1. Introduction
The World Health Organization (WHO) has reported that pneumonia continues to be a significant cause of mortality in the pediatric age group for children under five years of age, where it accounts for nearly one-fifth of childhood deaths worldwide [1]. In this report, we provide the most up to date guidelines for the management of community-acquired pneumonia (CAP) in infants and children aged over 90 days. The current recommendations given by the Pediatric Infectious Diseases Society and Infectious Diseases Society of America 2011 Practice guidelines, WHO, and other international guidelines are considered as well as regional variations in susceptibility patterns and resources [[1], [2], [3], [4], [5], [6]]. Some published and unpublished antibiograms from several tertiary care organizations in the regions comprising Saudi Arabia, United Arab Emirates, Kuwait, and Oman have shown significant improvements in the susceptibility pattern of Streptococcus pneumoniae to penicillin for the last three years, so the current recommendations have been customized accordingly [2,3]. These guidelines cover the diagnosis and therapeutic options for the management of CAP in children. The definition of children-specific guidelines for the management of CAP can reduce morbidity and mortality. We present a clinical statement from the Saudi Pediatric Infectious Diseases Society (SPIDS) concerning the management of CAP in children.
2. Definition
-
A.
CAP: Defined as chest infection acquired outside a hospital or long-term care facility. CAP occurs within 48 hours of hospital admittance or in a patient presenting with pneumonia who has no features of healthcare-associated pneumonia (i.e., admission to hospital for two or more days within three months of infection; resident in long-term care facility; received recent chemotherapy, or wound care within the past 30 days of the current infection; or attended a hospital or hemodialysis clinic) [7].
-
B.
Complicated pneumonia: Chest infection associated with a complication such as parapneumonic effusion, empyema, lung abscess, or necrotizing pneumonia [4]. According to this guideline, we consider a patient with severe pneumonia (patient who requires intermediate or intensive care unit (ICU), nasal intermittent positive pressure ventilation, or invasive mechanical ventilation, or with fluid refractory shock) as a part of complicated pneumonia.
2.1. Outpatient vs. inpatient management
-
A.
Consider hospitalization for an infant or a child with suspected CAP if their condition is associated with one or more of the manifestations in Table 1 [8].
-
B.
An infant or a child with CAP should be admitted to an ICU or intermediate care unit with continuous cardiorespiratory monitoring if the illness is associated with any manifestation presented in Table 2 [8].
- C.
Table 1.
Criteria to consider hospitalization for pediatric CAP∗.
|
|
|
|
|
|
|
|
|
|
|
∗Adapted from Messinger AI, Kupfer O, Hurst A, Parker S. Management of Pediatric Community-acquired Bacterial Pneumonia. Pediatrics in review. 2017 Sep; 38(9):394 [8].
Table 2.
Criteria to Consider IMCU/ICU admission for Pediatric CAP∗.
|
|
∗Adapted with modification from Messinger AI, Kupfer O, Hurst A, Parker S. Management of Pediatric Community-acquired Bacterial Pneumonia. Pediatrics in review. 2017 Sep; 38(9):394 [8].
3. Diagnostic testing
-
A.
Complete blood count: Routine estimation of the complete blood count is not essential in all cases of suspected CAP managed in an outpatient setting but it may be helpful for the assessment of patients who require hospital admission.
-
B.
Acute-phase reactants such as the erythrocyte sedimentation rate and C-reactive protein can support a clinical assessment for managing the development strategy and in a response to therapy evaluation, particularly for complicated CAP.
-
C.Serum procalcitonin (PCT)
-
•PCT does not need to be measured routinely. However, if available, it may be helpful for differentiating the etiology of pneumonia and severity when used in addition to clinical, epidemiological, and other diagnostic testing.
- •
-
•PCT concentrations < 0.1 ng/mL have a very high negative predictive value, where they efficiently exclude typical bacterial CAP [9].
-
•
-
D.Blood culture
-
•Blood culture (outpatient): Blood culture is not necessary routinely unless the patient's condition is deteriorating post-antibiotic therapy.
-
•Blood culture (inpatient): Blood culture should be conducted for children who require hospitalization for assumed bacterial CAP.
-
•Sufficient volumes of blood will probably yield a pathogen, so the microbiology laboratory should be contacted for the recommended blood volume required according to the age of the patient [11].
-
•
-
E.Respiratory viral studies
-
•Tests for influenza virus infection and other respiratory viruses ((i.e., direct fluorescent antibody or enzyme immunoassay tests) should be utilized as a part of the assessment of children with CAP, if available.
-
•If available, nasopharyngeal aspiration for viral multiplex PCR may help to determine a viral cause and reduce the need for antibiotics.
-
•
-
F.Chest radiography
-
•Chest radiographs (outpatient): Posteroanterior chest radiographs are not required to confirm the CAP diagnosis but they should be performed for patients with suspected or recorded hypoxia or those with significant respiratory distress, as well as for patients who fail to respond to initial antimicrobial treatment to check for pneumonia complications.
-
•Chest radiographs (Inpatient): Perform chest radiographs for all patients hospitalized with CAP to outline the size and characteristics of parenchymal infiltrates, and to recognize complications of pneumonia that may require prompt intervention rather than antimicrobial therapy.
-
•Follow-up chest radiographs
-
oRepeat chest imaging in individuals who do not exhibit improvement within 48–72 hours following initiation of appropriate antimicrobial therapy.
-
oFor complicated pneumonia with parapneumonic effusion status post-therapeutic intervention, repeated imaging is not recommended daily if the patient is clinically stable.
-
oRepeat chest radiography if a patient with complicated pneumonia becomes unstable, worsens clinically, or has persistent fever for over 48–72 hours while receiving appropriate antimicrobial therapy.
-
o
-
•
4. Treatment
- Table 3 lists he preferred empiric therapeutic agents and alternative agents for children with CAP when managed as an outpatient.
Table 3.
Empiric therapy for pediatric community-acquired pneumonia (CAP) – outpatient.
| Site of Care (outpatient) |
Empiric Therapy for Presumed Bacterial Pneumonia |
Empiric Therapy for Presumed Atypical Pneumonia |
Empiric Therapy for Presumed Influenza Pneumonia |
||||
|---|---|---|---|---|---|---|---|
| Antibiotic | Route | Regimen | Antibiotic | Route | Regimen | Antiviral | |
| Initial therapy | Amoxicillin | ∗∗PO | 90 mg/kg/day divided 3 times daily | Azithromycin | PO | Given as a single daily dose; 10 mg/kg on day 1; 5 mg/kg on days 2–5 | Oseltamivir |
| Alternative | ∗Amoxicillin clavulanate | PO | Amoxicillin component, 90 mg/kg/day divided 3 times daily | Clarithromycin | PO | 15 mg/kg/day in 2 doses for 7–14 days | |
| Or ∗Cefuroxime |
PO | 30mg/kg/day divided q12h | Erythromycin | PO | 40mg/kg/day in 4 doses | ||
∗Consider in children whom not completing Haemophilus influenzae type b (Hib) vaccines series (three doses). ∗∗PO Orally. Doses was adapted from 2017 Nelson's Pediatric Antimicrobial Therapy, 23rd Ed.
- Table 4 lists the preferred empiric therapeutic agents and alternative agents for children with CAP when managed as an inpatient.
Table 4.
Empiric therapy for pediatric community-acquired pneumonia (CAP) – inpatient.
| Site of Care (Inpatient). | Empiric Therapy for Presumed Bacterial Pneumonia |
Empiric Therapy for Presumed Atypical Pneumonia |
Empiric Therapy for Presumed Influenza Pneumonia |
|||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic | Route | Regimen | Antibiotic | Route | Regimen | Antiviral | ||
| Uncomplicated CAP. | Initial therapy | Ampicillin | IV | 200 mg/kg/day divided every 6 hr. | Azithromycin | IV/PO | Given as a single daily dose; 10 mg/kg on day 1; 5 mg/kg on days 2 to ss 5 | Oseltamivir |
| Or Penicillin G | IV | 250,000-400,000 U/kg/day divided every 4–6h | ||||||
| Alternative | *Amoxicillin clavulanate | IV | 90 mg/kg/day divided every 8 hr. | Clarithromycin | PO | 15 mg/kg/day in 2 doses for 7–14 days. | ||
| Or *Cefuroxime | IV | 150 mg/kg/day divided every 8 hr. | Or Erythromycin | IV/PO | 40mg/kg/day in 4 doses | |||
| Complicated CAP. | Ceftriaxone | IV | 75mg/kg/day every 24 hr. | Same Empiric Therapy for Presumed Atypical Pneumonia in Uncomplicated Pneumonia. | ||||
| **Clindamycin | IV | 40mg/kg/day divided every q8h | ||||||
| Or**Vancomycin | IV | 60mg/kg/day divided every q8h | ||||||
Doses was adapted from 2017 Nelson's Pediatric Antimicrobial Therapy, 23rd Ed.
*Consider in children whom not completing Haemophilus influenzae type b (Hib) vaccines series (three doses).
** Consider if infection suspected due to community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) especially in cases of Necrotizing pneumonia, Sepsis, Concurrent skin infection due to MRSA, and/or Previous MRSA colonization. IV Intravenously; PO Orally.
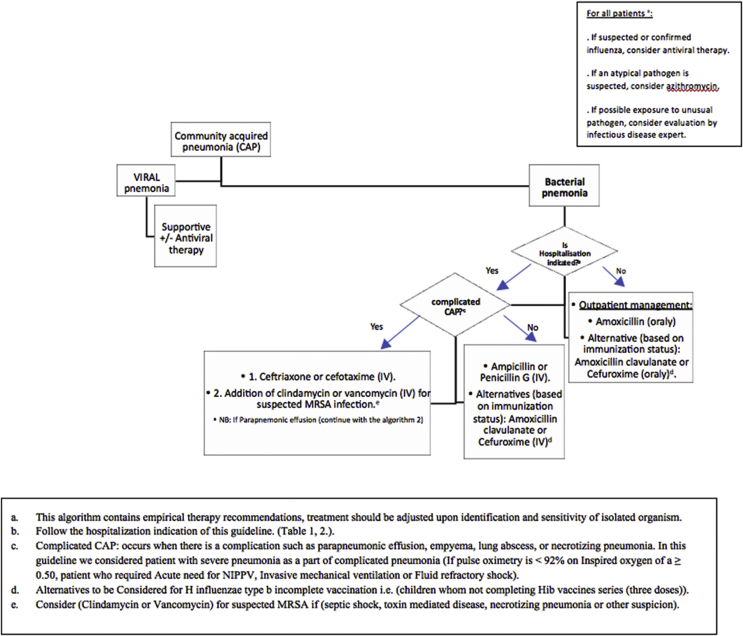
- Fig. 1 shows the SPIDS recommendations for the management of CAP in children aged >3 months.
-
A.Anti-infective therapy (outpatient)
-
•Antibiotic treatment is not required regularly for preschool aged children with CAP because most cases are due to viral infections.
- •
-
•SPIDS recommends amoxicillin-clavulanate or second-generation cephalosporin (cefuroxime) as an alternative for inappropriately immunized infants and children, i.e., children who have not completed Haemophilus influenzae type b (Hib) vaccine series (three doses).
-
•Consider macrolide antibiotics for the treatment of individuals (primarily school-aged children and adolescents) with suspected atypical pneumonia.
-
•Influenza antiviral therapy should be ordered as soon as possible for children with CAP consistent with clinical influenza virus infection [5].
-
•
-
B.Anti-infective therapy (inpatient)
-
•SPIDS recommends penicillin G or ampicillin for an infant or child admitted to a hospital ward with CAP.
-
•Amoxicillin-clavulanate or second-generation cephalosporin (cefuroxime) should be considered as an alternative for inappropriately immunized infants and children, i.e., children who have not completed Hib vaccine series (three doses).
-
•Consider a third-generation parenteral cephalosporin (ceftriaxone or cefotaxime) for hospitalized infants and children admitted with complicated pneumonia.
-
•Add macrolide in addition to a B-lactam antibiotic for hospitalized child with suspected atypical pneumonia.
-
•Clindamycin or vancomycin should be provided in addition to B-lactam therapy if clinical, laboratory, or imaging characteristics are consistent with infection caused by Staphylococcus aureus.
-
•
-
C.Measures to minimize antibiotic resistance
-
•Limiting antibiotic exposure whenever possible is highly recommended.
-
•Using the most narrow-spectrum antibiotic for the suspected or identified pathogen is a primary goal of therapy.
-
•Treating for the shortest possible duration will minimize antibiotic exposure and selection for resistance.
-
•
-
D.Duration of antimicrobial therapy
-
•SPIDS recommends treatment courses of 5–7 days for most cases of mild, uncomplicated disease managed on an outpatient basis [10].
-
•SPIDS recommends a treatment duration of 7–10 days for more severe uncomplicated cases.
-
•Long treatment courses may be required for complicated CAP (see parapneumonic effusion management).
-
•Infections caused by specific pathogens, especially community-associated methicillin-resistant Staphylococcus aureus, may require more prolonged treatment than those due to Streptococcus pneumoniae.
- •
-
•
-
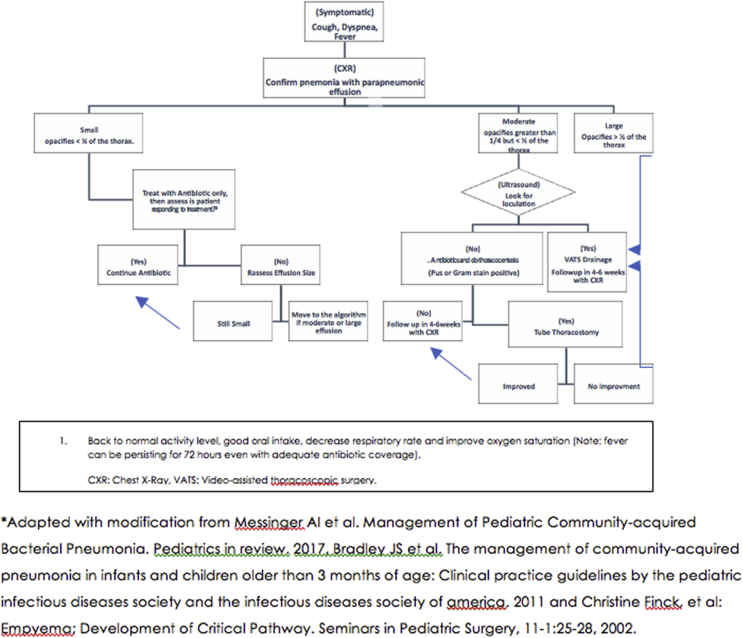
E.Parapneumonic effusion management
- •
-
•Patients with clinically significant hypoxemia, hypercapnia, and positive pressure requirements, or with complete respiratory failure requiring intubation and ventilation will probably benefit from drainage [8].
-
•Other indications for drainage of the pleural space include the finding of thick pus during diagnostic thoracentesis, presence of fever and systemic illness after 5–7 days of therapy, presence of effusion for more than 10 days, and in cases of toxic shock with Staphylococcus aureus, debulking disease and thus toxins [8].
-
•If pleural fluid aspiration is successful, then consider bacteriological examinations (Gram staining and culture).
-
•Pleural fluid white blood cell count with white cell differential analysis are recommended mainly to help differentiate bacterial, mycobacterial, and malignant etiologies.
-
•Analyses of pleural liquid parameters, i.e., pH, glucose, protein, and lactate dehydrogenase, rarely change the management plan and they are generally NOT suggested [4].
-
•Consider removing a chest tube in the absence of an intrathoracic air leak and when pleural fluid drainage is less than 1 mL/kg/24 hours [4].
-
•Antibiotic therapy for parapneumonic effusions:
-
oEmpirical therapy should be applied as suggested for cases with complicated CAP. The appropriate regimen may need to be adjusted according to the susceptibility pattern if the organism is isolated from blood or pleural fluid culture.
-
oThe period of antibiotic treatment depends on the effectiveness of drainage and the clinical response by each child with complicated CAP. In most cases, antibiotic therapy for 2–4 weeks is sufficient.
-
o
Fig. 1.
Saudi Pediatric Infectious Diseases Society recommendations for the management of community-acquired pneumonia in children aged >3 months.
Fig. 2.
Saudi Pediatric Infectious Diseases Society recommendations for the management of pneumonia with parapneumonic effusion.
- Fig. 2 [13,14], shows the SPIDS recommendations for the management of pneumonia with parapneumonic effusion.
-
F.Clinical course and follow-up for uncomplicated CAP
-
•Clinical improvement usually noted in the first three days after starting appropriate antibiotic treatment.
-
•Failure to respond to initial therapy after three days of antibiotics requires clinical, radiological, and laboratory reassessment to determine the current severity of the illness and to anticipate progression in order to determine whether higher levels of care or support are needed.
-
•
-
G.Discharge criteria discharge criteria from healthcare institutes are as follows.
-
•Improvement in the general clinical condition in the form of good activity, appetite, baseline respiratory status, and fever resolved for at least one day.
- •
-
•Compliance with oral antimicrobial thereby if it needs to be continued after discharge.
-
•
5. Prevention
-
A.
SPIDS recommends strict adherence to the Saudi national immunization program.
-
B.
SPIDS recommend annual influenza vaccine for all infants and children aged 6 months or older.
-
C.
SPIDS recommends respiratory syncytial virus (RSV) monoclonal antibody for high-risk infants to minimize the occurrence and sequelae of RSV infection.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.World Health Organization . World Health Organization; Geneva: 2014. Revised WHO classification and treatment of childhood pneumonia at health facilities–Evidence summaries. [PubMed] [Google Scholar]
- 2.Al-Waili B.R., Al-Thawadi S., Al Hajjara S. Impact of the revised penicillin susceptibility breakpoints for streptococcus pneumoniae on antimicrobial resistance rates of meningeal and non-meningeal pneumococcal strains. Ann Saudi Med. 2013;33(2):111. doi: 10.5144/0256-4947.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tariq Z., Uz W., Hassanein A.A., Hashmey R.H. Changes in susceptibility pattern of Streptococcus pneumonia at tawam hospital in Al ain, United Arab Emirates during (2004–2011) Pakistan Armed Forces Med. J. 2016;(66) [Google Scholar]
- 4.Bradley J.S., Byington C.L., Shah S.S., Alverson B., Carter E.R., Harrison C. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7) doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris M., Clark J., Coote N., Fletcher P., Harnden A., McKean British Thoracic Society Guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 6.Royal Children's Hospital, Melbourne, Australia . December 2016. Clinical Practice guidelines: community acquired pneumonia [internet] Internet, last updated. [Google Scholar]
- 7.American Thoracic Society Infectious Diseases Society of America: guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 8.Messinger A.I., Kupfer O., Hurst A., Parker S. Management of pediatric community-acquired bacterial pneumonia. Pediatr Rev. 2017;38(9):394. doi: 10.1542/pir.2016-0183. [DOI] [PubMed] [Google Scholar]
- 9.Stockmann C., Ampofo K., Killpack J., Williams D.J., Edwards K.M., Grijalva C.G. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J. Pediat. Infec. Dis. Soc. 2017:piw091. doi: 10.1093/jpids/piw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee C. Open forum infectious diseases. Oxford University Press US; 2016. Using procalcitonin to guide antibiotic therapy; p. ofw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Saux N., Robinson J.L. Uncomplicated pneumonia in healthy Canadian children and youth: practice points for management. Paediatr Child Health. 2015;20(8):441–445. doi: 10.1093/pch/20.8.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas-Reyes M.X., Granados Rugeles C. The Cochrane Library; 2006. Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudtson J., Grewal H. Pediatric empyema – an algorithm for early thoracoscopic intervention. J Soc Laparoendosc Surg: J Soc Laparoendosc Surg. 2004;8(1):31. [PMC free article] [PubMed] [Google Scholar]
- 14.Finck C., Wagner C., Jackson R., Smith S. Seminars in pediatric surgery. Elsevier; 2002. Empyema: development of a critical pathway; pp. 25–28. [DOI] [PubMed] [Google Scholar]