Abstract
Purpose:
To develop an accelerated cardiac perfusion pulse sequence and test whether it is capable of increasing the spatial coverage, generating high-quality images, and enabling quantification of myocardial blood flow (MBF).
Methods:
We implemented an accelerated first-pass cardiac perfusion pulse sequence by combining radial k-space sampling, compressed sensing (CS), and KWIC filtering. The proposed and clinical standard pulse sequences were evaluated in a randomized order in 13 patients at rest. For visual analysis, three readers graded the conspicuity of wall enhancement, artifact, and noise level on a 5-point Likert scale (overall score index = sum of three individual scores). Resting MBF was calculated using a Fermi function model with and without KWIC filtering. The mean visual scores and MBF values were compared between sequences using appropriate statistical tests.
Results:
The proposed pulse sequence produced greater spatial coverage (6–8 slices) with higher spatial resolution (1.6×1.6×8mm3) and shorter readout duration (78 ms) compared to clinical standard (3–4 slices, 3×3×8mm3, 128ms, respectively). The overall image score index between accelerated (11.1 ± 1.3) and clinical standard (11.2 ± 1.3) was not significantly different (p=0.64). The mean resting MBF values with KWIC filtering (0.9 – 1.2 mL/g/min across different slices) were significantly lower (p < 0.0001) than those without KWIC filtering (3.1 – 4.3 mL/g/min), and agreed better with values reported in literature.
Conclusion:
An accelerated, first-pass cardiac perfusion pulse sequence with radial k-space sampling, CS, and KWIC filtering is capable of increasing the spatial coverage, generating high-quality images, and enabling quantification of MBF.
Introduction
Coronary artery disease (CAD) is a leading cause of death in the United States (1). First-pass cardiac perfusion MRI (2) with administration of gadolinium-based contrast agents (GBCA) and vasodilator stress is a well-established imaging test for diagnosing obstructive CAD and has been shown to be as accurate as cardiac SPECT (3). Currently, extensive research efforts are ongoing to improve myocardial coverage (4–6), minimize dark rim artifacts (DRA) (7,8), quantify myocardial blood flow (MBF) (9,10), and generate high-quality images.
The aforementioned limited myocardial coverage is a concern for first-pass cardiac perfusion MRI. For instance, a standard perfusion MRI scan typically acquires three short-axis slices. During pharmacologic stress or tachycardia, the patient’s heart rate may rise and limit the number of slices that may be sampled per heartbeat. Limited myocardial coverage decreases the diagnostic confidence, because it may fail to sample ischemic myocardium and provide little margin of error to read through image artifacts (e.g. DRA). One approach to increasing the spatial coverage is to speed-up the scan with acceleration methods such as k-t SENSE (11), k-t PCA (12), BLOSM (13) and compressed sensing (CS) (5,14). In this study, we elected to use CS to accelerate perfusion MRI because it achieves high acceleration rates.
While visual diagnosis is the clinical norm for evaluating cardiac perfusion MRI, quantitation of MBF has the potential to improve accuracy in diagnosis (9), particularly in the setting of multi-vessel disease (10) and microvascular disease such as in women’s heart disease, and it may play an important role in risk stratification (15). Quantitative analysis of perfusion MRI requires an accurate arterial input function (AIF), which is typically measured in the left ventricular (LV) blood pool. Proven approaches to measuring AIF accurately include the dual-bolus (16) and dual-imaging methods (17,18), both of which are designed to avoid a nonlinear relationship between the LV blood pool signal and GBCA concentration, particularly for high GBCA concentration values. A dual-bolus technique is not practical in a clinical setting, because it requires accurate preparation of two different concentrations at equal volumes and a complex setup to prevent backflow in the injector apparatus (19). A dual-imaging approach requires extensive pulse sequence development to include a dedicated AIF acquisition where all of its parameters are known (20). In this study, we elected to use a synthetic dual-imaging approach in conjunction with image acceleration provided by CS.
This study sought to increase the spatial coverage and quantify MBF using a synergistic combination of the following advanced methods: (a) radial k-space sampling and CS (14,21,22) to increase the spatial coverage and (b) k-space weighted image contrast (KWIC) filtering (23) reconstruction to retrospectively choose an arbitrary saturation-recovery time to the center of k-space (TS) and perform simultaneous reconstruction with a short TS for AIF assessment and a long TS for maximal tissue enhancement (i.e. dual-imaging). Similar to the method described by the University of Utah group (24,25), our method uses KWIC filtering and signal modeling of radial k-space sampling. In contrast to the method used by the Utah group, our method uses accelerated acquisition (30 rays vs. 96 rays by the Utah group) and a single ray to determine the signal (1 ray for AIF and 15 rays for wall enhancement vs. 24 rays by the Utah group). Unlike the model-based image reconstruction method described by Tran-Gia et al. (26), our method performs conventional image reconstruction for visual analysis by readers and then uses signal modeling during post-processing to quantify MBF. The purpose of this study is to develop an accelerated cardiac perfusion pulse sequence and test whether it is capable of increasing the spatial coverage, generating high-quality images for visual analysis, and enabling quantification of MBF.
Methods
Numerical Phantom Experiment
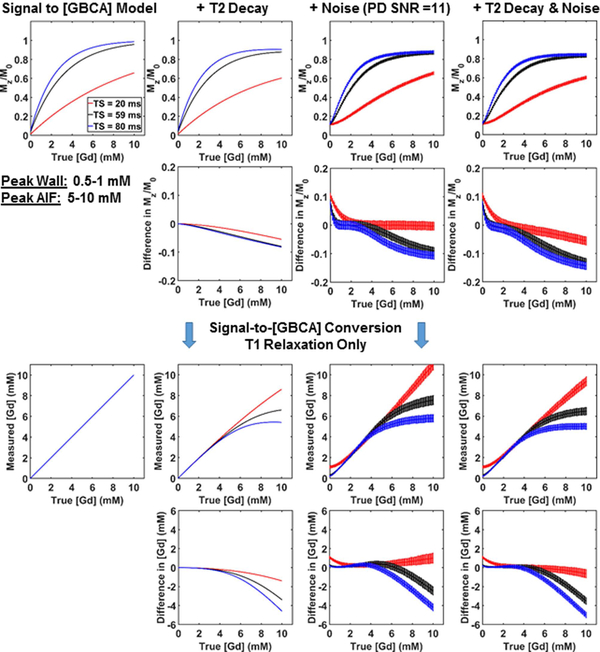
We performed a theoretical analysis to estimate the impact of noise and T2 decay on accuracy. While T2* is more relevant for gradient echo readout, we elected to model T2 instead since T2* depends on a variety of factors that would be difficult to model accurately. For this experiment, we assumed signal-to-noise ratio (SNR) of the proton density image acquired with 12° flip angle to be 11, which is a conservative estimate for patients imaged at 1.5T. This value corresponds to the maximum intrinsic SNR for T1-weighted images when TS approaches infinity. For each iteration, we defined the region of interest with 100 voxels representing the left ventricular cavity of a short-axis plane and computed the mean values. This experiment was repeated 10,000 times to plot the average trends (i.e. Monte Carlo), where reported values represent the average and standard deviation of mean values from all iterations. For GBCA concentration ([GBCA]) ranging from 0 to 10 mM (0.1 steps), assuming fast water exchange, we calculated the longitudinal relaxation rate (R1) = R1,0 + r1 [GBCA], where R1,0 is native longitudinal relaxation rate of blood = 0.6667 s−1 (i.e. native T1 = 1.5 s) and r1 is the longitudinal relaxivity = 5.3 L/(mmol*s) of blood with gadobutrol (27). We also calculated the transverse relaxation rate (R2) = R2,0 + r2 [GBCA], where R2,0 is native transverse relaxation rate of blood = 4 s−1 (i.e. native T2 = 250 ms) and r2 is the transverse relaxivity = 5.4 L/(mmol*s) of blood with gadobutrol (27). For each T1 and T2 derived from ground truth [GBCA], the T1-weighted signal described by Eq.5 in Kholmovski et al.(24) was normalized with a scale ranging from 0 to 1 by setting the proton density or equilibrium magnetization (M0) equal to 1. Next, the T1-weighted signal was attenuated by multiplying it to exp(−TE/T2), where TE = 1.5 ms. White Gaussian noise with mean = 0 and standard deviation = 0.15 was added to the T1 weighted signal such that the SNR equals to 11 when TS approaches infinity (i.e. proton density). We applied two additional steps prior to converting signal to [GBCA]. First, to mimic a standard MR image reconstruction, we applied a magnitude operation to the T1 weighted signal, leading to Rician noise statistics for signal values close to zero. Second, we enforced normalized T1 weighted signal to be ≤ 1 by replacing values greater than 1 to be equal to 1, leading to a downward shift in signal values close to 1 due to an asymmetric contribution of noise. This second step is necessary since normalized T1 weighted signal greater than 1 will produce a spurious result. Finally, the normalized signal with T2 decay and noise added was converted to R1 and then [GBCA] using our signal modeling that accounts for only T1 relaxation.
Study Population
We prospectively enrolled 13 patients (mean age = 44 ± 18 years; 8 men and 5 women) undergoing a clinically indicated cardiac MRI with administration of GBCA for assessment of myocardial infiltration or viability. Because these clinical MRI scans did not involve a dynamic contrast-enhanced MRI, they provided us an opportunity to add two resting perfusion scans for research without significantly altering the clinical MRI protocol. All subjects provided written informed consent. This study was performed in accordance with protocols approved by our institutional review board and was Health Insurance Portability and Accountability Act (HIPAA) compliant. See Supporting Information Table S1 for relevant clinical profiles.
MRI Hardware
MRI was performed on two 1.5T whole-body MRI scanners (MAGNETOM Aera & Avanto, Siemens Healthineers, Erlangen, Germany) equipped with a gradient system capable of achieving a maximum gradient strength of 45 mT/m and a slew rate of 200 T/m/s. Imaging was performed with body matrix and spine array coils with 18 and 30 coil elements on the Avanto and Aera, respectively.
Pulse Sequence
We modified an ultra-fast gradient echo (i.e. TurboFLASH) pulse sequence to employ radial k-space sampling with the 7th Fibonacci sequence of golden angles, which is equal to 23.628° (28), and compared its performance against a prototype cardiac perfusion MRI pulse sequence using a TurboFLASH readout with Cartesian k-space sampling. This prototype sequence is referred to as clinical standard throughout. The proposed and standard cardiac rest perfusion MRI scans were performed 5 min apart in a randomized order, where each scan was performed with administration of 0.1 mmol/kg of gadobutrol at 4–5 mL/s via a power injector. Gadobutrol was diluted with equal volume of saline and this mixture was administered via an antecubital IV injection followed by a 20 mL of saline flush. Patients were given instructions to breathe shallow throughout the perfusion scans.
Imaging parameters for the standard perfusion MRI sequence included: mean FOV = 380 mm x 305 mm, mean spatial resolution = 3 mm x 3 mm, slice thickness = 8 mm, acquisition matrix = 128 × 102, TE/TR = 1.14/2.5 ms, readout duration = 128 ms, TS = 136 ms, 2-fold acceleration with TGRAPPA (29), receiver bandwidth = 750 Hz/pixel, 3–4 short-axis planes per heartbeat, 65 repetitions, and flip angle = 15°.
The relevant imaging parameters for the proposed perfusion pulse sequence included: FOV = 300 mm × 300 mm, acquisition matrix = 192 × 192, nominal spatial resolution = 1.6 mm × 1.6 mm, slice thickness = 8 mm, flip angle = 12°, TE/TR = 1.5/2.6 ms, receiver bandwidth = 700 Hz/pixel, readout duration = 78 ms (30 rays), radio-frequency field insensitive saturation pulse (30), nominal TS = 59 ms (for the 16th ray), 75 repetitions, and 6–8 (3–5 short-axis and 3 long-axis) slices per heart beat. For the accelerated rest perfusion scans, we additionally acquired proton density images without applying the saturation pulse for the first two heart beats, in order to normalize the T1-weighted signal by M0, as previously reported (31). Immediately after the perfusion scan, over 1 heart beat, we acquired 30 calibration rays per slice (a pair with opposite polarities per angle; 12° angular steps), in order to correct for gradient delays, as previously described (32). We designed the pulse sequence with a short readout duration (78 ms) to make our pulse sequence relatively insensitive to cardiac and respiratory motion.
KWIC Filtering
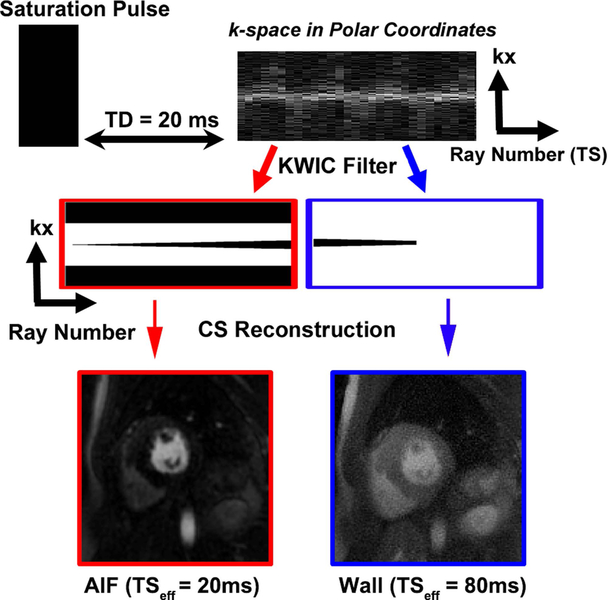
In radial sampling, the center of k-space is sampled by each ray and therefore does not provide a unique TS. To compensate for this problem, we applied a KWIC filter to retrospectively choose an arbitrary TS. To accurately quantify AIF, images with a short TS were reconstructed using a KWIC filter where the k-space center was retained for the first ray (i.e. effective TS = 20 ms as shown in Figure 1) and omitted for succeeding rays. For myocardial wall assessment, images were reconstructed using another KWIC filter where the k-space center was retained for the last fifteen rays (i.e. effective TS = 80 ms as shown in Figure 1). This scheme enabled us to reconstruct an AIF image with TS = 20 ms and myocardial wall enhancement images with TS = 80 ms from the same data set.
Figure 1:
A schematic showing how KWIC filtering is used to synthetically replicate dual imaging. This is accomplished by multiplying the k-space in polar coordiates with two different KWIC filters. To accurately quantify AIF, images with TS=20 ms were reconstructed using a KWIC filter where the k-space center data was retained for the first ray and omitted for all the other rays (red). For myocardial wall enhancement assessment, images were reconstructed using another KWIC filter with TS = 80 ms, where the k-space center data was retained for the last fifteen rays and omitted for all the other rays (blue). Following CS reconstruction, AIF images with TS = 20 ms and wall enhancement images with TS = 80 ms are produced. For the KWIC filters, black and white correspond to zero and one, respectively.
Image Reconstruction
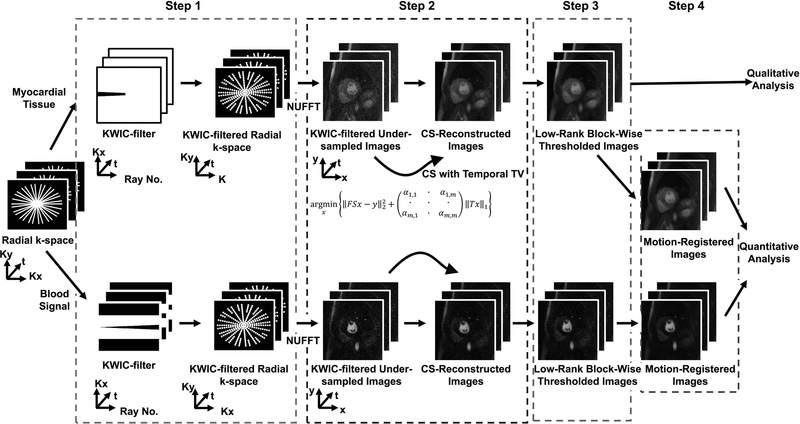
A schematic of the reconstruction pipeline is shown in Figure 2. Note, the reconstruction is performed using 4 successive steps (Figure 2A), where reconstruction parameters were derived empirically based on visual inspection of training data sets.
Figure 2:
A schematic of the image reconstruction pipeline involving 4 steps. In step 1, a KWIC filter was applied to the radial k-space. For the AIF image reconstruction, the k-space center was retained for the first ray and omitted for all the other rays. For the myocardial wall image reconstruction, the k-space center was retained for the last fifteen rays. In step 2, the undersampled images were reconstructed using CS with temporal total variation (TTV) as the sparsifying transform (30 iterations). A different regularization weight was used for the foreground and background. In step 3, three iterations of low-rank block-wise thresholding was performed to suppress the residual artifacts and noise. In step 4, in order to generate MBF maps, we performed motion correction using ANTS. For dynamic display of results after each of 3 steps (TTV, TTV + low-rank block-wise, TTV + low-rank block-wise + motion correction) in the reconstruction pipeline, see Supporting Information Video S1.
In step 1, a KWIC filter was applied to the undersampled k-space. For the AIF image reconstruction, a KWIC filter was applied such that the k-space center of first ray was retained. Because the signal is relatively low for the first ray, we elected to zero out the outer 50% of k-space to reduce aliasing artifacts. For the myocardial wall image reconstruction, another KWIC filter was applied such that the k-space center of last fifteen rays is retained. Additionally, AIF images were reconstructed without KWIC filtering to evaluate the impact of KWIC filtering on MBF quantification.
In step 2, CS was used to enforce sparsity along the temporal dimension by using temporal total variation (TTV) as the sparsifying transform with empirically derived regularization weights for the foreground and background:
where F is the undersampled nonuniform fast Fourier transform (NUFFT) operator, S is the coil sensitivities in x-y space, x represents the estimated dynamic images being reconstructed, y is the undersampled multi-coil k-space data, α is a matrix of regularization weights defined with different weights for the foreground and the background, T is the finite difference operator which is applied along the time dimension and m is a m-by-m square mask used to distinguish the foreground from the background. For the mask, the foreground was defined as signal intensity greater than 10% of the maximum intensity of the time-averaged image (33). To standardize the regularization weights across different rest perfusion datasets, the normalized regularization weight for the foreground was optimized as 10% of the maximum intensity of a low-resolution proton density image (34). For the background, the normalized regularization weight was set as the maximum intensity of a low-resolution proton density image. To minimize the effect of streaking artifacts on the regularization weight, a low-resolution proton density image (e.g. outer 50% of k-space zeroed) was reconstructed solely for this purpose using NUFFT (35). The CS reconstruction termination criterion was 30 iterations. The CS reconstruction code will be made available upon request.
In step 3, the resulting images were further denoised using three iterations of low-rank block-wise thresholding. An image block of 8×8 was used, and the normalized threshold value was set to 20% of the absolute maximum value.
In step 4, the resulting images were registered through time using the Advanced Normalization ToolS (ANTS) package (36). The details of the motion correction parameters used is provided in the Appendix.
For dynamic display of results after each of 3 steps following KWIC filtering with TS = 80 ms (TTV, TTV + low-rank block-wise, TTV + low-rank block-wise + motion correction) in the reconstruction pipeline, see Supporting Information Video S1.
Visual Analysis
We used images without motion correction for visual analysis, because our readers do not use motion correction in clinical practice. Twenty six rest perfusion datasets (2 rest perfusion scans for each of 13 patients), grouped as a set of 3–4 short-axis planes, were randomized and de-identified for independent evaluation by 3 readers using a 5-point Likert scale for the following three categories: conspicuity of wall enhancement (1: nondiagnostic; 2: poor; 3: clinically acceptable; 4:good; 5: excellent), noise and artifact levels (1: nondiagnostic; 2: severe; 3: moderate; 4: mild; 5: minimal). The first reader (DCL) is a cardiologist with 16 years of clinical experience in reading cardiovascular MRI. The second (PJA) and third readers (MJB) are radiology and cardiology trainees, respectively, each with 1 year of clinical experience reading cardiovascular MRI. Prior to independent blinded review, training data sets were scored by consensus of all reviewers to calibrate their scores. The three readers were blinded to each other’s scores, pulse sequence type, and clinical history when grading the images. An overall image score index was computed by summing the three individual scores (ranging from 3–15).
MBF Quantification Using Bloch Equation Based Signal Modeling
We quantified resting MBF from the proposed perfusion scans because they contain AIF images. All data analysis was performed in MATLAB (R2016A, The MathWorks, Inc, Natick, United States). T1-weighted images were normalized by the proton density image, in order to correct for the unknown M0 (31) and conveniently scale the longitudinal magnetization (Mz) as a fraction of M0. For the AIF, the signal-time curves were generated by placing a region of interest in the basal plane of the LV blood pool. For the myocardial wall enhancement, the signal-time information was generated on a pixel-by-pixel basis. As described in the simulation section, to avoid spurious results, we replaced Mz > M0 to be equal to M0. Assuming fast water exchange and ignoring the effects of noise and T2 decay, we converted the normalized signal (Mz/M0) to GBCA concentration using a Bloch equation based signal modeling (see Figure 3, top row, column 1) of saturation recovery with radial k-space sampling (24,25) and gadobutrol’s longitudinal relaxivity value 5.3 and 5.2 L/(mmol*s) for the blood and myocardium, respectively (27). Subsequently, a Fermi function (16) was used to deconvolve the AIF and myocardial GBCA concentration vs. time curves and estimate absolute MBF on a pixel-by-pixel basis. During image analysis, we accounted for the bolus arrival time by manually translating the AIF curve through time. The fitting for the Fermi function deconvolution was mostly automated, however, the foot of arrival of GBCA concentration in the myocardium was manually specified in cases where the automated fitting was poor. The peak AIF values derived from the basal slice were recorded, and the average MBF over the entire myocardium was recorded per plane.
Figure 3:
Numerical phantom results with concentration values ranging from 0 to 10 mM. (Row 1) Plots of normalized signal as a function of ground truth [GBCA]: T1 relaxation only (column 1), T2 decay added condition (column 2), noise added condition (column 3), and T2 decay and noise added condition (column 4). (Row 2) The corresponding errors in normalized signal. (Row 3) The corresponding plots of measured [GBCA] as a function of ground truth [GBCA]. Each curve was derived from our signal modeling that only accounts for T1 relaxation. (Row 4) The corresponding erros in [GBCA]. Errors induced by noise and T2 decay in normalized signal and measured [GBCA] are smaller for higher TS values (59 and 80 ms) than TS = 20 ms at low concentration values between 0.5 to 1 mM representing peak enhancement in the myocardium, whereas errors in normalized signal and measured [GBCA] are smaller for TS = 20 ms than higher TS values (59 and 80 ms) at high concentration values between 5 to 10 mM representing peak blood enhancement in the left ventricular cavity (i.e. AIF).
Statistical Analysis
The statistical analyses were conducted by one investigator (NKN). A Kolmogorov-Smirnov test was performed to test the null hypothesis that each variable is normally distributed at the 5% significance level. A paired t-test was performed for normal distribution, whereas the Wilcoxon signed rank test was performed for non-normal distribution. Assuming non-normal distribution, the Wilcoxon signed rank test was used to compare the mean reader scores between clinical standard and accelerated rest perfusion scans. Assuming normal distribution, a paired t-test was used to compare the mean AIF and MBF values between with and without KWIC filtering and the mean overall image score indices between the accelerated and standard sequences. Also, assuming normal distribution, we computed the intraclass correlation coefficient (ICC) to infer inter-reader variability. A p < 0.05 was considered statistically significant for each statistical test.
Results
Numerical Phantom Experiments
Figure 3 shows plots of normalized signal and measured [GBCA] as a function of ground truth [GBCA] for three different TS values (20, 59, and 80 ms) for the following four conditions: (a) an ideal T1 relaxation without noise or T2 decay, (b) T2 decay added, (c) noise added, and (d) T2 decay and noise added. Errors induced by noise and T2 decay in normalized signal and measured [GBCA] are smaller for higher TS values (59 and 80 ms) than TS = 20 ms at low concentration values between 0.5 to 1 mM representing peak enhancement in the myocardium, whereas errors in normalized signal and measured [GBCA] are smaller for TS = 20 ms than higher TS values (59 and 80 ms) at high concentration values between 5 to 10 mM representing peak enhancement in the left ventricular cavity (i.e. AIF).
Patient Study
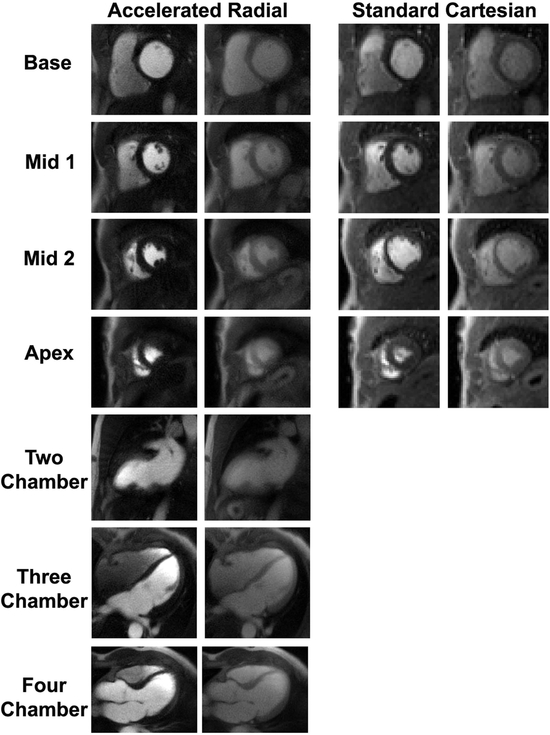
The order of the rest perfusion scans was almost equally split between the standard and accelerated pulse sequences, where accelerated went first, seven out of thirteen cases. Representative rest perfusion images obtained using the standard and accelerated pulse sequences at peak LV blood pool and myocardial enhancement are shown in Figure 4 (see corresponding Supporting Information Video S2 and Supporting Information Video S3 for dynamic display). The proposed pulse sequence enabled extensive myocardial coverage (6–8 slices) with higher spatial resolution (1.6 mm × 1.6 mm × 8 mm ) and shorter readout duration (78 ms) than the clinical standard sequence (3–4 slices, 3 mm × 3 mm × 8 mm, 128 ms, respectively).
Figure 4:
Representative cardiac perfusion images obtained using the accelerated radial sequence (columns 1–2) and the standard perfusion sequence (columns 3–4) at peak enhancement in the blood pool (columns 1 and 3) and myocardial wall (columns 2 and 4). Using the proposed sequence, there was time left in the cardiac cycle to acquire three additional long-axis images. For dynamic display, see Supporting Information Video S2 for standard images and Supporting Information Video S3 for accelerated images.
The visual scores are summarized in Supporting Information Table S2. The overall image score index was not significantly different (p = 0.64) between accelerated (11.1 ± 1.3) and clinical standard (11.2 ± 1.3). The ICC between the three raters was 0.76 for the standard sequence and 0.61 for the accelerated sequence. The median conspicuity (3.7; range: 2.0–4.3 for accelerated vs. 3.7; range: 2.7–4.3 for clinical standard, p = 0.43), artifact (4.0; range: 3.3–4.3 for accelerated vs. 4.0; range: 3.0–4.3 for clinical standard, p = 0.74), and noise (3.3; range: 2.5–4.3 for accelerated vs. 3.7; range: 2.7–4.3 for clinical standard, p = 0.63) scores were not significantly different between the clinical standard and proposed sequences. It should be noted that the median visual scores for the proposed scan were deemed clinically acceptable for all three categories (≥ 3.0).
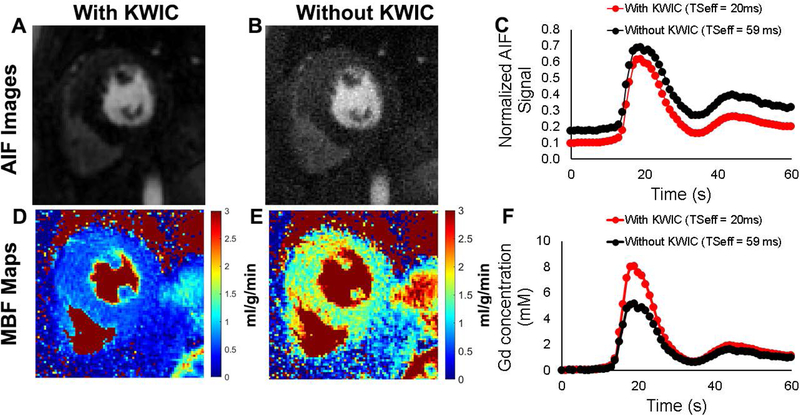
Figure 5 shows example AIF images and the corresponding resting MBF maps obtained with (TS = 20 ms) and without (TS = 59 ms) KWIC filtering, as well as their corresponding normalized signal-time and AIF curves. Averaging the results over 13 patients (see Supporting Information Table S3), the mean peak AIF concentration with KWIC filtering (9.2 ± 1.6 mM) was significantly higher (p< 0.0001) than without KWIC filtering (3.1 ± 1.2 mM), and the mean resting MBF values for all patients with KWIC filtering (ranging from 0.9 to 1.2 mL/g/min across different slice locations) were significantly lower (p < 0.001) than those values without KWIC filtering (ranging from 3.1 to 4.3 mL/g/min across different slice locations).
Figure 5:
Representative arterial input function (AIF) images reconstructed with (A) and without (B) KWIC filtering. (C) The corresponding normalized signal-time curves with (red) and without the KWIC filtering (black). The corresponding MBF maps with (D) and without KWIC filtering (E). (F) The corresponding AIF curves with (red) and without the KWIC filtering (black).
Discussion
In this study, we developed a novel cardiac perfusion pulse sequence using a combination of radial sampling, CS and KWIC filtering reconstruction and subsequently tested it to determine whether it is capable of increasing myocardial coverage, generating high-quality images for visual analysis, and permitting quantification of MBF. Compared with a clinical standard pulse sequence, the proposed pulse sequence produced overall visual score index that was not significantly different, while essentially doubling the myocardial coverage and increasing the spatial resolution. Compared with resting MBF values reported in literature (example 1: 0.61–1.1 mL/g/min averaging 363 subjects overall from 23 cardiac PET studies (37); example 2: 0.95–1.03 mL/g/min based on a state-of-the-art dual-imaging and reconstruction method (20)), the resting MBF values with KWIC filtering (TS = 20 ms for AIF, TS = 80 ms for wall enhancement) agreed better than without KWIC filtering (TS = 59 ms for AIF and wall enhancement). Our mean peak AIF of 9.2 mM following 0.10 mmol/kg administration of gadobutrol is comparable with peak AIF of 4.4 mM following 0.05 mmol/kg administration of gadobutrol reported by Kellman et al. (20).
This study addresses the challenges associated with achieving a good balance between competing factors: a) increasing the myocardial coverage with high spatial resolution, b) high image quality, and c) accurate MBF quantification. Achieving a good balance is technically challenging. For example, acceleration techniques are needed to increase the myocardial coverage, at the expense of low SNR due to undersampling and use of a shorter TS. Tailoring the image acquisition to enable MBF quantification requires a dedicated AIF acquisition (e.g. dual-imaging) per cardiac cycle, which translates to less time available for sampling the myocardial enhancement images per cardiac cycle. Increasing the spatial resolution requires increasing the readout duration per image, which translates to less myocardial coverage per cardiac cycle. It is fundamentally challenging to achieve high accuracy defined as less than 10% error over a wide range of GBCA concentration (0 – 10 mM) expected in first-pass cardiac perfusion MRI. We systematically engineered the pulse sequence and image reconstruction pipeline to achieve a good balance between these competing factors. While a dual imaging pulse sequence reduces error for peak AIF values, it should be noted that accuracy depends on a variety of factors, including noise and T2 decay (see Fig.3). One approach to minimizing the impact of noise and T2 decay is to perform first-pass cardiac perfusion MRI at 3T and use a short TE, respectively.
The proposed pulse sequence features several advantages that have clinical implications. First, more complete myocardial coverage is important for improving the diagnostic confidence, because it will lead to a more complete assessment of the ischemic burden and provide a greater margin of error to read through image artifacts (e.g. DRA). Second, generating high-quality images is important since visual diagnosis is the clinical norm at most clinical practices. Third, enabling quantification of MBF is important for diagnosing multi-vessel and microvascular diseases. In addition, MBF could serve as a useful metric for monitoring patients following revascularization or medical therapy, as well as comparing across patients. Fourth, the proposed sequence is less sensitive to cardiac and respiratory motion because of the short readout duration (78 ms).
This study includes several limitations that warrant discussion. First, we did not compare our accelerated perfusion sequence to other investigational accelerated perfusion sequences (5,11–14). It is difficult to directly compare first-pass perfusion MRI scans due to a number of considerations, including multiple GBCA injections and unavailability of investigational pulse sequences. We elected to compare our sequence to a standard pulse sequence since it is commercially available. Second, for each patient, we performed two resting perfusion MRI scans with a 5 min gap between them. This decision was made to allow partial clearance of GBCA after the first scan without compromising the timing of clinically ordered LGE scan. Regrettably, despite best efforts, the second rest perfusion scan was impacted by the residual GBCA from the first rest perfusion acquisition. This translated to higher tissue enhancement in the second rest perfusion scan in all patients and partial LGE effects for the second rest perfusion scans in one patient with myocardial infarction. This problem was partially mitigated by randomizing the pulse sequence order such that this effect on the reader scores washes out. Third, this study did not evaluate the accuracy of MBF values obtained from the proposed accelerated sequence with either the previously described dual-bolus or dual-imaging method. It is impractical to add a dual-bolus method to a clinical cardiac MRI without significantly altering the clinical workflow. It requires significant pulse sequence development work to implement a dual-imaging sequence with exact imaging parameters (20), which is beyond the scope of this study. The resting MBF values with KWIC filtering agree with resting MBF values reported in literature (20,37). Nonetheless, future studies are warranted to validate the accuracy of MBF derived from our pulse sequence. Fourth, we did not evaluate the performance of our sequence during vasodilator stress. Future stress perfusion studies are warranted to fully evaluate the performance of our pulse sequence in patients with suspected CAD. Fifth, the clinical standard and proposed pulse sequences had different imaging parameters. As such, these findings need to be interpreted after accounting for the differences in imaging parameters (spatial resolution, flip angle, and TS values). While our proposed method produced higher spatial resolution, the standard method produced higher contrast-to-noise ratio (CNR) due to longer TS and higher flip angle. Our visual scores suggest that those differences in spatial resolution (higher for ours) and CNR (higher for standard) may have canceled each other during visual evaluation. In practice it is difficult to match both the spatial resolution and CNR between standard and accelerated perfusion MRI scans without introducing a bias.
Conclusion
This study describes a novel approach that combines radial k-space sampling, CS, and KWIC filtering for improving the myocardial coverage, generating high-quality images, and enabling quantification of MBF. Further studies are warranted to evaluate the diagnostic performance in a diverse set of patients suspected with CAD at peak vasodilation and rest.
Supplementary Material
Supporting Information Table S1: Clinical characteristics of 13 patients enrolled for this study. Only one patient presented with LGE in the septal wall due to myocardial infarction. The remaining twelve patients did not show LGE during cardiac MRI. A detailed list of clinical profiles is not relevant for this study and is thus omitted due to space constraint.
Supporting Information Table S2: Summary of the visual assessment scores. P < 0.05 corresponds to significant difference. The overall image score index is reported as mean ± standard deviation. The wall enhancement, artifact, and noise scores are presented as median (range).
Supporting Information Table S3: Mean resting MBF measurements in the basal, mid-ventricular and apical slice locations with and without KWIC filtering. Values represent mean ± standard deviation. P < 0.05 corresponds to significant difference.
Supplementary Video S1. Movie display of accelerated radial perfusion images at three different stages following KWIC filtering (temporal TV, temporal TV + low-rank block wise, temporal TV + low rank block wise + motion correction) of CS reconstruction pipleline shown in Figure 2.
Supplementary Video S2. Movie display of clinical standard perfusion images shown in Figure 4 (columns 3–4).
Supplementary Video S3. Movie display of accelerated radial perfusion images shown in Figure 4 (columns 1–2).
Acknowledgments
The authors thank Ms. Louise M. Collins for assistance with IRB. The authors also thank funding support from the National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954).
Grant Support: This work was supported in part by funding from the National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954)
Appendix
Registration
Motion-registration was performed using the Advanced Normalization ToolS (ANTS) package (36). To increase the computational speed, registration was restricted to a fixed mask around the heart. Preprocessing was performed semi-automatically to select a mask around the heart. Using ANTS, the images were registered to a reference fixed frame, which was automatically chosen to be a frame with maximum blood pool contrast. The registration was performed using the antsRegistration command. Preprocessing steps before the registration included histogram-matching and intensity-truncation. Histogram-matching was performed for each fixed and moving image pair to prevent alteration of the signal intensity due to application of the registration transforms. The image intensities were truncated to restrict extreme values due to bad coils. The registration parameters were chosen to minimize motion due to respiration while preventing over-smoothing of the images. A multivariate image registration approach was used (38). From each image, two images were created: 1) a SUSAN image, derived using a noise reduction filtering procedure while preserving structure (39) and 2) a Laplacian-based edge-detection image derived from SUSAN image. A deformable B-spline Syn pairwise registration was performed using the SUSAN and Laplacian images and three similarity metrics. A cross correlation (CC) metric (window radius = 6 voxels) was used with the original imaging data and a Demons metric was used with the SUSAN and Laplacian filtered images (38). A multi-resolution approach consisting of three levels was used and the resolution was doubled for each successive level. The specific parameters used for the registration are shown below:
antsRegistration --dimensionality 2
-z 0
--output [outputN4,%s]
-x[mask,mask]
-winsorize-image-intensities [0,20000]
--use-histogram-matching 1
-t BSplineSyN[0.1,2×2,0×0] -m CC[ %s,%s , 1, 6]
-m Demons[%s,%s,1,1] -m Demons[%s,%s,1,1]
-s 2×1×0 -f 4×2×1 -c [100×50×25,1e-8,10]
Footnotes
Conference Meeting:
This work was presented in part at the 2018 ISMRM
References
- 1.CDC NUCoD-oCWOD, released 2015. Data are from the Multiple Cause of Death Files, 1999–2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed Feb. 3, 2015.
- 2.Atkinson DJ, Burstein D, Edelman RR. First-pass cardiac perfusion: evaluation with ultrafast MR imaging. Radiology 1990;174(3 Pt 1):757–762. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood JP, Maredia N, Radjenovic A, Brown JM, Nixon J, Farrin AJ, Dickinson C, Younger JF, Ridgway JP, Sculpher M, Ball SG, Plein S. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison A, Adluru G, Damal K, Shaaban AM, Wilson B, Kim D, McGann C, Marrouche NF, DiBella EV. Rapid ungated myocardial perfusion cardiovascular magnetic resonance: preliminary diagnostic accuracy. J Cardiovasc Magn Reson 2013;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010;64(3):767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plein S, Ryf S, Schwitter J, Radjenovic A, Boesiger P, Kozerke S. Dynamic contrast-enhanced myocardial perfusion MRI accelerated with k-t sense. Magn Reson Med 2007;58(4):777–785. [DOI] [PubMed] [Google Scholar]
- 7.Sharif B, Dharmakumar R, LaBounty T, Arsanjani R, Shufelt C, Thomson L, Merz CN, Berman DS, Li D. Towards elimination of the dark-rim artifact in first-pass myocardial perfusion MRI: removing Gibbs ringing effects using optimized radial imaging. Magn Reson Med 2014;72(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motwani M, Jogiya R, Kozerke S, Greenwood JP, Plein S. Advanced cardiovascular magnetic resonance myocardial perfusion imaging: high-spatial resolution versus 3-dimensional whole-heart coverage. Circ Cardiovasc Imaging 2013;6(2):339–348. [DOI] [PubMed] [Google Scholar]
- 9.Mordini FE, Haddad T, Hsu LY, Kellman P, Lowrey TB, Aletras AH, Bandettini WP, Arai AE. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc Imaging 2014;7(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AR, Antkowiak PF, Nandalur KR, West AM, Salerno M, Arora V, Christopher J, Epstein FH, Kramer CM. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol 2010;56(7):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitanis V, Manka R, Boesiger P, Kozerke S. Accelerated cardiac perfusion imaging using k-t SENSE with SENSE training. Magn Reson Med 2009;62(4):955–965. [DOI] [PubMed] [Google Scholar]
- 12.Vitanis V, Manka R, Giese D, Pedersen H, Plein S, Boesiger P, Kozerke S. High resolution three-dimensional cardiac perfusion imaging using compartment-based k-t principal component analysis. Magn Reson Med 2011;65(2):575–587. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Salerno M, Yang Y, Epstein FH. Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: block low-rank sparsity with motion-guidance (BLOSM). Magn Reson Med 2014;72(4):1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182–1195. [DOI] [PubMed] [Google Scholar]
- 15.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124(20):2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian TF, Rettmann DW, Aletras AH, Liao SL, Taylor JL, Balaban RS, Arai AE. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology 2004;232(3):677–684. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Axel L. Multislice, dual-imaging sequence for increasing the dynamic range of the contrast-enhanced blood signal and CNR of myocardial enhancement at 3T. J Magn Reson Imaging 2006;23(1):81–86. [DOI] [PubMed] [Google Scholar]
- 18.Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging 2004;20(1):39–45. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee N, Benefield BC, Harris KR, Fluckiger JU, Carroll T, Lee DC. An empirical method for reducing variability and complexity of myocardial perfusion quantification by dual bolus cardiac MRI. Magn Reson Med 2017;77(6):2347–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, Ugander M, Xue H. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 2017;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif B, Dharmakumar R, LaBounty T, Shufelt C, Thomson LE, Merz NB, Berman DS, Li D. Eliminating dark-rim artifacts in first-pass myocardial perfusion imaging. Journal of Cardiovascular Magnetic Resonance 2013;15(S1):O3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison A, Adluru G, Damal K, Shaaban AM, Wilson B, Kim D, McGann C, Marrouche NF, DiBella EV. Rapid ungated myocardial perfusion cardiovascular magnetic resonance: preliminary diagnostic accuracy. J Cardiovasc Magn Reson 2013;15(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song HK, Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 2000;44(6):825–832. [DOI] [PubMed] [Google Scholar]
- 24.Kholmovski EG, DiBella EV. Perfusion MRI with radial acquisition for arterial input function assessment. Magn Reson Med 2007;57(5):821–827. [DOI] [PubMed] [Google Scholar]
- 25.Kim TH, Pack NA, Chen L, DiBella EV. Quantification of myocardial perfusion using CMR with a radial data acquisition: comparison with a dual-bolus method. J Cardiovasc Magn Reson 2010;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran-Gia J, Lohr D, Weng AM, Ritter CO, Stab D, Bley TA, Kostler H. A model-based reconstruction technique for quantitative myocardial perfusion imaging. Magn Reson Med 2016;76(3):880–887. [DOI] [PubMed] [Google Scholar]
- 27.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005;40(11):715–724. [DOI] [PubMed] [Google Scholar]
- 28.Wundrak S, Paul J, Ulrici J, Hell E, Geibel MA, Bernhardt P, Rottbauer W, Rasche V. Golden ratio sparse MRI using tiny golden angles. Magn Reson Med 2016;75(6):2372–2378. [DOI] [PubMed] [Google Scholar]
- 29.Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA). Magn Reson Med 2005;53(4):981–985. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Oesingmann N, McGorty K. Hybrid adiabatic-rectangular pulse train for effective saturation of magnetization within the whole heart at 3 T. Magn Reson Med 2009;62(6):1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cernicanu A, Axel L. Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Acad Radiol 2006;13(6):686–693. [DOI] [PubMed] [Google Scholar]
- 32.Block K, Uecker M. Simple method for adaptive gradient-delay compensation in radial MRI. In: Proceedings of the 19rd Annual Meeting of ISMRM, Montreal, Quebec, Canada 2011 Abstract No. 2816. [Google Scholar]
- 33.Ilicak E, Cetin S, Bulut E, Oguz KK, Saritas EU, Unal G, Cukur T. Targeted vessel reconstruction in non-contrast-enhanced steady-state free precession angiography. NMR Biomed 2016;29(5):532–544. [DOI] [PubMed] [Google Scholar]
- 34.Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella EV, Sodickson DK, Otazo R, Kim D. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn Reson Med 2013;70(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. J Magn Reson 2007;188(2):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroeder W, Ibanez L, Ng L, Cates J. The ITK Software Guide. The Insight Consortium 2003. [Google Scholar]
- 37.Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, Cerqueira MD, deKemp RA, DePuey EG, Dilsizian V, Dorbala S, Ficaro EP, Garcia EV, Gewirtz H, Heller GV, Lewin HC, Malhotra S, Mann A, Ruddy TD, Schindler TH, Schwartz RG, Slomka PJ, Soman P, Di Carli MF, Directors SCCBo, Directors ABo. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 2018;59(2):273–293. [DOI] [PubMed] [Google Scholar]
- 38.Tustison NJ, Yang Y, Salerno M. Advanced Normalization Tools for Cardiac Motion Correction Statistical Atlases and Computational Models of the Heart - Imaging and Modelling Challenges; 2015; Cham: Springer International Publishing; p 3–12. (Statistical Atlases and Computational Models of the Heart - Imaging and Modelling Challenges). [Google Scholar]
- 39.Smith SM, Brady JM. SUSAN—A New Approach to Low Level Image Processing. International Journal of Computer Vision 1997;23(1):45–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1: Clinical characteristics of 13 patients enrolled for this study. Only one patient presented with LGE in the septal wall due to myocardial infarction. The remaining twelve patients did not show LGE during cardiac MRI. A detailed list of clinical profiles is not relevant for this study and is thus omitted due to space constraint.
Supporting Information Table S2: Summary of the visual assessment scores. P < 0.05 corresponds to significant difference. The overall image score index is reported as mean ± standard deviation. The wall enhancement, artifact, and noise scores are presented as median (range).
Supporting Information Table S3: Mean resting MBF measurements in the basal, mid-ventricular and apical slice locations with and without KWIC filtering. Values represent mean ± standard deviation. P < 0.05 corresponds to significant difference.
Supplementary Video S1. Movie display of accelerated radial perfusion images at three different stages following KWIC filtering (temporal TV, temporal TV + low-rank block wise, temporal TV + low rank block wise + motion correction) of CS reconstruction pipleline shown in Figure 2.
Supplementary Video S2. Movie display of clinical standard perfusion images shown in Figure 4 (columns 3–4).
Supplementary Video S3. Movie display of accelerated radial perfusion images shown in Figure 4 (columns 1–2).