Abstract
The Bacillus Calmette–Guérin (BCG) vaccine contains live attenuated Mycobacterium bovis; was first used in humans to prevent tuberculosis (TB) in 1921. The World Health Organization (WHO) established the Expanded Program on Immunization in 1974 to ensure that all children have access to routinely recommended vaccines including BCG. Each year 120 million doses of BCG vaccine are administered worldwide. Intradermal BCG vaccine gives rise to a classic primary complex that consists of a cutaneous nodule at the site of injection and subclinical involvement of the regional lymph nodes, which is self-limiting and requires no treatment.
However, ipsilateral regional lymph node enlargement may follow BCG vaccine and is considered as the most common complication, some progress to suppuration. Rarely a disseminated BCG infection may develop in immunocompromised individuals resulting in a devastating outcome. Within the last decades, variable strategies have been applied in treating lymphadenitis related to BCG vaccine, ranging from observation, anti-mycobacterial therapy, aspiration, incision and drainage to lymph node surgical excision.
We are presenting these guidelines that intended to optimize and standardize management of various types of BCG related lymph adenitis in children. They are based upon the best available evidence in literature beside our experience in this field.
Keywords: Bacillus Calmette-Guérin (BCG), Lymphadenitis, Management, Disseminated BCG infection, Guidelines
1. Purpose of the guidelines
To standardize a clinical practice for classification and management of BCG related lymphadenitis in children, extrapolated from the relevant literature besides our accumulative experience in this field, through evaluation of benefits and harms of alternative care options. These guidelines intended to provide an outcome with maximum benefits and minimum risks, reduce the inappropriate variation in clinical practice, eliminate the unnecessary interventions, promote efficient use of resources and support the decision making processes for the best interest of children presenting with BCG related lymphadenitis.
2. Introduction
BCG vaccine developed by Albert Calmette and Camille Guerin in France between 1908 and 1921 contained a live attenuated Mycobacterium bovis. Currently, there are multiple strains of BCG vaccines in use around the world produced by different manufacturers.
BCG is now used worldwide in childhood immunization programs with approximately 100 million newborns being vaccinated each year [1]. In Saudi Arabia, BCG was introduced initially for population at risks in 1964 and later for all newborns in 1970 [2].
Efficacies of BCG vaccines ranges from 0 to 80% in studies of different populations throughout the world [3]. The main role of the BCG vaccines is to protect vaccinees, especially infants and children against disseminated TB and tuberculous meningitis with an estimated efficacy of 78% and 64% respectively [4], [5]. The current estimated incidence of TB in Saudi Arabia is 14/100,000 [6]. Globally the incidence of BCG adverse reaction differs between regions, ranging between 0.5–100 per 1000 vaccinations [7], [8], [9] with the most common presentation is regional lymphadenitis, mainly non-suppuartive lymphadenitis. A rare complication is a disseminated disease, in less than one in a million of vaccinated individuals [7], [10].
In Saudi Arabia, a raised incidence in BCG related lymphadenitis from zero to 10.4 per 1000 vaccinations was linked to the introduction of the Danish strain(SSI 1331), currently in use since November 2005 [11], leading to more awareness about this complication [12] and raising the need to establish national guidelines with the best management approach and outcome.
3. Classification of BCG related lymphadenitis
The term BCG lymphadenitis applies when lymph node(s) have become large enough to be easily palpable and a cause of concern for the parents [13], [14], likely with a diameter greater than or equal to 1 cm.
3.1. Regional BCG related lymphadenitis
The term regional lymphadenitis may apply when there is a BCG vaccine at one arm with ipsilateral regional lymph node(s) involvement. Laboratory and radiological investigations are not routinely recommended in a thriving child with unremarkable physical examination and no evidence of immunodeficiency in the family history.
The flowing features are in favor of regional BCG related lymphadenitis rather than other pathology [14], [15], [16]:
-
1)
BCG vaccination at the ipsilateral arm.
-
2)
Onset between 2 weeks and 6 months, most patients present within 2–4 months after BCG vaccination.
-
3)
Child age not more than 2 years
-
4)
Absence of systemic manifestations such as fever and weight loss.
-
5)
Absence of tenderness over the lymph node(s).
-
6)
Axillary lymph node is mostly involved, although supraclavicular or cervical may be involved in isolation or association with axillary lymphadenopathy.
-
7)
Unremarkable physical examination, e.g. no distant lymphadenopathy or organomegaly.
3.1.1. Non suppurative lymphadenitis
This form represents the majority of BCG related lymphadenitis.
Its typically develops 2 weeks to 6 months post immunization and tends to affect the axillary lymph node(s). Supraclavicular and cervical lymph nodes can be involved, in isolation or in addition to the axillary lymph node(s) [17], [18], usually it regress with time and some may progress to suppuration.
3.1.2. Suppurative lymphadenitis
The suppurative form is marked by the progressive enlargement of regional lymph node(s) with collection of suppurative material and fluctuation associated with erythema and edema. If untreated, suppurative lymphadenitis frequently ruptures with sinus formation, resulting in prolonged course of illness and scaring sequels [14], [15].
3.2. Disseminated BCG infectio
The term disseminated BCG infection considered when there is involvement of distant anatomical site(s) beyond BCG administration site and ipsilateral lymph node(s) [19]. Synonyms of disseminated BCG infection are disseminated/systemic BCG disease or BCGosis.
Disseminated BCG infection is the most serious complication of BCG vaccination. Fatal infection has occurred at a rate of 0.06–1.56 cases per million doses [12]; these deaths occurred primarily among immunocompromised persons. Immune response to Mycobacterial infection starts with macrophages through phagocytosis and followed by cell mediated immunity. A granuloma formation is associated with production of chemokines and cytokines. Both tumor necrosis factor (TNF) and interferon gamma (IFN-γ) are the cornerstone cytokines involved in activation of macrophage host defense mechanisms [1].
The disseminated BCG infection has been reported in immunocompromised children with primary immunodeficiency (PID), including severe combined immunodeficiency (SCID) [12], [20], complete Di George syndrome (cDGS), chronic granulomatous diseases (CGD), the Mendelian susceptibility to mycobacterial disease (MSMD) (e.g. IFN-γ receptor 1/2 deficiencies, IL-12/23 receptor β1 chain deficiency, IL-12p40 deficiency, S TAT1 deficiency and NEMO deficiency and acquired immune deficiency syndrome (AIDS) [12], [21].
3.2.1. Clinical presentation of disseminated BCG infection
The following findings may indicate disseminated BCG infection:
-
1)
Systemic manifestations: fever, anemia, loss or poor weight gain.
-
2)
Distant lymph node(s) enlargement, beyond the ipsilateral lymph node(s) such as right axillary or inguinal.
-
3)
Cutaneous lesions or abscesses beyond the region of vaccination [22].
-
4)
Hepatosplenomegaly.
-
5)
Bone tenderness or swelling reflecting underlying osteomyelitis [23], [24].
-
6)
Signs of meningeal involvement, such as seizures [20].
3.2.2. Investigations for disseminated BCG infection
The investigations should be obtained with guidance of pediatric immunology and infectious diseases specialists. These may include identification of the M. bovis from the patient's organs by culture and/or standard PCR, as well as typical histopathological changes with granulomatous inflammation, in addition to identifying the specific underlying immunodeficiency status. Radiological investigations such as bone scan and abdominal Computed Tomography (CT) scan may be beneficial in evaluating the site(s) and extent of dissemination.
4. Factors associated with increased BCG lymphadenitis incidence
4.1. Vaccine related factors
-
1)
Residual virulence of the BCG strain: BCG strains from different pharmaceutical manufacturers are known to have different reactogenicity profile [12], [14], [25], [26].
-
2)
Viability of final vaccine product: This is related to the quality of the administered vaccine and is affected by storage conditions such as the cold chain [14], [25]. Live particles concentration in the vaccines ranges from 50,000 to 3 million per dose, according to the BCG strain [1].
4.2. Administration related factors
-
1)
Subcutaneous injection: Subcutaneous administration has been associated with a higher frequency of BCG vaccine complications in comparison with correctly administered intradermal injection [14], [25].
-
2)
Dose of BCG injection: The higher the dose of BCG injection the more risk of developing vaccine adverse effects including lymphadenitis [14], [25], [27].
4.3. Host related factors
5. Management of BCG related lymphadenitis
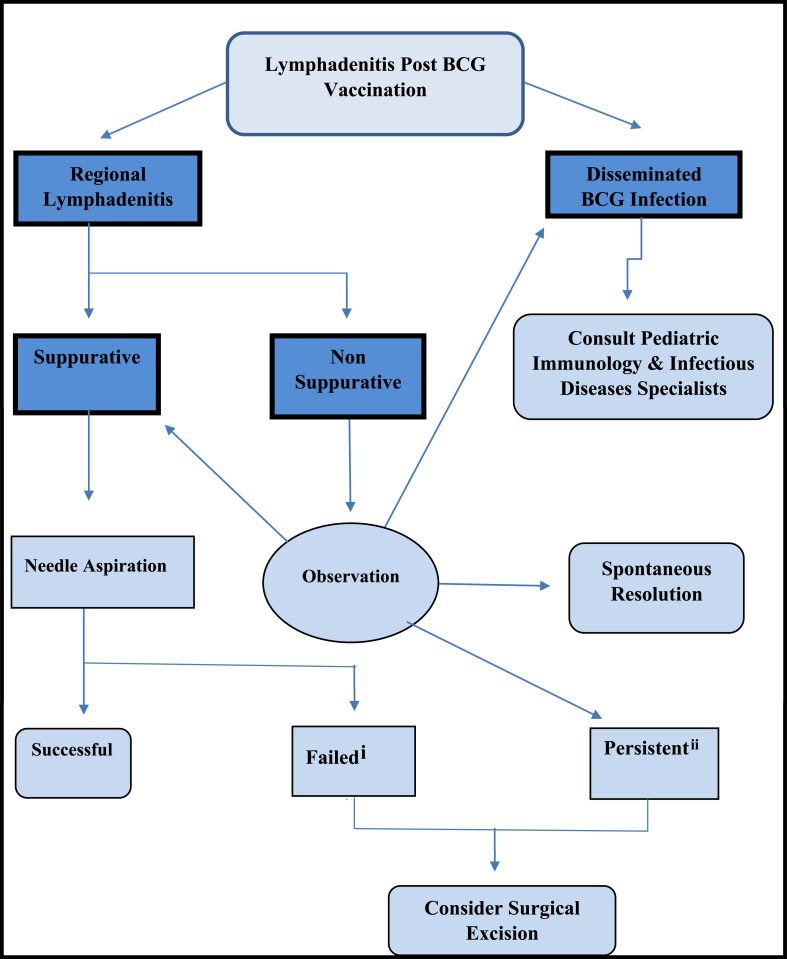
5.1. Management of BCG related lymphadenitis algorithm
-
i
failed initial aspiration, failed repeated aspiration or re-accumulation after ≥2 aspirations
-
ii
persistent ≥ 6–9 months, with size ≥3 cm
5.2. Management of non-suppurative lymphadenitis
The non suppurative form is a relatively benign clinical course where most of the nodes resolve spontaneously within 4–6months. Antimycobacterial therapy generally have no effect on the course [29], rather reassurance and regular follow up are needed.
The following are recommended clues for the initial assessment and subsequent evaluation with follow up visits:
-
1.
Detailed clinical history to rule out immunodeficiency.
-
2.
Growth and development assessment.
-
3.
Size and consistency of the lymph node(s).
-
4.
Symptoms or signs of dissemination (e.g. distant lymphadenopathy, hepatosplenomegaly, skin lesions etc.)
-
5.
Address family concerns and emphasize on importance to proceed with vaccination schedule (unless specific contraindications).
If the node(s) show no signs of regression and remained persistently enlarged for more than 6–9 months with a size of 3 cm or more, it is unlikely to regress spontaneously [18], [28], [30], [31], therefore surgical excision may be considered [16], [29], [31].
5.3. Management of suppurative lymphadenitis
5.3.1. Role of antimicrobial therapy
Antimycobacterial drugs cannot prevent suppuration nor shorten the duration of healing, therefore are not recommended [16], [20], [29], [32].
Antibiotic therapy is indicated for treatment of pyogenic lymphadenitis, as a result of secondary infection with pyogenic bacteria such as Streptococcus pyogenes or Staphylococcus aureus.
5.3.2. Needle aspiration
When suppurative BCG lymphadenitis left untreated, it will progress to spontaneous rupture/drainage and sinus formation. This leads to slow healing over several months and probable keloid formation. Moreover, it is associated with an unpleasant cosmetic outcome. In contrast, needle aspiration can shorten the duration of healing, and lessens the complications [8], [14], [16], [29], [30], [33], [34], [35], [36].
Sometimes repeated aspirations are required for optimal management. For ease of evacuation of thick inflammatory materials, a wider bore needles are preferred [35].
Needle aspiration is considered to be a safer option when compared with surgical excision, which likely will require general anesthesia in young infants. Incision and drainage should be avoided due to the risk of persistent draining wound, delayed wound healing and unpleasant cosmetic outcome with scar formation [14], [35], [36].
Generally it's not recommended to send the aspirated fluid for bacterial or mycobacterial examination unless expecting a pyogenic infection or suspecting an associated disseminated disease to help in antimicrobial selection.
5.3.3. Surgical excision
Surgical excision should be considered for children who failed needle aspiration or failed to respond to repeated needle aspirations more than twice, after balancing the risks of general anesthesia and potential surgical complications [14], [16], [33], [36].
5.4. Management of disseminated BCG infection
5.4.1. Immunology workup to rule out immunodeficiency and optimizing treatment
Health care provider should suspect immunodeficiency in all cases with disseminated disease, therefore pediatric immunology service should be consulted to rule out any underlying immunodeficiency disease (e.g. SCID, CGD and IFN-γ/IL-12 pathway defects) and to help in optimizing treatment of the underlying immunodeficiency status e.g. Hematopoietic stem cell transplantation (HSCT) in patients with SCID [28], [37], [38].
5.4.2. Antimycobacterial treatment
Antimycobacterial therapy should be started in consultation with pediatric infectious diseases specialists.
Which agents, number and duration of antimycoabcetrial drugs depends on the microbiological susceptibility testing, underlying immunodeficiency, degree of dissemination and clinical response. Generally a minimum of four medications are required, with duration of therapy vary from months to years depending on the response and underlying immune defects.
M. bovis are intrinsically resistant to pyrazinamide. BCG strains have different susceptibility patterns [39], an example is the Danish strain (SSI 1331), currently used in Saudi Arabia, have low-level resistance to isoniazid that may not be of clinical significance [39], [40] on the other hand it is resistant to ethionamide [39]. These differences should be taken into consideration when selecting empirical therapy
6. Conclusions
-
1)
Non suppuartive lymphadenitis is a clinical diagnosis that requires close observation and follow-up. Antimycobactrial therapy are not recommended while surgical excision may be considered if the node remained persistently enlarged more than 6–9 months with a size of 3 cm or more.
-
2)
Suppurative lymphadenitis is a clinical diagnosis that requires needle aspiration while incision and drainage is not recommended. Surgical excision is considered upon failed aspiration(s). Sending aspirated fluid for microbiological examination is generally not indicated unless expecting a disseminated or pyogenic infection.
-
3)
Disseminated BCG infection requires consultation with pediatric immunology and infectious diseases specialists for the optimum management.
-
4)
Based on available data on the BCG vaccine with a low efficacy, serious complications and the high incidence of primary immunodeficiency disorders among Saudi children, it is wise to consider delay the timing of BCG vaccination in our community.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Contributor Information
Nagi Elsidig, Email: nagiamin16@hotmail.com.
Dayel Alshahrani, Email: daalshahrani@kfmc.med.sa.
Mohammed Alshehri, Email: malshehri@kfmc.med.sa.
Sami Alhajjar, Email: hajjar@kfshrc.edu.sa.
Suliman Aljummah, Email: jumaah@kfshrc.edu.sa.
Ibrahim Bin Hussain, Email: dribrahim1382@gmail.com.
Mohammad Alshaalan, Email: shaalanm1@ngha.med.sa.
Fahad Alzamil, Email: fzamil@ksu.edu.sa.
Abdularahman Alodyani, Email: aalodyani@yahoo.com.
Fahad Aljobair, Email: fjobair@gmail.com.
References
- 1.Observed Rate of Vaccine Reactions, Bacille Calmette Guerin (BCG) Vaccine 2012. World Health Organization (WHO), Global Vaccine Safety, Immunization, Vaccines and Biologicals.
- 2.Mahmoud Refqi, Emam Fahmi. General Directorate of Health Affairs, Qassim Region, Public Health Administration; Qassim, Saudi Arabia: 2011. Childhood immunization program, surveillance report. [Google Scholar]
- 3.Andersen P., Doherty T.M. The success and failure of BCG – implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005 Aug;3(8):656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 4.Colditz G.A., Brewer T.F., Berkey C.S., Wilson M.E., Burdick E., Fineberg H.V. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 5.Expanding immunization coverage. Immunization, vaccines and biological. WHO/UNICEF. [Updated 2014; Accessed 2014 September 4]. Available from URL: http://www.unicef.org/immunization/index_coverage.html.
- 6.World Health Organization (WHO) estimates of tuberculosis incidence by country. 2013. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733758290 [Google Scholar]
- 7.Lotte A., Wasz-Höckert O., Poisson N., Dumitrescu N., Verron M., Couvet E. BCG complications. Adv Tuberc Res. 1984;21:107–193. [PubMed] [Google Scholar]
- 8.Suliman O.M., Ahmed M.J., Bilal J.A. Clinical characteristics and needle aspiration management of Bacillus Calmette-Guérin lymphadenitis in children. Saudi Med J. 2015 Mar;36(3):280–285. doi: 10.15537/smj.2015.3.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ru Sorense. Clinical presentation of tuberculosis in patient with immunodeficiency. Pediatr Infect Dis J. 1989;8:201–206. Annals of Pediatric Surgery, Vol 5, No 3, July 2009, PP 187–193. [PubMed] [Google Scholar]
- 10.Grange J.M. Complications of Bacille Calmette-Guérin (BCG) vaccination and immunotherapy and their management. Commun Dis Public Health. 1998;1:84–88. [PubMed] [Google Scholar]
- 11.Alrabiaah A.A., Alsubaie S.S., Bukhari E.I., Gad A., Alzamel F.A. Outbreak of Bacille Calmette-Guérin-related lymphadenitis in Saudi children at a university hospital after a change in the strain of vaccine. Ann Saudi Med. 2012 Jan-Feb;32(1):4–8. doi: 10.5144/0256-4947.2012.4. PubMed PMID: 22156633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Jumaah Suliman, Al Hajjar Sami, Mousab Hamoud Al. Bacille Calmette-Guérin vaccination in Saudi Arabia: benefits versus risks. Ann Saudi Med. 2012;32(1):1–3. doi: 10.5144/0256-4947.2012.1. PMID: 22156632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goraya J.S., Virdi V.S. Bacille Calmette-Guérin lymphadenitis. Postgrad Med J. 2002 Jun;78(920):327–329. doi: 10.1136/pmj.78.920.327. PMCID: PMC1742390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan W.M., Kwan Y.W., Leung C.W. Management of Bacillus Calmette-Guérin lymphadenitis. HK J Paediat. 2011;16:85–94. (new series) [Google Scholar]
- 15.Goraya J.S., Virdi V.S. Treatment of Calmette-Guérin bacillus adenitis: a metaanalysis. Pediatr Infect Dis J. 2001 Jun;20(6):632–634. doi: 10.1097/00006454-200106000-00020. PubMed PMID: 11419511. [DOI] [PubMed] [Google Scholar]
- 16.Sataynarayana S., Mathur A.D., Verma Y., Pradhan S., Bhandari M.K. Needle aspiration as a diagnostic tool and therapeutic modality in suppurative lymphadenitis following Bacillus Calmette Guerin vaccination. J Assoc Physicians India. 2002 Jun;50:788–791. PubMed PMID: 12240843. [PubMed] [Google Scholar]
- 17.Al-Salem A.H., Kothari M.R., AlHani H.M., Oquaish M.M., Khogeer S.S., Desouky M.S. Safety of intradermal Bacillus Calmette-Guerin vaccine for neonates in Eastern Saudi Arabia. Saudi Med J. 2012 Feb;33(2):172–176. PubMed PMID: 22327758. [PubMed] [Google Scholar]
- 18.Bukhari E., Alzahrani M., Alsubaie S., Alrabiaah A., Alzamil F. Bacillus Calmette-Guerin lymphadenitis: a 6-year experience in two Saudi hospitals. Indian J Pathol Microbiol. 2012 Apr-Jun;55(2):202–205. doi: 10.4103/0377-4929.97869. PubMed PMID: 2277164. [DOI] [PubMed] [Google Scholar]
- 19.Hesseling A.C., Rabie H., Marais B.J., Manders M., Lips M., Schaaf H.S. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;(42):548–558. doi: 10.1086/499953. pmid: 16421800. Infection. 1991 Nov-Dec; 19(6):414-6. [DOI] [PubMed] [Google Scholar]
- 20.Sohail Shagufta, Afzal Muhammad, Anwar Vaqas, Shama Quratulain. Disseminated Bacille calmette – guerin (BCG) disease in an infant with severe combined immunodeficiency: S259–S261. J Coll Physicians Surg Pak. 2014;24 [PubMed] [Google Scholar]
- 21.Galal N., Boutros J., Marsafy A., Kong X.F., Feinberg J., Casanova J.L. Mendelian susceptibility to mycobacterial disease in egyptian children. Mediterr J Hematol Infect Dis. 2012;4(1) doi: 10.4084/MJHID.2012.033. e2012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gantzer A., Neven B., Picard C., Brousse N., Lortholary O., Fraitag S. Severe cutaneous bacillus Calmette-Guérin infection in immunocompromised children: the relevance of skin biopsy. J Cutan Pathol. 2013;40(1):30–37. doi: 10.1111/cup.12048. [DOI] [PubMed] [Google Scholar]
- 23.Oyachi N., Obana K., Suzuki T., Kimura S., Chino K., Oyama T. Costal BCG osteomyelitis developing 1 year after BCG vaccination. Pediatr Int. 2013 Oct;55(5):641–643. doi: 10.1111/ped.12072. PubMed PMID: 24134752. [DOI] [PubMed] [Google Scholar]
- 24.Chan P.K., Ng B.K., Wong C.Y. Bacille Calmette-Guérin osteomyelitis of the proximal femur. Hong Kong Med J. 2010 Jun;16(3):223–226. PubMed PMID: 20519760. [PubMed] [Google Scholar]
- 25.Milstien J.B., Gibson J.J. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990;68:93–108. [PMC free article] [PubMed] [Google Scholar]
- 26.Hengster P., Schnapka J., Fille M., Menardi G. Occurrence of suppurative lymphadenitis after a change of BCG vaccine. Arch Dis Child. 1992;67:952–955. doi: 10.1136/adc.67.7.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladeira I., Carvalho I., Correia A., Carvalho A., Duarte R. BCGitis in children. Rev Port Pneumol. 2014;20(3):172–173. doi: 10.1016/j.rppneu.2013.12.003. Epub 2014/02/18. [DOI] [PubMed] [Google Scholar]
- 28.Ying W., Sun J., Liu D., Hui X., Yu Y., Wang J. Clinical characteristics and immunogenetics of BCGosis/BCGitis in Chinese children: a 6 year follow-up study. PloS One. 2014;9(4):e94485. doi: 10.1371/journal.pone.0094485. Epub 2014/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuello-García C.A., Pérez-Gaxiola G., Jiménez Gutiérrez C. Treating BCG-induced disease in children. Cochrane Database Syst Rev. 2013 Jan 31;1:CD008300. doi: 10.1002/14651858.CD008300.pub2. Review. PubMed PMID: 23440826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassana Rasha H., Abel-Elah Alyb Kamal, Kandila Shaimaa M., El Maysaa, Sayed Zaki Post-Bacillus Calmette-Gué rin lymphadenitis in Egyptian children: an outbreak. Ann Pediatr Surg. 2012;8:69–73. [Google Scholar]
- 31.Daei Parizi M., Kardoust Parizi A., Izadipour S. Evaluating clinical course of BCG lymphadenitis and factors affect on it during a 5-year period in Kerman, Iran. J Trop Pediatr. 2014;60(2):148–153. doi: 10.1093/tropej/fmt100. Epub 2013/12/12. [DOI] [PubMed] [Google Scholar]
- 32.Nazir Z., Qazi S.H. Bacillus Calmette-Guerin (BCG) lymphadenitis-changing trends and management. J Ayub Med Coll Abbottabad. 2005 Oct-Dec;17(4):16–18. PubMed PMID: 16599027. [PubMed] [Google Scholar]
- 33.Abdulhameed Fatma D., Hummaida Tarig I. Surgical management of BCG vaccine – induced regional lymph nodes adverse effects. Ann Pediatr Surg. July 2009;5(3):187–193. [Google Scholar]
- 34.Caglayan S., Arikan A., Yaprak I., Aksoz K., Kansoy S. Management of suppuration in regional lymph nodes secondary to BCG vaccination. Acta Paediatr Jpn. 1991 Dec;33(6):699–702. doi: 10.1111/j.1442-200x.1991.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 35.Juzi J.T., Sidler D., Moore S.W. Surgical management of BCG vaccine-induced regional axillary lymphadenitis in HIV-infected children. S Afr J Surg. 2008 May;46(2):52–55. PubMed PMID: 18686936. [PubMed] [Google Scholar]
- 36.Pal Subrata, Chakarabarti1 Srabani, Prakash Phukan Jyoti, Biswas Sudhanya, Sinha Anuradha, Sinha Rajani. Role of needle aspiration in diagnosis and management of suppurative Bacille Calmette–Guerin adenitis: an institutional study of 30 cases. J Laboratory Physicians. Jan-Jun 2015;7(1) doi: 10.4103/0974-2727.154782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roesler J., Horwitz M.E., Picard C., Bordigoni P., Davies G., Koscielniak E. Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr. 2004;145(6):806–812. doi: 10.1016/j.jpeds.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Shahmohammadi Soheila, Mohammad J.S., Mohammad S.R. BCG-osis after BCG vaccination in immunocompromised children: case series and review. J Pediatr Rev. 2014;2(1):47–54. [Google Scholar]
- 39.Ritz Nicole, Tebruegge Marc, Connell Tom G., Sievers Aina, Robins-Browne Roy, Curtis Nigel. Susceptibility of mycobacterium bovis BCG vaccine strains to antituberculous antibiotics. Antimicrob Agents And Chemother. Jan. 2009:316–318. doi: 10.1128/AAC.01302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Description of BCG VACCINE SSI, www.ssi.dk.