Abstract
Background
Active management of the third stage of labour involves giving a prophylactic uterotonic, early cord clamping and controlled cord traction to deliver the placenta. With expectant management, signs of placental separation are awaited and the placenta is delivered spontaneously. Active management was introduced to try to reduce haemorrhage, a major contributor to maternal mortality in low‐income countries. This is an update of a review last published in 2015.
Objectives
To compare the effects of active versus expectant management of the third stage of labour on severe primary postpartum haemorrhage (PPH) and other maternal and infant outcomes.
To compare the effects of variations in the packages of active and expectant management of the third stage of labour on severe primary PPH and other maternal and infant outcomes.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov and the World health Organization International Clinical Trials Registry Platform (ICTRP), on 22 January 2018, and reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing active versus expectant management of the third stage of labour. Cluster‐randomised trials were eligible for inclusion, but none were identified.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias, carried out data extraction and assessed the quality of the evidence using the GRADE approach.
Main results
We included eight studies, involving analysis of data from 8892 women. The studies were all undertaken in hospitals, seven in higher‐income countries and one in a lower‐income country. Four studies compared active versus expectant management, and four compared active versus a mixture of managements. We used a random‐effects model in the analyses because of clinical heterogeneity. Of the eight studies included, we considered three studies as having low risk of bias in the main aspects of sequence generation, allocation concealment and completeness of data collection. There was an absence of high‐quality evidence according to GRADE assessments for our primary outcomes, which is reflected in the cautious language below.
The evidence suggested that, for women at mixed levels of risk of bleeding, it is uncertain whether active management reduces the average risk of maternal severe primary PPH (more than 1000 mL) at time of birth (average risk ratio (RR) 0.34, 95% confidence interval (CI) 0.14 to 0.87, 3 studies, 4636 women, I2 = 60%; GRADE: very low quality). For incidence of maternal haemoglobin (Hb) less than 9 g/dL following birth, active management of the third stage may reduce the number of women with anaemia after birth (average RR 0.50, 95% CI 0.30 to 0.83, 2 studies, 1572 women; GRADE: low quality). We also found that active management of the third stage may make little or no difference to the number of babies admitted to neonatal units (average RR 0.81, 95% CI 0.60 to 1.11, 2 studies, 3207 infants; GRADE: low quality). It is uncertain whether active management of the third stage reduces the number of babies with jaundice requiring treatment (RR 0.96, 95% CI 0.55 to 1.68, 2 studies, 3142 infants, I2 = 66%; GRADE: very low quality). There were no data on our other primary outcomes of very severe PPH at the time of birth (more than 2500 mL), maternal mortality, or neonatal polycythaemia needing treatment.
Active management reduces mean maternal blood loss at birth and probably reduces the rate of primary blood loss greater than 500 mL, and the use of therapeutic uterotonics. Active management also probably reduces the mean birthweight of the baby, reflecting the lower blood volume from interference with placental transfusion. In addition, it may reduce the need for maternal blood transfusion. However, active management may increase maternal diastolic blood pressure, vomiting after birth, afterpains, use of analgesia from birth up to discharge from the labour ward, and more women returning to hospital with bleeding (outcome not pre‐specified).
In the comparison of women at low risk of excessive bleeding, there were similar findings, except it was uncertain whether there was a difference identified between groups for severe primary PPH (average RR 0.31, 95% CI 0.05 to 2.17; 2 studies, 2941 women, I2 = 71%), maternal Hb less than 9 g/dL at 24 to 72 hours (average RR 0.17, 95% CI 0.02 to 1.47; 1 study, 193 women) or the need for neonatal admission (average RR 1.02, 95% CI 0.55 to 1.88; 1 study, 1512 women). In this group, active management may make little difference to the rate of neonatal jaundice requiring phototherapy (average RR 1.31, 95% CI 0.78 to 2.18; 1 study, 1447 women).
Hypertension and interference with placental transfusion might be avoided by using modifications to the active management package, for example, omitting ergot and deferring cord clamping, but we have no direct evidence of this here.
Authors' conclusions
Although the data appeared to show that active management reduced the risk of severe primary PPH greater than 1000 mL at the time of birth, we are uncertain of this finding because of the very low‐quality evidence. Active management may reduce the incidence of maternal anaemia (Hb less than 9 g/dL) following birth, but harms such as postnatal hypertension, pain and return to hospital due to bleeding were identified.
In women at low risk of excessive bleeding, it is uncertain whether there was a difference between active and expectant management for severe PPH or maternal Hb less than 9 g/dL (at 24 to 72 hours). Women could be given information on the benefits and harms of both methods to support informed choice. Given the concerns about early cord clamping and the potential adverse effects of some uterotonics, it is critical now to look at the individual components of third‐stage management. Data are also required from low‐income countries.
It must be emphasised that this review includes only a small number of studies with relatively small numbers of participants, and the quality of evidence for primary outcomes is low or very low.
Plain language summary
Delivering the placenta in the third stage of labour
What is the issue?
The aim of this Cochrane Review was to look at different ways of delivering the placenta after the birth of the baby; expectant, active or mixed management. We asked, what are the benefits and harms for all women, but specifically for women at low risk of severe bleeding (haemorrhage)? We collected and analysed all relevant studies to answer this question (22 January 2018).
Why is this important?
Once a baby is born, the womb (uterus) continues to contract, causing the placenta to separate from the wall of the uterus. The mother then delivers the placenta, or 'after‐birth'. This is called expectant management of third stage of labour. Active management of third stage involves three components: 1) giving a drug (a uterotonic) to help contract the uterus; 2) clamping the cord early (usually before, alongside, or immediately after giving the uterotonic); 3) traction is applied to the cord with counter‐pressure on the uterus to deliver the placenta (controlled cord traction). Mixed management uses some, but not all, of the three components. Active management was introduced to try to reduce severe blood loss at birth. This is a major cause of women dying in low‐income countries where women are more likely to be poorly nourished, anaemic and have infectious diseases. In high‐income countries, severe bleeding occurs much less often, yet active management has become standard practice in many countries.
What evidence did we find?
We found eight studies that contributed data and involved 8892 women and their babies. All studies were undertaken in hospital settings, seven in higher‐income countries and one in a lower‐income country. Four studies compared active with expectant management and four compared active with mixed management.
Overall, the quality of the evidence was generally low or very low and we need more data to be confident in the findings. For all women, irrespective of their risk of severe bleeding, active management may reduce severe bleeding and anaemia. However, it also may reduce the baby’s birthweight and increase the mother's blood pressure, afterpains, vomiting, and the number of women returning to hospital with bleeding. Findings were similar for women at low risk of bleeding, though it was unclear if there was any difference in the incidence of severe bleeding or anaemia.
What does this mean?
Women should be given information before they give birth to help them make informed choices. Some adverse effects experienced by mothers may possibly be avoided by using specific drugs. Delaying cord clamping may benefit the baby by preventing the reduction in birthweight from early cord clamping, but more research is needed. Also, it may be that just giving a uterotonic might reduce severe bleeding, without using the other parts of active management. More research is needed, particularly in low‐income countries.
Summary of findings
Summary of findings for the main comparison. Active versus expectant management of the third stage of labour (all women).
| Active versus expectant management of the third stage of labour (all women) | ||||||
|
Population: all women who expected a vaginal birth at 24 weeks' gestation or later and their babies Setting: UK and Ireland, hospital setting. The countries were classified as 'higher‐income' and 'lower‐income', with the border between lower‐middle‐income and upper‐middle‐income being the cut‐off. All studies included in this main analysis were undertaken in higher‐income countries (defined according to World Bank definitions 2018). Intervention: active management of the third stage of labour Comparison: expectant management of the third stage of labour | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Expectant management of the third stage of labour | Active management of the third stage of labour | |||||
|
Severe primary PPH (clinically estimated or measured blood loss ≥ 1000 mL at time of birth) |
24 per 1000 | 8 per 1000 (3 to 21) | RR 0.34 (0.14 to 0.87) | 4636 (3 studies) | ⊕⊝⊝⊝ Very lowa | |
|
Very severe primary PPH (clinically estimated or measured blood loss ≥ 2500 mL at time of birth) |
See comment | See comment | Not estimable | 0 (0 studies) | See comment | No data |
| Maternal mortality | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No data |
| Maternal Hb < 9 g/dL 24‐72 hours postpartum | 71 per 1000 | 36 per 1000 (21 to 59) | RR 0.50 (0.3 to 0.83) | 1572 (2 studies) | ⊕⊕⊝⊝ Lowb | |
| Admission to SCBU/NICU | 52 per 1000 | 42 per 1000 (31 to 58) | RR 0.81 (0.60 to 1.11) | 3207 (2 studies) | ⊕⊕⊝⊝ Lowc | |
| Neonatal jaundice requiring phototherapy or exchange transfusion | 49 per 1000 | 47 per 1000 (27 to 83) | RR 0.96 (0.55 to 1.68) | 3142 (2 studies) | ⊕⊝⊝⊝ Very lowd | |
| Neonatal polycythaemia treated with dilutional exchange transfusion | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; Hb: haemoglobin; NICU: neonatal intensive care unit; PPH: primary postpartum haemorrhage; RR: risk ratio; SCBU: special care baby unit | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRisk of bias: of the three studies providing data for this outcome, all are at low risk of bias for sequence generation (selection bias) and allocation concealment (selection bias). All are at high risk of bias for lack of blinding for clinicians and women and all are unclear for blinding of outcome assessment. Two studies are at low risk of bias for incomplete outcome data (attrition bias), and one is unclear. Two are at high risk of selective reporting (reporting bias) and one is unclear. One study is at high risk of other bias and two are unclear (see 'Risk of bias' tables and Figure 2). Downgraded 1. Inconsistency: there is some overlap of confidence intervals of the three studies, however, Tau² = 0.38, the P value for the Chi² test of heterogeneity is 0.08 and I² = 60%. These suggest the presence of heterogeneity, which cannot be explained by any of the subgroups or sensitivity analyses performed. Downgraded 1. Indirectness: directly answers the question. Imprecision: total (cumulative) sample size 4636 is less than the optimal information size of 18,590 (assuming α = 0.05, 1‐β = 0.80, relative risk reduction (RRR) of 25% from control event rate). Events = 88, Downgraded 1. Publication bias: assessment of funnel plot asymmetry not performed due to fewer than 10 studies included for this outcome.
bRisk of bias: of the two studies providing data for this outcome, both are at low risk of bias for sequence generation (selection bias) and one is at low risk of bias for allocation concealment (selection bias) and the other is unclear. Both are at high risk of bias for lack of blinding of clinicians and women, both are unclear for blinding of outcome assessment (although unlikely to affect Hb measurements). One is at high risk of bias for incomplete outcome data (attrition bias) while the other is unclear. One is high risk of bias for selective reporting (reporting bias), the other is unclear. Both studies are at high risk of other bias (see 'Risk of bias' tables and Figure 2). Downgraded 1. Inconsistency: the confidence intervals of the two studies overlap. Tau² = 0.02, the P value for the Chi² test of heterogeneity is 0.31 and I² = 3%. Although Tau² is non‐zero, tests suggest an absence of unexplained heterogeneity. Indirectness: directly answers the question. Imprecision: total (cumulative) sample size 1572 is less than the optimal information size of 5804 (assuming α = 0.05, 1‐β = 0.80, RRR of 25% from control event rate). Events = 94. Downgraded 1. Publication bias: assessment of funnel plot asymmetry not performed due to fewer than 10 studies included for this outcome.
cRisk of bias: of the two studies providing data for this outcome, both are at low risk of bias for sequence generation (selection bias) and allocation concealment (selection bias). Both are at high risk of bias for lack of blinding of clinicians and women, both are unclear for blinding of outcome assessment. One is at low risk of bias for incomplete outcome data (attrition bias), the other is unclear. Both are at high risk of bias for selective reporting (reporting bias) and both are at high risk for other biases (see 'Risk of bias' tables and Figure 2). Downgraded 1. Inconsistency: the confidence intervals of the two studies overlap. Heterogeneity: Tau² = 0.00; Chi² P value = 0.40, I² = 0%. This suggests an absence of unexplained heterogeneity. Indirectness: directly answers the question. Imprecision: total (cumulative) sample size 3207 is less than the optimal information size of 8066 (assuming α = 0.05, 1‐β = 0.80, RRR of 25% from control event rate). Events = 152. Downgrade 1 Publication bias: assessment of funnel plot asymmetry not performed due to fewer than 10 studies included for this outcome.
dRisk of bias: of the two studies providing data for this outcome, both are at low risk of bias for sequence generation (selection bias) and allocation concealment (selection bias). Both are at high risk of bias for lack of blinding of clinicians and women, both are unclear for blinding of outcome assessment. One is low risk of bias for incomplete outcome data (attrition bias), the other is unclear. Both are at high risk of bias for selective reporting (reporting bias) and both are at high risk of other biases (see 'Risk of bias' tables and Figure 2). Downgraded 1. Inconsistency: there is some overlap of confidence intervals of the two studies. However, Tau² = 0.11, P value for heterogeneity = 0.09 and I² = 66%, which suggest the presence of heterogeneity that cannot be explained by any of the subgroups or sensitivity analyses performed. Downgraded 1. Indirectness: directly answers the question. Imprecision: total (cumulative) sample size 3142 is less than the optimal information size of 8584 (assuming α = 0.05, 1‐β = 0.80, RRR of 25% from control event rate). Events = 149. Downgraded 1. Publication bias: assessment of funnel plot asymmetry not performed due to fewer than 10 studies included for this outcome.
Background
Description of the condition
The third stage of labour is the time from the birth of the baby to the expulsion of the placenta and membranes. Once the baby is born, the uterus continues to contract and reduce in size. There is a lack of full understanding of the physiology of the third stage of labour, but recent work using ultrasonography has demonstrated that the process of placental separation has three distinct phases (Herman 2002). The first, or latent phase, consists of strong uterine contractions, which lead to thickening of the uterine muscle, thus causing a shearing force to occur between the elastic uterine wall and the more rigid placenta (Herman 2002). Continued contractions lead to gradual separation of the placenta, commencing at one of the poles (most commonly the lower) and spreading slowly during the contraction or detachment phase until full separation occurs. This is followed by delivery of the placenta in the expulsion phase (Herman 2002). Muscle fibres surrounding the maternal vessels contract to prevent excessive bleeding (Inch 1985), and the mother's coagulation system is activated temporarily (Bonnar 1970).
There is always some blood loss during the third stage of labour as the placenta separates and is delivered, but what might be considered a normal amount of loss is the subject of debate (Gyte 1992). Nevertheless, some women can suffer from considerable blood loss during or after the third stage of labour. This can be a primary haemorrhage (within the first 24 hours; Mousa 2014), or a secondary haemorrhage (between 24 hours and six weeks; McDonald 2003). Postpartum haemorrhage (PPH) is commonly defined as a blood loss in excess of 500 mL (WHO 2003), with severe haemorrhage being a loss of 1000 mL or more and very severe haemorrhage being a loss of 2500 mL or more (Bloomfield 1990; Greer 1998; Penney 2005). However, the impact of blood loss at birth on an individual woman can vary considerably and will depend not only on the volume of blood lost, but also on her general state of health, the speed of the loss, her haemoglobin (Hb) levels at the time and her coagulation system. It is well documented that blood loss is consistently under‐ or over‐estimated by clinicians (Razvi 2008), although many centres do try to measure and record blood loss accurately. In well‐nourished women, some consider that, in general, there is little impact from a blood loss of 500 mL (Bloomfield 1990), this being equivalent to a routine blood donation (Burnley 2006), but in women in low‐income countries who may be poorly nourished and anaemic, this loss can cause considerable morbidity or mortality. It has been estimated that at least 25% of maternal deaths in a number of countries are due to haemorrhage; most due to PPH (Abouzaher 1998; Khan 2006). The vast majority of these happen in the developing world, and PPH is the leading cause of maternal mortality in sub‐Saharan Africa (Lazarus 2005). However, a study in Mexico (Romero‐Gutierrez 2007), reported that while the leading cause of maternal death was haemorrhage, two‐thirds of bleeding‐related deaths resulted from placental abruption, placenta accreta, placenta praevia, and peripartum hysterectomy, rather than uterine atony (poor contraction of the muscles in the uterus). Significant morbidity does occur, though, from major bleeding due to uterine atony, which is far more common than the other causes of bleeding listed above. The seriousness with which PPH is viewed by professionals is evidenced in joint policy statements between the International Confederation of Midwives (ICM) and the International Federation of Gynaecology and Obstetrics (FIGO) (ICM‐FIGO 2003; ICM‐FIGO 2006), and the World Health Organization (WHO 2003), all of which have recommended active management of the third stage of labour. Debate continues among women and practitioners on the optimum method of management of the third stage of labour to balance the benefits and harms.
There are two distinct approaches to the clinical management of the third stage of labour: expectant and active management. However, a third approach is sometimes used that consists of a combination of components of both expectant and active management: this has been referred to as 'mixed management' or the 'piecemeal approach' (Prendiville 1989). Expectant, active and mixed management approaches, and comparisons of different types of active management, have been the subject of a number of critical reviews (Elbourne 1995; Gyte 1994; Maughan 2006; McDonald 2007a; Prendiville 1989; Prendiville 1996; Soltani 2008).
Description of the intervention
(a) Expectant management of the third stage of labour
Expectant management is also known as conservative or physiological management and is popular in some northern European countries (Nordstrom 1997), and in New Zealand (Dixon 2013). It is also practised on occasion in midwife‐led units and in home births in the UK and Ireland (Begley 2009; Blackburn 2008; Fry 2007; Kanikosmay 2007), and is the usual practice when birthing at home or in the community in some low‐income countries. The main principle of expectant management is a 'hands off' approach, where signs of placental separation are awaited and the placenta is birthed spontaneously or with the aid of gravity, maternal pushing (Begley 2012; Inch 1985) or, sometimes, nipple stimulation (Inch 1985) hence:
a prophylactic uterotonic agent is not administered;
ideally, the umbilical cord is neither clamped nor cut until the placenta has been delivered but, at a minimum, caregivers have waited until cord pulsation has ceased; and
the placenta is delivered spontaneously with the aid of gravity and sometimes by maternal effort (Begley 2012; Rogers 1998).
There can be variations within expectant management. For example, some caregivers will wait for the placenta to be delivered before clamping and cutting the cord whilst others, for convenience, just wait until pulsation has finished. Breastfeeding or other means of stimulating the physiological release of oxytocin, such as nipple stimulation, is sometimes also used (Bullough 1989), but is not an essential component of expectant management. Some 'expert' midwives will use gentle traction on the cord once the placenta is seen to be in the vagina (Begley 2012), with good results.
(b) Active management of the third stage of labour
In active management of the third stage of labour, the clinician intervenes by using the following package of interventions (Prendiville 1989):
the routine administration of a prophylactic uterotonic drug just before, with, or immediately after, the birth of the baby;
early cord clamping and cutting* (i.e. prior to, alongside, or immediately after administration of an oxytocic, which is before cord pulsation ceases); and
controlled cord traction to deliver the placenta.
*current WHO recommendations (WHO 2014), are to delay cord clamping, and the National Institute for Health and Clinical Excellence (NICE) now recommends "deferred" cord clamping (NICE 2014).
These interventions are implemented routinely and prophylactically in an attempt to reduce the blood loss associated with the third stage of labour and to reduce the risk of PPH. There are many possible variations with this package of interventions.
There are different uterotonic drugs that can be used, for example, oxytocin (intravenous (IV) or intramuscular (IM)); syntometrine (IM); ergometrine (IV or IM); misoprostol (IM; Liabsuetrakul 2018; McDonald 2007b; Su 2012; Tunçalp 2012; Westhoff 2013), carbetocin, or paired combinations of these drugs (Gallos 2018). There is also debate over the route of administration and dosage of the drugs used. Recent guidelines from WHO, FIGO, ICM and NICE all recommend the use of 10 IU (international units) of oxytocin IM ((ICM‐FIGO 2003; NICE 2014; WHO 2012). Misoprostol is potentially the most important uterotonic for use in some low‐income countries because it is stable at ambient temperatures and is inexpensive (Parsons 2007). However, it does have adverse side effects (Mousa 2014), such as shivering, nausea and headaches, and it has been shown to be less effective than other agents (Tunçalp 2012). A recent network analysis, however, suggests that the three most effective drugs for preventing PPH of 500 mL or more are ergometrine and oxytocin, carbetocin, and misoprostol combined with oxytocin (Gallos 2018).
There are differing timings for giving the prophylactic uterotonic drug, for example, with the crowning of the baby's head; with the birth of the anterior shoulder; immediately after the birth of the baby; after the birth of the baby but before the placenta is delivered (Harris 2004), and after the placenta is delivered (Winter 2007). The timing of administration of uterotonic drugs is the subject of another Cochrane Review (Soltani 2010).
There can be variation in the time when the cord is clamped and cut; this can be immediately the baby is born; within a set time after the birth, for example, within 30 seconds or a minute; or anytime before umbilical cord pulsation ceases (McDonald 2013; Rabe 2012; Van Rheenan 2007).
There are also different timings for the initiation of controlled cord traction, such as waiting for signs of placental separation or not (McDonald 2003).
There can also be a delay in using the whole package of active management until after cord pulsation ceases, which has been described as ‘delayed active management’ (Gyte 2006).
Some guidelines (e.g. ICM‐FIGO 2003), add uterine massage to the active management package although there is little evidence to support this (Abdel‐Aleem 2010).
Placental cord drainage is sometimes used with active management of the third stage. This involves releasing the clamp on the maternal end of the umbilical cord to allow the blood from the placental side to drain, thus reducing the size of the placenta and thereby hoping to help separation and reduce the chance of a retained placenta (Prendiville 1989; Soltani 2011).
Some of these variations in the components of active management of the third stage of labour may no longer be considered good practice (e.g. early cord clamping), but may, nonetheless, be used in included studies identified for this review.
(c) Mixed management of the third stage of labour
Mixed management of the third stage of labour, (or 'combined' or 'piecemeal' management), which consists of a mixture of some of the components of both active and expectant management of the third stage, but without exclusively containing all the components of either. Although active management of the third stage is usually recommended (ICM‐FIGO 2006; NICE 2014; WHO 2003), there are many variations, and in practice some women may actually receive mixed management (Harris 2006; Mercer 2000). Mixed management of the third stage might include, for example: (1) early uterotonic administration, cord clamping after pulsation ceases and controlled cord traction; or (2) delayed uterotonic administration until cord pulsation ceases, then cord clamping and controlled cord traction. These forms of mixed management of the third stage are of interest because of the evidence of benefits from delayed cord clamping for the baby (McDonald 2013; Mercer 2008; Rabe 2012).
How the intervention might work
Expectant management
Expectant management of the third stage relies on the natural contractions of the uterus, stimulated by a surge of physiological oxytocin at birth, and anything that interferes with this oxytocin release may reduce the effectiveness of the physiological process in the third stage (Inch 1985). Release of oxytocin can, for example, be inhibited by anxiety through the excess release of adrenaline (Buckley 2004).
Hence, expectant management of the third stage of labour is commonly only considered appropriate following a labour where there has been no interference with the natural release of oxytocin, for example, where oxytocin augmentation, induction, epidural or narcotic analgesia, or both, have not been used (Buckley 2004; Fry 2007); but some will consider that these aspects still need to be assessed in well‐designed studies. This type of labour is more likely when the woman has positive psychological support from her midwife, or other trained supporter, who encourages her to listen to her body's messages about movement, positioning, hydration and nutrition (Bohren 2017; Buckley 2004; Sandall 2016).
Active management
In active management of the third stage of labour, it is suggested that the prophylactic administration of a uterotonic will reduce bleeding and the risk of severe haemorrhage (Greer 1998; Prendiville 1989). The role of early cord clamping and controlled cord traction in the reduction of bleeding is less clear, but it is thought that once the uterotonic drug has been administered, it is important to deliver the placenta quickly to prevent it being retained. Applying a clamp to the cord thus gives the caregiver something to grasp in order to deliver the placenta quickly by applying controlled cord traction. Active management of the third stage has been standard practice in many parts of the world for many years (Prendiville 1989). Recently, however, arguments have been put forward for a delay in cord clamping, pointing out that it is not an evidence‐based part of the package of active management (Weeks 2007). A Cochrane Review found that neither early nor late cord clamping showed any significant difference in PPH rates (McDonald 2013).
A number of Cochrane Reviews have been conducted examining different aspects of active management of the third stage of labour. These include reviews on prophylactic oxytocin in the third stage of labour (Westhoff 2013); prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour (McDonald 2007b); prophylactic use of ergot alkaloids in the third stage of labour (Liabsuetrakul 2018); prostaglandins for preventing PPH (Tunçalp 2012); oxytocin agonists for preventing PPH (Su 2012); timing of cord clamping in term infants (McDonald 2013) and timing of cord clamping in preterm infants (Rabe 2012).
Potential adverse effects
Interventions used in active management of the third stage have some adverse effects, due mainly to the uterotonic drugs used and to the common practice of early clamping of the cord.
Uterotonic drugs can increase the risk of hypertension, nausea and vomiting for women (Maughan 2006), and which appear to be related to the use of ergometrine‐based drugs. Active management in many countries has moved away from ergometrine‐based uterotonics, for this reason, and possibly also due to clinicians' fear of retained placenta, although a review of ergometrine‐based drugs compared with other uterotonics showed no difference in rates of manual removal of placentae (McDonald 2007b).
The potential consequences for the newborn infant of active management of the third stage of labour relate mainly to the timing of cord clamping. The effects on the neonate of early versus deferred cord clamping have been explored in Cochrane and other systematic reviews (Hutton 2007; McDonald 2013; Rabe 2012). Early cord clamping reduces the volume of placental blood transfusion and thus reduces the baby's blood volume at birth by about 20% for term infants (RCOG 2009; Werner 2005). This results in lower blood haematocrit (HCT) levels and Hb concentrations after birth in term infants but the long‐term importance of this effect is unknown (Hutton 2007; McDonald 2013; Prendiville 1989; Van Rheenan 2007). Potentially, placental transfusion may be more important for infants born in low‐ and middle‐income settings where iron‐deficiency anaemia exacerbated by nutritional and infectious insults may have substantial and long‐term adverse effects on growth and development (Van Rheenan 2007). For preterm infants, another specific concern is the effect of postnatal placental transfusion on neonatal haemodynamic transition processes. The Cochrane Review of early versus delayed cord clamping for preterm infants found some evidence that infants who had early cord clamping had a higher risk of hypotension treated with volume‐transfusion and of intraventricular haemorrhage (Rabe 2012).
In contrast, early cord clamping also results in lower postnatal levels of plasma bilirubin and a lower incidence of neonatal jaundice that requires phototherapy (McDonald 2013; Rabe 2012). Treatment of neonatal jaundice may result in mother‐infant separation that delays the initiation and establishment of breastfeeding and disrupts early neonatal metabolic adaptation (Mercer 2001). For infants born in low‐ or middle‐income settings, or in rural or remote settings distant from healthcare facilities, the need for phototherapy (or its lack of availability) may be of greater clinical importance.
If uterotonic drugs are administered before delivery of the infant, for example, inadvertently prior to the birth of an undiagnosed twin, then disruption of the placental‐uterine wall interface and interruption of placental‐umbilical blood flow may cause acute perinatal asphyxia compromising neonatal cardio‐respiratory transition. Newborn infants compromised at birth are more likely to need transition support (cardio‐respiratory resuscitation). If an asphyxial insult has been severe or prolonged (for example, if exacerbated by obstructed labour such as shoulder dystocia) then other potential consequences may include neonatal encephalopathy, with its associated risk of mortality and long‐term neurodevelopmental morbidity.
Why it is important to do this review
We undertook this review because of the need to determine if active, expectant management, or a mixed management package, was most likely to be of overall benefit. It is important to assess the impact of all these forms of care on both the mother and baby. We believe that this review is highly relevant to families and clinicians, as women frequently enquire about the differences in third‐stage management during the antenatal period. This is an update of review last published in 2015 (Begley 2015).
Objectives
To compare the effects of active versus expectant management of the third stage of labour on severe primary postpartum haemorrhage (PPH) and other maternal and infant outcomes.
To compare the effects of variations in the packages of active and expectant management of the third stage of labour on severe primary PPH and other maternal and infant outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised, and quasi‐randomised, controlled trials of active versus expectant management of the third stage of labour. Cluster‐randomised trials were eligible for inclusion, but none were identified.
Types of participants
All women who expected a vaginal birth at 24 weeks' gestation or later. We looked at women in higher‐income settings separately from women in lower‐income settings.
Types of interventions
(a) Active management of the third stage of labour, which is here defined as the package of interventions comprising:
the administration of a prophylactic uterotonic just before, with, or immediately after the birth of the baby;
early cord clamping and cutting (from immediately after the birth of the baby's head in the case of a nuchal cord, or immediately after the birth of the baby to, usually, within a minute of birth);
controlled cord traction to aid the delivery of the placenta.
(b) Expectant management of the third stage of labour, which is here defined as:
no prophylactic administration of a uterotonic;
the umbilical cord is neither clamped nor cut until the placenta has been delivered or until cord pulsation has ceased; and
the placenta is delivered spontaneously with the aid of gravity and sometimes by maternal effort.
None of the components of active management, described above, are employed routinely.
(c) Mixed management of the third stage of labour consists of a mixture of some of the components of both active and expectant management of the third stage, but without exclusively containing all the components of either (Table 2).
1. Terms and definitions used in this review.
| Terms | Definitions used in this review |
| Expectant management of third stage of labour |
|
| Active management of third stage of labour |
|
| Mixed management of third stage of labour | A mixture of some of the components of both active and expectant management of third stage of labour, but without exclusively containing all the components of either. There can be a number of different mixed third stage managements, for example:
|
| Early prophylactic uterotonic | Prophylactic uterotonic drug administered just before, with, or immediately after, the birth of the infant |
| Delayed prophylactic uterotonic | Prophylactic uterotonic drug administered after the cord pulsation has ceased |
| Early cord clamping | The application of a clamp to the umbilical cord within 60 seconds of the birth of the infant (McDonald 2013) |
| Delayed cord clamping | The application of a clamp to the umbilical cord more than 1 minute after birth or when cord pulsation has ceased (McDonald 2013) |
| Sarnat staging for hypoxic ischaemic encephalopathy (Sarnat 1976) |
|
Comparisons
Active versus expectant management of the third stage of labour: all women
Active versus expectant management of the third stage of labour: women at low risk of bleeding
Active versus mixed management of the third stage of labour, with early prophylactic uterotonic administration, delayed cord clamping and controlled cord traction
Active versus mixed management of the third stage of labour, with delayed prophylactic uterotonic administration, delayed cord clamping and controlled cord traction
Active versus mixed management of the third stage of labour, with delayed prophylactic uterotonic administration, delayed cord clamping, no controlled cord traction
Expectant versus mixed management of the third stage of labour, with early prophylactic uterotonic administration, delayed cord clamping and controlled cord traction
Expectant versus mixed management of the third stage of labour, with delayed prophylactic uterotonic administration, delayed cord clamping and controlled cord traction
Expectant versus mixed management of the third stage of labour, with delayed prophylactic uterotonic administration, delayed cord clamping, no controlled cord traction
Active versus mixed management of the third stage of labour with uterotonic after placental delivery, immediate cord clamping, no controlled cord traction
Active versus mixed management of the third stage of labour with no routine uterotonic, early cord clamping, no controlled cord traction
Active versus mixed management of the third stage of labour with no routine uterotonic, early cord clamping, and controlled cord traction
We included comparisons three to eight because of the review team's awareness of these different forms of clinical management of the third stage of labour and following the results of two reviews that indicated the benefits of delaying cord clamping for the baby (McDonald 2013; Rabe 2012). There are other variations of mixed management that could also be considered, for example, variations in controlled cord traction (Hofmeyr 2015), but we considered the above to be the most commonly used and thus important to review. We included comparisons nine to 11 because we added studies that had used these comparisons to this review update.
Types of outcome measures
We selected outcome measures in order of importance with due recognition of the core data set of outcome measures identified by Devane 2007.
Primary outcomes
Maternal
*Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss greater than or equal to 1000 mL)
*Very severe primary PPH at time of birth (clinically estimated or measured blood loss greater than or equal to 2500 mL)
Maternal mortality
Maternal Hb concentration less than 9 g/dL 24 to 72 hours postpartum
Infant
Admission to neonatal special care or intensive care unit
Neonatal jaundice requiring phototherapy or exchange transfusion
Neonatal polycythaemia treated with dilutional exchange transfusion
Secondary outcomes
Maternal
*Severe primary PPH after delivery of placenta and up to 24 hours (clinically estimated or measured blood loss greater than or equal to 1000 mL)
*Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss greater than or equal to 1000 mL)
*Primary blood loss equal to or greater than 500 mL at time of birth (clinically estimated or measured)
*Primary blood loss equal to or greater than 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured)
*Primary blood loss equal to or greater than 500 mL at time of birth and up to 24 hours (clinically estimated or measured)
*Mean blood loss (mL) at time of birth (clinically estimated or measured)
*Mean blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured).
*Mean blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured)
Maternal blood transfusion
Clinical signs of severe blood loss at the time of birth, for example, woman feeling breathless, weak, faint, pale, exhausted
Therapeutic uterotonics during the third stage or within the first 24 hours, or both
Mean length of the third stage (minutes)
Manual removal of the placenta as defined by study authors
Diastolic blood pressure greater than 90 mmHg between birth of baby and discharge from the labour ward
Vomiting between birth of baby and discharge from the labour ward
Any analgesia between birth of the baby and discharge from the labour ward
Women's assessment of pain during the third stage as reported by study authors
Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks)
Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital
Surgical evacuation of retained products of conception
Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period
Infant
Apgar score less than 7 at five minutes
Birthweight
Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1)
Neonatal mortality (not pre‐specified)
Intraventricular haemorrhage ‐ papillae grade III/IV (for infants born before 34 weeks' gestation only)
Number of infants who received a red blood cell transfusion
Infant Hb level at 24 to 72 hours
Infant Hb level at three to six months
Infant iron indices (ferritin) at three to six months
Exclusive breastfeeding at discharge
Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months
*All PPH amounts and mean blood losses are now expressed at three time periods, 'at the time of the birth', 'after delivery of the placenta and up to 24 hours', and 'at the time of birth and up to 24 hours'.
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (22 January 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains studies identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen search results and review the full text of all relevant study reports identified through the searching activities described above. Based on the intervention described, the Information Specialist assigns each study report a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and then adds it to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing study reports (22 January 2018) using the search methods described in Appendix 1.
Searching other resources
We retrieved additional relevant references cited in papers identified through the above search strategy and assessed their suitability for inclusion in the review.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Begley 2015.
For this update, we used the following methods for assessing the five reports that we identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
We obtained all potentially eligible studies identified by the search strategy as full‐text papers and two review authors assessed each study for potential inclusion. We resolved any disagreements through discussion with at least one additional review author. None of the potentially eligible studies required translation. We sought and received additional information from Yildirim 2016.
Data extraction and management
We used the data extraction form designed for the previous version of this review (Begley 2015), to extract data. Two review authors extracted the data independently from the included study using the form. We resolved discrepancies through discussion with at least one additional review author. In the previous version of this review, two review authors (Gillian Gyte (GG), Declan Devane (DD)), and a member of the Cochrane Pregnancy and Childbirth Group's staff independently reviewed Begley's paper (Begley 1990), and the lead author of this review was not involved in any discussions of the paper's inclusion, or assessment of its risk of bias status. We used the Review Manager software (Review Manager 2014), to enter all data, which were checked independently.
Assessment of risk of bias in included studies
Two review authors (GG and LB) independently assessed risk of bias as a measure of methodological quality of included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved discrepancies through discussion with at least one additional review author. When information regarding any of the criteria was unclear, we contacted the authors of the original reports to provide further details. Where these data were unobtainable, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by sensitivity analysis. We used the following criteria in the assessment of bias.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
It is not possible to blind participants or personnel in these trials, as the fact that a uterotonic has been given (rather than a placebo, or nothing) is usually apparent to both women (who feel a strong contraction or pain) and clinicians (who can see or feel a strongly contracted uterus) following injection of a uterotonic. In addition, it is clear, in many cases, to both women and clinicians if early versus late cord clamping is practised or if cord traction versus maternal effort is used. It is usually possible to blind technicians who conduct laboratory tests, but this does not always happen so cannot be presumed to be so.
We assessed the methods as:
high risk of bias for participants;
high risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. It is usually possible to blind technicians who conduct laboratory tests, but this does not always happen so cannot be presumed to be so.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete data collection (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study and for each outcome the completeness of data, including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or was sought and supplied by the trial authors, we re‐included missing data in the analyses. No studies required re‐analysis with the original allocated treatment groups being restored to their correct groups. Following these steps, studies were assessed as:
low risk of bias ‐ less than 10% attrition at any stage, or 10% to 15% attrition in small sections of data, equal in both groups and due to natural fall‐out of long‐term follow‐up;
high risk of bias ‐ more than 20% attrition, or more than 15% exclusion at any stage when the reason for missing data was likely to be related to true outcomes;
unclear risk of bias.
Acknowledging that with long‐term follow‐up, complete data are difficult to attain, we discussed whether missing data greater than 20% might (a) be reasonably expected, and (b) impact on outcomes; if the latter, we excluded such studies. We subjected studies where attrition levels were unclear, or missing data greater than 15% occurred, to sensitivity analysis.
(5) Selective reporting bias
We describe for each included study how we examined the possibility of selective outcome reporting bias and we assessed reporting methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would be expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We also assessed and describe for each included study any important concerns we had about other possible sources of bias (e.g. specific study design, trial stopped early; extreme baseline imbalances). We thus assessed studies as being:
low risk of bias;
high risk of bias (problems detailed);
unclear risk of bias.
(7) Overall risk of bias
We made explicit judgements about whether or not studies were, overall, at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions with reference to (1) to (6) above (Higgins 2017). As necessary, we explored the impact of the level of bias through undertaking sensitivity analyses.
Assessment of the quality of the evidence using GRADE
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison (GRADE 2013).
Maternal *severe primary PPH at time of birth (clinically estimated or measured blood loss greater than or equal to 1000 mL)
*Very severe primary PPH at time of birth (clinically estimated or measured blood loss greater than or equal to 2500 mL)
Maternal mortality
Maternal Hb concentration less than 9 g/dL 24 to 72 hours postpartum
Infant admission to neonatal special care or intensive care unit
Neonatal jaundice requiring phototherapy or exchange transfusion
Neonatal polycythaemia treated with dilutional exchange transfusion
We used the GRADEpro Guideline Development Tool (GRADEpro GDT 2015), to import data from Review Manager 5 (Review Manager 2014), in order to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) as outcomes were measured in the same way between studies. We planned to use the standardised mean difference (SMD) to combine studies that measured the same outcome, but used different scales, but this was not required.
Unit of analysis issues
Cluster‐randomised trials
We identified no cluster‐randomised trials in this review.
In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised studies. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (section 16.3.4 or 16.3.6), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the study (if possible), from a similar study or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised studys, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This is not an eligible study design for this review.
Dealing with missing data
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised, and there was sufficient information in the study report or in information obtained from the study authors, we planned to restore them to the correct group and analyse accordingly (i.e. intention‐to‐treat (ITT) analysis). No studies required re‐analysis with the original allocated treatment groups being restored to their correct groups. We used the number of women randomised minus the number of participants known to have missing data as the denominators. Where loss to follow‐up was greater than 20%, or where study authors had excluded participants at a level greater than 15% and for reasons that were deemed to impact on outcomes, we excluded that study.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003), and Chi² statistics. We regarded heterogeneity as substantial if the Tau² was greater than zero and either an I² statistic was greater than 30% or there was a low P value (< 0.10) in the Chi² test for heterogeneity (Deeks 2017).
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would have performed exploratory analyses to investigate it (Sterne 2017).
Where we suspected reporting bias (see 'Selective reporting bias' above), we contacted study authors asking them to provide missing outcome data. If this had not been possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (Review Manager 2014). We used random‐effects meta‐analyses for combining data because we considered that there was clinical heterogeneity sufficient to expect that the underlying treatment effects would differ between studies. We treated the random‐effects summary as the average of the range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between studies. If we had considered that the average treatment effect was not clinically meaningful, we would not have combined studies. We have presented the results as the average treatment effect with its 95% CI, and the estimates of Tau², P value for the Chi² test and I² statistic (Deeks 2017). We found significant clinical and methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between studies, which supported our choice of random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We did not undertake any interaction tests (Deeks 2001), as we were unable to conduct subgroup analyses as planned, due to lack of usable data. We had planned the following subgroup analyses:
spontaneous versus operative vaginal birth;
nulliparous versus multiparous women;
lower‐income versus higher‐income setting;
full‐term versus preterm birth (including outcomes specific to preterm babies).
For this update, we deleted the fifth subgroup analysis, ‘low risk of PPH versus high risk of PPH’, as we realised that the analysis we had undertaken of the low‐risk group was in fact a separate comparison rather than a subgroup analysis. We decided to split the countries into 'higher‐income' and 'lower‐income', based on the World Bank definitions, with the border between lower‐middle‐income and upper‐middle‐income being the cut‐off. All the included studies in the main analysis were undertaken in higher‐income countries (defined according to World Bank definitions 2018).
Sensitivity analysis
We performed sensitivity analysis based on study quality, separating high‐quality studies from studies of lower quality. 'High quality' was, for the purposes of this sensitivity analysis, defined as a study having adequate sequence generation, allocation concealment and an attrition rate of less than 20%, given the stated importance of attrition as a quality measure (Tierney 2005).
Results
Description of studies
Results of the search
See: Figure 1.
1.
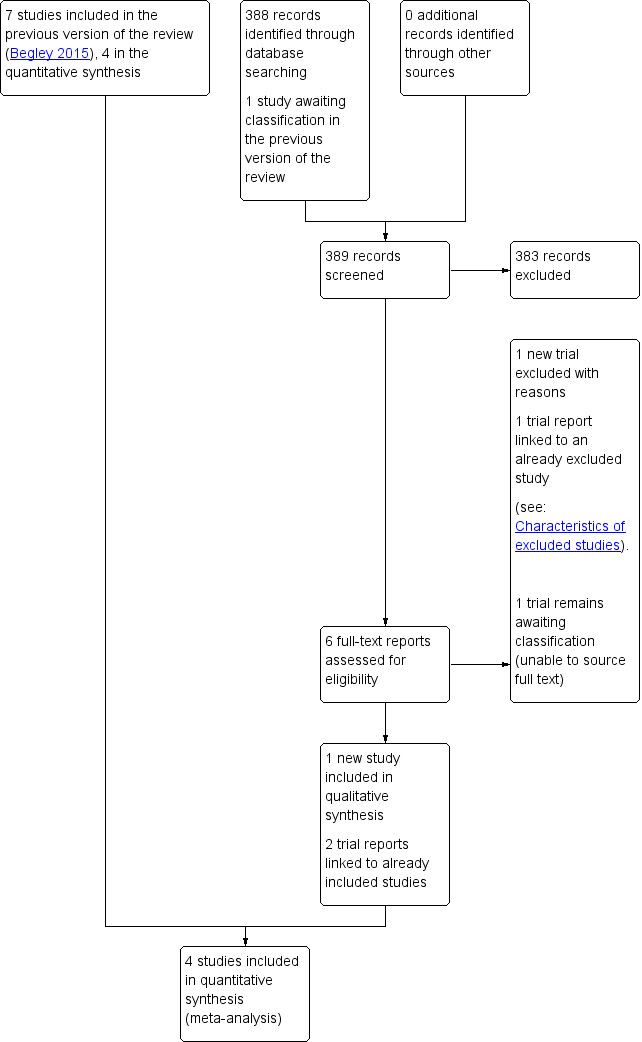
Study flow diagram
The updated 2018 search retrieved five records to assess and we also re‐assessed one report awaiting classification in the previous section of the review (Rosario 1973). We included one new study (Yildirim 2016), excluded one study (Neri‐Mejia 2016) and linked three clinical trials registry reports to studies already assessed (Hoffman 2006; Jangsten 2011; Rogers 1998). We are still unable to source the full‐text of Rosario 1973.
Included studies
We included eight studies involving 8892 women (analysed) (Begley 1990; Jangsten 2011; Jerbi 2007; Khan 1997; Prendiville 1988; Rogers 1998; Thilaganathan 1993; Yildirim 2016). Included studies were conducted in the UK (Prendiville 1988; Rogers 1998; Thilaganathan 1993), Abu Dhabi (Khan 1997), Ireland (Begley 1990), Sweden (Jangsten 2011), Tunisia (Jerbi 2007), and Turkey (Yildirim 2016) . All studies took place in hospital settings. (See Characteristics of included studies.)
Four studies (4829 women) compared active versus expectant management (Begley 1990; Prendiville 1988; Rogers 1998; Thilaganathan 1993), and four studies (4063 women) compared active versus mixed management (Jangsten 2011; Jerbi 2007; Khan 1997; Yildirim 2016). In all studies, participants were healthy pregnant women expected to give birth vaginally. Four studies included only women classified as being at low risk of bleeding or its effects (Begley 1990; Rogers 1998; Thilaganathan 1993; Yildirim 2016), and four (Jangsten 2011; Jerbi 2007; Khan 1997; Prendiville 1988) included women irrespective of their risk of bleeding.
Studies were conducted from January 1986 to January 1987 (Prendiville 1988); from October 1987 to October 1988 (Begley 1990); from January 1988 to February 1990 (Thilaganathan 1993); June 1993 to December 1995 (Rogers 1998); January to June 1995 (Khan 1997); February to March 2005 (Jerbi 2007); November 2006 to April 2008 (Jangsten 2011); and “in 2010” (Yildirim 2016). Funding sources were; the Maternity and Child Division at the World Health Organization, Geneva, and support for the National Perinatal Epidemiology Unit from the DHSS (Prendiville 1988); the Research and Development Trust of the Coombe Hospital (Begley 1990); the Public Health and Operational Research Committee of the Anglia and Oxford Regional Health Authority, and support for the National Perinatal Epidemiology Unit from the DHSS (Rogers 1998); Research and Development Board in Göteborg and Bohuslän Baby Bag and the SU foundation (Jangsten 2011); and Kanuni Sultan Suleyman Education and Research Hospital (Yildirim 2016). Three studies gave no information on funding (Jerbi 2007; Khan 1997; Thilaganathan 1993). Only two studies gave any declarations of interest (Jangsten 2011; Yildirim 2016), both stating that there were none. We noted considerable differences in the protocols for both active and expectant management in the various studies (Table 3).
2. Varying managements used in studies in the main analysis compared with the study's planned regime of management.
| Study | Active management protocol | Expectant management protocol | Active management used | Expectant management used |
| Begley 1990 |
|
|
|
|
| Prendiville 1988 |
|
|
|
|
| Rogers 1998 |
|
|
|
|
| Thilaganathan 1993 |
|
|
No information | No information |
| BP: blood pressure; CCT: controlled cord traction; IM: intramuscular; IV: intravenous | ||||
Interventions in the ‘active’ management groups
The studies used various uterotonic regimens. These were intravenous (IV) ergometrine 0.5 mg (Begley 1990), intramuscular (IM) syntometrine (5 units oxytocin + 0.5 mg ergometrine; Thilaganathan 1993), IM syntometrine (5 units oxytocin + 0.5 mg ergometrine) or IM 10 units oxytocin if the woman had raised blood pressure (Prendiville 1988; Rogers 1998), IM 10 units oxytocin for all women (Khan 1997; Yildirim 2016), IV oxytocin 5 units (Jerbi 2007) and IV oxytocin 10 units (Jangsten 2011). The descriptions of the timing of administration of uterotonic agent also varied and included, “at the delivery of the anterior shoulder”, “as soon as possible after birth of anterior shoulder”, “immediately after the birth of the anterior shoulder” (which in practice probably equates to the same time), “immediately following birth”, “as soon as baby is born” (which is, in practice, very similar in timing to the preceding descriptions, perhaps 10 to 20 seconds later), "within the first minute after delivery" and "within 2 minutes of birth".
All studies stated that the cord was clamped and cut either within 30 seconds or “immediately” or "early", which in practice is likely to be approximately similar timing. All studies attempted controlled cord traction once the uterus was contracted. Two studies included maternal effort as an option (Jangsten 2011; Rogers 1998), and one included fundal pressure (Jerbi 2007).
Protocols in the ‘expectant’ management groups
In all studies, no uterotonic was to be given routinely prior to delivery of the placenta. However, in one study, an IV infusion of oxytocin 10 units in 500 mL normal saline was given slowly to all women following delivery of the placenta (Khan 1997). One study administered 2 mL of placebo (saline solution) intravenously within two minutes (Jangsten 2011). Practice varied widely as to how many women did, in fact, receive a uterotonic, either prophylactically: 0% (Begley 1990), 2.5% (Rogers 1998), and 20% (Jangsten 2011; Prendiville 1988), and/or as a treatment 9% (Yildirim 2016), 14% (Begley 1990), 21% (Rogers 1998), 30% (Prendiville 1988), and 38% (Jangsten 2011), with no information given in the other three studies.
In four studies, clinicians were asked to try not to cut or clamp the cord until after pulsation ceased (Begley 1990; Prendiville 1988; Rogers 1998; Thilaganathan 1993), although this was achieved in only 42% to 70% of participants. In one study the cord was clamped after "cord pulsation had slowed down " (Yildirim 2016). In three studies, the cord was to be clamped and cut after birth of the baby (Jangsten 2011; Jerbi 2007; Khan 1997). Maternal effort was to be used in six studies (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998; Thilaganathan 1993, Yildirim 2016), with the option in some of gentle controlled cord traction once the placenta had separated (Begley 1990), or assisting the placenta out once it was felt in the vagina (Thilaganathan 1993). Maternal effort was used by 32% to 88% of participants, across the six studies. One study used controlled cord traction with gentle fundal pressure (Jerbi 2007), and another used uterine massage after placental delivery (Jangsten 2011).
Given the differences in uterotonics used in the active groups and the wide variation in the proportion of women in expectant management groups who actually received a uterotonic, it was decided to use a random‐effects model due to the degree of clinical heterogeneity.
Excluded studies
We excluded 10 studies (Abdel‐Aleem 2010; Deneux‐Tharaux 2013; Gulmezoglu 2012; Hoffman 2006; Kashanian 2010; Magann 2006; Muller 1996; Ramirez 2001; Vasegh 2005Neri‐Mejia 2016 (see Characteristics of excluded studies)). One study was only available as a conference abstract with no information on the number of women randomised to each group, and the authors of the previous version of the review had been unable to obtain further information from the study authors (Muller 1996). Although we were able to contact one of the authors, we obtained no further useful information. One study assessed the timing for manual removal of the placenta, so did not fit the criteria for inclusion (Magann 2006). We excluded the third study because of the high number of women excluded after randomisation (48%) (Kashanian 2010). We excluded the fourth study (Hoffman 2006), due to concerns regarding the number of women withdrawn, after randomisation, due to caesarean section. Only a conference abstract was available, but we obtained further information on methodology from the authors. We excluded the fifth study due to insufficient information on the numbers included in each of the three arms, and the method of management for the expectant arm (Ramirez 2001). We excluded Vasegh 2005 due to insufficient information in the published study and inability to elicit a response from the authors. One study (Neri‐Mejia 2016), evaluated three different types of oxytocin administration (IM, IV and infusion) and two studies that looked at active management with or without controlled cord traction (Deneux‐Tharaux 2013; Gulmezoglu 2012), were excluded as we deemed them more appropriate for inclusion in the Cochrane Review on controlled cord traction (Hofmeyr 2015).
Risk of bias in included studies
Other than for the medication, it is not possible to blind personnel and participants to active or expectant management of the third stage. None of the studies used a placebo, and so we assessed all studies as high risk of bias for blinding. Of the eight studies included, we considered three studies as having low risk of bias in the main aspects of sequence generation, allocation concealment and completeness of data collection (Begley 1990; Jangsten 2011; Rogers 1998), these being our criteria for overall quality for sensitivity analyses. We assessed one study as having low risk of bias in sequence generation and allocation concealment, and high risk of bias for lack of blinding and other biases (Prendiville 1988). We considered one study at high risk of bias for completeness of data collection, lack of blinding and other biases and 'unclear' for allocation concealment and selective reporting (Thilaganathan 1993), and assessed one study as unclear on five of the assessment criteria (Khan 1997). We assessed one study as unclear for sequence generation and blinding of outcome assessment, at high risk of bias for allocation concealment, blinding of participants, selective reporting and other biases, but acceptable for completeness of data (Jerbi 2007), and the final study was unclear on four of the assessment criteria, at high risk of bias for blinding of participants but had a low risk of bias for sequence generation and completeness of data collection (Yildirim 2016). See Figure 2 and Figure 3 for a summary of risk of bias assessments.
2.
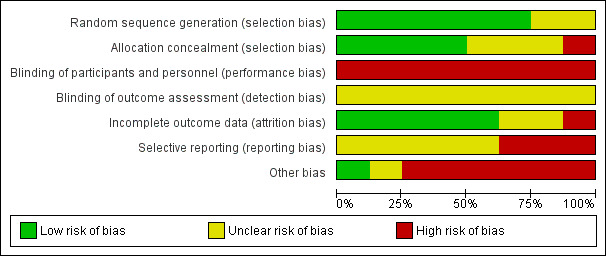
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
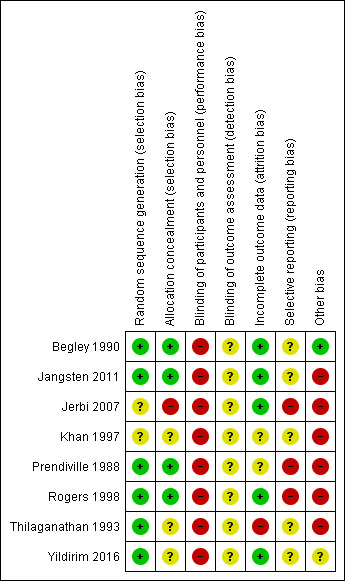
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six studies used adequate sequence generation using random‐number tables or computer random‐number generators (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998; Thilaganathan 1993; Yildirim 2016) and in two the method was unclear (Jerbi 2007; Khan 1997). We judged allocation concealment as low risk of bias in four studies (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998), and as unclear risk in three (Khan 1997; Thilaganathan 1993; Yildirim 2016). One study did not conceal allocation and so we assessed it as high risk of bias (Jerbi 2007).
Blinding
Blinding was not possible when assessing the management of third stage of labour, for either women or clinicians (Characteristics of included studies). The assessment of many outcomes, particularly blood loss, could, therefore, have been unconsciously affected by people's beliefs. Having chosen maternal Hb less than 9 g/dL as a hard outcome relating to blood loss at the protocol stage, we have now also included the mean postnatal Hb values to help in understanding the blood loss estimations. Haemoglobin assessment would usually be performed by a technician who would be blinded to the study allocation.
Begley 1990 and Jangsten 2011, the Dublin and Swedish studies respectively, measured blood loss, but all the other studies estimated it, and are therefore open to subjective inaccuracies, which should, however, have been the same across both groups; in addition, both blood loss estimation and measurement were open to bias. For certain outcomes such as Hb concentration, which could be measured by a blinded outcome assessor, we attempted to assess how such blinding had occurred. In practice, we found that almost all studies did not mention how they blinded such assessors.
Incomplete outcome data
Five studies presented complete outcome data (Begley 1990; Jangsten 2011; Jerbi 2007; Rogers 1998; Yildirim 2016), with acceptable levels of attrition except for some follow‐up measures, such as postnatal Hb levels. However, one of these studies excluded women post randomisation (2.2%) who had a postpartum haemorrhage (PPH) due to deep vaginal lacerations and so we assessed it as being at unclear risk of bias (Yildirim 2016). We considered one study at high risk of bias for complete data in that it was not clear how many women were randomised, and an unknown number of women were withdrawn following randomisation, due to caesarean section, operative delivery and cervical tears (Thilaganathan 1993). One study had high levels of missing data for some outcomes, for example, 19% of Hb results missing in the active arm and 18% in the expectant, but for other outcomes data were more complete and so we assessed this study as being at unclear risk of bias (Prendiville 1988). In the remaining study, it was also unclear how many data were missing (Khan 1997). In all three of these studies (Khan 1997; Prendiville 1988; Thilaganathan 1993), and in the study that excluded women with deep vaginal lacerations (Yildirim 2016), the denominator used was the number given by study authors as taking part in the study after withdrawals had been made.
Selective reporting
We did not assess any studies as free of selective reporting bias. We categorised five as 'unclear' (Begley 1990; Jangsten 2011; Khan 1997; Thilaganathan 1993; Yildirim 2016), as it was not apparent from the published papers that they had reported all outcomes, and we were unable to check study protocols. We judged three studies as high risk of reporting bias due to some outcomes being reported which were not listed in the methods section (Jerbi 2007; Prendiville 1988; Rogers 1998).
Other potential sources of bias
We judged one study as free of other apparent sources of bias (Begley 1990). One of the remaining studies (Prendiville 1988), included women at increased risk of PPH (high parity, all age groups, previous PPH, epidural, long labour, operative delivery). This was also a problem with another study (Khan 1997). Women at increased risk of PPH will have a higher blood loss, by definition, using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even of low‐risk women, which may result in higher intervention (mixed management) rates. In Prendiville 1988, 50% of the expectant management group received an oxytocic, a proportion of intervention incompatible with the philosophy of expectant management. In one study, although 11,000 women were available, of whom at least half would usually be considered potentially eligible, only 1802 were entered into the study (Jangsten 2011). The majority were excluded due to "excessive workload". This has the potential to have biased the study, as midwives would have had the choice of not asking the women to participate and may unconsciously have not offered participation to some women who they felt were not suitable for expectant management. Jerbi 2007 reported that women were allocated to the two groups after placental delivery, yet they described the active management group as having had oxytocin with the anterior shoulder. One other study had no power calculation, did not use a null hypothesis, and the study groups were quite different sizes (103 and 90). In addition, they described the variables age, birthweight and parity as equal between the groups but did not give any details (Thilaganathan 1993).
Midwives in all studies, (except Yildirim 2016), were more used to using active than expectant management, which is likely to have had an influence on results in the expectant arm. This influence may have been that they a) reverted to a type of active management, potentially reducing blood loss and narrowing the difference between study arms in terms of blood loss outcomes or b) used mixed management, which, from the data, was more likely to increase blood loss or c) would have conducted a type of expectant management that was not ideal and resulted in increased blood loss in the expectant arm. Rogers 1998 administered a questionnaire to 92 of the 153 midwives prior to the study commencement, which showed that 84% felt "very confident" of active management, whilst only 41% were "very confident" of expectant management. Similarly, Prendiville 1988 states that, before the study commenced, the midwives were trained in the use of expectant management. Only six (13%), however, said that they were very confident in using expectant management before the study started and 22 (46%) afterwards. In addition, of 49 midwives responding to a questionnaire regarding this study, 30 (61%) had never managed a third stage expectantly. Among the remaining 19, only one had practised expectant management as defined in the report (Harding 1989). In Begley 1990, the PPH rate in the expectant arm fell during the study from 21% in the pilot study and 12% over the first four months, to 7% in the last six months, as midwives developed their skill (Begley 1990). In contrast to training in the use of expectant management, additional information provided by the lead author of one study (Yildirim 2016), noted that they had to train practitioners for "the active management protocol (especially for controlled cord traction)". The other studies did not provide any information on skill levels, nor on whether practitioners had training in both expectant and active management of third stage of labour.
Finally, Prendiville 1988 changed the protocol after 425 births, but included all births in the results, which may have affected the findings.
Effects of interventions
See: Table 1
The review includes eight studies involving 8892 women. We used a random‐effects model for pooling data because of clinical heterogeneity seen in the included studies. In the forest plots, for six of the outcomes the "Favours expectant" label is on the left rather than the right. This was dictated by whether we are reporting negative (e.g. PPH) or positive (e.g. breastfeeding) outcomes.
1. Active versus expectant management of third stage of labour: all women (four studies, 4829 women)
This comparison included four studies (Begley 1990; Prendiville 1988; Rogers 1998; Thilaganathan 1993). Three studies included only women at low risk of bleeding (Begley 1990; Rogers 1998; Thilaganathan 1993) and one study included women irrespective of risk of bleeding (Prendiville 1988). We assessed two studies as being of high quality (Begley 1990; Rogers 1998); one raised concerns regarding high risk of bias in terms of midwives' comfort with expectant management, and other possible biases (Prendiville 1988); and we considered one study to have high risk of bias in terms of incomplete outcome data and selective reporting bias (Thilaganathan 1993). We used random‐effects meta‐analyses due to the clinical heterogeneity involved. The random‐effects summary gives an average for 'active' methods versus 'expectant' methods, and it is important to note that the treatment effect found by comparing any two specific techniques may differ from this. For a number of outcomes, there was very little statistical heterogeneity found (Tau² = 0 and I² = 0%), so there appears to be a single common treatment effect for these outcomes. We have assessed the overall quality of the evidence using GRADE (GRADE 2013; Table 1).
Primary outcomes
For women
Compared with expectant management, it is uncertain whether active management reduces the incidence of severe PPH:
severe primary PPH, 1000 mL or more at time of birth (average risk ratio (RR) 0.34, 95% confidence interval (CI) 0.14 to 0.87, 3 studies, 4636 women, random‐effects (Tau² = 0.38, Chi² P = 0.08, I² = 60%) very low‐quality evidence; Analysis 1.1).
1.1. Analysis.
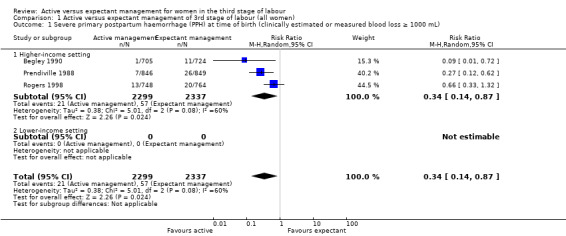
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 1 Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
However, active management may reduce the number of women who are anaemic after birth:
maternal Hb less than 9 g/dL at 24 to 72 hours (average RR 0.50, 95% CI 0.30 to 0.83, 2 studies, 1572 women, random‐effects (Tau² = 0.02, Chi² P = 0.31, I² = 3%) low‐quality evidence; Analysis 1.4).
1.4. Analysis.
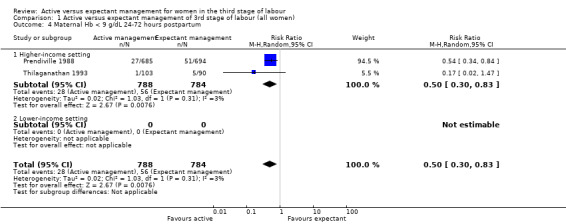
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 4 Maternal Hb < 9 g/dL 24‐72 hours postpartum.
None of the studies reported on the other primary maternal outcomes of very severe primary PPH (≥ 2500 mL), except Begley 1990, who found no instances of such extreme blood loss in either group, or maternal mortality.
For babies
Compared with expectant management, active management may make little or no difference to:
admission to neonatal special care or intensive care unit (average RR 0.81, 95% CI 0.60 to 1.11, 2 studies, 3207 infants, random‐effects (Tau² = 0.00, Chi² P = 0.40, I² = 0%) low‐quality evidence; Analysis 1.5);
1.5. Analysis.
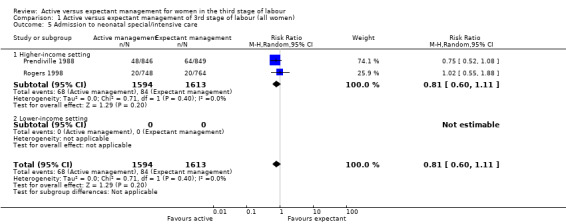
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 5 Admission to neonatal special/intensive care.
and it is uncertain whether active management reduces or increases the number of babies with jaundice requiring treatment:
neonatal jaundice requiring phototherapy or exchange transfusion (average RR 0.96, 95% CI 0.55 to 1.68, 2 studies, 3142 infants, random‐effects (Tau² = 0.11, Chi² P = 0.09, I² = 66%) very low‐quality evidence; Analysis 1.6).
1.6. Analysis.
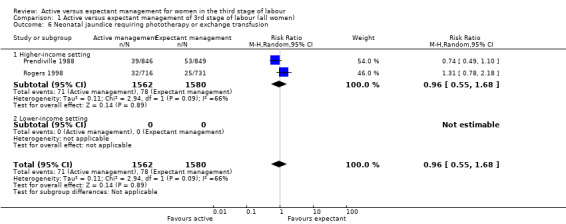
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 6 Neonatal jaundice requiring phototherapy or exchange transfusion.
None of the studies reported on the other primary neonatal outcome of neonatal polycythaemia treated with dilutional exchange transfusion.
It should be noted that the evidence presented on the primary outcomes selected is based on results of a small number of studies with relatively small numbers of participants. The lack of consistent quality of evidence for these outcomes should be borne in mind when considering the overall results (see Table 1).
Secondary outcomes
Compared with expectant management, active management (with high‐quality evidence) reduces:
mean maternal blood loss (mean difference (MD) in mL −78.80, 95% CI −95.96 to −61.64, 2 studies, 2941 women, random‐effects (Tau² = 34.93, Chi² P = 0.26, I² = 22%) Analysis 1.13).
1.13. Analysis.
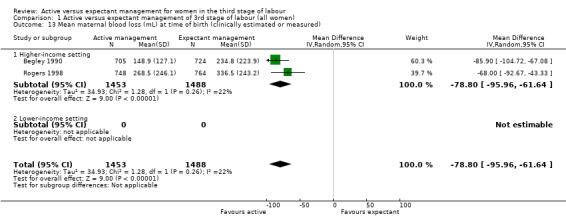
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured).
Also, active management (with moderate‐quality evidence) probably reduces:
primary blood loss 500 mL or more, clinically estimated or measured at birth (average RR 0.34, 95% CI 0.27 to 0.44, 3 studies, 4636 women, random‐effects (Tau² = 0.02, Chi² P = 0.23, I² = 32%) Analysis 1.10);
therapeutic uterotonics during the third stage and/or within the first 24 hours (average RR 0.19, 95% CI 0.15 to 0.23, 4 studies, 4829 women, random‐effects (Tau² = 0.00, Chi² P = 0.47, I² = 0%) Analysis 1.18);
mean birthweight (MD in g −76.90, 95% CI −108.51 to −45.30, 2 studies, 3207 infants, random‐effects (Tau² = 0.00, Chi² P = 0.58, I² = 0%) Analysis 1.30).
1.10. Analysis.
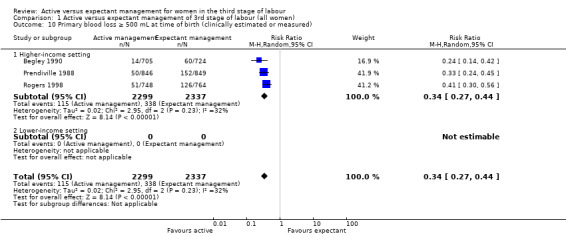
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
1.18. Analysis.
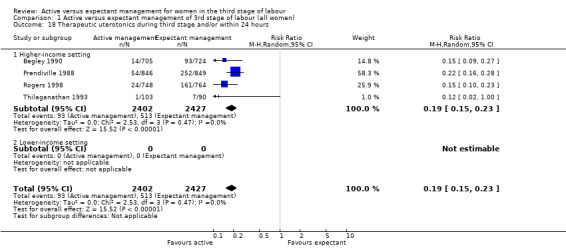
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
1.30. Analysis.
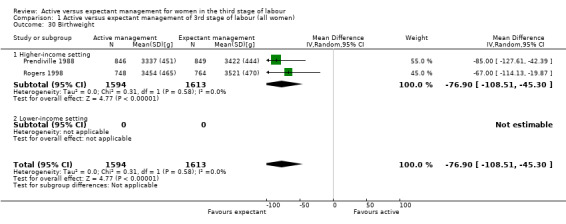
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 30 Birthweight.
In addition, active management (with low‐quality evidence) may reduce:
maternal blood transfusion (average RR 0.35, 95% CI 0.22 to 0.55, 4 studies, 4829 women, random‐effects (Tau² = 0.00, Chi² P = 0.46, I² = 0%) Analysis 1.16).
1.16. Analysis.
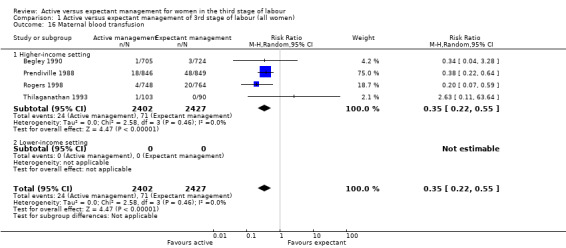
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 16 Maternal blood transfusion.
Compared with expectant management, active management (with low‐quality evidence) may increase:
postnatal diastolic blood pressure more than 90 mmHg up to discharge from labour ward (average RR 4.10, 95% CI 1.63 to 10.30, 3 studies, 4636 women, random‐effects (Tau² = 0.32, Chi² P = 0.14, I² = 49%) Analysis 1.21);
vomiting from birth of baby to discharge from labour ward (average RR 2.47, 95% CI 1.36 to 4.48, 3 studies, 4636 women, random‐effects (Tau² = 0.14, Chi² P = 0.09, I² = 59%) Analysis 1.22);
administration of any analgesia from birth up to discharge from labour ward (RR 2.53, 95% CI 1.34 to 4.78, 1 study, 1429 women; Analysis 1.23);
afterpains (RR 2.53, 95% CI 1.34 to 4.78, 1 study, 1429 women; Analysis 1.28);
return to hospital as an in‐ or outpatient because of bleeding (outcome not pre‐specified) (average RR 2.21, 95% CI 1.29 to 3.79, 2 studies, 2941 women, random‐effects (Tau² = 0.00, Chi² P = 0.82, I² = 0%) Analysis 1.40);
postnatal maternal Hb (outcome not pre‐specified) (MD 0.52, 95% CI 0.44 to 0.60, 3 studies, 4062 women, random‐effects (Tau² = 0.00, Chi² P = 0.66, I² = 0%) Analysis 1.41).
1.21. Analysis.
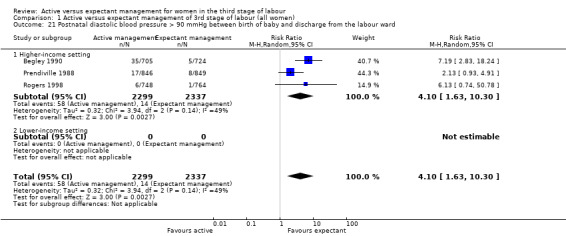
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
1.22. Analysis.
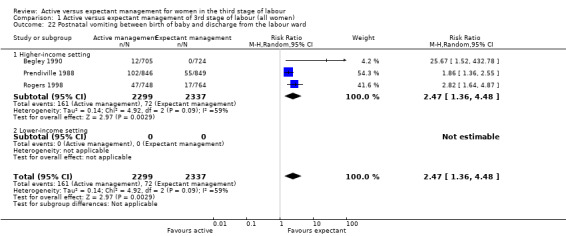
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 22 Postnatal vomiting between birth of baby and discharge from the labour ward.
1.23. Analysis.
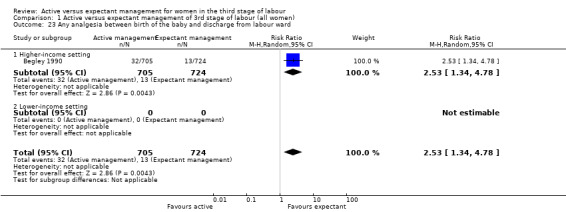
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 23 Any analgesia between birth of the baby and discharge from labour ward.
1.28. Analysis.
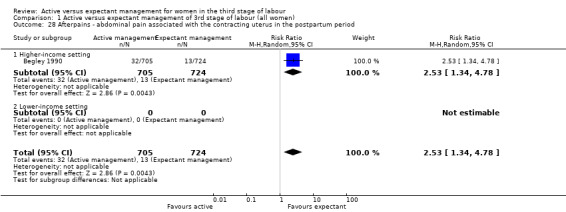
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period.
1.40. Analysis.
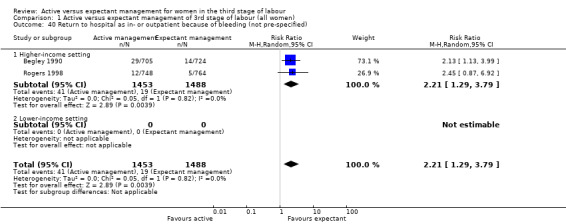
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified).
1.41. Analysis.
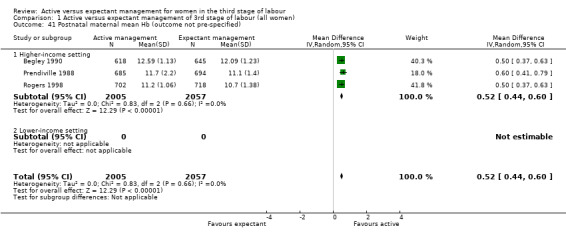
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 41 Postnatal maternal mean Hb (outcome not pre‐specified).
Compared with expectant management, active management (with moderate‐quality evidence) probably makes little or no difference to:
exclusive breastfeeding at discharge (RR 1.01, 95% CI 0.96 to 1.07, 1 study, 1695 women; Analysis 1.38).
1.38. Analysis.
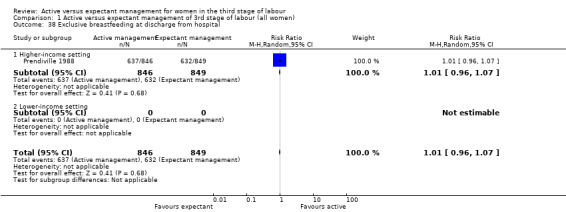
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 38 Exclusive breastfeeding at discharge from hospital.
Also, active management (with low‐quality evidence) may make little or no difference to:
mean length of the third stage in minutes (MD −0.30, 95% CI −1.87 to 1.27, 1 study, 1429 women; Analysis 1.19);
secondary blood loss/any vaginal bleeding needing treatment after 24 hours and before six weeks (average RR 1.10, 95% CI 0.40 to 2.99, 3 studies, 4636 women, random‐effects (Tau² = 0.67, Chi² P = 0.0005, I² = 87%) Analysis 1.25);
surgical evacuation of retained products of conception (average RR 0.74, 95% CI 0.32 to 1.71, 3 studies, 4636 women, random‐effects (Tau² = 0.26, Chi² P = 0.15, I² = 47%) Analysis 1.27).
1.19. Analysis.
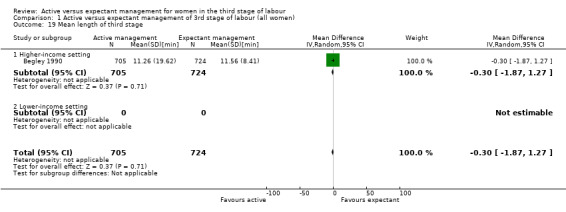
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 19 Mean length of third stage.
1.25. Analysis.
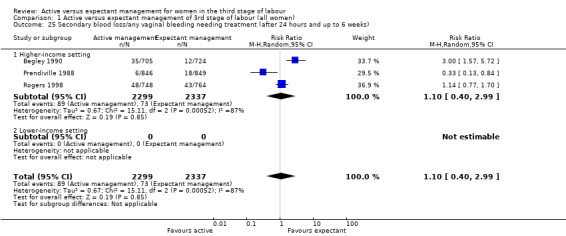
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks).
1.27. Analysis.
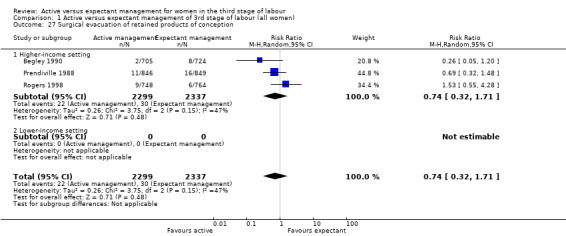
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 27 Surgical evacuation of retained products of conception.
In addition, we are uncertain if active management (with very low‐quality evidence) improves the incidence of:
manual removal of placenta (average RR 1.78, 95% CI 0.57 to 5.56, 4 studies, 4829 women, random‐effects (Tau² = 0.82, Chi² P = 0.01, I² = 73%) Analysis 1.20);
Apgar scores less than 7 at five minutes (RR 1.00, 95% CI 0.38 to 2.66, 1 study, 1695 infants; Analysis 1.29).
1.20. Analysis.
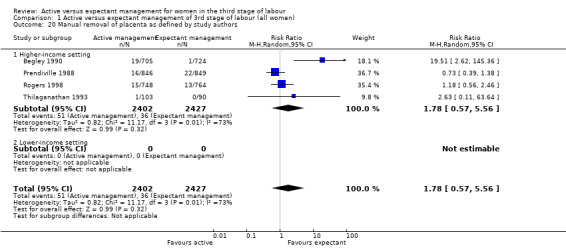
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 20 Manual removal of placenta as defined by study authors.
1.29. Analysis.
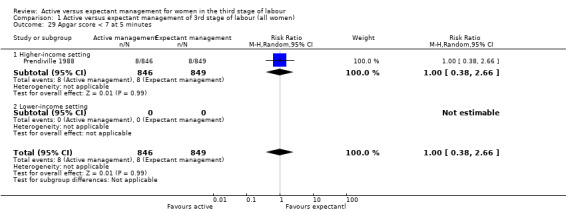
Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 29 Apgar score < 7 at 5 minutes.
Authors of the included studies did not assess any of the review's other secondary outcomes.
2. Active versus expectant management of third stage of labour: women at low risk of bleeding (three studies, 3134 women)
This comparison included three studies (Begley 1990; Rogers 1998; Thilaganathan 1993). We considered two studies to be of high methodological quality (Begley 1990; Rogers 1998) and one study to have high risk of bias in terms of incomplete outcome data and selective reporting bias (Thilaganathan 1993). All meta‐analyses used random‐effects meta‐analyses due to the clinical heterogeneity involved. For a number of outcomes, there was very little heterogeneity found (Tau² = 0 and I² = 0%), so there appears to be a single common treatment effect for these outcomes.
Primary outcomes
For women at low risk of PPH: compared with expectant management, it is uncertain whether active management (with very low‐quality evidence) reduces:
severe primary PPH, 1000 mL or more at time of birth (average RR 0.31, 95% CI 0.05 to 2.17, 2 studies, 2941 women, random‐effects (Tau² = 1.46, Chi² P = 0.06, I² = 71%) Analysis 2.1);
maternal Hb less than 9 g/dL at 24 to 72 hours (RR 0.17, 95% CI 0.02 to 1.47, 1 study, 193 women; Analysis 2.4).
2.1. Analysis.
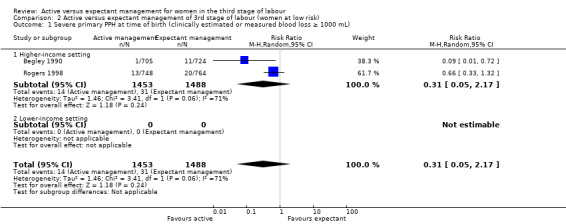
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
2.4. Analysis.
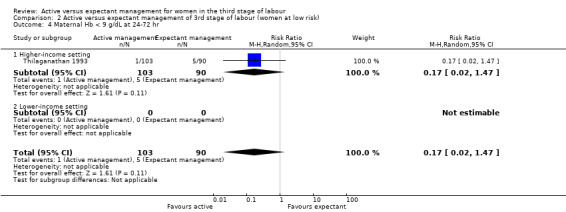
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 4 Maternal Hb < 9 g/dL at 24‐72 hr.
None of the studies reported the other primary outcomes of: very severe primary PPH (≥ 2500 mL) except Begley 1990, who found no instances of such extreme blood loss in either group, or maternal mortality.
Also, active management (with low‐quality evidence) may make little or no difference to:
neonatal jaundice requiring phototherapy or exchange transfusion (RR 1.31, 95% CI 0.78 to 2.18, 1 study, 1447 infants; Analysis 2.6).
2.6. Analysis.
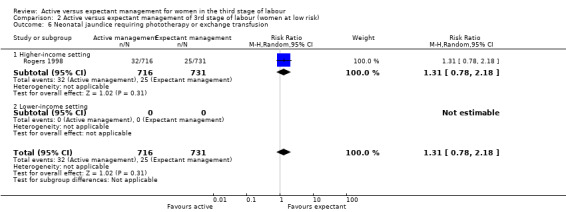
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 6 Neonatal jaundice requiring phototherapy or exchange transfusion.
In addition, it is uncertain if active management (with very low‐quality evidence) makes any difference to:
admission to neonatal special care or intensive care unit (RR 1.02, 95% CI 0.55 to 1.88, 1 study, 1512 infants; Analysis 2.5).
2.5. Analysis.
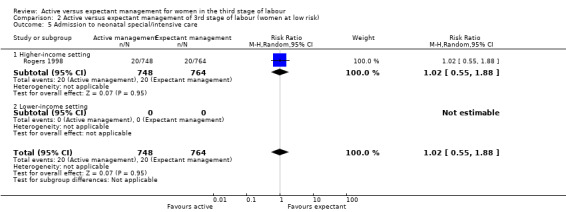
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 5 Admission to neonatal special/intensive care.
None of the studies reported the other primary outcome of neonatal polycythaemia.
Secondary outcomes
For women at low risk of PPH: compared with expectant management, active management (with moderate‐quality evidence) probably reduces:
therapeutic uterotonics during the third stage and/or within the first 24 hours (average RR 0.15, 95% CI 0.11 to 0.21, 3 studies, 3134 women, random‐effects (Tau² = 0.00, Chi² P = 0.98, I² = 0%) Analysis 2.18).
2.18. Analysis.
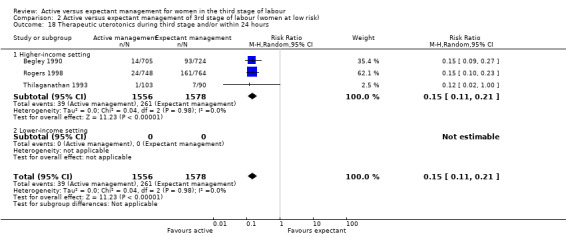
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
Also, active management (with low‐quality evidence) may reduce:
primary blood loss 500 mL or more, clinically estimated or measured at time of birth (average RR 0.33, 95% CI 0.20 to 0.56, 2 studies, 2941 women, random‐effects (Tau² = 0.10, Chi² P = 0.10, I² = 63%) Analysis 2.10);
mean maternal blood loss (mL) (MD −78.80, 95% CI −95.96 to −61.64, 2 studies, 2941 women, random‐effects (Tau² = 34.93, Chi² P = 0.26, I² = 22%) Analysis 2.13);
maternal blood transfusions (average RR 0.30, 95% CI 0.10 to 0.88, 3 studies, 3134 women, random‐effects (Tau² = 0.14, Chi² P = 0.32, I² = 11%) Analysis 2.16);
mean birthweight in g (MD −67.00, 95% CI −114.13 to −19.87, 1 study, 1512 infants; Analysis 2.30).
2.10. Analysis.
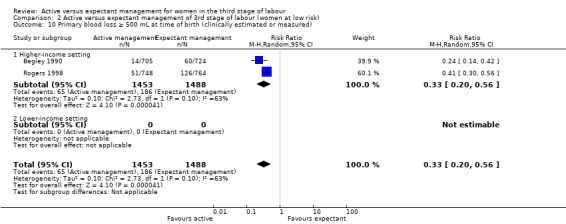
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
2.13. Analysis.
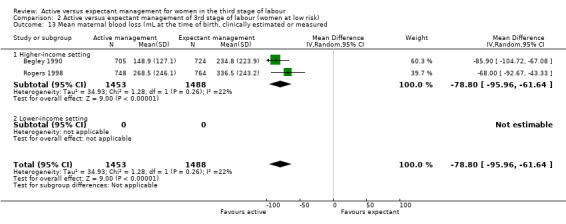
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 13 Mean maternal blood loss (mL at the time of birth, clinically estimated or measured.
2.16. Analysis.
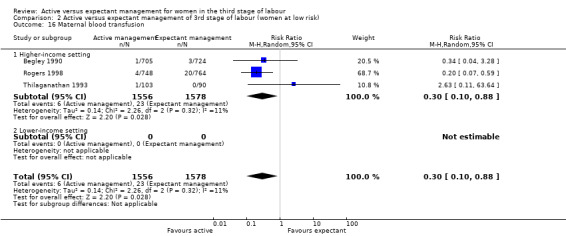
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 16 Maternal blood transfusion.
2.30. Analysis.
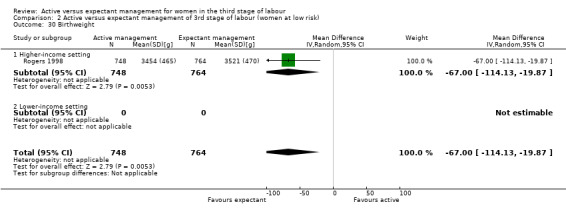
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 30 Birthweight.
Compared with expectant management, active management (with moderate‐quality evidence) probably increases:
postnatal maternal mean Hb (outcome not pre‐specified) (MD in g/dL 0.50, 95% CI 0.41 to 0.59, 2 studies, 2683 women, random‐effects (Tau² = 0.00, Chi² P = 1.00, I² = 0%) Analysis 2.41).
2.41. Analysis.
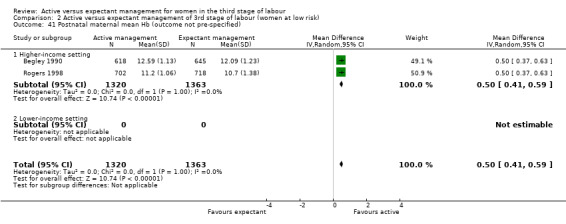
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 41 Postnatal maternal mean Hb (outcome not pre‐specified).
Also, active management (with low‐quality evidence) may increase:
postnatal diastolic blood pressure more than 90 mm Hg (average RR 7.00, 95% CI 2.99 to 16.43, 2 studies, 2941 women, random‐effects (Tau² = 0.00, Chi² P = 0.89, I² = 0%) Analysis 2.21);
administration of any analgesia between birth of the baby and discharge from labour ward (RR 2.53, 95% CI 1.34 to 4.78, 1 study, 1429 women; Analysis 2.23);
afterpains (RR 2.53, 95% CI 1.34 to 4.78, 1 study, 1429 women; Analysis 2.28);
return to hospital as an in‐ or outpatient because of bleeding (outcome not pre‐specified) (average RR 2.21, 95% CI 1.29 to 3.79, 2 studies, 2941 women, random‐effects (Tau² = 0.00, Chi² P = 0.82, I² = 0%) Analysis 2.40).
2.21. Analysis.
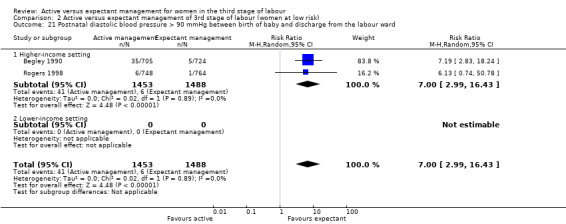
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
2.23. Analysis.
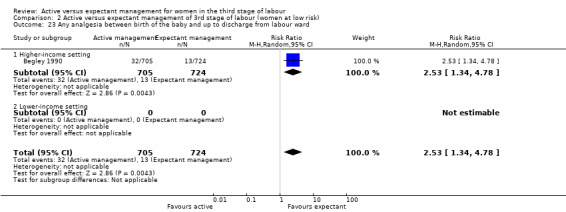
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 23 Any analgesia between birth of the baby and up to discharge from labour ward.
2.28. Analysis.
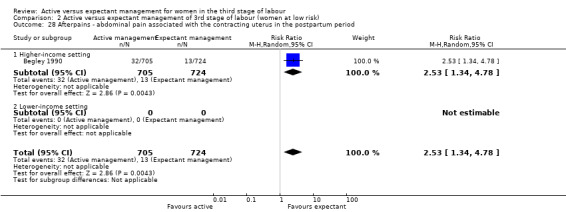
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period.
2.40. Analysis.
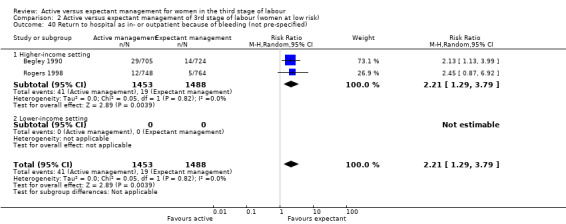
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified).
In addition, active management (with moderate‐quality evidence) probably slightly increases:
secondary blood loss/any vaginal bleeding needing treatment after 24 hours and before six weeks (average RR 1.78, 95% CI 0.69 to 4.60, 2 studies, 2941 women, random‐effects (Tau² = 0.39, Chi² P = 0.01, I² = 84%) Analysis 2.25).
2.25. Analysis.
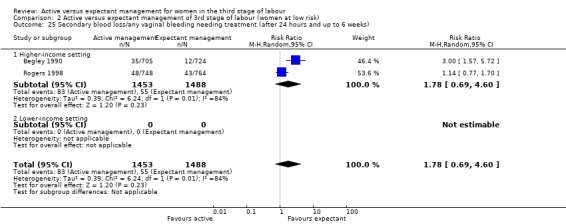
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks).
However, active management (with low‐quality evidence) may make little or no difference to:
length of the third stage in minutes (MD −0.30, 95% CI −1.87 to 1.27, 1 study, 1429 women; Analysis 2.19);
manual removal of placenta (average RR 3.58, 95% CI 0.42 to 30.61, 3 studies, 3134 women, random‐effects (Tau² = 2.58, Chi² P = 0.02, I² = 75%) Analysis 2.20);
postnatal vomiting (average RR 5.63, 95% CI 0.69 to 46.08, 2 studies, 2941 women, random‐effects (Tau² = 1.60, Chi² P = 0.12, I² = 60%) Analysis 2.22);
surgical evacuation of retained products of conception (average RR 0.69, 95% CI 0.12 to 3.98, 2 studies, 2941 women, random‐effects (Tau² = 1.17, Chi² P = 0.06, I² = 72%) Analysis 2.27).
2.19. Analysis.
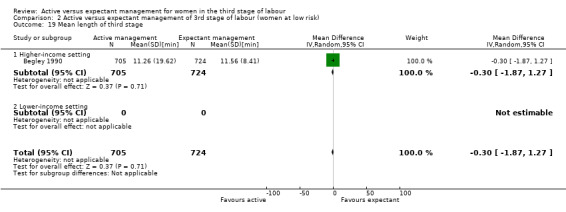
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 19 Mean length of third stage.
2.20. Analysis.
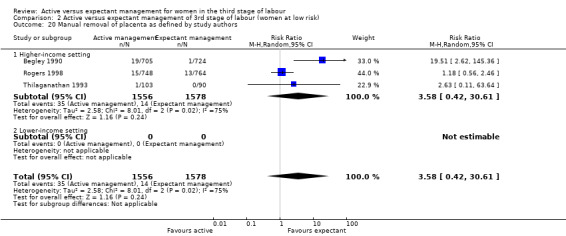
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 20 Manual removal of placenta as defined by study authors.
2.22. Analysis.
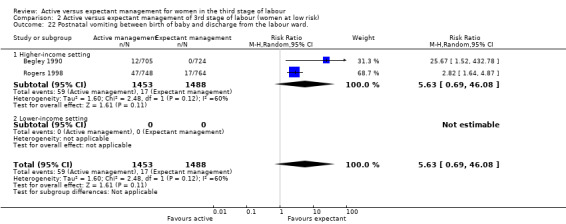
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 22 Postnatal vomiting between birth of baby and discharge from the labour ward..
2.27. Analysis.
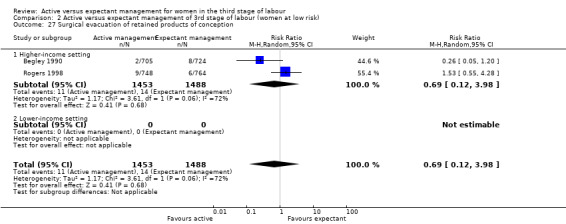
Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 27 Surgical evacuation of retained products of conception.
Authors of the included studies did not assess any of the review's other secondary outcomes.
3. Active versus mixed management of third stage: all women, early uterotonic, delayed cord clamping, controlled cord traction (no studies)
There were no studies that assessed this comparison.
4. Active versus mixed management of third stage of labour: all women, delayed uterotonic, delayed cord clamping, controlled cord traction (no studies)
There were no studies that assessed this comparison.
5. Active versus mixed management of third stage of labour: all women, delayed uterotonic, delayed cord clamping, no controlled cord traction (one study, 654 women)
This comparison included one study (Yildirim 2016).
Primary outcomes
The study did not report any of the review's primary outcomes: severe primary PPH (≥ 1000 mL time of birth); very severe primary PPH (≥ 2500 mL); maternal mortality; maternal Hb less than 9 g/dL at 24 to 72 hours; admission to neonatal special care or intensive care unit; neonatal jaundice requiring phototherapy or exchange transfusion; neonatal polycythaemia treated with dilutional exchange transfusion. The study authors' own primary outcome was one of this review's secondary outcomes.
Secondary outcomes
Compared with mixed management, active management (with low‐quality evidence) may slightly reduce:
mean length of the third stage in minutes (MD −5.15, 95% CI −5.71 to −4.59, 1 study, 654 women; Analysis 5.10);
postnatal maternal mean Hb (MD in g/dL 0.69, 95% CI 0.58 to 0.80, 1 study, 654 women; Analysis 5.14).
5.10. Analysis.
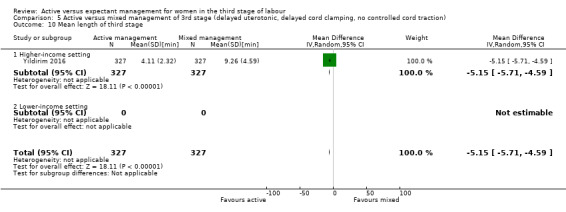
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 10 Mean length of third stage.
5.14. Analysis.
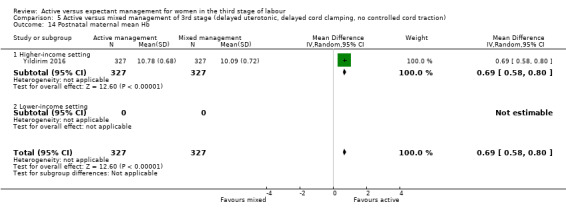
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 14 Postnatal maternal mean Hb.
Also, active management (with low‐quality evidence) ‐ may slightly increase:
birthweight in g (MD 68, 95% CI 23.87 to 112.13, 1 study, 654 infants; Analysis 5.13).
5.13. Analysis.
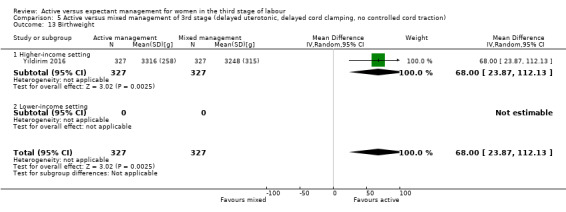
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 13 Birthweight.
Compared with mixed management, it is uncertain if active management (with very low‐quality evidence) makes any difference, improves, or reduces:
maternal blood transfusions (RR 0.75, 95% CI 0.17, 3.32, 1 study, 654 women; Analysis 5.8);
therapeutic uterotonics during the third stage and/or within the first 24 hours (RR 0.90, 95% CI 0.55 to 1.48, 1 study, 654 women; Analysis 5.9);
manual removal of placenta (RR 0.67, 95% CI 0.11 to 3.96, 1 study, 654 women; Analysis 5.11);
surgical evacuation of retained products of conception (RR 7.00, 95% CI 0.36 to 134.98, 1 study, 654 women; Analysis 5.12).
5.8. Analysis.
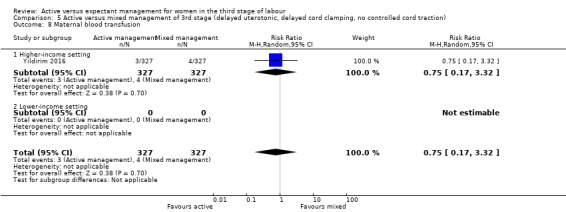
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 8 Maternal blood transfusion.
5.9. Analysis.
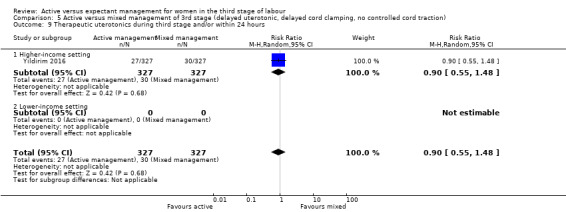
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 9 Therapeutic uterotonics during third stage and/or within 24 hours.
5.11. Analysis.
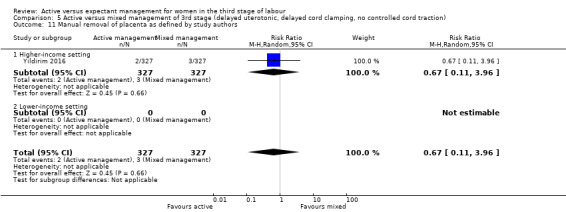
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 11 Manual removal of placenta as defined by study authors.
5.12. Analysis.
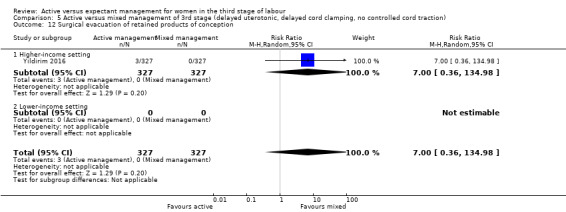
Comparison 5 Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction), Outcome 12 Surgical evacuation of retained products of conception.
The authors of this study did not assess any of the review's other secondary outcomes.
6. Expectant versus mixed management of third stage of labour: all women, early uterotonic, delayed cord clamping, controlled cord traction (no studies)
There were no studies that assessed this comparison.
7. Expectant versus mixed management of third stage of labour: all women, delayed uterotonic, delayed cord clamping, controlled cord traction (no studies)
There were no studies that assessed this comparison.
8. Expectant versus mixed management of third stage of labour: all women, delayed uterotonic, delayed cord clamping, no controlled cord traction (no studies)
There were no studies that assessed this comparison.
9. Active versus mixed management of third stage of labour: all women, immediate cord clamping, no controlled cord traction, uterotonic after placental delivery (one study, 1648 women; comparison not pre‐specified)
This comparison included one study with 1648 women (Khan 1997). We had not pre‐specified this comparison.
Primary outcomes
Compared with mixed management, it is uncertain whether active management (with very low‐quality evidence) reduces:
severe primary PPH, blood loss1000 mL or more at time of birth (RR 0.23, 95% CI 0.09 to 0.55, 1 study, 1648 women; Analysis 9.1).
9.1. Analysis.
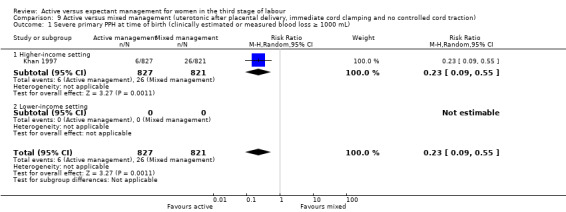
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
The study did not report the other primary outcomes of: very severe primary PPH (≥ 2500 mL); maternal Hb less than 9 g/dL at 24 to 72 hours; maternal mortality, neonatal jaundice requiring phototherapy or exchange transfusion; or neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, it is uncertain whether active management (wit−h very low‐quality evidence) reduces:
primary blood loss of 500 mL or more, clinically estimated or measured at time of birth (RR 0.53, 95% CI 0.38 to 0.74, 1 study, 1648 women; Analysis 9.10);
therapeutic uterotonics during the third stage and/or within the first 24 hours (RR 0.45, 95% CI 0.26 to 0.77, 1 study, 1648 women; Analysis 9.18);
length of the third stage in minutes (MD −10.00, 95% CI −10.24 to −9.76, 1 study, 1648 women; Analysis 9.19).
9.10. Analysis.
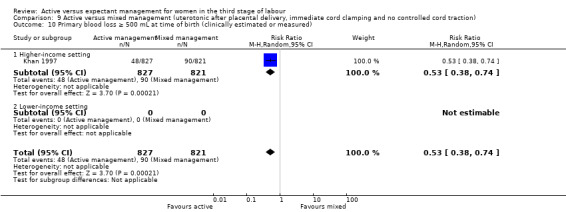
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
9.18. Analysis.
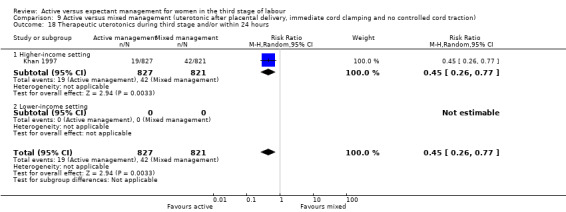
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
9.19. Analysis.
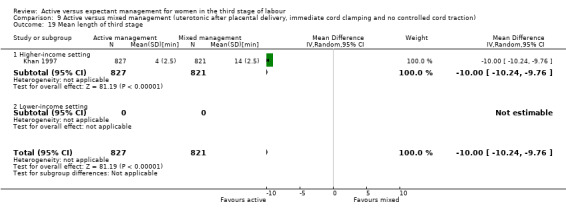
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 19 Mean length of third stage.
Also, it is uncertain if active management (with very low‐quality evidence) reduces or improves:
maternal blood transfusions (RR 0.25, 95% CI 0.03 to 2.22, 1 study, 1648 women; Analysis 9.16);
clinical signs of severe blood loss (RR 0.25, 95% CI 0.05 to 1.17, 1 study, 1648 women; Analysis 9.17).
9.16. Analysis.
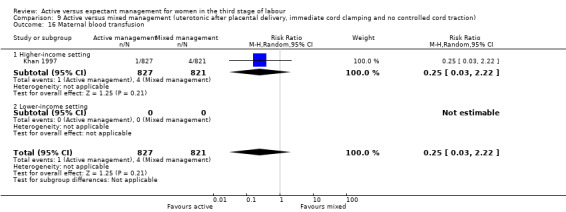
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 16 Maternal blood transfusion.
9.17. Analysis.
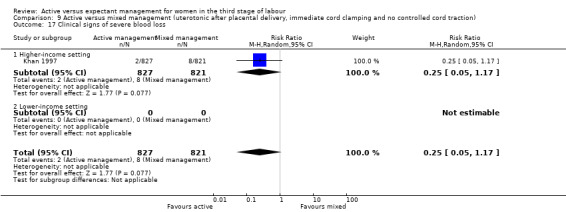
Comparison 9 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) , Outcome 17 Clinical signs of severe blood loss.
Authors of the included study did not assess any of the review's other secondary outcomes.
10. Active versus mixed management of third stage of labour: all women, no routine uterotonic, immediate cord clamping, no controlled cord traction (one study, 1631 women, comparison not pre‐specified)
This comparison included one study (Jangsten 2011). We judged this study to be at low risk of bias for sequence generation, allocation concealment and incomplete outcome data, unclear for selective reporting bias, but high risk of bias for other biases (see Characteristics of included studies).
Primary outcomes
Compared with mixed management, it is uncertain if active management (with very low‐quality evidence) reduces:
severe primary PPH at time of birth of 1000 mL or more (RR 0.78, 95% CI 0.48 to 1.24, 1 study, 1621 women; Analysis 10.1).
10.1. Analysis.
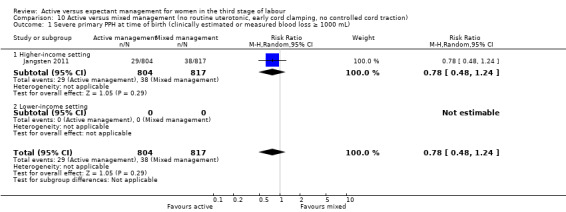
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
Also, active management (with very low‐quality evidence) may slightly improve:
maternal Hb less than 9 g/dL at 24 to 72 hours (RR 1.23, 95% CI 0.75 to 2.01, 1 study, 1631 women; Analysis 10.4).
10.4. Analysis.
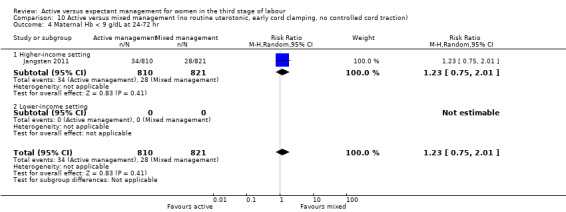
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 4 Maternal Hb < 9 g/dL at 24‐72 hr.
The study did not report the other primary outcomes of: very severe primary PPH (≥ 2500 mL); maternal mortality; neonatal jaundice requiring phototherapy or exchange transfusion; neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, active management (with moderate‐quality evidence) probably reduces:
therapeutic uterotonics during the third stage and/or within the first 24 hours (RR 0.39, 95% CI 0.33 to 0.48, 1 study, 1631 women; Analysis 10.18);
severe primary PPH at time of birth and up to two hours after, clinically estimated or measured blood loss of 1000 mL or more (RR 0.60, 95% CI 0.47 to 0.78, 1 study, 1621 women, Analysis 10.44); we had not pre‐specified this outcome);
mean maternal blood loss after delivery of the placenta and up to two hours (outcome not pre‐specified) (MD in mL −49.00, 95% CI −75.52 to −22.48, 1 study, 1621 women; Analysis 10.45).
10.18. Analysis.
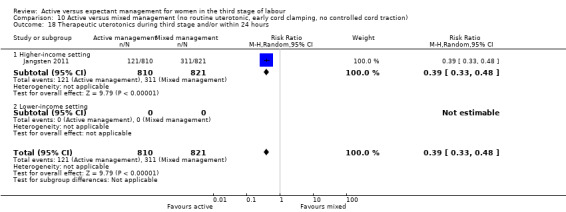
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
10.44. Analysis.
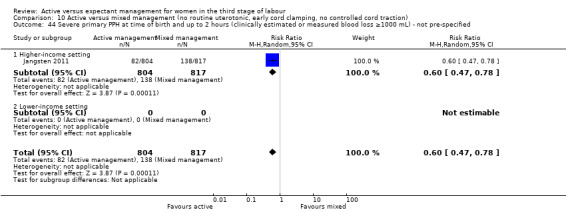
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 44 Severe primary PPH at time of birth and up to 2 hours (clinically estimated or measured blood loss ≥1000 mL) ‐ not pre‐specified.
10.45. Analysis.
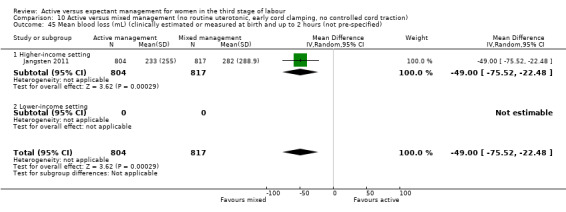
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 45 Mean blood loss (mL) (clinically estimated or measured at birth and up to 2 hours (not pre‐specified).
Also, active management (with low‐quality evidence) may reduce:
primary blood loss of 500 mL or more at time of birth, clinically estimated or measured at birth (RR 0.51, 95% CI 0.40 to 0.66, 1 study, 1621 women; Analysis 10.10);
mean maternal blood loss at time of birth (MD in mL −94.00, 95% CI −126.57 to −61.43, 1 study, 1621 women; Analysis 10.13);
mean length of third stage in minutes (MD −1.60, 95% CI −3.08 to −0.12; Analysis 10.19);
severe primary PPH after delivery of placenta and up to two hours, clinically estimated or measured blood loss of 1000 mL or more (RR 0.54, 95% CI 0.39 to 0.74, 1 study, 1621 women; Analysis 10.43); we had not pre‐specified this outcome.
10.10. Analysis.
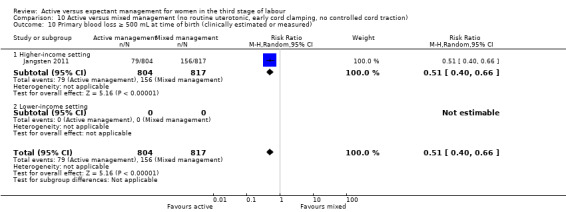
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
10.13. Analysis.
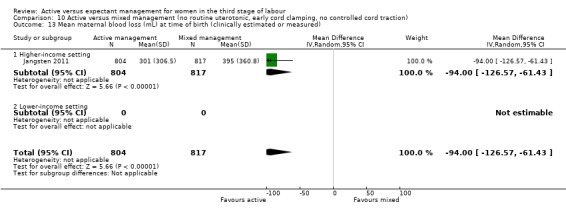
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured).
10.19. Analysis.
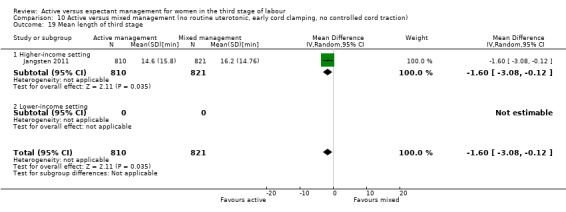
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 19 Mean length of third stage.
10.43. Analysis.
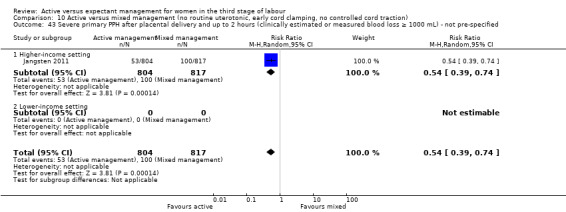
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 43 Severe primary PPH after placental delivery and up to 2 hours (clinically estimated or measured blood loss ≥ 1000 mL) ‐ not pre‐specified.
Compared with mixed management, active management (with low‐quality evidence) may increase:
postnatal maternal mean Hb (MD in g/dL 0.28, 95% CI 0.14 to 0.42, 1 study, 1631 women; Analysis 10.42); we had not pre‐specified this outcome.
10.42. Analysis.
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 42 Postnatal maternal mean Hb (outcome not pre‐specified).
Compared with mixed management, active management (with low‐quality evidence) may make little or no difference to:
mean birthweight (MD in g 15.00, 95% CI ‐28.88 to 58.88; Analysis 10.31).
10.31. Analysis.
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 31 Birthweight.
Also, it is uncertain whether active management (with very low‐quality evidence) improves or reduces:
maternal blood transfusion (RR 0.79, 95% CI 0.43 to 1.46; Analysis 10.16);
manual removal of placenta (RR 1.25, 95% CI 0.71 to 2.21; Analysis 10.20).
10.16. Analysis.
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 16 Maternal blood transfusion.
10.20. Analysis.
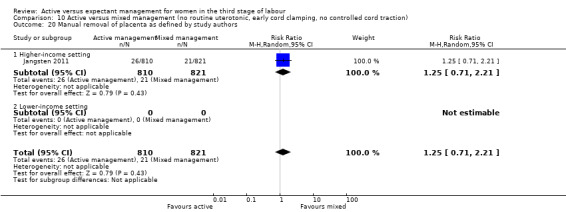
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 20 Manual removal of placenta as defined by study authors.
The study did not assess any of the review's other secondary outcomes, including, afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period. However, we assessed two similar, non‐prespecified outcomes (Jangsten 2011), and active management (with low‐quality evidence) may reduce:
afterpains at two hours after birth (RR −2.80, 95% CI −4.62 to −0.98, 1 study, 1425 women; Analysis 10.28);
afterpains the day after birth (RR −3.00, 95% CI −5.33 to −0.67, 1 study, 1336 women; Analysis 10.29).
10.28. Analysis.
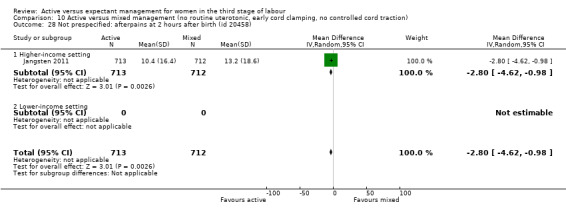
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 28 Not prespecified: afterpains at 2 hours after birth (id 20458).
10.29. Analysis.
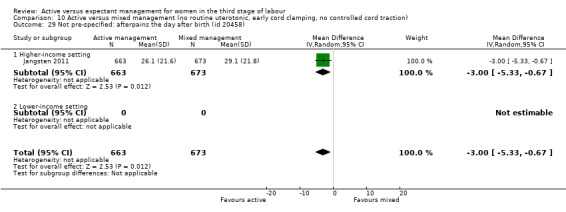
Comparison 10 Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction), Outcome 29 Not pre‐specified: afterpains the day after birth (id 20458).
11. Active versus mixed management of third stage of labour: all women, no routine uterotonic, immediate cord clamping, controlled cord traction (one study, 130 women, comparison not pre‐specified)
This comparison included one study (Jerbi 2007).
Primary outcomes
Compared with mixed management, it is uncertain whether active management (with very low‐quality evidence) reduces:
maternal Hb less than 9 g/dL at 24 to 72 hours (RR 0.80, 95% CI 0.34 to 1.90; Analysis 11.4).
11.4. Analysis.
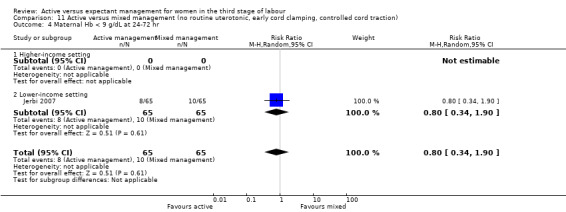
Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, controlled cord traction), Outcome 4 Maternal Hb < 9 g/dL at 24‐72 hr.
The study did not report on the other primary outcomes of: severe primary PPH (≥ 1000 mL time of birth); very severe primary PPH (≥ 2500 mL); maternal mortality; neonatal jaundice requiring phototherapy or exchange transfusion; neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, it is uncertain if active management (with very low‐quality evidence) reduces:
mean length of the third stage in minutes (MD −8.12 95% CI −9.72 to −6.52; Analysis 11.19).
11.19. Analysis.
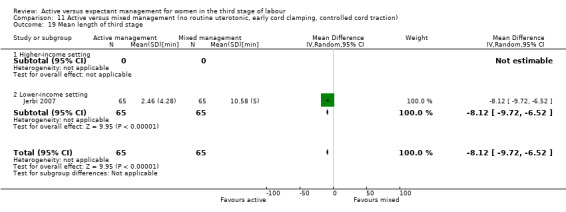
Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, controlled cord traction), Outcome 19 Mean length of third stage.
Also, it is uncertain if active management (with very low quality evidence) makes any difference to the incidence of:
manual removal of placenta (RR 1.00, 95% CI 0.06 to 15.65; Analysis 11.20).
11.20. Analysis.
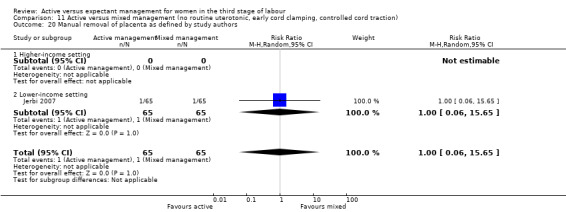
Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, controlled cord traction), Outcome 20 Manual removal of placenta as defined by study authors.
The study did not assess any of the review's other secondary outcomes.
Sensitivity analysis
We undertook sensitivity analyses including only the four studies with adequate sequence generation, allocation concealment and complete outcome reporting (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998). Removing the studies at high risk of bias did not change the overall findings.
Discussion
This review includes eight studies conducted in hospital settings in five countries involving 8892 women. None of the included studies reported any maternal deaths, nor any very severe (greater than 2500 mL) postpartum haemorrhages (PPH), and also no neonatal mortality. It should be noted that the random‐effects summaries presented are the average effects found for 'active' versus 'expectant' management. Thus, it may not necessarily be true that all methods of active management will have the reported size of advantage in terms of PPH, or other outcomes, over all methods of expectant management.
Summary of main results
Active versus expectant management of the third stage of labour in women, irrespective of their risk of bleeding
It is uncertain whether active management in hospitals in higher‐income settings leads to a reduction in severe primary PPH of 1000 mL or more, and it may reduce the number of women with maternal Hb less than 9 g/dL at 24 to 72 hours postnatal. Indices of maternal blood loss may be improved; for example, mean Hb may be higher by 0.5 g/dL in the active group. However, the average difference may not be clinically important, as routine blood donation reduces Hb levels by approximately 0.6 g/dL (Burnley 2006), without ill effects, and postnatal women undergo a diuresis postnatally that reverses the haemodilution of pregnancy, thus increasing their Hb levels within a few days after birth (Hytten 2001; Taylor 1981). The more clinically important effects are the possible reduction in severe PPH rate, need for transfusion and uterotonic therapy, which suggests (with very low‐quality) its effectiveness in lessening the severe bleeding that can prove life‐threatening if left untreated.
However, active management (with moderate‐quality evidence) probably reduces average birthweight for the baby (possibly due to decreased placental transfusion at birth arising from the early cord clamping component, Farrar 2009a), and (with low‐quality evidence) may increase the incidence of maternal postpartum diastolic blood pressure greater than 90 mmHg (possibly due to the use of ergometrine‐based uterotonics), afterpains, need for postpartum analgesia in the labour ward, and women having to return to hospital because of bleeding. Using data from only the high‐quality studies showed similar results.
The main findings may also be presented using the 'number needed to treat for an additional harmful outcome'. There would be one fewer severe PPH for every 66 women who had active management (95% CI 44 to 127) and one woman fewer with a postnatal Hb under 9 g/dL for every 28 women who had active management (95% CI 17 to 73). Conversely, there would be one more woman with a diastolic blood pressure greater than 90 mmHg for every 52 women who had active management (95% CI 38 to 83) and one more woman who had to return to hospital because of bleeding for every 65 women who had active management (95% CI 39 to 192).
Although this analysis considers women irrespective of risk of bleeding, the studies mainly included women at low risk of bleeding, with just one out of the four studies specifically including women of any risk. There were no specific data on women considered to be at high risk of bleeding.
Overall, active management may reduce the risk of severe bleeding, and it would be important to investigate if this benefit arose from the uterotonic component of the active management alone. The negative effects of active management appear, in the main, to be due either to 1) the administration of a specific uterotonic (e.g. hypertension due to ergometrine‐containing preparations and hypotension due to intravenous oxytocin boluses (Lewis 2007)), or 2) possibly to controlled cord traction leading to retained shreds of membrane or placenta, thus causing the increased incidence of return to hospital due to bleeding, or 3) early cord clamping leading to a 20% reduction in the baby's blood volume. Different uterotonics will have differing effects, and clinicians will need to assess the optimum one to use according to the circumstances. Recent international guidelines have turned to intramuscular oxytocin as a uterotonic that provides effective prophylaxis but without the associated side effects (ICM‐FIGO 2003; NICE 2014; WHO 2012) and a recent network meta‐analysis has suggested that the most effective drugs for preventing PPH of 500 mL of more could be ergometrine and oxytocin, carbetocin, or misoprostol combined with oxytocin (Gallos 2018). However, most of these studies group together intramuscular and intravenous oxytocin, and a recent randomised study suggests that the intravenous route may be more effective (Adnan 2018). In this Cochrane Review, of the eight studies, three administered the drug intravenously and five intramuscularly. Thus some of the oxytocics may have been less effective and so reduced the overall efficacy of the prophylaxis. The possible increased incidence of women in the active management group having to return to hospital due to bleeding is of concern, as such bleeding takes place away from immediate access to medical assistance. This would, again, be of greater significance for women in low‐income countries.
The probable reduction in the average birthweight of babies following active management is possibly due to a reduction in placental transfusion following early cord clamping (McDonald 2013; Rabe 2012; RCOG 2009). The evidence from the studies we identified is that the average volume of transfused blood was 77 mL (45 to 108 mL) based on differences in the birthweight between the groups. This estimate is consistent with historical data (Yao 1974), and also with more recent data (Farrar 2009a). Farrar’s small study (n = 26) weighing babies at birth on accurate scales to calculate placental transfusion showed that 79 mL (interquartile range 50 to 163 mL) of extra blood was transfused to babies following vaginal births and 84 mL (interquartile range 59 to 165 mL) following caesarean births. Placental transfusion for most of these babies was completed by about three minutes after birth, but transfusion continued for up to five minutes for some babies (Farrar 2009a). As all babies in this study breathed normally at birth, further studies are necessary to ascertain whether or not the cord remains pulsating for longer if the baby has not commenced respiration.
With expectant management, term infants receive about 80 mL more blood from placental transfusion than with active management, with its early cord clamping ‐ thus adding about 20% more to the infant's blood volume (Werner 2005). This may be associated with a lower incidence of anaemia in infancy (Cernadas 2006; Chaparro 2006; McDonald 2013; Van Rheenan 2007). It is possible that anaemia in early infancy may have adverse effects for the infant's longer‐term growth and development, particularly for infants born in low‐income countries. Although it is also plausible that placental transfusion with expectant management may increase the risk of neonatal jaundice requiring phototherapy (McDonald 2013), there was no evidence for this in the two studies that assessed this outcome. Finally, it needs to be considered that in the context of the administration of a powerful uterotonic, early cord clamping may protect the baby by preventing a sudden increase of blood volume into the transitional circulation, that might disrupt physiological processes such as duct closure, lung fluid reabsorption and cerebral haemodynamic autoregulation. This may be particularly important to infants who are slow to breathe (Mercer 2008). This problem can be avoided by giving the uterotonic drug immediately following the deferred cord clamping. The included studies did not report on these specific neonatal adverse outcomes, but this review did not find evidence that admission rates to special care baby units (SCBU) or neonatal intensive care units (NICU) were affected by the types of third‐stage management included (average RR 0.81, 95% CI 0.60 to 1.11, 2 studies, 3207 babies).
In the three studies that documented both severe PPH (≥ 1000 mL) and number of blood transfusions (Begley 1990; Prendiville 1988; Rogers 1998), it is noted that a total of 78 out of the 4636 women had a severe PPH, whereas 94 received a blood transfusion, perhaps indicating an over‐use of this treatment in a healthy population, under‐estimation of blood loss, or undetected low antenatal haemoglobins in some women. Given that a woman’s body is well prepared for normal blood loss at birth by the haemodilution of pregnancy (Mims 2005), and that 600 mL to 750 mL of diluted blood is equivalent to a routine blood donation, it is possible that the impact of blood losses less than 750 mL are not severe in normal, healthy women, but this needs investigation. In addition, the decrease in plasma volume in the early days after birth increases postnatal Hb concentration in a reversal of haemodilution (Hytten 2001; Mims 2005; Taylor 1981).
Active versus expectant management of the third stage of labour in women at low risk of bleeding
In hospitals in higher‐income settings, we found that it was uncertain whether active management (with very low‐quality evidence) reduced maternal primary PPH greater than 1000 mL, although the number of women was insufficient to assess this outcome with confidence (two studies, 2941 women) and further studies would be needed to study this association.
One small study (n = 193) compared the number of women with Hb levels less than 9 g/dL postnatally (Thilaganathan 1993) but the high risk of bias identified means that we cannot rely on the findings. None of the studies assessed Apgar scores less than 7 at five minutes. Other indices of maternal blood loss (with low‐quality evidence) may be reduced with the use of active management in this population. Active management again resulted (with moderate‐quality evidence) in a probable lower birthweight (possibly due to reduced placental transfusion), and (with low‐quality evidence) might result in an increase in the incidence of postpartum diastolic blood pressure greater than 90 mmHg, afterpains, need for postpartum analgesia in the labour ward, and having to return to hospital as an in‐ or outpatient because of bleeding.
Mixed managements around the timing of cord clamping in combination with the timing of uterotonic drug
Active versus mixed management of third stage of labour: all women, delayed prophylactic uterotonic administration, delayed cord clamping, no controlled cord traction (one study, 654 women)
The one study included (Yildirim 2016), was of low quality (unclear on four of the assessment criteria, and at high risk of bias for lack of blinding). The women were all at low risk of bleeding. The results indicated that, compared with mixed management, active management (with low‐quality evidence) may slightly reduce mean length of the third stage, and slightly increase birthweight. It is uncertain if active management (with very low‐quality evidence) makes any difference, improves, or reduces rates of maternal blood transfusions, therapeutic uterotonics during the third stage or within the first 24 hours, manual removal of placenta, or surgical evacuation of retained products of conception.
Active versus mixed management of the third stage of labour: all women, immediate cord clamping, no controlled cord traction and uterotonic after placental delivery (one study, 1648 women, comparison not pre‐specified)
The one study (Khan 1997), assessing this comparison was of uncertain quality (sequence generation was unclear). The results indicated that it is uncertain whether active management (with very low‐quality evidence) showed a reduction in severe primary PPH. It is also uncertain whether active management (with very low‐quality evidence) showed a reduction in the rate of blood loss greater than 500 mL, use of therapeutic uterotonics postpartum and length of the third stage, and it is uncertain if active management (with very low‐quality evidence) reduces or improves the number of blood transfusions (Figure 2).
Active versus mixed management of third stage of labour: all women, no routine uterotonic, immediate cord clamping, no controlled cord traction (one study, 1631 women, comparison not pre‐specified)
The one study included was of high quality (Jangsten 2011). Compared with mixed management, it is uncertain if active management (with very low‐quality evidence) reduces severe primary PPH or rates of maternal Hb less than 9 g/dL at 24 to 72 hours. Active management (with low‐quality evidence) may reduce the rate of blood loss greater than 500 mL and mean length of the third stage, may make little or no difference to mean birthweight, and (with moderate‐quality evidence) probably reduces the use of therapeutic uterotonics during the third stage. It is uncertain whether active management (with very low‐quality evidence) improves or reduces the number of maternal blood transfusions or rate of manual removal of placenta.
Active versus mixed management of third stage of labour: all women, no routine uterotonic, immediate cord clamping, controlled cord traction (one study, 130 women, comparison not pre‐specified)
The one study included was of moderate quality (Jerbi 2007). The results indicated that it is uncertain whether active management (with very low‐quality evidence) reduces maternal Hb less than 9 g/dL at 24 to 72 hours, compared to mixed management. It is uncertain if active management (with very low‐quality evidence) showed a reduction in mean length of the third stage, or makes any difference to the incidence of rates of manual removal of placenta.
Overall completeness and applicability of evidence
Four studies compared active versus expectant management of the third stage of labour and four compared active versus mixed management. All were conducted in hospitals, seven in higher‐income settings, and one in a lower‐income setting (n = 130). Four studies involved women at low risk of bleeding and four included women irrespective of their risk of bleeding. Given these factors, the results of the meta‐analysis can only be applied to care given in higher‐income settings. As only four studies (two of uncertain quality), compared active versus mixed management, and all mixed managements differed, we are unable to draw firm conclusions on any apparent differences between these two managements. Also, for many of the outcomes there is heterogeneity in the treatment effects, so there is no information as to the specific factors that might affect the difference between active and expectant management.
Quality of the evidence
The evidence presented on the primary outcomes selected is based on results of a small number of studies with relatively small numbers of participants. The lack of consistent high‐quality evidence for these outcomes should be borne in mind when considering the overall results (see Table 1).
We assessed the methodological quality of the included studies as ‘high quality’ in terms of sequence generation, allocation concealment and complete outcome data for three studies (Begley 1990; Jangsten 2011; Rogers 1998). We undertook sensitivity analysis by study quality to assess for any substantial difference in the main results. This made little difference to the overall findings except to indicate that there was no high‐quality evidence for the results of comparisons between active management and mixed management of the third stage of labour.
In all studies, there is the problem of assessment of blood loss by clinicians where no blinding is possible. However, studies used other indices of blood loss, such as postnatal Hb, which may have been assessed by technicians blinded to treatment allocation. For this reason, we included an outcome that had not been pre‐specified, mean postnatal Hb, in addition to comparing the number of women with Hb levels less than 9 g/dL at 24 to 72 hours, which not all studies had measured.
Adherence to intervention protocols and, in particular, to the management proposed for the two study arms was mixed (Table 3). In the three high‐quality studies (Begley 1990; Jangsten 2011; Rogers 1998), the majority of women in the active management arm (93% to 100%) received a uterotonic as directed. In the expectant management arm, however, practice varied widely, with 86% receiving "no uterotonic" in one study (Begley 1990), 76.5% in another (Rogers 1998), and 62% in the third (Jangsten 2011). Only 50% of women in the expectant management arm in one study (Prendiville 1988), received "no uterotonic", with no information given in the other three studies. The intervention protocol for the expectant arm of Prendiville 1988 did allow for administration of a uterotonic if necessary, as it stated, “Try not to give a uterotonic”; however, the actual proportion that received the drug appears incompatible with a philosophy of expectant management. In addition, the fact that the administration of the same uterotonic that constituted the main treatment in active management to half of the 'expectant' management group as well, does raise questions as to the usefulness of the findings. The resulting 'mixed' management may also increase levels of blood loss and other complications, as changing the study protocol after the first 425 births to exclude participants when mixed management had to be used (due to cutting the cord early because of nuchal cord, concerns about meconium or baby needing resuscitation) resulted in a decrease in PPH rate.
In addition, Prendiville 1988 included women at increased risk of bleeding. The total numbers involved are not clear, but 84 (5%) had previous third‐stage problems, 212 (13%) had an epidural in labour, and 230 (14%) had assisted births. As these women are likely to have a higher blood loss when expectant management is used, clinicians experiencing this may respond by anxiety in subsequent births using expectant management, even for women at low risk of bleeding, and be more likely to give a uterotonic early; this may have increased the intervention or 'mixed management' rates in women allocated to expectant management in this study (30% received uterotonic for treatment, and 20% prophylactically), and would also have decreased the opportunity for those midwives to keep up their skill level in expectant management.
A second study, comparing active with mixed management, also included women at increased risk of bleeding (Jangsten 2011), with similar possible results as above: 7% (n = 131) had a caesarean section, 714 (40%) had an epidural in labour, and 148 (8%) had a ventouse birth.
The skill of the midwives in both forms of care in all studies is of interest. The midwives in Rogers 1998 were said to be “similarly confident” in active and expectant management. However, the questionnaire administered to 92 of the 153 midwives prior to the study commencement showed that, whereas 84% felt “very confident” of active management, only 41% were “very confident” of expectant management. Similarly, Prendiville 1988 states that, before the study commenced, the researchers sought the advice of midwives in the UK who were “known to practise physiological management” and then used this advice to train the midwives. Only six (13%) of the midwives in this study, however, said that they were very confident of physiological management before the study started and 22 (46%) afterwards. Harding 1989 found that, of 49 midwives responding to a questionnaire regarding this study, 30 (61%) had never managed a third stage physiologically. Among the remaining 19, only one had practised physiological management as defined in the study. Those in Begley 1990 were also more used to active management and the PPH rate in the expectant arm fell during the study from 21% in the pilot study to 7% in the last six months, as they developed their skills. Yildirim 2016 was unique in that the authors had to train clinicians for the active management protocol, particularly in how to perform controlled cord traction, as it was not common practice in the hospital at the time.
Midwives’ skill and the accurate measurement of blood loss obviously has an impact on documented blood loss in the third stage of labour. For example, the mean (estimated) blood loss in the active arm of Rogers 1998 (269 mL, standard deviation (SD) 246), was actually greater than the mean (measured) blood loss in the expectant arm of Begley 1990 (235 mL, SD 224). This indicates that results of all these studies need to be examined in conjunction with local practice, to ascertain whether or not similar results will be found in different settings.
Finally, Prendiville 1988 changed the protocol after 425 births, but included all births in the results, which may have affected the findings.
We examined the quality of the body of evidence for our four primary outcomes for which we found data (severe primary PPH (≥ to 1000 mL at time of birth), maternal Hb < 9 g/dL 24 to 72 hours postpartum, admission to SCBU or NICU, neonatal jaundice requiring phototherapy or exchange transfusion) using GRADE. The GRADE assessments were of either low or very low quality. Downgrading decisions were based on inconsistency in two of the outcomes, and risk of bias and imprecision in all four ‐ see Table 1.
Potential biases in the review process
All the authors of this review have an interest in third‐stage management, and each brings different views on the methods that might be used. C Begley conducted one of the studies included in the review, and is a member of a team who conducted a systematic review for the International Confederation of Midwives (ICM) on expectant management of the third stage of labour. G Gyte has written on the third stage and is a involved in a study on timing of cord clamping in preterm birth, and other members have written on third‐stage management. W McGuire is also involved in further studies on timing of cord clamping. A Weeks is involved in running clinical studies of the timing of cord clamping and misoprostol for third‐stage prophylaxis in low‐resource settings. However, the review authors’ views differed and, during the review process, discussion and consensus was necessary to reach a final conclusion acceptable to all. Although all humans bring potential biases to any endeavour, we did try to ensure that our eventual conclusion arose solely from the data. Feedback from an international audience will serve to improve the next review update.
Agreements and disagreements with other studies or reviews
Previous version of this review
Our assessment of the evidence differs from the first published version of this review (Prendiville 2000), which concluded that active management was "superior" to expectant management. For the main comparison, we have no additional studies but we analysed four studies (Jangsten 2011; Jerbi 2007; Yildirim 2016; Khan 1997), in different categories of 'active compared with mixed management', which were not included in the main analysis. We have, however, used the recent methodology introduced for Cochrane Reviews, which assesses risk of bias in the individual studies more carefully than in the past (Higgins 2017). We also chose to use a random‐effects model for analysis due to the clear variations in the specific forms of both active and expectant management used in the included studies (clinical heterogeneity). Our findings, therefore, differ for some outcomes because of this decision (particularly for the group of women at low risk of bleeding) but we have reported Tau² and I² statistic, regardless of level of heterogeneity. We believe the evidence shows both benefits and harms for active management, and believe it is now critical to look at the advantages and disadvantages of the individual components of third‐stage management to see if the benefits can be achieved with fewer harms.
The previous authors of this review recommended active management of the third stage due to the benefit identified in terms of reduced incidence of severe bleeding. We agree that bleeding is a very important component when balancing the benefits and harms of active compared to expectant management of the third stage of labour. However, we consider that the number of harms caused by active management also deserve consideration. In particular, the increased rate of hypertension, increased numbers of mothers returning to hospital due to bleeding, and the possible decrease in average blood volume of newborns reflected in the lower birthweight for babies where the mother has received active management of the third stage, are of concern. In the population of women at low risk of bleeding, such harms are of more concern, as there was no statistical evidence that severe bleeding was reduced by active management. Further studies would be needed to confirm if there is a difference or not. Our analysis of women at low risk of bleeding differs from the previous version of the review in that we excluded the Prendiville data (Prendiville 1988), as we considered there to be a high risk of bias because the randomisation was not stratified by high and low risk of bleeding and there were no specific data in the published study to enable us to use the data had we chosen to do so.
Other systematic reviews on active versus expectant management of the third stage of labour
Active management in the included studies involved, in general, clamping and cutting the cord soon after the baby’s birth. A number of women in the expectant management groups also had their baby’s cord clamped and cut before pulsation had ceased. Another Cochrane Review has recommended that, in future, the umbilical cord should not be clamped and cut until after pulsation has ceased, regardless of the type of management used (McDonald 2013); if this change in practice is implemented it may negate the harms of active management due to early cord clamping. However, the optimum timing of the administration of the uterotonic still needs to be determined clearly. Despite the evidence from the Cochrane Review regarding timing of cord clamping (McDonald 2013), a survey of 1176 members of the Royal College of Obstetricians and Gynaecologists (RCOG) and 1445 members of the Royal College of Midwives (RCM) in the UK found that the majority (94% and 71%, respectively) 'always' or 'usually' use active management, and 73% and 40% clamp the cord within 20 seconds (Farrar 2009b). It would thus appear that there is considerable variation in practice and not all practitioners use the complete package of ‘active’ or ‘expectant’ management as described in these studies.
Drawing on evidence from the Cochrane Review on 'Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour' (McDonald 2007b), and other research on ergot compounds (Maughan 2006), we would expect that omitting the ergot component of the prophylactic uterotonic drug used as part of active management of the third stage should reduce the adverse effects of hypertension identified for active management.
Guidelines issued by various policy‐making bodies promote different aspects of third‐stage management. The RCM provides information on the benefits and harms of both methods, drawing on the published literature, including the previous version of the Cochrane Review. They recommend providing information for women and state that expectant/physiological management of the third stage can be seen as the logical ending to a normal labour (RCM 2008). The National Institute for Health and Clinical Excellence (NICE) contains recommendations on the management of the third stage of labour in their guideline on intrapartum care (NICE 2014). Using the previous Cochrane Review and other published research evidence, NICE conclude that active management of the third stage is to be recommended, including the use of oxytocin (10 international units by intramuscular injection), deferred clamping and cutting of the cord and controlled cord traction. They recommend that women should be told the benefits and harms of both active and expectant management. It is recommended that women who request expectant management and are at low risk of PPH "should be supported in their choice" (NICE 2014 p 183), but the guideline does not acknowledge the findings of the previous Cochrane Review, in women at low risk of PPH, that show no difference in severe PPH or rates of maternal Hb less than 9 g/dL at 24 to 72 hours postnatal between those receiving expectant or active management. The World Health Organization (WHO) also bases its recommendations on the previous Cochrane Review, promoting the offering of active management to all women, provided they are cared for by skilled attendants (WHO 2012). The International Confederation of Midwives states that, "Every midwife is required to attend the birth of the placenta without the aid of uterotonics" and that knowledge of expectant/physiological management of the third stage is "a basic midwifery competency". It further recommends that "when a woman makes an active choice to experience a non‐interventionist placental birth, the midwife will ensure the woman and her family have all the information necessary on which to make the decision" (ICM 2008).
Two issues bear further discussion in relation to published work: the experience of midwives in using expectant management and the use of expectant management of the third stage when the first and second stages of labour have not been normal. The RCM defines a normal birth as one where a woman commences, continues and completes labour physiologically at term (RCM 2004 Part 4e). Anecdotally, midwives experienced in expectant management say that only women who have had a normal, physiological labour and birth should have expectant management of the third stage. It has also been suggested in the literature that women who require induction or augmentation of labour with oxytocin (Sheiner 2005), or misoprostol (Phillip 2004), are prone to higher blood loss postpartum. In three of the four studies included in this review, high percentages of women had received a uterotonic for induction or acceleration of labour, and in all of them expectant management was not the norm for the midwives involved. It was recommended at the time of the first Cochrane Review on this topic that studies be conducted in areas where midwives were skilled at using expectant or physiological management. The Netherlands and New Zealand are two such places, and observational studies emanating from these countries are worth examining as their results indicate no increase in blood loss in conjunction with the use of expectant management.
In Holland, a descriptive study was conducted to determine the incidence and risk factors for PPH in 3464 nulliparous women giving birth vaginally. The women were stratified for high and low risk factors for PPH, with 1416 stratified as low risk (41%) (Bais 2004). Approximately 50% of these women received prophylactic oxytocin and 50% did not, with no significant difference found in blood loss (Bais 2004). The New Zealand College of Midwives conducted a population‐based, retrospective cohort study, which reported on the management provided by midwives during the third stage of labour (NZCM 2009). The study included 33,752 women who experienced a expectant/physiologically normal labour and birth. Almost half of the women (48.1%) had expectant management in the third stage while 51.9% had an actively managed third stage. Women who had expectant management were more likely to have a blood loss of less than 500 mL (96.3%) than those who had active management (93.1%). Mean blood loss was 213.6 mL (95% CI 211.6 mL to 215.5 mL) for the expectant management group and 241.6 mL (95% CI 239.4 mL to 243.8 mL) for active management. Similar findings were seen in an Irish study comparing midwife‐led with consultant‐led care (Begley 2009; Begley 2011a). Thirty per cent (n = 136) of the 446 women who received midwife‐led care throughout their pregnancy and birth, and received no epidurals or oxytocin in labour, had expectant management. None of these 136 women had a PPH, whereas the PPH rate for the women having active management was 1% (Begley 2009). A more recent study in the same Irish midwife‐led unit (MLU), over a six‐year period, showed that, of 1878 women birthing in the MLU, 50% had physiological management of the third stage compared with 5% of 900 similarly low‐risk women who were transferred to the hospital labour ward and had a spontaneous vaginal birth. The PPH rates were 2.9% and 7.2%, respectively (Dencker 2017).
Although these are observational data, there is an interesting link here between normal blood loss and expectant management of the third stage, when such management follows a physiologically normal first and second stage, and care is given by midwives skilled in the technique. This is an area that would benefit from further research.
Authors' conclusions
Implications for practice.
Active management of the third stage of labour in hospitals in higher‐income settings may bring benefits to women of mixed levels of risk of bleeding in terms of reducing mean maternal blood loss and probably reducing blood loss (greater than 500 mL) although it is uncertain if it reduces severe blood loss (greater than 1000 mL). It probably reduces the use of therapeutic uterotonics, but it may also cause harm, such as postnatal hypertension, pain and return to hospital due to bleeding. In addition, active management is probably associated with a reduction in birthweight possibly reflecting a reduction in neonatal blood volume due to early cord clamping.
In women at low risk of bleeding, we found that it was uncertain whether active management reduced severe blood loss (greater than 1000 mL) although there was still a reduction in other indices of maternal blood loss (low‐quality evidence). Active management again resulted in a probable lower birthweight (moderate‐quality evidence) and an increase in the incidence of postpartum diastolic blood pressure greater than 90 mmHg (with low‐quality evidence), afterpains, need for postpartum analgesia in the labour ward, and having to return to hospital as an in‐ or outpatient because of bleeding. It must be emphasised that this review includes only a small number of studies with relatively small numbers of participants, and the quality of evidence for primary outcomes is low or very low.
In the context of these studies, in higher‐income settings with high levels of clinician expertise and adequate access to emergency care, healthy women do not appear to suffer unduly from the results of above average blood loss (about 500 mL) that does not reach the level of severe (greater than 1000 mL) primary postpartum haemorrhage (PPH; Bloomfield 1990). There are both benefits and harms from active management of the third stage and it is also unclear whether all three components of the active management package are required to gain the benefit of reduced PPH.
Healthcare providers could, therefore, present information to all women in the antenatal period on the advantages and disadvantages of both methods of third‐stage management to facilitate their discussion and informed choice of care. This information could include not only the benefits of active management (reduces the risk of severe blood loss and postnatal anaemia in women at mixed risk of bleeding) but also the harms to the mother (increases the risk of hypertension if using ergot compounds, increases afterpains, need for analgesia, and bleeding following discharge). In addition, information regarding the effects on the baby of early versus deferred cord clamping could be provided whilst acknowledging the uncertainty deferred cord clamping brings due to the lack of evidence around optimal timing of the prophylactic uterotonic.
Although the studies in the review did not assess women at increased risk of bleeding specifically, it can be deduced from Prendiville 1988 that for these women the benefit of reduced blood loss is likely to outweigh the harms. This may lead clinicians to suggest active management of the third stage with a prophylactic uterotonic that contains no ergot, and also deferred cord clamping, though women's choice should always be respected.
Women at low risk of bleeding could be informed of the potential benefits and adverse effects of both expectant and active management of the third stage of labour, and how adverse effects can be minimised. When expectant management is used, it is important that the option of using a uterotonic (non‐ergot based initially) as treatment at any time is available if excess bleeding occurs. These results cannot, and should not, be extrapolated to other contexts such as low‐income countries where access to care is often severely restricted, or those countries with insufficient trained clinicians or inadequate emergency care
Implications for research.
In the next update of this review we will assess the use of the agreed core outcome set on PPH (Meher 2019). Future studies could consider the results of the Cochrane Review on timing of cord clamping (McDonald 2013), and consider inclusion of the element of leaving the cord unclamped until it has stopped pulsating (or has gone 'white', indicating the vein has emptied) in their protocols for both active and expectant management. Individual components of both types of management could be examined in further studies. All future studies could aim to include maternal, fetal and infant outcomes, as listed in this review, or in the core outcome set on PPH, or both (Meher 2019), as far as is practicable. Studies are needed in low‐income or resource‐constrained countries. Studies could establish whether, in order to reduce bleeding for the mother, a uterotonic drug is what is needed rather than all three components of active management of the third stage. Studies similar to two recent studies that looked at active management with or without controlled cord traction (Deneux‐Tharaux 2013; Gulmezoglu 2012), will assist in answering this question.
It is also critical to establish whether or not a routine uterotonic is really necessary for healthy women at low risk of bleeding, and what is the optimum time for administration (i.e. before or after cord clamping). The concept of 'secondary prophylaxis' used when necessary in conjunction with expectant management, where the uterotonic is given at the first sign of excessive blood loss (e.g. 350 mL), deserves further exploration as a way of reducing the rate of side effects for healthy women. In addition, it is important to consider the adverse effects on the mother's blood pressure, pain, and returning to hospital after the birth because of bleeding.
Future studies may compare different types of expectant management; for example, comparing expectant management with expectant management followed by uterotonic, or expectant management using maternal effort only with expectant management using maternal effort and gentle cord traction. Given the possibility that women who require induction or augmentation of labour with a uterotonic are prone to higher postpartum blood loss, researchers could consider if such women should be excluded from future studies including an expectant management arm, as the natural release of oxytocin may be inhibited by the administration of exogenous oxytocics or other uterotonics.
Feedback
Mc'Alpine, 31 August 2002
Summary
I have some questions. In the four included studies, how many women were in each study and when were the studies done? Was a comparison made between maternity hospitals, birth centres, and home delivery? For postpartum haemorrhage of more than 500 mls, what does "relative risk 0.38, 95% confidence interval 0.32 to 0.46" mean in terms of numbers?
Why do you conclude that active management should be the 'routine' management of choice in a maternity hospital? What are the implications for other settings?
[Summary of comments from Elizabeth McAlpine, August 2002]
Reply
Reply from Cecily Begley in December 2009: The number of women in each study can be found in the analyses graphs in this updated review, and the overall numbers of studies and women included for each outcome are also reported in the text. Dates for the studies are included in the references. All the studies were in hospital settings and we found no studies of midwifery‐led birth centres or home births. We have included this information on study settings in the ‘Characteristics of Included studies’ and at appropriate places in the text of the review. Our estimate of the relative risk of blood loss greater than 500 mL is slightly different from the previous version of the review because we have used a random‐effects analysis. We also report the ‘Numbers needed to treat’ under ‘Summary of main results’ for the outcomes of severe primary PPH and postnatal Hb < 9 g/dL; we hope this addresses the meaning of the relative risk and confidence intervals for these outcomes. Our conclusions differ from the previous review as we have included additional outcomes which show a balance of benefits and harms and we have used new systematic review methodology. Our findings refer to hospital settings because this is where the included studies were undertaken. We cannot provide evidence‐based information for other settings.
Contributors
Cecily Begley
Matthews, December 2004
Summary
My anecdotal observation, having changed my practice to include physiological management of the third stage, is that women who choose this option have a decrease in the amount of lochia postpartum and a shorter duration of vaginal discharge. I have not seen any studies that could confirm or refute this.
[Comment received from Mary Jo Matthews, December 2004]
Reply
We have included in the update of the review the outcome ‘Amount of lochia > 24 hours and up to discharge from hospital [mL]’; however, none of the included studies assessed this outcome, so we have, therefore, no evidence to confirm or refute this observation.
Contributors
Cecily Begley
Van Wyk, 4 March 2009
Summary
This review was last updated in 2000, and comments sent in 2002 and 2004 have not been addressed. The authors stated in 2007 that an update is in progress. We are concerned about the validity of the review findings.
[Summary of feedback from Susan van Wyk on behalf of the Masters Clinical Epidemiology class, Stellenbosch University, February 2009]
Reply
A new team took over this review in December 2008. We have now updated this review and addressed these comments from 2002 and 2004. Our conclusions differ from the previous review as we have included additional outcomes which show a balance of benefits and harms and we have used new systematic review methodology. The delay has arisen from our needing to produce a new protocol for the review prior to undertaking the update.
Contributors
Reply from Cecily Begley, December 2009
What's new
| Date | Event | Description |
|---|---|---|
| 22 January 2018 | New search has been performed | Search updated and five new trial reports identified. Three were clinical trials registry reports of studies already assessed. We excluded one new trial in comparison 5. We are still unable to source the full‐text of Rosario 1973. One new study has been included in this update (Yildirim 2016). All details were updated in line with the new Cochrane requirements. We changed one section of risk of bias for two papers and updated the 'Summary of findings' table footnotes with this information, and included the setting. Dr Linda Biesty joined the team. We had erroneously referred to the comparison, 'women at low risk of excessive bleeding' as a "subgroup" in the text, so we altered this. |
| 22 January 2018 | New citation required but conclusions have not changed | Main conclusions have not changed. Language to reflect the GRADE assessments of the quality of the evidence was added, as was a sentence on the conclusions in relation to the comparison of women at low risk of excessive bleeding. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 20 October 2014 | New search has been performed | Search updated. We included a further publication on one of the already included studies and now include data on after‐pains for this comparison (Jangsten 2011). We updated the risk of bias for all included studies to assess both performance bias and detection bias. We have included a 'Summary of findings' table in this update. |
| 20 October 2014 | New citation required but conclusions have not changed | Three references identified and one included for this update (a new paper from a trial already included) and two were excluded. |
| 17 August 2011 | New citation required but conclusions have not changed | Deirdre Murphy and Susan MacDonald stepped down as authors. Andrew Weeks joined the team. |
| 17 August 2011 | New search has been performed | Search updated in February 2011: two new studies included (Jangsten 2011; Jerbi 2007); four excluded (Abdel‐Aleem 2010; Hoffman 2006; Ramirez 2001; Vasegh 2005); and one added to Studies awaiting classification. Protocol section updated ‐ seeDifferences between protocol and review. |
| 10 May 2010 | Feedback has been incorporated | Feedback on the previous version of this review added, along with replies from the new review team. |
Acknowledgements
We acknowledge the work of the previous review team, upon which the protocol was based (Prendiville 2000), and also the contribution of Deirdre Murphy and Sue McDonald in the previous versions of this review. We thank the study authors who provided additional information on request: Beck (on behalf of Muller), Begley, Hoffman, Rogers/Elbourne, Thilaganathan, Yildirim.
Particular thanks to Therese Dowswell, who contributed greatly to data extraction and tables on a previous version.
Thanks to Nazie AmirAnsari and Alireza Karbalaei who translated Vasegh 2005 and Emily Lemon who translated Muller 1996.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods ‐ ICTRP and ClinicalTrials.gov
ICTRP
postpartum h(a)emorrhage
third AND stage AND labo(u)r AND bleeding
active AND expectant AND third
active AND expectant AND labo(u)r
ClinicalTrials.gov
Advanced search
Interventional studies | postpartum hemorrhage
Interventional studies | third stage | labor
Interventional studies | bleeding | labor
Data and analyses
Comparison 1. Active versus expectant management of 3rd stage of labour (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.87] |
| 1.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.87] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL 24‐72 hours postpartum | 2 | 1572 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.83] |
| 4.1 Higher‐income setting | 2 | 1572 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.83] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 2 | 3207 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.11] |
| 5.1 Higher‐income setting | 2 | 3207 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.11] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 2 | 3142 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 6.1 Higher‐income setting | 2 | 3142 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.27, 0.44] |
| 10.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.27, 0.44] |
| 10.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | ‐78.80 [‐95.96, ‐61.64] |
| 13.1 Higher‐income setting | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | ‐78.80 [‐95.96, ‐61.64] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 16.1 Higher‐income setting | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 16.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.15, 0.23] |
| 18.1 Higher‐income setting | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.15, 0.23] |
| 18.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.87, 1.27] |
| 19.1 Higher‐income setting | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.87, 1.27] |
| 19.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by study authors | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.57, 5.56] |
| 20.1 Higher‐income setting | 4 | 4829 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.57, 5.56] |
| 20.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [1.63, 10.30] |
| 21.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [1.63, 10.30] |
| 21.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.36, 4.48] |
| 22.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.36, 4.48] |
| 22.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.1 Higher‐income setting | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women's assessment of pain during third stage as reported by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.40, 2.99] |
| 25.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.40, 2.99] |
| 25.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 27.1 Higher‐income setting | 3 | 4636 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 27.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.1 Higher‐income setting | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 1 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.38, 2.66] |
| 29.1 Higher‐income setting | 1 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.38, 2.66] |
| 29.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 2 | 3207 | Mean Difference (IV, Random, 95% CI) | ‐76.90 [‐108.51, ‐45.30] |
| 30.1 Higher‐income setting | 2 | 3207 | Mean Difference (IV, Random, 95% CI) | ‐76.90 [‐108.51, ‐45.30] |
| 30.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage ‐ Papille grade III/IV ‐ (for infants born before 34 weeks' gestation only) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3‐6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 1 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 38.1 Higher‐income setting | 1 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 38.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified) | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre‐specified) | 3 | 4062 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.44, 0.60] |
| 41.1 Higher‐income setting | 3 | 4062 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.44, 0.60] |
| 41.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Active versus expectant management of 3rd stage of labour (women at low risk).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 2.17] |
| 1.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 2.17] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.47] |
| 4.1 Higher‐income setting | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.47] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 1 | 1512 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.55, 1.88] |
| 5.1 Higher‐income setting | 1 | 1512 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.55, 1.88] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 1 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.78, 2.18] |
| 6.1 Higher‐income setting | 1 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.78, 2.18] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 10.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 10.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL at the time of birth, clinically estimated or measured | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | ‐78.80 [‐95.96, ‐61.64] |
| 13.1 Higher‐income setting | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | ‐78.80 [‐95.96, ‐61.64] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.88] |
| 16.1 Higher‐income setting | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.88] |
| 16.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.21] |
| 18.1 Higher‐income setting | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.21] |
| 18.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.87, 1.27] |
| 19.1 Higher‐income setting | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.87, 1.27] |
| 19.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by study authors | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [0.42, 30.61] |
| 20.1 Higher‐income setting | 3 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [0.42, 30.61] |
| 20.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [2.99, 16.43] |
| 21.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [2.99, 16.43] |
| 21.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward. | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.69, 46.08] |
| 22.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.69, 46.08] |
| 22.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and up to discharge from labour ward | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.1 Higher‐income setting | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women's assessment of pain during third stage as reported by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.69, 4.60] |
| 25.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.69, 4.60] |
| 25.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.98] |
| 27.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.98] |
| 27.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.1 Higher‐income setting | 1 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 1 | 1512 | Mean Difference (IV, Random, 95% CI) | ‐67.0 [‐114.13, ‐19.87] |
| 30.1 Higher‐income setting | 1 | 1512 | Mean Difference (IV, Random, 95% CI) | ‐67.0 [‐114.13, ‐19.87] |
| 30.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage ‐ Papille grade III/IV ‐ (for infants born before 34 weeks' gestation only) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants exposed to one or more red blood cell transfusions | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3‐6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified) | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.1 Higher‐income setting | 2 | 2941 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre‐specified) | 2 | 2683 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.41, 0.59] |
| 41.1 Higher‐income setting | 2 | 2683 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.41, 0.59] |
| 41.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Active versus mixed management of 3rd stage (early uterotonic, delayed cord clamping, controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal blood transfusion | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.17, 3.32] |
| 8.1 Higher‐income setting | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.17, 3.32] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Therapeutic uterotonics during third stage and/or within 24 hours | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.55, 1.48] |
| 9.1 Higher‐income setting | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.55, 1.48] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Mean length of third stage | 1 | 654 | Mean Difference (IV, Random, 95% CI) | ‐5.15 [‐5.71, ‐4.59] |
| 10.1 Higher‐income setting | 1 | 654 | Mean Difference (IV, Random, 95% CI) | ‐5.15 [‐5.71, ‐4.59] |
| 10.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Manual removal of placenta as defined by study authors | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.96] |
| 11.1 Higher‐income setting | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.96] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Surgical evacuation of retained products of conception | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.36, 134.98] |
| 12.1 Higher‐income setting | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.36, 134.98] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Birthweight | 1 | 654 | Mean Difference (IV, Random, 95% CI) | 68.0 [23.87, 112.13] |
| 13.1 Higher‐income setting | 1 | 654 | Mean Difference (IV, Random, 95% CI) | 68.0 [23.87, 112.13] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Postnatal maternal mean Hb | 1 | 654 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.58, 0.80] |
| 14.1 Higher‐income setting | 1 | 654 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.58, 0.80] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Expectant versus mixed management (early uterotonic, delayed cord clamping, controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Expectant versus mixed management (delayed uterotonic, delayed cord clamping, controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Expectant versus mixed management (delayed uterotonic, delayed cord clamping, no controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no controlled cord traction) .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.55] |
| 1.1 Higher‐income setting | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.55] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.74] |
| 10.1 Higher‐income setting | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.74] |
| 10.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.22] |
| 16.1 Higher‐income setting | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.22] |
| 16.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.17] |
| 17.1 Higher‐income setting | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.17] |
| 17.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 18.1 Higher‐income setting | 1 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 18.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1648 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐10.24, ‐9.76] |
| 19.1 Higher‐income setting | 1 | 1648 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐10.24, ‐9.76] |
| 19.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women's assessment of pain during third stage as reported by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage ‐ Papille grade III/IV ‐ (for infants born before 34 weeks' gestation only) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3‐6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre‐specified) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Active versus mixed management (no routine uterotonic, early cord clamping, no controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.24] |
| 1.1 Higher‐income setting | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.24] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.75, 2.01] |
| 4.1 Higher‐income setting | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.75, 2.01] |
| 4.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.66] |
| 10.1 Higher‐income setting | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.66] |
| 10.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | ‐94.0 [‐126.57, ‐61.43] |
| 13.1 Higher‐income setting | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | ‐94.0 [‐126.57, ‐61.43] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.43, 1.46] |
| 16.1 Higher‐income setting | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.43, 1.46] |
| 16.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.33, 0.48] |
| 18.1 Higher‐income setting | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.33, 0.48] |
| 18.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.08, ‐0.12] |
| 19.1 Higher‐income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.08, ‐0.12] |
| 19.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by study authors | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.71, 2.21] |
| 20.1 Higher‐income setting | 1 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.71, 2.21] |
| 20.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women's assessment of pain during third stage as reported by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Not prespecified: afterpains at 2 hours after birth (id 20458) | 1 | 1425 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐4.62, ‐0.98] |
| 28.1 Higher‐income setting | 1 | 1425 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐4.62, ‐0.98] |
| 28.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Not pre‐specified: afterpains the day after birth (id 20458) | 1 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐5.33, ‐0.67] |
| 29.1 Higher‐income setting | 1 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐5.33, ‐0.67] |
| 29.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Birthweight | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 15.0 [‐28.88, 58.88] |
| 31.1 Higher‐income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 15.0 [‐28.88, 58.88] |
| 31.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Intraventricular haemorrhage ‐ Papille grade III/IV ‐ (for infants born before 34 weeks' gestation only) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Number of infants who received a red blood cell transfusion. | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant Hb level at 3‐6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Postnatal maternal mean Hb (outcome not pre‐specified) | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.14, 0.42] |
| 42.1 Higher‐income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.14, 0.42] |
| 42.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 43 Severe primary PPH after placental delivery and up to 2 hours (clinically estimated or measured blood loss ≥ 1000 mL) ‐ not pre‐specified | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 43.1 Higher‐income setting | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 43.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 44 Severe primary PPH at time of birth and up to 2 hours (clinically estimated or measured blood loss ≥1000 mL) ‐ not pre‐specified | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.47, 0.78] |
| 44.1 Higher‐income setting | 1 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.47, 0.78] |
| 44.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45 Mean blood loss (mL) (clinically estimated or measured at birth and up to 2 hours (not pre‐specified) | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | ‐49.0 [‐75.52, ‐22.48] |
| 45.1 Higher‐income setting | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | ‐49.0 [‐75.52, ‐22.48] |
| 45.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Active versus mixed management (no routine uterotonic, early cord clamping, controlled cord traction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24‐72 hr | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.34, 1.90] |
| 4.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lower‐income setting | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.34, 1.90] |
| 5 Admission to neonatal special/intensive care | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐8.12 [‐9.72, ‐6.52] |
| 19.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Lower‐income setting | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐8.12 [‐9.72, ‐6.52] |
| 20 Manual removal of placenta as defined by study authors | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.65] |
| 20.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Lower‐income setting | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.65] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women's assessment of pain during third stage as reported by study authors | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains ‐ abdominal pain associated with the contracting uterus in the postpartum period | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage ‐ Papille grade III/IV ‐ (for infants born before 34 weeks' gestation only) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3‐6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in‐ or outpatient because of bleeding (not pre‐specified) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 Higher‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Lower‐income setting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre‐specified) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 Higher‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 Lower‐income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Begley 1990.
| Methods | RCT with randomisation of individual women | |
| Participants |
Irish hospital setting. High‐income country Inclusion criteria: all women at low risk of haemorrhage (< 35 years; parity < 5; 1st stage of labour < 15 hours; no previous history of PPH; Hb > 11 g/dL (or 10.6 g/dL for capillary sample)) with singleton, cephalic presentation, 35 to 36 weeks at recruitment; no medical complications that would contraindicate ergometrine or would increase the risk of bleeding (cardiac disease, use of heparin, hypertension), and expected to give birth vaginally 1429 women randomised out of 2901 eligible Exclusion criteria: women with hypertension in pregnancy or 1st or 2nd stage; epidural anaesthesia (included in separate study); APH; 1st stage > 15 hours; OVB; women attending private care Clinician responsible for third stage: midwives |
|
| Interventions |
Intervention: active management of third stage (N = 705)
For retained placenta: 1 h after birth:
Comparison: expectant management of 3rd stage (N = 724)
Special circumstances: if baby’s cord is clamped and cut before pulsation ceases (due to cord round neck, asphyxia, etc) do not give ergometrine. Milk any placental blood into bowl and discard it. Watch for signs of placental separation and deliver placenta by CCT. Retained placenta > 1 h after birth:
Data entered into comparisons 1 and 2 |
|
| Outcomes |
Pre‐specified outcomes: manual removal of placenta; PPH (> 500 mL); mean blood loss; length 3rd stage; Hb < 10 g/dL at 48‐72 h; and difference between 32 weeks and 48‐72 h PP: PP blood transfusions; side effects 1‐2 h post birth; PP complications; breastfeeding; serum prolactin; women’s views. No neonatal outcomes (Information from Oxford Database of Perinatal Trials registration sheet) and from Begley 1990): morbidity; blood loss during 3rd stage; method of placental delivery; complications occurring in first 1‐2 h post birth (haemorrhage, nausea, vomiting, raised BP, pain); Hb on 3rd postnatal day; prolactin levels on 3rd postnatal day, duration of breastfeeding |
|
| Notes | Between 1 October 1987 and 31 October 1988, 2901 women were deemed eligible for initial inclusion, 2650 agreed to take part. 1221 of these were excluded prior to randomisation because of epidural (399); OVB (354); CS (132); rapid birth (95); hypertension (77); missed (53); low Hb (40); woman’s request (28); miscellaneous (23); breech (20) Actual management used in the active arm: all given IV ergometrine 0.5 mg before delivery of placenta; 89% cord clamped and cut; 93% CCT and 5% maternal effort; 7% upright and 93% recumbent Actual management used in the expectant arm: 14% got ergometrine for treatment, not prophylactically, 6 (0.83%) before placenta delivered; cord left unclamped till pulsation ceased 42%; placenta delivered by maternal effort 32% and CCT 66%; 11% upright Dates of study: 1 October 1987‐31 October 1988 Funding sources: "This study was funded by the Research and Development Trust of the Coombe Hospital" Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unpublished information from study author: random number tables were used (Fleiss 1981). The 1st number was selected from the table by a disinterested observer and the numbers were allocated in blocks of 100 following in sequence |
| Allocation concealment (selection bias) | Low risk | Quote: “...a numbered, sealed envelope containing the randomly allocated group was stapled to the woman’s chart in readiness for admission... The envelope remained sealed until the women was in second stage of labour and the midwife was certain a normal delivery would ensue. The envelope was then opened...” Quote: "When a woman was excluded from the study, her envelope was returned, unopened, to the researcher. All returned envelopes were re‐allocated in numerical order prior to starting the next batch of 100 envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind women or clinicians in this study. The outcome assessor was often the caregiver for many important outcomes, e.g. blood loss and PPH. Even though blood loss was measured and not estimated, there may have been bias in measuring. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Describes the differences in measuring blood loss in non‐blinded staff and attempts to standardise methods. Hb measurement was conducted by assessors blinded to allocation (personal communication) thus some outcomes were blinded but for some it was not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some missing data for some postnatal Hb measurements (618 out of 705 in the active group (12% attrition) and 645 out of 724 in the expectant (11% attrition) ITT not mentioned but no loss to follow‐up for outcomes measured during labour. Some outcome data are taken from the unpublished thesis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods are reported, but no protocol for the study is available. |
| Other bias | Low risk | No significant difference in baseline characteristics, but more women in the physiological arm had pethidine in labour (46% compared with 52%, P = 0.05). This may impact on outcomes in the physiological arm where the sight and sound of the baby may be the stimulus for the hormonal release needed for natural 3rd stage and pethidine may impact here. 1st and 2nd stage management similar and no obvious differences overall. |
Jangsten 2011.
| Methods | RCT with randomisation of individual women | |
| Participants |
Setting: university hospital, Sweden. High‐income country Inclusion criteria: healthy women with normal pregnancies, a gestational age of 34‐43 weeks, singleton, cephalic presentation and expected vaginal birth (included ventouse deliveries) Exclusion criteria: non‐Swedish speaking, previous PPH, elective CS, pre‐eclampsia, grand multiparity (> 5) or IUFD Subgroups: high‐income, not low‐risk Clinician responsible for third stage: midwives |
|
| Interventions |
Experimental intervention: active management of third stage (N = 903, but analysed 810)
Control/comparison intervention: mixed management of 3rd stage (N = 899, but analysed 821) Description: mixed: (no routine uterotonic; early cord clamping, no CCT)
Data used in comparison 11 |
|
| Outcomes |
Pre‐specified outcomes: primary outcome was the incidence of blood loss > 1000 mL during the 3rd stage of labour. Other outcomes: Hb at 24 h and women's views Reported outcomes: blood loss > 1000 mL and > 500 mL during the 3rd stage of labour, blood loss > 1000 mL and > 500 mL in first 2 h PP, Hb at 24 h, change in Hb from antenatal to 24 h postnatal, retained placenta/retained part of placenta or membranes, additional uterotonics, duration of 3rd stage. Blood transfusion, units transfused, experience of mothers, afterpains Outcomes obtained by email response 28 April 2011: manual removal of placenta alone, cross‐over in additional uterotonics, Hb < 9 g/dL at 24‐48 h, length of 3rd stage > 60 min Outcomes obtained by email response 26 May 2011 clarification of cross‐over in additional uterotonics, blood loss > 500 mL and > 1000 mL during 3rd stage of labour separated from blood loss > 500 mL and > 1000 mL in first 2 h PP |
|
| Notes | We wrote to the study author for additional information which was provided as follows: "In the active group, 41 women had extra Synt, therapeutically, before the placenta (some went on to have a second dose, and/or Methergin) = 41 767 had no extra Synt before the placenta. Of these, 26 had a second dose after the placenta was delivered, 6 had a second dose of Synt and a dose of Methergin and 48 had Methergin but no Synt = 80 IN TOTAL, IN THE ACTIVE GROUP, 121 WOMEN HAD THERAPEUTIC UTEROTONICS. In the expectant group, 160 women had a dose of Synt, therapeutically, before the placenta (some went on to have a second dose, and/or Methergin) = 160 655 had no Synt before the placenta. Of these, 102 had a dose of Synt after the placenta was delivered, 32 had a dose of Synt and a dose of Methergin and 17 had Methergin but no Synt. = 151 IN TOTAL, IN THE EXPECTANT GROUP, 311 WOMEN HAD THERAPEUTIC UTEROTONICS." In further correspondence, the author replied: "Blood loss before and during placenta delivery: IN ACTIVE GROUP: 0 to 500 mL = 86.57% (n = 696) > 500 mL = 13.43% (n = 108) and 0 to 1000 mL = 96.39% (n = 775) > 1000 mL = 3.61% (n = 29) Blood loss before and during placenta delivery: IN EXPECTANT GROUP: 0 to 500 mL = 76.25% (n = 623) > 500 mL = 23.75% (n = 194) and 0 to 1000 mL = 95.3% (n = 779) > 1000 mL = 4.65% (n = 38)" And also: Question: Number of women having retained placenta and manual removal of placenta (excluding those who had retained pieces of membrane, etc, if possible) Answer: "in total 47 manual removal of the placenta were performed. In active group 26 in expectant group 21." Question: Number of maternal haemoglobin <9gm/dl at 24‐48 hrs Answer: "In total there were 62 women with Hb < 90. In active group 34 women, and in expectant group 28 women" Question: Number of lengths of third stage > 60 minutes. Answer: "In total there were 54 women with third stge that exceede 60 min. In active group 31 women and in expectant group 23 women." Question: Was 'mean blood loss before placenta' actually 'blood loss up to and including expulsion of placenta'? Answer: "mean bloodloss before placenta was expelled is refered to as before and during expulsion." Dates of study: November 2006‐April 2008 Funding sources: "The study was supported by grants from the Research and Development Board in Göteborg and Bohuslän Baby Bag and the SU foundation". Declarations of interest: "The authors have no conflicts of interest and are independent from industrial funding" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the computer‐generated randomisation group were prepared in consecutive order and kept in another unit. At randomisation, midwives phoned the staff at the other unit who opened the sealed envelopes and disclosed the assigned intervention and study number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Received an injection in both groups to “blind”, but expectant group were asked to push and did not have CCT, active group had CCT Clinicians not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinicians not blinded and clinicians involved in many important outcomes decisions/assessments, e.g. blood loss, therapeutics uterotonics, blood transfusions. Other outcomes could have been blinded, e.g. Hb |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 903 women randomised to the active group and 899 women randomised to the mixed management group:
Overall 10% and 9% The analysis was ITT, apart from above exclusions |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods are reported, but no protocol for the study is available. |
| Other bias | High risk | There were more inductions in the active group (10% versus 7%). Describe any differential diagnosis: none Although 11,000 women were potentially eligible, of whom at least half would usually be considered eligible, only 1802 were entered into the study. Numbers excluded due to ineligibility are not recorded, and other reasons given are "excessive workload" or "admission in advanced labour". Email response from Jangsten 28 April 2001: "the hard workload was one reason that the women were not included and that all eligible women were not asked to participate. Few women refused in participating but I don't have the number". This has the potential to have biased the study, as midwives would have had the choice of not asking the women to participate and may unconsciously have not offered participation to some women who they felt were not suitable for physiological management. |
Jerbi 2007.
| Methods | RCT of individual women in low‐income setting | |
| Participants |
Setting: Sousse, Tunisia. Low‐income country Women with singleton pregnancies expecting to give birth vaginally Exclusion criteria: placenta praevia, APH, non‐cephalic presentation, intrauterine death, parity > 5, uterine fibroids, anticoagulation therapy, history of PPH, history of CS |
|
| Interventions |
Intervention: active management of 3rd stage (N = 65)
Comparison: mixed management of 3rd stage (N = 65)
Data included in comparison 12 |
|
| Outcomes | Pre‐specified: reduction in HCT and Hb | |
| Notes | We contacted the study authors again on 14 March 2011, for further information on the management in the comparison arm, the methodology they used and data obtained. Reply received 19 March 2011. No publication has emanated, no further data were provided, but methodology was clarified Dates of study: February‐March 2005 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | As per www.randomization.com (not stated in publication but information provided by study author on 19 March 2011) |
| Allocation concealment (selection bias) | High risk | Not concealed in any way (not stated in publication, but in information provided by study author on 19 March 2011) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. Unblinded assessment made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss described in publication and confirmed by information from study author on 19 March 2011. Study authors also provided information that the analysis was by "intention to treat" |
| Selective reporting (reporting bias) | High risk | No study protocol found. Some outcomes reported but not noted in methods of paper |
| Other bias | High risk | Women reported to have been allocated to groups after placental delivery yet active management group supposedly had oxytocin with the anterior shoulder |
Khan 1997.
| Methods | RCT with randomisation of individual women | |
| Participants |
Abu Dhabi hospital setting. High‐income country Inclusion criteria: all women expected to give birth vaginally. 1657 women randomised out of a possible 4239 Exclusion criteria: refusal or CS in second stage (9 excluded, final sample 1648) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 827)
Comparison: mixed management of 3rd stage (N = 821)
In both groups, if placenta not delivered after 30 min, CCT or digital removal attempted, with IV oxytocin infusion if bleeding present Data included in comparison 10 |
|
| Outcomes |
Primary: PPH Secondary: duration of 3rd stage, retained placenta, shock, blood transfusion, methylergonovine or 15‐methyl‐a‐prostaglandin to control haemorrhage |
|
| Notes | Not readily comparable to other studies as IV oxytocin infusion given to all women in expectant management group after delivery of placenta. This is the practice in the USA but not elsewhere Dates of study: January‐June 1995 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail |
| Allocation concealment (selection bias) | Unclear risk | Numbered, sealed, opaque envelopes. However, if sequence generation is not random then allocation cannot be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Attempted with 2nd MW recording blood loss – however, no guarantee that the 1st MW could/would not have altered the amount of blood in the receptacle, so not any better than just 1 clinician measuring |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided Presumed blinded, but unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2582 excluded prior to randomisation due to refusal 9 excluded after randomisation due to emergency CS |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods are reported, but there is no protocol for the study available |
| Other bias | High risk | 2582 out of 4239 refused to participate. Those that did agree may have been biased. It is unknown whether or not the midwives had sufficient training in physiological 3rd stage before the study started. This study has been criticised for including all women (including high parity, all age groups, previous PPH, epidural, long labour, operative delivery) and not confining inclusion criteria to women who were low risk. Women at high risk of PPH will have a higher blood loss using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even of low‐risk women, which may result in higher intervention (mixed management) rates. Also, the minimal intervention (control) group had the cord clamped and cut immediately after delivery, which is suspected to lead to an increase in blood loss |
Prendiville 1988.
| Methods | RCT with randomisation of individual women | |
| Participants |
UK hospital setting. High‐income country Inclusion criteria: all women expected to give birth vaginally. 1695 women randomised out of a possible 4709 Exclusion criteria: refusal, cardiac disease, APH, non‐cephalic presentation, multiple pregnancy, IUFD, if clinician had good reason not to include women. After the first 5 months, exclusions included women with ritodrine given 2 h before birth; anticoagulant treatment; any condition needing a particular management of 3rd stage (e.g. meconium‐stained liquor, dural tap) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 846)
Comparison: expectant management of 3rd stage (N = 849)
Data included in comparison 1 |
|
| Outcomes | Pre‐specified outcomes: PPH (and “more objective measures of blood loss”, presumably Hb); length 3rd stage; need for therapeutic oxytocics; manual removal placenta; ERPC; side effects of oxytocics (nausea, vomiting, headaches, hypertension); Apgar scores; PCV; SCBU; jaundice; breastfeeding. Views of a subsample of women | |
| Notes |
Actual management used in the active arm: 99% given prophylactic uterotonic before delivery of placenta; 99% cord clamped and cut before delivery of placenta; 99% CCT; 26% upright Actual management used in the expectant arm: 30% received uterotonic for treatment, and 20% prophylactically; cord left unclamped till pulsation ceased 48%; placenta delivered by maternal effort 60% and CCT 40%; 49% upright Dates of study: 1 January 1986‐31 January 1987 Funding sources: “The maternity and child division at the World Health Organisation, Geneva, provided some additional funds. The national perinatal epidemiology unit is supported by the DHSS” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description of the randomisation given. However, verbal assurances from study authors that sequence generation was random |
| Allocation concealment (selection bias) | Low risk | Quote: “On admission to the labour ward... Correspondingly numbered, sealed opaque envelopes were placed in the woman’s notes...” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the women or the clinicians in any of the studies. Quote: “We were concerned that clinical estimates of blood loss might also be subject to systematic bias between the two study groups as the observer could not be blinded to the management allocated. We therefore studied three maternal haematological variables – namely, postpartum (24‐48 hrs) haemoglobin concentration ≤ 90g/L, mean postpartum packed cell volume and mean change in haemoglobin concentration between about 34 weeks gestation and post partum”. Primary outcome though is still PPH > 500 mL which is subject to systematic bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data for some outcomes, e.g. 19% of Hb results missing in active arm and 18% in the physiological Apparently no women were excluded after randomisation but 182 are described as having not entered in the study due to the cord being cut early for fetal safety reasons. The allocation details, however, state that when the clinician was ready to prepare for delivery, the envelope was opened and "all women for whom an envelope was opened were deemed to have entered the study and were followed up". The envelope would have been opened before any neonatal need for attention became apparent |
| Selective reporting (reporting bias) | High risk | No study protocol found. A large number of outcomes reported but not noted in methods of paper |
| Other bias | High risk | Protocol was modified after 5 months (425 births), due to high blood loss in expectant management group, to allow women in the control arm who needed some active management to be switched to fully active management. However, data for the first 5 months were still included in analysis. Study was stopped early because of potential harm. Sample size was meant to be 3900 but stopped after 1695. 30 women in the control group gave a late maternal refusal, whereas only 1 in the experimental group did so. The outcomes of these women are included in analysis. It is questioned whether the midwives had sufficient training in physiological 3rd stage before the study started. Harding et al found that, of 49 midwives responding to a questionnaire, only 1 had practised physiological management as defined in the study. Only 6 (13%) of the midwives said that they were very confident of physiological management before the study and 22 (46%) afterwards (Harding 1989; Prendiville 1988 paper). This study has been criticised for including all women (including high parity, all age groups, previous PPH, epidural, long labour, operative delivery) and not confining inclusion criteria to women who were low risk. Women at high risk of PPH will have a higher blood loss using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even of low‐risk women, which may result in higher intervention (mixed management) rates. Only 47% (403/849) of women in physiological arm received the full physiological package (a problem with other studies also). But, in particular, 168/849 = 20% had prophylactic oxytocic, which is a large number for a “prophylactic” treatment as opposed to one in response to clinical need. In addition, 252 (30%) had a uterotonic as a treatment, so in total, 50% of the expectant management group received an oxytocic. However, 99% (838/846) of women in active management group received allocated management. |
Rogers 1998.
| Methods | RCT with randomisation of individual women in balanced blocks, with allocation to 1 of 2 delivery postures within each arm | |
| Participants |
UK hospital setting. High‐income country Inclusion criteria: 1512 women at low risk of PPH giving birth at study hospital (including water births) Exclusion criteria: placenta praevia, previous PPH, APH after 20 weeks’ gestation, Hb < 10 g/dL or MCV < 75 fL, non‐cephalic presentation, multiple pregnancy, intrauterine death, epidural anaesthesia, parity > 5, uterine fibroid, oxytocin augmentation infusion, anticoagulation therapy, intended instrumental or OVB, duration of gestation < 32 weeks, (plus any other contraindication, in clinician’s view) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 748) 2 arms: active management ‐ upright position (N = 374); active management ‐ supine position (N = 374)
Control: expectant management of 3rd stage (N = 764) 2 arms: expectant management ‐ upright position (N = 381); expectant management ‐ supine position (N = 383)
Data included in comparisons 1 and 2 |
|
| Outcomes | Pre‐specified outcomes: PPH (> 500 mL) as assessed/estimated by the attending MW (used for power calculation); severe PPH 1000 mL), blood transfusion, iron tablets postnatally, Hb at 24‐48 h P/N, self‐completed questionnaire on maternal fatigue and depression at 6 weeks P/N, nausea, vomiting, headache, hypertension, manual removal of placenta, ERPC, neonatal outcomes, views of mothers and staff | |
| Notes |
Actual management used in the active arm: 699 (93.4%) had full active management; 95% given prophylactic uterotonic before delivery of placenta; 93% cord clamped before pulsation ceased; 46% CCT; 44% upright Actual management used in the expectant arm: 488 (63.9%) had full expectant management; 21% received oxytocic for treatment, and 2.5% prophylactically; cord left unclamped till pulsation ceased 70%; placenta delivered by CCT 12%; 43% upright The setting is described as one where the midwives were “similarly confident” in active and expectant management. However, the questionnaire administered to 92 of the 153 midwives prior to the study commencement showed that, whereas 84% felt “very confident” of active management, only 41% were “very confident” of expectant management. Maternal mean Hb levels were reported with SEs and so we calculated SDs Dates of study: June 1993‐December 1995 Funding sources: “The study was supported by a grant from the Public Health and Operational Research Committee of the Anglia and Oxford Regional Health Authority. The NPEU is supported by the Department of Health”. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Variable sized balanced blocks “...randomisation envelopes were prepared in advance..” in an external academic unit ‐ National Perinatal Epidemiology Unit, Oxford |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes stored on the ward. Entry to the study occurred when an envelope was opened. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Partly blinded. The technicians who did antenatal and postnatal blood tests were unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available on 1507 out of 1512 at discharge (< 0.5% attrition, approximately equal losses in both groups) At 6 weeks' follow‐up < 5% attrition |
| Selective reporting (reporting bias) | High risk | No study protocol found. A large number of outcomes reported but not noted in methods of paper |
| Other bias | High risk |
Also:
|
Thilaganathan 1993.
| Methods | RCT with randomisation of individual women | |
| Participants |
UK hospital setting. High‐income country Inclusion criteria: women at "low risk of PPH” (defined only by the exclusion criteria for study) and at term (37‐42 weeks). 193 women randomised, from an unknown population Exclusion criteria: grand multiparity; malpresentation, multiple pregnancy; previous CS or PPH; APH; pregnancy‐induced hypertension and IUFD Then after randomisation: women who had had augmentation, instrumental or OVB, 3rd degree tear and cervical laceration |
|
| Interventions |
Intervention: active management of 3rd stage (N = 103):
Comparison: expectant management of 3rd stage (N = 90): presumed no oxytocic, though not stated in the published paper Authors’ information by letter states:
Both groups: if placenta not delivered in 30 min, bladder emptied and medical assistance sought. If delivery not imminent manual removal performed. Medical assistance sought for any excessive blood loss Data included in comparisons 1 and 2 |
|
| Outcomes |
Pre‐specified outcomes: estimated blood loss; Hb in labour and 3rd postnatal day; length of 3rd stage; complications Reported outcomes: as above plus therapeutic uterotonics, blood transfusion |
|
| Notes | Drop in Hb is not calculated correctly. Maternal mean postnatal Hb reported as median and range. Active: 11.7 g/dL (10.7‐12.6 g/dL) and expectant 11.7 g/dL (10.9‐12.6 g/dL) Worrying problems with methodology and analysis Dates of study: January 1988‐February 1990 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...randomly allocated using standard randomisation tables...” |
| Allocation concealment (selection bias) | Unclear risk | Not described. Not clear when randomisation occurred Quote: “...the midwife responsible for the management of her patient was not aware of the proposed allocation until her patient was entered into the study” (authors' information states: "randomised in the late 1st stage of labour when it was apparent that they were likely to delivery normally") |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not clear how many were randomised. P. 20 states “A total of 193 women completed the study AND had all results available for complete analysis”. This could mean that a larger number were included but that some of their results were missing, and they were therefore excluded. This could lead to significant bias. It is very unlikely that all participants received the allocated management, yet this is not presented. The study groups were also very different sizes (103 and 93), which sounds unlikely. Women withdrawn after randomisation for operative delivery, third‐degree tears and cervical lacerations. Numbers were not given; there is a significant risk of bias here. It is not stated in the published paper whether or not ITT analysis was used, but the response to Diana Elbourne’s letter of April 1991 states that they did not analyse on "intention to treat" as it would not answer the aims of this preliminary study. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods are reported, but there is no protocol available for the study |
| Other bias | High risk | Variables age, birthweight and parity said to be equal between the groups but no details given. No power calculation done. Not a null hypothesis The study groups were very different sizes (103 and 90) |
Yildirim 2016.
| Methods | RCT with randomisation of individual women | |
| Participants |
Setting: Istanbul Bakirkoy Maternity and Children's Hospital, Turkey. Upper‐middle‐income country (World Bank), so classed as higher‐income setting Inclusion criteria: absence of risk factors for PPH; gestational age of 36–42 weeks; a singleton pregnancy; live fetus; cephalic presentation; expected fetal birthweight of 2500–4500 g; maternal age of < 40 years; parity (min‐max) 0–3 Exclusion criteria: acute fetal distress, conversion to abdominal delivery during labour, need for labour augmentation, persistent high BP (> 140/90 mm Hg), placenta praevia, ablatio placenta or uterine bleeding of any other cause encountered during pregnancy or labour; previous CS; uterine scar; PPH in previous pregnancies; hydramnios; symptoms of maternal infection; drug use in labour; abnormal placentation (accreta, increta or percreta), coagulation defects, forceps or vacuum extraction, Hb concentration of < 8 g/dL; use of anticoagulants and tocolytics during pregnancy, multiple gestations, any known uterine malformations, and deep vaginal lacerations |
|
| Interventions |
Intervention: active management of the 3rd stage Total number randomised: n = 333, but 327 were analysed
Control: expectant management of the 3rd stage ('mixed management' in our review) Total number randomised: n = 336, but 327 were analysed
|
|
| Outcomes | Prepartum Hb and HCT levels, postpartum Hg‐HCT levels, reductions in Hg levels of > and < 3 g/dL, prolonged 3rd stage of labour, transfusion requirement, requirement of additional uterotonics, additional interventions including manual removal of the placenta, and the requirement of additional surgery or curettage | |
| Notes |
Dates of study: 2010 Funding sources: Kanuni Sultan Suleyman Education and Research Hospital (email from lead author) Declarations of interest: “The authors declare that they have no conflicts of interest with any third party and that they have no financial disclosure to be made” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The abstract reports “women were randomly assigned” however, no description is given in relation to this process. We contacted the lead author who responded: “We prepared 1000 sealed opaque envelopes assigned for each group; 500 were tagged with ‘active management’, and the other 500 with ‘expectant management’. We shuffled the envelopes and put them in a box… Allocation took place when vaginal delivery was imminent, at the end of the second stage of labor by pulling out one of the envelopes out of the box randomly.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocation concealment was made using sealed opaque envelopes and took place when vaginal delivery was imminent, at the end of the second stage of labor.” Additional information from the lead author stated, quote: “We shuffled the envelopes and put them in a box. Allocation took place when vaginal delivery was imminent, at the end of the second stage of labor by pulling out one of the envelopes out of the box randomly….We tagged papers with active or expectant management. Then we put them in to the envelopes (with nothing written on the outside). Thus, the performer was not able to see which protocol was written inside the envelope.” Although the sequence generated was random, the envelopes were not sequentially numbered and so the random sequence could not be guaranteed as per the Cochrane Handbook of Systematic Reviews of Interventions, which says, “If investigators use envelopes, they should develop and monitor the allocation process to preserve concealment. In addition to use of sequentially numbered, opaque, sealed envelopes, they should ensure that the envelopes are opened sequentially, and only after the envelope has been irreversibly assigned to the participant." (Higgins 2017) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and clinicians cannot be blinded to the different management of care associated with active and expectant management. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “only the analyser of the outcome data was blinded to the study groups because the performer could not be blinded to the allocated management protocol”. However, we deemed this domain to be of unclear risk of bias as non‐blinded clinicians were involved in many important outcome decisions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 women out of 669 women (2.2%) were excluded after randomisation due to deep vaginal lacerations. |
| Selective reporting (reporting bias) | Unclear risk | No protocol for the study is available. All outcomes reported in the methods are reported plus one outcome (atony) reported but not mentioned in methods. |
| Other bias | Unclear risk | Baseline characteristics were similar (age, gestational week, body mass index, parity, episiotomy, and mean length of labour). However, there is little methodological information in the publication on which to judge potential for bias – mostly the information is about clinical care. The study authors do not identify the profession of the clinicians, nor is it clear if the healthcare practitioners received any education/training in relation to expectant and active management of the 3rd stage of labour before this published study. However, the lead author provided clarification on this reporting: “We had to train the performers for the active management protocol (especially for the controlled cord traction) which was not a common practice in our hospital then.” Exclusion of women with PPH due to deep vaginal lacerations (6/333 in active management group and 9/336 in expectant management group), although numbers are low, this could contribute to bias. |
APH: antepartum haemorrhage; BP: blood pressure; CCT: controlled cord traction; CS: caesarean section; ERPC: evacuation of retained products of conception; fL: femtolitre; GA: general anaesthesia; g/dL: grams/decilitre; Hb: haemoglobin; HCT: haematocrit (= packed cell volume); IM: intramuscular; ITT: intention‐to‐treat; IU: international units; IUFD: intrauterine fetal death; IV: intravenous; MCV: mean corpuscular volume; mL: millilitre; MW: midwife; OVB: operative vaginal birth; PCV: packed cell volume (= haematocrit); P/N: postnatal; PPH: postpartum haemorrhage; PP: postpartum; RCT: randomised controlled trial; SCBU: special care baby unit; SD: standard deviation; SE: standard error; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aleem 2010 | This study looked at uterine massage vs active management vs active management plus uterine massage, so it was not a comparison of active and expectant management within the definitions used in the review. |
| Deneux‐Tharaux 2013 | This study was an RCT of active management with and without CCT, so it was not a comparison of active and expectant management within the definitions used in the review. |
| Gulmezoglu 2012 | This study was an RCT of active management with and without CCT, so it was not a comparison of active and expectant management within the definitions used in the review. |
| Hoffman 2006 | Conference abstract available only, but further information on methodology obtained from study authors. Concerns re number of women withdrawn, after randomisation, due to CS |
| Kashanian 2010 | 48% of participants excluded in both arms following randomisation |
| Magann 2006 | This study looked at different times of undertaking manual removal of placenta to try to reduce PPH, so it was not a comparison of active and expectant management within the definitions used in the review. |
| Muller 1996 | French conference abstract only, no full publication identified. The translation provided no information on the number of women randomised to each group and so it was not possible to use these data. Previous review authors wrote for further information but had no response. We wrote and received a response from the co‐author, but no further details to add to the published information. |
| Neri‐Mejia 2016 | An RCT of 3 different types of oxytocin administration (IM, IV and infusion) |
| Ramirez 2001 | Insufficient information on the numbers included in each of the 3 arms, and the method of management for the expectant arm. |
| Vasegh 2005 | Insufficient information |
CCT: controlled cord traction; CS: caesarean section; IM: intramuscular;IV: intravenous; PPH: postpartum haemorrhage; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Rosario 1973.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting a copy of the paper |
Differences between protocol and review
We did not carry out additional searching as proposed. We decided after looking at the variation in the interventions and controls used in the studies that we had clinical heterogeneity between the studies (Table 2) and so we have used a random‐effects meta‐analysis throughout the review.
We have changed the labels on the secondary outcomes of 'Primary postpartum haemorrhage (> 500 mL)' and 'Secondary postpartum haemorrhage (> 500 mL)' to 'Primary maternal blood loss > 500 mL' and 'Secondary maternal blood loss > 500 mL' respectively. This is because we believe that in research the term 'haemorrhage' should be reserved for excessive blood loss. We have also included reference to the mean Hb values in order to provide an outcome that was calculated by blinded personnel.
We have modified the wording in the methods sections for 'Assessment of heterogeneity', 'Assessment of reporting biases' and 'Data synthesis' to update them with the new methods being used by the group, developed in conjunction with the group's statisticians, Simon Gates and Richard Riley. We have used these new methods in the review.
We decided to reduce the number of outcomes, in line with the Cochrane Handbook of Systematic Reviews of Interventions' recommendations. We made the following changes to outcomes.
Nine primary outcomes reduced to seven
Clinical signs of severe blood loss at the time of birth, e.g. woman feeling breathless, weak, faint, pale, exhausted: moved to secondary outcomes list.
Evidence of acidaemia indicated by a pH less than seven or base deficit greater than 12 mmol/L in umbilical arterial cord blood, or (c) neonatal blood sample in first hour of life, or both: removed.
Apgar score less than 7 at five minutes, and neonatal ('hypoxic ischaemic') encephalopathy assessed using Sarnat staging: moved to secondary outcomes list.
Changes to primary outcomes
All PPH amounts and mean blood losses are now expressed at three time periods "at the time of the birth", "after delivery of placenta and up to 24 hours", and "at the time of birth and up to 24 hours". This was done because it was noted that the two primary outcomes "Severe primary PPH (clinically estimated or measured blood loss greater than or equal to 1000 mL at time of birth and up to 24 hours)" and "Very severe primary PPH (clinically estimated or measured blood loss greater than or equal to 2500 mL at time of birth and up to 24 hours)", which were based on the international definition of postpartum haemorrhage (PPH) could, in fact, provide misleading results if study authors measured or estimated blood loss at birth, and over a period of some hours in the first 24 hours, and added all amounts together to provide an overall PPH rate. While this estimate could also be useful, it raises the PPH rate artificially in comparison with studies that do not do this. Accordingly, we have changed the first two primary outcomes to “Severe primary postpartum haemorrhage (clinically estimated or measured blood loss greater than or equal to 1000 mL at time of birth)” and “Very severe primary PPH (clinically estimated or measured blood loss greater than or equal to 2500 mL at time of birth)” and have included the original definitions as secondary outcomes.
"Maternal Hb concentration less than 9 g/dL 24 to 48 hours postpartum" changed to "Maternal Hb concentration less than 9 g/dL 24 to 72 hours postpartum" ‐ as Hb levels may be taken within the first three days postnatal, rather than the first two.
Secondary outcomes deleted (to reduce number of outcomes)
Iron therapy during the puerperium.
Length of the third stage greater than or equal to 60 minutes.
Nausea between birth of baby and discharge from the labour ward.
Headache between birth of baby and discharge from the labour ward.
Maternal views of third‐stage management (assessed using a validated questionnaire).
Maternal Hb concentration less than 9 g/dL postdischarge and up to six weeks.
Sequelae of PPH (length of stay; infection; re‐admission).
Infant Hb level and iron indices beyond three months.
Changes to secondary outcomes
"Administration of oral or rectal analgesia (e.g. paracetamol, codeine, non‐steroidals) between birth of the baby and discharge from the labour ward" and "Administration of opiate analgesia between birth of the baby and discharge from the labour ward" combined as "Administration of any analgesia between birth of the baby and discharge from the labour ward".
"Secondary blood loss equal to or greater than 500 mL (clinically estimated or measured after 24 hours and before six weeks)" and "Any vaginal bleeding needing treatment (after 24 hours and before six weeks)" and "Uterotonic treatment after 24 hours and before six weeks" combined as "Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks)".
"Infant Hb level (Hb) at 24 to 48 hours" changed to "at 24 to 72 hours", as Hb levels may be taken within the first three days postnatal, rather than the first two.
"Intraventricular haemorrhage (preterm infants): (i) grade III/IV; (ii) all grades (Sarnat 1976)" changed to "Papille grade III/IV intraventricular haemorrhage (for infants born before 34 weeks' gestation only)".
"Transfusion requirements (preterm infants): (i) number of infants exposed to one or more red blood cell transfusions; (ii) number of transfusions per infant; (iii) number of donors to whom the infant was exposed" changed to "Number of infants who received a red blood cell transfusion".
"Breastfeeding at discharge from hospital and at interval assessments until six months" changed to "Exclusive breastfeeding at discharge from hospital".
New secondary outcomes included
"Neonatal mortality" included
Search methods
For the 2018 update we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Types of interventions
We included the following three comparisons as we added studies that had used them to this review update (2018).
Comparison 9: active versus mixed management of the third stage of labour with uterotonic after placental delivery, immediate cord clamping and no controlled cord traction Comparison 10: active versus mixed management of the third stage of labour with no routine uterotonic, early cord clamping, no controlled cord traction. Comparison 11: active versus mixed management of the third stage of labour with no routine uterotonic, early cord clamping, controlled cord traction.
We deleted the fifth subgroup analysis ‘low risk of PPH versus high risk of PPH’ as we realised that the analysis of studies undertaken with women in the low risk group was in fact a separate comparison.
We changed the original subgroups of 'high‐income country' and 'low‐income country' to 'higher‐income setting' and 'lower‐income setting', based on the World Bank definitions, with the border between the lower‐middle‐income and upper‐middle‐income being the cut‐off point (World Bank definitions 2018).
Contributions of authors
Cecily Begley (CB) drafted the background section and all other authors contributed to editing the text. All review authors contributed to the drafting of the inclusion criteria for the review. Gill Gyte (GG) and Declan Devane (DD) added the methodology section with other authors commenting. CB and GG abstracted and pooled data. William McGuire checked data entry, DD prepared the 'Summary of findings' table, which was checked by Andrew Weeks. GG, DD and CB wrote the results section with other authors commenting. CB and GG wrote the discussion and implications sections, with input from all authors. For this update, GG and LB updated the RoB sections, DD and LB updated the methods, AW and CB updated the data entry and analysis, CB and WM updated the SoF table. Cecily Begley is the guarantor of the review.
Sources of support
Internal sources
(GG) The University of Liverpool, UK.
External sources
-
(GG) National Institute for Health Research, UK.
2009 Version of this review was supported by the NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS02
Human Reproduction Programme. World Health Organization. Geneva, Switzerland.
Declarations of interest
Cecily Begley: was the lead researcher on the 'Dublin trial' (Begley 1990). Gill Gyte, Declan Devane, and a member of the Cochrane Pregnancy and Childbirth Group's staff independently reviewed Begley's paper and agreed inclusion in the review. GG and DD extracted data.
Gill Gyte: I have written extensively on third‐stage management and was a co‐applicant on a five year study of care at preterm birth which included a pilot randomised controlled trial of delayed cord clamping with immediate neonatal care with cord intact versus early cord clamping (funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme [RPPG‐0609‐10107]). I am currently a Public and Patient Involvement representative on the Trial Management Group on a Health Technology Assessment funded clinical trial of carboprost versus oxytocin as first line treatment of primary postpartum haemorrhage, led by Professor Andrew Weeks based at University of Liverpool, UK. I also received royalties from John Wiley & Sons in respect of ‘A Cochrane Pocketbook – Pregnancy and Childbirth’ Hofmeyr GJ et al. 2008.
Declan Devane: was a member of the Data Monitoring Board for the Cord pilot trial ‐ immediate versus deferred cord clamping for very preterm birth (before 32 weeks' gestation). (The study is not included in this review.)
William McGuire: none known
Andrew Weeks: has been on a programme grant related to the timing of cord clamping, as well as investigating the use of misoprostol for postpartum haemorrhage prophylaxis in rural Uganda (Weeks AD, Ditai J, Ononge S, Faragher B, Frye LJ, Durocher J, Mirembe FM, Byamugisha J, Winikoff B, Alfirevic Z. The MamaMiso study of self‐administered misoprostol to prevent bleeding after childbirth in rural Uganda: a community‐based, placebo‐controlled randomised trial. BMC Pregnancy Childbirth. 2015 Sep 14;15:219). He is also one of nine designers of a small resuscitation trolley (the BASICS trolley; Weeks AD, Watt P, Yoxall CW, Gallagher A, Burleigh A, Bewley S, Heuchan AM, Duley L. Innovation in immediate neonatal care: development of the Bedside Assessment, Stabilisation and Initial Cardiorespiratory Support (BASICS) trolley. BMJ Innov. 2015 Apr;1(2):53‐58) that allows neonatal resuscitation with an intact cord and the inventor of the PPH Butterfly, a device to allow minimally invasive uterine compression to treat postpartum haemorrhage (Cunningham C, Watt P, Aflaifel N, Collins S, Lambert D, Porter J, Lavender T, Fisher T, Weeks A. PPH Butterfly: a novel device to treat postpartum haemorrhage through uterine compression. BMJ Innov. 2017 Feb;3(1):45‐54.).
Linda M Biesty: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Begley 1990 {published and unpublished data}
- Begley CM. Comparative Studies in the Third Stage of Labour [thesis]. Dublin: Trinity College, University of Dublin, Ireland, 1989. [Google Scholar]
- Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3‐17. [DOI] [PubMed] [Google Scholar]
- Begley CM. A comparison of physiological and pharmacological methods of managing the third stage of labour. Personal communication 1987.
- Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60‐72. [DOI] [PubMed] [Google Scholar]
Jangsten 2011 {published and unpublished data}
- Jangsten E, Bergh I, Mattsson LA, Hellstrom AL, Berg M. Afterpains: a comparison between active and expectant management of the third stage of labor. Birth 2011;38(4):294‐301. [DOI] [PubMed] [Google Scholar]
- Jangsten E, Mattsson LA, Lyckestam I, Hellstrom AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG 2011;118(3):362‐9. [DOI] [PubMed] [Google Scholar]
- NCT01221051. A comparison of active and expectant management of the third stage of labor. clinicaltrials.gov/ct2/show/NCT01221051 (first received 14 October 2010).
Jerbi 2007 {published data only}
- Jerbi M, Hidar S, Elmoueddeb S, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2007;96(3):198‐9. [DOI] [PubMed] [Google Scholar]
Khan 1997 {published data only}
- Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomised controlled trial. American Journal of Obstetrics and Gynecology 1997;177(4):770‐4. [DOI] [PubMed] [Google Scholar]
Prendiville 1988 {published data only}
- Elbourne DR, Harding J. The Bristol third stage trial. Proceedings of Research and the Midwives Conference; 1989; Manchester, UK. 1989:19‐31.
- Harding JE, Elbourne DR, Prendiville WJ. Views of mothers and midwives participating in the Bristol randomized, controlled trial of active management of the third stage of labor. Birth 1989;16:1‐6. [DOI] [PubMed] [Google Scholar]
- Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297:1295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 1998 {published data only}
- ISRCTN63422923. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. isrctn.com/ISRCTN63422923 (first received 23 January 2014).
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial [see comments]. Lancet 1998;351(9104):693‐9. [DOI] [PubMed] [Google Scholar]
- Wood J, Rogers J, Elbourne D, McCandlish R, Truesdale A. The Hinchingbrooke third stage trial. International Confederation of Midwives 24th Triennial Congress; 1996 May 26‐31; Oslo, Norway. 1996:140.
Thilaganathan 1993 {published data only}
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19‐22. [DOI] [PubMed] [Google Scholar]
Yildirim 2016 {published data only}
- Yildirim D, Ozyurek SE, Ekiz A, Eren EC, Hendem DU, Bafali O, et al. Comparison of active vs. expectant management of the third stage of labor in women with low risk of postpartum hemorrhage: a randomized controlled trial. Ginekologia Polska 2016;87(5):399‐404. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Aleem 2010 {published data only}
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Deneux‐Tharaux 2013 {published data only}
- Deneux‐Tharaux C, Sentilhes L, Maillard F, Closset E, Vardon D, Lepercq J, et al. Effect of routine controlled cord traction as part of the active management of the third stage of labour on postpartum haemorrhage: multicentre randomised controlled trial (TRACOR). BMJ (Clinical Research Ed.) 2013;346:f1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulmezoglu 2012 {published data only}
- Gulmezoglu AM, Lumbiganon P, Landoulsi S, Widmer M, Abdel‐Aleem H, Festin M, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non‐inferiority trial. [Erratum appears in Lancet. 2012 May 5;379(9827):1704]. Lancet 2012;379(9827):1721‐7. [DOI] [PubMed] [Google Scholar]
- Gulmezoglu AM, Widmer M, Merialdi M, Qureshi Z, Piaggio G, Elbourne D, et al. Active management of the third stage of labour without controlled cord traction: a randomized non‐inferiority controlled trial. Reproductive Health 2009;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2006 {published data only}
- Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S107. [Google Scholar]
- Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S82. [Google Scholar]
- NCT00473707. A randomized trial of active versus expectant management of the third stage of labor. clinicaltrials.gov/show/NCT00473707 (first received 15 May 2007).
Kashanian 2010 {published data only}
- Kashanian M, Fekrat M, Masoomi Z, Ansari NS. Comparison of active and expectant management on the duration of the third stage of labour and the amount of blood loss during the third and fourth stage of labour: a randomised controlled trial. Midwifery 2010;26(2):241‐5. [DOI] [PubMed] [Google Scholar]
Magann 2006 {published data only}
- Magann EF, Doherty DA, Briery CM, Niederhauser A, Chauhan SP, Morrison JC. Obstetric characteristics for a prolonged third stage of labor and risk for postpartum hemorrhage. Gynecologic and Obstetric Investigation 2008;65(3):201‐5. [DOI] [PubMed] [Google Scholar]
- Magann EF, Doherty DA, Briery CM, Niederhauser A, Morrison JC. Timing of placental delivery to prevent post‐partum haemorrhage: lessons learned from an abandoned randomised clinical trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46(6):549‐51. [DOI] [PubMed] [Google Scholar]
Muller 1996 {published data only}
- Muller R, Beck G. Active management of the third stage of labour. 19th Swiss Congress of the Swiss Society of Gynecology and Obstetrics; 1996 June; Interlaken, Switzerland. 1996.
Neri‐Mejia 2016 {published data only}
- Neri‐Mejia M, Pedraza‐Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306‐13. [PubMed] [Google Scholar]
Ramirez 2001 {published data only}
- Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? preliminary results [abstract]. Journal of Perinatal Medicine 2001;Suppl 1(Pt 2):364. [Google Scholar]
Vasegh 2005 {published data only}
- Vasegh FR, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labor. HAYAT: The Journal of Tehran Faculty of Nursing & Midwifery 2005;10(23):102. [Google Scholar]
References to studies awaiting assessment
Rosario 1973 {published data only}
- Rosario YP Do, Jain CK. Active management of third stage of labour. Journal of Obstetrics and Gynaecology of India 1973;23(1):66‐9. [Google Scholar]
Additional references
Abouzaher 1998
- Abouzaher C. Antepartum and postpartum haemorrhage. In: Murray CJL, Lopez AD editor(s). Health Dimensions of Sex and Reproduction. Boston: Harvard University Press, 1998:172‐4. [Google Scholar]
Adnan 2018
- Adnan N, Conlan‐Trant R, McCormick C, Boland F, Murphy DJ. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ 2018;362:k3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bais 2004
- Bais J, Eskes M, Pel M, Bonsel G, Bleker O. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;115(2):166‐72. [DOI] [PubMed] [Google Scholar]
Begley 2009
- Begley C, Devane D, Clarke M. An Evaluation of Midwifery‐Led Care in the Health Service Executive North Eastern area: the Report of the MidU Study . Dublin: Health Service Executive, December 2009. [Google Scholar]
Begley 2011a
- Begley C, Devane D, Clarke M, McCann C, Hughes P, Reilly M, et al. Comparison of midwife‐led and consultant‐led care of healthy women at low risk of childbirth complications in the Republic of Ireland: a randomised trial. BMC Pregnancy and Childbirth 2011;11:85‐94. [DOI: 10.1186/1471-2393-11-85] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2012
- Begley C, Guilliland K, Dixon L, Reilly M, Keegan C. Irish and New Zealand midwives' expertise in expectant management of the third stage of labour: the 'MEET' study. Midwifery 2012;28:733‐9. [DOI: 10.1016/j.midw.2011.08.008] [DOI] [PubMed] [Google Scholar]
Blackburn 2008
- Blackburn S. Physiological third stage of labour and birth at home. In: Edwins J editor(s). Community Midwifery Practice. Oxford: Blackwell Publishing, 2008. [Google Scholar]
Bloomfield 1990
- Bloomfield TH, Gordon H. Reaction to blood loss at delivery. Journal of Obstetrics and Gynaecology 1990;10(Suppl 2):S13‐S16. [Google Scholar]
Bohren 2017
- Bohren MA, Hofmeyr GJ, Sakala C, Fukuzawa RK, Cuthbert A. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD003766.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnar 1970
- Bonnar J, McNicol GP, Douglas AS. Coagulation and fibrinolysis mechanisms during and after normal childbirth. British Medical Journal 1970;2(103):200‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckley 2004
- Buckley SJ. Undisturbed birth ‐ nature's hormone blueprint for safety, ease and ecstasy. MIDIRS Midwifery Digest 2004;14(2):203‐9. [Google Scholar]
Bullough 1989
- Bullough CH, Msuku RS, Karonde L. Early suckling and postpartum haemorrhage: controlled trial in deliveries by traditional birth attendants. Lancet 1989;2(8662):522‐5. [DOI] [PubMed] [Google Scholar]
Burnley 2006
- Burnley M, Roberts CL, Thatcher R, Doust JH, Jones AM. Influence of blood donation on O2 uptake on‐kinetics, peak O2 uptake and time to exhaustion during severe‐intensity cycle exercise in humans. Experimental Physiology 2006;91:499‐509. [DOI] [PubMed] [Google Scholar]
Cernadas 2006
- Cernadas JM, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C, et al. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized, controlled trial. Pediatrics 2006;117(4):e779‐e786. [DOI] [PubMed] [Google Scholar]
Chaparro 2006
- Chaparro CM, Neufeld LM, Tena Alavez G, Eguia‐Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet 2006;367(9527):1997‐2004. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dencker 2017
- Dencker A, Smith V, McCann C, Begley C. Midwife‐led maternity care in Ireland – a cohort study. BMC Pregnancy and Childbirth 2017;17:101‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devane 2007
- Devane D, Begley C, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth 2007;34(2):164‐72. [DOI] [PubMed] [Google Scholar]
Dixon 2013
- Dixon L, Tracy S, Guilliland K, Fletcher L, Hendry C, Pairman S. Outcomes of physiological and active third stage labour care amongst women in New Zealand. Midwifery 2013;29:67–74. [DOI: 10.1016/j.midw.2011.11.003] [DOI] [PubMed] [Google Scholar]
Elbourne 1995
- Elbourne D. Care in the third stage of labour. In: Robinson S, Thomson AM editor(s). Midwives, Research and Childbirth. Vol. 4, London: Chapman & Hall, 1995:192‐207. [Google Scholar]
Farrar 2009a
- Farrar D, Airey R, Tuffnell D, Law G, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with the cord intact. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(Suppl 1):Fa7. [DOI] [PubMed] [Google Scholar]
Farrar 2009b
- Farrar D, Airey R, Tuffnell D, Duley L. Care during the third stage of labour: a postal survey of obstetricians and midwives. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(Suppl 1):Fa40. [Google Scholar]
Fleiss 1981
- Fleiss JL. Statistical Methods for Rates and Proportions. 2nd Edition. New York: John Wiley & Sons, 1981. [Google Scholar]
Fry 2007
- Fry J. Physiological third stage of labour: support it or lose it. British Journal of Midwifery 2007;15(11):693‐5. [Google Scholar]
Gallos 2018
- Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011689.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (Editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. gdt.gradepro.org/app/handbook/handbook.html.
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greer 1998
- Greer I, Lang G, Patel N. The management of postpartum haemorrhage: a clinical practice guideline for professionals involved in maternity care in Scotland. SPCERH publication 6. www.abdn.ac.uk/spcerh/pubs.shtml#2002 1998 (accessed 2008).
Gyte 1992
- Gyte G. The significance of blood loss at delivery. MIDIRS Midwifery Digest 1992;2(1):88‐92. [Google Scholar]
Gyte 1994
- Gyte GM. Evaluation of the meta‐analyses on the effects, on both mother and baby, of the various components of 'active' management of the third stage of labour. Midwifery 1994;10(4):183‐99. [DOI] [PubMed] [Google Scholar]
Gyte 2006
- Gyte G. The third stage of labour. Part 2: active management of third stage. National Childbirth Trust New Digest 2006;36:22‐8. [Google Scholar]
Harding 1989
- Harding JE, Elbourne DR, Prendiville WJ. Views of mothers and midwives participating in the Bristol randomized, controlled trial of active management of the third stage of labor. Birth 1989;16:1‐6. [DOI] [PubMed] [Google Scholar]
Harris 2004
- Harris T. Care in the third stage of labour. In: Henderson C, MacDonald S editor(s). Mayes Midwifery. Edinburgh: Bailliere Tindall, 2004:507‐23. [Google Scholar]
Harris 2006
- Harris T. An explanation for third stage practice variation: the theory of contingent decision making. Normal Labour and Birth: 3rd Research Conference; 2006 June 7‐9; Grange‐over‐Sands, England, UK. 2006.
Herman 2002
- Herman A, Zimerman A, Arieli S, Tovbin Y, Bezer M, Bukovsky I, et al. Down‐up sequential separation of the placenta. Ultrasound in Obstetrics & Gynecology 2002;19:278‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook. London: Cochrane.
Hofmeyr 2015
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2007
- Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full‐term neonates: systematic review and meta‐analysis of controlled trials. JAMA 2007;297:1241‐52. [DOI] [PubMed] [Google Scholar]
Hytten 2001
- Hytten F. The physiology of the puerperium. In: Chamberlain G, Steer P editor(s). Turnbull's Obstetrics. 3rd Edition. Edinburgh: Churchill Livingstone, 2001:635‐46. [Google Scholar]
ICM 2008
- International Confederation of Midwives. Role of the Midwife in Physiological Third Stage Labour: Position Statement. The Hague: International Confederation of Midwives, 2008. [Google Scholar]
ICM‐FIGO 2003
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Management of the third stage of labour to prevent post‐partum haemorrhage. Joint statement. internationalmidwives.org/assets/uploads/documents/FIGO/PPH%20Joint%20Statement.pdf 2008 (accessed 26 January 2018).
ICM‐FIGO 2006
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Prevention and treatment of post‐partum haemorrhage: new advances for low resource settings. Joint statement. www.figo.org/docs/PPH%20Joint%20Statement%202%20English.pdf 2006 (accessed 26 January 2018).
Inch 1985
- Inch S. Management of third stage of labour ‐ another cascade of intervention?. Midwifery 1985;1(2):114‐22. [Google Scholar]
Kanikosmay 2007
- Kanikosmay F. Third stage: the why of physiological practice. Midwives, the official journal of the Royal College of Midwives 2007;10(9):422‐5. [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. [DOI] [PubMed] [Google Scholar]
Lazarus 2005
- Lazarus JV, Lalonde A. Reducing postpartum haemorrhage in Africa. International Journal of Gynecology & Obstetrics 2005;88(1):89‐90. [DOI] [PubMed] [Google Scholar]
Lewis 2007
- Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers' Lives: reviewing maternal deaths to make motherhood safer‐ 2003‐2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH, 2007. [Google Scholar]
Liabsuetrakul 2018
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD005456.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maughan 2006
- Maughan KL, Heim SW, Galazka SS. Preventing postpartum hemorrhage: managing the third stage of labor. American Family Physician 2006;73(6):1025‐8. [PubMed] [Google Scholar]
McDonald 2003
- McDonald S. Physiology and management of the third stage of labour. In: Fraser DM, Cooper MA editor(s). Myles Textbook for Midwives. Edinburgh: Churchill Livingstone, 2003:507‐30. [Google Scholar]
McDonald 2007a
- McDonald S. Management of the third stage of labour. Journal of Midwifery and Women's Health 2007;52(3):254‐61. [DOI] [PubMed] [Google Scholar]
McDonald 2007b
- McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2013
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004074.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2019
- Meher S, Cuthbert A, Kirkham JJ, Williamson P, Abalos E, Aflaifel N, et al. Core outcome sets for prevention and treatment of postpartum haemorrhage: an international Delphi consensus study. BJOG 2019;126(1):83‐93. [DOI: ] [DOI] [PubMed] [Google Scholar]
Mercer 2000
- Mercer JS, Nelson CC, Skovgaard RL. Umbilical cord clamping: beliefs and practices of American nurse‐midwives. Journal of Midwifery & Women's Health 2000;45(1):58‐66. [DOI] [PubMed] [Google Scholar]
Mercer 2001
- Mercer JS. Current best evidence: a review of the literature on umbilical cord clamping. Journal of Midwifery & Women's Health 2001;46(6):402‐14. [DOI] [PubMed] [Google Scholar]
Mercer 2008
- Mercer J, Skovgaard R, Erickson‐Owens D. Fetal to neonatal transition: first do no harm. In: Downe S editor(s). Normal Childbirth: Evidence and Debate. 2nd Edition. Edinburgh: Churchill Livingstone, 2008:149‐74. [Google Scholar]
Mims 2005
- Mims MP, Prchal JT. Hematology during pregnancy . In: Lichtman MA, Williams WJ, Beutler E, Kaushansky K, Kipps TJ, Seligsohn U, et al. editor(s). Williams Haematology. 7th Edition. New York: McGraw Hill Medical, 2005:101‐10. [Google Scholar]
Mousa 2014
- Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Intrapartum care for healthy women and babies. Intrapartum Care for Healthy Women and Babies, Guideline CG190. London: National Institute for Health and Care Excellence, 2014. [Google Scholar]
Nordstrom 1997
- Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology 1997;104(7):781‐6. [DOI] [PubMed] [Google Scholar]
NZCM 2009
- New Zealand College of Midwives. Third stage management practices of midwife lead maternity carers: an analysis of the New Zealand College of Midwives Midwifery Database Information 2004‐2008. Christchurch: New Zealand College of Midwives, 2009. [Google Scholar]
Parsons 2007
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology 2007;29(9):711‐8. [DOI] [PubMed] [Google Scholar]
Penney 2005
- Penney G, Adamson L, Kernaghan D. Scottish confidential audit of severe maternal morbidity. Second Annual Report 2004. Aberdeen. Scottish Programme for Clinical Effectiveness in Reproductive Health. www.abdn.ac.uk/spcerh/pubs.htm#2005 2005 (accessed 2005).
Phillip 2004
- Phillip H, Fletcher H, Reid M. The impact of induced labour on postpartum blood loss. Journal of Obstetrics and Gynaecology 2004;24(1):12‐5. [DOI] [PubMed] [Google Scholar]
Prendiville 1989
- Prendiville WJ, Elbourne DR. Care during the third stage of labour. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:1145‐69. [Google Scholar]
Prendiville 1996
- Prendiville WJ. The prevention of post partum haemorrhage: optimising routine management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:19‐24. [DOI] [PubMed] [Google Scholar]
Rabe 2012
- Rabe H, Diaz‐Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003248.pub3] [DOI] [PubMed] [Google Scholar]
Razvi 2008
- Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;36(2):152‐4. [DOI] [PubMed] [Google Scholar]
RCM 2004
- Royal College of Midwives. Normal Childbirth: Position Statement. London: Royal College of Midwives, 2004. [Google Scholar]
RCM 2008
- Royal College of Midwives. Third Stage of Labour: Midwifery Practice Guideline. London: RCM, 2008. [Google Scholar]
RCOG 2009
- Duley LMM, Weeks AD, Hey EN, Drife JO. Clamping of the umbilical cord and placental transfusion. RCOG Scientific Advisory Committee, Opinion Paper 14 May 2009, issue www.rcog.org.uk/clamping‐umbilical‐cord‐and‐placental‐transfusion.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero‐Gutierrez 2007
- Romero‐Gutierrez G, Espitia‐Vera A, Ponce‐Ponce de Leon AL, Huerta‐Vargas LF. Risk factors of maternal death in Mexico. Birth 2007;34(1):21‐5. [DOI] [PubMed] [Google Scholar]
Sandall 2016
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife‐led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD004667.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electrographic study. Archives of Neurology 1976;33:696. [DOI] [PubMed] [Google Scholar]
Sheiner 2005
- Sheiner E, Sarid L, Levy A, Seidman DS, Hallak M. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population‐based study. Journal of Maternal‐Fetal & Neonatal Medicine 2005;18(3):149‐54. [DOI] [PubMed] [Google Scholar]
Soltani 2008
- Soltani H. Global implications of evidence based practice: management of the third stage of labour. Midwifery 2008;24:138‐42. [DOI: 10.1016/j.midw.2008.03.002] [DOI] [PubMed] [Google Scholar]
Soltani 2010
- Soltani H, Hutchon DR, Poulose TA. Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD006173] [DOI] [PubMed] [Google Scholar]
Soltani 2011
- Soltani H, Poulose TA, Hutchon DR. Placental cord drainage after vaginal delivery as part of the management of the third stage of labour. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD004665.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Su 2012
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 1981
- Taylor DJ, Phillips P, Lind T. Puerperal haematological indices. British Journal of Obstetrics and Gynaecology 1981;88(6):601‐6. [DOI] [PubMed] [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34:79‐87. [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rheenan 2007
- Rheenen P, Moor L, Eschbach S, Grooth H, Brabin B. Delayed cord clamping and haemoglobin levels in infancy: a randomised controlled trial in term babies. Tropical Medicine & International Health 2007;12(5):603‐16. [DOI] [PubMed] [Google Scholar]
Weeks 2007
- Weeks A. Umbilical cord clamping after birth. BMJ 2007;335:312‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2005
- Werner EJ. Disorders of the fetomaternal unit. In: Alarcon PA, Werner EJ editor(s). Neonatal Hematology. Cambridge: Cambridge University Press, 2005:10‐39. [Google Scholar]
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2007
- World Health Organization. Reducing the global burden: postpartum hemorrhage. Making Pregnancy Safer. World Health Organization. Geneva: World Helath Organization, 2007. [Google Scholar]
WHO 2012
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. Guideline: Delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
Winter 2007
- Winter C, Macfarlane A, Deneux‐Tharaux C, Zhang W‐H, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG 2007;114:845‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 1974
- Yao AC, Lind J. Placental transfusion. American Journal of Diseases of Children 1974;127:128‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Begley 2010
- Begley CM, Gyte GML, Murphy DJ, Devane D, McDonald SJ, McGuire W. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007412.pub2] [DOI] [PubMed] [Google Scholar]
Begley 2011b
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007412.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2015
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Prendiville 2000
- Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000007] [DOI] [PubMed] [Google Scholar]


