak JS, Fookes RE, Niang M, Butterworth AE, Sturrock RF, Doenhoff MJ, 1997. Schistosoma mansoni: maturation rate and drug susceptibility of different geographical isolates. Exp. Parasitol 86, 29–36. 10.1006/expr.1997.4149 [DOI] [PubMed] [Google Scholar]
- [8].Ismail M, Botros S, Metwally A, William S, Farghally A, Tao L-F, Day TA, Bennett JL 1999. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg 60, 932–935. [DOI] [PubMed] [Google Scholar]
- [9].William S, Sabra A, Ramzy F, Mousa M, Demerdash Z, Bennett JL, Day TA, Botros S, 2001. Stability and reproductive fitness of Schistosoma mansoni isolates with decreased sensitivity to praziquantel. Int. J. Parasitol 31, 1093–1100. 10.1016/S0020-7519(01)00215-6 [DOI] [PubMed] [Google Scholar]
- [10].Botros S, Sayed H, Amer N, El-Ghannam M, Bennett JL, Day TA, 2005. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int. J. Parasitol 35, 787–791. 10.1016/j.ijpara.2005.02.005 [DOI] [PubMed] [Google Scholar]
- [11].Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DMS, Colley DG, Black CL, Secor WE, Mkoji GM, Loker ES, 2009. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis 18, e504 10.1371/journal.pntd.0000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fallon PG, Doenhoff MJ, 1994. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg 51, 83–88. 10.4269/ajtmh.1994.51.83 [DOI] [PubMed] [Google Scholar]
- [13].Coeli R, Baba EH, Araujo N, Coelho PMZ, Oliveira G, 2013. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Negl. Trop. Dis 7, e2596 10.1371/journal.pntd.0002596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mwangi IN, Sanchez MC, Mkoji GM, Agola LE, Runo SM, Cupit PM, Cunningham C, 2014. Praziquantel sensitivity of Kenyan Schistosoma mansoni isolates and the generation of a laboratory strain with reduced susceptibility to the drug. Int. J. Parasitol. Drugs Drug Resist 4, 296–300. 10.1016/j.ijpddr.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pinto-Almeida A, Mendes T, Armada A, Belo S, Carrilho E, Viveiros M, Afonso A, 2015. The role of efflux pumps in Schistosoma mansoni praziquantel resistant phenotype. PLoS One 10, e0140147 10.1371/journal.pone.0140147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Couto FF, Coelho PMZ, Araújo N, Kusel JR, Katz N, Jannotti-Passos LK, Mattos ACA, 2011. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz 106, 153–157. 10.1590/S0074-02762011000200006 [DOI] [PubMed] [Google Scholar]
- [17].Kasinathan RS, Morgan WM, Greenberg RM, 2010. Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel. Mol. Biochem. Parasitol 173, 25–31. 10.1016/j.molbiopara.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kasinathan RS, Sharma LK, Cunningham C, Webb TR, Greenberg RM, 2014. Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile schistosomes to praziquantel. PLoS Negl. Trop. Dis 8, e3265 10.1371/journal.pntd.0003265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hines-Kay J, Cupit PM, Sanchez MC, Rosenberg GH, Hanelt B, Cunningham C, 2012. Transcriptional analysis of Schistosoma mansoni treated with praziquantel in vitro. Mol. Biochem. Parasitol 186, 87–94. 10.1016/j.molbiopara.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sanchez MC, Krasnec KV, Parra AS, von Cabanlong C, Gobert GN, Umylny B, Cupit PM, Cunningham C, 2017. Effect of praziquantel on the differential expression of mouse hepatic genes and parasite ATP binding cassette transporter gene family members during Schistosoma mansoni infection. PLoS Negl. Trop. Dis 11, e0005691 10.1371/journal.pntd.0005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cupit PM, Cunningham C, 2015. What is the mechanism of action of praziquantel and how might resistance strike? Future Med. Chem 7, 701–705. 10.4155/fmc.15.11 [DOI] [PubMed] [Google Scholar]
- [22].Lamberton PHL, Faust CL, Webster JP, 2017. Praziquantel decreases fecundity in Schistosoma mansoni adult worms that survive treatment: evidence from a laboratory life-history trade-offs selection study. Infect. Dis. Poverty 6, 110 10.1186/s40249-017-0324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bolger AM, Lohse M, Usadel B, 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Langmead B, Salzberg SL, 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li B, Dewey CN, 2011. RSEM: accurate transcript quantification from RNA Seq data with or without a reference genome. BMC Bioinformatics 12, 323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Robinson MD, McCarthy DJ, Smyth GK 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, Haag JD, Gould MN, Stewart RM and Kendziorski C, 2013. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 29, 1035–1043. 10.1093/bioinformatics/btt087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Varet H, Brillet-Guéguen L, Coppée JY, Dillies MA, 2016. SARTools: a DESeq2-and edgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS One 11, e0157022 10.1371/journal.pone.0157022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A, 2008. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36, 3420–3435. 10.1093/nar/gkn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al-Shahrour F, Díaz-Uriarte R, Dopazo J, 2004. FatiGO; a web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics 20, 578–580. 10.1093/bioinformatics/btg455 [DOI] [PubMed] [Google Scholar]
- [32].Boissier J, Moné H, 2000. Experimental observations on the sex ratio of adult Schistosoma mansoni, with comments on the natural male bias. Parasitology 121, 379–383. [DOI] [PubMed] [Google Scholar]
- [33].Andrews P, Thomas H, Pohlke R, Seubert J, 1983. Praziquantel. Med. Res. Rev 3, 147–200. [DOI] [PubMed] [Google Scholar]
- [34].Shaw MK, Erasmus DA 1987. Schistosoma mansoni: structural damage and tegumental repair after in vivo treatment with praziquantel. Parasitology 94, 243–254. [DOI] [PubMed] [Google Scholar]
- [35].LoVerde PT, 2002. Presidential address. Sex and schistosomes: an interesting biological interplay with control implications. J. Parasitol 88, 3–13. 10.1645/0022-3395(2002)088[0003:PASASA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- [36].Liu Y, Zhou J, White KP, 2014. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics 30, 301–304. 10.1093/bioinformatics/btt688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].You H, McManus DP, Hu W, Smout MJ, Brindley PJ, Gobert GN 2013. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 9, e1003254 10.1371/journal.ppat.1003254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Croisille Y, Junera H, Meusy J-J, Charniaux-Cotton H, 1974. The female-specific protein (Vitellogenic Protein) in Crustacea with particular reference to Orchestia gammarella (Amphipoda). Amer. Zool 14, 1219–1228. [Google Scholar]
- [39].Chan JD, Cupit PM, Gunaratne GS, McCorvy JD, Yang Y, Stoltz K, Webb TR, Dosa PI, Roth BL, Abagyan R, Cunningham C, Marchant JS, 2017. The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat. Commun 8, 1910 10.1038/s41467-017-02084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pinto-Almeida A, Mendes T, Ferreira P, Belo S, Anibal F.d.F., Allegretti SM, Carrilho E, Afonso A, 2018. Comparative proteomics reveals characteristic proteins on praziquantel-resistance in Schistosoma mansoni. bioRxiv. [Google Scholar]
- [41].Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, Marcatto-Maggi AL, Nobre-Santana S, Troiani AR, Cioli D, Valle C 2008. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp. Parasitol 119, 332–335. doi: 10.1016/j.exppara.2008.03.012 [DOI] [PubMed] [Google Scholar]
- [42].Vasiliou V, Vasiliou K, Nebert DW, 2009. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics 3, 281–290. 10.1186/1479-7364-3-3-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Greenberg RM, 2013. ABC multidrug transporters in schistosomes and other parasitic flatworms. Parasitol. Int 62, 647–653. 10.1016/j.parint.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roquis D, Lepesant JMJ, Villafan E, Boissier J, Vieira C, Cosseau C, Grunau C, 2014. Exposure to hycanthone alters chromatin structure around specific gene functions and specific repeats in Schistosoma mansoni. Front. Genet 5, 207 10.3389/fgene.2014.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cioli D, Pica-Mattoccia L, Archer S, 1989. Resistance of schistosomes to hycanthone and oxamniquine. Mem. Inst. Oswaldo Cruz 84, 38–45. 10.1590/S0074-02761989000500005 [DOI] [PubMed] [Google Scholar]
- [46].Pica-Mattoccia L, Dias LC, Moroni R, Cioli D 1993. Schistosoma mansoni: genetic complementation analysis shows that two independent hycanthone/oxamniquine-resistant strains are mutated in the same gene. Exp. Parasitol 77, 445–9. 10.1006/expr.1993.1104 [DOI] [PubMed] [Google Scholar]
- [47].Valentim CL, Cioli D, Chevalier FD, Cao X, Taylor AB, Holloway SP, Pica-Mattoccia L, Guidi A, Basso A, Tsai IJ, Berriman M, Carvalho-Queiroz C, Almeida M, Aguilar H, Frantz DE, Hart PJ, LoVerde PT, Anderson TJ. 2013. Genetic and molecular basis of drug resistance and species-specific drug action in schistosome parasites. Science 342, 1385–1389. doi: 10.1126/science.1243106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.