Abstract
Background:
Severe cutaneous adverse reactions (SCARs) are frequent in inpatient oncology. Early intervention may reduce morbidity, mortality, and hospitalization costs, however current clinical and histologic features are unreliable SCAR predictors. There is a need to identify rational markers of SCARs that could lead to effective therapeutic interventions.
Objective:
To characterize the clinical and serologic features of hospitalized patients with cancer who developed SCARs.
Methods:
Retrospective review of 49 hospitalized cancer patients with a morbilliform rash and recorded testing for serum cytokines (IL-6, IL-10, TNF-α) or elafin, and prior dermatology consultation. Patients were categorized as having a ‘simple’ morbilliform rash without systemic involvement or ‘complex’ morbilliform rash with systemic involvement.
Results:
Fifteen out of 49 patients (30.6%) were deceased at 6 months from time of dermatologic consultation. Elafin, IL-6, and TNF-α were significantly higher in patients who died compared to patients who were still alive at 6 months. IL-6 and IL-10 were significantly higher in patients with a drug-related ‘complex’ rash.
Limitations:
Retrospective design, limited sample size, high-risk patient population.
Conclusion:
In cancer patients with SCARs, elafin, IL-6, and TNF- α may predict a poor outcome. Agents directed towards these targets may represent rational treatments for the prevention of fatal SCARs.
Keywords: Severe cutaneous adverse reaction, cytokine, drug reaction, drug reaction with eosinophilia and systemic symptoms, drug induced hypersensitivity syndrome, graft versus host disease, drug rash
Capsule Summary
• Cancer patients have increased risk of severe cutaneous adverse reactions, without reliable biomarkers to identify predisposition for associated morbidity and mortality.
• In hospitalized cancer patients with morbilliform rash, elafin, IL-6, TNF-α were associated with mortality. IL-6, IL-10 were associated with drug-related systemic involvement. These biomarkers may guide future therapeutic research.
Introduction
Severe cutaneous adverse reactions (SCARs) to drugs, which encompass a spectrum of entities including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug-induced hypersensitivity syndrome (DIHS)/ drug reaction with eosinophilia and systemic syndrome (DRESS) are associated with significant morbidity, mortality, and hospitalization costs.1,2 Incidence ranges from 2 to 7 per million cases per year for SJS and TEN to 1 case per 1,000 to 10,000 drug exposures for DRESS.3–5 Prompt recognition and treatment of SCARs is critical, as these patients can rapidly develop multiorgan dysfunction or failure without treatment.6
Several studies have demonstrated an increased risk of SJS/TEN in active cancer patients, which may be attributed to the role of the immune system in the development of SCARs as well as exposure to multiple medications.7–9 Furthermore, cancer patients have a significantly higher risk of mortality with SJS/TEN compared to non-cancer patients.10 Several factors have been proposed to explain this elevated risk, including an immunocompromised status, malnutrition, toxicity from chemotherapeutic or immunotherapy agents, and organ dysfunction from malignancy, although the exact mechanisms remain to be elucidated. In addition to having an elevated risk of SCARs, patients with hematologic malignancy and history of hematopoietic stem cell transplant (HSCT) are also at risk for graft versus host disease (GVHD). GVHD and SCARs can be difficult to distinguish given their similar clinical presentations.
Diagnosis of SCARs largely relies on clinical assessment. Furthermore, prediction of progression of a simple drug rash into a systemic reaction can be difficult, as clinical morphology of the rash, histopathology, and standard laboratory values are often insufficient to predict outcome.11–13 There is a need to identify reliable markers that can help anticipate those patients with SCARs who are at increased risk of progression and possible death. In cancer patients, identification of high-risk patients has important implications, including earlier treatment and ability to resume cancer treatment. The objective of this study was to identify clinical and serologic features of hospitalized patients with cancer who developed SCARs.
Methods
This was a retrospective cohort study approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC). A database query of adult patients with cancer who were hospitalized between August 1, 2016 and July 31, 2017 and had ICD 9 or 10 codes for rash (R23, R21, 693, 692, 695, 690–698, L20-L30, L51, L43.2, T88.7, L55–59), recorded testing for serum cytokines (interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α)) or serum elafin, and prior dermatology consultation revealed 191 eligible patients (Figure 1). Given the limited impact of skin biopsy and serum studies on diagnosis and management of morbilliform rash14,15, and recent FDA approval of anti-IL-6 receptor antibody tocilizumab for cytokine release syndrome, with utility in pro-inflammatory disorders16–20, select biomarker levels are obtained at our institution as standard of care for patients presenting with possible drug eruption in order to better understand the disease course and as a potential therapeutic target for intervention. All data was retrospectively collected.
Figure 1.
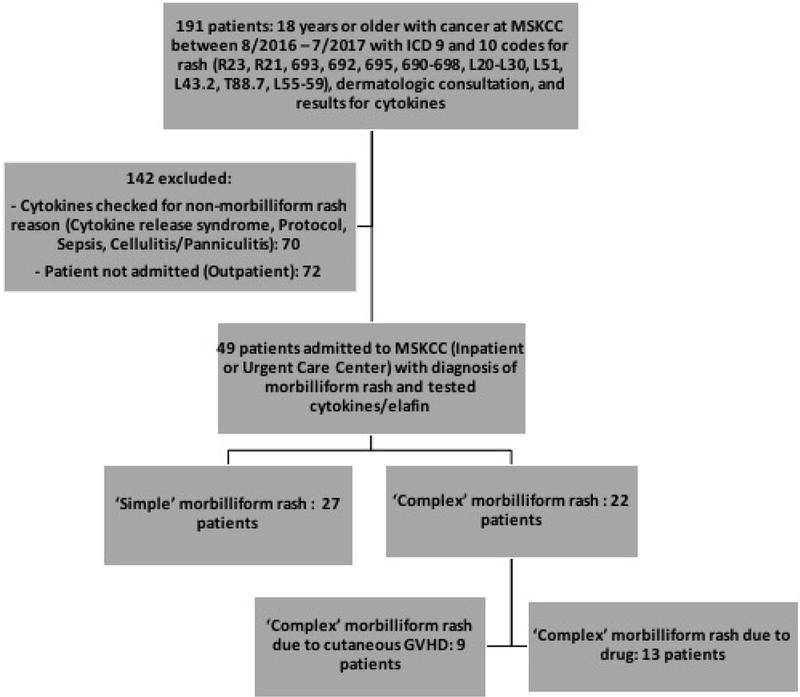
Flowchart of patient selection.
One hundred forty-two patients were excluded: 70 patients were excluded because cytokines were checked for a reason other than a morbilliform rash (i.e., cytokine release syndrome, study protocol, sepsis, or cellulitis/panniculitis), and 72 patients were excluded because they were not admitted to the hospital (i.e., the patient was seen as an outpatient).
Forty-nine patients were admitted as an inpatient or seen at the urgent care center at MSKCC with a diagnosis of morbilliform rash and tested for cytokines or elafin. Chart review was performed for all patients to assign to ‘simple’ and ‘complex’ morbilliform rash groups. ‘Simple’ morbilliform rash was defined as a rash with no systemic involvement, or spontaneous resolution of rash with remote systemic involvement (i.e., transient elevation in liver transaminases or bilirubin that returned to baseline), or limited course of rash that did not require systemic therapy. ‘Complex’ morbilliform rash was defined as a SCAR with systemic organ involvement requiring systemic therapy with a prolonged duration of the rash.
For each patient, a modified RegiSCAR score21 was calculated based on the following items: fever ≥ 38.5°C; peripheral eosinophilia ( ≥ 700/mm3 or ≥ 10%, or ≥ 1500/mm3 or ≥ 20%); atypical lymphocytes; rash ≥ 50% of body surface area with facial edema, purpura, infiltration, or desquamation; organ involvement; disease duration > 15 days; at least 3 biological investigations (e.g., blood cultures, viral serology, biopsy) performed and negative to rule out an alternative diagnosis. Comprehensive metabolic panel, including glomerular filtration rate (GFR), blood urea nitrogen (BUN), creatinine (Cr), transaminases, and total bilirubin and urine eosinophils, were also reviewed. For all laboratory values, only results within 7 days of cytokine testing were used in the analysis for consistency and to minimize the impact of events unrelated to the rash. Reference values for cytokines are determined by our institution’s laboratory and are as follows: IL-10 ≤ 18 pg/mL, IL-6 ≤ 5 pg/mL, TNF-α ≤ 22 pg/mL. Elafin is an elastase inhibitor overexpressed in epithelial tissues upon inflammation or injury22, and has been found to be a diagnostic and prognostic plasma biomarker in cutaneous GVHD.23 Elafin has not been formally validated in this patient population; therefore, there is no diagnostic threshold.
Descriptive statistics and graphical methods were used to assess distributions of patient and medical test characteristics. Chi-square tests and Fisher’s Exact test were used to assess the association between rash type (‘simple’ versus ‘complex’) and nominally scaled patient and medical test characteristics. Wilcoxon rank-sum tests were used to assess differences in continuously scaled variables by rash type. All analyses were performed with STATA 12 software (StataCorp LP, College Station, TX).
Results
Patient Characteristics and Laboratory Values
Of the 49 patients with cancer and morbilliform rash who were admitted to the inpatient or urgent care center units and received dermatology consultation, 27 patients had a ‘simple’ morbilliform rash without systemic involvement, and 22 had a ‘complex’ morbilliform rash with systemic involvement (Figure 1). Of the 22 ‘complex’ morbilliform rash patients, 9 were cutaneous manifestations of GVHD (of which 7 were acute GVHD, 1 was late onset acute GVHD, and 1 was on the clinical spectrum of GVHD with engraftment syndrome). The remaining 13 ‘complex’ rashes were secondary to drug exposure. Demographic and other characteristics of ‘simple’ and ‘complex’ morbilliform rash patients are shown in Table 1. Most patients were admitted as an inpatient to the hospital (N=41) vs. Urgent Care Center (N=8). For both ‘simple’ and ‘complex’ rash patients, there were more patients with hematologic malignancy (N=18, 16, respectively) compared to solid organ malignancy (N=9, 6, respectively). Fifteen of the 49 patients (30.6%) were deceased at 6 months from the time of dermatologic consultation. Causes of death included organ failure, sepsis, and other multifactorial cancer-related causes.
Table 1.
Characteristics of hospitalized cancer patients and ‘simple’ morbilliform rash vs. ‘complex’ systemic morbilliform rash.
| Simple morbilliform rash (n=27) |
Complex morbilliform rash (n=22) |
p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Patients (N), gender | |||
| Female | 15 (55.6) | 12 (54.6) | 0.94 |
| Male | 12 (44.4) | 10 (45.4) | |
| Location | |||
| Inpatient | 22 (81.5) | 19 (86.4) | 0.65 |
| Urgent Care Center (ED) | 5 (18.5) | 3 (13.6) | |
| Cancer Diagnosis | |||
| Hematologic malignancy | 18 (66.7) | 15 (68.2) | 0.54 |
| Solid organ malignancy | 9 (33.3) | 6 (27.3) | |
| Both Solid and Hematologic | 0 (0) | 1 (4.6) | |
| Hematologic malignancy | 18 | 16 | |
| AML | 8 (29.6) | 6 (27.3) | -- |
| ALL | 2 (7.4) | 0 | -- |
| Multiple myeloma | 3 (11.0) | 2 (9.1) | -- |
| CML | 0 | 2 (9.1) | -- |
| CMML | 1 (3.7) | 0 | -- |
| Myelofibrosis | 1 (3.7) | 0 | -- |
| Myelodysplastic syndrome | 0 | 2 (9.1) | -- |
| DLBCL | 1 (3.7) | 2 (9.1) | -- |
| Mantle cell lymphoma | 1 (3.7) | 0 | -- |
| Gray zone Lymphoma | 1 (3.7) | 0 | -- |
| Indolent B-cell lymphoma | 0 | 1 (4.5) | -- |
| ATLL | 0 | 1 (4.5) | -- |
| Solid organ malignancy | 9 | 6 | |
| Melanoma | 2 (7.4) | 1 (4.5) | -- |
| Colon | 2 (7.4) | 1 (4.5) | -- |
| Ovarian | 2 (7.4) | 1 (4.5) | -- |
| Breast | 1 (3.7) | 0 | -- |
| Urothelial | 1 (3.7) | 0 | -- |
| Prostate | 1 (3.7) | 0 | -- |
| Renal | 0 | 1 (4.5) | -- |
| CTCL | 0 | 1 (4.5) | -- |
| Sarcoma | 0 | 1 (4.5) | -- |
| Status | |||
| Alive | 19 | 10 | |
| Deceased | 8 | 12 | |
| Modified RegiSCAR (median) | 1.5 | 3.0 | <0.001* |
| Atypical lymphocytes (N) | 5 (18.5) | 4 (18.2) | 0.98 |
| T>38C (N) | 7 (25.9) | 4 (18.2) | 0.52 |
|
Rash (>50%, +purpura/edema/scale), median |
1 | 2 | 0.006* |
| Eos score (0–2), median | 0 | 1 | 0.001* |
| Internal organs involved | |||
| 0 | 16 (59.3) | 2 (9.1) | 0.001 |
| 1 | 10 (37.0) | 16 (72.7) | |
| 2 | 1 (3.7) | 4 (18.2) | |
| Decreased GFR relative to baseline (N) | 4 (14.8) | 4 (18.2) | 0.75 |
| Presence of urine eosinophils (N) | 0 | 3 (13.6) | 0.05 |
|
Elevated transaminases relative to baseline (N) |
3 (11.1) | 16 (72.7) | <0.001 |
| Elevated total bilirubin relative to baseline (N) | 6 (22.2) | 8 (36.4) | 0.28 |
|
Skin biopsy supportive of drug reaction (N) |
10 (37.0) | 13 (59.1) | 0.12 |
| Resolution > 15 days (N) | 10 (37.0) | 19 (86.4) | <0.001 |
|
At least 3 negative biological investigations to exclude alternate dx (N) |
27(100) | 22 (100) | 1.0 |
| WBC/µL (median) | 3,420 | 8,550 | 0.05* |
| CTCAE v4.03 Grade (median) | 3 | 3 | 0.26* |
| Cytokines/Biomarkers | |||
| Elafin ng/mL median | 17.9 | 25.5 | 0.22 |
| IL-6 pg/mL median | 16 | 26 | 0.11 |
| IL-10 pg/mL median | 19.5 | 31 | 0.07 |
| TNF-α pg/mL median | 12 | 18 | 0.03 |
Based on the two-sample Wilcoxon rank-sum test
Median modified RegiSCAR score was 3 in ‘complex’ rash patients and 1.5 in ‘simple’ rash patients (p<0.001, range −1 to 5). ‘Complex’ rash patients were significantly more likely to have a rash covering >50% of body surface area with purpura, edema, or scale (p=0.006), peripheral eosinophilia (p=0.001), internal organ involvement (p=0.001), and resolution of rash longer than 15 days (p<0.001). Relative to baseline levels, ‘complex’ rash patients had significant elevations in transaminases (p<0.001). Median white blood cell (WBC) count per µL was 8,550 in ‘complex’ morbilliform rash patients compared to 3,420 in ‘simple’ rash patients (p=0.05). The median values for all cytokines (elafin, IL-6, IL-10, and TNF-α) were higher in the complex rash group compared to simple rash group, although only TNF-α reached statistical significance (p=0.03). Median neutrophil-to-lymphocyte ratio (NLR) was higher in ‘simple’ rash patients (8.5) compared to ‘complex’ rash patients (6.6).
Among the variables included in the modified RegiSCAR score in Table 1, only elevated bilirubin relative to baseline was significantly associated with death at 6 months from time of dermatologic consultation. Furthermore, this was an inverse association, with 55.3% of patients alive at 6 months having elevated bilirubin relative to baseline, compared to 17.7% in those who died (p=0.01).
Cytokines and Organ Involvement
Median IL-6 level was significantly higher in patients with elevated bilirubin compared to patients with bilirubin in normal limits (63.5 vs. 22, p< 0.05). Median IL-10 was higher in patients with elevated transaminases, although it did not reach statistical significance (31 vs. 19.5, p<0.10). IL-6, IL-10, TNF-α, and elafin were not associated with peripheral eosinophilia or renal dysfunction, as measured by decreased GFR relative to baseline.
Cytokines and All-Cause Mortality
Median values for elafin, IL-6, IL-10, and TNF-α for patients who were alive (N=34) versus deceased (N=15) at 6 months from time of dermatologic consultation are shown in Figure 2. Elafin, IL-6, and TNF-α were significantly higher in patients deceased at 6 months (p=0.029, p=0.002, p=0.04, respectively) compared to patients who were alive.
Figure 2.
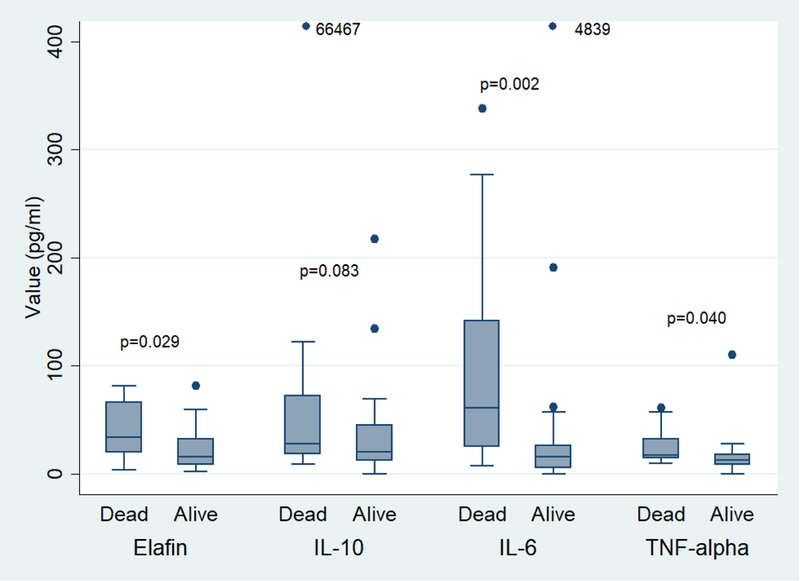
Cytokines and all-cause mortality. Mortality defined as status at 6 months from time of dermatologic consultation. 66467 and 4839 refer to cytokine values that were much higher than the y-axis of the graph.
Cytokines and Progression to Complex Morbilliform Rash
As shown in Figure 3, ‘complex’ rash patients (due to drug or GVHD) had a higher median IL-6 value compared to ‘simple’ rash patients, although it did not reach statistical significance (p=0.06). Patients with ‘complex’ morbilliform rash due to drug (Figure 4) had a significantly higher median IL-10 and IL-6 value compared to the group of patients with a ‘simple’ rash or with ‘complex’ rash due to GVHD (p=0.03 and p=0.05, respectively).
Figure 3.
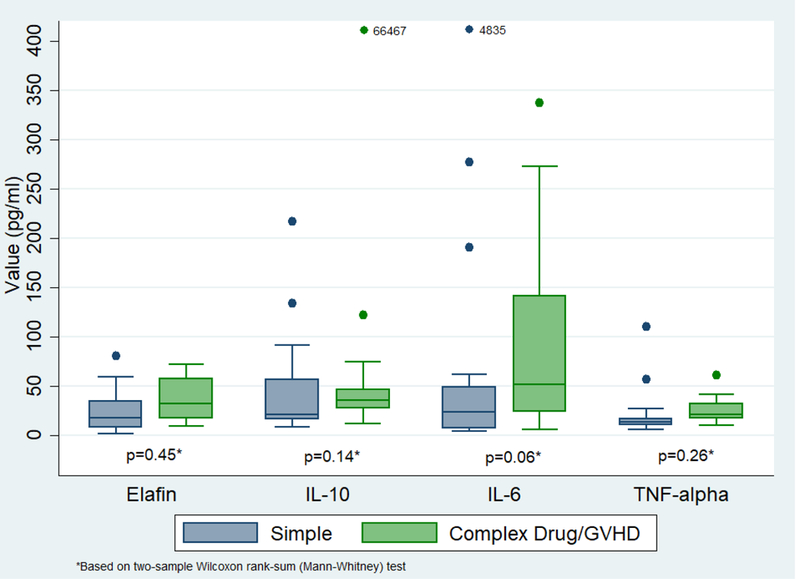
Cytokines in ‘simple’ rash vs. ‘complex’ morbilliform rash due to drug or GVHD. 66467 and 4839 refer to cytokine values that were much higher than the y-axis of the graph.
Figure 4.
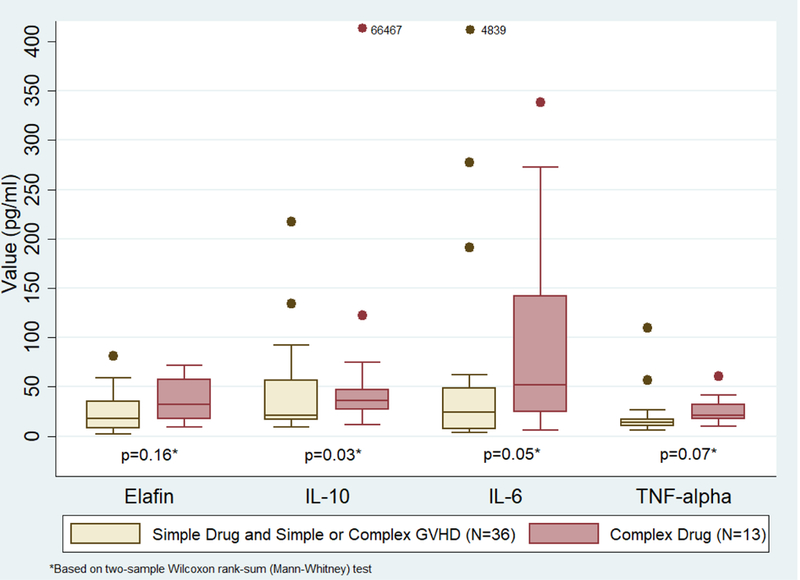
Cytokines in ‘simple’ rash and ‘complex’ morbilliform rash due to GVHD vs. ‘complex’ morbilliform rash due to drug only. 66467 and 4839 refer to cytokine values that were much higher than the y-axis of the graph.
Discussion
In this study, elafin, TNF-α, and IL-6 were significantly associated with all-cause mortality in hospitalized cancer patients who developed SCARs. This is the first study to report elafin levels in a cohort of patients with SCARs. Elafin is undetectable in normal skin, but overexpressed in wound healing, inflammatory disorders such as psoriasis, Sweet syndrome, Behcet syndrome, and neutrophil-mediated vasculitis, in skin with actinic damage, and in alveolar injury.24–29 It may be released in response to tissue degradation by neutrophil infiltration and in response to IL-1 and TNF-α.28,30 In patients with acute GVHD, high cutaneous elafin expression was associated with significantly decreased two-year overall survival compared to low elafin.31 A recent case report found elevated elafin expression in a post-HSCT patient initially thought to have bullous GVHD, but later favored to have TEN given the overall clinical picture.32 While GVHD and drug-related SCARs are difficult to distinguish clinically, our results suggest that elafin may be a useful biomarker to identify patients with a suspected diagnosis of SCAR or GVHD who are at increased risk of death within 6 months. Additionally, recombinant human elafin has shown efficacy in mitigating or preventing epithelial lung injury.33,34 Given its broad anti-inflammatory activity, elafin’s potential as a therapeutic agent for SCARs should be further explored.
TNF-α was also significantly associated with all-cause mortality. Elevated TNF-α has been found in SCARs such as AGEP, SJS, TEN as well as GVHD.35–37 Furthermore, the successful use of TNF-α inhibitors such as infliximab and etanercept has been reported for the treatment of AGEP, SJS, TEN and DRESS.37–41 Notably, infliximab is already used to treat ipilimumab-induced severe colitis in cancer patients42; TNF-α may serve as a similar potential therapeutic target in SCARs in the cancer population.
We found IL-6 to be statistically associated with higher all-cause mortality, and significantly elevated in ‘complex’ drug-related rash patients compared to ‘simple’ drug or GVHD rash and ‘complex’ GVHD rash patients. IL-6 promotes an inflammatory state by stimulating the acute phase responses and inhibiting the production of regulatory T-cells that are induced by TGF-β.43,44 In a study of patients who presented with clinical symptoms suggestive of an adverse drug reaction or viral infection, IL-6 levels were found to be significantly elevated in SJS, TEN, and DRESS patients compared to healthy controls.45 Elevated IL-6 production is also associated with increased incidence and severity of GVHD.16 Blockade of the IL-6 receptor with tocilizumab or siltuximab has been shown to attenuate the pathologic damage caused by IL-6 mediated processes such as GVHD, cytokine release syndrome, and psoriasis.16–18 Targeted therapy with tocilizumab has shown efficacy and is FDA-approved for the treatment of cytokine release syndrome following chimeric antigen receptor (CAR) T-cell therapy.17,19 Tocilizumab has also been successfully used for anti-PD-1 inhibitor-associated cytokine release syndrome and for skin GVHD with a cytokine pattern resembling cytokine release syndrome.20,46 Furthermore, anti-IL-6 receptor antibodies suppress T-cell activation through inhibition of IL-2 production and induction of regulatory T cells and effectively treat other IL-6 mediated syndromes, suggesting a potentially novel therapeutic role in drug eruptions associated with IL-6 elevations.47
We also found significantly elevated IL-10 levels in patients who ultimately developed a ‘complex’ drug-related SCAR compared to patients with a ‘simple’ rash due to drug or GVHD and ‘complex’ GVHD rash patients. IL-10 is important in maintaining the integrity of tissue epithelia48, and has an anti-inflammatory role in the immune response: it is chemotactic for peripheral CD8+ T-cells, and inhibits the production of inflammatory cytokines, such as IL-6 and TNF-α.49 Elevated IL-10 has been found in patients with acute GVHD, SJS, and TEN.36,49 Thought to originate from activated keratinocytes in TEN, elevated IL-10 may reflect a defense mechanism against drug-specific cytotoxic T-cells that are activated during the disease process.50 In GVHD, whether IL-10 is protective or reflects a compensatory response is less clear. Further research is needed to explore the significance and utility of IL-10 as a therapeutic agent in these disease entities.
An additionally notable study finding is the higher median NLR in ‘simple’ rash patients compared to ‘complex’ rash patients. While NLR has garnered recent interest for its prognostic role, particularly in solid organ malignancies51, our findings show that NLR may have limited utility in a patient population with higher proportion of hematologic malignancies. Moreover, we did not find significant associations of established clinical markers, such as rash BSA or internal organ involvement, with all-cause mortality in this patient cohort. These findings support the need for alternative biomarkers such as the cytokines evaluated. A recent analysis of inpatient dermatologic consultations at a cancer hospital found that nearly half of consultations were for patients with underlying hematologic malignancies, with significantly longer hospital stays for these patients compared to patients not consulted by dermatology.52
Limitations of this study include its retrospective design, as well as a limited sample size. All cases were recruited from a tertiary referral cancer center. As mentioned previously, cancer patients have a higher risk of mortality with SJS/TEN compared to non-cancer patients. A larger, prospective study examining the association of cytokines with SCARs is needed, as well as longitudinal assessment of cytokine levels to assess their prognostic significance. This exploratory analysis presents potential therapeutic targets in a high-risk patient population, for whom a ‘complex’ rash can disrupt and delay treatment of underlying disease.
Conclusion
In hospitalized cancer patients presenting with morbilliform rash, elafin, IL-6, and TNF-α may have an important role in identifying patients at higher risk of mortality. IL-10 may be a useful diagnostic marker for drug-related morbilliform rash with systemic organ involvement. Further research is needed to elucidate the potential utility of these cytokines as therapeutic targets and of elafin as a therapeutic agent.
Acknowledgments
Funding sources: Dr. Markova is supported by a Dermatology Foundation Career Development Award. This study was also funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center.
Abbreviations
- SCAR
severe cutaneous adverse reaction
- SJS
Stevens-Johnson syndrome
- TEN
toxic epidermal necrolysis
- DIHS
drug-induced hypersensitivity syndrome
- DRESS
drug reaction with eosinophilia and systemic syndrome
- GVHD
graft versus host disease
- IL-6
interleukin-6
- IL-10
interleukin-10
- TNF-α
tumor necrosis factor alpha
- GFR
glomerular filtration rate
- BUN
blood urea nitrogen
- Cr
creatinine
- WBC
white blood cell
- NLR
neutrophil-to-lymphocyte ratio
Footnotes
Conflicts of interest disclosure: The authors have no relevant conflicts of interest to disclose.
IRB approval status: Approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. The New England journal of medicine. 1994;331(19):1272–1285. [DOI] [PubMed] [Google Scholar]
- 2.Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). The British journal of dermatology. 2007;157(5):989–996. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173.e171–113; quiz 185–176. [DOI] [PubMed] [Google Scholar]
- 4.Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. The British journal of dermatology. 2003;149(5):1018–1022. [DOI] [PubMed] [Google Scholar]
- 5.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. The British journal of dermatology. 2013;169(5):1071–1080. [DOI] [PubMed] [Google Scholar]
- 6.Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet (London, England). 2017;390(10106):1996–2011. [DOI] [PubMed] [Google Scholar]
- 7.Frey N, Jossi J, Bodmer M, et al. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in the UK. The Journal of investigative dermatology. 2017;137(6):1240–1247. [DOI] [PubMed] [Google Scholar]
- 8.Gillis NK, Hicks JK, Bell GC, Daly AJ, Kanetsky PA, McLeod HL. Incidence and Triggers of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a Large Cancer Patient Cohort. The Journal of investigative dermatology. 2017;137(9):2021–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. The Journal of investigative dermatology. 2008;128(1):35–44. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Lee YY, Su SC, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with malignancies. The British journal of dermatology. 2015;173(5):1224–1231. [DOI] [PubMed] [Google Scholar]
- 11.Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145(1):67–72. [DOI] [PubMed] [Google Scholar]
- 12.Yang MS, Kang MG, Jung JW, et al. Clinical features and prognostic factors in severe cutaneous drug reactions. International archives of allergy and immunology. 2013;162(4):346–354. [DOI] [PubMed] [Google Scholar]
- 13.Shiohara T, Kano Y, Hirahara K, Aoyama Y. Prediction and management of drug reaction with eosinophilia and systemic symptoms (DRESS). Expert opinion on drug metabolism & toxicology. 2017;13(7):701–704. [DOI] [PubMed] [Google Scholar]
- 14.Marra DE, McKee PH, Nghiem P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft-versus-host disease. J Am Acad Dermatol. 2004;51(4):543–546. [DOI] [PubMed] [Google Scholar]
- 15.Weaver J, Bergfeld WF. Quantitative analysis of eosinophils in acute graft-versus-host disease compared with drug hypersensitivity reactions. The American Journal of dermatopathology. 2010;32(1):31–34. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayasekera P, Parslew R, Al-Sharqi A. A case of tumour necrosis factor-alpha inhibitor-and rituximab-induced plantar pustular psoriasis that completely resolved with tocilizumab. Br J Dermatol. 2014;171(6):1546–1549. [DOI] [PubMed] [Google Scholar]
- 19.Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellwig Y, Yoo YE, Ress ML, et al. Fulminant skin GvHD with a cytokine pattern resemblant of cytokine release syndrome successfully treated with multimodal immunosuppression including tocilizumab. Pediatr Blood Cancer. 2015;62(11):2033–2035. [DOI] [PubMed] [Google Scholar]
- 21.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? The British journal of dermatology. 2007;156(3):609–611. [DOI] [PubMed] [Google Scholar]
- 22.Wiedow O, Schroder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. The Journal of biological chemistry. 1990;265(25):14791–14795. [PubMed] [Google Scholar]
- 23.Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Science translational medicine. 2010;2(13):13ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka N, Fujioka A, Tajima S, Ishibashi A, Hirose S. Elafin is induced in epidermis in skin disorders with dermal neutrophilic infiltration: interleukin-1 beta and tumour necrosis factor-alpha stimulate its secretion in vitro. The British journal of dermatology. 2000;143(4):728–732. [DOI] [PubMed] [Google Scholar]
- 25.Schalkwijk J, van Vlijmen IM, Alkemade JA, de Jongh GJ. Immunohistochemical localization of SKALP/elafin in psoriatic epidermis. The Journal of investigative dermatology. 1993;100(4):390–393. [DOI] [PubMed] [Google Scholar]
- 26.Muto J, Kuroda K, Wachi H, Hirose S, Tajima S. Accumulation of elafin in actinic elastosis of sun-damaged skin: elafin binds to elastin and prevents elastolytic degradation. The Journal of investigative dermatology. 2007;127(6):1358–1366. [DOI] [PubMed] [Google Scholar]
- 27.Muto J, Fujimoto N, Ono K, et al. Deposition of elafin in the involved vascular wall of neutrophil-mediated cutaneous vasculitis. Journal of the European Academy of Dermatology and Venereology: JEADV. 2016;30(9):1544–1549. [DOI] [PubMed] [Google Scholar]
- 28.van Bergen BH, Andriessen MP, Spruijt KI, van de Kerkhof PC, Schalkwijk J. Expression of SKALP/elafin during wound healing in human skin. Archives of dermatological research. 1996;288(8):458–462. [DOI] [PubMed] [Google Scholar]
- 29.Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. American journal of respiratory cell and molecular biology. 1994;11(6):733–741. [DOI] [PubMed] [Google Scholar]
- 30.Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNF-alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Archives of dermatological research. 2000;292(4):180–187. [DOI] [PubMed] [Google Scholar]
- 31.Bruggen MC, Petzelbauer P, Greinix H, et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. The Journal of investigative dermatology. 2015;135(4):999–1006. [DOI] [PubMed] [Google Scholar]
- 32.Chen A, Chao K, Rodriguez L, Munday W, Worswick S. Toxic epidermal necrolysis versus cutaneous Graft-Versus-Host Disease in a hematopoietic stem cell transplant recipient: the role of elafin. Leukemia & lymphoma. 2018:1–3. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Zhou XD, Xu XY, Yang J. Recombinant human elafin protects airway epithelium integrity during inflammation. Molecular biology reports. 2010;37(6):2981–2988. [DOI] [PubMed] [Google Scholar]
- 34.Small DM, Zani ML, Quinn DJ, et al. A functional variant of elafin with improved antiinflammatory activity for pulmonary inflammation. Molecular therapy: the journal of the American Society of Gene Therapy. 2015;23(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine JE. Implications of TNF-alpha in the pathogenesis and management of GVHD. International journal of hematology. 2011;93(5):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia O, Delgado L, Barbosa IL, Campilho F, Fleming-Torrinha J. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47(1):58–62. [DOI] [PubMed] [Google Scholar]
- 37.Gencoglan G, Tosun M, Aktepe F. The molecular mechanism of etanercept, an anti-tumour necrosis factor-alpha receptor-fusion protein, in the treatment of acute generalized exanthematous pustulosis. The Journal of dermatological treatment. 2009;20(4):241–245. [DOI] [PubMed] [Google Scholar]
- 38.Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71(2):278–283. [DOI] [PubMed] [Google Scholar]
- 39.Kavala M, Zindanci I, Turkoglu Z, et al. Acute generalized exanthematous pustulosis induced by etanercept: another dermatologic adverse effect. Case reports in dermatological medicine. 2013;2013:601412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. The British journal of dermatology. 2002;146(4):707–709. [DOI] [PubMed] [Google Scholar]
- 41.Leman RE, Chen L, Shi X, Rolimpandoei SP, Ling X, Su Y. Drug reaction with eosinophilia and systemic symptoms (DRESS) successfully treated with tumor necrosis factor-alpha inhibitor. JAAD case reports. 2017;3(4):332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(6):836–842. [DOI] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. [DOI] [PubMed] [Google Scholar]
- 44.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Reviews of physiology, biochemistry and pharmacology. 2003;149:1–38. [DOI] [PubMed] [Google Scholar]
- 45.Shiohara T, Mizukawa Y, Aoyama Y. Monitoring the acute response in severe hypersensitivity reactions to drugs. Current opinion in allergy and clinical immunology. 2015;15(4):294–299. [DOI] [PubMed] [Google Scholar]
- 46.Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64(12). [DOI] [PubMed] [Google Scholar]
- 47.Yoshida H, Hashizume M, Suzuki M, Mihara M. Anti-IL-6 receptor antibody suppressed T cell activation by inhibiting IL-2 production and inducing regulatory T cells. Eur J Pharmacol. 2010;634(1–3):178–183. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71–109. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, He D, Tang X, Zhang X. Chemokine expression in diverse nonimmediate drug hypersensitivity reactions: focus on thymus activation-regulated chemokine, cutaneous T-cell-attracting chemokine, and interleukin-10. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2014;113(2):204–208. [DOI] [PubMed] [Google Scholar]
- 50.Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. The Journal of investigative dermatology. 2004;123(5):850–855. [DOI] [PubMed] [Google Scholar]
- 51.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 52.Phillips GS, Freites-Martinez A, Hsu M, et al. Inflammatory dermatoses, infections, and drug eruptions are the most common skin conditions in hospitalized cancer patients. J Am Acad Dermatol. 2018;78(6):1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]


