Abstract
Background and Objectives
This case-control study assessed the prevalence of sleep disorders among Egyptian children with chronic kidney disease (CKD), either maintained or not maintained on hemodialysis (HD), and compared them with healthy age and sex-matched children.
Patients and methods
The total study population included 95 children, 54 of whom were CKD patients, 22 maintained on HD and 32 not maintained on HD; 41 healthy children of matched age and sex composed the control group.
Subjective impairment of sleep quality was assessed using the Arabic version of the Children's Sleep Habits Questionnaire (CSHQ). Daytime sleepiness and restless leg syndrome (RLS) were assessed using a pediatric modification of the Epworth sleepiness scale (ESS) and RLS Questionnaire, respectively.
Results
Sleep disturbances were detected in 75.9% of the studied children with CKD: 81.8% in children with CKD undergoing dialysis, and 71.8% in children with CKD not on dialysis. Excessive daytime sleepiness (EDS) and RLS symptoms were reported in 22% and 20.4% of the studied children with CKD, respectively.
Conclusions
Sleep disturbances are very common among children with CKD. Sleep disturbances in patients with CKD include restless legs syndrome (RLS), excessive daytime sleepiness (EDS), sleep-disordered breathing (SDB), behavioral insomnias, and parasomnias.
Keywords: CKD, Hemodialysis, Children, Sleep disorders, Restless-leg syndrome
Abbreviations: CKD, chronic kidney disease; HD, Hemodialysis; eGFR, estimated glomerular filtration rate; CSHQ, Children's Sleep Habits Questionnaire; ESS, Epworth sleepiness scale; EDS, Excessive daytime sleepiness; RLS, restless leg syndrome; BMI, body mass index; ESRD, End stage renal disease; Hb, Hemoglobin; Ca, Total serum calcium; P, Serum phosphorus; SDB, sleep-disordered breathing
1. Introduction
An adequate amount and quality of sleep are essential for normal growth, development, and overall health of children. Disturbed sleep can adversely affect a child's daytime function, resulting in behavioral and emotional problems as well as decrements in daytime alertness and cognitive performance [1].
Sleep disorders are important but often overlooked health problem in children with chronic kidney disease (CKD) [2]. To date, a small number of studies have focused on sleep disturbances in children with CKD. Additionally, the underlying mechanisms of sleep problems in CKD remain unclear. The aim of this study was to assess the prevalence and risk factors of sleep disorders among Egyptian children with chronic kidney disease (CKD), either maintained or not maintained on hemodialysis (HD), and to compare them with healthy children.
2. Patients and methods
The study was approved by the Institutional Review Board of Faculty of Medicine, Tanta University, and informed consent was obtained from all participants prior to inclusion.
The total study population included 95 patients, 54 of whom were CKD patients; 22 patients were maintained on HD, and 32 patients were not maintained on HD. Another 41 healthy children of matched age and sex served as the control group. Participants were recruited from the outpatient nephrology clinic and hemodialysis unit in the Department of Pediatrics, Faculty of Medicine, Tanta University Hospital, AlGharbia, Egypt from June 2014 to September 2015.
Children younger than 6 years of age were excluded, as they could not reliably comprehend the questionnaire about RLS. Children on sedative or hypnotic medications or suffering from any other chronic illness were also excluded.
The stages of CKD were determined based on the eGFR using the Schwartz formula [3].
National Kidney Foundation Disease Outcomes Quality Initiative (K/DOQI) CKD stages: [4]
-
1.
Stage 1: eGFR ≥ 90 mL/min/1.73 m2
-
2.
Stage 2: eGFR = 60–89 mL/min/1.73 m2
-
3.
Stage 3: eGFR = 30–59 mL/min/1.73 m2
-
4.
Stage 4: eGFR = 15–29 mL/min/1.73 m2
-
5.
Stage 5: eGFR < 15 mL/min/1.73 m2, or receiving dialysis.
Subjective impairment of sleep quality was assessed using the Children's Sleep Habits Questionnaire (CSHQ) [5]. The children's mothers completed the Arabic version of the CSHQ [6]. The CSHQ was translated, modified, and validated for this population and culture. It is a retrospective, 33-item parent questionnaire grouped into eight subscales reflecting the following sleep domains: bedtime resistance (5 items), sleep onset delay (1 item), sleep duration (3 items), sleep anxiety (3 items), night waking (3 items), parasomnias (7 items), sleep-disordered breathing (3 items), and daytime sleepiness (8 items) [7].
The total sleep disturbance score included all of the items from the eight subscales; the parents were asked to recall sleep behaviors occurring over a “typical” recent week. Items were rated on a three-point scale: “usually” if the sleep behavior occurred 5–7 times/week, “sometimes” 2–4 times/week, and “rarely” for zero to one time/week. Each question was scored from 1 to 3, (one being rarely, 2 being sometimes, and 3 being usually) and then summed to create a total sleep score (with the total ranging from 33 to 99). Some items were reversed to make a higher score indicative of more disturbed sleep. Higher scores indicated a poorer quality of sleep. A total sleep score of 41 on the CSHQ is reported as the clinical cutoff for the identification of probable sleep problems [5].
A pediatric modification of the Epworth sleepiness scale (ESS) was used to assess daytime sleepiness, in which subjects were asked to rate on a scale of 0–3, how likely they would be to fall asleep in eight situations [8]. Excessive daytime sleepiness (EDS) was defined as an ESS score ≥ 11.
The restless leg syndrome (RLS) Questionnaire, derived from the standard criteria for the diagnosis of RLS in children and adolescents [9], was administered to determine the prevalence of RLS. Patients were given a positive diagnosis of RLS if they had a positive response to four standard questions: (1) an urge to move due to uncomfortable sensations in the legs, (2) uncomfortable sensations that are relieved by movement, (3) symptoms worsen during rest or inactivity, and (4) symptoms worsen in the evening. The questionnaire was filled out by the parent in the presence of the child for children aged 6–12 years and independently by older children and adolescents.
2.1. Statistical analysis
Descriptive statistics were calculated as the mean ± SD for continuous variables and as numbers and percentages for categorical variables using SPSS V.16. Group differences were tested using the chi-square test for categorical variables and one-way ANOVA for continuous variables. ANOVA was followed by a Tukey HSD (Honestly Significant Difference) post hoc test. Correlation coefficients and linear and logistic regression models were used to evaluate the association between sleep disturbance and variables such as age, BMI, and eGFR. All tests were two-sided, and differences were considered to be significant at P < .05.
3. Results
Studied groups' characteristics and demographic data are presented in Table 1. The total study population included 95 patients, with 54 CKD patients, 22 patients maintained on HD (n 22; M/F 12:10) and 32 CKD patients not maintained on HD (n 32; M/F 19:13); 41 healthy children of matched age and sex served as a control group (n 41; M/F 23:18). End stage renal disease (ESRD) patients were undergoing regular HD three times/week for 3–4 h using bicarbonate dialysis to maintain a minimum Kt/V index of 1.2 per session.
Table 1.
Studied groups' characteristics and demographic data.
| Parameters | Group 1 CKD-dialysis patients |
Group 2 CKD-non-dialysis patients |
Group 3 Healthy controls |
P value |
|---|---|---|---|---|
| Number | 22 | 32 | 41 | |
| Sex (Male/Female) | 12/10 | 19/13 | 23/18 | .86 |
| Male (%) | 54.5 | 59.3 | 56.1 | |
| Age (years) Mean (SD) | 10.70 (2.83) | 9.31 (2.71) | 9.64 (3.43) | .25 |
| Weight (kg) Mean (SD) | 26.93 (11.64) | 29.87 (9.66) | 33.26 (13.03) | .20 |
| BMI Mean (SD) | 16.83 (3.44) | 18.68 (2.99) | 19.15 (3.62) | .24 |
| Height (cm) Mean (SD) | 124.60 (17.68) | 126.30 (14.45) | 129.27 (16.83) | .36 |
| Estimated GFR (ml/min/1.73 m2) Mean (SD) | 10.69 (2.33) | 82.08 (17.83) | 103.80 (8.07) | <.001 |
| Duration of HD (months) Mean (SD) Kt/V Mean (SD) |
26.77 (25.87) 1.13 (0.085) |
CKD: Chronic kidney disease, BMI: Body mass index, GFR: Glomerular filtration rate, HD: Hemodialysis.
The studied groups' laboratory data are presented in Table 2. Serum creatinine, hemoglobin, calcium, and phosphorus were significantly higher in CKD children on hemodialysis compared to non–dialysis-dependent CKD children and controls (P < .05). However, there was no significant difference between non–dialysis-dependent CKD children and controls (P > .05).
Table 2.
Studied groups' laboratory data.
| Parameters | Group 1 CKD-dialysis patients |
Group 2 CKD-non-dialysis patients |
Group 3 Healthy controls |
P value |
|---|---|---|---|---|
| Creatinine (mg/dL) Mean (SD) | 5.92 (1.16) | 0.97 (0.19) | 0.64 (0.12) | <.001 |
| Hemoglobin (g/dL) Mean (SD) | 10.45 (1.44) | 11.26 (0.87) | 12.00 (0.81) | <.001 |
| Total Ca (mg/dL) Mean (SD) | 9.65 (0.89) | 10.17 (0.57) | 10.41 (0.46) | <.001 |
| P (mg/dL) Mean (SD) | 5.35 (0.75) | 4.29 (0.47) | 4.33 (0.59) | <.001 |
Ca: Total serum calcium, P: Serum phosphorus.
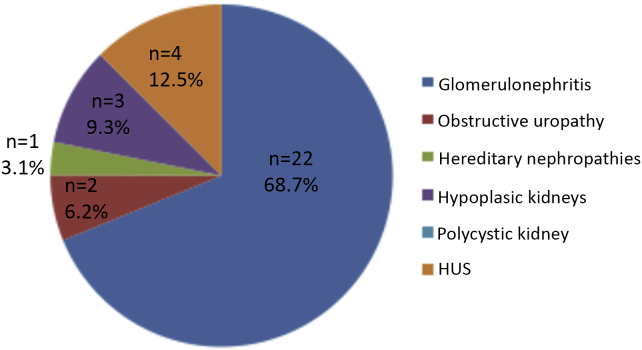
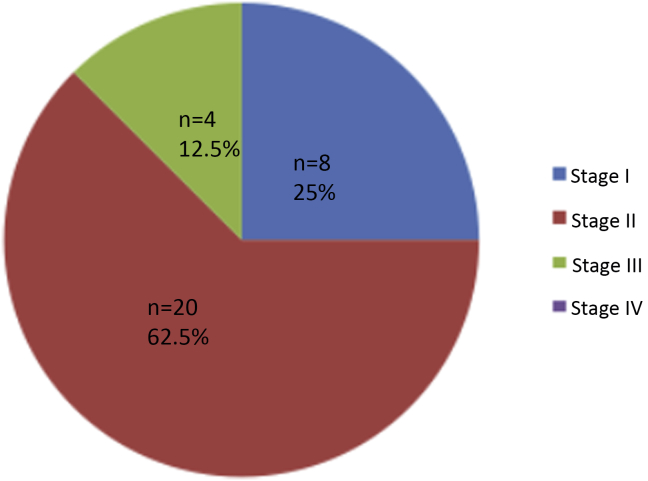
Figure 1, Figure 2 show the causes of CKD in the studied children. Glomerulonephritis was the most common cause, accounting for 45.4% in dialysis-dependent and 68.7% in non-dialysis-dependent children with CKD. Fig. 3 displays the stages of CKD in non-dialysis-dependent CKD patients; 62.5% of the studied non-dialysis-dependent CKD patients had Stage 2 disease.
Figure 1.
Causes of CKD in dialysis patients.
Figure 2.
Causes of CKD in non-dialysis patients.
Figure 3.
Stages of CKD in non-dialysis patients.
The Children's Sleep Habits Questionnaire (CSHQ) total and subscale scores are presented in Table 3. The total CSHQ scores were significantly higher in both groups of CKD children compared to controls (P < .05), while no significant difference was observed between CKD children on hemodialysis and non–dialysis-dependent patients (P = .054).
Table 3.
The Children's Sleep Habits Questionnaire (CSHQ): total and subscales scores in the studied groups.
| CSHQ subscales scores (Rang, mean(SD) |
Group 1 CKD-dialysis patients |
Group 2 CKD-non-dialysis patients |
Group 3 Healthy controls |
P value |
|---|---|---|---|---|
| 1. Bedtime resistance | 6–14 9.45 (2.06) |
6–12 8.06 (2.29) |
6–8 6.02 (0.93) |
<.001 |
| 2. Sleep onset delay | 1–3 2.45 (0.59) |
1–3 1.5 (0.56) |
1–2 1.14 (0.35) |
<.001 |
| 3. Sleep anxiety | 4–9 5.31 (1.76) |
4–7 5.12 (1.38) |
4–6 3.78 (0.68) |
<.001 |
| 4. Sleep duration | 3–8 5.4 (1.4) |
4–7 5.12 (0.83) |
3–6 4.04 (0.86) |
<.001 |
| 5. Night waking | 3–7 5.03 (1.37) |
3–6 4.27 (0.98) |
3–5 4.0 (0.77) |
<.001 |
| 6. Parasomnias | 7–16 11.40 (2.51) |
7–13 9.65 (1.71) |
7–10 8.41 (0.77) |
<.001 |
| 7. Sleep-disordered breathing | 3–6 4.2 (0.71) |
3–4 3.53 (0.50) |
3–4 3.31 (0.47) |
.036 |
| 8. Daytime sleepiness | 10–22 14.77 (3.95) |
8–15 10.93 (1.91) |
8–12 8.97 (0.93) |
<.001 |
| CSHQ Total scores | 41–78 54.81 (9.45) |
35–64 50.18 (7.98) |
34–48 39.70 (4.31) |
<.001 |
| CSHQ score >41 n (%) |
18 (81.8%) | 23 (71.8%) | 8 (19.5%) | <.001 |
Approximately 75.9% of the studied children with CKD scored above the CSHQ clinical cutoff score for disordered sleep: 81.8% in dialysis patients and 71.8% in non-dialysis patients.
The sleep duration and sleep anxiety scores on the CSHQ were significantly higher in both groups of CKD children compared to controls (P < .05), while no significant difference was observed between CKD children on hemodialysis and non–dialysis-dependent CKD patients (P > .05). The subscale scores of bedtime resistance, sleep onset delay, daytime sleepiness, and parasomnia were significantly higher in CKD children on hemodialysis than in non–dialysis-dependent CKD children (P < .05), while both groups of children with CKD had scores significantly higher than controls (P < .05). Sleep-disordered breathing and night waking were significantly higher in CKD children on hemodialysis compared to non–dialysis-dependent CKD children and controls (P < .05). However, no significant difference was observed between non–dialysis-dependent CKD children and controls (P > .05).
The Epworth sleepiness scale (ESS) results of the studied groups are presented in Table 4. The ESS score was significantly higher in CKD children on hemodialysis compared to those not on dialysis (P < .05). Both groups of CKD children had ESS scores that were significantly higher than those of controls (P < .05). An ESS score ≥11 was reported in 22% of the studied children with CKD: 45.5% in dialysis patients and 6.25% in non-dialysis patients compared to 2.4% of the children in the control group. EDS was significantly more prevalent in the in dialysis patients (P < .001).
Table 4.
The Epworth sleepiness scale (ESS) scores of the studied groups.
| ESS scores | Group 1 CKD-dialysis patients |
Group 2 CKD-non-dialysis patients |
Group 3 Healthy controls |
P value |
|---|---|---|---|---|
| Range | 5–18 | 3–14 | 2–14 | |
| Mean (SD) | 11.72 (3.48) | 7.25 (2.67) | 5.07 (2.47) | <.001 |
| ESS score ≥ 11 | ||||
| n (%) | 10 (45.5%) | 2 (6.25%) | 1 (2.4%) | <.001 |
| Total n | 22 | 32 | 41 | |
The RLS symptoms in the studied groups are presented in Table 5. RLS symptoms were reported in 20.4% of the studied children with CKD: 36.4% in dialysis patients and 9.4% in non-dialysis patients. RLS symptoms were reported in only 2.4% of the children in the control group. The prevalence of RLS was significantly higher in the in dialysis patients (P < .001).
Table 5.
Restless legs syndrome (RLS) symptoms in the studied groups.
| RLS n (%) | Group 1 CKD-dialysis patients |
Group 2 CKD-non-dialysis patients |
Group 3 Healthy controls |
P value |
|---|---|---|---|---|
| Yes | 8 (36.4%) | 3 (9.4%) | 1 (2.4%) | |
| No | 14 (63.6) | 29 (90.6%) | 40 (97.6%) | <.001 |
| Total | 22 | 32 | 41 |
3.1. The association between sleep disorders and demographic and laboratory data in children with CKD
There was a significant negative correlation between CSHQ scores and eGFR (r = −0.28, P = .04) and Ca (r = −0.384, P = .004), with no significant correlation between the score and age (r = −0.7, P = .5), BMI (r = −0.158, P = .125), or Hb (r = −0.23, P = .089). There was a significant positive correlation between serum phosphorus (r = 0.52, P < .001) and CSHQ scores.
Linear regression revealed a significant positive association between the serum phosphorus and CSHQ scores; every increase in serum P by 1 mg/dL was associated with an increase in CSHQ scores by 6.865. A significant negative association was found between the GFR and CSHQ scores, a decrease in GFR by 10 ml/min/1.73 m2 was associated with an increase in CSHQ scores by 0.65. Linear regression revealed no significant association between CSHQ and age, BMI, Hb, or Ca.
There was a significant positive correlation between the ESS score and serum P (r = 0.6, P < .001) and serum creatinine (r = 0.555, P < .001), with a significant negative correlation between the ESS score and GFR (r = −0.659, P < .001), with no significant correlation between the ESS score and age, Hb or BMI. Linear regression revealed a significant negative association between GFR and ESS. Every decrease in GFR by 1 ml/min/1.73 m2 was associated with an increase of 0.079 in ESS. Linear regression revealed no significant association between ESS and age, Hb, BMI, or serum phosphorus.
Logistic regression revealed a significant negative association between GFR and RLS (P < .05); a decrease in GFR by 1 ml/min/1.73 m2 was associated with an increase in the probability of RLS by 49%. No association was observed between RLS and other variables, e.g., age, Hb, serum Ca, or serum phosphorus.
4. Discussion
In the present study, symptoms of sleep disturbances were detected in 75.9% of the studied children with CKD and in 19.5% of the normal children in the control group. Sleep disturbances were more frequent in the hemodialysis group compared with non-dialysis CKD patients, with a prevalence of 81.8% in hemodialysis patients and 71.8% in non-dialysis patients.
The high prevalence of sleep disturbances in our study is similar to that reported in previous studies among children with CKD; sleep disorders were reported in 32%–85% of children with CKD [10], [11], [12], [13], [14]: 77–85% in dialysis patients [10], [11], [14], and 32–50% in non-dialysis patients [11], [12], [13].
Sleep disturbances detected in our patients with CKD included RLS, EDS, sleep-disordered breathing (SDB), behavioral insomnias, and parasomnias. Bedtime resistance, sleep onset delay, daytime sleepiness, and parasomnia were more reported in hemodialysis-dependent than non-dialysis-dependent CKD children; both groups had higher scores than the control group. Children with CKD displayed more sleep anxiety and shorter sleep duration than normal children, with no difference between hemodialysis and non–dialysis-dependent CKD children.
We observed SDB and night waking in HD patients, but not in non–dialysis-dependent CKD children. Both pediatric and adult studies have shown that SDB is common among advanced CKD (stages 4–5) and HD patients [10], [11], [14], [15], [16]. The non-dialysis-dependent CKD children in our study did not include stage IV CKD because of the lack of a transplantation program at Tanta University Hospital; therefore, most stage IV CKD patients are followed in other centers for preemptive transplantation. Furthermore, stage III CKD was observed in our study in only 12.5% of non-dialysis-dependent children, which may explain why SDB was not reported in our patients with non-dialysis-dependent CKD. Similarly, Sinha et al [12] reported SDB in only 6% of non–dialysis-dependent CKD children; most of the children studied were stage 1 and 2, while stage 4 was observed in only 2%.
We reported EDS in 22% of the studied children with CKD, with a prevalence of 45.5% in dialysis patients and 6.25% in non-dialysis patients. EDS was reported in only 2.4% of the children in the control group. RLS symptoms were detected in 20.4% of the studied children with CKD: 36.4% in dialysis patients and 9.4% in non-dialysis patients. RLS symptoms were reported in only 2.4% of the control group.
Our findings are consistent with those of previous studies among children with CKD; EDS symptoms were reported in 29–60% of children with CKD: 60% in dialysis patients [10], [11], [14] and 29–39.7% in non-dialysis patients [11], [17]. RLS symptoms were reported in 10%–35% of children with CKD [14], [17], [18].
The prevalence of sleep disorders among adults with CKD ranges from 40% to 80% [19], [20], [21], and RLS symptoms are reported in 20%–40% of adult patients with CKD [22], [23], [24].
The variation in prevalence of sleep disturbances in children with CKD between the studies is likely due to different populations being studied, either dialysis-dependent or non-dialysis-dependent; differences between the number of studied subjects; the method of diagnosis of sleep disorders, whether based on questionnaires or polysomnography (PSG); and the type of questionnaire used to assess sleep disturbances. Sinha et al [12] and Roumelioti et al [13] reported disordered sleep in 37% and 12% of the studied CKD children, respectively. The lower prevalence of sleep problems in these studies may be related to under-detection of sleep problems due to not using validated sleep questionnaires. In addition, those authors studied only non-dialysis-dependent CKD children and did not include dialysis patients.
Behavioral sleep problems (i.e., bedtime resistance, sleep onset delay, shorter sleep duration, and night awakenings) were observed among children with CKD in our study, suggesting that behavioral and psychological factors may contribute to the occurrence of sleep problems among children with CKD. Depression and anxiety have been documented as major determinants of sleep quality and disturbances in adult populations with CKD [25]. Adult studies have suggested that the etiologies of sleep problems in patients with CKD and those on chronic intermittent daytime hemodialysis are different. Functional and psychological factors may play a more prominent role in the non-dialysis-dependent CKD group, while intrinsic sleep disruption (arousals, apneas, and limb movements) secondary to the effects of chronic intermittent dialysis may play a more significant role in the dialysis population. This postulated different mechanism may explain the presence of sleep disorders in patients with early CKD [26] in which psychosocial factors could be important contributors.
4.1. Associations between CKD-related factors and sleep disorders
We found a significant positive association between the serum phosphorus and CSHQ scores and a significant negative association between the GFR and CSHQ scores, suggesting that lower GFR and hyperphosphatemia are risk factors for sleep disturbances in children with CKD. Consistent with our study, the positive association between sleep disturbances and hyperphosphatemia in CKD has been documented in a number of adult studies [27], [28], [29]. In contrast to our findings, Sinha et al [12] found no correlation between the stage of CKD and the prevalence of sleep problems; they did not observe any differences in the incidence of sleep disorders across the stages of CKD.
Additionally, lower GFR was associated with increased RLS and EDS in our study. Similarly, Roumelioti et al [13] found that lower GFR in pediatric CKD patients was associated with increased daytime sleepiness. In contrast, Riar et al [18] reported that RLS was more prevalent in adolescents, but he found no significant association between sleep symptoms and CKD stage.
Several uremic and non-uremic factors may explain the relatively high frequency of sleep problems in children with CKD. The increased prevalence of sleep disorders in CKD children undergoing dialysis suggests that the dialysis procedure may be a factor in sleep disorders by causing insufficient sleep. In addition, metabolic factors, including uremia, hyperphosphatemia, hyperparathyroidism, and iron status, can affect sleep in CKD patients [27], [28], [29], [30]. Low production of melatonin and circadian rhythm disruption observed in patients with chronic renal failure may also be causative factors for sleep disturbances [31], [32].
Congenital abnormalities, such as renal hypoplasia or dysplasia, and obstructive uropathy represent the most common causes of CKD in children younger than 5 years of age. While in CKD children older than 5 years of age, acquired diseases, such as glomerulonephritis, and inherited disorders, such as hereditary nephropathies predominate [33]. In our study, we excluded children less than 6 years of age, as they could not reliably comprehend the questionnaire about RLS, which may explain why glomerulonephritis was the most common cause of CKD in our studied patients.
There were limitations in this study. The questionnaire results were not corroborated with objective measurements of sleep disturbances, such as polysomnography. A second problem was the small sample size. Therefore, future studies should incorporate multi-site patients, using both subjective and objective measures such as polysomnography for assessing sleep disorders in children with CKD.
A strength of our study is that we studied both non-dialysis and dialysis-dependent children with CKD; consequently, we were able to compare the prevalence of sleep disorders across the full spectrum of chronic kidney disease. In addition, the study was a case-control study allowing comparisons with age and sex-matched healthy children as controls. Furthermore, the diagnosis of sleep disorders was based on structured interviews using a validated sleep questionnaire.
5. Conclusion
Sleep disturbances are very common in children with CKD compared to healthy children. Sleep disturbances in patients with CKD include restless legs syndrome (RLS), excessive daytime sleepiness (EDS), sleep-disordered breathing (SDB), behavioral insomnias, and parasomnias. Therefore, it is important for practitioners caring for children with CKD to anticipate and screen for treatable sleep disorders. Early recognition and treatment of sleep disturbances may have a positive impact on the growth and development of children with CKD.
Ethical approval
“All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.”
An informed consent was obtained from parents of all children participating in the study. The privacy rights of human subjects must always be observed.
Funding
None.
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Contributor Information
Amira H. Darwish, Email: dradarwish2012@gmail.com.
Hend Abdel-Nabi, Email: hend.abdelnabi@yahoo.com.
References
- 1.Beebe D.W. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am. 2011;58:649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stabouli S., Papadimitriou E., Printza N., Dotis J., Papachristou F. Sleep disorders in pediatric chronic kidney disease patients. Pediatr Nephrol. 2016;31(8):1221–1229. doi: 10.1007/s00467-015-3237-9. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Kidney Foundation K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2012;39:S1–S266. [PubMed] [Google Scholar]
- 5.Owens J.A., Spirito A., McGuinn M. The children's sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 6.Asaad T., Kahla O. Elnahda, El- Fagala; Egypt: 2001. Psychometric sleep assessments instruments: An Arabic version for sleep evaluation. [Google Scholar]
- 7.Goodlin-Jones B.L., Sitnick S.L., Tang K., Liu J., Anders T.F. The children's sleep habits questionnaire in toddlers and preschool children. J Dev Behav Pediatr. 2008;29:82–88. doi: 10.1097/dbp.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- 8.Melendres M.C., Lutz J.M., Rubin E.D., Marcus C.L. Daytime sleepiness and hyperactivity in children with suspected sleep disordered breathing. Pediatrics. 2004;114:768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 9.Allen R., Picchietti D., Hening W., Trenkwalder C., Walters A.S., Montplaisi J. Restless legs syndrome: diagnostic criteria, special consideration, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop to the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Davis I.D., Baron J., O'Riordan M.A., Rosen C.L. Sleep disturbances in pediatric dialysis patients. Pediatr Nephrol. 2005;20:69–75. doi: 10.1007/s00467-004-1700-0. [DOI] [PubMed] [Google Scholar]
- 11.Davis I.D., Greenbaum L.A., Gipson D., Wu L.L., Sinha R., Matsuda- Abedini M. Prevalence of sleep disturbances in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2012;27:451–459. doi: 10.1007/s00467-011-2010-y. [DOI] [PubMed] [Google Scholar]
- 12.Sinha R., Davis I.D., Matsuda-Abedini M. Sleep disturbances in children and adolescents with non–dialysis-dependent chronic kidney disease. Arch Pediatr Adolesc Med. 2009;163(9):850–855. doi: 10.1001/archpediatrics.2009.149. [DOI] [PubMed] [Google Scholar]
- 13.Roumelioti M.E., Schneider M.F., Gerson A.C., Hooper S., Benfield M., Warady B.A. Sleep and fatigue symptoms in children and adolescents with CKD: a cross sectional analysis from the chronic kidney disease in children (CKiD) study. Am J Kidney Dis. 2010;55:269–280. doi: 10.1053/j.ajkd.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Refaey A.M., Elsayed R.M., Sarhan A., Bakr A., Hammad A., Elmougy A. Sleep quality assessment using polysomnography in children on regular hemodialysis. Saudi J Kidney Dis Transpl. 2013;24:714–718. doi: 10.4103/1319-2442.113862. [DOI] [PubMed] [Google Scholar]
- 15.Amin R., Sharma N., Al-Mokali K., Sayal P., Al-Saleh S., Narang I. Sleep-disordered breathing in children with chronic kidney disease. Pediatr Nephrol. 2015;30:2135–2143. doi: 10.1007/s00467-015-3155-x. [DOI] [PubMed] [Google Scholar]
- 16.Roumelioti M.E., Buysse D.J., Sanders M.H., Strollo P., Newman A.B., Unruh M.L. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6(5):986–994. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Applebee G.A., Guillot A.P., Schuman C.C., Teddy S., Attarian H.P. Restless legs syndrome in pediatric patients with chronic kidney disease. Pediatr Nephrol Berl Ger. 2009;24:545–548. doi: 10.1007/s00467-008-1057-x. [DOI] [PubMed] [Google Scholar]
- 18.Riar S.K., Leu R.M., Turner-Green T.C., Rye D.B., Kendrick-Allwood S.R., McCracken C. Restless legs syndrome in children with chronic kidney disease. Pediatr Nephrol. 2013;28:773–795. doi: 10.1007/s00467-013-2408-9. [DOI] [PubMed] [Google Scholar]
- 19.Losso R.L., Minhoto G.R., Riella M.C. Sleep disorders in patients with end-stage renal disease undergoing dialysis: comparison between hemodialysis, continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Int Urol Nephrol. 2015;47(2):369–375. doi: 10.1007/s11255-014-0860-5. [DOI] [PubMed] [Google Scholar]
- 20.Iliescu E.A., Yeates K.E., Holland D.C. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transpl. 2004;19:95–99. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 21.Merlino G., Piani A., Dolso P., Adorati M., Cancelli I., Valente M. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transpl. 2006;21(1):184–190. doi: 10.1093/ndt/gfi144. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh D., Siddiqui S., Geddes C.C. Restless legs syndrome in patients on dialysis. Am J Kidney Dis. 2004;43(5):763–771. doi: 10.1053/j.ajkd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Gigli G.L., Adorati M., Dolso P., Piani A., Valente M., Brotini S. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5(3):309–315. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Rijsman R.M., DeWeerd A.W., Stam C.J., Kerkhof G.A., Rosman J.B. Periodic limb movement disorder and restless legs syndrome in dialysis patients. Nephrology. 2004;9:353–361. doi: 10.1111/j.1440-1797.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 25.Roumelioti M.E., Argyropoulos C., Buysse D.J., Nayar H., Weisbord S.D., Unruh M.L. Sleep quality, mood, alertness and their variability in CKD and ESRD. Nephron Clin Pract. 2010;114:c277–c287. doi: 10.1159/000276580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Santo R.M., Bartiromo M., Cesare C.M., Cirillo M. Sleep disorders occur very early in chronic kidney disease. J Nephrol. 2008;21(Suppl. 13):S59–S65. [PubMed] [Google Scholar]
- 27.Unruh M., Hartunian M., Chapman M., Jaber B.L. Sleep quality and clinical correlates in patients on maintenance hemodialysis. Clin Nephrol. 2003;59:280–288. doi: 10.5414/cnp59280. [DOI] [PubMed] [Google Scholar]
- 28.Sabry A.A., Abo-Zenah H., Wafa E., Mahmoud K., El-Dahshan K., Hassan A. Sleep disorders in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(2):300–305. [PubMed] [Google Scholar]
- 29.Ezzat H., Mohab A. Prevalence of sleep disorders among ESRD patients. Ren Fail. 2015;37(6):1013–1019. doi: 10.3109/0886022X.2015.1044401. [DOI] [PubMed] [Google Scholar]
- 30.Roger S.D., Harris D.C.H., Stewart J.H. Possible relation between restless legs and anemia in renal dialysis patients. Lancet. 1991;337(8756) doi: 10.1016/0140-6736(91)93248-8. [DOI] [PubMed] [Google Scholar]
- 31.Koch B.C., van der Putten K., Van Someren E.J., Wielders J.P., Ter Wee P.M., Nagtegaal J.E. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study) Nephrol Dial Transpl. 2010;25:513–519. doi: 10.1093/ndt/gfp493. [DOI] [PubMed] [Google Scholar]
- 32.Russcher M., Koch B.C., Nagtegaal J.E., van Ittersum F.J., Pasker-de Jong P.C., Hagen E.C. Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): a randomized controlled trial. Br J Clin Pharmacol. 2013;76:668–679. doi: 10.1111/bcp.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreedharan R., Avner E.D. Chronic kidney disease. In: Beherman R.E., Kliegman R.M., Jensen H.B., editors. Nelson textbook of pediatrics. 20th ed. Saunders Company; Philadelphia: 2015. pp. 2543–2546. [Google Scholar]