Abstract
Background and objectives
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) infections among previously healthy persons in community settings, without exposure to health care facilities, has been noted recently. Colonization rates of community-associated MRSA (CA-MRSA) have been reported to range from 0 to 9.2 percent. The nose and open skin areas are considered the most important sites for colonization. The aim of our study was to assess the prevalence and to describe the antibiotic susceptibility pattern of CA-MRSA among outpatient children.
Patients and methods
We prospectively screened every third consecutive child presenting to our pediatric emergency department of King Saud Medical City, a 275 bed tertiary care teaching hospital in Riyadh, Saudi Arabia, from March through July 2015.
Results
We analyzed a total of 830 screening results (n = 478 males, 57.6%). Most of the screened patients were from Riyadh (n = 824, 99.3%). A total of 164 (19.8%) were found to be colonized with S. aureus, and of these 38 (4.6%) with MRSA. Thus, the MRSA rate amongst all S. aureus carriers was 23.2%. All MRSA were susceptible to vancomycin, (94.7%) were susceptible to linezolid, (65.8%) to clindamycin, and (89.5%) to trimethoprim/sulfamethoxazole.
Conclusion
The rate of MRSA carriage among children in Riyadh province was within the range reported internationally. As the MRSA rate among S. aureus infected children was 23.2%, empirical MRSA coverage should be considered in children with suspected S. aureus infections.
Keywords: Community-associated, MRSA, Prevalence, Saudi Arabia
1. Introduction
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) infections among previously healthy persons in community settings, without exposure to health care facilities, has been noted recently [1], [2]. Community-associated (CA)-MRSA strains and healthcare-associated (HA)-MRSA strains differ in terms of epidemiology, microbiology, and clinical manifestations [3]. The rapid spread of CA-MRSA has been characterized by outbreaks of cutaneous infections in healthy individuals, but can also cause soft tissue and bone infections, sepsis and endocarditis, as well as necrotizing, frequently lethal pneumonia, especially after influenza infection.
Colonization rates of CA-MRSA have been reported to range from 0 to 9.2 percent [4]. The nose and open skin areas (e.g., wounds and device exit sites) are considered the most important sites for colonization [5], [6]. Nasal carriage of MRSA is an important risk factor for subsequent MRSA infection and transmission of this pathogen [7].
Several studies have shown that the prevalence of CA-MRSA varies geographically. Global outbreaks have been reported from the United States (US) and New Zealand [8]. In a population-based surveillance study of three US communities in 2001–2002, 8%–20% of all MRSA isolates were CA-MRSA by definition, with the highest incidence among children <2 years old [9]. King et al also demonstrated that approximately two-thirds of all community-associated S. aureus skin infections in Atlanta were due to MRSA clones 300 [10].
The highest rates of CA-MRSA carriage (>50%) are reported in North and South America, Asia, and Malta. Intermediate rates (25–50%) are reported in China, Australia, Africa, and some European countries, such as Portugal (49%), Greece (40%), Italy (37%) and Romania (34%). Other European countries, including the Netherlands and Scandinavian Countries, have generally low prevalence rates. However, epidemiological data from separate studies are often not comparable, owing to differences in study design and populations sampled [11].
Although several studies have reported the prevalence of MRSA nasal carriage in patients in health care settings [12], [13], this subject has been rarely investigated in healthy individuals in the broader community [14]. In Saudi Arabia, CA-MRSA was assessed by Fawzia et al in outpatient children at a university hospital from 2005 to 2008, where they found that 29.8% of clinical S. aureus isolates were CA-MRSA [15]. Of these cases, 64.7% were not associated with known risk factors. However, they evaluated clinical isolates rather than conducting active screening for MRSA. Another study on the prevalence of MRSA in the Western region of Saudi Arabia found that the prevalence of MRSA was 39.5% [16]. Infection was commonly associated with wound, skin, and soft tissue infections.
Currently, no data in Saudi Arabia on MRSA carriage in children is available. We aimed to assess the prevalence and to describe the antibiotic susceptibility pattern of MRSA among outpatient children in King Saud Medical City, a 1400 bed tertiary care hospital with a 275-bed children's hospital in Riyadh, Saudi Arabia.
2. Patients and methods
The study was approved by Research Advisory Council at this center. Informed consent was obtained from all legal guardians of participants.
2.1. Study design
We prospectively screened the anterior nares of children presenting to our pediatric emergency department of King Saud Medical City, a 275 bed tertiary care teaching hospital in Riyadh, Saudi Arabia, from March through July 2015.
Children under the age of 14 years who presented to the emergency department were eligible for the study. Patients who were hospitalized during the 12 months prior to their emergency visit, and those who were known MRSA colonizers, were excluded from the study. Thus, all newly detected MRSA colonizers in our study were defined as CA-MRSA.
Specimens were taken from each third consecutive patient meeting the inclusion criteria, and each patient was enrolled only once during the study period.
The attending emergency physician collected data on patient demographics (age, gender and history of recent hospitalization, antibiotic use and MRSA carriage).
2.2. Laboratory methods
A swab from each opening of the anterior nares were collected using pre-moistened sterile cotton swabs with normal saline solution, and were transported in Amie's transport medium.
Nasal swabs were processed within 2 h of collection and primary plating was done on mannitol salt agar (MSA) without oxacillin to screen for S. aureus. After inoculation, plates were incubated at 35 °C in oxygen and read after 24 and 48 h. Each distinctive morphotype of mannitol fermenting colonies (yellow colonies) was selected from the MSA plate and sub-cultured on a 5% sheep blood agar plate. The colony was confirmed as S. aureus by catalase, slide coagulase (Staphaurex, Remel) and DNase tests. BD Phoenix™ Automated Microbiology System (Becton Dickinson, Franklin Lakes, New Jersey) was used for identification and antibiotics susceptibility testing for S. aureus species, which included testing for inducible clindamycin resistance. Tested antibiotics included: penicillin, oxacillin, erythromycin, clindamycin, sulfamethaxazole, vancomycin, linezolid, rifampin, ciprofloxacin and daptomycin. Any S. aureus that was resistant to oxacillin and/or cefoxitin was defined as MRSA.
2.3. Statistical analysis
The carriages rate was reported as the percentage of positive cases with S. aureus and MRSA colonization, respectively, out of the total number of patients sampled. Antibiotic susceptibility for patients colonized with MRSA was reported as the percentage of susceptible isolates out of total positive cases. We compared sex and age as risk factors for carriage using Chi-square statistics and Mann–Whitney U-test for non-normally distributed continuous data, respectively. A P < .05 was considered statistically significant. Statistical analyses were conducted using the SPSS version 18 (Chicago, IL).
3. Results
830 consented children were enrolled, (57.6%) were male. Most of the screened patients were from Riyadh (n = 824, 99.3%). A minority were from Asir (N = 4, 0.5%) and Alqasim (n = 2, 0.2%). A total of 164 (19.8%) were found to be colonized with S. aureus, and of those 38 (4.6%) with MRSA (Table 1). Thus, the MRSA rate amongst all S. aureus carriers was 23.2%.
Table 1.
S. aureus and MRSA carriage by sex.
| Overall |
Males |
Females |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Total | 830 (100) | 478 (100) | 352 (100) |
| S. aureus carriage | 164 (19.8) | 103 (21.5) | 61 (17.3) |
| MRSA carriage | 38 (4.6) | 23 (4.8) | 15 (4.3) |
The carriage rates were similar among males and females with an odds ratio (OR) of 1.31 (95% confidence interval (CI) 0.92–1.86; P = .131) for S. aureus carriage and 1.14 (0.58–2.21; P = .708) for MRSA, respectively.
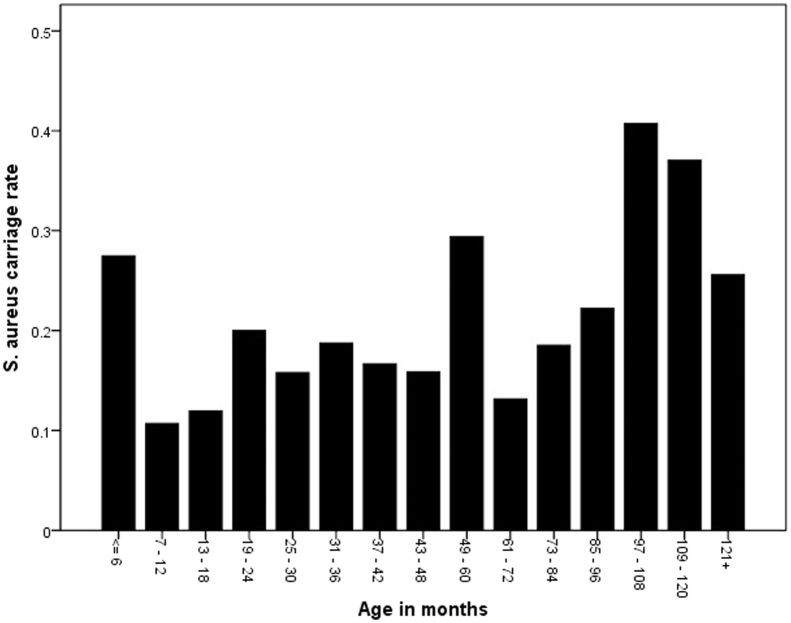
The age of the patients ranged from 1 month to 13 years. The median age was 34 months (interquartile range (IQR) 12–68.25 months). The median age of S. aureus carriers was significantly higher (41.5 months, IQR 14.25–84.0) than in non-carriers (31 months, IQR 12.0–63.25) using the Mann–Whitney U-test (P = .045). The distribution suggests a higher rate in the very young that drops to a lower level and then increases in older children (Fig. 1). Although the median age in MRSA carriers was higher than in non-carriers (60.0 versus 33.5 months), this difference was not statistically significant (P = .246).
Figure 1.
S. aureus carriage by age group.
Of the 38 cases colonized with MRSA, all isolates (100%) were vancomycin susceptible. Susceptibility rates for other antibiotics were 94.7% for linezolid, 65.8% for clindamycin and 89.5% for trimethoprim/sulfamethoxazole, 55.3% for Erythromycin, 84.2% for Ciprofloxacin, 97.3% for Rifampin and 94.7% for Daptomycin (Table 2). Seven (18.4%) isolates were clindamycin inducible resistant.
Table 2.
Antibiotic susceptibility for MRSA.
| Antibiotic | No. of sensitive isolates | Percentage |
|---|---|---|
| Vancomycin | 38 | 100% |
| Linezolid | 36 | 94.7% |
| Clindamycin | 25 | 65.8% |
| Trimethoprim–sulfamethoxazole | 34 | 89.5% |
| Erythromycin | 21 | 55.3% |
| Ciprofloxacin | 32 | 84.2% |
| Rifampin | 37 | 97.4% |
| Daptomycin | 36 | 94.7% |
4. Discussion
Since CA-MRSA was first reported in 1993, it continues to emerge, and nowadays, in many areas, it is more common than HA-MRSA strains [17]. We are not aware of any data on the prevalence of MRSA colonization among children in Saudi Arabia published to date. We found that 19.8% of children without previous hospital admission or known MRSA carriage who presented to our tertiary care emergency department were S. aureus carriers, and that 4.6% were colonized with MRSA, which is within the international reported range [4]. Given that we excluded children with a previous hospital admission and previously known MRSA carriers, this rate reflects the CA-MRSA rate in children presenting to our institution.
The MRSA rate among all S. aureus carriers was found to be 23.2%, consistent with the reported rate from infected children elsewhere [15]. Furthermore, we found no difference in S. aureus or MRSA prevalence rates between males and females. However, S. aureus carriers were older than non-carriers.
Given the higher percentage of MRSA among S. aureus carriers, MRSA coverage should be considered for all suspected moderate to severe Staphylococcal infections. All MRSA isolates were susceptible to vancomycin, and most of the isolates were susceptible to rifampin, linezolid, trimethoprim/sulfamethoxazole, daptomycin and ciprofloxacin. However, we found relatively low susceptibility rates to clindamycin and erythromycin (65.8% and 55.3% respectively) that would no longer justify the use of these antibiotics for empiric MRSA coverage. Vancomycin is the recommended empirical choice. However failure rate of vancomycin in CA-MRSA endocarditis is high even in combination therapy with gentamicin, and/or rifampin, particularly if vancomycin MIC is 2 μg/ml or more [18], [19], [20]. Daptomycin or linezolid as well as trimpethoprim/sulfamethoxazole could be used as an alternative treatment [21].
Other empirical choices include Lonezolid for CA-MRSA pneumonia and other moderate to severe infections apart from endocarditis or endovasculitis, and Trimethoprim–sulfamethoxazole for milder infections. These recommendations should be revised periodically based on annual antibiogram data.
To the best of our knowledge, this is the first study assessing the MRSA carriage rate in children in a Saudi Arabia setting. We collected data prospectively in a significant number of patients. However, this study also has a number of limitations. Firstly, it was a single center study and the rates may be different in other regions of Saudi Arabia. Furthermore, we conducted the study in a tertiary care emergency department setting, and the rates may be different in other settings, e.g. in patients seen by family physician.
Larger epidemiological studies would be needed to get a broader understanding of the prevalence and risk factors for MRSA, and in particular CA-MRSA carriage in the different provinces of Saudi Arabia.
5. Conclusion
The rate of CA-MRSA carriage among children in Riyadh province was within the range reported internationally. As the MRSA rate among children colonized with S. aureus was 23.2%, empiric MRSA coverage should be considered in patients with suspected moderate to severe S. aureus infections.
Conflict of interest
None.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Centers for Disease Control and Prevention Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. Morb Mortal Wkly Rep. 1999;48:707–710. [PubMed] [Google Scholar]
- 2.Naimi T.S., LeDell K.H., Como-Sabetti K., Borchardt S.M., Boxrud D.J., Etienne J. Comparison of community- and health care—associated methicillin-resistant Staphylococcus aureus infections. J Am Med Assoc. 2003;290(22):2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 3.Millar B.C., Loughrey A., Elborn J.S., Moore J.E. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA) J Hosp Infect. 2007;67(2):109–113. doi: 10.1016/j.jhin.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S.S., Ray P., Aggarwal A., Das A., Sharma M. A community-based study on nasal carriage of Staphylococcus aureus. Indian J Med Res. 2009;130(6):742–748. [PubMed] [Google Scholar]
- 5.Coia J.E., Duckworth G.J., Edwards D.I., Farrington M., Fry C., Humphreys H. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006;63(Suppl. 1):S1–S44. doi: 10.1016/j.jhin.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Scanvic A., Denic L., Gaillon S., Giry P., Andremont A., Lucet J. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32(10):1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 7.Muto C.A., Jernigan J.A., Ostrowsky B.E., Richet H.M., Jarvis W.R., Boyce J.M. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 8.Rybak M.J. Resistance to antimicrobial agents: an update. Pharmacotherapy. 2004;24:203s–215s. doi: 10.1592/phco.24.18.203S.52236. [DOI] [PubMed] [Google Scholar]
- 9.Fridkin S.K., Hageman J.C., Morrison M., Sanza L.T., Como-Sabetti K., Jernigan J.A. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352 doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 10.King M.D., Humphrey B.J., Wang Y.F., Kourbatova E.V., Ray S.M., Blumberg H.M. Emergence of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 11.Global-epidemiology-of-mrsa/(Global epidemiology of MRSA|Hand-in-Scan http://www.handinscan.com/global-epidemiology-of-mrsa/, [accessed 13.08.15].
- 12.Warren D.K., Guth R.M., Coopersmith C.M. Epidemiology of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27:1032–1040. doi: 10.1086/507919. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz P., Hortal J., Giannella M., Barrio J.M., Rodríguez-Créixems M., Pérez M.J. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infect. 2008;68(1):25–31. doi: 10.1016/j.jhin.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Kuehnert M.J., Kruszon-Moran D., Hill H.A., McQuillan G., McAllister S.K., Fosheim G. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193(2):172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 15.Al-Otaibi Fawzia Eida, Bukhari Elham Essa. Community acquired MRSA in outpatient children assisted at university hospital in Saudi Arabia 2005–2008. J Pediatr Infect Dis. 2010;5:369–376. 369. [Google Scholar]
- 16.El Amin N.M., Faidah H.S. MRSA in western region of Saudi Arabia, prevalence and antibiotics susceptibility pattern. Ann Saudi Med. 2012;32(5):513–516. doi: 10.5144/0256-4947.2012.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Tawfiq J.A. Incidence and epidemiology of methicillin-resistant Staphylococcus aureus infection in a Saudi Arabian Hospital, 1999–2003. Infect Control Hosp Epidemiol. 2006 Oct;27(10):1137–1139. doi: 10.1086/507971. [DOI] [PubMed] [Google Scholar]
- 18.Rybak M.J., Lomaestro B.M., Rotschafer J.C., Moellering R.C., Craig W.A., Billeter M. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 19.van Hal S.J., Paterson D.L. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405–410. doi: 10.1128/AAC.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakoulas G., Moise-Broder P.A., Schentag J., Forrest A., Moellering R.C., Jr., Eliopoulos G.M. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore C.L., Osaki-Kiyan P., Haque N.Z., Perri M.B., Donabedian S., Zervos M.J. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54:51–58. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]