Abstract
Cystic fibrosis (CF) is a lethal, monogenic disorder that affects multiple organ systems of the body. The incidence has been described before in the Middle East to be 1 in 2000 to 1 in 5800 live births, and the median survival was estimated to be from 10 to 20 years of age.
The present article attempts to revisit various facets of this disease and specifically highlights the most important lacunae that exist in treating CF. In addition, it also tries to emphasize the steps in improving the median survival of patients with CF, in these countries.
Keywords: Cystic fibrosis, P. aeruginosa, CFTR, Middle East, Treatment, Survival
Abbreviations: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CFRD, cystic fibrosis related diabetes; FEV1, forced expiratory volume in 1 second; P. aeruginosa, Pseudomonas aeruginosa
1. Introduction
Cystic fibrosis (CF) is a lethal, monogenic disorder that affects multiple organ systems of the body [1]. As per a Cystic Fibrosis Foundation annual report, 60,000 to 70,000 people across the world suffer from CF [2]. However, this estimate is based on the data available from the existing registries in developed countries (primarily the United States, Europe, and Australia).
Symptoms in patients with CF include accumulation of thick mucus, recurrent lung infections, persistent cough at times associated with phlegm with or without hemoptysis, wheezing, poor growth/weight gain (in spite of a good appetite), frequent greasy, bulky stools or difficulty in bowel movements, and electrolyte imbalance particularly in countries with warm weather [3], [4]. The estimated frequency of CF per live birth in Caucasians is believed to be 1 in 2000–2500 children. Determining the precise magnitude of prevalence of CF in South East Asian and Middle East countries remains elusive. Though the prevalence of CF has been estimated to fall in the range of 1 in 30,000 to 1 in 50,000, incidence of the disease has been reported in the range of 1 in 2000 to 1 in 5800 live births (4–13). Based on this, research suggests that these regions may harbor a very large proportion of CF patients in the world [13]. As per a recent report on extrapolated CF prevalence rates, it is projected that 34,357 patients in India and 41,898 patients in China may have CF [13]. The high prevalence of CF in these countries may be accountable due to the large population (>1 billion) of these countries. These prevalence extrapolations for CF are only rough estimates derived by applying the prevalence rates from the United States (or a similar country) to the population of other countries, and therefore may not reflect the actual prevalence of CF [13].
Similarly, determining the precise magnitude of the prevalence of CF in the Middle East countries remains difficult. Though the prevalence of CF has been estimated to fall in the range of 1 in 2500–5000. Based on this, It is projected that with a population of 27 million in Saudi Arabia (14), and around 18 live birth/1000, at least 800–1000 cases may have CF (Table 1) [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. These prevalence extrapolations for CF are only rough estimates and could be increased due to consanquinity (50% in the general population and 85% in CF families) [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], and therefore may magnify the actual prevalence of CF (Table 1) [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19].
Table 1.
Milestones of cystic fibrosis discovery in the Arab countries.
| Year (reference) | Country | Comment |
|---|---|---|
| 1958 [5] | Lebanon | First CF in Arabs |
| 1977 [6] | Iraq | First report |
| 1981 [7] | Kuwait | After death 2:1056, 1/3500 birth/year |
| 1984 [8] | Jordan | A pilot study (post-mortem) |
| 1985 [9] | Bahrain | 19 incidence 1:5800, 80% consanguineous marriages |
| 1986 [10] | Saudi Arabia | First case report |
| 1991 [11] | UAE | First report |
| 2001 [12] | Qatar | In a large Bedouin family |
The median survival in some Arab countries was estimated to be from 10 to 20 years of age [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] for many reasons such as: Delayed diagnosis due to decreased awareness of the variable presentation of the disease. Un-availabity of diagnostic tool such as quantitative sweat chloride test (e.g in the city of Jeddah, Saudi Arabia, with 5 million dwellers, only two centers with proper sweat chloride measurement's tool) [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Delayed institution of treatment due to delayed referral to a specialized CF center [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Early pseudomonas colonization at 3 years compared to 7 years in the western countries [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Poor compliance to treatment and chest physiotherapy, poor distribution of CF specialized centers, Delayed nutritional rehabilitation [4], [16].
Even after more than 7 decades since CF is known, there are many aspects of the disease which still remain enigmatic [20]. The present review attempts to revisit various facets of this disease and specifically highlights the most important lacunae that exist in treating CF. Further, it aims to emphasize the precarious consequences, if this disease is ignored or left untreated. Therefore early diagnosis of the disease is the important first step towards improved care. In addition, this review also captures the steps toward improvement of median survival in Arab countries.
2. Pathophysiology of CF
Patients with CF inherit a defective gene in each chromosome (i.e. 2 defective copies) that encodes the protein “CF transmembrane conductance regulator” (CFTR). The CFTR gene, which is located on chromosome 7, spans ∼250 kB in length, and translates into a protein of 1480 amino acids. The protein is a mucosal surface chloride channel present at the epithelial junctions and submucosal glands, and its dysregulation/failure in anion transport causes the disease manifestations [21], [22], [23].
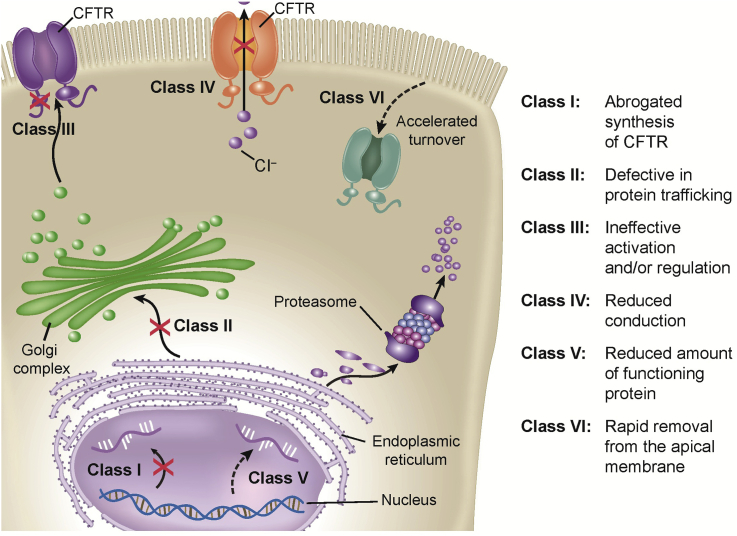
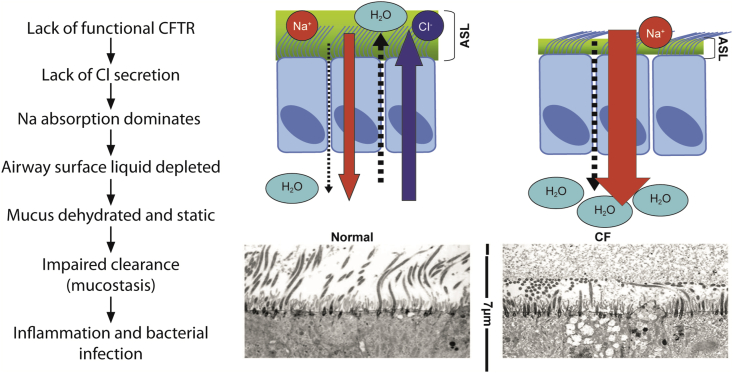
Approximately 1800 known mutations have been identified for CF gene, which gives rise to disease phenotypes [2]. Increasing attempts have been made to classify the known mutations. Six different classes of mutations are described based on the fate of the CFTR protein (Table 2 and Fig. 1). These reported gene defects lead to altered protein synthesis such that there is insufficient, adequately active CFTR at the cell surface. This causes the body to secrete unusually thick and sticky mucus (Fig. 2).
Table 2.
Spectrum of most common mutations or genotype reported in the cystic fibrosis patients [21].
| Class | Effect | % of CF population | Mutations | |||
|---|---|---|---|---|---|---|
| 1 | Defective CFTR protein and does not reach the cell surface | 88.5% | G542X 1717-8G->A 1898+3A->G |
711+1G->T W1282X CFTRdel19 |
1609delCA 1782delA 936delTA |
R1162X Q890X |
| 2 | Total loss of protein because of incorrect processing of CFTR | 2.4% | F508del | N1303K | 1507del | R1066C |
| 3 | Deregulates the ion channel | 4.4% | D1270N | G551D | ||
| 4 | Reduced ion fluxes and altered selectivity | L206W D836Y | R334W P205S |
R117H | R347H | |
| 5 | Functional proteins with normal chloride channel activity but reduced rate of synthesis | 2789+5G->A | 1811 + 1,6 | 3849 + 10kbC->T | ||
| 6 | Reduced expression of mutated CFTR protein because of rapid removal from the apical membrane | 3272 + 26G->A | kbA->G | |||
Figure 1.
Overview of types of functional mutations in CFTR.
Figure 2.
Comparison of normal vs defective epithelial fluid secretion in patients with cystic fibrosis.
The thick mucus clogs the lungs (pulmonary manifestation) leading to a vicious cycle of infection, inflammation, and lung tissue destruction, ultimately leading to life-threatening lung infections [21], [22]. The secreted mucus also clogs the pancreatic ducts, thereby obstructing the flow of bile digestive enzymes (which help in digestion and absorption of the food, especially of fats) to the small intestine. This leads to multitudes of disease conditions such as pancreatic insufficiency, gall stones, cysts, and chronic digestive problems (maldigestion and malabsorption) [23], [24], [25], [26], [27], [28], [29]. In patients with CF, epithelial anion transport fails, causing functional disruption in a number of organs besides the airways, including the digestive system, exocrine pancreas, gall bladder, reproductive tracts, and the sweat ducts [21], [23]. The types of complications in patients with CF largely depend on the degree and the type of CFTR mutation.
Diagnosis: CF is typically diagnosed based on typical clinical signs and symptoms and confirmed with high sweat chloride test >60 mmol/liter and or CFTR detection of 2 pathogenic mutations [1], [2], [3]. Reports from a retrospective study showed that diagnosis of CF in Saudi Arabia was done based on the typical clinical symptoms and high sweat chloride test [29]. In the past, newborn screening for meconium albumin using the BM test strip was used in Jordan to identify CF cases that were subsequently confirmed using the sweat chloride test [30].
The most common CFTR described in the United States and Europe is F508del, which constitutes 50%–85% of the CF population (Table 2), whereas those described in the Middle East were different due to consanguinity (Table 3, Table 4) [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36].
Table 3.
Important milestones in clinical and mutational findings in cystic fibrosis in the Middle East.
| Country | Year | Authors (reference) | No. of patients | Major CFTR mutations |
|---|---|---|---|---|
| UAE | 1994 | Frossard et al. [26] | 17 | S549R F508del |
| Lebanon | 1997 | Desgeorges et al. [27] | 20 | F508del W1282X N1303K |
| Saudi Arabia | 1997 | El-Harith et al. [28] | 15 | 3120+1G→A N1303K 1548delG |
| 1998 | Banjar et al. [29] | 70 | F508del I1234V N1303K R553K 3120+1G→A |
|
| 2000 | Kambouris et al. [31] | 70 | H139L S549R |
|
| Palestine | 1999 | Cahana et al. [32] | 70 | 3120 + LKdel8.6kg |
| Jordan | 2000 | Rawashdeh [33] | 202 | F508del |
| Qatar | 2001 | Abdul Wahab [34] | 29 | I1234V |
| Bahrain | 2002 | Eskandarani [35] | 19 | 2043delG |
| Tunisia | 2005 | Messadoud [36] | 390 | ΔF508, W1282, N1303K |
Legend: NO- Number of patients, UAE- United Arab Emirates, CFTR- Cystic fibrosis transmembrane regulator.
Table 4.
Distribution of Common CFTR in KSA Total of 272 patients.
| Mutation | Type | Exon/intron | Pts | % of total | No. of homoz | Homoz % of +CFTR (230) | No. of heteroz | Heteroz % of +CFTR (230) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1548delG | Novel | Exon 10 | 47 | 20% | 36 | 16% | 11 | 5% |
| 2 | F508del | Caucasian | Exon 10 | 29 | 12% | 28 | 12% | 1 | 0.5% |
| 3 | I1234V | African | Exon 19 | 28 | 11% | 28 | 12% | ||
| 4 | 3120+1G→A | African/ Greek |
Intron 16 | 24 | 10% | 24 | 10% | ||
| 5 | H139L | Novel | Exon 4 | 21 | 9% | 18 | 8% | 3 | 1% |
| 6 | 711+1G→A | Novel | Intron 5 | 20 | 8% | 16 | 7% | 4 | 2% |
| 7 | N1303K | European | Exon 21 | 7 | 3% | 7 | 3% | ||
| 8 | S549R | UAE (France) | Exon 12 | 7 | 3% | 7 | 3% | ||
| 9 | 2043delG | Bahrain | Exon 13 | 6 | 2% | 5 | 2% | 1 | 0.5% |
| 10 | 1507del 9 | Novel | Exon 9 | 5 | 2% | 5 | 2% | ||
| 11 | Others | 36 | 15% | 36 | 16% | ||||
| 12 | Not Identified | 11 | 5% | ||||||
| Total | 241 | 100% | 210 | 91% | 20 | 9% |
Legend: KSA- Kingdom of Saudi Arabia, Pts-number of Patients, No-number, Homoz- Homozgous, CFTR- Cystic fibrosis transmembrane regulator gene mutations, Heteroz- Heterozygous.
F508del presents in small numbers 10–20% in the Gulf area, compared to 30–50 in North African countries [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Novel mutations characterized most Arab countries due to numerous communities (cosmopolitan) especially in Lebanon [27], and Jordan [33]. Consanguinous intermarriages in the Gulf area is high (80–96%) which was expected to play a major factor in perpetuating many rare mutations as in S549R in United Arab Emirates (UAE, 19%) [26], (3849 + 10KbC > T) in 26% in UAE [26], and (I1134V) 83% in Qatar [34].
In Saudi Arabia (Author's personal experience) (Table 4), reports in CFTR detection showed: 89% of CFTR alleles, have been identified [28], [29], [30], [31]. F508del constitutes 12% of CFTR mutation. 1548 delG is the most common Saudi CFTR mutation identified (20%). 1548 delG, F508del, I1234V, 3120+1G > A, H139L, 711+1G > A, N1303K, S549R, 2043delG, 1507del 9 are the most common CFTR mutations of Saudi ethnic origin (80%). Screening for the previously mentioned 10 mutations would identify 80% of C.F. alleles (Table 4) [28], [29], [30], [31].
3. Strategies for treating CF
3.1. Currently established conservative therapies for treating CF
The currently available therapies principally aim at maintaining efficient lung function, clearing airways of mucus, administering nutritional therapy (i.e. enzyme, multivitamin, and mineral supplements) to maintain adequate growth, and managing complications [37]. Although these therapies have been highly successful in managing the consequences of the gene defect, they are palliative in nature.
Treatment guidelines have been developed in the United States [38] and Europe [39], with the scope to enhance care of CF patients, by sharing best practice. To the authors' knowledge, in the absence of regional or local guidelines, in other regions these recommendations serve as an example.
3.2. Gene therapy
The discovery of CFTR gene in 1989 spurred a great amount of interest among the researchers [24]. It was reckoned that replacement of CFTR gene during the neonatal period before the onset of airway damage might provide the much awaited cure to patients with CF [21]. However, effective gene delivery with aerosolized adenovirus vector could not be achieved due to the predominant presence of the adenoviral receptor at the entry on the basolateral surface of the epithelial cells [40].
3.3. Potentiators and correctors
Potentiators are compounds that enhance the channel gating characteristics of mutant CFTR via direct interaction [24]. Potentiators function by increasing the amount of time the CFTR channel is open, allowing for more Cl− to pass through the pores [24]. Despite the discovery of many potentiators by high-throughput method, the precise mechanism of action remains unclear [41]. Various clinical trials are currently underway which in due course may demonstrate the clinical benefits of these molecules in the treatment of CF. Ivacaftor, a CFTR potentiator, has shown efficacy and safety compared with placebo in CF patients with a G551D mutation [42], [43]. Ivacaftor is approved in Europe, United States, and Canada for the treatment of CF patients greater than or equal to 6 years of age who have a G551D mutation [42]. Ivacaftor treatment is not effective in CF patients with homozygous F508del-CFTR mutation, which is the most common mutation in Caucasian CF patients [44].
Similarly, compounds that correct the processing and facilitate the movement of mutant F508 CFTR (primarily class II mutations) out of the endoplasmic reticulum into the apical plasma membranes of epithelial cells are termed correctors. However, like the potentiators, the mechanism of action of correctors remains unknown. Lumacaftor (VX-809), an investigational corrector, has been shown to be effective in enhancing CFTR function in patients with F508del-CFTR [45]. A long-term phase III roll-over study of treatment with lumacaftor in combination with ivacaftor is ongoing; however, the study does not include CF patients from the Middle East region [46]. Molinski et al studied the molecular consequences of the c.3700 A > G (p.Ile1234Val) mutation in CF, which is characterized by defective folding and processing, similar to the F508del-CFTR [47]. Though rare in the west, this mutation is relatively common in the Middle East (12.3%) and is the second most common mutation in some Middle East countries [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [47]. Molinski et al hypothesized that the correction of the defective protein through lumacaftor may have a significant impact on medical treatment of CF in the Middle East.
3.4. Airways clearance
Congestion of the airways by viscous mucus is most common in patients with CF [48]. Clearance of the clogged airways is achieved by combination therapy that includes bronchodilators, postural drainage, and chest physical therapy [37]. Several mucoactive drugs that affect the physical characteristics or the quantity of mucus produced are known which fall under 2 broad categories of mucolytics and mucokinetics [49].
Dornase alfa, a recombinant human DNAse I, is a mucolytic drug which provides potential benefits by reducing the adhesiveness and viscoelasticity of CF sputum [50]. The results from clinical studies demonstrate that aerosolized dornase alfa significantly improves lung function achieving a 6%–7% increase from baseline in forced expiratory volume in 1 s (FEV1) after 6 months therapy [51], [52]. It has been reported that when the treatment is stopped, the respiratory function deteriorates below their previous values [53].
Hypertonic saline is a mucokinetic drug often been used for improving lung function by mucus clearance [54]. It is a highly concentrated salt solution (∼7%) which increases the ion concentration of airway surface liquid and osmotically draws fluid into the airway lumen. This results in replenishment of fluid layer and accelerated mucus clearance [55]. Inhaled/nebulized hypertonic saline has demonstrated modest improvement in lung function and reduction in pulmonary exacerbations without substantial adverse events [56], [57], [58]. Few studies have demonstrated improvements in respiratory function, the effects were found to be transient, and therefore, predicted to be of limited therapeutic benefit [59]. Some reports also recommend the use of bronchodilators prior inhalation of hypertonic saline to avoid transient narrowing of the airways, coughing, and pharyngeal discomfort [60], [61].
Mannitol dry powder (400 mg twice daily) is a recently developed product with mucolytic effect through osmotic mechanism. Mannitol is approved in Europe, for treatment of adult CF patients (above 18 years of age) as an add-on therapy to standard of care. In phase III trials it has been shown that 26 weeks treatment with mannitol dry powder improves lung function and reduces exacerbation rates [62].
In addition to the use of mucoactive drugs, physicians also recommend manual therapies/physiotherapy such as postural drainage, percussion and vibration or breathing through positive expiratory pressure device [60], [61], [62], [63]. These methods enable mucus clearance by accelerated expiratory flow, reducing airway obstruction, and improving rheology of mucus [64], [65]. Patients are also advised to do regular physical exercises in addition to chest physiotherapy from an early age for efficient maintenance of respiratory function. Increased physical exercise strengthens the respiratory muscles and also helps to clear the tenacious mucus secretion that blocks the airways [66].
3.5. Bronchodilators
Bronchodilator treatment is the most commonly used therapy being prescribed in ∼80% of patients with CF [67], [68], [69]. Although bronchodilators are usually given to overcome airways obstruction and hyper-responsiveness in CF patients because of inhaled mucolytic and/or antibiotic therapy, the response to inhaled bronchodilators is variable both between and within patients [67], [68], [69], [70]. However, long-term studies failed to demonstrate sustained clinical benefits [70]. Although their use in CF remains controversial, EU and US guidelines recommend regular use of inhaled β2-adrenergic bronchodilators to improve and maintain efficient lung function, and also can be used before inhaled antibiotics such as tobramycin and aztreonam [38], [39], [69].
3.6. Antibiotics in the treatment of CF
The significant improvement in the prognosis of CF can be largely attributed to the arsenal of newer antibiotics that combat with the pathogenic organisms [38], [39]. Treatment with specific antibiotic remains the mainstay of CF treatment. It is highly recommended to be used in conjunction with other therapies such as physiotherapy and treatment with bronchodilators to promote clearance of the bronchial mucus [38], [39]. Patients with CF often present with recurrent infections with Haemophilus influenza (H. influenzae), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa). H. influenzae and S. aureus are usually the first pathogens encountered in childhood which is later substituted by P. aeruginosa. An array of antibiotics is usually prescribed to treat these bacterial infections. However, the choice of antibiotics largely depends on the presence of specific pathogen in the respiratory tract. It is reported that overall 54.4% of the CF patients are infected with P. aeruginosa [2]. Chronic infections with P. aeruginosa result in rapid decline in lung function and increased need for antibiotic treatment and hospitalization [71]. Therefore, treatment with effective antibiotic regimen targeting this pathogen holds key in the successful management of CF. Aerosol antibiotics, such as colistin, tobramycin, and aztreonam are recommended to be used as regular maintenance therapy in the treatment of pseudomonas infection [38], [39]. Although oral antibiotics (fluoroquinolones, amoxicillin, cephalexin, or trimethoprim) may be prescribed for patients with less severe exacerbations, P. aeruginosa related exacerbations are commonly treated with combinations of intravenous (IV) antibiotics for 1–3 weeks [25], [38], [39]. Despite frequent intravenous therapy, patients often continue to present with deteriorating respiratory function and eventually succumb to lung disease [72].
Inhaled antibiotics have been increasingly used to treat persistent airway infection in people with CF. Treatment with aerosolized antibiotics has been advocated for the management of the chronic P. aeruginosa infection. The CF pulmonary guidelines published recently recommend the use of aerosolized/inhaled tobramycin with or without dornase alfa in the treatment of P. aeruginosa infections in CF [38], [39].
Tobramycin is an aminoglycoside antibiotic produced by Streptomyces tenebrarius, and is indicated for the management of CF patients infected with P. aeruginosa [73]. It primarily disrupts protein synthesis by bacteria, leading to altered cell membrane permeability, progressive disruption of the cell envelope, and eventual cell death [74]. Tobramycin has demonstrated in-vitro activity against a wide spectrum of gram-negative organisms including P. aeruginosa.
Tobramycin is also available as a specifically developed formulation for administration by nebulization. Evidence from clinical and pharmacological studies have demonstrated that tobramycin primarily concentrates in the airways following administration [75]. Inhaled tobramycin significantly improves lung function and reduces rate of exacerbations in patients with CF. Inhaled tobramycin is strongly recommended for chronic use in patients of ≥6 years of age with moderate-to-severe lung disease and with P. aeruginosa persistently present in cultures of the airways [38], [39], [76]. The quality of the evidence for the use of inhaled tobramycin in patients with mild disease is limited by the number of studies [38], [39], [58].
Macrolides antibiotics such as azithromycin are used at anti-inflammatory doses to treat CF patients. However, there is limited clinical evidence to support their use. A clinical trial has shown that long-term use of azithromycin in CF patients, ≥6 years of age, significantly reduced pulmonary exacerbations and the number of additional courses of oral antibiotics, irrespective of the infectious status [76]. Another randomized controlled trial demonstrated improvements in lung function after treatment with azithromycin compared with placebo [77].
Maintenance therapy with long-term macrolides has been proved to be beneficial in patients with CF [78]. However, long-term use of macrolides has shown evidences of emergence of bacterial resistance in CF patients [79], [80]. Of particular recent concern is the potential of increased colonization of CF patients with nontuberculous mycobacteria [81].
3.7. Pancreatic enzyme therapy
Patients with CF and signs and symptoms of maldigestion due to pancreatic insufficiency are usually prescribed with pancreatic enzymes replacements to aid digestion [82]. It has been reported that ∼15% of patients with CF possess sufficient exocrine pancreatic function that enables normal nutrient absorption [83]. Although studies endorse normal pancreatic function in “pancreatic sufficient” CF patients, quantitative assessment studies in these patients have demonstrated impaired pancreatic ductal enzyme secretions [84], [85]. Pancreatic enzyme preparations of porcine origin have been conventionally used to treat this type of maldigestion. Most marketed enzyme preparations come in special enteric-coated capsules that consist of desiccated porcine pancreatic extracts [25].
3.8. Nutritional support/high energy supplements
Besides the conservative treatment of CF, supplementation with appetite-rich food is essential for improved functional capacity and quality of life [86]. Nutritional supplementation particularly with fat-soluble vitamins A, E, K, and sometimes D and minerals constitute an integral part of treatment. The need for lipid-soluble vitamins can be assessed directly by the measurement of vitamin A, D, E levels [87].
4. Clinical implications of untreated CF
CF is a multi-manifestation disorder that affects respiratory, gastrointestinal, endocrine, sweat gland, and reproductive systems [74]. Improper or non-treatment of underlying disease may lead to several clinical implications.
4.1. Respiratory tract implications
Airways disease remains one of the worsening aspects in the course of CF. Most of the morbidity and mortality associated with CF result from progressive lung disease, upper airway infection, and chronic sinusitis [88]. CF patients acquire lung infection, which triggers the onset of inflammatory cascade. The hallmark of CF lung disease is bronchiectasis. This paves the way for the establishment of persistent infection by pathogens such as S. aureus, H. influenzae, and P. aeruginosa ultimately resulting in severely compromised airway defenses [53]. In few patients, diffuse bronchopulmonary disease is due to refractory Burkholderia cepacia, which further makes therapeutic management more cumbersome [88]. The progression of airways disease in patients with CF leads to eventual acute respiratory failure because of diffuse bronchopulmonary disease, pneumonia, or acute hemoptysis [86]. Supporting this, a US study reported that in ∼80% of patients with CF, cardiorespiratory complications are the most common cause of death [89].
4.2. Pancreatic duct implications
The epithelial layers of the pancreatic ducts and the gastrointestinal tract have abundant ion exchange channels and CFTR levels similar to those found in the airway epithelium [20], [90]. In patients with pancreatic manifestations of CF, the pancreas is compromised at birth [20]. Concretion of pancreatic ducts by thick, viscid mucus blocks digestive enzymes from entering the gut leads to poor digestion and malabsorption of food [91]. Pancreatic exocrine insufficiency contributes to growth retardation and delayed puberty in children and low body weight in adults [92]. It has been well documented that CF is the major cause of pancreatic exocrine failure in early childhood. In adults, it is commonly associated with chronic pancreatitis [92].
4.3. Gastrointestinal implications
Absence or abridged form of CFTR reduces fluid secretion in the gut [20]. The intestinal content in these patients contains minimal water content and becomes impacted. This may result in meconium ileus at birth and distal intestinal obstruction syndrome later in life [93]. Altered or malabsorption of the essential nutrients in the gut because of lack of digestive enzymes causes the nutrients to be excreted in the feces. This mainly leads to malnutrition and retarded growth and development [77]. In addition, people with CF experience other gastrointestinal symptoms such as severe gastroesophageal reflux disease, intestinal blockage by intussusception, and constipation [94]. Malnutrition is another common symptom in patients with untreated or poorly controlled CF that affects growth and reduces physical activity [95], [96]. The prevalence of malnutrition/hyponutrition in CF has been reported to be high although it varies in different studies [97]. Negative energy balance is considered as central to the development of malnutrition [98].
4.4. Rectal prolapse in CF
Rectal prolapse is another clinical implication of CF (characterized by protrusion of the rectal mucous membrane through the anus) encountered in both pediatric and adult patients [99]. The incidence of rectal prolapse is high among male infants with CF than in adults with a peak incidence between 1 and 3 years [102], [99], [100], [101]. In a study reported by Stern et al, rectal prolapse was found to occur in 18.5% of CF patients [97]. It further reported that though rectal prolapse preceded diagnosis of CF, physicians rarely appreciate its importance as a symptom of this disease [103].
4.5. CF-related diabetes
Diabetes, referred to as CF-related diabetes (CFRD), is a very common complication that develops over time in many patients with CF [104] CFRD is distinct as it usually combines the characteristics of both type 1 and type 2 diabetes [104]. Reported prevalence of diabetes in children with CF < 10 years of age is low. However, the prevalence increases significantly with age, with 50% of patients developing CFRD by the age of 30 years [105]. Few studies have demonstrated a plausible relationship between CFRD and poor nutritional and respiratory status [107], [106]. In patients with severely impaired insulin secretion, a more rapid deterioration in the respiratory status is observed [108]. Similar to rectal prolapse, the worsening in respiratory status may also predate the diagnosis of CFRD [109].
4.6. Implications of CF on liver
Complications of liver disease represent the third most frequent cause of disease-related death in patients with CF [77], [110]. CF-associated liver disease is presently classified among genetic cholangiopathies, which results because of lack or dysfunction of the CFTR at the apical membrane of bile duct cells [111]. Defective CFTR protein in a subset of CF patients leads to thickened bile secretions. This further chokes the bile ducts leading to liver and biliary complications [77], [111]. Although the biliary tract manifestation is usually clinically evident, the involvement of liver may only be apparent at the end stages [111]. Of the wide spectrum of hepatobiliary complications, progression of focal biliary cirrhosis to multilobular cirrhosis and potentially end-stage liver disease is the most clinically relevant problem in patients with CF [112].
4.7. Implications on the reproductive system
More than 95% of males with CF are infertile. This infertility is the result of obstructive azoospermia caused by maldevelopment of the mesonephric ducts, resulting in either agenesis or atresia of the epididymis, vas deferens, or seminal vesicles [114], [113]. Other factors that may complement infertility in males with CF are the low serum levels of vitamins A and E or interference with the normal transport of the sperm [115]. Although not so frequent, impaired fertility is also seen in females with CF. Infertility characterized by thickened cervical mucus in females may be a consequence of the presence of thick mucus in the genital tract that barricades sperm penetration [114]. In severe cases, malnutrition disrupts ovulation and causes amenorrhea [116].
4.8. Socioeconomic implications of CF
CF poses significant financial burden on the patient and the society on the whole [117]. Socioeconomic status remains an important risk factor in CF management, as seen in many chronic diseases, [119], [118]. The following factors profoundly impact the outcomes in CF patients: poor socioeconomic status, mental trauma in caregivers/patient, employment status, and education.
5. Barriers in treating CF
Following are the few barriers that affect the treatment in patients with CF.
5.1. Late diagnosis of CF
CF is detected with increasing frequency in older children, adolescents, and even young adults [120]. The clinical diagnosis of CF is often delayed because the early symptoms are minimal or nonspecific [120]. Considerable delays in diagnosis of children with CF occur as the disease is identified solely based on clinical presentation [120]. Another important reason responsible for protracted diagnosis of CF is lack of training among respiratory physicians [13].
5.2. Adherence
Treatment adherence is crucial in patients with CF. Reasons for poor adherence could be due to lack of access to care, insufficient patient education, and high treatment burden. Poor adherence is a notable problem, especially during adolescence [121]. Poor adherence leads to serious consequences, which includes increased morbidity and mortality, reduced quality of life, and greater health care costs. Although the rates of adherence to treatment for CF are generally low, they vary significantly between the different types of treatment [122]. The rates of adherence are particularly poor in pediatric population than in adults with CF. For example, the adherence rates for children with CF are estimated to be 40%–47% for chest physical therapy [124], [123], whereas adherence to dietary recommendations is even lower, ranging from 16% to 20% [125]. Briesacher et al have shown that high adherence to inhaled tobramycin solution is associated with a lower risk of hospitalization and also leads to a lower outpatient service cost, than patients with low adherence [126].
5.3. Limited number of CF testing centers in the Middle East countries
Decreased access to care is another impediment that adversely affects the health-related outcomes in many patients with CF [119], [118]. Further, the limited number of dedicated CF diagnostic centers makes it one of the challenging diseases to treat [13]. The scenario can be well visualized by an example that in a city like Riyadh, the commercial capital of Saudi Arabia with 5 million dwellers, has only 2 CF diagnostic centers [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [127].
5.4. Lack of proper medical care and patient education
Therapeutic insufficiency is the main cause of precocious complications and poor prognosis in patients with CF [128]. It is now generally agreed that a team of trained experienced health professionals in a specialist CF center with more than 50 patients can best provide this type of care. CF is a complex disease with numerous medical, psychiatric, and social consequences. For patients and their families, it means a lifetime of adjustments. Lack of public awareness can make the family feel isolated in its attempts to cope. Patients with CF often need caregivers with the knowledge and sensitivity to provide appropriate palliative care when required.
5.5. Cost and availability of pharmaceutical drugs
Management of CF is very expensive, with best survival rates seen when patients are treated in specialized CF centers. Costs of care increase with the age of patients, mainly because of recurrent deteriorations in the lung function and colonization with the pathogens such as P. aeruginosa [129]. The majority of patients with CF are often required to be on continuous medication to control the unabated infections [117]. Apart from direct costs for medication, laboratory analyses and costs of health care visits further add to the economic burden [117].
5.6. Poverty
The links between poverty and CF are extensive, strong, and pervasive. In spite of availability of sustainable treatment options, people hardly utilize them because of their exorbitant cost. People with CF and very poor affordability are even unaware of the disease and are often deprived quality treatment. In a disorder such as CF, with an equal incidence in different social groups, a report suggested that survival is strongly influenced by social factors [130].
5.7. Improved therapies should be made available
The quality of life and longevity in patients with CF can be prolonged if diagnosis of CF is established in the early stages. This will enable initiation of appropriate therapeutic regimen to commence as soon as a diagnosis is made [131]. Recent advancements have transformed CF from a disease characterized by death in early childhood to a chronic illness, with most patients living to adulthood. Despite this progress, many patients with CF eventually succumb primarily because of infection of the airways and lung failure. There is still a lack of treatment modalities that can offer complete cure. Hence, future research is warranted to equip the biological arsenal so that CF-related complications including deaths because of CF may be significantly curtailed.
5.8. Recommendations
A holistic approach comprising of the following recommendations may be appropriate in the treatment of CF: Improve awareness of the disease, and its variable presentation, Early referral to CF specialized centers, make the diagnostic sweat chloride test measurement to be available in most major hospitals for most populated cities, Early diagnosis and early institution of treatment, Early nutritional rehabilitation, improve adherence to treatment and air way clearance techniques, and to make most of the CF drugs available to most CF centers.
Acknowledgments
The authors would like to thank Siddharth Vishwakarma, Novartis Healthcare Pvt. Ltd., Hyderabad, for providing medical writing/editorial assistance. Hanaa Banjar and Gerhild Angyalosi contributed to all the sections equally. They reviewed the draft in its entirety and provided critical comments for intellectual content and flow. This study was funded by Novartis Pharma AG.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Conflict of Interest
Dr. Hanaa Banjar has nothing to report. Dr. Gerhild Angyalosi is in employee of Novartis Pharma AG, Switzerland
References
- 1.Becq E. Cystic fibrosis transmembrane conductance regulator modulators for personalized drug treatment of cystic fibrosis. Drug. 2010;70(3):241–259. doi: 10.2165/11316160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.CFF Patient Registry annual data report 2012. http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf [accessed 01.09.15] Available from:
- 3.The basics of cystic fibrosis: understanding symptoms and causes. [accessed 09.01.15]; Available from: www.cftrscience.com/pdfs/Flip_Chart_Patient.pdf.
- 4.Banjar H. Overview of cystic fibrosis: patients aged 1-12 years in a tertiary care center in Saudi Arabia. Middle East Pediatr. 1999;4(2):44–49. [Google Scholar]
- 5.Salam M.Z. Cystic fibrosis of the pancreas in an oriental child. Ann Paediatr. 1958;190(4):252–255. [PubMed] [Google Scholar]
- 6.Hassani M.A. Cystic fibrosis in Iraqi children. J Trop Pediatr Environ Child Health. 1977;23(3):136–137. [PubMed] [Google Scholar]
- 7.Aluwihare A.P., Ali S.M., Ahmad M.S., Mirghani G.A., Mohacsy J. Cystic fibrosis in Kuwait. Lancet. 1981;2(8254):1056. doi: 10.1016/s0140-6736(81)91260-5. [DOI] [PubMed] [Google Scholar]
- 8.Kamal M.F., Nazer H. Cystic fibrosis in Jordan: a pilot study. Ann Trop Paediatr. 1984;4(4):243–246. doi: 10.1080/02724936.1984.11748345. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mahroos F. Cystic fibrosis in bahrain incidence, phenotype, and outcome. J Trop Pediatr. 1998;44(1):35–39. doi: 10.1093/tropej/44.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Abdullah M.A. Cystic fibrosis in Saudi Arabia: a case report. Saudi Med J. 1986;(7) 189–891. [Google Scholar]
- 11.Aziz S.A.A. 1991. Cystic fibrosis in the UAE Emirates Med J; pp. 202–204. (9) [Google Scholar]
- 12.Abdul Wahab A., Dawod S., Tal Thani G. Cystic fibrosis in a large kindred family in Qatar. Ann Trop Paediatr. 2000;20(3):203–207. doi: 10.1080/02724936.2000.11748135. [DOI] [PubMed] [Google Scholar]
- 13.Imbesi P. Thought rare, cystic fibrosis now rising in India. Indus Bus J. 2006 http://www.rightdiagnosis.com/c/cf/stats-country.htm Available from: [Google Scholar]
- 14.Census shows Kingdom’s population at more than 27 million, Saudi Gazette, Wednesday, 24 November 2010, available from: (http://www.saudigazette.com.sa/index.cfm?method=home.regcon&contentID=2010112487888&archiveissuedate=24/11/2010).
- 15.Banjar H., Mogarri I. Demographic and clinical data of cystic fibrosis (CF) patients in a tertiary care center in Saudi Arabia. Emir Med J. Dec. 1998;16(3):166–169. [Google Scholar]
- 16.Banjar H. Microbiological data of cystic fibrosis patients in a tertiary care center in Saudi Arabia. Kuwait Med J. Sept. 2004;36(3):179–181. [Google Scholar]
- 17.Banjar H. Cystic fibrosis: presentation with other diseases, the experience in Saudi Arabia. J Cyst Fibros. 2003;2:155–159. doi: 10.1016/S1569-1993(03)00058-4. [DOI] [PubMed] [Google Scholar]
- 18.Banjar H. Nutritional data of cystic fibrosis patients. Bahrain Med Bull. 2004;26(3):91–94. [Google Scholar]
- 19.Banjar H. Morbidity and mortality data of cystic fibrosis patients. Saudi Med J. 2003;24(7):730–735. [PubMed] [Google Scholar]
- 20.Davis P.B. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 21.Cuthbert A.W. New horizons in the treatment of cystic fibrosis. Br J Pharmacol. 2011;163(1):173–183. doi: 10.1111/j.1476-5381.2010.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Gracia J., Mata F., Alvarez A., Casals T., Gatner S., Vendrell M. Genotype-phenotype correlation for pulmonary function in cystic fibrosis. Thorax. 2005;60(7):558–563. doi: 10.1136/thx.2004.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 24.Kreindler J.L. Cystic fibrosis: exploiting its genetic basis in the hunt for new therapies. Pharmacol Ther. 2010;125(2):219–229. doi: 10.1016/j.pharmthera.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguiness K., Casey S., Fulton J., Luder E., McKenna A., Hazle L. Pancreatic enzyme replacement in people with cystic fibrosis. Cyst Fibros Found Factsheet. 2006 http://www.cff.org/UploadedFiles/LivingWithCF/StayingHealthy/Diet/EnzymeReplacement/Nutrition-Pancreatic-Enzyme-Replacement.pdf [accessed 09.01.15] Available from: [Google Scholar]
- 26.Frossard P.M., John A., Dawson K. Cystic fibrosis in the United Arab Emirates: II-Molecular genetic analysis emirates Med J. 1994;12(12):249–254. [Google Scholar]
- 27.Desgeorges M., Megarbane A., Guittard C., Carles S., Loiselet J., Demaille J. Cysticc fibrosis in Lebanon: distribution of CFTR mutations among Arab communities. Hum Genet. 1997;100(2):279–283. doi: 10.1007/s004390050505. [DOI] [PubMed] [Google Scholar]
- 28.El-Harith E.A., Dork T., Stuhrmann M., Abu-Srair H., al-Shahri A., Keller K.M. Novel and characteristic CFTR mutations in Saudi Arab children with severe cystic fibrosis. J Med Genet. 1997;34(12):996–999. doi: 10.1136/jmg.34.12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banjar H., Mogarri I., Meyer B.F., Al-Hamed M., Kambouris M. Genetic and clinical data of cystic fibrosis patients in a tertiary care center in Saudi Arabia. Kuwait Med J. 1998;30(4):312–316. [Google Scholar]
- 30.Nazer H. Cystic fibrosis in Jordan–incidence and prevalence. Trans R Soc Trop Med Hyg. 1986;80(2):348. doi: 10.1016/0035-9203(86)90057-x. [DOI] [PubMed] [Google Scholar]
- 31.Kambouris M., Banjar H., Moggari I., Nazer H., Al-Hamed M., Meyer B.F. Identification of novel mutations in Arabs with cystic fibrosis and their impact on the cystic fibrosis transmembrane regulator mutation detection rate in Arab populations. Eur J Pediatr. 2000;159(5):303–309. doi: 10.1007/s004310051277. [DOI] [PubMed] [Google Scholar]
- 32.Laufer-Cahana A., Lerer I., Sagi M., Rachmilewitz-Minei T., Zamir C., Rivlin J.R. Cystic fibrosis mutations in Israeli Arab patients. Hum Mutat. 1999;14(6):543. doi: 10.1002/(SICI)1098-1004(199912)14:6<543::AID-HUMU16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Rawashdeh M., Manal H. Cystic fibrosis in Arabs: a prototype from Jordan. Ann Trop Paediatr. 2000;20(4):283–286. doi: 10.1080/02724936.2000.11748148. [DOI] [PubMed] [Google Scholar]
- 34.Abdul Wahab A., Al Thani G., Dawod S.T., Kambouris MAl, Hamed M. Heterogeneity of the cystic fibrosis phenotype in a large kindred family in Qatar with cystic fibrosis mutation (I1234V) J Trop Pediatr. 2001;47(2):110–112. doi: 10.1093/tropej/47.2.110. [DOI] [PubMed] [Google Scholar]
- 35.Eskandarani H.A. Cystic fibrosis transmembrane regulator gene mutations in Bahrain. J Trop Pediatr. 2002;48(6):348–350. doi: 10.1093/tropej/48.6.348. [DOI] [PubMed] [Google Scholar]
- 36.Messaoud T., Bel Haj Fredj S., Bibi A., Elion J., Ferec C., Fattoum S. Molecular epidemiology of cystic fibrosis in Tunisia. Ann Biol Clin Paris. 2005;63(6):627–630. [PubMed] [Google Scholar]
- 37.Sharma G. The pathophysiology, diagnosis, and investigation of cystic fibrosis. Bus Brief U. S Respir Care. 2006:64–71. [Google Scholar]
- 38.Mogayzel P.J., Jr., Naureckas E.T., Robinson K.A., Mueller G., Hadjiliadis D., Hoag J.B. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 39.Doring G., Flume P., Heijerman H., Elborn J.S., Consensus Study G Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11(6):461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Walters R.W., Grunst T., Bergelson J.M., Finberg R.W., Welsh M.J., Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274(15):10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 41.Pedemonte N., Tomati V., Sondo E., Galietta L.J. Influence of cell background on pharmacological rescue of mutant CFTR. Am J Physiol Cell Physiol. 2010;298(4):C866–C874. doi: 10.1152/ajpcell.00404.2009. [DOI] [PubMed] [Google Scholar]
- 42.Davies J.C., Wainwright C.E., Canny G.J., Chilvers M.A., Howenstine M.S., Munck A. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187(11):1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies J., Sheridan H., Bell N., Cunningham S., Davis S.D., Elborn J.S. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1(8):630–638. doi: 10.1016/S2213-2600(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 44.Flume P.A., Liou T.G., Borowitz D.S., Li H., Yen K., Ordonez C.L. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142(3):718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren H.Y., Grove D.E., De La Rosa O., Houck S.A., Sopha P., Van Goor F. VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell. 2013;24(19):3016–3024. doi: 10.1091/mbc.E13-05-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A phase 3 rollover study of lumacaftor in combination with ivacaftor in subjects 12 years and older with cystic fibrosis.
- 47.Molinski S.V., Gonska T., Huan L.J., Baskin B., Janahi I.A., Ray P.N. Genetic, cell biological, and clinical interrogation of the CFTR mutation c.3700 A>G (p.Ile1234Val) informs strategies for future medical intervention. Genet Med. 2014;16(8):625–632. doi: 10.1038/gim.2014.4. [DOI] [PubMed] [Google Scholar]
- 48.Bryson H.M., Sorkin E.M., alfa Dornase. A review of its pharmacological properties and therapeutic potential in cystic fibrosis. Drugs. 1994;48(6):894–906. doi: 10.2165/00003495-199448060-00006. [DOI] [PubMed] [Google Scholar]
- 49.Hurt K., Bilton D. Inhaled mannitol for the treatment of cystic fibrosis. Expert Rev Respir Med. 2012;6(1):19–26. doi: 10.1586/ers.11.87. [DOI] [PubMed] [Google Scholar]
- 50.Hodson M.E. Aerosolized dornase alfa (rhDNase) for therapy of cystic fibrosis. Am J Respir Crit Care Med. 1995;151(3 Pt 2):S70–S74. doi: 10.1164/ajrccm/151.3_Pt_2.S70. [DOI] [PubMed] [Google Scholar]
- 51.Harms H.K., Matouk E., Tournier G., von der Hardt H., Weller P.H., Romano L. Multicenter, open-label study of recombinant human DNase in cystic fibrosis patients with moderate lung disease. DNase International study Group. Pediatr Pulmonol. 1998;26(3):155–161. doi: 10.1002/(sici)1099-0496(199809)26:3<155::aid-ppul1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The pulmozyme study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 53.Zach M.S. The role of recombinant human DNase in the treatment of patients with cystic fibrosis: many promises, more problems. Thorax. 1996;51(7):750–755. doi: 10.1136/thx.51.7.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donaldson S.H., Bennett W.D., Zeman K.L., Knowles M.R., Tarran R., Boucher R.C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 55.Robinson M., Hemming A.L., Regnis J.A., Wong A.G., Bailey D.L., Bautovich G.J. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax. 1997;52(10):900–903. doi: 10.1136/thx.52.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elkins M.R., Robinson M., Rose B.R., Harbour C., Moriarty C.P., Marks G.B. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 57.Ballmann M., von der Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross-over pilot study in patients with cystic fibrosis. J Cyst Fibros. 2002;1(1):35–37. doi: 10.1016/s1569-1993(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 58.Tarran R., Grubb B.R., Parsons D., Picher M., Hirsh A.J., Davis C.W. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8(1):149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 59.Elkins M.R., Bye P.T. Inhaled hypertonic saline as a therapy for cystic fibrosis. Curr Opin Pulm Med. 2006;12(6):445–452. doi: 10.1097/01.mcp.0000245714.89632.b2. [DOI] [PubMed] [Google Scholar]
- 60.Bye P.T., Elkins M.R. Other mucoactive agents for cystic fibrosis. Paediatr Respir Rev. 2007;8(1):30–39. doi: 10.1016/j.prrv.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Bilton D., Bellon G., Charlton B., Cooper P., De Boeck K., Flume P.A. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. J Cyst Fibros. 2013;12(4):367–376. doi: 10.1016/j.jcf.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Kuys S.S., Hall K., Peasey M., Wood M., Cobb R., Bell S.C. Gaming console exercise and cycle or treadmill exercise provide similar cardiovascular demand in adults with cystic fibrosis: a randomised cross-over trial. J Physiother. 2011;57(1):35–40. doi: 10.1016/S1836-9553(11)70005-4. [DOI] [PubMed] [Google Scholar]
- 63.Dasgupta B., Brown N.E., King M. Effects of sputum oscillations and rhDNase in vitro: a combined approach to treat cystic fibrosis lung disease. Pediatr Pulmonol. 1998;26(4):250–255. doi: 10.1002/(sici)1099-0496(199810)26:4<250::aid-ppul3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.App E.M., Kieselmann R., Reinhardt D., Lindemann H., Dasgupta B., King M. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest. 1998;114(1):171–177. doi: 10.1378/chest.114.1.171. [DOI] [PubMed] [Google Scholar]
- 65.De Abreu e Silva F.A., Dodge J.A. Guidelines for the diagnosis and management of cystic fibrosis. WHO Hum Genet Programme Int Cyst Fibros Assoc. 1996 http://www.cfww.org/docs/who/2002/guidelines_for_the_diagnosis_and_management_of_cf.pdf [accessed 09.01.15] Available from: [Google Scholar]
- 66.Brand P.L. Bronchodilators in cystic fibrosis. J R Soc Med. 2000;93(Suppl. 38):37–39. [PMC free article] [PubMed] [Google Scholar]
- 67.Colombo J.L. Long-acting bronchodilators in cystic fibrosis. Curr Opin Pulm Med. 2003;9(6):504–508. doi: 10.1097/00063198-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Flume P.A., O'Sullivan B.P., Robinson K.A., Goss C.H., Mogayzel P.J., Jr., Willey-Courand D.B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 69.Nixon G.M., Armstrong D.S., Carzino R., Carlin J.B., Olinsky A., Robertson C.F. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138(5):699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 70.FitzSimmons S.C. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122(1):1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 71.Neu H.C. Tobramycin: an overview. J Infect Dis. 1976;(134 Suppl):S3–S19. doi: 10.1093/infdis/134.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 72.Bryan L.E., editor. Aminoglycoside resistance. Antimicrobial drug resistance. Academic Press; Orlando, FL: 1984. pp. 241–277. [Google Scholar]
- 73.TOBI (tobramycin) solution April 2014. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=94f9e516-6bf6-4e30-8dde-8833c25c2560 [accessed 26.12.14] Available from:
- 74.Clement A., Tamalet A., Leroux E., Ravilly S., Fauroux B., Jais J.P. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax. 2006;61(10):895–902. doi: 10.1136/thx.2005.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saiman L., Marshall B.C., Mayer-Hamblett N., Burns J.L., Quittner A.L., Cibene D.A. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 76.Solidoro P., Braido F., Boffini M., Corsico A.G. New life for macrolides. Minerva Med. 2013 Dec 11 [Epub ahead of print] [PubMed] [Google Scholar]
- 77.Phaff S.J., Tiddens H.A., Verbrugh Haott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J Antimicrob Chemother. 2006;57(4):741–746. doi: 10.1093/jac/dkl014. [DOI] [PubMed] [Google Scholar]
- 78.Olivier K.N., Weber D.J., Wallace R.J., Jr., Faiz A.R., Lee J.H., Zhang Y. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 79.Renna M., Schaffner C., Brown K., Shang S., Tamayo M.H., Hegyi K. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest. 2011;121(9):3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durie P., Kalnins D., Ellis L. Uses and abuses of enzyme therapy in cystic fibrosis. J R Soc Med. 1998;91(Suppl. 34):2–13. doi: 10.1177/014107689809134s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harper T.B., 3rd Relationship of nutrition and pulmonary function in cystic fibrosis. J Pediatr. 1983;103(1):164–165. doi: 10.1016/s0022-3476(83)80807-5. [DOI] [PubMed] [Google Scholar]
- 82.Gaskin K.J., Durie P.R., Lee L., Hill R., Forstner G.G. Colipase and lipase secretion in childhood-onset pancreatic insufficiency. Delineation of patients with steatorrhea secondary to relative colipase deficiency. Gastroenterology. 1984;86(1):1–7. [PubMed] [Google Scholar]
- 83.Kopelman H., Corey M., Gaskin K., Durie P., Weizman Z., Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95(2):349–355. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- 84.Grossman S., Grossman L.C. Pathophysiology of cystic fibrosis: implications for critical care nurses. Crit Care Nurse. 2005;25(4):46–51. [PubMed] [Google Scholar]
- 85.Olveira GOlveira C. Nutrition, cystic fibrosis and the digestive tract. Nutr Hosp. 2008;23(Suppl 2):71–86. [PubMed] [Google Scholar]
- 86.Goss C.H., Burns J.L. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitchell R., Kumar V., Robbins S.L., Abbas A.K., Fausto N. 2007. Robbins basic pathology. [Google Scholar]
- 88.Taylor C.J., Baxter P.S., Hardcastle J., Hardcastle P.T. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988;29(7):957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor C.J., Aswani N. The pancreas in cystic fibrosis. Paediatr Respir Rev. 2002;3(1):77–81. doi: 10.1053/prrv.2002.0183. [DOI] [PubMed] [Google Scholar]
- 90.Turck D., Michaud L., Wizla-Derambure N. Digestive diseases and nutrition in cystic fibrosis. Rev Prat. 2003;53(2):151–157. [PubMed] [Google Scholar]
- 91.Khoshoo V., Udall J.N., Jr. Meconium ileus equivalent in children and adults. Am J Gastroenterol. 1994;89(2):153–157. [PubMed] [Google Scholar]
- 92.Malfroot A., Dab I. New insights on gastro-oesophageal reflux in cystic fibrosis by longitudinal follow up. Arch Dis Child. 1991;66(11):1339–1345. doi: 10.1136/adc.66.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levy L.D., Durie P.R., Pencharz P.B., Corey M.L. Effects of long-term nutritional rehabilitation on body composition and clinical status in malnourished children and adolescents with cystic fibrosis. J Pediatr. 1985;107(2):225–230. doi: 10.1016/s0022-3476(85)80130-x. [DOI] [PubMed] [Google Scholar]
- 94.Pencharz P.B., Durie P.R. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clin Nutr. 2000;19(6):387–394. doi: 10.1054/clnu.1999.0079. [DOI] [PubMed] [Google Scholar]
- 95.Cohen-Cymberknoh Malena, Shoseyov David, Kerem Eitan. Managing cystic fibrosis. Am J Respir Crit Care Med. 2011;183(No. 11):1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 96.Shepherd R.W., Cleghorn G., Ward L.C., Wall C.R., Holt T.L. Nutrition in cystic fibrosis. Nutr Res Rev. 1991;4(1):51–67. doi: 10.1079/NRR19910007. [DOI] [PubMed] [Google Scholar]
- 97.Siafakas C., Vottler T.P., Andersen J.M. Rectal prolapse in pediatrics. Clin Pediatr (Phila) 1999;38(2):63–72. doi: 10.1177/000992289903800201. [DOI] [PubMed] [Google Scholar]
- 98.Shalaby R., Ismail M., Abdelaziz M., Ibrahem R., Hefny K., Yehya A. Laparoscopic mesh rectopexy for complete rectal prolapse in children: a new simplified technique. Pediatr Surg Int. 2010;26(8):807–813. doi: 10.1007/s00383-010-2620-7. [DOI] [PubMed] [Google Scholar]
- 99.Puri B. Rectal prolapse in children: laparoscopic suture rectopexy is a suitable alternative. J Indian Assoc Pediatr Surg. 2010;15(2):47–49. doi: 10.4103/0971-9261.70634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qvist N., Rasmussen L., Klaaborg K.E., Hansen L.P., Pedersen S.A. Rectal prolapse in infancy: conservative versus operative treatment. J Pediatr Surg. 1986;21(10):887–888. doi: 10.1016/s0022-3468(86)80015-x. [DOI] [PubMed] [Google Scholar]
- 101.Stern R.C., Izant R.J., Jr., Boat T.F., Wood R.E., Matthews L.W., Doershuk C.F. Treatment and prognosis of rectal prolapse in cystic fibrosis. Gastroenterology. 1982;82(4):707–710. [PubMed] [Google Scholar]
- 102.Brunzell C., Hardin D., Moran A., Schindler T., Schissel K. 4th ed. 2008. Cystic fibrosis foundation. Man aging cystic fibrosis-related diabetes (CFRD). An instruction guide for patients and families.http://www.cff.org/uploadedfiles/livingwithcf/stayinghealthy/diet/diabetes/cfrd-manual-4th-edition.pdf Available from: [Google Scholar]
- 103.Lanng S. Glucose intolerance in cystic fibrosis patients. Paediatr Respir Rev. 2001;2(3):253–259. doi: 10.1053/prrv.2001.0148. [DOI] [PubMed] [Google Scholar]
- 104.Peraldo M., Fasulo A., Chiappini E., Milio C., Marianelli L. Evaluation of glucose tolerance and insulin secretion in cystic fibrosis patients. Horm Res. 1998;49(2):65–71. doi: 10.1159/000023128. [DOI] [PubMed] [Google Scholar]
- 105.Rosenecker J., Hofler R., Steinkamp G., Eichler I., Smaczny C., Ballmann M. Diabetes mellitus in patients with cystic fibrosis: the impact of diabetes mellitus on pulmonary function and clinical outcome. Eur J Med Res. 2001;6(8):345–350. [PubMed] [Google Scholar]
- 106.Rolon M.A., Benali K., Munck A., Navarro J., Clement A., Tubiana-Rufi N. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr. 2001;90(8):860–867. [PubMed] [Google Scholar]
- 107.Milla C.E., Warwick W.J., Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 108.Herrmann U., Dockter G., Lammert F. Cystic fibrosis-associated liver disease. Best Pract Res Clin Gastroenterol. 2010;24(5):585–592. doi: 10.1016/j.bpg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13(6):529–536. doi: 10.1097/MCP.0b013e3282f10a16. [DOI] [PubMed] [Google Scholar]
- 110.Feranchak A.P. Hepatobiliary complications of cystic fibrosis. Curr Gastroenterol Rep. 2004;6(3):231–239. doi: 10.1007/s11894-004-0013-6. [DOI] [PubMed] [Google Scholar]
- 111.Moyer K., Balistreri W. Hepatobiliary disease in patients with cystic fibrosis. Curr Opin Gastroenterol. 2009;25(3):272–278. doi: 10.1097/MOG.0b013e3283298865. [DOI] [PubMed] [Google Scholar]
- 112.Kaplan E., Shwachman H., Perlmutter A.D., Rule A., Khaw K.T., Holsclaw D.S. Reproductive failure in males with cystic fibrosis. N Engl J Med. 1968;279(2):65–69. doi: 10.1056/NEJM196807112790203. [DOI] [PubMed] [Google Scholar]
- 113.Welsh M.J., Tsui L.-C., Boat T.F., Beaudet A.L. Cystic fibrosis. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The metabolic and molecular bases of inherited disease. 7th edition. McGraw-Hill; New York, NY: 1995. [Google Scholar]
- 114.Denning C.R., Sommers SCQuigley H.J., Jr. Infertility in male patients with cystic fibrosis. Pediatrics. 1968;41(1):7–17. [PubMed] [Google Scholar]
- 115.Gilljam M., Antoniou M., Shin J., Dupuis A., Corey M., Tullis D.E. Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest. 2000;118(1):85–91. doi: 10.1378/chest.118.1.85. [DOI] [PubMed] [Google Scholar]
- 116.Schechter M.S., Shelton B.J., Margolis P.A., Fitzsimmons S.C. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 117.Adler N.E., Boyce W.T., Chesney M.A., Folkman S., Syme S.L. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- 118.Mackenbach J.P., Kunst A.E., Cavelaars A.E., Groenhof F., Geurts J.J. Socioeconomic inequalities in morbidity and mortality in western Europe. The EU working group on socioeconomic Inequalities in Health. Lancet. 1997;349(9066):1655–1659. doi: 10.1016/s0140-6736(96)07226-1. [DOI] [PubMed] [Google Scholar]
- 119.Steinraths M., Vallance H.D., Davidson A.G. Delays in diagnosing cystic fibrosis: can we find ways to diagnose it earlier? Can Fam Physician. 2008;54(6):877–883. [PMC free article] [PubMed] [Google Scholar]
- 120.Bregnballe V., Schiotz P.O., Boisen K.A., Pressler T., Thastum M. Barriers to adherence in adolescents and young adults with cystic fibrosis: a questionnaire study in young patients and their parents. Patient Prefer Adherence. 2011;5:507–515. doi: 10.2147/PPA.S25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masterson T.L., Wildman B.G., Newberry B.H., Omlor G.J. Impact of age and gender on adherence to infection control guidelines and medical regimens in cystic fibrosis. Pediatr Pulmonol. 2011;46(3):295–301. doi: 10.1002/ppul.21366. [DOI] [PubMed] [Google Scholar]
- 122.Passero M.A., Remor B., Salomon J. Patient-reported compliance with cystic fibrosis therapy. Clin Pediatr (Phila) 1981;20(4):264–268. doi: 10.1177/000992288102000406. [DOI] [PubMed] [Google Scholar]
- 123.Quittner A.L., Espelage D.L., Ievers-Landis C., Drotar D. Measuring adherence to medical treatments in childhood chronic illness: considering multiple methods and sources of information. J Clin Psychol Med Settings. 2000;(7):41–54. [Google Scholar]
- 124.Anthony H., Paxton S., Bines J., Phelan P. Psychosocial predictors of adherence to nutritional recommendations and growth outcomes in children with cystic fibrosis. J Psychosom Res. 1999;47(6):623–634. doi: 10.1016/s0022-3999(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 125.Briesacher B.A., Quittner A.L., Saiman L., Sacco P., Fouayzi H., Quittell L.M. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11:5. doi: 10.1186/1471-2466-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stark L.J., Jelalian E.D., L M . In: Cystic fibrosis. Handbook of pediatric psychology. R.M.C, editor. Guilford Press; , New York: 1995. [Google Scholar]
- 127.Banjar H., Mogarri I., Kambouris M. Geographic distribution of cystic fibrosis transmembrane regulator gene in Saudi Arabia. Ann Trop Pediatr. March 1999;19(1):69–73. doi: 10.1080/02724939992671. [DOI] [PubMed] [Google Scholar]
- 128.Khemiri M., Ben Rhouma A., Bouzid S., Messaoud T., Guesmi M., Hamzaoui M. Clinical characteristics and outcome of cystic fibrosis: report of 16 cases. Tunis Med. 2008;86(6):567–572. [PubMed] [Google Scholar]
- 129.Baumann U., Stocklossa C., Greiner W., von der Schulenburg J.M., von der Hardt H. Cost of care and clinical condition in paediatric cystic fibrosis patients. J Cyst Fibros. 2003;2(2):84–90. doi: 10.1016/S1569-1993(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 130.Britton J.R. Effects of social class, sex, and region of residence on age at death from cystic fibrosis. BMJ. 1989;298(6672):483–487. doi: 10.1136/bmj.298.6672.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris L.J., Mascia A.V., Farnsworth P.B. Cystic fibrosis: making a correct and early diagnosis. J Fam Pract. 1978;6(4):749–756. [PubMed] [Google Scholar]