Abstract
Hepatitis B virus (HBV) infection is a lifelong dynamic disease that can be controlled with treatment but cannot yet be cured. Risk of end-stage liver disease and hepatocellular carcinoma (HCC) increases with ongoing inflammation and HBV viremia. Initial treatments consist of tenofovir or entecavir. Patients who require treatment include those with chronic hepatitis, cirrhosis, HCC, or HIV coinfection; patients receiving immunosuppressive treatments; and women in the third trimester of pregnancy who have elevated HBV DNA level. A number of virologic and host immune approaches are being investigated with the aim of achieving HBV eradication. This article summarizes an IAS-USA webinar given by Marion G. Peters, MD, on June 14, 2018.
Keywords: HBV, hepatitis B, serum markers, chronic hepatitis, HCC, cirrhosis, tenofovir, entecavir, immunosuppression, pregnancy, viral eradication
Hepatitis B Virus (HBV) infection is a lifelong dynamic disease that changes over time. Risk of end-stage liver disease and cancer increases with ongoing inflammation and HBV viremia in adults. Fibrosis can be reversible, and treatment can decrease fibrosis progression. At present, chronic HBV infection can be controlled but not cured. Reactivation can occur even in patients who have lost hepatitis B surface antigen (HBsAg).
HBV Serum Markers
In the serum, for every intact HBV virion (Dane particle), the sub-particles HBsAg are present in vast numbers. Thus, HBV DNA can be below limit of detection, with HBsAg present in circulation. With regard to serologic markers in HBV infection, HBsAg indicates acute or chronic infection and is the first serologic marker to appear. Infection is considered chronic if it persists for greater than 6 months. The presence of HBV e-antigen (HBeAg) indicates active replication of virus. Its absence can indicate absence of ongoing replication; absence can also indicate mutations in the pre-core region of the e-antigen that prevent production of e-antigen. Antibody to HBV core antigen (anti-HBc) is present in infection (IgM in acute infection) and with past exposure to HBV. It may be found alone when antibody to HBsAg (anti-HBs) wanes. Anti-HBs indicates recovery or immunity to HBV. It is detectable after immunity conferred by HBV vaccination, and is occasionally seen in chronic carriers. Anti-HBe antibody (anti-HBe) generally indicates that virus is no longer replicating. However, it can be found in patients with HBeAg mutations (e-antigen-negative patients) who have active disease.1
Management according to serologic findings can be summarized as follows. Patients who are HBsAg-positive and anti-HBc-positive should be referred to care for HBV infection. Patients who are anti-HBs-positive and anti-HBc-positive have evidence of past infection; in these cases, infection is latent but can reactivate in immunocompromised patients, with re-emergence of HBsAg. Patients who are HBsAg-, anti-HBs-, and anti-HBc-negative lack evidence of immunity and should be vaccinated. Patients who are only anti-HBs-positive are immune or vaccinated.
Control of HBV
Control of HBV infection includes control of inflammatory components, indicated by normalization of serum alanine aminotransferase (ALT) level and normal liver biopsy; virologic control, indicated by reduction in HBV DNA; and immune control, indicated by seroconversion from HBeAg-positive to anti-HBe-positive and anti-HBs-positive to HBsAb-positive status.
The 2017 European Association for the Study of the Liver (EASL) guidelines2 differentiate chronic infection from chronic hepatitis, as shown in Table; in essence, chronic infection is having virus present in serum, and chronic hepatitis is present when there is evidence of liver damage.2 Among HBeAg-seropositive patients, those with chronic infection and those with chronic hepatitis have elevated HBsAg and elevated HBV DNA levels. ALT levels are normal in those with chronic infection and elevated in those with chronic hepatitis. Among HBeAg-sero-negative patients, HBsAg level is low in those with chronic infection and intermediate in those with chronic hepatitis, with HBV DNA level being low in the former but elevated in the latter and ALT level being normal and elevated, respectively. In brief, patients with chronic infection have normal ALT level and no or low-level liver damage, whereas those with chronic hepatitis have elevated HBV DNA and ALT levels and evidence of ongoing necroinflammatory disease in the liver. Treatment is directed at this latter group.
Table 1.
Chronic Infection and Chronic Hepatitis Characteristics in Individuals who Are Hepatitis B Virus e-Antigen (HBeAg)-Seropositive (top) and HBeAg-Seronegative (bottom). Adapted from the European Association for the Study of the Liver 2017 guidelines.2
| HBeAg-Seropositive Individuals | ||
| Chronic Infection | Chronic Hepatitis | |
| HBsAg | High | High/intermediate |
| HBeAg | Positive | Positive |
| HBV DNA | >107 IU/mL | 104–107 IU/mL |
| ALT | Normal | Elevated |
| Liver disease | None/minimal | Moderate/severe |
| Old terminology | Immune tolerant | Immune reactive HBeAg-positive |
| HBeAg-Seronegative Individuals | ||
| Chronic Infection | Chronic Hepatitis | |
| HBsAg | Low | Intermediate |
| HBeAg | Negative | Negative |
| HBV DNA | <2000 IU/mL | >2000 IU/mL |
| ALT | Normal | Elevated |
| Liver disease | None | Moderate/severe |
| Old terminology | Inactive carrier | HBeAg-negative chronic hepatitis |
Abbreviations: hepatitis B virus surface antigen indicates HBsAg; hepatitis B virus e-antigen, HBeAg; hepatitis B virus, HBV; alanine aminotransferase, ALT
Who Should Be Treated
Patients with HBV infection who should be treated include those with chronic hepatitis (ie, have elevated ALT and HBV DNA levels), those with cirrhosis (ie, any level ALT, detectable HBV DNA), those with hepatocellular carcinoma (HCC), those with HIV coinfection, those on chemotherapy or biologic response modifiers, and women in the third trimester of pregnancy if HBV DNA is greater than 200,000 1U/mL. Monitoring HBV monoinfection includes measuring serum ALT and HBV DNA levels every 3 months; if levels are normal for 1 year, they can then be measured every 6 months. It should be noted that older patients may have cirrhosis with normal ALT levels. If ALT level increases, closer monitoring should be resumed and the potential need for therapy discussed. Treatment should be considered if HBV DNA level becomes elevated. Asian men aged 40 years or older, Asian women aged 50 years or older, sub-Saharan African people older than 20 years, and those with family history of HCC should be monitored for HCC.
It should be noted that an elevated ALT level can be found in patients with HBV infection due to metabolic syndrome and non-alcoholic fatty liver disease. Patients with HBV infection plus obesity or metabolic syndrome have a higher risk of cirrhosis than those with HBV infection alone. If HBV DNA level is low or undetectable in these patients, abnormal ALT level may be due to non-alcoholic fatty liver disease. Weight gain or central adiposity (rather than body mass index, since this may be normal in some patients— eg, patients of Asian decent) may increase suspicion that ALT level is increasing in this context; however, liver biopsy may be needed to determine whether the ALT level increase is associated with HBV-related damage or steatosis. Statins may also lead to increases in ALT level (with some data indicating that these drugs may reduce risk for HCC). There are no contraindications for use of statins in individuals with HBV infection or for other drugs used in treatment of metabolic syndrome (eg, antihypertensive agents or antidiabetic agents, including metformin).
HBV Treatments
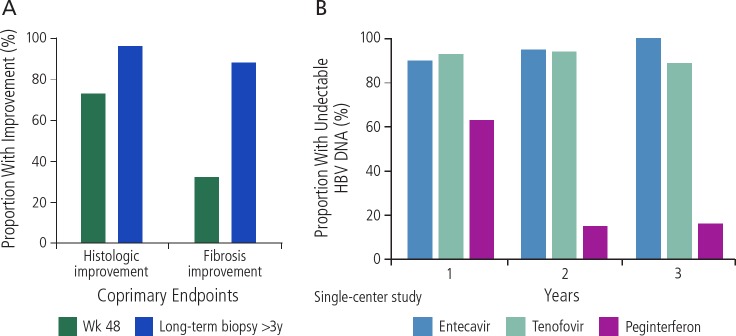
Currently approved treatments for HBV infection consist of interferon alfa-2b, lamivudine, adefovir, entecavir, peginterferon alfa-2a, telbivudine, tenofovir disoproxil (TDF), and tenofovir alaf-enamide (TAF). The only 2 first-line agents are entecavir and tenofovir (as TDF or TAF). Figure 1A provides data for the efficacy of the first-line treatments by showing histology and fibrosis improvements with entecavir therapy.3 As can be seen, histologic and fibrosis improvement were observed in 96% and 88% of patients with long-term treatment (biopsy at >3 years). Figure 1B shows data from different studies on the efficacy of entecavir and tenofovir (compared with peginterferon) in reducing HBV DNA to undetectable levels over the course of 3 years of treatment.4–11
Figure 1.
A: Histologic and fibrosis improvement with long-term entecavir treatment. Adapted from Chang et al.3 B: Proportions of hepatitis B virus (HBV) e-antigen-seronegative patients with reduction in HBV DNA to undetectable levels. Trials are not head-to-head comparisons and were conducted in different patient populations using different trial designs. Adapted from Lok et al, Marcellin et al, Baqai et al, Marcellin et al, Lau et al, Lim et al, Chang et al, Marcellin et al.4,5,7–11,21
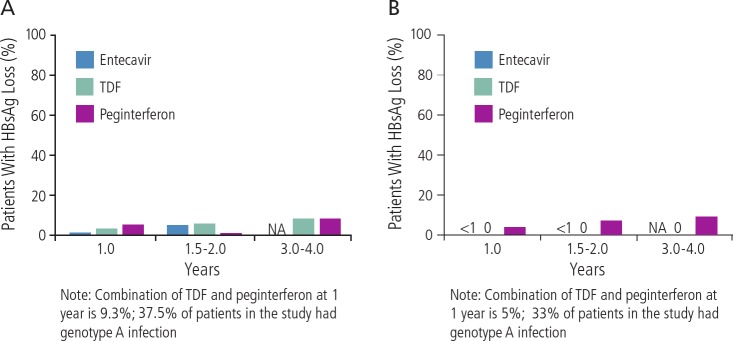
As shown in Figure 2A and B, neither entecavir nor tenofovir treatment results in loss of HBsAg in the vast majority of HBeAg-seropositive12–19 or HBeAg-negative patients,5,14,17,20,23 with sustained undetectable HBV DNA. A recent study showed loss of HBsAg in 9% of patients receiving combined peginterferon and tenofovir at 3 years (although 33% of patients in the study had HBV genotype A, which is particularly responsive to interferon). In general, the likelihood of clearance of HBsAg with entecavir or tenofovir is approximately less than 1% per year of treatment.
Figure 2.
Loss of hepatitis B virus (HBV) surface antigen (HBsAg) in (A) HBV e-antigen (HBeAg)-seropositive patients and (B) HBeAg-seronegative patients, with sustained undectable HBV DNA levels in separate trials with extended tenofovir disoproxil fumarate (TDF) or entecavir treatment, or 1 year of peginterferon treatment. Trials are not head-to-head comparisons and were conducted in different patient populations using different trial designs. NA indicates not applicable. Adapted from Marcellin et al, Gish et al, Marcellin et al, Buster et al, Heathcote et al, Marcellin et al, Heathcote et al, Janssen et al, Marcellin et al, Shouval et al, Chang et al, Brunetto et al, Lai et al.5,12,14–19,21–23,34,35
There are 3 types of “cure” that can be considered in the context of HBV infection. Functional cure consists of clinical resolution of infection that is sustained off drug treatment, with no inflammation present, as indicated by normal ALT level and negative liver biopsy, loss of HBsAg, and potential gain of anti-HBs. Complete cure is virologic cure, consisting of all the elements of functional cure plus loss of covalently closed circular (ccc) HBV DNA in the liver. A third or interim goal of therapy is achieving an inactive state, in which there is no inflammation (normal ALT level, negative biopsy), low or undetectable HBV DNA level, and HBsAg-seropositivity. This state is what is now our best treatment success—a patient still has HBV infection, but infection is inactive.
HBV cccDNA in host hepatocytes is the template for transcription and production of new virions. Persistence of HBV cccDNA can result in reactivation of infection in “recovered” patients who have lost HBsAg but still have anti-HBs and anti-HBc detectable in serum—eg, in immunosuppressed patients such as those with HIV infection, those receiving immunosuppressive therapy, and those undergoing hematopoietic stem cell transplantation. There is a high rate of HBV reactivation in immunosuppressed patients, including: during chemotherapy, in patients with HIV infection after immune reconstitution, after organ transplantation and stem cell transplantation, and with use of biologic response modifiers such as rituximab (anti-CD20) and tumor necrosis factor-α inhibitors.
Rituximab and stem cell transplantation are the most potent reactivators of HBV.24,25 All patients with HBV infection should be tested prior to chemotherapy or other immunosuppressive treatments for HBsAg, anti-HBs, and anti-HBc status. For those who are anti-HBs-seropositive, antiviral therapy should be started before immunosuppressive treatment. For those who are anti-HBc-seropositive but HBsAg-seronegative, anti-HBV therapy should be administered preemptively for rituximab or stem cell transplantation; preemptive anti-HBV therapy should be considered for other forms of chemotherapy, with close monitoring for HBV DNA in those not given anti-HBV therapy.26 All patients receiving anti-HBc-positive livers receive anti-HBV therapy. For other organ transplant, consultation with hepatology is recommended for management and follow-up.
HBV and HCC
Increased risk of progression to HCC in HBV infection has been associated with: male sex; younger age at the time of infection; excess alcohol consumption and non-alcoholic fatty liver disease; high HBV DNA level; coinfection with HIV, HCV, or hepatitis D virus (HDV); and infection with HBV genotypes C and Aa. Screening for HCC involves ultrasound performed and alpha fetoprotein level determined every 6 months, with use of computed tomography or magnetic resonance imaging to identify HCC if a lesion is detected during screening.
The few available data on survival in patients with HCC with or without HIV coinfection suggest poorer outcomes in those with HIV infection. One study, in which patients were followed up every 6 months with ultrasound and alpha fetoprotein testing, showed 2-year survival of 44% in those with HIV infection and 60% in those without (P = 0.2)27 Common to all studies has been a younger age at HCC diagnosis in patients with HIV infection, with a predominance of data also indicating more aggressive HCC in those with HIV infection.
HBV and HIV
Coinfection with HIV has been found to increase risk of HBV chronicity (ie, reduce likelihood of HBsAg clearance in unvaccinated patients), increase antiretroviral-related hepatotoxicity, and increase risk of end-stage liver disease. Patients with coinfection have poorer hospital outcomes and higher risk of progression to cirrhosis, HCC, and death than patients with either HIV or HBV monoinfection.28–30
Flares in patients with HBV/HIV co-infection are common. Many HIV medications and many over-the-counter medications are hepatotoxic, and other causes of ALT elevations in patients with HBV/HIV coinfection should be sought. Flares may also be associated with use of antiretroviral therapy without anti-HBV therapy and with stopping of antiretroviral therapy. Atypical serologic findings occur in patients with HIV coinfection during antiretroviral therapy. For example, reverse seroconversion may occur, in which patients who were anti-HBc-seropositive only become HBs-seropositive. All individuals with HBV/HIV coinfection require screening for HCC.
HBV and Pregnancy
All pregnant women should be tested for HBsAg. Infants born to mothers who are HBsAg-seropositive should receive both hepatitis B immune globulin (HB1G) and hepatitis B vaccine within 12 hours of birth, with subsequent vaccination at 1 then 3 or 6 months. More than 90% of children will then be immune to HBV infection. Vaccine failure has been associated with inadequate therapy (not receiving HB1G, not receiving follow-up vaccination) and high HBV DNA (>200,000 1U/mL) in the mother.
A study of HBV immunoprophylaxis in 1242 pregnant women with HBV infection showed an overall failure rate of 4.9%, with failure rate increasing with increasing maternal HBV DNA level.31 No cases of mother-to-child transmission occurred for 174 mothers with HBV DNA levels of less than 6 million log10 copies/mL (approximately 200,000 1U/mL); transmission rates were 3.0% among 298 with levels of 6.0 to 6.9; 5.5% among 531 with levels of 7.0 to 7.9; and 9.6% among 531 with levels of 8.0 million log10 copies/mL or higher.
Current recommendations are to assess HBV DNA level in all pregnant women at 26 to 28 weeks of pregnancy to determine need for treatment in the mother. It should be noted that many pregnant women with chronic HBV genotye B infection are immune-tolerant (high HBV-DNA level with normal ALT level) because they are young women. Treatment consists of tenofovir-based therapy during the third trimester. Entecavir is not recommended due to the finding of toxicity in animal studies. Lamivudine and telbivudine are not generally recommended because of the risk of emergence of resistance mutations. Women with levels below 20,000 1U/mL do not require treatment. Treatment should be considered in those with levels above 20,000 1U/mL but less than 200,000 1U/mL if the mother has active disease (ie, per usual treatment indications). Mothers with levels above 200,000 1U/mL should receive treatment up to delivery (or up to 1 month post-partum).32,33 All of the anti-HBV drugs are excreted in breast milk at low levels, but breastfeeding is not contraindicated in any current guidelines; prescribing information for the drugs do contain a warning about breastfeeding.
Conclusions
Individuals with HBV infection should receive anti-HBV treatment if they have elevated ALT level and elevated HBV DNA level. Treatment should also be given to patients who have cirrhosis and detectable HBV DNA, those who are going to receive chemotherapy or immunosuppressive therapy, and those with HIV infection. Comorbidities occur in patients with HBV infection, and patients need to be evaluated for HIV, HCV, HDV, and hepatitis A virus infection, and metabolic syndrome. Patients with HBV infection should report to their primary care practitioner any new diagnoses or planned therapies so that considerations for anti-HBV therapy can be made; this is particularly important if the patient is to receive high-dose steroids, chemotherapy, or rituximab, or if the patient is pregnant or wishes to become pregnant. Patients should undergo screening for HCC with imaging and alpha fetoprotein testing every 6 months. Candidates for screening include all Asian men aged over 40 years, Asian women aged over 50 years, sub-Saharan African people older than 20 years of age, all patients with HIV infection, all patients with cirrhosis, and patients with a first-degree relative with HCC.
A number of approaches are being investigated with the aim of achieving eradication of HBV. Virologic approaches include entry inhibitors, agents that block cccDNA, transcription inhibitors, RNA interference, HBV capsid inhibitors, polymerase inhibitors, and secretion inhibitors. Host immune approaches include interferons, toll-like receptor inhibitors, immune checkpoint inhibitors (such as the programmed cell death 1 [PD-1] and programmed death ligand 1 [PD-L1] inhibitors used in cancer therapy), interleukin-7, therapeutic vaccines (eg, immune complex vaccines, nasal HBV vaccines, DNA vaccines, T cell vaccines, adenovirus-based vaccines), and yeast-based vaccines. Several of these agents are being tested in humans, with many more in the pipeline. It is clear that combination approaches will be needed to achieve eradication and much work remains to be done to identify promising strategies. However, there is good likelihood of exciting data in this regard in the not too distant future.
Footnotes
Presented by Marion G. Peters, MD, in June 2018. First draft preparedfrom transcripts by Matthew Stenger. Reviewed and edited by Dr Peters in January 2019.
Financial affiliations in the past 12 months: Dr Peters has no relevant financial affiliations to disclose. Her spouse is employed by Hoffman-La Roche.
References
- 1. Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol. 2004;2:87–106. [DOI] [PubMed] [Google Scholar]
- 2. Wendon J, Cordoba J, Dhawan A, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–1081. [DOI] [PubMed] [Google Scholar]
- 3. Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010; 52(3):886–893. [DOI] [PubMed] [Google Scholar]
- 4. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3): 661–662. [DOI] [PubMed] [Google Scholar]
- 5. Marcellin P, Buti M, Krastev Z, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-negative patients with chronic hepatitis B (study 102), preliminary analysis. Hepatology. 2008;370A–371A.18220278
- 6. Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009; 29(2):242–247. [DOI] [PubMed] [Google Scholar]
- 7. Baqai SF, Yi DH, Gish RG. Profound virologic response in chronic hepatitis B (CHB) patients treated with entecavir (ETV) in HBeAg positive and negative disease. Hepatology. 2009;530A. [Google Scholar]
- 8. Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigennegative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136(7):2169–2179. [DOI] [PubMed] [Google Scholar]
- 9. Lau GK, Piratvisuth T, Thongsawat S, et al. Durability of response to peginterferon alfa-2a (40 KD) (PEGASYS) in Asian patients with HBeAg-positive chronic hepatitis B: 12 month follow-up data from a large, randomized study (abstract 49). Program and Abstracts From the 2006 Shanghai-Hong Kong International Liver Congress. March 25–28, 2006; Shanghai, China.
- 10. Lim SG, Lai C-L, Gane E, et al. The antiviral efficacy of telbivudine is consistent across hepatitis B patient subgroups: results from the GOLBE study (abstract). Program and Abstracts From the Shanghai-Hong Kong International Liver Congress. March 25–28, 2006; Shanghai, China.
- 11. Chang TT, Chao YC, Sollano J, et al. Entecavir (ETV) treatment through 96 weeks results in substantial virologic and biochemical improvement and HBeAg seroconversion in HBeAg chronic hepatitis B (CHB) patients (abstract). Program and Abstracts From the Shanghai-Hong Kong International Liver Congress. March 2528, 2006; Shanghai, China
- 12. Gish RG, Lok AS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133(5):1437–1444. [DOI] [PubMed] [Google Scholar]
- 13. Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. [DOI] [PubMed] [Google Scholar]
- 14. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–2455. [DOI] [PubMed] [Google Scholar]
- 15. Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135(2):459–467. [DOI] [PubMed] [Google Scholar]
- 16. Heathcote EJ, Gane EJ, DeMan RA, et al. Two year tenofovir disoproxil fumarate (TDF) treatment and adefovir dipivoxil (ADV) switch data in HBeAg-positive patients with chronic hepatitis B (study 103), preliminary analysis. Hepatology. 2008;376A. [Google Scholar]
- 17. Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–144. [DOI] [PubMed] [Google Scholar]
- 18. Heathcote EJ, Gane EJ, de Man RA, et al. Three years of tenofovir disoproxil (TDF) treatment in HBeAg-positive patients (HBeAg+) with chronic hepatitis B (study 103), preliminary analysis. Hepatology. 2009;533A–534A.19115220
- 19. Janssen HL, Van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. [DOI] [PubMed] [Google Scholar]
- 20. Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011–1020. [DOI] [PubMed] [Google Scholar]
- 21. Marcellin P, Buti M, Krastev Z, et al. Three years of tenofovir disoproxil fumarate (TDF) treatment in HBeAg-negative patients with chronic hepatitis B (study 102); preliminary analysis. Hepatology. 2009; 532A–533A.
- 22. Shouval D, Lai CL, Chang TT, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50(2):289–295. [DOI] [PubMed] [Google Scholar]
- 23. Brunetto MR, Oliveri F, Colombatto P, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139(2):483–490. [DOI] [PubMed] [Google Scholar]
- 24. Loomba R, Rowley A, Wesley R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148(7):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013; 57(3):209–214. [DOI] [PubMed] [Google Scholar]
- 26. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim C, Goutte N, Gervais A, et al. Standardized care management ensures similar survival rates in HIV-positive and HIVnegative patients with hepatocellular car-cinoma. J Acquir Immune Defic Syndr. 2012; 61(5):581–587. [DOI] [PubMed] [Google Scholar]
- 28. Thio CL, Seaberg EC, Skolasky R Jr., et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349): 1921–1926. [DOI] [PubMed] [Google Scholar]
- 29. Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356(14): 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajbhandari R, Jun T, Khalili H, Chung RT, Ananthakrishnan AN. HBV/HIV coinfection is associated with poorer outcomes in hospitalized patients with HBV or HIV. J Viral Hepat. 2016;23(10):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han GR, Zhao W, Xu CL, Ge CY, Jiang HX, Pan C. Risk factors associated with perinatal infection of HBV in infants who born to HBsAg and HBeAg positive mothers. Hepatology. 2011;444A. [Google Scholar]
- 32. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60(2):468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang TT, Gish RG, de MR, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–1010. [DOI] [PubMed] [Google Scholar]
- 35. Lai CL, Shpizen S, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1074–1076. [DOI] [PubMed] [Google Scholar]