Abstract
Background and objectives
To evaluate whether initial urinalysis (UA) and urinary nitrite results can be used as a proxy for choosing empiric antibiotic therapy.
Materials and methods
A retrospective study was conducted in an urban inner city community hospital in New York City (NYU Woodhull Medical Center). We reviewed the charts of patients seen in the Emergency Department and Pediatric Clinic who had a diagnosis of urinary tract infection (UTI) during a 3 year time period (January 2010–December 2012). Statistical analysis was performed using SPSS 20.0 statistical software.
Results
Between January 2010 and December 2012, a total of 378 patients had a diagnosis of UTI. Seventy-five (19.8%) were males and 203 (80.2%) were females. Of the 378 patients with a diagnosis of UTI, the most common isolated pathogen was Escherichia coli, which was detected in 283 (74.9%) isolates. Other bacteria included Klebsiella spp 30 (7.9%), Proteus 21 (5.6%), Enterococcus 14 (3.7%), and others 30 (7.9%). The resistance rate was higher in the nitrite positive group for the following antibiotics: TMP/SMX and ampicillin with or without sulbactam. No significant correlation was found with the remaining studied antibiotics. No significant correlation was found between leukoesterase and the resistance patterns in all of the studied antibiotics, except cefazolin.
Conclusion
Urinary nitrite results are not helpful in choosing an initial antibiotic to treat a UTI. Leukocytosis in the blood or urine or the presence of a fever cannot be used to predict bacterial resistance. The use of nitrofurantoin or cephalexin for the treatment of cystitis was optimum, and in the presence of negative leukoesterase, nitrofurantoin was preferable to cephalexin.
Keywords: Pediatrics, Infectious disease-bacteria, Renal, Emergency department management
Abbreviations: UTI, urinary tract infection; CBC, complete blood count; LE, leukocyte esterase; UA, urinalysis
1. Introduction
Urinary tract infections (UTIs) occur in 1–3% of girls and 1% of boys. In girls, the first UTI usually occurs by the age of 5 years, with peaks during infancy and toilet training. In boys, most UTIs occur during the 1st year of life. The prevalence of UTIs varies with age. During the 1st year of life, the male: female ratio is 2.8–5.4: 1. Beyond 1–2 years, there is a female preponderance, with a female: male ratio of 10: 1 [1].
The association between urinary nitrite and UTIs was first reported in 1914 and has frequently been the object of investigation [2]. The advantages of utilizing urinary nitrites are its low cost, the rapidity with which the results are available, and its ability to categorize patients into two distinct groups, nitrite positive or negative [3], [4].
Knowledge of the spectrum of pathogens and their patterns of resistance in the population allows the clinician to empirically select an effective agent [5]. Whether the absence of urine nitrites predicts resistance to common antibiotics that are used for the treatment of uncomplicated UTIs has been poorly investigated. Furthermore, the results from the few studies that have investigated this correlation are conflicting [6], [7], [8], [9].
2. Materials and methods
2.1. Setting
Woodhull Medical Health and Mental Center is an inner city community hospital in New York, NY that is affiliated with NYU, School of Medicine.
2.2. Study design
This is a retrospective, descriptive, and analytical study that was conducted in an urban inner city community hospital in New York City (NYU Woodhull Medical and Mental Health Center). Charts were reviewed for all patients seen in the Emergency Department and Pediatric Unit who had a diagnosis of a UTI during a 3-year period (Jan 2010–Dec 2012).
Urine cultures were sent either by urine catheterization or clean mid-stream catch depending on whether the patient was toilet trained or not. As a general practice in our institution, a culture obtained by a urinary bag is not sent due to the high likelihood of contamination. This study received approval from our Institutional Review Board of NYU Langone Medical Center before chart review commenced.
2.3. Data storage and statistical analysis
The chart numbers were obtained from the medical record department at Woodhull Medical and Mental Health Center. All of the charts were reviewed through the use of the electronic medical record system QUADRAMED to obtain demographic, clinical, and laboratory information from the patients.
The data were stored on NYU REDCap (secure data base on NYU Onsite) on a secured computer in Woodhull Medical Center. Each subject was entered into the REDcap database as a subject number (1, 2,3, etc.). We used SSPS statistical software, version 20, to analyze our data. Chi-square and Fisher Exact tests were used. P value < .05 was considered statistically significant.
3. Inclusion and exclusion criteria
We collected the records of all pediatric patients aged 1 day to 18 years of age who had a final discharge diagnosis of UTI, cystitis, or pyelonephritis. Only patients who had a single urine pathogen of >105 colony-forming units (CFU) per milliliter were included. Patients who had a diagnosis of UTI, pyelonephritis, or cystitis were excluded if no urine culture was sent, if the culture was negative, or grew more than a single pathogen.
4. Measurements
The following information was collected from the medical records: demographic information (age and sex), history of subjective or documented fever, urinalysis (UA) findings (urine white blood cell [WBC], leukocyte esterase [LE], and nitrite), leukocytosis in the complete blood count (CBC), causative organism, and susceptibility pattern to antibiotics. For the purpose of the study, leukocytosis was defined as a WBC in the complete blood count of more than 15,000 regardless of the subject's age. Positive WBCs in the urine was defined as the presence of >5 WBC/power field. Sensitivity information was gathered for each bacterium. A bacterium was considered to be resistant to a specific antibiotic if the report read either resistance or intermediate.
5. Results
We studied the sensitivity pattern to the following antibiotics: ampicillin, ampicillin/sulbactam, cefazolin, cefuroxime, cefotaxim, ceftazidim, cefepime, ciprofloxacin, nitrofurantoin, gentamicin, piperacillin/tazobactam, trimethoprim/sulfamethoxazole, and imipenem.
Between January 2010 and December 2012, a total of 378 patients had a diagnosis of UTI. Seventy-five (19.8%) were males, and 303 (80.2%) were females. Fifty (36.8%) males and 86 (63.2%) females were less than 2 years of age. In the 2–13 years of age group, there were 23 males (13.1%) and 153 (86.9%) females. Of those older than 13 years of age, 2 (3%) were males and 64 (97%) were females. The age distribution of the isolates differed significantly by gender (P value < .0005).
With regard to the UA results, nitrite was positive in 38% and negative in 62% of patients. Negative nitrite results were more common in patients less than 2 years of age (P value of .034). Bacteria were positive in 86.2% and negative 13.8% of patients. LE was positive 82.7% and negative 17.3% of patients. CBC was obtained in 37.6% of patients. Leukocytosis was present in 44.4% of patients.
Of the 378 patients with a diagnosis of UTI, the most common isolated pathogen was Escherichia coli, which was detected in 283 (74.9%) isolates. Other bacteria included Klebsiella spp 30 (7.9%), Proteus 21 (5.6%), Enterococcus 14 (3.7%), and others 30 (7.9%). Urine nitrite was tested in 371 patients and was positive in 141(38%) and negative in 230 (62%). WBCs in the urine were evaluated in 307 samples and were positive in 247 (80.4%) and negative in (19.6%). LE was positive in 307 (82.7%) and negative in 64 (17.3%) samples. Bacteria was identified in the UA in 263 (86.2%) and was negative in 42 (13.8%) patients. A CBC was obtained in 142 (37.6%) patients, while 236 (62.4%) patients had no CBC results. Among the patients who had results from a CBC, 63 (44.4%) had leukocytosis and 79 (55.6%) had no leukocytosis. Fever was present in 148 (45.4%) patients and was absent in 178 (54.6%).
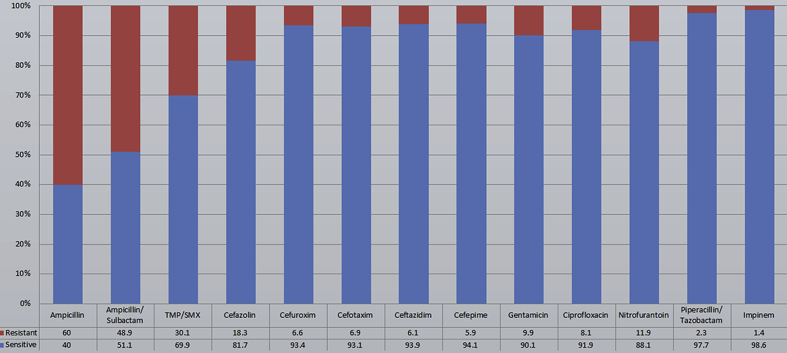
Fig. 1 shows the sensitivity pattern to all of the isolated pathogens.
Figure 1.
Sensitivity pattern for all isolated pathogens.
The correlation between the nitrite results and resistance patterns to common antibiotics that may be used to treat UTI is shown in Table 1.
Table 1.
The correlation between nitrite results and resistant patterns to common antibiotics that may be used to treat UTIs.
| Antibiotic | Sensitive | Resistant | P Value |
|---|---|---|---|
| Ampicillin | |||
| Positive Urine Nitrite | 44 (31.4%) | 96 (68.6%) | .007 |
| Negative Urine Nitrite | 103 (45.8%) | 122 (54.2%) | |
| Ampicillin/Sulbactam | |||
| Positive Urine Nitrite | 60 (42.9%) | 80 (57.1%) | .012 |
| Negative Urine Nitrite | 122 (56.5%) | 94 (43.5%) | |
| Cefazolin | |||
| Positive Urine Nitrite | 106 (80.3%) | 26 (19.7%) | .46 |
| Negative Urine Nitrite | 176 (83.4%) | 35 (16.6%) | |
| Cefuroxime | |||
| Positive Urine Nitrite | 105 (92.1%) | 9 (7.9%) | .38 |
| Negative Urine Nitrite | 176 (94.6%) | 10 (5.4%) | |
| Cefotaxime | |||
| Positive Urine Nitrite | 129 (92.8%) | 10 (7.2%) | .79 |
| Negative Urine Nitrite | 202 (93.5%) | 14 (6.5%) | |
| Ceftazidime | |||
| Positive Urine Nitrite | 131 (92.9%) | 10 (7.1%) | .44 |
| Negative Urine Nitrite | 204 (94.9%) | 11 (5.1%) | |
| Cefepime | |||
| Positive Urine Nitrite | 124 (93.2%) | 9 (10%) | .52 |
| Negative Urine Nitrite | 204 (94.9%) | 11 (5.1%) | |
| Ciprofloxacin | |||
| Positive Urine Nitrite | 125 (93.3%) | 9 (6.7%) | .51 |
| Negative Urine Nitrite | 200 (91.3%) | 19 (8.7%) | |
| Nitrofurantoin | |||
| Positive Urine Nitrite | 114 (91.9%) | 10 (8.1%) | .16 |
| Negative Urine Nitrite | 187 (87%) | 28 (13%) | |
| Gentamicin | |||
| Positive Urine Nitrite | 122 (91%) | 12 (9%) | .68 |
| Negative Urine Nitrite | 200 (89.7%) | 23 (10.3%) | |
| Impinem | |||
| Positive Urine Nitrite | 131 (98.5%) | 2 (1.5%) | .63 |
| Negative Urine Nitrite | 212 (98.6%) | 3 (1.4%) | |
| Piperacillin/Tazobactam | |||
| Positive Urine Nitrite | 129 (97%) | 4 (3%) | .36 |
| Negative Urine Nitrite | 211 (98.1%) | 4 (1.9%) | |
| TM/SMX | |||
| Positive Urine Nitrite | 81 (60.4%) | 53 (39.6%) | .005 |
| Negative Urine Nitrite | 163 (74.8%) | 55 (25.2%) | |
The correlation between the LE results and resistance patterns is shown in Table 2.
Table 2.
The correlation between leukoesterase results and resistant patterns.
| Antibiotic | Sensitive | Resistant | P Value |
|---|---|---|---|
| Ampicillin | |||
| Positive Urine Leukoesterase | 123 (40.6%) | 180 (59.4%) | .73 |
| Negative Urine Leukoesterase | 24 (38.7%) | 38 (61.3%) | |
| Ampicillin/Sulbactam | |||
| Positive Urine Leukoesterase | 148 (49.8%) | 149 (50.2%) | .27 |
| Negative Urine Leukoesterase | 34 (57.6%) | 25 (42.4%) | |
| Cefazolin | |||
| Positive Urine Leukoesterase | 241 (84.3%) | 45 (15.7%) | .026 |
| Negative Urine Leukoesterase | 41 (71.9%) | 16 (28.1%) | |
| Cefuroxime | |||
| Positive Urine Leukoesterase | 243 (94.6%) | 14 (5.4%) | .12 |
| Negative Urine Leukoesterase | 38 (88.4%) | 5 (1.6%) | |
| Cefotaxime | |||
| Positive Urine Leukoesterase | 281 (94%) | 18 (6%) | .2 |
| Negative Urine Leukoesterase | 50 (89.3%) | 6 (10.7%) | |
| Ceftazidime | |||
| Positive Urine Leukoesterase | 281 (94.6%) | 16 (5.4%) | .36 |
| Negative Urine Leukoesterase | 54 (91.5%) | 5 (8.5%) | |
| Cefepime | |||
| Positive Urine Leukoesterase | 275 (94.5%) | 16 (5.5%) | .65 |
| Negative Urine Leukoesterase | 53 (93%) | 4 (7%) | |
| Ciprofloxacin | |||
| Positive Urine Leukoesterase | 271 (91.9%) | 24 (8.1%) | .75 |
| Negative Urine Leukoesterase | 54 (93.1%) | 4 (6.9%) | |
| Nitrofurantoin | |||
| Positive Urine Leukoesterase | 255 (90.1%) | 28 (9.9%) | .084 |
| Negative Urine Leukoesterase | 46 (82.1%) | 10 (17.9%) | |
| Gentamicin | |||
| Positive Urine Leukoesterase | 268 (90.5%) | 28 (9.5%) | .63 |
| Negative Urine Leukoesterase | 54 (88.5) | 7 (11.5%) | |
| Impinem | |||
| Positive Urine Leukoesterase | 288 (99%) | 3 (1%) | .15 |
| Negative Urine Leukoesterase | 55 (96.5%) | 2 (3.5%) | |
| Piperacillin/Tazobactam | |||
| Positive Urine Leukoesterase | 285 (97.9%) | 6 (2.1%) | .51 |
| Negative Urine Leukoesterase | 55 (96.5%) | 2 (3.5%) | |
| TM/SMX | |||
| Positive Urine Leukoesterase | 202 (68.5%) | 93 (31.5%) | .44 |
| Negative Urine Leukoesterase | 42 (73.7%) | 15 (26.3%) | |
The correlation between the urine nitrite results and different age groups is shown in Table 3.
Table 3.
Comparing urinary nitrite results in different age groups.
| Age groups | Urinary Nitrite |
||
|---|---|---|---|
| Negative | Positive | Total | |
| <2 Years | 76 (65%) | 41 (35%) | 117 |
| 2–13 Years | 115 (61%) | 74 (39%) | 189 |
| >13 Years | 39 (60%) | 26 (40%) | 65 |
| Total | 230 (62%) | 141 (38%) | 371 |
| P Value .034 | |||
6. Discussion
Urine culture is the gold standard for the diagnosis of UTI. However, the result of the culture is not readily available to the clinician in the Emergency Department. Thus, this study was conducted to determine whether the initial UA and urine nitrite results could serve as a guide in choosing the most appropriate empirical antibiotic. Several uropathogens, such as E. coli, Klebsiella, and Proteus, can reduce nitrate to nitrite, whereas others do not have this ability.
Several factors can lead to a false negative nitrite result, including a short time between urine collection and testing, the amount of bacteriuria, a urine pH less than 6.0, organisms that further reduce nitrites to ammonia and dilute urine, and the presence of blood, urobilinogen, medications, or ascorbic acid [5]. A nitrite test is not a sensitive marker for children, particularly infants, who empty their bladders frequently. Therefore, a negative nitrite test result has little value in ruling out a UTI. Moreover, not all urinary pathogens reduce nitrate to nitrite [6].
Very few studies have investigated the relationship between urinary nitrite results and the selection of initial antibiotics. Weiz and his colleagues [6] suggested that a negative urine nitrite test is a possible indicator that a microorganism is resistant to the first and third-generation of cephalosporins. However, Grant et al [8] concluded that the detection of urine nitrites should not influence the use of first-generation cephalosporins for urinary tract infections. Larson et al [9] reported that no significant difference was observed between the rates of TMP-SMX resistance in both negative and positive nitrite groups. Mahyar et al [7] studied a large number of antibiotics, such as gentamycin, amikacin, nalidixic acid, ampicillin, and nitrofurantoin, in addition to first and third-generation cephalosporin. However, they found no correlation between urinary nitrite results and bacterial resistance to antimicrobial drugs. In our study, we evaluated the significance of urine nitrites in relation to a larger group of antimicrobial agents, some of which have not previously been studied.
In the past, sulphonamides were the drug of choice for outpatient management of UTIs. However, most hospitals switched to a first or a third generation cephalosporin due to the emerging resistance to sulphonamides. Unfortunately, resistance to first and now even third generation cephalosporins is increasingly reported in the literature, especially in developing countries. This mandates local evaluations of susceptibility patterns to common oral antibiotics used in the treatment of UTIs [10], [11], [12], [13].
Consistent with the vast majority of studies, we found that E. coli was the most commonly isolated bacteria (74.9%). This supports the fact that most UTIs are ascending infections. The bacteria arise from the faecal flora, colonize the perineum, and enter the bladder via the urethra [1], [5], [10], [14]. Based on expert opinion, the threshold of 20% as the resistance prevalence at which the agent is no longer recommended for empirical treatment of UTI is a widely accepted practice [15].
7. Bacterial susceptibility pattern
In our institution, we generally use cephalexin as an empirical antibiotic for the treatment of most uncomplicated UTIs diagnosed in the ED.
In our study, the resistance rate was higher in the nitrite positive group for TMP/SMX and ampicillin with or without sulbactam. However, because the resistance rate was >20%, even in the nitrite negative group, these above mentioned antibiotics are not appropriate for empirical treatment in our institution. In all of the studied cephalosporins, the resistance rate was lower in nitrite negative group (0.7–5.1), but these differences are not significant. However, for nitrofurantoin, gentamicin, ciprofloxasin, and piperacillin/tazobactam, the resistance rates were higher in the nitrite negative group (1.3–4.9), although these differences were not significant. No difference was found with imipenem. Thus, urinary nitrite results are not helpful in choosing an initial antibiotic to treat a UTI.
No significant correlation was found between the LE and resistance patterns in all of studied antibiotics, except for cefazolin, which had a resistance rate of 16% in the LE positive group that was much higher (28.1%) in the LE negative group. Thus, if LE is negative in initial UA, a first generation cephalosporin should not be used as an empirical therapy in our population.
No correlation was found between the presence of fever or leukocytosis and resistance patterns.
In our patients, bacteria were highly resistant to TMP/SMX, reaching a rate of 30.1%. However, first generation cephalosporins (we used cefazolin as a proxy for first generation cephalosporin) are still acceptable as a first line empirical therapy, with an overall resistance rate of 18.3%. Consistent with previous studies, nitrofurantoin was very effective among other oral antibiotics, with a resistance rate of 11.9%, The American Academy of Pediatrics recommends that ciprofloxacin be limited to urinary tract infections caused by Pseudomonas aeruginosa or other multidrug-resistant and Gram-negative bacteria (the FDA licensed ciprofloxacin for complicated E. coli urinary tract infections and pyelonephritis attributable to E. coli in patients 1–17 years of age) (evidence grade II-2) [16].
Nitrofurantoin is an oral antibiotic that is used in the treatment and prevention of lower UTIs. Nitrofurantoin only achieves antibiotic concentrations in the urine with low circulating blood levels and poor tissue penetration making it unsuitable for the treatment of upper UTIs. Other contraindications are renal failure and neonates and children with G6PD deficiency [17].
8. Limitations
A potential selection bias arises from our definition of a positive culture as > 100 K CFUs of bacteria. Some other studies have used lower thresholds. However, we focused on a pediatric population with incontrovertible UTIs. Reducing the CFU threshold may have included some patients without UTIs. Another limitation is that we studied in vitro susceptibility, which may not always reflect in vivo susceptibility. In addition, this was a retrospective study, which has known limitations.
9. Conclusion
Due to the high resistance of bacteria to TMP/SMX and ampicillin with or without sulbactam, urinary nitrite results were not helpful in predicting bacterial resistance. LE, leukocytosis in blood or urine and fever were also of little value in the prediction of bacterial resistance. We recommend the use of nitrofurantoin or cephalexin in the treatment of cystitis. If LE is negative, nitrofurantoin is preferable to cephalexin. Second or third generation cephalosporins are appropriate antibiotics in the management of complicated UTI or when pyelonephritis is highly suspected.
Funding
None.
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Elder J.S. Urinary tract infections. In: Kliegman R., Santon B.F., Schor N.F., Geme J.W., Behrman R.E., editors. Nelson textbook of Pediatrics. ed 19. Sauders; Philadelphia, PA: 2011. pp. 1829–1834. [Google Scholar]
- 2.James G.P., Paul K.L., Fuller J.B. Urinary nitrite and urinary-tract infection. AJCP. 1978;70:671–678. doi: 10.1093/ajcp/70.4.671. [DOI] [PubMed] [Google Scholar]
- 3.Lenke R.R., Van Dorsten P.J. The efficacy of the nitrite test and microscopic urinalysis in predicting urine culture results. Am J Obstet Gynecol. 1981;140:427–429. doi: 10.1016/0002-9378(81)90039-9. [DOI] [PubMed] [Google Scholar]
- 4.Lohr J.A., Portilla M.G., Geuder T.G., Dunn M.L., Dudley S.M. Making a presumptive diagnosis of urinary tract infection by using a urinalysis performed in an on-site laboratory. J Pediatr. 1993;122:22–25. doi: 10.1016/s0022-3476(05)83481-x. [DOI] [PubMed] [Google Scholar]
- 5.McLoughlin T.G., Jr., Joseph M.M. Antibiotic bresistance patterns of uropathogens in pediatric emergency department patients. Acad Emerg Med. 2003;10:347–351. doi: 10.1111/j.1553-2712.2003.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 6.Weiz D., Seabrook J.A., Lim R.K. Urinary nitrite is a significant predictor of pediatric UTI susceptibility to first and third–generation cephalosporins. J Emerg Med. 2008;39:6–12. doi: 10.1016/j.jemermed.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Mahyar A., Ayazi P., Froozesh M., Daneshi-Kohan M.M., Barikani A. Can urinary nitrite results be used to conduct antimicrobial option for urinary tract infection in children? Iran J Pediatr. 2012;22:237–240. [PMC free article] [PubMed] [Google Scholar]
- 8.Grant D.C., Chan L., Waterbrook A. Urine nitrite not correlated with bacterial resistance to cephalosporins. J Emerg Med. 2005;28:321–323. doi: 10.1016/j.jemermed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Larson M.J., Brooks C.B., Leary W.L., Lewis L.M. Can urinary nitrite results be used to guide antimicrobial choice for urinary tract infection? J Emerg Med. 1997;15:435–438. doi: 10.1016/s0736-4679(97)00069-3. [DOI] [PubMed] [Google Scholar]
- 10.Prais D., Straussberg R., Avitzur Y., Nussinovitchet M., Harel L., Amir J. Bacterial susceptibility to oral antibiotics in community acquired urinary tract infection. Arch Dis Child. 2003;88:215–218. doi: 10.1136/adc.88.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldraich N.P., Manfroi A. Febrile urinary tract infection: Escherichia coli susceptibility to oral antimicrobials. Pediatr Nephrol. 2002;17:173–176. doi: 10.1007/s00467-001-0808-8. [DOI] [PubMed] [Google Scholar]
- 12.Sedighi I., Solgi A., Amanati A., Alikhani M.Y. Choosing the correct empirical antibiotic for urinary tract infection in pediatric: surveillance of antimicrobial susceptibility pattern of Escherichia coli by E-Test method. Iran J Microbiol. 2014;6:387–391. [PMC free article] [PubMed] [Google Scholar]
- 13.Yolbaş I., Tekin R., Kelekci S., Tekin A., Okur M.H., Ece A. Community-acquired urinary tract infections in children: pathogens, antibiotic susceptibility and seasonal changes. Eur Rev Med Pharmacol Sci. 2013;17:971–976. [PubMed] [Google Scholar]
- 14.Edlin R., Shapiro D., Hersh A., Copp H. Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J Urol. 2013:222–227. doi: 10.1016/j.juro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K., Hooton T.M., Naber K.G., Wullt B., Colgan R., Miller L.G. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:103–120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Infectious Diseases The use of systemic fluoroquinolones. Pediatrics. 2006;118:1287–1292. doi: 10.1542/peds.2006-1722. [DOI] [PubMed] [Google Scholar]
- 17.Ramlakhan S., Singh V., Stone J., Ramtahal A. Clinical options for the treatment of urinary tract infections in children. Clin Med Insights Pediatr. 2014;8:31–37. doi: 10.4137/CMPed.S8100. [DOI] [PMC free article] [PubMed] [Google Scholar]