Abstract
OBJECTIVE:
Our objective was to compare the accuracy of preoperative PET/CT and contrast-enhanced CT in detecting cervical nodal metastases in patients treated with neck dissection and to scrutinize the ability of each modality to determine nodal stage.
STUDY DESIGN:
Case series with chart review.
SETTING:
Montefiore Medical Center, Bronx, NY 10467.
SUBJECTS AND METHODS:
Patients who underwent neck dissection at our institution for primary treatment of head-and-neck-squamous-cell-carcinoma and had received preoperative PET/CT and CECT were included in this study. Imaging studies were reinterpreted by three specialists within the field and compared for inter-reader agreement. Concordance between radiology and histopathology was measured using neck levels and sides, along with patient nodal stage. Sensitivity, specificity, accuracy, PPV, NPV, and agreement coefficients were calculated.
RESULTS:
Seventy-three patients were included in the study. Sensitivity was 0.69 and 0.94 (level and side) for PET/CT versus 0.53 and 0.66 for CECT (p=0.056, p=0.001). Specificity was 0.86 and 0.56 for PET/CT versus 0.91 and 0.76 for CECT (p=0.014, p=0.024). No significant difference was found in overall accuracy (p=0.33, p=0.88). The overall agreement percentages between N-stage called by imaging modality and pathology were 52 and 55% for PET/CT and CECT, respectively.
CONCLUSION:
No significant difference in sensitivity was found between PET/CT and CECT. CECT was found to have superior specificity compared to PET/CT. The information gleaned from each modality in the pretreatment evaluation of HNSCC appears to be complimentary.
Keywords: head and neck squamous cell carcinoma, PET/CT, contrast-enhanced CT, nodal staging, preoperative imaging, neck dissection
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) accounts for an estimated 650,000 new cancer cases and 350,000 deaths annually world-wide.1 For affected patients, lymph node status is one of the most important prognosticators. The presence of cervical lymph node metastases is associated with a decrease in long-term survival and warrants escalated treatment plans.2,3 In order to stage and treat patients appropriately, prompt, accurate tumor evaluation and localization is required.4
Imaging is invaluable in the detection of metastases to the cervical lymph nodes.5 In patients with clinically negative neck examinations, elective neck therapy is advised if risk of occult metastasis exceeds 15 to 20%.6,7 Unfortunately, the rate of undetected metastatic disease with conventional imaging modalities is estimated to be >30%.7,8 In order to reduce the number of unnecessary neck dissections, yet appropriately employ these treatments when warranted, radiological advances seek to improve upon the ability to accurately rule-in or rule-out cervical metastatic disease.
Contrast-enhanced CT (CECT) and MRI are established modalities in the pre- therapeutic staging of HNSCC, characterizing tumor size, vessel infiltration, and cervical lymph node metastases.2,9 Some propose CECT as first-line, owing to its high reliability and accessibility to assess the upper aero-digestive anatomy and cervical nodal basins simultaneously.10 Others propose the newer FDG PET combined with low-dose non-contrast CT (PET/CT) as the more accurate modality.5,11–19 However, using PET/CT alone for preoperative planning can be problematic due to limited resolution and high false positive rates.20 Thus, our objectives were: 1) to assess and compare the accuracy of PET/CT and CECT in detecting cervical nodal metastases, and 2) to scrutinize the ability of each modality, and the combination of these modalities, to accurately determine nodal stage.
MATERIALS AND METHODS
Study Patients
A retrospective chart review of all adult patients with HNSCC who underwent neck dissection for initial treatment between 2006 and 2014, and received both PET/CT and CECT preoperatively, was approved by the Montefiore Medical Center institutional review board. Two hundred-fourteen patients received neck dissection as initial treatment, and 86 were evaluated preoperatively with both imaging modalities within 6 months of surgery. Other exclusion criteria included: partial treatment prior to imaging (n=2), incomplete pathology report (n=1), presence of HIV infection (n=1), incomplete study (base of skull to lower mandible, n=1), or irretrievable studies (PACS archiving error, n=8). In total, the study cohort was comprised of 73 patients.
Imaging
For 18F-FDG-PET imaging, all patients were asked to fast for 4+ hours prior to intravenous administration of a weight-adjusted dose of 5.18MBq/kg (0.14mCi/kg) 18FDG. Images from the base of skull to mid-thighs were acquired on a Philips Gemini TF TOF PET/CT scanner (Philips Medical Systems, Cleveland, Ohio) with a spatial resolution of 5mm in the center of the field of view, with emission scan acquired at 100s-per-frame in 3D acquisition mode. CT scanning was performed in spiral mode from mid-thighs to the base-of-skull at 100mAs and 120keV. No contrast medium was used during CT scan. Proprietary vendor-provided software was used for image reconstruction with LISTMODE ordered subset/expectation maximization reconstruction algorithm (3 iterations, 33 subsets). Low-dose CT scan was used for non-uniform attenuation correction. Scatter and random coincidence corrections were performed by vendor-provided programming.
The patients underwent CECT with various systems over an 8-year period. The units included a 64 multi-detector CT scanner (LightSpeed Advantage, GE Healthcare), and a 16/64 multi-detector scanner (Philips Brilliance). The region of interest extended from the base-of-skull to the upper-mediastinum. Scanning parameters were as follows: section thickness, 0.0625mm; standard field of view by patient dimensions; voltage, 120 kV; weight-based tube current; and matrix 512×512. Images were reconstructed with a slice thickness of 3mm in soft tissue and bone windows (per department protocols). A bolus of contrast material (iohexol, Omnipaque 300, GE Health care; iopromide, Ultravist 300, Bayer HealthCare; and iodixanol, Visipaque 320, GE Healthcare) was administered intravenously at a rate of 2–3mL/s with a scan delay of 90s.
Surgery and Histopathology
All patients underwent primary tumor resection and neck dissection. A selective neck dissection—involving I/II/III, II/III, II/III/IV, II/III/IV/VI, other combination—or radical neck dissection was planned after review of the primary tumor site, pathology, imaging data, clinical exam, and stage at the HNSCC multidisciplinary tumor board, according to the American Joint Committee on Cancer (AJCC) guidelines.21 As per operative reports, cervical lymph nodes were resected en-bloc with intraoperative labeling of the appropriate cervical level.22 Lymph nodes were dissected from specimens and stained with hematoxylin and eosin for histologic examination. Per institutional standard of care, one slide was made for each lymph node. Subsequently, the size, location, and number of metastatic nodes were documented in pathology reports.
Image Interpretation
PET/CT images were interpreted by three board-certified nuclear medicine physicians (RN, AV, TA). All cervical lymph nodes with increased focal tracer uptake, compared to background and blood pool activity with asymmetric distribution, were considered suspicious for metastatic involvement. Standardized-uptake-value based criteria were not instituted.
CECT images were interpreted by three CAQ-certified (certificates of added qualifications) neuroradiologists (KS, EG, MN). Any cervical lymph node satisfying at least one of the following criteria was considered suspicious for metastatic involvement: necrosis; extra-nodal-extension; round or irregular shape with enhancement, size >0.5cm (shortest axial diameter); round or irregular shape without enhancement, size >1.0cm; oval shape with enhancement, size >1.0cm; oval shape without enhancement, size >1.5cm; or grouping of two or more lymph nodes.23,24
Cervical regions were divided into 8 levels on each side (IA, IB, IIA, IIB, III, IV, VA, VB) according to consensus definitions set forth by the American Head and Neck Society 22 and by Som et al. 25 When lymph nodes were determined positive for metastasis by imaging criteria, both corresponding neck level and side were marked positive for statistical analysis. PET/CT and CECT imaging results were independently compared to histopathology results.
Staging
Using morphological data from PET/CT or CECT studies, a preoperative imaging-based nodal stage (iN) was assigned to each patient according to AJCC criteria.26 For each modality, the consensus iN stage for a given patient was the iN stage designated by 2 or more readers. The gold standard for comparison was the final nodal stage (N) as determined by histopathology. Agreement between iN and N stages was assessed using Cohen’s kappa coefficient.
Statistical Analysis
Inter-reader agreement for PET/CT and CECT readers was assessed using Cohen’s kappa coefficient. Independent coefficients were calculated for each possible pair of PET/CT readers and CECT readers, as well as a coefficient describing the collective agreement (among all 3 readers) using a variation of the kappa coefficient. For each imaging modality, the neck level or neck side was considered positive for statistical analyses if at least 2 of the 3 readers agreed on metastatic involvement.
Concordance between imaging and histopathology was evaluated based on the aforementioned anatomic neck level, independent of side. Additionally, an independent analysis evaluated the concordance for neck side alone, better controlling for intraoperative variability. The sensitivity, specificity, overall accuracy, positive predictive value, and negative predictive value in detecting metastatic lymph nodes were calculated for each modality. A two-sample proportion test was used to compare the corresponding proportions for PET/CT and CECT. A p-value of 0.05 or less was considered statistically significant.
RESULTS
Patients received preoperative PET/CT and CECT a median of 23 (range 1–104 days) and 31 days (range 6–191 days) prior to surgery, respectively. The clinicopathologic characteristics of the cohort are presented in Table 1. In total, 579 neck level specimens corresponding with 116 neck sides were sent to pathology. 83 of 579 (14%) resected neck levels and 53 of 116 (46%) resected neck sides showed histological evidence of metastatic involvement. The most common primary tumor site was the oral cavity (49%), followed by the oropharynx (21%). By pathology, 27 (37%) patients were staged N0, 9 (12%) were staged N1, 35 (48%) were staged N2, and 2 (3%) were staged N3. Fifty-six (77%) patients had overall stage III or IV disease at the time of treatment. The preoperative iN stage designations assigned by each PET/CT and CECT reader are presented in Table 2.
Table 1.
Patient Characteristics
| Parameter | n (% of total) |
|---|---|
| Patients | 73 (100) |
| Neck Levels | 579 |
| Neck Sides | 116 |
| Age: | |
| ≥60 | 51 (70.0) |
| <60 | 22 (30.0) |
| Gender: | |
| Male | 59 (80.8) |
| Female | 14 (19.2) |
| Primary Site: | |
| Oral Cavity | 36 (49.3) |
| Oropharynx | 15 (20.5) |
| Hypopharynx | 6 (8.2) |
| Larynx | 13 (17.8) |
| Other | 3 (4.1) |
| Overall Pathology Stage: | |
| I | 6 (8.2) |
| II | 10 (13.7) |
| III | 11 (15.1) |
| IV | 45 (61.6) |
| Undetermined | 1 (1.4) |
| Pathology Nodal Stage: | |
| N0 | 27 (37.0) |
| N1 | 9 (12.3) |
| N2 | 35 (47.9) |
| N2a | 3 (4.1) |
| N2b | 21 (28.8) |
| N2c | 11 (15.1) |
| N3 | 2 (2.7) |
None
Table 2.
Nodal stage designation by histopathology, 18F-FDG PET/CT, and CECT respectively
| Nodal Stage | Pathology n (% of total) |
PET/CT#1* n (% of total) |
PET/CT#2 n (% of total) |
PET/CT#3 n (% of total) |
CECT#1 n (% of total) |
CECT#2 n (% of total) |
CECT#3 n (% of total) |
|---|---|---|---|---|---|---|---|
| N0 | 27 (37) | 10 (14) | 14 (19) | 11 (15) | 27 (37) | 28 (38) | 28 (38) |
| N1 | 9 (12) | 17 (23) | 16 (22) | 20 (27) | 14 (19) | 12 (16) | 12 (16) |
| N2 | 35 (48) | 43 (59) | 40 (55) | 39 (53) | 29 (40) | 30 (41) | 30 (41) |
| N3 | 2(3) | 3(4) | 3(4) | 3(4) | 3(4) | 3 (4) | 3 (4) |
| Total | 73 (100) | 73 (100) | 73 (100) | 73 (100) | 73 (100) | 73 (100) | 73 (100) |
N: Nodal stage designated by histopathology
Each numbered modality (i.e. #1, #2, etc.) refers to an individual physician reader Table 3:
Of 73 patients, 44 (60%) underwent bilateral neck dissection, and 29 (40%) underwent unilateral neck dissection. Of 27 N0 patients without evidence of cervical metastasis on pathology, 14 (52%) patients received ipsilateral neck dissection, and 13 (48%) received bilateral neck dissection. Of 58 patients with primary tumor laterality, 33 (57%) had ipsilateral lymph node disease, 6 (10%) had bilateral involvement, 2 (3%) had contralateral nodal metastases without ipsilateral disease, and 17 (29%) had no cervical lymph node involvement.
When assessing concordance between imaging and pathology by neck level, PET/CT was found to correctly identify occult metastases in 57 of 83 (69%) positive neck levels, while CECT was found to identify 44 of 83 (53%) positive neck levels (Table 3). Absence of occult metastasis was correctly reported in 424 of 496 (86%) and 450 of 496 (91%) negative neck levels, using PET/CT and CECT, respectively. No significant difference was found between PET/CT and CECT in terms of sensitivity (p=0.056). Although CECT was more specific (p=0.014), there was no significant difference in overall accuracy (p=0.33). In one patient with a false positive PET/CT, the presence of a fatty hilum on CECT accurately ruled out metastatic disease (Figure 1). However, in another patient, fatty hilum incorrectly ruled out a level IB lymph node found to be positive for metastatic disease on both PET/CT and pathology (Figure 2).
Table 3.
Assessment of the performance of 18F-FDG PET/CT and CECT for identifying metastatic cervical lymph node disease
| Imaging Modality | TP (n) | FP (n) | FN (n) | TN (n) | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Neck Level (n = 579) | |||||||||
| PET/CT | 57 | 72 | 26 | 424 | 69 (57, 78) | 86 (82, 88) | 83 (80, 86) | 44 (36, 53) | 94 (92, 96) |
| CECT | 44 | 46 | 39 | 450 | 53 (42, 64) | 91 (88, 93) | 85 (82, 89) | 49 (38, 60) | 92 (89, 94) |
| p value | 0.056 | 0.014 | 0.334 | 0.583 | 0.231 | ||||
| Neck Side (n= 116) | |||||||||
| PET/CT | 50 | 28 | 3 | 35 | 94 (83, 99) | 56 (43, 68) | 73 (64, 81) | 64 (52, 74) | 92 (78, 98) |
| CECT | 35 | 15 | 18 | 48 | 66 (52, 78) | 76 (64, 86) | 72 (62, 79) | 70 (55, 82) | 73 (60, 83) |
| p value | 0.001 | 0.024 | 0.883 | 0.619 | 0.034 | ||||
TP: True Positive
FP: False Positive
FN: False Negative
TN: True Negative
PPV: Positive Predictive Value
NPV: Negative Predictive Value
Figure 1:

Figure 1a: 18F-FDG PET/CT shows submandibular gland labeled by red cross. Suspicious lymph node is anterolateral to the red cross. Images represent a false positive detection of a preoperative metastatic cervical lymph node by 18F-FDG PET/CT. Although abnormal focal tracer uptake is found on imaging, no evidence of metastatic disease was found on histopathology. Figure 1b: CECT shows the same patient with normal anatomy, a large lymph node with fatty hilum, read correctly as negative for metastatic disease.
Figure 2:
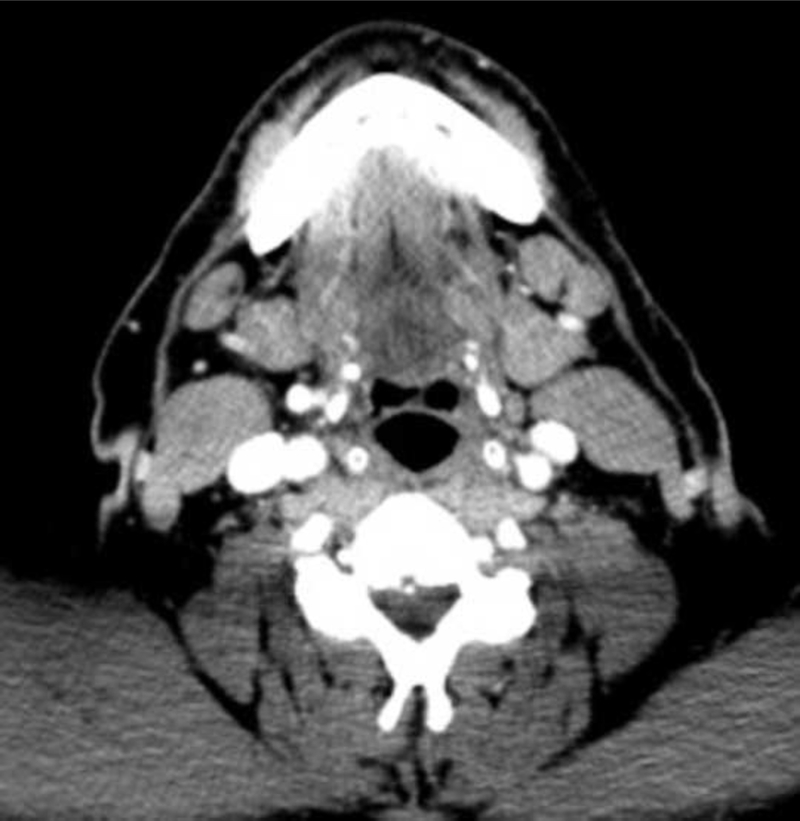
Figure 2a: 18F-FDG PET/CT shows the accurate detection of a preoperative metastatic cervical lymph node. Abnormal focal tracer uptake is found on imaging, corresponding to histopathology findings consistent with metastasis to cervical level 1B. Figure 2b: CECT of the same patient shows a normal lymph node with fatty hilum per criteria, therefore demonstrating a false negative finding.
When an independent analysis was performed evaluating neck laterality rather than nodal station, PET/CT was found to correctly identify the presence of occult metastases in 50 of 53 (94%) positive neck sides, while CECT was found to correctly identify 35 of 53 (66%) positive neck sides (Table 3). Absence of cancer involvement was correctly reported in 35 of 63 (56%) and 48 of 63 (76%) negative neck sides, using PET/CT and CECT, respectively. Of the 38 neck sides determined negative by PET/CT, only 3 were subsequently found to be positive for disease on pathologic evaluation, yielding a false negative rate of 8%. PET/CT was found to be more sensitive (p=0.001) in this analysis although remained less specific than CECT (p=0.024). Overall accuracy between the two modalities did not differ significantly (p=0.88).
Of the 27 N0 patients who had no evidence of cervical metastasis on pathology, 16 (59%) patients were assigned a preoperative stage of iN1 or higher by PET/CT, whereas 8 (30%) patients were assigned a higher preoperative stage by CECT. The nodal staging assignments yielded by PET/CT and CECT for each pathology stage are further described in Table 4. The overall agreement percentages between iN stage called by imaging modality compared to pathology N stage were 52 and 55% for PET/CT and CECT, respectively (Table 5). When preoperative iN and pathology N stage assignments were discordant, 37% of patients were overstaged by PET/CT data, whereas 19% of patients were overstaged by CECT. 26% of patients were understaged by CECT as compared to 11 % by PET/CT.
Table 4.
Evaluation of agreement between staging based on pathology and staging based on 18F-FDG PET/CT and CECT
| PET/CT: | N0 | N1 | N2a | N2b | N2c | N3 | NC | Total | %C | |
|---|---|---|---|---|---|---|---|---|---|---|
| PATH | ||||||||||
| N0 | 11 | 7 | 0 | 1 | 4 | 0 | 4 | 27 | 41 | |
| N1 | 0 | 4 | 0 | 2 | 2 | 0 | 1 | 9 | 44 | |
| N2a | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | |
| N2b | 0 | 2 | 0 | 9 | 8 | 1 | 1 | 21 | 43 | |
| N2c | 0 | 1 | 0 | 1 | 9 | 0 | 0 | 11 | 82 | |
| N3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 100 | |
| Total | 11 | 16 | 0 | 14 | 23 | 3 | 6 | 73 | ||
| %C | 100 | 25 | 0 | 64 | 39 | 67 | PA: 52% |
| CECT: | N0 | N1 | N2a | N2b | N2c | N3 | NC | Total | %C | |
|---|---|---|---|---|---|---|---|---|---|---|
| PATH | ||||||||||
| N0 | 19 | 4 | 0 | 1 | 3 | 0 | 0 | 27 | 70 | |
| N1 | 3 | 4 | 0 | 1 | 1 | 0 | 0 | 9 | 44 | |
| N2a | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | |
| N2b | 4 | 3 | 0 | 12 | 1 | 1 | 0 | 21 | 57 | |
| N2c | 2 | 1 | 0 | 5 | 3 | 0 | 0 | 11 | 27 | |
| N3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 100 | |
| Total | 28 | 13 | 0 | 21 | 8 | 3 | 0 | 73 | ||
| %C | 68 | 31 | 0 | 57 | 38 | 67 | PA: 55% |
%C: Percent Correct
NC: No consensus preoperative stage assignment between 3 readers
PA: Overall percent agreement
Table 5.
Percent agreement between nodal staging called by imaging modality compared to pathology gold standard
| −4 | −3 | −2 | −1 | TRUE | 1 | 2 | 3 | 4 | |
| PET/CT | 6% | 4% | 4% | 23% | 52% | 5% | 3% | 3% | 0% |
| CECT | 4% | 3% | 3% | 10% | 55% | 12% | 4% | 7% | 3% |
| OVERSTAGED | TRUE | UNDERSTAGED | |||||||
| PET/CT | 37% | 52% | 11% | ||||||
| CECT | 19% | 55% | 26% | ||||||
Column headers are measurements of the deviation between stage (N0, N1, N2a, N2b, N2c, N3) on imaging versus pathology
Kappa coefficient values demonstrated moderate agreement (κ=0.53–0.68) for PET/CT and almost perfect agreement (κ=0.88–1.00) for CECT, assessing inter-reader agreement for positive determinations of cervical lymph node metastases by neck level and by neck side. Assessing inter-reader agreement for preoperative iN stage assignments, demonstrated moderate agreement (κ = 0.46–0.71) for PET/CT and almost perfect agreement (κ = 0.85–0.94) for CECT.
DISCUSSION
In the present study, we compared the accuracy of PET/CT and CECT in detecting occult metastases to cervical lymph nodes in previously untreated patients with HNSCC. We assessed the clinical usefulness of the two modalities for nodal staging by comparing preoperative iN stage assignments to pathology N stage assignments as the gold standard. Our results add to the limited literature on the efficacy of PET/CT in preoperative nodal staging: PET/CT stages nodal involvement more accurately than conventional imaging;13 PET/CT may be more useful than CECT for nodal staging;27 PET/CT leads to upstaging and changes in patient management.28 Our results cannot confirm that PET/CT overstages cervical lymph node disease. Moreover, the data shows that PET/CT offers no overall accuracy advantage over CECT in the preoperative staging of HNSCC. Although concordance between preoperative iN stages and pathology N stages were ~50% for both PET/CT and CECT, 37% of calls were overstaged by PET/CT while 19% were overstaged by CECT. Neither modality should be used in isolation to preoperatively stage cervical lymph node involvement. Rather, the development of criteria incorporating information gleaned from both PET/CT and CECT may improve the accuracy of preoperative cervical lymph node assessment.
In our study, all imaging studies were evaluated by 3 independent radiologists to evaluate the accuracy and reproducibility of all PET/CT and CECT results. PET and PET/CT are known to have excellent inter-reader agreement for the detection of distant metastasis and for the assessment of response to therapy in patients with HNSCC.29,30 However, ours compares the inter-reader agreement for PET/CT and CECT for the detection and staging of preoperative cervical lymph node metastasis. Our findings suggest that CECT has higher inter-reader agreement (0.85–1 ) than PET/CT (0.46–0.71 ) for both staging as well as neck-level and neck-side evaluation. These data may suggest that heuristics for identifying metastatic lymph nodes for CECT are more standardized, while standards for PET/CT require further development. Several studies propose various standardized uptake value (SUV) cut-offs (2.0–3.5) during PET/CT evaluations to help prognosticate pre-treatment metastatic disease or monitor post-treatment cervical lymph nodes.31–33 Others propose a system of graded size-based SUVmax cut-offs,13 but additional inter-institutional investigation will be necessary to confirm universal utility of an SUV cut-off in nodal evaluation.
Our findings suggest that PET/CT is more sensitive than CECT in detecting the presence of cervical lymph node metastases only when ruling out disease to a given neck side, which is in accordance with previous studies.5,11–19 In particular, PET/CT was 28% more sensitive than CECT in detecting neck sides that contained metastatic lymphadenopathy (p=0.001). 50 of 53 (94%) positive neck sides were correctly identified by PET/CT. Of 38 neck sides deemed negative by PET/CT, only 3 were found to have evidence of disease, yielding a false negative rate of 8%, whereas the false negative rate for CECT on neck side analysis was 27% (18 of 66). The ability to deem a neck free of disease with high confidence would be clinically relevant, reducing the number of unproductive nodal dissections or radiation therapy. However, no overall difference in sensitivity was found between the two modalities, as evidenced by the lack of statistical significance on neck level based analysis (p=0.056). Neither modality was particularly sensitive in detecting neck levels containing metastatic lymphadenopathy (69% for PET/CT and 53% for CECT). PET/CT had a 19% higher NPV than CECT, with pretest prevalence at 46% of dissected neck sides (p=0.034). Both morphological and functional imaging modalities are limited in their ability to detect micrometastases in individual lymph nodes at specific neck levels, as shown in previous studies.17,34 In their own 70-person cohort and review of prior studies, Nahmias et al. reported that PET/CT lacked the sensitivity to clinically help the surgeon, due to a high rate of false negative nodes.35 Our data largely support these findings, with the exception that PET/CT was effective in ruling out nodal involvement of a neck side.
Our findings also suggest that CECT is more specific than PET/CT. Although prior studies have shown that CECT is more specific than PET/CT in the detection of extra-nodal extension,36 our study found that CECT was 5% (by neck level) and 20% (by neck side) more specific than PET/CT in the overall identification of metastatic lymph nodes (p=0.014, p=0.024). On neck side analysis, CECT was found to have a false positive rate of 30% (15 of 50), compared to 36% (28 of 78) for PET/CT. Nearly 60% of N0 patients would have been overstaged (iN1 or higher) by PET/CT data. As the more conservative modality, CECT is more accurate in confirming the presence of metastasis than PET/CT, contrary to previous studies.17,19 Nevertheless, both modalities were found to have high false positive rates in detecting metastatic disease to a given neck side. The major limitation of PET/CT is well known: inflammatory lymph nodes may have as much tracer uptake as metastatic lymph nodes.37 Morphological data from CECT studies can supplement the PET/CT evaluation, ultimately helping to delineate metastatic from benign lymph. Recent prospective studies have suggested that PET/CT may have stronger prognostic value than conventional imaging modalities when used to stage patients.19,38 This effect was unable to be measured retrospectively but should be further validated.
Our study is limited as a single-institution retrospective study. Due to the retrospective nature, a standardized intraoperative protocol for labeling nodes from cervical levels could not be implemented. Another limitation is that patients with a variety primary tumor sites, with corresponding metastatic potentials, were included. In order to compare PET/CT and CECT, 98 patients were excluded from the original cohort if only one modality was used in the preoperative evaluation, potentially introducing selection bias. Because 10 CECT studies and 4 PET/CT studies were performed over 60 days prior to surgery, median times were calculated so as not to over weight these studies. The complete temporal distribution of imaging studies performed prior to neck dissection can be found in Supplemental Figure 1 (available online only). Median time between PET/CT and surgery was 1 week shorter than the median time between CECT and surgery (p=0.001). The accuracy of both modalities are likely underestimated and conservative by including studies performed up to 27 weeks prior to surgery. It may be true that the accuracy of these imaging modalities could be improved using a strategy that incorporates pre-test probabilities for cervical lymph node metastasis, based on a combination of clinical data (site, stage, clinical exam, etc.). Such a study would require a larger cohort and be validated using one or several external datasets. Lastly, the scope of our study was narrow and our analyses did not incorporate routinely used methods of preoperative staging such as clinical stage with physical exam, ultrasound-guided fine needle aspiration or core needle biopsy, sentinel node biopsy, or focused magnetic resonance imaging. A multi-center prospective design would better lend itself to researching these preoperative options.
CONCLUSION
In conclusion, PET/CT was more sensitive than CECT in excluding metastatic involvement of a given neck side. However, no difference in overall sensitivity was found between the two modalities. CECT was found to have superior specificity compared to PET/CT. Broadly speaking, the information gleaned from PET/CT and CECT for pretreatment evaluation of HNSCC appears to be complimentary, suggesting a benefit of using contrast to improve upon the diagnostic yield of PET/CT studies. Continued refinement of the application and reporting for these modalities, for example greater standardization of the criteria for reporting suspicious cervical lymph nodes on PET/CT, may improve the ability to accurately assess cervical lymph nodes in patients with HNSCC.
Supplementary Material
ABBREVIATION KEY
- HNSCC
Head and neck squamous cell carcinoma
- CECT
Contrast-enhanced CT
- AJCC
American Joint Committee on Cancer
- iN
Imaging-based nodal stage
- N
Pathology-based nodal stage
- SUV
standardized uptake value
Footnotes
PRESENTATIONS
Eastern Neuroradiological Society 27th Annual Meeting. Newport, RI. September 2015.
American Society of Neuroradiology 54th Annual Meeting. Washington, D.C. May 2016.
No sponsorships or competing interests have been disclosed for this article.
REFERENCES
- 1.Argiris Ax, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack MG, Rieger J, Baghi M, et al. Cervical lymph nodes. Eur J Radiol. 2008;66:493–500. [DOI] [PubMed] [Google Scholar]
- 3.Kohler HF, Kowalski LP. How many nodes are needed to stage a neck? A critical appraisal. Euro Arch Otorhinolaryngol. 2010;267:785–91. [DOI] [PubMed] [Google Scholar]
- 4.Quon A, Fischbein NJ, McDougall IR, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med. 2007;48 Suppl 1: 58S–67S. [PubMed] [Google Scholar]
- 5.Roh JL, Yeo NK, Kim JS, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43:887–93. [DOI] [PubMed] [Google Scholar]
- 6.Snow GB, Patel P, Leemans CR, et al. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol. 1992;249:187–94. [DOI] [PubMed] [Google Scholar]
- 7.Pitman KT. Rationale for elective neck dissection. Am J Otolaryngol. 2000;21:31–7. [DOI] [PubMed] [Google Scholar]
- 8.Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160–7. [DOI] [PubMed] [Google Scholar]
- 9.Weber AL, Romo L, Hashmi S. Malignant tumors of the oral cavity and oropharynx: clinical, pathologic, and radiologic evaluation. Neuroimaging Clin N Am. 2003;13:443–64. [DOI] [PubMed] [Google Scholar]
- 10.Monnet O, Cohen F, Lecorroller T, et al. [Cervical lymph nodes]. J Radiol. 2008;89:1020–36. [DOI] [PubMed] [Google Scholar]
- 11.Yoo J, Henderson S, Walker-Dilks C. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer. Clin Oncol (R Coll Radiol). 2013;25:e33–66. [DOI] [PubMed] [Google Scholar]
- 12.Ng SH, Yen TC, Chang JT, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol. 2006;24:4371–6. [DOI] [PubMed] [Google Scholar]
- 13.Murakami R, Uozumi H, Hirai T, et al. Impact of FDG-PET/CT imaging on nodal staging for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007;68:377–82. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki Y, Saitoh M, Notani K, et al. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med. 2008;22:177–84. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues RS, Bozza FA, Christian PE, et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J Nucl Med. 2009;50:1205–13. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Kim JS, Doo H, et al. Combined [18F]fluorodeoxyglucose positron emission tomography and computed tomography for detecting contralateral neck metastases in patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47:376–80. [DOI] [PubMed] [Google Scholar]
- 17.Roh JL, Park JP, Kim JS, et al. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology. 2014;271:153–61. [DOI] [PubMed] [Google Scholar]
- 18.Rohde M, Dyrvig AK, Johansen J, et al. 18F-fluoro-deoxy-glucose-positron emission tomography/computed tomography in diagnosis of head and neck squamous cell carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2014;50:2271–9. [DOI] [PubMed] [Google Scholar]
- 19.Park JT, Roh JL, Kim JS, et al. F FDG PET/CT versus CT/MR Imaging and the Prognostic Value of Contralateral Neck Metastases in Patients with Head and Neck Squamous Cell Carcinoma. Radiology. 2015:150959. [DOI] [PubMed] [Google Scholar]
- 20.Cammaroto G, Quartuccio N, Sindoni A, et al. The role of PET/CT in the management of patients affected by head and neck tumors: a review of the literature. Eur Arch Otorhinolaryngol. 2016;273:1961–73. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, American Joint Committee on Cancer., American Cancer Society AJCC cancer staging handbook: from the AJCC cancer staging manual, 7th ed. New York: Springer; 2010. p. xix, 718 p. [Google Scholar]
- 22.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–8. [DOI] [PubMed] [Google Scholar]
- 23.van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379–84. [DOI] [PubMed] [Google Scholar]
- 24.Anzai Y, Brunberg JA, Lufkin RB. Imaging of nodal metastases in the head and neck. J Magn Reson Imaging. 1997;7:774–83. [DOI] [PubMed] [Google Scholar]
- 25.Som PM, Curtin HD, Mancuso AA. An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classifications. Arch Otolaryngol Head Neck Surg. 1999;125:388–96. [DOI] [PubMed] [Google Scholar]
- 26.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6 ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 27.Jeong HS, Baek CH, Son YI, et al. Use of integrated 18F-FDG PET/CT to improve the accuracy of initial cervical nodal evaluation in patients with head and neck squamous cell carcinoma. Head Neck 2007;29:203–10. [DOI] [PubMed] [Google Scholar]
- 28.Nair S, Mohan S, Nilakantan A, et al. Impact of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography scan on initial evaluation of head and neck squamous cell carcinoma: our experience at a tertiary care center in India. World J Nucl Med. 2015;14:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senft A, de Bree R, Golding RP, et al. Interobserver variability in chest CT and whole body FDG-PET screening for distant metastases in head and neck cancer patients. Mol Imaging Biol. 2011;13:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus C, Ciarallo A, Tahari AK, et al. Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J Nucl Med. 2014;55:1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joo YH, Yoo Ie R, Cho KJ, et al. Prognostic value of preoperative 18F-FDG PET/CT for primary head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2014;271:1685–91. [DOI] [PubMed] [Google Scholar]
- 32.Joo YH, Yoo IR, Cho KJ, et al. The value of preoperative 18F-FDG PET/CT for the assessing contralateral neck in head and neck cancer patients with unilateral node metastasis (N1–3). Clin Otolaryngol. 2014;39:338–44. [DOI] [PubMed] [Google Scholar]
- 33.Chan JY, Sanguineti G, Richmon JD, et al. Retrospective review of positron emission tomography with contrast-enhanced computed tomography in the posttreatment setting in human papillomavirus-associated oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2012;138:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoder H, Carlson DL, Kraus DH, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47:755–62. [PubMed] [Google Scholar]
- 35.Nahmias C, Carlson ER, Duncan LD, et al. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg. 2007;65:2524–35. [DOI] [PubMed] [Google Scholar]
- 36.Lee JR, Choi YJ, Roh JL, et al. Preoperative Contrast-Enhanced CT Versus (1)(8)F-FDG PET/CT Evaluation and the Prognostic Value of Extranodal Extension for Surgical Patients with Head and Neck Squamous Cell Carcinoma. Ann Surg Oncol. 2015;22 Suppl 3:S1020–7. [DOI] [PubMed] [Google Scholar]
- 37.Fukui MB, Blodgett TM, Snyderman CH, et al. Combined PET-CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics. 2005;25:913–30. [DOI] [PubMed] [Google Scholar]
- 38.Ryu IS, Roh JL, Kim JS, et al. Impact of (18)F-FDG PET/CT staging on management and prognostic stratification in head and neck squamous cell carcinoma: A prospective observational study. Eur J Cancer. 2016;63:88–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


