Abstract
Thermophilic bacterial communities generate thick biofilm on carbon steel API 5LX and produce extracellular metabolic products to accelerate the corrosion process in oil reservoirs. In the present study, nine thermophilic biocorrosive bacterial strains belonging to Bacillus and Geobacillus were isolated from the crude oil and produced water sample, and identified using 16S rRNA gene sequencing. The biodegradation efficiency of hydrocarbons was found to be high in the presence of bacterial isolates MN6 (82%), IR4 (94%) and IR2 (87%). During the biodegradation process, induction of the catabolic enzymes such as alkane hydroxylase, alcohol dehydrogenase and lipase were also examined in these isolates. Among them, the highest activity of alkane hydroxylase (130 µmol mg−1 protein) in IR4, alcohol dehydrogenase (70 µmol mg−1 protein) in IR2, and higher lipase activity in IR4 (60 µmol mg−1 protein) was observed. Electrochemical impedance spectroscopy and X-ray diffraction data showed that these isolates oxidize iron into ferrous/ferric oxides as the corrosion products on the carbon steel surface, whilst the crude oil hydrocarbon served as a sole carbon source for bacterial growth and development in such extreme environments.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1604-0) contains supplementary material, which is available to authorized users.
Keywords: Biocorrosion, Biodegradation, Carbon steel, Oil reservoir, Thermophilic bacteria
Introduction
The oil reservoirs consist of multiphase fluids including crude oil, gas and water with extreme conditions of temperature, pressure and salinity (Li et al. 2007). Crude oil is a mixture of various aliphatic and aromatic hydrocarbons, which account for 75% of its total content along with resins, asphalts and other contents (Hao et al. 2004; Wang et al. 2012). Crude oil, produced water and gas surroundings of oil reservoir facilities encompass diverse microbial communities. The action of microorganisms leads to a negative impact on the oil superiority and the infrastructure of the production and transporting facilities. The crude oil biodegradation in the oil reservoir leads to the development of ‘heavy oil’ which is economically very low in price, thus making the recovering process hard (Head et al. 2003; Stevenson et al. 2011; Elumalai et al. 2017a). Microbial communities have the aptitude to influence the corrosion mechanisms in oil reservoir ecosystems by promoting growth and development, utilizing vital resources such as organic carbon from crude oil, inorganic mineral from metal surface-related electron donors and acceptors (Coetser and Cloete 2005; Pedersen 2013; Liang et al. 2016; Aktas et al. 2017; Hernsdorf et al. 2017; Elumalai et al. 2017b). Many researchers have carried out culture-dependent studies in oil reservoir samples to examine their culturable microbial communities, including manganese, iron oxidizers/reducers, sulphate and nitrate reducers (Stevenson et al. 2011; Magot et al. 2000). Many indigenous microbial communities contribute to an electrochemical process of microbiologically influenced corrosion (MIC), in which the microorganisms can participate and influence the corrosion reactions (Pillay and Lin 2013; Hajj et al. 2013; Elumalai et al. 2017b; Narenkumar et al. 2018). This pose a remarkable risk to the oil reservoir-associated facilities and surrounding environment due to the oil leakage. About 20–30% of the corrosion problems in the oil reservoir are due to the microbial action (Duncan et al. 2009; Stipanicev et al. 2014; Parthipan et al. 2018a). The interaction between the microorganisms and metal surface due to the electrochemical activity leads to passive layer disruptions, scale formation and under deposit corrosion in pipelines (Hamilton 2003; Beech and Sunner 2004; Parthipan et al. 2018b).
Thermophilic bacteria can tolerate up to 55 °C in oil reservoir such as Moorella sp., Gelria sp., and Pseudomonas sp. that have the ability to degrade n-alkanes under thermophilic conditions (Zhou et al. 2005, 2013). Geobacillus sp. can use a wide range of hydrocarbons including, aliphatic hydrocarbons, naphthalene, pentadecane, hexadecane and heptadecane as sole carbon sources under chlorate-reducing, nitrate-reducing, and methanogenic conditions at 50 °C (Sorkhoh et al. 1993; Zheng et al. 2011; Bao et al. 2014; Shen et al. 2015; Parthipan et al. 2017a; Elumalai et al. 2017a). During biodegradation of crude oil, the temperature helps to increase the rate of hydrocarbon degradation (Shimura et al. 1999). Many thermophilic bacteria from genus Bacillus, Thermus, Thermococcus, Thermotoga, Mycobacterium and Geobacillus were reported to degrade the hydrocarbons (Feitkenhauer et al. 2003; Chamkha et al. 2008; Hesham et al. 2012). In hydrocarbon biodegradation, the metabolic enzyme activity is considered to be a vital parameter for the oxidation of n-alkanes (Watkinson and Morgan 1990; Sikkema et al. 1995; Elumalai et al. 2017a; Parthipan et al. 2017b). Multiple genes for alkane hydroxylase can utilize versatile alkanes (Van Beilen et al. 2002).
Many hydrocarbon-degrading bacteria are involved in microbial corrosion of carbon steel API 5LX in petroleum-transporting pipelines/storage tanks (Mortazavia et al. 2013; Kristensen et al. 2015; Shekoohiyan et al. 2016; Parthipan et al. 2017c, 2018c). Over the metal surface, the presence of microorganisms leads to the formation of biofilm due to the accumulation of microbial metabolites termed as extracellular polymeric substance (EPS), consisting of lipopolysaccharides, nucleic acids, proteins, and carbohydrates (Beer et al. 1994; Li et al. 2015; Narenkumar et al. 2016). The physiochemical properties between metal and microbe interaction are due to the electrical charge and hydrophobic phenomena. In addition, EPS can modify the morphology and chemistry of the metal surface which lead to the formation of complexes with a redox potential on the metal surface. Such modification of physiochemical properties on metal surface due to microbial actions on the environment, the metabolism electrochemically enhances the corrosion process (Hamilton 2003; Alabbas et al. 2012).
MIC is initiated with small pit formation due to microbial action (Parthipan et al. 2017d). Slime-forming aerobic bacteria, Pseudomonas, form thin films with corrosion deposits on metal surfaces (Little and Ray 2002; Pillay and Lin 2013; Rajasekar et al. 2017). Some of the iron-oxidizing bacterial genus such as Gallionella, Sphaerotilus, Crenothrix, Leptothrix, Bacillus, Clonothrix and Lieskeella were identified and reported as corrosion-causing bacteria (Mishra and Singh 2012; Parthipan et al. 2017e). Thermophilic bacteria have the ability to promote biofilm development and cause MIC on metal surfaces. The biodegradation potentialities of the thermophilic bacterial communities on microbial corrosion of carbon steel in oil reservoir are less studied so far. So, the present study deals with the isolation of the thermophilic bacterial strains from the oil reservoir samples (crude oil and produced water) and examining their potentiality of biodegradation/biocorrosion behaviour in carbon steel API 5LX. This study would contribute in understanding the involvement of thermophilic bacterial species towards microbial corrosion and biodegradation.
Materials and methods
Site description and reservoir conditions
Oil reservoir was located in the Cauvery river basin, Karaikal, India (latitude 10.7694° and longitude 79.6155°). The oil reservoir in the Cauvery basin has been flooded with crude oil, produced water and methane gas for the past 30 years. Two reservoirs AKM 08 (station-I) and KMP 12 (station-II) were selected (based on their severe corrosion problems among other wells) for collection of crude oil and produced water samples. The depth of the both reservoirs was in the range of 2200–2700 m below the sea level and temperature ranged from 45 to 55 °C. The crude oil and produced water mixture were collected using sterile 1-L sample containers (ten numbers) to fulfil capacity from both well-heads after 5–7-min flushing. The containers were tightly sealed and kept in an ice box and immediately transported to the laboratory for further analysis. The crude oil and produced water were separated using a separator funnel. Crude oil API (American Petroleum Institute) gravity should be 960–9800 kg m−3. Physicochemical characteristics of oil reservoir-produced water were obtained from the oil company and verified by inductively coupled plasma mass spectrometry (ICPMS), and are shown in Supplementary Table S1.
Isolation of bacteria
The collected crude oil and produced water samples were serially diluted (10−3–10−6) using 60% sodium chloride solution. 1 mL of each dilution was poured directly into the sterile Petri dishes followed by pouring of selective medium (iron agar, manganese agar and Thiobacillus agar). The composition of each selective media was described as earlier (Rajasekar et al. 2007a, b). The poured plates were incubated under aerobic conditions at 50 °C for 2–5 days. After incubation, bacteria were enumerated and isolated. The isolated colonies were streaked onto the respective medium to obtain pure culture. Partial identification was carried out using morphological and biochemical tests as described earlier (Rajasekar et al. 2007a, b).
Identification of bacteria by 16S rRNA gene sequencing
1 mL of overnight grown bacterial culture was used to isolate the genomic DNA as described by Rajasekar et al. (2010). The isolated DNA was amplified with 16S rRNA gene using universal primers 518F (5′-CCAGCAGCCGCGGTAATACG-3′) and 800R (5′-TACCAGGGTATCTAATCC-3′). The process conditions of PCR were performed with a 50 µL reaction mixture encompassing of 2 µL DNA (10 ng) as the template, forward and reverse primers (0.5 µM), and 1.5 mM of MgCl2 and 50 µM of dNTPs along with 1 µL of TaqDNA polymerase and buffer as suggested by the manufacturer (Himedia, Mumbai). PCR was carried out with a Mastercycler Personal (Eppendorf) with the following programme: initial denaturation at 95 °C for 1 min; 40 cycles of denaturation (3 min at 95 °C), annealing (1 min at 55 °C), and extension (2 min at 72 °C); followed by the final extension (at 72 °C for 5 min). The amplified product was purified by purification kit (Himedia, Mumbai). Then, amplified DNA was employed for 16S rRNA gene sequencing and the similarity analyses (BLAST program) as described earlier (Rajasekar et al. 2010).
Biodegradation study and analytical methods
Bushnell–Hass (BH) medium was utilized for biodegradation experiments. Selected isolates MN6, IR4 and IR2 were pre-cultured overnight at 50 °C in sterile BH broth with glucose as carbon source. Biodegradation study initiated with the inoculation of individual isolates (initial load 2.1 × 106 CFU mL−1) in sterile 100 mL BH broth in a 250-mL flask with 1% of filtre sterilized crude oil. The sterility of the crude oil was checked by plating method. The BH medium with sterile crude oil served as an abiotic control. All the experiments were done in triplicates and incubated aerobically on orbital shaker (150 rpm) at 50 °C for 15 days. The optimum growth rate of bacterial culture during the biodegradation study was monitored using UV spectrophotometer (JASCO V-630) at OD540. Characterization of total hydrocarbon fractions and changes in the functional group during biodegradation were analyzed by gas chromatographic–mass spectrometry (GC–MS) model Agilent 7890A, Restek Rxi-5 MS (30 m × 0.25 mm × 0.25 µm). Phytone (C20) was used as an internal standard for GC-MS and Fourier-transform infrared spectrum (FTIR, Perkin Elmer version 10.4.00), respectively. The detailed procedures of GC–MS, FTIR and biodegradation efficiency were carried out as described earlier (Rajasekar et al. 2007a, b). The extra-cellular enzymes, viz., alkane hydroxylase, alcohol dehydrogenase and lipase assay, were estimated according to previous literature (Mishra and Singh 2012).
Biocorrosion, electrochemical studies and surface analysis
As described earlier by Rajasekar et al. (2007a, b), carbon steel API 5LX ((in weight %) of 0.07 C, 1.36 Mn, 0.008 Ti, 0.003 S, 0.004 P and balance Fe) was used for biocorrosion studies. The coupons were machined to the small squares with size of 2.5 cm × 2.5 cm with 0.4 cm thickness and used for weight loss study. For electrochemical impedance spectroscopy (EIS) studies, carbon steel coupon was embedded with epoxy resin with an exposed area of 1.0 cm2 (10 mm × 10 mm × 1 mm) used. Coupons were prepared as described earlier (Rajasekar et al. 2010). Weight loss and EIS coupons positioned in a 1000-mL Erlenmeyer flask with 500 mL of sterile autoclaved produced water acted as control system I. Similar to system I, other three systems were inoculated with 2 mL (about 106 CFU mL−1) of individual strains such as MN6, IR4 and IR2, and marked as the experimental system II, III and IV, respectively. All experiments were carried out in triplicates and incubated at 50 °C for 15 days in static and aerobic conditions. Total viable bacterial count was enumerated during the corrosion study using standard plate count method. At the end of biocorrosion study, weight loss coupons were removed and pickled in cleaning solutions as described by American society for testing and materials (ASTM) standard G1-81. The corrosion rates of each system were calculated according to ASTM standard (Parthipan et al. 2017e). Corrosion products were collected from each coupon and were subjected to X-ray diffraction (XRD, BRUKER-8030, Germany) analysis for determining the nature of oxides. At end of the study, the working electrode (1-cm2 coupon) was removed from each system and further analyzed using EIS (CHI608E-CH Electrochemical analyser). At steady-state conditions, open circuit potential (OCP) and impedance were carried out in a frequency range of 0.1–105 Hz using a 10 mV min−1. The potentiodynamic polarization measurements were carried out by polarizing in range between − 200 and 200 mV at scan rate of 0.002 V s−1 with respect to corrosion potential (Ecorr). The measurements were made in a conventional three-electrode electrochemical cell coupled with a potentiostat (Xu et al. 2013).
Results and discussion
Bacterial analysis
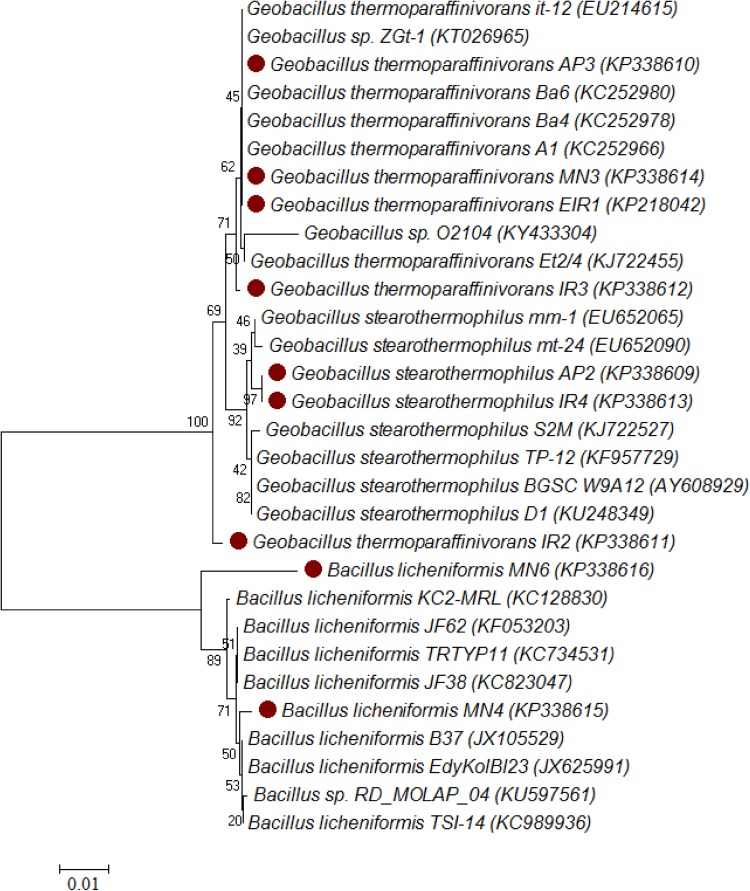
Both iron and manganese agar plates have considerable numbers of the bacterial colonies in the both sampling sites as mentioned in the Supplementary Table S2. All the isolated strains showed good growth at 50 °C within 24–72 h. Biochemical characterization studies revealed that all the isolates belong to the genera Geobacillus and Bacillus. These strains can grow in halophilic conditions (produced water contains high amount of chloride ranging from 59,303 to 86,269 mg L−1). Totally, 44% of the strains (four strains including EIR1, IR2, IR3, and IR4) were identified in station-I and 56% (five strains including IR5, IR6, MN2, MN4 and MN6) were identified in station-II as shown in Supplementary Table S3. Gram-positive bacterial species were found to be dominantly present in both oil reservoir stations, which may due to the capability of endospore formation by the Gram-positive bacteria (bacilli). The phylogenetic tree was constructed based on the neighbour-joining analysis of the 16S rRNA gene sequences as shown in Fig. 1. Two strains of Bacillus species were identified as B. licheniformis isolated from iron and manganese agar, respectively. All the isolates have the aptitude to grow in minimal media with crude oil as sole carbon source.
Fig. 1.
Neighbour-joining tree based on 16S rRNA gene sequences, showing phylogenetic relationships between sequences of the bacterial phylum Firmicutes. Red dot indication refers to identified thermophilic isolates. Numbers at nodes specify bootstrap values > 50% from 1000 replicates. GenBank accession numbers are presented in parentheses. The scale bar represents sequence divergence
According to 16S rRNA gene sequencing studies, thermophilic isolates exhibited 100% of DNA homology to B. licheniformis MN6 (KP338616), G. stearothermophilus IR4 (KP338613) and G. thermoparaffinivorans IR2 (KP338611). These results suggest an interspecies level of relatedness between the identified groups (Nazina et al. 2001). Meintanis et al. (2006) pointed that around 154 thermophilic bacteria were identified in the soil, sea water and sediment samples collected from volcano of Santorini at Nea Kameni Island, and those isolates were subjected to screening for alkane hydroxylase (alkJ) gene. This report confirmed the role of specific gene (alkJ) that played a key role in degradation of alkanes. Among 154 strains reported, ten strains had these genes. Besides, the dominant strains, Geobacillus sp. and Bacillus sp. were identified in high-temperature oil field for the first time in Indian oil reservoir (Marchant and Banat 2010). Occurrence of microbes in oil facilities are the one of the key factors for biodegradation and corrosion problems.
Biodegradation study
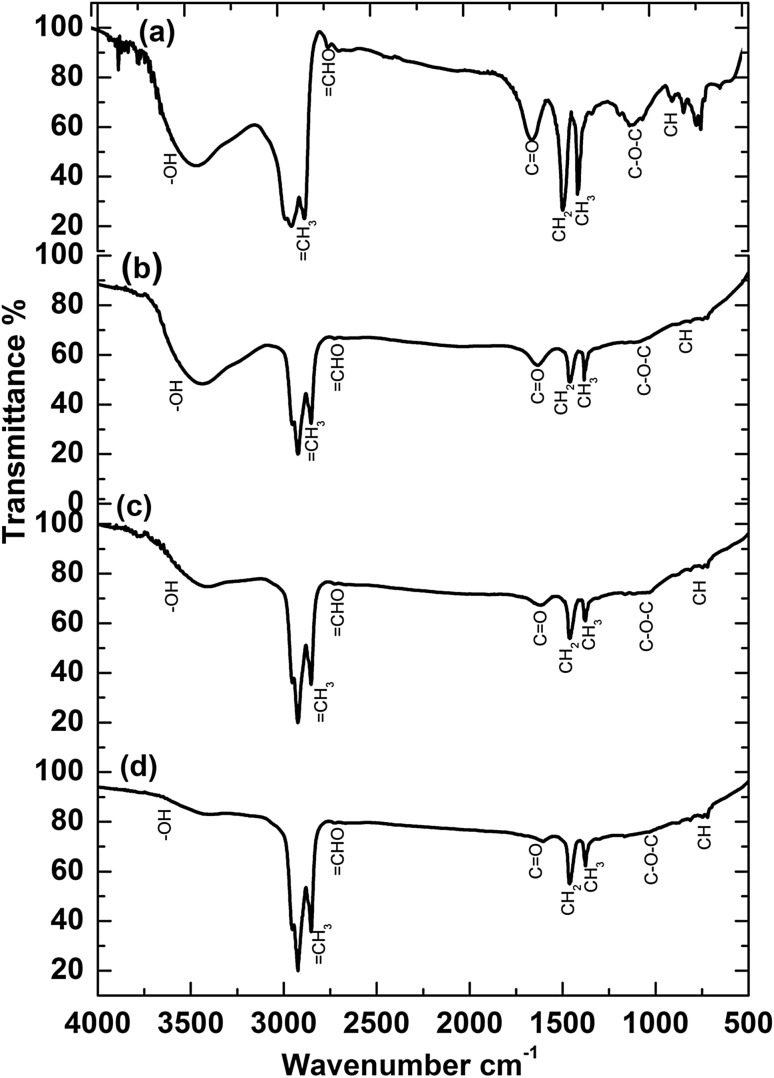
Thermophilic bacterial isolates utilized crude oil as sole carbon source with an increase in growth and cell proliferation as shown in Fig. S1. Especially, the strain IR4 had the highest growth compared to others. FTIR spectra (Fig. 2a) of crude oil in control system showed characteristic bands at 2850 cm−1 (=CH3 stretching mode), 2714 cm−1 (=CHO in aldehydes), 1634 cm−1 (C=O in tertiary amide), 1449 cm−1 (CH2 in aliphatic compounds), 1374 cm−1 (CH3 in aliphatic compounds), 1069 cm−1 (C–O–C aliphatic ethers) and 877 cm−1 (CH out of plane), respectively. Figure 2b–d shows the FTIR spectrum after biodegradation of crude oil in the presence of MN6, IR4 and IR2. Based on the FTIR spectrum, it was noticed that the spectra of metabolites (in terms of functional groups) greatly differed in the presence of bacteria compared to control. The decreased intensity of broad peaks at 877–1634 cm−1 showed the major metabolites corresponding to CH3 and CH2 groups of aliphatic compounds, C=O in tertiary amide, =CH3 and =CHO in aldehydes were degraded by MN6, IR4 and IR2 strains. GC–MS chromatogram of the crude oil degradation is shown in Fig. S2. The GC retention data of the crude oil matching to structural assignations were done after a library search of a database and the mass spectra interpretation is presented in Fig. S2a–d. GC–MS profile of crude oil biodegradation with bacterial strains MN6, IR4 and IR2 (Supplementary Table S4) showed the biodegradation efficiency (BE) of the individual strains as 82%, 94%, and 87% for MN6, IR4 and IR2 respectively. The GC–MS analyses of degraded crude oil samples showed multiple peaks that are grouped based on the carbon numbers present in each compound. Some known compounds were also identified in this study and confirmed after matching with library.
Fig. 2.
FTIR spectra of crude oil biodegradation by thermophilic bacteria (a) control, (b) B. licheniformis MN6, (c) G. stearothermophilus IR4 and (d) G. thermoparaffinivorans IR2
In the presence of thermophilic bacterial strains, significant changes in the functional groups of hydrocarbon compound suggested the effective degradation of both aliphatic and aromatic hydrocarbons by thermophilic bacterial strains. GC–MS analysis too confirmed the complete degradation of light and heavy chain hydrocarbons by MN6, IR4 and IR2 strains. Involvement of bacterial enzymes leads to the degradation of short-chain hydrocarbons by microbes, thus rendering early solubility of the crude oil. The turbidity of the culture too confirmed the growth of the cells in the presence of crude oil. The ‘degraded’ crude oil products were found along with bacterial emulsifying agents (Parthipan et al. 2017e). Viable bacterial count data too indicated that the 1% of crude oil had a positive effect on bacterial growth. The thermophilic bacterial strains, G. stearothermophilus and Bacillus sp., isolated from Volcano Island have been reported to effectively degrade the hydrocarbon in extreme environment. Previously, Geobacillus sp. and Bacillus sp. have been reported to be present in oil-polluted soil and petroleum reservoirs, and were found to utilize crude oil as a sole carbon and energy sources (Maugeri et al. 2002).
Role of degradative enzymes in the biodegradation of crude oil
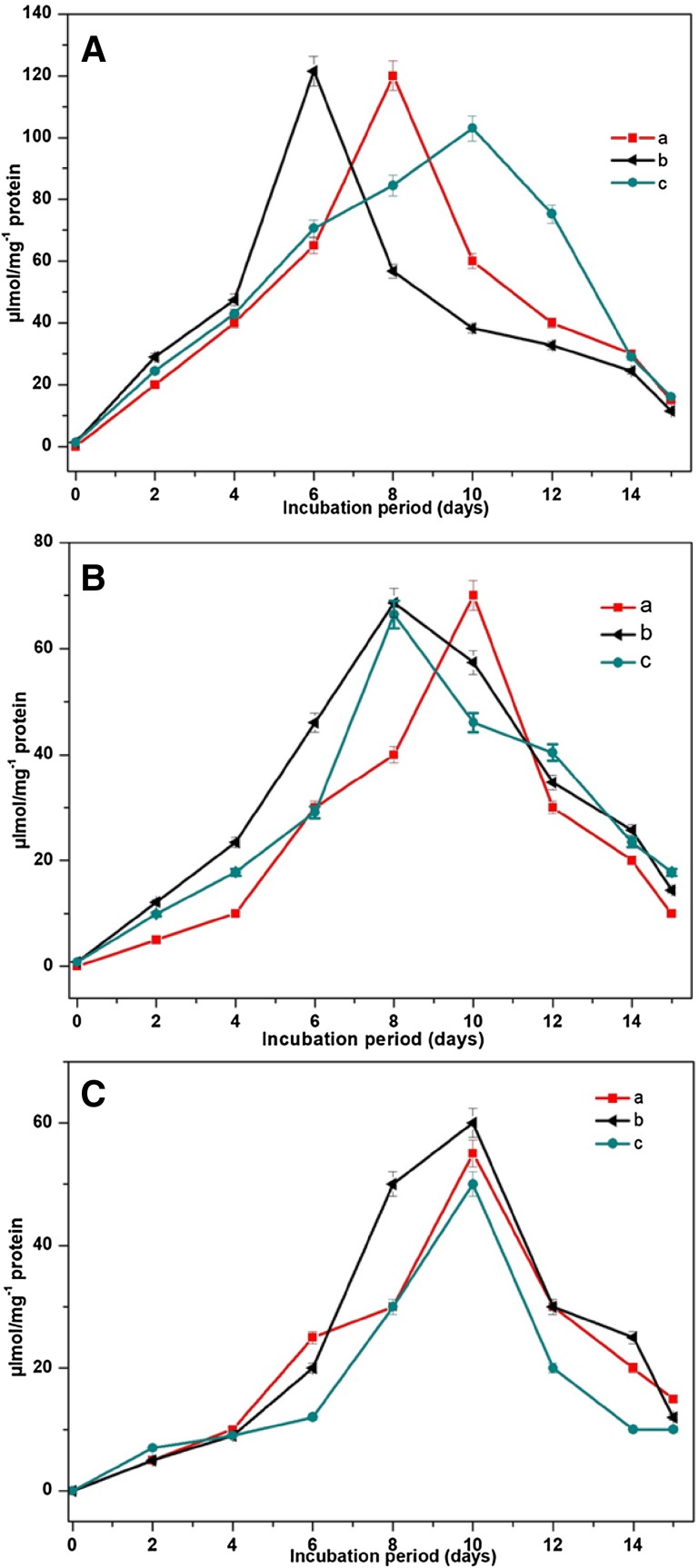
Figure 3A shows the induction of alkane hydroxylase (120 µmol mg−1 protein) by Bacillus licheniformis MN6, 130 µmol mg−1 protein by Geobacillus stearothermophilus IR4 and 110 µmol mg−1 protein by Geobacillus thermoparaffinivorans IR2 between 6 and 10 days of crude oil degradation. Alcohol dehydrogenase-induced degradation of crude oil by three bacterial strains is shown in Fig. 3B. The intensity of peaks was found to be proportional to the activity of alcohol dehydrogenase during degradation of crude oil with different incubation periods. Maximum alcohol dehydrogenase activity was found in the presence of Bacillus licheniformis MN6 (70 µmol mg−1 protein) followed by Geobacillus stearothermophilus IR4 (60 µmol mg−1 protein) and Geobacillus thermoparaffinivorans IR2 (58 µmol mg−1 protein). Likewise, Lipase enzyme was also found to be produced by all the three bacterial strains throughout degradation process. On analyzing the level of enzyme induction, it was initially slow and later increased rapidly after 5th day of incubation. Figure 3C shows fascinatingly high crude oil degradation in MN6-induced lipase (55 µmol mg−1 protein), IR4 showed 60 µmol mg−1 protein and IR2 showed 50 µmol mg−1 protein. The induction of alkane hydroxylase was noted to have a direct impact on the degradation process of crude oil during different incubation periods. Amongst all other three enzymes, alkane hydroxylase enzyme activity was noted to be highly induced, thus confirming their key role in the degradation process than other enzymes (Wu et al. 2014; Parthipan et al. 2017b).
Fig. 3.
Quantification enzyme assay A alkane hydroxylase, B alcohol dehydrogenase and C lipase activity during crude oil biodegradation by thermophilic bacterial isolates: (a) B. licheniformis MN6, (b) G. stearothermophilus IR4 and (c) G. thermoparaffinivorans IR2. Error bars on the graph signify the standard deviation (SD) of triplicate samples (P < 0.002)
An interesting observation of higher degradation capability of hydrocarbon by IR4 was due to the presence of thermo-stable enzyme. These enzymes were reported to play an important role in the degradation of hydrocarbons (Marchant and Banat 2010; Wolicka and Borkowski 2012). Shorter chain n-alkanes were degraded completely compared to heavy chains such as n-heptadecane and n-octadecane (Zhang et al. 2012; Liu et al. 2015). Biodegradation efficiency of MN6 and IR2 showed higher n-alkane degradation rate than branched alkanes with higher carbon compounds (C26). Our observation was supported by earlier report (Cunha et al. 2006) on the capability of enteric bacterium, Serratia marcescens, to degrade diesel/naphtha hydrocarbon in oil facilities (Rajasekar et al. 2007a, b).
Biocorrosion study and surface analysis
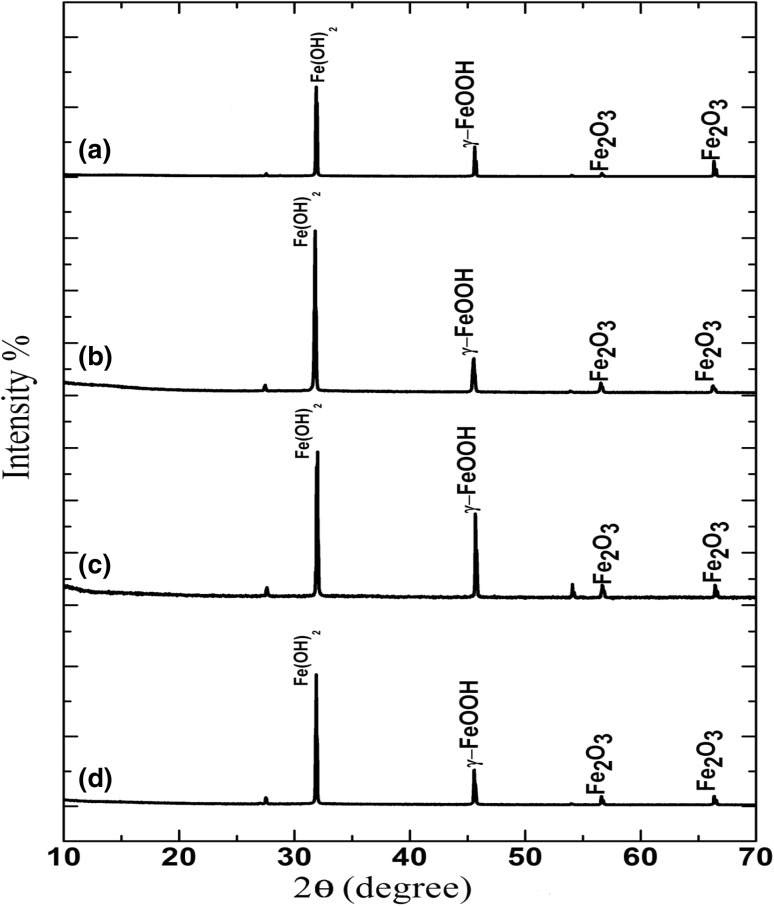
Bacterial isolates showed an excellent growth in carbon steel surface (Fig. S3). It revealed that all the strains can form biofilm on carbon steel surface. Based on the weight loss experiment, the corrosion rate of carbon steel in the presence/absence of corrosive bacterial system was recorded. The corrosion rates in control were noted as 0.95 ± 0.1, MN6 1.70 ± 0.1, IR4 2.12 ± 0.2 and IR2 1.59 ± 0.2 mm year−1 in system I, II, III and IV, respectively. The XRD spectra of the corrosion product in control system and other bacterial systems are presented in Fig. 4. The X-ray diffraction peaks at 2Ѳ = 27.4°, 31.9°, 45.6°, 56.3° and 66.1° revealed the existence of Fe(OH2), FeO(OH) and ע-FeOOH, respectively. The XRD pattern of the corrosion product in the presence of thermophilic bacterial strains MN6, IR4 and IR2 are shown in Fig. 4b–d. It can be clearly noted that all the peak intensities of system-III were increased than other systems, which indicates that the corrosion rate is higher in the presence of strain, IR4. The presence of thermophilic bacterial species in produced water can form biofilms, thus accelerating the corrosion reaction.
Fig. 4.
XRD characterization of the corrosion product obtained from the carbon steel API 5LX by thermophilic bacteria isolates (a) control, (b) B. licheniformis MN6, (c) G. stearothermophilus IR4 and (d) G. thermoparaffinivorans IR2
Immersion of carbon steel in produced water with bacterial strains leads to metal deterioration due to microbial action. Aerobic thermophilic bacterial strains increase the corrosion rate compared to mesophilic bacteria with increasing of exposure time (Almeida and Franca 1999). The XRD data revealed that the corrosion reaction of carbon steel API 5LX occurs as follows: in solution phase, oxygen molecule gets reduced into OH− ions, and the iron gets oxidized to ferrous ions (Fe2+) and reacts with the dissolved oxygen to form lipidocrocite (ע-FeOOH). After the formation of ע-FeOOH on metal surface, the reaction slows down the reactive iron and limits the oxygen diffusion (Elumalai et al. 2017b). Initial corrosion product, Fe(OH)2, gets oxidized by bacterial strains to form lepidocrocite FeOOH and ferric oxides in oxygen environment, thus forming a crystalline phase with rhombic structure on metal surface (Hajj et al. 2013). XRD patterns revealed the presence of Fe(OH)2 in bacterial systems, thus confirming the of corrosion accelerated by bacterial metabolites.
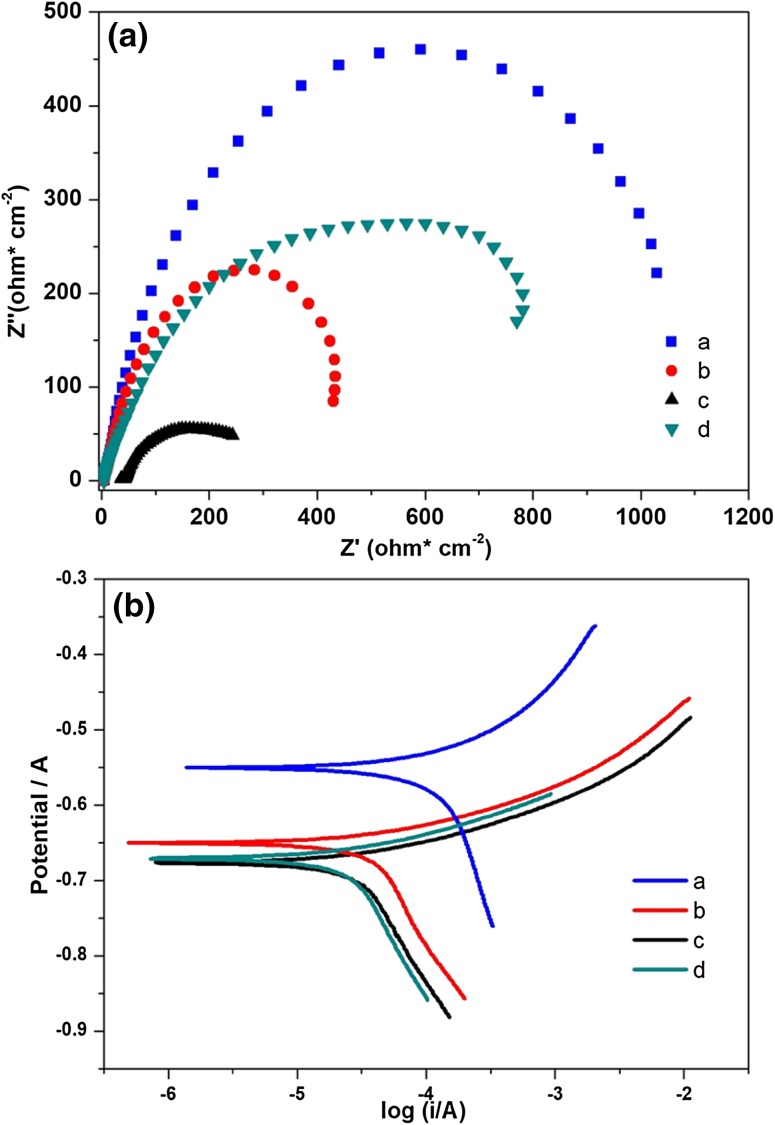
Electrochemical studies
Figure 5A shows the impedance spectra of Nyquist plot for carbon steel API 5LX in control and experimental systems. The Nyquist plots in the presence of thermophilic bacteria are shown in Fig. 5A(b–d) corresponding to MN6, IR4 and IR2 strains. All the bacterial systems were observed as depressed semi-circular curves, thus representing the biofilm formation with pores of corrosion products on metal surface. The observation of depressed semicircle in Fig. 5A(b) indicated the activation control reaction occurred due to the interaction between metal and heterogeneous nature of bacterial biofilm on metal surface. The electrochemical parameters in the absence/presence of bacterial isolates are presented in Supplementary Table S5. According to obtained results, thermophilic bacterial system showed lower Rct values (7.78, 1.22 and 1.67Ω) than the control (41.93Ω). Figure 5B shows the Tafel polarization curves exposed to bacteria and control system, respectively. On visualizing, higher anodic current density was noted in the absence of bacteria than the bacterial system. Hence, it can be understood that the anodic dissolution occurred highly in the presence of bacteria. Tafel analysis data (Ecorr, Icorr, βa, βc) are shown in Supplementary Table S5. Increased corrosion current (Icorr) values of 28.18 µA cm−2, 39.81 µA cm−2 and 32.31 µA cm−2 of carbon steel API 5LX metal in all the bacterial systems II–IV were noted when compared to control system-I (17.82 µA cm−2). This could be due to the formation of corrosion on metal surface as reported by Xu et al. (2013) and Qu et al. (2014). Corrosion potential from Ecorr − 658 to − 677 mV exhibited the positive shift thus strongly supporting the activity of thermophilic bacteria on corrosion scale formation on a metal surface as shown in Fig. 5B.
Fig. 5.
Electrochemical study: A impedance plot, B potentiodynamic polarization curves of MIC on carbon steel API 5LX by thermophilic isolates: (a) control, (b) B. licheniformis MN6, (c) G. stearothermophilus IR4 and (d) G. thermoparaffinivorans IR2
Electrochemical studies reported that the lowest resistance in IR4 was due to the acceleration of higher corrosion by bacterial biofilm (Alabbas et al. 2012). This result was in concordance with the weight loss, XRD data, thus proving the acceleration of corrosion process by thermophilic bacterial strains on the metal surface. The bacteria-exposed metal surface contained the combination of iron oxides, Fe2O3 layer, which affected the charge transfer resistance. Ecorr typically showed a slightly higher electrochemical activity and enhanced redox quality on the metal surface, which were probably due to the bacterial metabolic activity, a most important phenomena in MIC investigation studies (Alabbas et al. 2012; Narenkumar et al. 2017). As seen in Fig. 5B, the anodic curves were almost same and cathodic curves were different in all bacterial system. It demonstrated that the cathodic hydrogen evolution process occurred at the metal surface leading to the bacterial consumption of hydrogen from iron along with water, thus contributing and accelerating the corrosion process. Further, these results indicated that electrochemical transfer processes on metal oxidation contributed to the electrochemical response (Davidova et al. 2012). Lenhart et al. (2014) suggested that the corrosion due to biofilm presence on the metal surface might be due to pitting or localized corrosion.
Conclusion
The biodegradation efficiency and biocorrosion behaviour of carbon steel API 5LX in produced water by B. licheniformis MN6, G. stearothermophilus IR4 and G. thermoparaffinivorans IR2 have been investigated. Among the isolates, IR4 was found to efficiently accelerate the corrosion process with a corrosion rate of 2.12 mm year−1 on carbon steel API 5LX. GC-MS analysis showed the BE of hydrocarbon was about 94%. Subsequently, the degradative enzymes such as alkane hydroxylase and alcohol dehydrogenase were found to play a vital role in the crude oil degradation process. EIS and XRD data too clearly supported the presence of oxidized ferrous ion into ferrous/ferric oxides as corrosion product. This work primarily focused on the culture-dependent method to implicate the importance of thermophilic bacteria strains towards biocorrosion of crude oil reservoir facility. These strains were also found to oxidize the iron and manganese into oxides and utilize the hydrocarbon as sole carbon source in oil reservoir environment. The understanding of the role of thermophiles in the oil reservoir conditions will assist in the selection of suitable biocides/inhibitor for control of biocorrosion in the oil reservoir and oil-contaminated environments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by Department of Biotechnology, Government of India (BT/RLF/Re-entry/17/2012), Science and Engineering Research Board, Department of Science and Technology (DST-SERB), Government of India (EEQ/2016/000449) and University Grants Commission (MRP-MAJOR-MICRO-2013-31825).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Byung-Taek Oh, Email: btoh@jbnu.ac.kr.
Aruliah Rajasekar, Email: rajasekargood@gmail.com, Email: rajasekargood@tvu.edu.in.
References
- Aktas DF, Sorrell KR, Duncan KE, Wawrik B, Callaghan AV, Suflita JM. Anaerobic hydrocarbon biodegradation and biocorrosion of carbon steel in marine environments: the impact of different ultra low sulfur diesels and bioaugmentation. Int Biodeterior Biodegrad. 2017;118:45–56. doi: 10.1016/j.ibiod.2016.12.013. [DOI] [Google Scholar]
- Alabbas FM, Williamson C, Bhola SM, Spear JR, Olson DL, Mishra B, Kakpovbia A. Influence of sulfate reducing bacterial biofilm on corrosion behaviour of low-alloy, high-strength steel (API-5L X80) Int Biodeterior Biodegrad. 2012;78:34–42. doi: 10.1016/j.ibiod.2012.10.014. [DOI] [Google Scholar]
- Almeida MAN, Franca FPD. Thermophilic and mesophilic bacteria in biofilms associated with corrosion in a heat exchanger. World J Microbiol Biotechnol. 1999;15:439–442. doi: 10.1023/A:1008963632593. [DOI] [Google Scholar]
- Bao M, Sun P, Yang X, Wang X, Wang L, Cao L, Li F. Biodegradation of marine surface floating crude oil in a large-scale field simulated experiment. Environ Sci Process Impacts. 2014;16:1948–1956. doi: 10.1039/c4em00166d. [DOI] [PubMed] [Google Scholar]
- Beech IB, Sunner J. Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol. 2004;15:181–186. doi: 10.1016/j.copbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Beer D, Stoodley P, Roe F, Lewandowski Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol Bioeng. 1994;23(9):1131–1138. doi: 10.1002/bit.260431118. [DOI] [PubMed] [Google Scholar]
- Chamkha M, Mnif S, Sayadi S. Isolation of a thermophilic and halophilic tyrosol-degrading Geobacillus from a Tunisian high temperature oil field. FEMS Microbiol Lett. 2008;283:23–29. doi: 10.1111/j.1574-6968.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- Coetser SE, Cloete TE. Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol. 2005;31(4):213–232. doi: 10.1080/10408410500304074. [DOI] [PubMed] [Google Scholar]
- Cunha CDD, Rosado AS, Sebastian GV, Seldin L, Weid IVD. Oil biodegradation by Bacillus strains isolated from the rock of an oil reservoir located in a deep-water production basin in Brazil. Appl Microbiol Biotechnol. 2006;73:949–959. doi: 10.1007/s00253-006-0531-2. [DOI] [PubMed] [Google Scholar]
- Davidova IA, Duncan KE, Perez-Ibarra B, Suflita JM. Involvement of thermophilic archaea in the biocorrosion of oil pipelines. Environ Microbiol. 2012;14:1762–1771. doi: 10.1111/j.1462-2920.2012.02721.x. [DOI] [PubMed] [Google Scholar]
- Duncan KE, Gieg LM, Parisi VA, Tanner RS, Tringe SG, Bristow J, Suflita JM. Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ Sci Technol. 2009;43:7977–7984. doi: 10.1021/es9013932. [DOI] [PubMed] [Google Scholar]
- Elumalai P, Parthipan P, Karthikeyan OP, Rajasekar A. Enzyme-mediated biodegradation of long-chain n-alkanes (C32 and C40) by thermophilic bacteria. 3 Biotech. 2017;7:116. doi: 10.1007/s13205-017-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elumalai P, Parthipan P, Narenkumar J, Sarankumar RK, Karthikeyan OP, Rajasekar A. Influence of thermophilic bacteria on corrosion of carbon steel in hyper chloride environment. Int J Environ Res. 2017;11(3):339–347. doi: 10.1007/s41742-017-0031-5. [DOI] [Google Scholar]
- Feitkenhauer H, Muller R, Markl H. Degradation of polycyclic aromatic hydrocarbons and long chain alkanes at 60–70°C by Thermus and Bacillus spp. Biodegradation. 2003;14:367–372. doi: 10.1023/A:1027357615649. [DOI] [PubMed] [Google Scholar]
- Hajj HE, Abdelouas A, Mendili YE, Karakurt G, Grambow B, Martin C. Corrosion of carbon steel under sequential aerobic–anaerobic environmental conditions. Corros Sci. 2013;76:432–440. doi: 10.1016/j.corsci.2013.07.017. [DOI] [Google Scholar]
- Hamilton WA. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling. 2003;19(1):65–76. doi: 10.1080/0892701021000041078. [DOI] [PubMed] [Google Scholar]
- Hao R, Lu A, Zeng Y. Effect on crude oil by thermophilic bacterium. J Pet Sci Eng. 2004;43:247–258. doi: 10.1016/j.petrol.2004.02.017. [DOI] [Google Scholar]
- Head IM, Jones DM, Larter SR. Biological activity in the deep subsurface and the origin of heavy oil. Nature. 2003;426:344–352. doi: 10.1038/nature02134. [DOI] [PubMed] [Google Scholar]
- Hernsdorf W, Amano Y, Miyakawa K, Ise K, Suzuki Y, Anantharaman KA, Probst K, Burstein D, Thomas BC, Banfield JF. Potential for microbial H2 and metal transformations associated with novel bacteria and archaea in deep terrestrial subsurface sediments. ISME J. 2017;11:1915–1929. doi: 10.1038/ismej.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesham AEL, Mohamed NH, Ismail MA, Shoreit AMA. 16S rRNA gene sequences analysis of Ficus elastica rubber latex degrading thermophilic Bacillus strain ASU7 isolated from Egypt. Biodegradation. 2012;23(5):717–724. doi: 10.1007/s10532-012-9547-8. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Johnsen AR, Christensen JH. Marine biodegradation of crude oil in temperate and Arctic water samples. J Hazard Mater. 2015;300:75–83. doi: 10.1016/j.jhazmat.2015.06.046. [DOI] [PubMed] [Google Scholar]
- Lenhart TR, Duncan KE, Beech IB, Sunner JA, Smith W, Bonifay V, Biri B, Suflita JM. Identification and characterization of microbial biofilm communities associated with corroded oil pipeline surfaces. Biofouling. 2014;30(7):823–835. doi: 10.1080/08927014.2014.931379. [DOI] [PubMed] [Google Scholar]
- Li H, Yang SZ, Mu BZ, Rong ZF, Zhan J. Molecular phylogenetic diversity of the microbial community associated with a high-temperature petroleum reservoir at an offshore oil field. FEMS Microbiol Ecol. 2007;60:74–84. doi: 10.1111/j.1574-6941.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Li JY, Mbadinga SM, Liu JF, Gu JD, Mu BZ. Analysis of bacterial and archaeal communities along a high-molecular-weight polyacrylamide transportation pipeline system in an oil-field. Int J Mol Sci. 2015;16:7445–7461. doi: 10.3390/ijms16047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Aktas DF, Aydin E, Bonifay V, Sunner J, Suflita JM. Anaerobic biodegradation of alternative fuels and associated biocorrosion of carbon steel in marine environments. Environ Sci Technol. 2016;50(9):4844–4853. doi: 10.1021/acs.est.5b06388. [DOI] [PubMed] [Google Scholar]
- Little B, Ray R. A perspective on corrosion inhibition by biofilms. Corrosion. 2002;58:424–428. doi: 10.5006/1.3277632. [DOI] [Google Scholar]
- Liu JF, Mbadinga SM, Yang SZ, Gu JD, Mu BZ. Chemical structure, property and potential applications of surfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int J Mol Sci. 2015;15:4814–4837. doi: 10.3390/ijms16034814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magot M, Ollivier B, Patel BKC. Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek. 2000;77:103–116. doi: 10.1023/A:1002434330514. [DOI] [PubMed] [Google Scholar]
- Marchant R, Banat IM. The genus Geobacillus and hydrocarbon utilization. In: Timmis KN, editor. Handbook of hydrocarbon and lipid microbiology. Berlin: Springer; 2010. pp. 1888–1894. [Google Scholar]
- Maugeri TL, Gugliandolo C, Caccamo D, Stackebrandt E. Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents. Syst Appl Microbiol. 2002;25:450–455. doi: 10.1078/0723-2020-00119. [DOI] [PubMed] [Google Scholar]
- Meintanis C, Chalkou KI, Kormas KA, Karagouni AD. Biodegradation of crude oil by thermophilic bacteria isolated from a volcano island. Biodegradation. 2006;17(2):105–111. doi: 10.1007/s10532-005-6495-6. [DOI] [PubMed] [Google Scholar]
- Mishra S, Singh SN. Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour Technol. 2012;111:148–154. doi: 10.1016/j.biortech.2012.02.049. [DOI] [PubMed] [Google Scholar]
- Mortazavia B, Horela A, Beazley MJ, Sobecky PA. Intrinsic rates of petroleum hydrocarbon biodegradation in Gulf of Mexico intertidal sandy sediments and its enhancement by organic substrates. J Hazard Mater. 2013;244:537–544. doi: 10.1016/j.jhazmat.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Narenkumar J, Madhavan J, Nicoletti M, Benelli G, Murugan K, Rajasekar A. Role of bacterial plasmid on biofilm formation and its influence on corrosion of engineering materials. J Bio Tribo Corros. 2016;2:24. doi: 10.1007/s40735-016-0054-z. [DOI] [Google Scholar]
- Narenkumar J, Parthipan P, Nanthini AUR, Benelli G, Murugan K, Rajasekar A. Ginger extract as green biocide to control microbial corrosion of mild steel. 3 Biotech. 2017;7:133. doi: 10.1007/s13205-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narenkumar J, Parthipan P, Madhavan J, Murugan K, Marpu SB, Suresh AK, Rajasekar A. Bioengineered silver nanoparticles as potent anti-corrosive inhibitor for mild steel in cooling towers. Environ Sci Pollut Res. 2018;25:5412–5420. doi: 10.1007/s11356-017-0768-6. [DOI] [PubMed] [Google Scholar]
- Nazina TN, Tourova TP, Poltaraus AB, Novikova EV, Grigoryan AE, Ivanova AA, Lysenko AM, Petrunyaka VV, Osipov GA, Belyaev SS, Ivanov MV. Taxonomic study of aerobic thermophilic bacilli descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int J Syst Evol Micrbiol. 2001;51:433–446. doi: 10.1099/00207713-51-2-433. [DOI] [PubMed] [Google Scholar]
- Parthipan P, Elumalai P, Karthikeyan OP, Ting YP, Rajasekar A. A review on biodegradation of hydrocarbon and their influence on corrosion of carbon steel with special reference to petroleum industry. J Environ Biotechnol Res. 2017;6(1):12–33. [Google Scholar]
- Parthipan P, Elumalai P, Sathishkumar K, Sabarinathan D, Murugan K, Benelli G, Rajasekar A. Biosurfactant and enzyme mediated crude oil degradation by Pseudomonas stutzeri NA3 and Acinetobacter baumannii MN3. 3 Biotech. 2017;7:278. doi: 10.1007/s13205-017-0902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthipan P, Ganesh Babu T, Anandkumar B, Rajasekar A. Biocorrosion and its impact on carbon steel API 5LX by Bacillus subtilis A1 and Bacillus cereus A4 isolated from Indian crude oil reservoir. J Bio Tribo Corros. 2017;3:32. doi: 10.1007/s40735-017-0091-2. [DOI] [Google Scholar]
- Parthipan P, Narenkumar J, Elumalai P, Preethi PS, Nanthini AUR, Agrawal A, Rajasekar A. Neem extract as a inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environments. J Mol Liq. 2017;240:121–127. doi: 10.1016/j.molliq.2017.05.059. [DOI] [Google Scholar]
- Parthipan P, Preetham E, Machuca LL, Rahman PKSM, Murugan K, Rajasekar A. Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front Microbiol. 2017;8:193. doi: 10.3389/fmicb.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthipan P, Elumalai P, Narenkumar J, Machuca LL, Murugan K, Karthikeyan OP, Rajasekar A. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int Biodeterior Biodegrad. 2018;132:66–73. doi: 10.1016/j.ibiod.2018.05.005. [DOI] [Google Scholar]
- Parthipan P, Sabarinathan D, Angaiah S, Rajasekar A. Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J Mol Liq. 2018;261:473–479. doi: 10.1016/j.molliq.2018.04.045. [DOI] [Google Scholar]
- Parthipan P, Elumalai P, Ting YP, Rahman PKSM, Rajasekar A. Characterization of hydrocarbon degrading bacteria isolated from Indian crude oil reservoir and their influence on biocorrosion of carbon steel API 5LX. Int Biodeterior Biodegrad. 2018;129:67–80. doi: 10.1016/j.ibiod.2018.01.006. [DOI] [Google Scholar]
- Pedersen K. Metabolic activity of subterranean microbial communities in deep granitic groundwater supplemented with methane and H(2) ISME J. 2013;7:839–849. doi: 10.1038/ismej.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay C, Lin J. Metal corrosion by aerobic bacteria isolated from stimulated corrosion systems: effects of additional nitrate sources. Int Biodeterior Biodegrad. 2013;83:158–165. doi: 10.1016/j.ibiod.2013.05.013. [DOI] [Google Scholar]
- Qu Q, He Y, Wang L, Xu H, Li L, Chen Y, Ding Z. Corrosion behavior of cold rolled steel in artificial seawater in the presence of Bacillus subtilis C2. Corros Sci. 2014;91:321–329. doi: 10.1016/j.corsci.2014.11.032. [DOI] [Google Scholar]
- Rajasekar A, Ponmariappan S, Maruthamuthu S, Palaniswamy N. Bacterial degradation and corrosion of naphtha in transporting pipeline. Curr Microbiol. 2007;55:374–381. doi: 10.1007/s00284-007-9001-z. [DOI] [PubMed] [Google Scholar]
- Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PK. Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol. 2007;85:1175–1188. doi: 10.1007/s00253-009-2289-9. [DOI] [PubMed] [Google Scholar]
- Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PKSM. Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol. 2010;85:1175–1188. doi: 10.1007/s00253-009-2289-9. [DOI] [PubMed] [Google Scholar]
- Rajasekar A, Xiao W, Sethuraman M, Parthipan P, Elumalai P. Airborne bacteria associated with corrosion of mild steel 1010 and aluminum alloy 1100. Environ Sci Pollut Res. 2017;24:8120–8136. doi: 10.1007/s11356-017-8501-z. [DOI] [PubMed] [Google Scholar]
- Shekoohiyan S, Moussavia G, Naddafi K. The peroxidase-mediated biodegradation of petroleum hydrocarbons in a H2O2-induced SBR using in-situ production of peroxidase: biodegradation experiments and bacterial identification. J Hazard Mater. 2016;313:170–178. doi: 10.1016/j.jhazmat.2016.03.081. [DOI] [PubMed] [Google Scholar]
- Shen T, Pi Y, Bao M, Xu N, Li Y, Lu J. Biodegradation of different petroleum hydrocarbons by free and immobilized microbial consortia. Environ Sci Process Impacts. 2015;17:2022–2033. doi: 10.1039/c5em00318k. [DOI] [PubMed] [Google Scholar]
- Shimura M, Dhar GM, Kimbara K, Nagato H, Kiyohara H, Hatta T. Isolation and characterization of a thermophilic Bacillus sp. JF8 capable of degrading polychlorinated biphenyls and naphthalene. FEMS Microbiol Lett. 1999;178:87–93. doi: 10.1111/j.1574-6968.1999.tb13763.x. [DOI] [PubMed] [Google Scholar]
- Sikkema J, De Bont JAM, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Revolut. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkhoh NA, Ibrahim AS, Ghannoum MA, Radwan SS. High-temperature hydrocarbon degradation by Bacillus stearothermophilus from oil-polluted Kuwaiti desert. Appl Microbiol Biotechnol. 1993;39:123–126. doi: 10.1007/BF00166860. [DOI] [Google Scholar]
- Stevenson BS, Drilling HS, Lawson PA, Duncan KE, Parisi VA, Suflita JM. Microbial communities in bulk fluids and biofilms of an oil facility have similar composition but different structure. Environ Microbiol. 2011;13(4):1078–1090. doi: 10.1111/j.1462-2920.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- Stipanicev M, Florin T, Loïc E, Omar R, Régine B, Magdalena S, Iwona B. Corrosion of carbon steel by bacteria from North Sea offshore seawater injection systems: laboratory investigation. Bioelectrochemistry. 2014;97:76–88. doi: 10.1016/j.bioelechem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Van Beilen JB, Smits TH, Whyte LG, Schorcht S, Rothlisberger M, Plaggemeier T, Engesser KH, Witholt B. Alkane hydroxylase homologues in gram positive strains. Environ Microbiol. 2002;4:676–682. doi: 10.1046/j.1462-2920.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- Wang LY, Duan RY, Liu JF, Yang SZ, Gu JD, Mu BZ. Molecular analysis of the microbial community structures in water-flooding petroleum reservoirs with different temperatures. Biogeosci Discuss. 2012;9:5177–5203. doi: 10.5194/bg-9-4645-2012. [DOI] [Google Scholar]
- Watkinson RJ, Morgan P. Physiology of aliphatic hydrocarbon degrading microorganisms. Biodegradation. 1990;1:79–92. doi: 10.1007/BF00058828. [DOI] [PubMed] [Google Scholar]
- Wolicka D, Borkowski A. Microorganisms and crude oil. In: Lomero-Zeron L, editor. Introduction to enhanced oil recovery (EOR) processes and bioremediation of oil-contaminated sites. Croatia: Intech; 2012. [Google Scholar]
- Wu B, Lan T, Lu D, Liu Z. Ecological and enzymatic responses to petroleum contamination. Environ Sci Process Impacts. 2014;16:1501–1509. doi: 10.1039/C3EM00731F. [DOI] [PubMed] [Google Scholar]
- Xu D, Li Y, Song F, Gu T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros Sci. 2013;77:385–390. doi: 10.1016/j.corsci.2013.07.044. [DOI] [Google Scholar]
- Zhang J, Zhang X, Liu J, Li R, Shen B. Isolation of a thermophilic bacterium, Geobacillus sp. SH-1, capable of degrading aliphatic hydrocarbons and naphthalene simultaneously, and identification of its naphthalene degrading pathway. Bioresour Technol. 2012;124:83–89. doi: 10.1016/j.biortech.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Zheng C, He J, Wang Y, Wang M, Huang Z. Hydrocarbon degradation and bioemulsifier production by thermophilic Geobacillus pallidus strains. Bioresour Technol. 2011;102:9155–9165. doi: 10.1016/j.biortech.2011.06.074. [DOI] [PubMed] [Google Scholar]
- Zhou C, Li X, Jiang S. Distribution and properties of high molecular weight hydrocarbons in crude oils and oil reservoir of Shengli oil field, China. J Pet Sci Eng. 2005;48:27–240. doi: 10.1016/j.petrol.2005.06.001. [DOI] [Google Scholar]
- Zhou F, Mbadinga SM, Liu JF, Gu JD, Mu BZ. Evaluation of microbial community composition in thermophilic methane-producing incubation of production water from a high-temperature oil reservoir. Environ Biotechnol. 2013;34(18):2681–2689. doi: 10.1080/09593330.2013.786135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.