Abstract
Obesity is generally considered an adult disease, although there has been a constant increase in the prevalence of overweight and obese children in the last few decades. Childhood obesity is not limited to developed countries, with increasing numbers being reported from developing countries as well as from Saudi Arabia. Young populations with obesity suffer from similar comorbidities as obese adults, including type 2 diabetes mellitus, dyslipidemia, obstructive sleep apnea, polycystic ovarian syndrome, pseudotumor cerebri, and fatty liver disease. Recent advances in weight loss surgery have given hope to obese adolescents who are refractory to lifestyle changes and low-calorie diet plans. This review emphasizes a holistic approach for obese adolescents and describes in detail a multidisciplinary team and their role in adolescent bariatric surgery. There are unique medical, psychological, and nutritional requirements during the pre-operative, immediate post-operative, and long-term phases to achieve a desirable outcome. Identification of an appropriate candidate for bariatric surgery is critical and must balance the risks and benefits of weight loss surgery. Different surgical procedures are available and should be tailored to the needs of the patient and the expertise of the surgeon.
Keywords: Adolescent, Obesity, Bariatric surgery
1. Introduction
The prevalence of obesity has been rising in children and adolescents in recent decades, and a sustained increase in severe obesity has been observed at a young age. Nearly 2% of children and adolescents are morbidly obese in the United States [1]. Young age obesity is multifactorial and can result due to genetic, environmental and social factors, and energy imbalance [2]. Weight reduction plans with lifestyle changes including a healthy and low-calorie diet, exercise and specific counseling for pediatric obese patients has modest results with minimal sustainability [3], [4]. Obesity-related comorbidities were once only considered to be associated with the adult obese population, but the adolescent obese population has exhibited an increase in the occurrence of type 2 diabetes mellitus, dyslipidemia, obstructive sleep apnea, polycystic ovarian syndrome, pseudotumor cerebri, and fatty liver disease [5]. In addition to medical comorbidities, youths with obesity are also at risk for psychiatric disorders such as depression, anxiety, and low self-esteem [6], and the probability of becoming an obese adult is much higher for obese children than for normal weight children. A body mass index (BMI) above the 99th percentile in children is strongly linked to a BMI above 30 kg/m2 as an adult [7]. Comorbidities and mortality are higher in adults who were obese adolescents compared to those who become obese as adults [8]. These factors have resulted in increased bariatric surgery in adolescents [9].
2. Epidemiology and definitions
The most common way to measure the body fat is to calculate the body mass index (BMI) because direct measurement of fat is difficult. BMI is not always a true measure of body fat, as athletes with significant muscle mass can have high BMIs but a very low body weight fat percentage. However, body fat content can be reliably predicted with BMI in children and adolescents [10]. Adult BMI classifies a body fat content of 25 kg/m2 and above as overweight; 30 kg/m2 and above as obese; 40 kg/m2 and above as morbidly obese; and 50 kg/m2 and above as super obese [11]. The Centers for Disease Control and Prevention provide BMI centile charts accounting for age, gender, and growth pattern. Children with BMI growth curves between 85th–94th percentiles are defined as overweight, and those in or above the 95th percentile are obese [11]. More severe obese adolescents are defined by an expert committee of the American Academy of Pediatrics, which states a BMI of 30–32 for 10–12 year old and a BMI of 34 for 14–16 year old as the 99th percentile, and any value above the 99th is defined as extreme obesity [12].
The global prevalence of childhood obesity was estimated in 2010, and 43 million children (35 million in developing countries) were estimated to be overweight and obese, with 92 million at risk of being overweight [13]. The National Health and Nutrition Examination Survey reported US data from 2009 to 2010 stating 31.8% of children age 2–19 had BMI above 85th percentile, 16.9% were above the 95th percentile, and 12.3% were above the 97th percentile [14].
The prevalence of childhood obesity in Saudi Arabia has been increasing in the last 2 decades, and the reported prevalence falls between that of developed and developing countries. A cross sectional national epidemiological survey of 12,701 children (boys 6281; girls 6420) was published in 2002, which reported that 10.7% of boys and 12.7% of girls were overweight, and 6.0% of boys and 6.74% of girls were obese [15]. One study published in 2007 compared the two data sets from 1988 to 2005 to analyze the trend of body fat and obesity in primary school boys [16]. A significant rise in body mass index (16.5 ± 2.1 to 18.0 ± 4.0 kg/m2; P < .005) was observed over this period. Another study in 2005 examined the frequency of overweight, obesity and severe obesity in Saudi children aged 5–18 [17] using the World Health Organization (WHO) 2007 reference to define overweight, obese, and severely obese children. There were 19,317 healthy children, of which 50.8% were boys. The prevalence of overweight, obesity, and severe obesity in different age groups was 23.1%, 9.3% and 2%, respectively.
3. Comorbid conditions with obesity
There is increasing evidence that pediatric obesity is associated with substantial medical and psychological comorbidity, similar to adults.
Type 2 diabetes mellitus (T2DM): Type 2 diabetes mellitus is linked to obesity, and as the prevalence of obesity rises, the incidence of type 2 diabetes mellitus has also increased in pediatric populations [18]. Children with T2DM are at higher risk for developing obesity-related problems early in life, including hypertension, dyslipidemia, fatty liver disease, and atherosclerosis [19].
Obstructive sleep apnea: Sleep disorders with symptoms of snoring, hypopnea, and apnea are highly associated with childhood obesity. Obstructive sleep apnea can cause variable degrees of fatigue, poor academic performance, hypertension, and ventricular dysfunction [20].
Non-alcoholic steatohepatitis: The incidence of non-alcoholic fatty liver disease is much higher in obese children compared to lean children. Of obese children, 38% exhibit steatosis compared to 5% of lean children, and non-alcoholic steatohepatitis is seen 9% in obese children and 1% of lean children [21].
Metabolic syndrome: Metabolic syndrome, including high waist circumference, dyslipidemia, hyperinsulinism, elevated inflammatory markers, and the presence of hypertension with ventricular hypertrophy has been reported in young obese adults. These factors are strong predictors of long-term cardiovascular morbidity [22].
Benign intracranial hypertension: Benign intracranial hypertension or pseudotumor cerebri is associated with high intracranial pressure without a mass lesion, has been associated with obesity and is resolved with weight loss management [23].
Quality of life: Obesity in adolescents has significant negative impact on quality of life with psychological consequences [24], [25]. One study of obese adolescents reported 53% suffer mild depression, 30% self-reported clinically significant depression, and 45% are clinically depressed based on a report from the mother [26].
A review of the published literature and best practice guidelines indicated reduction or resolution of these medical and psychological comorbidities after weight reduction from surgical treatment [27], [28], [29].
4. Multidisciplinary team development
The selection and preparation of patients for weight loss surgery requires a well-qualified and coordinated team of professionals. The development of a multidisciplinary team (MDT) and a clinical pathway has generated positive and sustained outcomes in children and adolescents undergoing bariatric surgery [30]. MDT and periodic rounds for each case provide an opportunity for holistic and patient family-based approach for selection as well as pre-surgical and post-surgical management [31].
An ideal MDT should contain four to five members (Table 1): a trained pediatric bariatrician or pediatric specialist with an interest and experience in pediatric obesity, an adult or pediatric surgeon with bariatric surgery and adolescent care experience, a certified pediatric dietician to address the pre and post-surgical diet plan, a pediatric psychologist with expertise in adolescent and family treatment and experience treating eating disorders, and a coordinator who is a registered nurse or social worker to maintain coordination in the MDT and between the patient/family and the health professionals with an aim of good compliance and follow up [28]. Other desirable members include a hepatologist with expertise in fatty liver disease, a pediatric endocrinologist with experience in diabetes and dyslipidemia, and an exercise physiologist [31].
Table 1.
Adolescent bariatric surgery multidisciplinary team.
| Mandatory members |
|
| Desirable members |
|
5. Patient selection (suitability) criteria
Careful and vigilant selection of candidates for bariatric surgery is vital for positive outcomes and the post-surgical well-being of the patient. Once a candidate fulfills the selection criteria, it is critical for the team of health professionals to prepare the patient for surgery and post-surgical care and to realistically explain weight reduction targets.
Published best practice guidelines, position statements, and clinical pathways based on emerging evidence of favorable bariatric surgery results in pediatric age groups have established criteria for child and adolescent bariatric surgery very similar to the adult criterion [30], [32], [33]. These criteria are as follows (Table 2):
-
•
Body mass index: A BMI higher than 35 kg/m2 in the presence of serious comorbid conditions such as type 2 diabetes mellitus, obstructive sleep apnea (AHI ≥ 15), pseudotumor cerebri, or non-alcoholic steatohepatitis. A BMI above 40 kg/m2 with mild comorbid conditions, such as mild OSA (AHI ≥ 5), insulin resistance, impaired fasting glucose, hypertension, dyslipidemia, and impaired quality of life.
-
•
Failed weight reduction: Candidate has registered for a well-organized weight reduction program with a dieticianpost, a behavioral, and physical therapist for 6 months, but it has failed to show a healthy reduction in his/her weight.
-
•
Tanner stage and skeletal maturity: Candidates should have reached a pubertal maturity of Tanner stage IV–V and achieved more than 95% of their adult height based on radiographic evidence.
-
•
Understanding lifestyle changes: Good candidates show comprehension and commitment to lifestyle changes, including the dietary habits required postoperatively to avoid complications and sustain weight loss.
-
•
Psychosocial compatibility: Various psychosocial aspects must be evaluated before a final decision can be made. The patient and family should exhibit mature decision making abilities, understand the risk and benefits of surgery, and have realistic weight loss expectations. The existence of strong family support and other social supports such as close friendships and a minimization of social isolation support a better outcome. The patient and family should demonstrate compliance with the pre-operative plan and motivation for the post-operative lifestyle. Any psychotic disorders require treatment and must be under control.
-
•
Contraindications: Candidates should be excluded if they exhibit correctable medical causes of obesity, substance abuse, medical or psychological disabilities that would impair compliance with the post-operative care plan and pregnancy or planning pregnancy 12–18 months after the surgery [20].
Table 2.
Adolescent bariatric surgery eligibility criteria.
| Candidates for adolescent bariatric surgery |
|
| Eligibility criteria |
|
| Contraindications |
|
BMI, body mass index; T2DM, type 2 diabetes mellitus; OSA, obstructive sleep apnea; BIH, benign intracranial hypertension; NASH, non-alcoholic steatohepatitis.
6. Pre-surgical assessment (preparation) for bariatric surgery
Every child/adolescent candidate for bariatric surgery should receive a detailed assessment, preparation, and counseling from each member of above mentioned multidisciplinary team.
-
•
Medical assessment: The pediatrician considered to be the MDT leader should perform the initial assessment while considering the requirements and complications of bariatric surgery and is obliged to address the comorbid conditions associated with obesity [31]. The burden of disease is determined with a full medical assessment and diagnostic work up (Table 3). A routine work up includes a complete blood count, renal and liver profile, fasting blood glucose and insulin, glycated hemoglobin (HbA1c), lipid profile, Vitamin D, parathyroid hormone, thyroid function test, ACTH, cortisol, blood group, and coagulation profile. Other micronutrients such as Vitamin B1, B6, B12, folate, zinc, magnesium, copper, and iron might also be screened.
Table 3.
Adolescent bariatric surgery medical screening.
| Laboratory tests |
|
| Radiological work up |
|
| Sleep study | Polysomnography |
The radiological work up includes abdominal ultrasound to screen for gallstones, and a pelvic ultrasound might be performed on girls above 12 years age. Skeletal maturity should be assessed with bone age using a left wrist X-ray. The bone mineral density measurement with dual-energy X-ray absorptiometric scan is important because of the high risk of reduced bone density after bariatric surgery.
Polysomnography is indicated if the history is suggestive of sleep apnea. Genetic studies can also be considered if the clinical examination is indicative of any syndrome or monogenic obesity [30], [34]. Prophylaxis for deep venous thrombosis with pharmacologic therapy and mechanical compression stockings should be advised if there is high risk for post-operative DVT [35].
-
•
Psychological assessment: The psychologist has a vital role preparing the patient for surgery. He/she identifies the strengths and barriers that will help achieve a positive surgical outcome. Multiple sessions and interviews should explore the patient, family, and their surroundings to create a holistic approach resolving psychosocial issues (Table 4).
Table 4.
Psychological and nutritional assessment domains for adolescent bariatric surgery.
| Psychological assessment |
|
| Nutritional assessment |
|
The psychological assessment consists of three components. 1. Evaluation of current family environment and stressors. 2. Evaluation of emotional maturity, cognitive function and comprehension of the surgery, and relevant recommendations. 3. Counseling for perioperative dietary and psychosocial changes [31].
Psychiatric disorders such as depression, low self-esteem, and anxiety are higher in obese youth [6]. Depression is reported to be 3–4 times higher in obese young candidates for bariatric surgery [36]. Overweight adolescents are also at higher risk for body image disorders [37]. The psychological assessment provides an opportunity to treat these functional disorders and also to provide behavioral therapy if any substance abuse has been documented by the patient or caregivers [38].
Special attention should be given to specific aspects related to weight management and surgery. These include [38]:
-
1.
Adherence to the treatment of medical comorbid conditions.
-
2.
Knowledge and expectations of bariatric surgery in terms of changes in lifestyle and magnitude of weight loss.
-
3.
History of dietary- and activity-based weight loss attempts.
-
4.
Eating habits and disorders such as binge eating and bulimia nervosa.
-
5.
Barriers to activity habits including financial restrains or lack of time.
-
6.
Motivation to change after surgery.
-
•
Nutritional assessment: Every child undergoing bariatric surgery and his/her family needs nutritional counseling and education for pre-operative weight loss and screening for micronutrient deficiencies, an immediate post-operative diet progression plan, and a long term post-operative diet and supplementation plan with special attention to bone health (Table 4) [39].
Nutritional management should begin 6 months prior to surgery with progressive family base structured meal plans. It is crucial that the family provide an encouraging and supportive environment that adheres to dietary recommendations. The restriction of calories to 1000 kcal with a carbohydrate-controlled liquid diet 10 days prior to surgery will deplete hepatic glycogen and shrink liver size, facilitating the surgical approach [31].
Obese adolescents might exhibit micronutrient deficiencies even prior to surgery due to unbalanced diet and poor food choices. It is advised to perform screening and supplementation if necessary for Vitamin B1, B12, iron, folate, and Vitamin D [39].
The post-operative diet progression plan varies with each surgical procedure, and it is critical to follow the recommended plan to avoid surgical complications. Centers offering bariatric surgery should have a written protocol in place. Patient understanding of the plan in advance can avoid psychological stress and improve the compliance [39].
Macronutrients, water, and proteins are commonly deficient after surgery. Dehydration can result due to restricted intake, diarrhea secondary to dumping syndrome or if concentrated forms of sugars (honey, jelly, or candy) are ingested. Young girls have a daily recommended intake of 2.3–2.7 L of water, and 3.3–3.7 L are recommended for boys [40]. Protein deficiency is more common in malabsorptive procedures such as biliopancreatic diversion, but it has also been observed in pure restrictive procedures as well. The adult recommendation for protein intake is 60–90 g per day for restrictive surgery and 80–120 g per day for malabsorptive procedures [41].
Micronutrient deficiencies are common after surgery and include both water- and fat-soluble vitamins. Regular screening (every 3 months to yearly) and supplementation of multivitamins with Vitamin B1, B6, B12, folate, iron, and Vitamin D is mandatory. The most common deficiencies are Vitamin D and iron. Iron and folate replacement is particularly important in young menstruating girls whose chances of pregnancy improve with weight loss [39].
Bone mineral density is also reduced after surgery due to the decrease in BMI, but low Vitamin D and high parathyroid hormone levels might further deteriorate bone health. In adults, Vitamin D replacement does not fully restore bone density, suggesting a role for other hormones such as ghrelin [42]. The minimum recommended dose of calcium is 1300 mg/day and Vitamin D is 600–2000 IU [43].
-
•
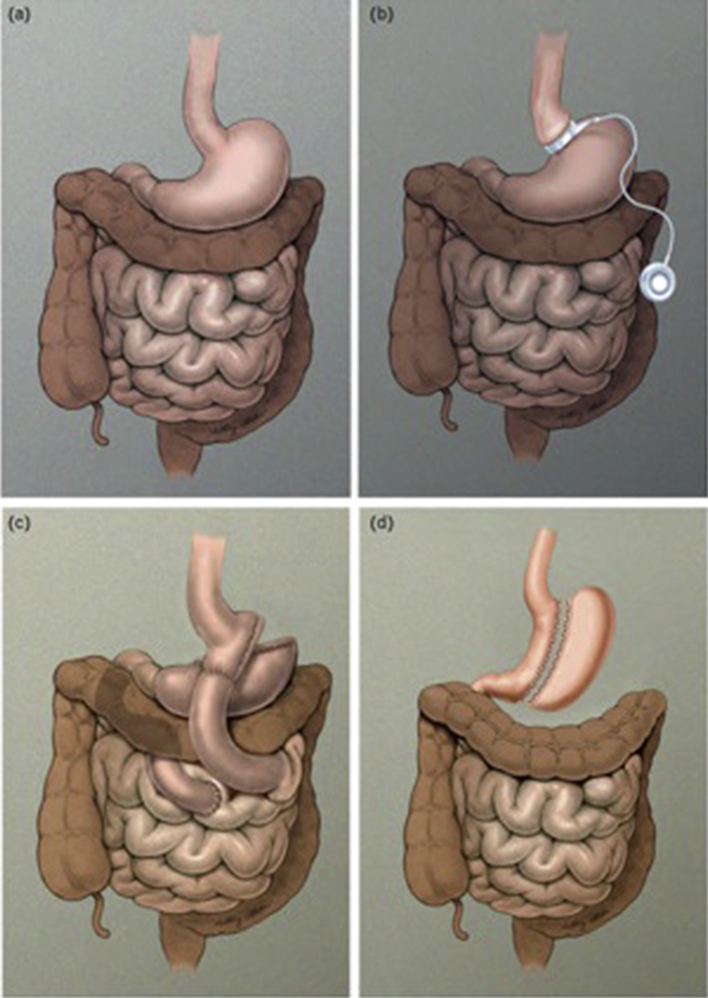
Surgical assessment and choice of procedure: The surgeon and the pediatric bariatrician make the final decision concerning surgery. He/she would choose one of the recommended procedures for adolescents according to his/her level of expertise and skill [31]. Surgical procedures (Fig. 1) are classified as purely restrictive, purely malabsorptive, or mixed. Malabsorptive procedures including duodenal switch and biliopancreatic diversion are not recommended in the pediatric age group because of complications and severe malabsorption [44].
Figure 1.
(a) Normal gastrointestinal anatomy, (b) adjustable gastric band (AGB), (c) Roux-en-Y gastric bypass, and (d) vertical sleeve gastrectomy.
Adjustable gastric banding is a reversible restrictive procedure, but it is not approved in the United States in patients under the age of 18 by The Food and Drug Administration (FDA) [27]. Laparoscopic sleeve gastrectomy is a primarily restrictive procedure, but it also helps reduce appetite because the ghrelin-producing segment of stomach is removed [45]. Sleeve gastrectomy is an irreversible procedure that has been shown to be safe and effective in adolescents [46], [47]. Roux-en-Y gastric bypass (RYGB) is an irreversible but mixed restrictive and malabsorptive procedure, and it is the first technique reported to be performed on adolescents in the 1970s [48]. This is most widely used procedure in adults and children. Meta-analysis for adolescents receiving RYGB has shown good safety and efficacy [49].
-
•
Consent: The risks and benefits associated with bariatric surgery must be explained to the patient and family separately to avoid coercion. Informed assent from the patient should be obtained with an evaluation of his/her understanding of the risks, benefits, and follow-up plan. The parents' consent should include a detailed discussion of medical and surgical treatment options. Every effort should be made to avoid any subtle or overt coercion [28].
7. Summary
Obesity prevalence is rising in pediatric populations. Bariatric surgery has shown promising results in terms of safety and efficacy, and there is a need to increase awareness among primary healthcare physicians to overcome the reluctance and barriers to bariatric surgery for the pediatric age group. A multidisciplinary approach, institutional protocols, and pathways can help patients and improve outcomes. Adolescents undergoing weight loss surgery require a smooth transition program in place.
Ethical clearance
Ethical clearance is not required for this review article.
Conflict of interest
None to be declared by the authors.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Skinner A., Skelton J.A. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 2.Inge T.H., Krebs N.F., Garcia V.F., Skelton J.A., Guice K.S., Strauss R.S. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Danielsson P., Kowalski J., Ekblom O., Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 4.Ebbeling C.B., Pawlak D.B., Ludwig D.S. Childhood obesity: public health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 5.Inge T.H., Miyano G., Bean J., Helmrath M., Courcoulas A., Harmon C.M. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–222. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 6.Zametkin A.J., Zoon C.K., Klein H.W., Munson S. Psychiatric aspects of child and adolescent obesity: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43:134–150. doi: 10.1097/00004583-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Freedman D.S., Mei Z., Srinivasan S.R., Berenson G.S., Dietz W.H. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150 doi: 10.1016/j.jpeds.2006.08.042. 12–17.e2. [DOI] [PubMed] [Google Scholar]
- 8.Vanhala M., Vanhala P., Kumpusalo E., Halonen P., Takala J. Relation between obesity from childhood to adulthood and the metabolic syndrome: population based study. BMJ. 1998;317:319. doi: 10.1136/bmj.317.7154.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelleher D.C., Merrill C.T., CottrellL T., Nadler E.P., Burd R.S. Recent national trends in the use of adolescent inpatient bariatric surgery: 2000 through 2009. Arch Pediatr Adolesc Med. 2012:1–7. doi: 10.1001/2013.jamapediatrics.286. [DOI] [PubMed] [Google Scholar]
- 10.Daniels S.R., Khoury P.R., Morrison J.A. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99(6):804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 11.Han T.S., Sattar N., Lean M. ABC of obesity. Assessment of obesity and its clinical implications. BMJ. 2006;333(7570):695–698. doi: 10.1136/bmj.333.7570.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow S.E., Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 13.de Onis Mercedes, Blössner Monika, Borghi Elaine. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 14.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. J Am Med Assoc. 2012;307(5):483–490Pubmed. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Hazmi Mohsen A.F., Warsy Arjumand S. The prevalence of obesity and overweight in 1-18 year old Saudi children. Ann Saudi Med. 2002;22(5–6):303–307. doi: 10.5144/0256-4947.2002.303. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hazzaa Hazzaa M. Prevalence and trends in obesity among school boys in central Saudi Arabia between 1988 and 2005. Saudi Med J. 2007;28(10):1569–1574. [PubMed] [Google Scholar]
- 17.El Mouzan Mohammad I., Foster Peter J., Al Herbish Abdullah S., Al Salloum Abdullah A., Al Omer Ahmad A., Qurachi Mansour M. Prevalence of overweight and obesity in Saudi children and adolescents. Ann Saudi Med. 2010 May-Jun;30(3):203–208. doi: 10.4103/0256-4947.62833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagot-Campagna A., Pettitt D.J., Engelgau M.M., Burrows N.R., Geiss L.S., Valdez R. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 19.Pinhas-Hamiel O., Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 20.Brandt M.L., Harmon C.M., Helmrath M.A., Inge T.H., McKay S.V., Michalsky M.P. Morbid obesity in pediatric diabetes mellitus: surgical options and outcomes. Nat Rev Endocrinol. 2010;6:637–645. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- 21.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 22.Morrison J.A., Friedman L.A., Harlan W.R., Harlan L.C., Barton B.A., Schreiber G.B. Development of the metabolic syndrome in black and white adolescent girls: a longitudinal assessment. Pediatrics. 2005;116:1178–1182. doi: 10.1542/peds.2004-2358. [DOI] [PubMed] [Google Scholar]
- 23.Chandra V., Dutta S., Albanese C.T., Shepard E., Farrales-Nguyen S., Morton J. Clinical resolution of severely symptomatic pseudotumor cerebri after gastric bypass in an adolescent. Surg Obes Relat Dis. 2007;3:198–200. doi: 10.1016/j.soard.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Fallon E.M., Tanofsky-Kraff M., Norman A.C., McDuffie J.R., Taylor E.D., Cohen M.L. Health-related quality of life in overweight and nonoverweight black and white adolescents. J Pediatr. 2005;147:443–450. doi: 10.1016/j.jpeds.2005.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss R.S., Pollack H.A. Social marginalization of overweight children. Arch Pediatr Adolesc Med. 2003;157:746–752. doi: 10.1001/archpedi.157.8.746. [DOI] [PubMed] [Google Scholar]
- 26.Zeller M.H., Saelens B.E., Roehrig H., Kirk S., Daniels S.R. Psychological adjustment of obese youth presenting for weight management treatment. Obes Res. 2004;12:1576–1586. doi: 10.1038/oby.2004.197. [DOI] [PubMed] [Google Scholar]
- 27.Thakkar R.K., Michalsky M.P. Update on bariatric surgery in adolescence. Curr Opin Pediatr. 2015 Jun;27(3):370–376. doi: 10.1097/MOP.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 28.Pratt Janey S.A., Lenders Carine M., Dionne Emily A., Hoppin Alison G., Hsu George L.K., Inge Thomas H. Best Practice updates for pediatric/adolescent weight loss surgery. Obesity. 2009;17:901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefater M.A., Jenkins T., Inge T.H. Bariatric surgery for adolescents. Pediatr Diabetes. 2013;14:1–12. doi: 10.1111/j.1399-5448.2012.00899.x. [DOI] [PubMed] [Google Scholar]
- 30.Alqahtani Aayed R., Elahmedi Mohamed O. Pediatric bariatric surgery: the clinical pathway. Obes Surg. 2015;25:910–921. doi: 10.1007/s11695-015-1586-x. [DOI] [PubMed] [Google Scholar]
- 31.Wulkan Mark L., Walsh Stephanie M. The multi-disciplinary approach to adolescent bariatric surgery. Semin Pediatr Surg. 2014;23:2–4. doi: 10.1053/j.sempedsurg.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Pratt J.S., Lenders C.M., Dionne E.A., Hoppin A.G., Hsu G.L., Inge T.H. Best practice updates for pediatric/adolescent weight loss surgery. Obes (Silver Spring) 2009;17:901–910. doi: 10.1038/oby.2008.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michalsky M., Reichard K., Inge T., American Society for Metabolic and Bariatric Surgery ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8:1–7. doi: 10.1016/j.soard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Hsia Daniel S., Fallon Sara C., Brandt Mary L. Adolescent bariatric surgery. Arch Pediatr Adolesc Med. 2012;166(8):757–766. doi: 10.1001/archpediatrics.2012.1011. [DOI] [PubMed] [Google Scholar]
- 35.Lawson M.L., Kirk S., Mitchell T., Chen M.K., Loux T.J., Daniels S.R., Pediatric Bariatric Study Group One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41(1):137–143. doi: 10.1016/j.jpedsurg.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Kilpatrick D.G., Ruggiero K.J., Aciemo R., Saunders B.E., Resnick H.S., Best C.L. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the national survey of adolescents. J Consult Clin Psychol. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 37.Tanofsky-Kraff M., Yanovski S.Z., Wilfley D.E., Marmaroush C., Morgan C.M., Yanovski J.A. Eating disordered behaviors, body fat, and psycho pathology in overweight and normal-weight children. J Consult Clin Psychol. 2004;72:53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin Heather, Smith Kevin, Ward Wendy L. Psychological assessment of the adolescent bariatric surgery candidate. Surg Obes Relat Dis. 2013;9:474–481. doi: 10.1016/j.soard.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Fullmer M.A., Abrams S.H., Hrovat K., Mooney L., Scheimann A.O., Hillman J.B. Nutritional strategy for adolescents undergoing bariatric surgery: report of a working group of the Nutrition Committee of NASPGHAN/NACHRI. J Pediatr Gastroenterol Nutr. 2012;54:125–135. doi: 10.1097/MPG.0b013e318231db79. [DOI] [PubMed] [Google Scholar]
- 40.US Department of Agriculture, National agricultural library. Dietary guidance. DRI tables. http://fnic.nal.usda.gov/nal_display/index.php?info_center1/44&tax_level1/43&tax_subject1/4256&topic_id1/41342&level3_id1/45140.
- 41.Mechanick J.I., Kushner R.F., Sugerman H.J., Gonzalez-Campoy J.M., Collazo-Clavell M.L., Spitz A.F. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obes (Silver Spring) 2009;17(Suppl. 1):S1–S70. doi: 10.1038/oby.2009.28. [DOI] [PubMed] [Google Scholar]
- 42.Pacifico L., Anania C., Poggiogalle E., Osborn J.F., Prossomariti G., Martino F. Relationships of acylated and des-acyl ghrelin levels to bone mineralization in obese children and adolescents. Bone. 2009;45:274–279. doi: 10.1016/j.bone.2009.04.204. [DOI] [PubMed] [Google Scholar]
- 43.Misra M., Pacaud D., Petryk A., Collett-Solberg P.F., Kappy M., Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 44.Marceau P., Marceau S., Biron S., Hould F.S., Lebel S., Lescelleur O. Long-term experience with duodenal switch in adolescents. Obes Surg. 2010;20:1609–1616. doi: 10.1007/s11695-010-0262-4. [DOI] [PubMed] [Google Scholar]
- 45.Frezza E.E., Chiriva-Internati M., Wachtel M.S. Analysis of the results of sleeve gastrectomy for morbid obesity and the role of ghrelin. Surg Today. 2008;38(6):481–483. doi: 10.1007/s00595-007-3648-8. [DOI] [PubMed] [Google Scholar]
- 46.Boza C., Viscido G., Salinas J., Crovari F., Funke R., Perez G. Laparoscopic sleeve gastrectomy in obese adolescents: results in 51 patients. Surg Obes Relat Dis. 2012;8:133–137. doi: 10.1016/j.soard.2011.11.021. discussion 137–139. [DOI] [PubMed] [Google Scholar]
- 47.Alqahtani A.R., Antonisamy B., Alamri H., Elahmedi M., Zimmerman V.A. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg. 2012;256:266–273. doi: 10.1097/SLA.0b013e318251e92b. [DOI] [PubMed] [Google Scholar]
- 48.Inge T.H., Zeller M.H., Lawson M.L., Daniels S.R. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr. 2005;147:10–19. doi: 10.1016/j.jpeds.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Treadwell J.R., Sun F., Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248(5):763–776. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]