Abstract
Objective:
Fatigue is a common side effect of cancer treatment, but there is considerable variability in fatigue severity and persistence among survivors. This study aimed to characterize longitudinal trajectories of fatigue after breast cancer treatment and to identify predictors of varying fatigue trajectories.
Methods:
Women (N=191) from the Mind-Body Study completed assessments after primary treatment for early-stage breast cancer and at regular follow-ups that occurred up to 6 years after treatment (M = 4.3 years). Growth mixture models were used to characterize fatigue trajectories, and demographic, medical, and biobehavioral risk factors were examined as predictors of trajectory group.
Results:
Five trajectories were identified, characterized as High, Recovery, Late, Low, and Very Low fatigue. The High and Recovery groups (40% of sample) evidenced elevated fatigue at post-treatment that declined in Recovery but persisted in the High group. In bivariate analyses, trajectory groups differed significantly on depressive symptoms, sleep disturbance, childhood adversity, body mass index, and the inflammatory marker soluble TNF receptor type II, which were higher in the High and/or Recovery groups. In multivariate models, depressive symptoms and childhood adversity distinguished High and Recovery from other groups. Rates of chemotherapy were higher in the Recovery than in the High or Late group, whereas rates of endocrine therapy were higher in the High than in the Recovery group.
Conclusions:
There are distinct longitudinal trajectories of fatigue after breast cancer treatment. Psychological factors are strongly associated with adverse fatigue trajectories, and together with treatment exposures may increase risk for cancer-related fatigue.
Keywords: fatigue, cancer, trajectory, biobehavioral, risk factor
Fatigue is one of the most common and distressing symptoms experienced by cancer patients and causes significant impairment in quality of life. On average, fatigue increases after treatment onset and declines in the months after treatment completion, as documented in longitudinal studies that report on mean levels of fatigue during and after treatment (Andrykowski, Donovan, Laronga, & Jacobsen, 2010; Dhruva et al., 2010; Donovan et al., 2004; Jacobsen et al., 1999; Nieboer et al., 2005). However, there is considerable individual variability in the longitudinal trajectory of cancer-related fatigue that may be obscured by a focus on average levels (Bower, 2014; Carlson, Waller, Groff, Giese-Davis, & Bultz, 2013; Dhruva et al., 2010). For example, some patients experience very little fatigue throughout treatment and into survivorship, whereas others have high fatigue that persists for years after treatment completion. Understanding the factors that contribute to this variability has important implications for the identification and treatment of at risk patients.
Outside of the cancer literature, investigators have captured individual differences in symptom trajectories using an analytic approach that identifies subgroups of patients who show similar change over time (growth mixture models; GMMs). This approach requires multiple assessments over time, but provides a much more nuanced and complete picture of the symptom experience. GMMs have been used to identify trajectories of psychological adjustment following stressful life events, focusing primarily on distress and depressive symptoms. These studies have identified several prototypical patterns of adjustment, including a “resilience” trajectory characterized by minimal disruption in functioning, a “recovery” trajectory characterized by initial disruption then return to baseline, a “delayed” trajectory characterized by increasing levels of disruption over time, and a “chronic” trajectory characterized by persistent high levels of disruption (Bonnano, 2004). This work has shed new light on individual variability in responses to stress and challenged previous conceptions of the ubiquity and severity of distress in the aftermath of stressful life experiences (Bonanno, Westphal, & Mancini, 2011).
Building on this work, investigators have begun to examine trajectories of depressive symptoms in cancer patients and survivors in the months after diagnosis and treatment. For example, Stanton and colleagues examined trajectories of depressive symptoms in 460 breast cancer patients assessed within 4 months after invasive breast cancer diagnosis and at six additional assessments across a 12 month follow-up (Stanton, Wiley, et al., 2015). They found four distinct trajectory groups, including women with chronically elevated symptoms of depression (38%), women who started with high symptoms then recovered (20%), and women with low (32%) and very low symptoms (11%) across the assessment period. These findings are generally consistent with other studies looking at trajectories of depression and distress in women with breast cancer, particularly the finding that approximately half of women report consistently low or very low symptoms of depression and a smaller group report elevated symptoms across assessment points (Avis, Levine, Case, Naftalis, & Van Zee, 2015; Dunn et al., 2011; Henselmans et al., 2010; Lam et al., 2010; Rottmann et al., 2016).
In contrast to depression, only a handful of studies have used this approach to identify individual differences in trajectories of cancer-related fatigue. Donovan and colleagues examined fatigue trajectories in 261 breast cancer patients who completed assessments after treatment and at 2, 4, and 6-month post-treatment follow-ups. They identified two distinct fatigue groups: one that reported consistently low fatigue (33%), and one that reported consistently high fatigue (67%) (Donovan, Small, Andrykowski, Munster, & Jacobsen, 2007). Women in the high fatigue group were more likely to be unmarried, have a lower income, a higher body mass index, engage in greater fatigue catastrophizing, and be lower in exercise participation; only body mass index and catastrophizing remained significant predictors in multivariate analysis. A study of 398 breast cancer patients assessed before surgery and monthly for 6 months also identified two fatigue groups, who were classified as high fatigue (61.5%) and low fatigue (38.5%)(Kober et al., 2016). In this study, the high fatigue group was younger, more educated, had more comorbidities and lower performance status, had more lymph nodes removed, and were more likely to receive chemotherapy than the low fatigue group. Further, polymorphisms in two cytokine SNPs (IL1B and IL10) were associated with being in the high fatigue group. A study of fatigue trajectories in 77 breast cancer patients undergoing chemotherapy identified three groups with different trajectories, including one with “high fatigue” (50%), one with “transient fatigue” (27%), and one with “low fatigue” (23%) (Junghaenel, Cohen, Schneider, Neerukonda, & Broderick, 2015). Demographic and medical variables were not associated with fatigue trajectories in this study, but those in the high fatigue group had poorer functioning across domains, including more depressed mood. Finally, a study of 290 breast cancer patients assessed before surgery and 4 and 8 month follow-ups found two fatigue groups, although here the majority of women fell into the low fatigue group (79%) rather than the high fatigue group (21%)(Bodtcher et al., 2015). In this study, low physical activity, receipt of chemotherapy, and anxiety were significantly associated with being in the high fatigue group.
These studies provide initial evidence for distinct subgroups of patients who experience different courses of fatigue during and in the months after cancer treatment. To our knowledge, no studies have examined individual differences in trajectories of fatigue beyond one year post-treatment. This is important given that research on longer-term survivors indicates that approximately 20–30% will experience elevated fatigue up to 10 years after treatment completion (Bower et al., 2006; Cella, Davis, Breitbart, & Curt, 2001), and that fatigue and other symptoms may emerge late in the survivorship period (Stanton, Rowland, & Ganz, 2015). Identification of patients who are at risk for persistent cancer-related fatigue, beyond the “expected” period of recovery (6–12 months post-treatment; (Goldstein et al., 2012)), will facilitate the deployment of targeted interventions to those most in need.
The current study sought to characterize trajectories of fatigue in women diagnosed with early-stage breast cancer who were followed for up to 6 years after treatment completion, and to identify factors that differentiated women in these trajectory groups. Cancer-related fatigue is multifactorial and may be influenced by demographic, medical, psychosocial, behavioral, and biological factors (Bower, 2014; C. Fagundes, LeRoy, & Karuga, 2015; Mitchell, 2010). Initial research on fatigue trajectories has identified a number of predictors of fatigue (Bodtcher et al., 2015; Donovan et al., 2007; Junghaenel et al., 2015; Kober et al., 2016), although none of these studies included a comprehensive assessment of biobehavioral risk factors. In particular, inflammatory processes have rarely been examined, despite evidence that genetic and circulating markers of inflammation are associated with elevated fatigue during and after cancer treatment (Bower, 2014; Lacourt & Heijnen, 2017; Saligan & Kim, 2012; Wang, Yin, Miller, & Xiao, 2017). In addition, none of these studies assessed childhood adversity, although stressful experiences in childhood have been linked with other fatigue-related disorders (Heim et al., 2006) and preliminary evidence supports an association with cancer-related fatigue (Bower, Crosswell, & Slavich, 2014; C. P. Fagundes, Lindgren, Shapiro, & Kiecolt-Glaser, 2012; Han et al., 2016; Witek, Tell, Albuquerque, & Mathews, 2013). Drawing from an empirically-based model of cancer-related fatigue (Bower, 2014), the current study examined key biobehavioral risk factors that have been associated with fatigue across the cancer continuum, including depression, sleep disturbance, and history of childhood adversity, as well as circulating concentration of inflammatory markers and variants in genes that regulate pro-inflammatory cytokine production.
METHODS
Participants:
The present data come from the Mind Body Study (MBS), a longitudinal observational study of women with early-stage breast cancer recruited from the Los Angeles community between 2007 and 2010. The design, eligibility criteria, recruitment, and assessment procedures used in the MBS are described in earlier publications (Ganz,Bower, et al., 2013; Ganz, Kwan, et al., 2013; Ganz et al., 2014). Briefly, women were recruited after diagnosis and completion of primary treatment (surgery, radiation, and/or chemotherapy) for Stage 0-IIIA breast cancer. In-person appointments were scheduled within 3 months after primary treatment but before initiation of endocrine therapy (ET) if prescribed (T1), and 6 months (T2) and 12 months (T3) later. Patients who completed the T3 assessment were invited to participate in a longitudinal follow-up study that included annual questionnaires administered by mail at 2 years (T4), 3 years (T5), and 4 years (T6) after T1. We also conducted a “final” in-person appointment (TF) that included completion of questionnaires and other assessments that were part of the parent MBS visits. The TF assessments were all conducted between March 2013 and July 2014 and were not tied to the timing of the T1 assessment. Some participants reached the TF assessment period before their T5 or T6 assessments; because the TF visit was the final assessment for this study, these women were never asked to complete the T5 or T6 questionnaires. A flow chart indicating the sequence of study assessments is shown in Figure 1. The UCLA Institutional Review Board approved the study, and all participants provided written informed consent.
Figure 1.
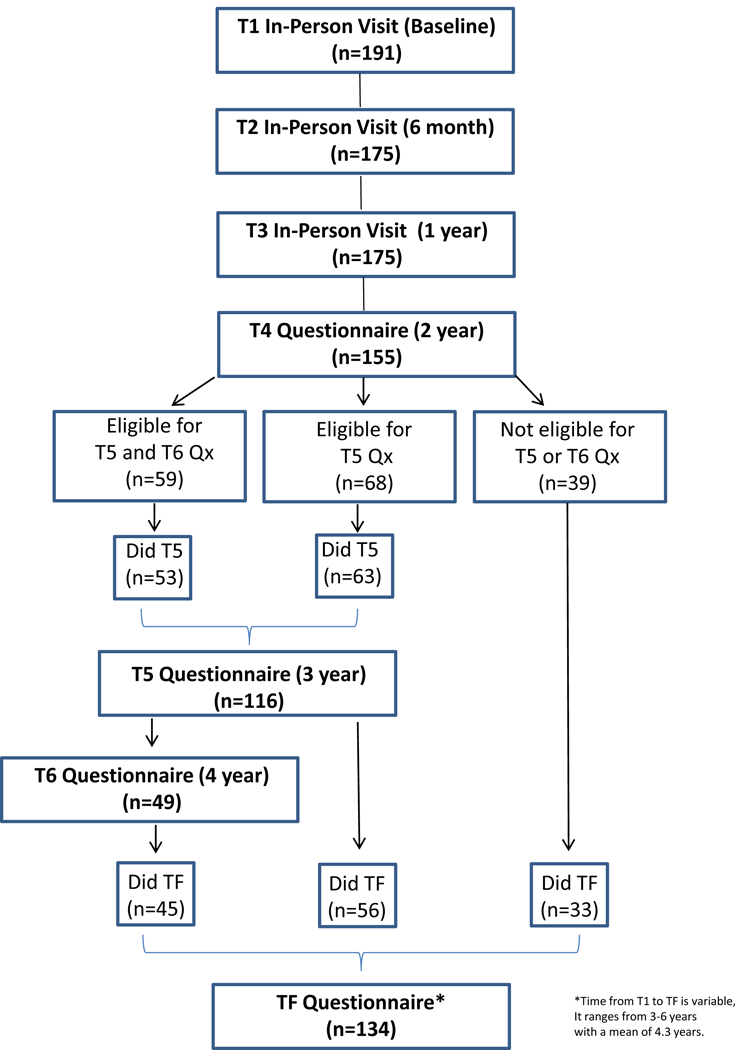
Flow chart indicating the number of patients who completed each assessment. Note that some patients were not eligible for the T5 and/or T6 assessments, as the final study assessment (TF) occurred before they reached these assessment points.
Measures:
Demographic, medical, and clinical information was obtained from self-report at T1 and from medical record abstraction. Fatigue was assessed at each assessment point with the Multidimensional Fatigue Symptom Inventory-Short Form, a valid and reliable measure of cancer-related fatigue (Stein, Jacobsen, Blanchard, & Thors, 2004; Stein, Martin, Hann, & Jacobsen, 1998). We focused on the four-item general fatigue subscale, which assesses feelings of being “fatigued”, “tired”, and “worn out” in the last 7 days on a 0–5 point scale. Depressive symptoms were assessed at T1 with the Beck Depression Inventory-II (BDI-II), a 21-item measure that assesses cognitive, affective, and vegetative symptoms of depression during the past two weeks with excellent reliability and validity (Beck, Steer, & Brown, 1996). For analyses, the two items on the BDI-II assessing fatigue/lack of energy were removed to minimize overlap with fatigue. Subjective sleep quality was assessed at T1 with the Pittsburgh Sleep Quality Index (Buysse et al., 1991), a 19-item questionnaire that assesses subjective sleep quality and disturbances over the past month. This questionnaire has high internal consistency, test-retest reliability, and diagnostic validity with polysomnography. For each of these scales, higher scores indicate more symptoms. Childhood adversity was assessed at T3 with the Risky Families Questionnaire, which was adapted from Felitti et al. (1998) by Taylor, Lerner, Sage, Lehman, and Seeman (2004). This 13-item scale assesses physical and emotional abuse and neglect and disorganization/conflict/chaos in the home between the ages of 5 and 15. The validity of this scale has been demonstrated through corroboration with in-person interviews (Taylor et al., 2004).
Plasma samples for circulating inflammatory markers were collected at T1 and assayed as previously described (Bower et al., 2011). Briefly, blood samples were collected into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80C for subsequent batch testing. We assessed four inflammatory markers that have been associated with behavioral symptoms in previous studies with cancer patients, including a subsample of women in the current study (Bower et al., 2011): soluble tumor necrosis factor (TNF) receptor type II (sTNF-RII), interleukin-1 receptor antagonist (IL-1ra), interleukin 6 (IL-6), and C-reactive protein (CRP). Plasma levels of all cytokines were determined by enzyme-linked immunosorbent assays according to manufacturer’s protocols, as previously described.
Genomic DNA was extracted from peripheral blood leukocytes collected at T1 and assayed by a commercial TaqMan Genotyping Assay (Applied Biosystems, Foster City, CA) performed on a iCycler real-time polymerase chain reaction instrument (BioRad, Hercules, CA) following manufacturer’s protocols, as previously described. We focused on SNPs in genes encoding cytokines that have been linked at the protein level to cancer-related fatigue, including IL1B −511 C>T (rs16944), IL6 −174 G>C (rs1800795), and TNF −308 G>A (rs1800629) (Barsevick, Frost, Zwinderman, Hall, & Halyard, 2010; Bower et al., 2013; Collado-Hidalgo, Bower, Ganz, Cole, & Irwin, 2006).
Data Analysis
GMMs were conducted to model fatigue trajectories (Duncan & Duncan, 1995; Jung & Wickrama, 2008). Time was coded continuously as the number of months since end of treatment (see Supplementary Figure S1). To identify the optimal trajectory shape and number of groups, it is common to try several models and compare fit indices to pick the optimal model. We tested models that varied on three features: (1) trajectory shape: linear, quadratic, and cubic splines; (2) random effects and covariance structures: fixed effects only, random intercepts only, uncorrelated random intercepts and slopes, and freely correlated random intercepts and slopes; and (3) number of groups: one to five group solutions. The residual variances and latent variable means were allowed to differ in each group (Wright & Hallquist, 2014). For latent class models and GMMs based on discrete time, tests of whether additional groups provide significantly better fit are available, such as the Vuong-Lo-Mendell-Rubin or bootstrapped likelihood ratio test. However, these tests are not currently available for GMMs based on continuous time. Therefore, the best fitting model from the 60 tested was selected based on three criteria: (1) whether the smallest group had at least 10 women, (2) sample size-adjusted BIC, and (3) corrected AIC. The sample size-adjusted BIC and corrected AIC are based on the absolute fit of the model and critically include a penalty for more complex models so that parsimonious models are favored.
Potential predictors of fatigue trajectory were examined by testing relations between trajectory groups and sociodemographic, disease and treatment-related, medical, and biobehavioral characteristics. First, bivariate relations were assessed using analysis of variance for continuous factors and chi-square tests for categorical factors. Predictors with p < .10 in bivariate tests or of a priori interest (i.e., receipt of endocrine therapy) were entered into a multinomial logistic regression to test their unique contributions.
GMMs were estimated using robust maximum likelihood with all available data using full information maximum likelihood estimation, which is efficient and produces unbiased estimates as long as the data are missing at random (Enders & Bandalos, 2001). Optimization was based on 500 initial stage starts and 100 final stage optimizations. Chi-square tests of statistical significance were based on 2 million Monte Carlo simulated samples (Hope, 1968), which allows accurate tests when some cell frequencies are zero. Analyses were conducted in R v3.3.1 with different GMM tested using the train LGMM function in MplusAutomation v0.7–0 (Hallquist & Wiley, in press) and Mplus v7.3 (Muthén & Muthén, 2012).
RESULTS
Sample Characteristics
A total of 191 women had questionnaire data available and were included in primary analyses. A flow chart indicating the number of women who completed questionnaires at each assessment is provided in Figure 1. Because the timing of the TF assessment was variable, this influenced the number of assessments that could be completed by an individual participant. In particular, the reduced sample size at T6 reflects that fact that most women had completed the TF assessment before they reached the T6 assessment. The mean follow-up period (from T1 to TF) was 4.3 years and ranged from 3–6 years.
Overall sample characteristics are provided in Table 1. On average, women in this study were 52 years old, non-Hispanic white, married, college-educated, and employed at study entry. The majority had lumpectomy with radiation therapy, approximately half received chemotherapy, and 70% received endocrine therapy.
Table 1.
Sample Characteristics Overall and by Trajectory Group
| Overall | Latent Trajectory Group | |||||
|---|---|---|---|---|---|---|
| Very Low / Low |
Late | Recovery | High | P Value |
||
| Age (years) | 51.75 (8.33) | 51.79 (8.57) | 52.93 (6.64) | 50.79 (9.50) | 52.14 (6.60) | .707 |
| Race/Ethnicity | .415 | |||||
| White | 151 (79.1) | 64 (76.2) | 29 (87.9) | 43 (81.1) | 15 (71.4) | |
| Non-White | 40 (20.9) | 20 (23.8) | 4 (12.1) | 10 (18.9) | 6 (28.6) | |
| Marital Status | .486 | |||||
| Married | 124 (65.3) | 55 (66.3) | 19 (57.6) | 38 (71.7) | 12 (57.1) | |
| Not Married | 66 (34.7) | 28 (33.7) | 14 (42.4) | 15 (28.3) | 9 (42.9) | |
| Education | .917 | |||||
| Less than college degree | 35 (18.3) | 15 (17.9) | 8 (24.2) | 9 (17.0) | 3 (14.3) | |
| College degree | 56 (29.3) | 27 (32.1) | 7 (21.2) | 16 (30.2) | 6 (28.6) | |
| More than college degree | 100 (52.4) | 42 (50.0) | 18 (54.5) | 28 (52.8) | 12 (57.1) | |
| Employment Status | .206 | |||||
| Employed full- or part-time | 123 (64.7) | 57 (68.7) | 23 (69.7) | 28 (52.8) | 15 (71.4) | |
| Not employed | 67 (35.3) | 26 (31.3) | 10 (30.3) | 25 (47.2) | 6 (28.6) | |
| Income Group | .125 | |||||
| < $100,000 | 75 (40.1) | 27 (33.3) | 11 (33.3) | 26 (49.1) | 11 (55.0) | |
| >=$100,000 | 112 (59.9) | 54 (66.7) | 22 (66.7) | 27 (50.9) | 9 (45.0) | |
| Surgery Type | .309 | |||||
| Lumpectomy | 126 (66.0) | 60 (71.4) | 18 (54.5) | 33 (62.3) | 15 (71.4) | |
| Mastectomy | 65 (34.0) | 24 (28.6) | 15 (45.5) | 20 (37.7) | 6 (28.6) | |
| Cancer Stage at Diagnosis | .136 | |||||
| 0 | 25 (13.1) | 9 (10.7) | 5 (15.2) | 5 (9.4) | 6 (28.6) | |
| 1 | 88 (46.1) | 40 (47.6) | 19 (57.6) | 21 (39.6) | 8 (38.1) | |
| 2+ | 78 (40.8) | 35 (41.7) | 9 (27.3) | 27 (50.9) | 7 (33.3) | |
| Treatment type | .082 | |||||
| Chemotherapy + Radiation | 78 (40.8) | 32 (38.1) | 10 (30.3) | 27 (50.9) | 9 (42.9) | |
| Chemotherapy only | 21 (11.0) | 7 (8.3) | 4 (12.1) | 10 (18.9) | 0 (0.0) | |
| Radiation only | 64 (33.5) | 33 (39.3) | 11 (33.3) | 12 (22.6) | 8 (38.1) | |
| Neither | 28 (14.7) | 12 (14.3) | 8 (24.2) | 4 (7.5) | 4 (19.0) | |
| Endocrine Therapy | .372 | |||||
| No | 55 (29.3) | 25 (30.5) | 9 (27.3) | 18 (34.6) | 3 (14.3) | |
| Yes | 133 (70.7) | 57 (69.5) | 24 (72.7) | 34 (65.4) | 18 (85.7) | |
| Number of Comorbidities | .270 | |||||
| None | 140 (73.3) | 66 (78.6) | 20 (60.6) | 39 (73.6) | 15 (71.4) | |
| 1+ | 51 (26.7) | 18 (21.4) | 13 (39.4) | 14 (26.4) | 6 (28.6) | |
| Body Mass Index (BMI) | 25.70 (5.38) | 24.68 (4.77)a | 25.84 (5.19) | 26.27 (5.70) | 28.23 (6.49)a | .044 |
| Childhood adversity (RF) | 28.06 (10.50) | 26.91 (9.43) | 24.78 (11.17)a | 31.02 (10.76)a | 31.16 (11.01) | .024 |
| Depressive symptoms (BDI-II) | 8.88 (6.86) | 5.91 (5.15)ac | 6.82 (5.78)bd | 12.77 (6.80)cd | 14.00 (7.11)ab | < .001 |
| Sleep disturbance (PSQI) | 7.61 (3.54) | 6.70 (3.48)ab | 7.39 (3.35) | 8.54 (3.38)b | 9.35 (3.51)a | .002 |
| CRP (log mg/L) | 0.04 (1.27) | −0.18 (1.24) | −0.03 (1.29) | 0.36 (1.24) | 0.24 (1.37) | .128 |
| IL1RA (log pg/ml) | 5.37 (0.50) | 5.30 (0.50) | 5.37 (0.47) | 5.44 (0.50) | 5.44 (0.57) | .447 |
| IL6 (log pg/ml) | 0.34 (0.56) | 0.34 (0.58) | 0.28 (0.59) | 0.35 (0.54) | 0.45 (0.50) | .810 |
| sTNFR2 (log pg/ml) | 7.70 (0.26) | 7.65 (0.27)a | 7.74 (0.27) | 7.78 (0.24)a | 7.64 (0.27) | .036 |
| IL6 −174 | .498 | |||||
| GG | 83 (48.0) | 37 (48.7) | 12 (40.0) | 26 (55.3) | 8 (40.0) | |
| GC | 67 (38.7) | 31 (40.8) | 11 (36.7) | 15 (31.9) | 10 (50.0) | |
| CC | 23 (13.3) | 8 (10.5) | 7 (23.3) | 6 (12.8) | 2 (10.0) | |
| TNF −308 | .153 | |||||
| GG | 131 (75.3) | 59 (76.6) | 17 (56.7) | 40 (85.1) | 15 (75.0) | |
| GA | 40 (23.0) | 16 (20.8) | 12 (40.0) | 7 (14.9) | 5 (25.0) | |
| AA | 3 (1.7) | 2 (2.6) | 1 (3.3) | 0 (0.0) | 0 (0.0) | |
| IL1B −511 | .586 | |||||
| CC | 21 (12.2) | 7 (9.1) | 3 (10.0) | 8 (17.4) | 3 (15.8) | |
| CT | 87 (50.6) | 43 (55.8) | 12 (40.0) | 23 (50.0) | 9 (47.4) | |
| TT | 64 (37.2) | 27 (35.1) | 15 (50.0) | 15 (32.6) | 7 (36.8) | |
Note. Values represent mean (standard deviation) for continuous variables and frequency (percent) for categorical variables. P Values refer to a test for bivariate relations between each variable and trajectory group. Pairwise comparisons were conducted for predictors that showed a significant overall effect; groups that differed significantly are indicated by the same letter. BDI-II = Beck Depression Inventory-II, PSQI = Pittsburgh Sleep Quality Index, RF = Risky Families Questionnaire, CRP = C-reactive protein, IL1RA = interleukin-1 receptor agonist, IL6 = interleukin-6, sTNFR2 = soluble receptor for tumor necrosis factor alpha type II. Note that scores on the BDI-II displayed in Table 1 represent the total score, including all items, though for analyses the two energy/fatigue items were deleted.
Identifying Fatigue Trajectories
The best fitting model based on both fit indices was the five group, quadratic time model with random intercepts only (see Supplementary Table S1). The average estimated probability of belonging to each group was high (.82) indicating that most women could be placed in one group with a high degree of certainty (see Supplementary Figure S2). The groups were labeled High (n = 21), characterized by persistently elevated fatigue; Recovery (n = 53), characterized by initially high but decreasing fatigue; Late (n = 33), characterized by initially low but gradually increasing fatigue; Low (n = 65), characterized by initially low and gradually decreasing fatigue; and Very Low (n = 19), characterized by persistently stable and low fatigue. The trajectory parameter estimates from the growth models for each group are in Table 2. The Low and Recovery groups demonstrated significant declines over time, while the Late group significantly increased. The Very Low and High groups were relatively stable. The estimated average trajectories for each group are shown in Figure 2. Mean fatigue levels at T1 are as follows: High group = 16.10, Recovery group = 16.00, Late group = 5.24, Low group = 5.24, and Very Low group = 2.53. Note that fatigue scores in the High and Recovery groups at baseline were approximately three times higher than non-cancer controls (mean for noncancer control group in validation sample = 5.06) (Stein et al., 1998). Supplementary Figure S3 shows the individual fatigue trajectories for each woman in each fatigue trajectory group. There were no large differences in the amount of missing data at each study assessment (T2 to TF) by fatigue trajectory group (graphed in Supplementary Figure S4) and chi-squared tests confirmed that there were no statistically significant differences in amount of missingness at any assessment (all p > .05).
Table 2.
Trajectories by Group from the Final Growth Mixture Model
| Term | Very Low b [95% CI] |
Low b [95% CI] |
Recovery b [95% CI] |
Late b [95% CI] |
High b [95% CI] |
|---|---|---|---|---|---|
| Intercept | 2.21* [0.50, 3.92] |
4.59*** [3.80, 5.38] |
9.26*** [7.97, 10.56] |
8.66*** [6.10, 11.22] |
18.08*** [17.23, 18.93] |
| Years since Treatment (linear slope) |
−0.05 [−0.25, 0.15] |
−0.48* [−0.84, −0.12] |
−1.82*** [−2.28, −1.36] |
0.86* [0.07, 1.65] |
0.34 [−0.12, 0.80] |
| Years since Treatment Squared (quadratic slope) |
0.10* [0.01, 0.18] |
0.05 [−0.10, 0.20] |
0.61*** [0.43, 0.78] |
−0.12 [−0.45, 0.22] |
−0.20 [−0.48, 0.09] |
| Residual Variance | 0.74** [0.23, 1.26] |
7.00*** [3.18, 10.82] |
15.28*** [11.39, 19.16] |
23.84*** [15.71, 31.96] |
8.70*** [5.32, 12.08] |
| N | 19 | 65 | 53 | 33 | 21 |
Note. Values are unstandardized regression coefficients estimated from the growth mixture model for each group with 95% confidence intervals in brackets. N = estimated number of women in each group. The intercept was centered at two-years post treatment.
p < .05,
p < .01,
p < .001
Figure 2.
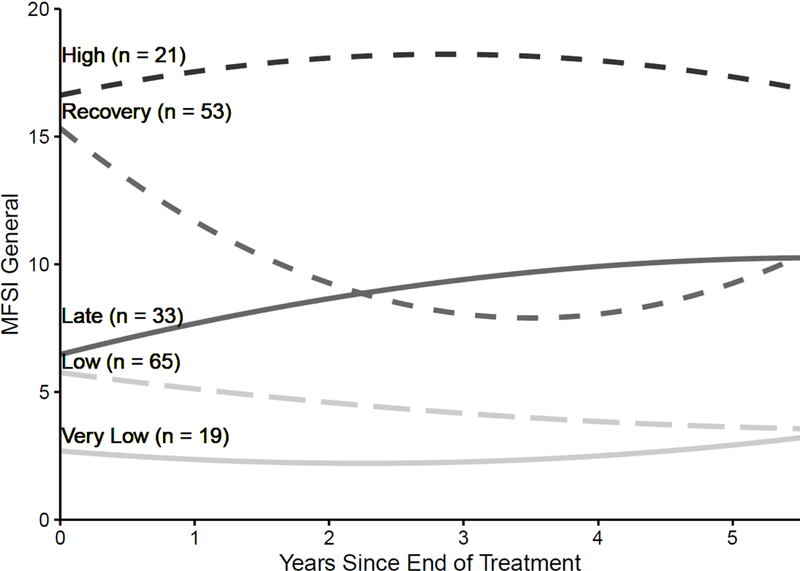
Estimated trajectories of fatigue in the 5 groups over the follow-up period.
Predicting Fatigue Trajectory Groups
Because both the Very Low and Low Groups had relatively low fatigue throughout, for simplicity they were collapsed and are simply called Low when testing predictors. Table 1 shows descriptive statistics for potential predictors by latent trajectory group along with tests for bivariate relations. There were significant associations between BMI, childhood adversity, depressive symptoms, subjective sleep quality, and sTNFR2 levels and fatigue group (all ps < .05) and a trend for cancer treatment (p < .10). The High and Recovery groups had the highest levels of depressive symptoms, childhood adversity, sleep disturbance, and BMI, whereas the Low group had the lowest levels of these predictors (except childhood adversity, which was lowest in the Late group). The High group also had the highest rate of endocrine therapy (85.7% received endocrine therapy). In contrast, the Recovery group had the highest rate of chemotherapy (69.8%) and the lowest rate of endocrine therapy (65.4%). Among the biological predictors, the inflammatory marker sTNF-RII was elevated in the Recovery group relative to the Low group.
To examine the unique predictive utility of these factors, they were entered along with an a priori predictor, endocrine therapy, simultaneously into a multinomial logistic regression predicting trajectory group. For the regression analysis, cancer treatment was coded as chemotherapy (+/− radiation), radiation alone, or neither. Likelihood ratio tests of the overall significance of each predictor revealed that only T1 depressive symptoms was uniquely significant overall, with trends for cancer treatment and childhood adversity (Table 3). Results for individual pairwise contrasts between trajectory groups in the multivariate model are presented in detail in Table 3 and summarized here. In terms of treatment exposures, women who received chemotherapy (with or without radiation therapy) were significantly more likely to be in the Recovery group than either the High or Late group. In contrast, those who received endocrine therapy were more likely to be in the High group than the Recovery group. In terms of psychological factors, women with higher levels of depressive symptoms at T1 were more likely to be in the High or Recovery groups than the Low group, and women with higher levels of childhood adversity were more likely to be in the High or Recovery group than the Late group. No factor distinguished the Low from the Late group.
Table 3.
Multinomial Logistic Regression Analyses Predicting Latent Trajectory Group
| Low v High |
Late v High |
Recovery v High |
Low v Recovery |
Late v Recovery |
Low v Late |
P Value | |
|---|---|---|---|---|---|---|---|
| Treatment (ref=neither) | .089 | ||||||
| Chemotherapy+ | 6.48 [0.90, 46.61] |
1.63 [0.20, 13.34] |
37.85** [2.75, 520.80] |
0.17 [0.02, 1.81] |
0.04* [0.00, 0.54] |
3.87 [0.82, 19.10] |
|
| Radiation Only | 2.52 [0.39, 16.27] |
0.93 [0.12, 6.97] |
10.51 [0.75, 148.14] |
0.24 [0.02, 2.68] |
0.09 [0.01, 1.17] |
2.72 [0.58, 12.80] |
|
| Endocrine Therapy | 0.16 [0.03, 1.01] |
0.33 [0.05, 2.44] |
0.14* [0.02, 0.85] |
1.21 [0.42, 3.52] |
2.44 [0.59, 10.07] |
0.50 [0.14, 1.75] |
.136 |
| BMI | 0.56* [0.32, 1.01] |
0.74 [0.40, 1.37] |
0.70 [0.39, 1.26] |
0.80 [0.49, 1.32] |
1.05 [0.60, 1.84] |
0.76 [0.46, 2.02] |
.274 |
| Depressive symptoms | 0.33** [0.15, 0.72] |
0.35* [0.15, 0.84] |
0.73 [0.37, 1.43] |
0.46* [0.25, 0.84] |
0.48 [0.22, 1.02] |
0.96 [0.48, 2.31] |
.014 |
| Childhood adversity | 0.65 [0.32, 1.32] |
0.40* [0.17, 0.92] |
0.85 [0.42, 1.72] |
0.77 [0.49, 1.19] |
0.47* [0.24, 0.90] |
1.64 [0.89, 3.04] |
.087 |
| Sleep disturbance | 0.60 [0.27, 1.31] |
0.79 [0.33, 1.85] |
0.86 [0.38, 1.95] |
0.69 [0.42, 1.15] |
0.91 [0.49, 1.69] |
0.76 [0.45, 1.29] |
.365 |
| sTNFR2 (log pg/ml) | 1.23 [0.61, 2.49] |
1.95 [0.90, 4.21] |
1.65 [0.80, 3.42] |
0.75 [0.46, 1.22] |
1.18 [0.65, 2.15] |
0.63 [0.38, 1.06] |
.169 |
Note. Values are odds ratios. For continuous variables (BMI, BDI, Risky Families, PSQI, sTNFR2), predictors were standardized. In this table, Low = membership in the Very Low or Low fatigue groups. Due to missing data on individual predictors, N = 150. P values indicate the multivariate statistical significance of a predictor independent of all other factors in the model.
p < .05,
p < .01
DISCUSSION
This study examined trajectories of post-treatment fatigue in women with early-stage breast cancer who were followed for up to 6 years after treatment. We identified 5 distinct trajectories, which generally represent four different patterns of fatigue. The majority of women (44%) had either low or very low levels of fatigue throughout the study period. This is clearly a low risk group, whose fatigue levels are comparable to women of similar age with no cancer history (Stein et al., 1998). A Late group (17%) had low fatigue at the initial assessment but gradually increased over the follow-up period. A Recovery group (28%) had high fatigue at the initial post-treatment assessment that declined over the follow-up period, but never reached the level of the Low/Very Low group. Finally, a small High group (11%) of women had elevated fatigue across the study period.
These trajectories identify several at risk groups of women, particularly those in the High and Recovery groups whose fatigue levels were approximately 3 times higher than non-cancer controls at post-treatment (Stein et al., 1998). Psychological factors emerged as the strongest predictors of membership in these groups, particularly depressive symptoms and childhood adversity, which discriminated the High and Recovery groups from the Low and Late groups in bivariate and multivariate analyses. Women completed the measure of depressive symptoms (BDI-II) within 3 months after treatment completion, when symptoms are typically declining (Henselmans et al., 2010). However, the mean score for women in the High group was 14, which falls into the “mild” range of major depression (Beck, Steer, Brown, & G.K, 1996) and is elevated both in this sample and relative to other samples of women with breast cancer (who typically score below clinical thresholds; (Stanton & Bower, 2015)). Fatigue and depression are closely related in the context of cancer (Jacobsen, Donovan, & Weitzner, 2003) and previous studies have shown that history of depression (Andrykowski, Schmidt, Salsman, Beacham, & Jacobsen, 2005) and depressive symptoms predict post-treatment fatigue (Dhruva et al., 2010; Geinitz et al., 2004). Overall, these findings highlight the importance of depression as a risk factor for persistent fatigue in the years after breast cancer treatment.
Our findings also identify childhood adversity as a risk factor for persistent fatigue. Women in the High and Recovery groups reported higher levels of abuse, neglect, and household conflict and disorganization during their childhood years than women in the Very Low/Low and Late groups, and relative to other samples of breast cancer survivors (McFarland et al., 2016). Childhood adversity is known to predict a range of poor mental and physical health outcomes in adulthood (Felitti et al., 1998), and a handful of studies have documented links with cancer-related fatigue (Bower et al., 2014; C. P. Fagundes et al., 2012; Han et al., 2016; Witek et al., 2013). The mechanisms through which childhood adversity influences fatigue in cancer patients have not been determined, but may include alterations in neural, neuroendocrine, immune, and/or behavioral processes of potential relevance for fatigue (Heim et al., 2009; Kuhlman, Chiang, Horn, & Bower, 2017; Lupien, McEwen, Gunnar, & Heim, 2009; Miller, Chen, & Parker, 2011).
Treatment exposures were also uniquely associated with fatigue trajectories, and specifically discriminated between the High and Recovery groups. Here, the pattern of results suggested that chemotherapy may have contributed to the acute elevations in fatigue observed in the Recovery group (who had the highest rate of chemotherapy exposure), whereas endocrine therapy may have contributed to the persistent fatigue observed in the High group (who had the highest rate of endocrine therapy exposure). Another potential at risk group is the Late group, who demonstrated an increase in fatigue over time and eventually reached the level seen in the Recovery group. However, risk factors for membership in this group were not evident in this sample. Of note, neither demographic nor medical factors, including comorbidities, were associated with fatigue trajectories.
In terms of biological risk factors, results of bivariate analyses showed that one of the inflammatory markers assessed, soluble TNF receptor type II, was elevated in the Recovery group relative to those in the Very Low/Low group. We have previously shown an association between fatigue and sTNF-RII in a subset of this cohort at the T1 assessment, particularly among women treated with chemotherapy (Bower et al., 2011). The current results are consistent with these earlier findings and suggest that chemotherapy-related elevations in sTNF-II are associated with higher levels of fatigue in the immediate aftermath of treatment. We did not find an association between cytokine-related genetic variants and fatigue trajectories, in contrast with our earlier findings showing an association between high expression variants in proinflammatory cytokine genes and immediate post-treatment fatigue at T1 in this cohort (Bower et al., 2013).
To our knowledge, only three previous studies have examined trajectories of cancer-related fatigue in the months after cancer treatment (Bodtcher et al., 2015; Donovan et al., 2007; Kober et al., 2016). All identified two trajectories, high and low, though the percentage of patients falling into the two groups differed across samples. The longer follow-up in the current study may have provided more of an opportunity to identify distinct trajectories, including changes in fatigue that became evident several years after treatment completion. The trajectory groups identified here are more consistent with those observed in studies of adjustment to bereavement and other stressful life events described by Bonanno (Bonanno et al., 2011). In particular, the Low/Very Low fatigue group resembles the “resilient” profile, which is characterized by minimal symptoms, whereas the High and Recovery groups resemble the “chronic” and “recovering” profiles, respectively. Bonanno (2011) suggests that there are multiple, independent predictors of resilient outcomes which may vary based on timing and context, consistent with our results. There may also be common risk and resilience factors across stressors and potentially, across outcomes. Thus, research on adjustment to cancer may benefit from incorporating predictors identified in the broader literature on adjustment to stressful life events; similarly, research on trajectories of adjustment to stress could be enhanced by considering predictors identified in the context of cancer as well as outcomes that are important in this group, including fatigue.
A notable strength of the study is the long follow-up, with women an average of 4.3 years after treatment at the final study assessment compared to 6- and 8-months in previous studies. However, the sample sizes in individual fatigue trajectory groups were modest (19 to 65), resulting in limited power to detect baseline factors that distinguish trajectory groups. Other treatment or sociodemographic factors may have emerged as significant predictors in a larger sample. On a related note, although this study included key biological and behavioral variables associated with fatigue in previous research, we did not assess risk factors such as physical activity and fatigue catastrophizing that may also play an important role in persistent fatigue (Bodtcher et al., 2015; Donovan et al., 2007). Further, the sample was relatively homogeneous, reflecting the geographic area from which women were recruited and assessed. The extent to which these results will hold for other populations requires careful consideration in future research. Finally, there was a substantial amount of missing data at later study assessments (T4 to TF); however, there was no evidence that missingness differed between fatigue trajectory classes. Furthermore, retention was excellent (>90%) for the first three study assessments, and most classes evidenced stable trajectories or (in the case of the Recovery class) had most change occur in the first two years after treatment completion, where data were relatively complete.
Overall, these findings highlight substantial individual variability in response to cancer diagnosis and treatment and identify distinct subgroups of patients with very different experiences of fatigue in the years post-treatment. Psychological factors increase a woman’s risk of experiencing fatigue, both in the immediate aftermath of treatment and over the following years. Treatment exposures also play a role, and may interact with host vulnerabilities to promote and maintain fatigue in otherwise healthy survivors. Future research should interrogate the pathways linking risk factors with cancer-related fatigue, which will facilitate the development and deployment of targeted interventions for those most at risk. For example, we have recently shown that the association between childhood trauma and depressive symptoms in women with breast cancer is mediated by deficits in mindfulness and optimism (Kuhlman, Boyle, et al., 2017), suggesting a potential target for intervention (i.e., mindfulness-based therapy). Similarly, determination of risk factors and mechanisms for cancer-related fatigue could be used to help screen and provide early intervention for vulnerable patients, which should reduce the burden of fatigue in the growing population of breast cancer survivors.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01-CA109650), the Cousins Center for Psychoneuroimmunology, and the Breast Cancer Research Foundation (BCRF). PAG is a member of the scientific advisory board of the BCRF.
REFERENCES
- Andrykowski MA, Donovan KA, Laronga C, & Jacobsen PB (2010). Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer, 116(24), 5740–5748. doi: 10.1002/cncr.25294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, & Jacobsen PB (2005). Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol, 23(27), 6613–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis NE, Levine BJ, Case LD, Naftalis EZ, & Van Zee KJ (2015). Trajectories of depressive symptoms following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev, 24(11), 1789–1795. doi: 10.1158/1055-9965.EPI-15-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsevick A, Frost M, Zwinderman A, Hall P, & Halyard M (2010). I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual.Life Res, 19(10), 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996). BDI-II Manual (Vol. 2). San Antonio: The Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). BDI-II manual. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- Bodtcher H, Bidstrup PE, Andersen I, Christensen J, Mertz BG, Johansen C, & Dalton SO (2015). Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual Life Res, 24(11), 2671–2679. doi: 10.1007/s11136-015-1000-0 [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Westphal M, & Mancini AD (2011). Resilience to loss and potential trauma. Annu Rev Clin Psychol, 7, 511–535. doi: 10.1146/annurev-clinpsy-032210-104526 [DOI] [PubMed] [Google Scholar]
- Bower JE (2014). Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol, 11(10), 597–609. doi: 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, & Slavich GM (2014). Childhood Adversity and Cumulative Life Stress: Risk Factors for Cancer-Related Fatigue. Clin Psychol.Sci, 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, & Belin TR (2006). Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer, 106(4), 751–758. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, & Cole SW (2013). Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol, 31(13), 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, & Cole SW (2011). Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol, 29(26), 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Hoch CC, Yeager AL, & Kupfer DJ (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14(4), 331–338. [PubMed] [Google Scholar]
- Carlson LE, Waller A, Groff SL, Giese-Davis J, & Bultz BD (2013). What goes up does not always come down: patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psychooncology, 22(1), 168–176. doi: 10.1002/pon.2068 [DOI] [PubMed] [Google Scholar]
- Cella D, Davis K, Breitbart W, & Curt G (2001). Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J.Clin.Oncol, 19(14), 3385–3391. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, & Irwin MR (2006). Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res, 12(9), 2759–2766. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, . . . Miaskowski C (2010). Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs, 33(3), 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, . . . McGrath P (2004). Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom.Manage, 28(4), 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Munster P, & Jacobsen PB (2007). Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol, 26(4), 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, & Duncan SC (1995). Modeling the processes of development via latent variable growth curve methodology. Structural Equation Modeling, 2(3), 187–213. [Google Scholar]
- Dunn LB, Cooper BA, Neuhaus J, West C, Paul S, Aouizerat B, . . . Miaskowski C (2011). Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol, 30(6), 683–692. doi: 10.1037/a0024366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457. [Google Scholar]
- Fagundes C, LeRoy A, & Karuga M (2015). Behavioral Symptoms after Breast Cancer Treatment: A Biobehavioral Approach. J Pers Med, 5(3), 280–295. doi: 10.3390/jpm5030280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Lindgren ME, Shapiro CL, & Kiecolt-Glaser JK (2012). Child maltreatment and breast cancer survivors: social support makes a difference for quality of life, fatigue and cancer stress. Eur.J Cancer, 48(5), 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, . . . Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am.J Prev.Med, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, . . . Cole SW (2013). Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behavior and Immunity, 30 Suppl, S99–108. doi: 10.1016/j.bbi.2012.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DH, . . . Belin TR (2013). Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst, 105(11), 791–801. doi: 10.1093/jnci/djt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Petersen L, Castellon SA, Bower JE, Silverman DH, Cole SW, . . . Belin TR (2014). Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol, 32(31), 3559–3567. doi: 10.1200/JCO.2014.56.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinitz H, Zimmermann FB, Thamm R, Keller M, Busch R, & Molls M (2004). Fatigue in patients with adjuvant radiation therapy for breast cancer: long-term follow-up. J Cancer Res Clin Oncol, 130(6), 327–333. doi: 10.1007/s00432-003-0540-9 [DOI] [PubMed] [Google Scholar]
- Goldstein D, Bennett BK, Webber K, Boyle F, de Souza PL, Wilcken NR, . . . Lloyd AR (2012). Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol, 30(15), 1805–1812. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, & Wiley JF (in press). MplusAutomation: An R package for facilitating large-scale latent variable analyses in Mplus. Structural Equation Modeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TJ, Felger JC, Lee A, Mister D, Miller AH, & Torres MA (2016). Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology, 25(2), 187–193. doi: 10.1002/pon.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nater UM, Maloney E, Boneva R, Jones JF, & Reeves WC (2009). Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch.Gen.Psychiatry, 66(1), 72–80. [DOI] [PubMed] [Google Scholar]
- Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, . . . Reeves WC (2006). Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Arch.Gen.Psychiatry, 63(11), 1258–1266. [DOI] [PubMed] [Google Scholar]
- Henselmans I, Helgeson VS, Seltman H, De Vries J, Sanderman R, & Ranchor AV (2010). Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol, 29(2), 160–168. [DOI] [PubMed] [Google Scholar]
- Hope AC (1968). A simplified Monte Carlo significance test procedure. Journal of the Royal Statistical Society. Series B (Methodological), 30(3), 582–598. [Google Scholar]
- Jacobsen PB, Donovan KA, & Weitzner MA (2003). Distinguishing fatigue and depression in patients with cancer. Semin.Clin.Neuropsychiatry, 8(4), 229–240. [PubMed] [Google Scholar]
- Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, & Lyman GH (1999). Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J.Pain Symptom.Manage ., 18(4), 233–242. [DOI] [PubMed] [Google Scholar]
- Jung T, & Wickrama K (2008). An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass, 2(1), 302–317. [Google Scholar]
- Junghaenel DU, Cohen J, Schneider S, Neerukonda AR, & Broderick JE (2015). Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy. Support Care Cancer, 23(9), 2579–2587. doi: 10.1007/s00520-015-2616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober KM, Smoot B, Paul SM, Cooper BA, Levine JD, & Miaskowski C (2016). Polymorphisms in Cytokine Genes Are Associated With Higher Levels of Fatigue and Lower Levels of Energy in Women After Breast Cancer Surgery. J Pain Symptom Manage, 52(5), 695–708 e694. doi: 10.1016/j.jpainsymman.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Boyle CC, Irwin MR, Ganz PA, Crespi CM, Asher A, . . . Bower JE (2017). Childhood maltreatment, psychological resources, and depressive symptoms in women with breast cancer. Child Abuse Negl, 72, 360–369. doi: 10.1016/j.chiabu.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci Biobehav Rev, 80, 166–184. doi: 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourt TE, & Heijnen CJ (2017). Mechanisms of Neurotoxic Symptoms as a Result of Breast Cancer and Its Treatment: Considerations on the Contribution of Stress, Inflammation, and Cellular Bioenergetics. Curr Breast Cancer Rep, 9(2), 70–81. doi: 10.1007/s12609-017-0245-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WW, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, . . . Fielding R (2010). Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology, 19(10), 1044–1051. doi: 10.1002/pon.1658 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev.Neurosci, 10(6), 434–445. [DOI] [PubMed] [Google Scholar]
- McFarland DC, Andreotti C, Harris K, Mandeli J, Tiersten A, & Holland J (2016). Early Childhood Adversity and its Associations With Anxiety, Depression, and Distress in Women With Breast Cancer. Psychosomatics, 57(2), 174–184. doi: 10.1016/j.psym.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol.Bull, 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SA (2010). Cancer-related fatigue: state of the science. PM.R, 2(5), 364–383. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LV, van der WE, . . . de Vries EG (2005). Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol, 23(33), 8296–8304. [DOI] [PubMed] [Google Scholar]
- Rottmann N, Hansen DG, Hagedoorn M, Larsen PV, Nicolaisen A, Bidstrup PE, . . . Johansen C (2016). Depressive symptom trajectories in women affected by breast cancer and their male partners: a nationwide prospective cohort study. J Cancer Surviv, 10(5), 915–926. doi: 10.1007/s11764-016-0538-3 [DOI] [PubMed] [Google Scholar]
- Saligan LN, & Kim HS (2012). A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun, 26(6), 830–848. doi: 10.1016/j.bbi.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, & Bower JE (2015). Psychological Adjustment in Breast Cancer Survivors. Adv Exp Med Biol, 862, 231–242. doi: 10.1007/978-3-319-16366-6_15 [DOI] [PubMed] [Google Scholar]
- Stanton AL, Rowland JH, & Ganz PA (2015). Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol, 70(2), 159–174. doi: 10.1037/a0037875 [DOI] [PubMed] [Google Scholar]
- Stanton AL, Wiley JF, Krull JL, Crespi CM, Hammen C, Allen JJ, . . . Weihs KL (2015). Depressive episodes, symptoms, and trajectories in women recently diagnosed with breast cancer. Breast Cancer Res Treat, 154(1), 105–115. doi: 10.1007/s10549-015-3563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, & Thors C (2004). Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage, 27(1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, & Jacobsen PB (1998). A multidimensional measure of fatigue for use with cancer patients. Cancer Pract, 6(3), 143–152. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, & Seeman TE (2004). Early environment, emotions, responses to stress, and health. Journal of Personality, 72(6), 1365–1394. [DOI] [PubMed] [Google Scholar]
- Wang T, Yin J, Miller AH, & Xiao C (2017). A systematic review of the association between fatigue and genetic polymorphisms. Brain Behav Immun, 62, 230–244. doi: 10.1016/j.bbi.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek JL, Tell D, Albuquerque K, & Mathews HL (2013). Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav.Immun, 30 Suppl, S149–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AG, & Hallquist MN (2014). Mixture modeling methods for the assessment of normal and abnormal personality, part II: longitudinal models. Journal of Personality Assessment, 96(3), 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


