Abstract
Background
Polycystic ovary syndrome (PCOS) is a common reproductive endocrinopathy in women of childbearing age, affecting 5–15% women in this age group. Suggestive cardinal features comprise hyperandrogenism, ovulatory dysfunction and/or polycystic ovary appearance. The gold standard radiological tool is the pelvic ultrasound (PUS) whose yield may be limited in overweight and obese adolescent girls.
Objective and hypotheses
To evaluate the contribution of pelvic MRI to the diagnosis of PCOS in a specific group of virginal overweight and obese adolescent girls.
Method
Eight adolescent girls seen for menstrual irregularities or amenorrhea, with features of hyperandrogenism were biochemically screened (LH, FSH, testosterone, S-DHEA, delta-4 androstenedione, 17 (OH) P, SHBG, TSH, free T4, prolactin and lipid profile, fasting blood sugar and HOMA-IR and HOMA-B). Each had PUS and/or pelvic MRI (PMRI) performed. Other causes of hyperandrogenism were excluded.
Imaging
PUS with the trans-abdominal transducer was attempted in only one patient Acuson© scanner, using 3.5–7.5 MHz transducer; PMRI was performed in all patients with phased array coil of 1.5 T Siemens MRI scanner, with T1 and T2-weighted axial and coronal images. PCOS was defined according to the Rotterdam PCOS consensus Workshop.
Results
Eight girls (mean age 14,6 ± 1.47 years) are reported, one was overweight (BMI Z-score > 1 SDS), seven others were obese (BMI Z-score > 2 SDS). Mean age at menarche was 11.58 ± 1.11 years, except for one who had not yet entered menarche. All had menstrual irregularities, acanthosis nigricans, acne, hirsutism, and biochemical characteristics of PCOS (high plasma androgens, insulin resistance, glucose/insulin ratio <4.5, decreased SHBG).
PUS was not contributive to the diagnosis of PCOS, whereas PMRI showed typical aspect (well delineated peripheral ovarian cysts), with increased ovarian volume and stroma.
Conclusion
Although PUS remains the gold standard for the diagnosis of PCOS in most cases, its limitations in overweight and obese girls are real and must be considered.
If utilization of endovaginal transducer not being feasible in young virginal girls, PMRI could be a useful alternative, allowing greater delineation of structural components of the ovary and better appreciation of both its volume and structural alterations.
Keywords: PCOS, Adolescents girls, Menstrual irregularities, Pelvic MRI, Diagnosis, Obesity, Overweight, Adolescents
1. Introduction
Polycystic ovary syndrome (PCOS) is a common heterogeneous and complex reproductive endocrinopathy affecting 5–15% women of child-bearing age in all ethnic and racial groups [1], [2], [3]. An incidence of 3% has been reported in a general population of Iranian adolescents, but higher incidence between 11% and 26% has also been reported by others [4], [5].
Although the exact cause of PCOS remains unknown, available evidence suggest that it could result from the interaction between heritable predisposition, intra and extrauterine environmental factors, insulin resistance variations, and defective changes in steroidogenesis/steroid metabolism, of which disordered gonadotropin secretion, insulin resistance and hyperinsulinemia, hyperandrogenism, ovarian dysfunction and follicular arrest are the more prominent features. The androgen overexposure hypothesizes that PCOS could originate in fetal life, with clinical expression becoming apparent only during adolescence when hypothalamic-pituitary-ovarian axis attains maturation [6], [7], [8], [9], [10].
Being the commonest cause of hyperandrogenism and menstrual irregularities in females, PCOS per se also carries the risks of leading to infertility, dysfunctional uterine bleeding, obesity, type 2 diabetes, hypertension, dyslipidemia and metabolic syndrome in affected individuals [10], [11], [12], [13].
The PCOS clinical spectrum is wide, and includes women free from evidence of both clinical and biochemical parameters, despite having dysfunctional polycystic ovaries. In the majority of patients, however, clinical cardinal features associate irregular menstrual cycles, acne, obesity, hirsutism, with signs of hyperinsulinism.
Affected individuals show, biochemical parameters of hyperandrogenism, and polycystic ovaries on radiological investigations. An early description of this syndrome was in adult patients, extrapolation of these clinical and biochemical features to the pediatric population raised a number of concerns [14].
In a recent publication, pediatric recommendations have emerged from the pediatric and adolescent medicine experts calling for caution, as characteristic features on which the diagnosis of PCOS is based may overlap with those found in normal pubertal development in adolescent girls. The experts also recommend that other causes of menstrual disorders be excluded and that a ≥2 years history of oligomenorrhoea is necessary before considering the diagnosis. They also recommend that obesity, insulin resistance, and hyperinsulinemia should not be used for the diagnosis. Lastly, in the absence of a definitive diagnosis, therapy to alleviate symptoms and prevent subsequent co-morbidities should be considered [15].
Overall, the diagnosis in both adolescents and adults females remains based on the Rotterdam consensus criteria. To be diagnosed PCOS, a patient must present at least two out of three of the following: chronic anovulation (presenting as oligo or amenorrhea), polycystic ovary morphology (on pelvic ultrasound) and clinical or biochemical signs of androgen excess [16].
In spite of abnormal ovarian morphology being part of diagnostic criteria, its isolated presence or absence is neither essential nor sufficient for the diagnosis of PCOS.
Radiologically, the gold standard tool for investigating ovarian morphology has, so far, been the pelvic ultrasound (PUS), accepted criteria of which, were derived from studies performed in a population of adult women [17].
Initial ovarian sonographic description of PCOS had emphasized on the distribution and size of antral follicles within an enlarged ovary [15], but this has been reviewed by subsequent studies, and the recent Task Force recommends to utilize either follicle number per ovary (≥25) when a sophisticated US transducer ≥ 8 MHz is available or, otherwise, an ovarian volume of ≥10 ml to define PCOS morphology [18].
Despite the lack of specific pediatric US criteria, cases of PCOS in children have been reported, based on those derived from adult studies [19], [20], [21].
It is noteworthy that in routine practice, trans-abdominal US, used for virginal patients, is only reliable in lean adolescents, whereas the trans-vaginal US which has better yield is not feasible. Trans-abdominal PUS per se, also, has several limitations that have been underlined previously by Yoo et al. namely the difficulty to obtain clear images in obese girls, and the confusion in interpreting reported results as most of them are based on ovarian volume rather than on antral follicles number, etc. [22].
The above-mentioned technical difficulties and limitations have led some researchers to consider pelvic MRI as a reliable alternative to PUS in the diagnosis of PCOS in some adolescent girls, with encouraging results. MRI as investigating modality offers the advantage of improved visualization of the ovarian anatomy and a clearer definition of its contents [22], [23], [24], [25].
We, hereby report our experience of abdominal MRI in the diagnosis of PCOS in overweight and obese adolescent virginal girls in whom trans-abdominal PUS was not feasible.
2. Population and methods
2.1. Population
The study population comprised eight adolescent girls recruited from the cohort of children attending our ediatric endocrinology clinics. Those included in the study had signs and symptoms of hyperandrogenism (acne, increased body odor, hirsutism, overweight or obesity, and menstrual irregularities) as main complaints.
Hirsutism was evaluated according to the Ferriman Gallway score, (with score ≥ 8 considered as significant) [26]. Overweight and obesity were defined according to the World Health Organization by BMI-for-age (Z-scores), with BMI calculated by dividing each individual's body weight in kilogram by the square of their height in meters (kg/m2). A child with a Z-score above 1 SDS was considered overweight and, and one with a Z-score over 2 SDS was categorized as obese.
To be investigated for PCOS, each patient had to have a history of irregular menstrual cycle (oligo or amenorrhea) lasting ≥ 6 months and having occurred at least two years after menarche or primary amenorrhea after excluding other causes. All eight patients were Tanner pubertal stage 5 [14].
Patients were biochemically screened (basal LH, FSH, testosterone, S-DHEA, delta-4 androstenedione, 17 (OH) P, TSH, FT4, SHBG, prolactin and plasma lipid profile, fasting blood sugar, fasting plasma insulin and HOMA-IR and HOMA-B were calculated). Blood samples were drawn in the morning between 08h00 and 09h00 a.m., after an overnight fasting period of 10–12 hours, through an indwelling catheter placed around 30 minutes before sampling. Other causes of hyperandrogenism such as adrenal hyperplasia and adrenal tumors were excluded. For one patient, we started imaging with PUS, the poor yield of which led us to go for pelvic MRI thereafter.
For the purpose of this study, we compared our findings to those reported by Cappa et al. with their control population serving as normal references [23].
2.2. Method
2.2.1. Imaging
PUS was performed with the transabdominal transducer (Acuson® scanner using 3.5–7.5 MHZ transducers), pelvic MRI was performed with phased array coil of 1.5 T Siemens MRI scanner, with T1 and T2-weighted axial and coronal images, 4 mm slices. Images capture and interpretation were performed by a senior radiologist with extensive experience in MRI technics in children and adults population.
(Dr. SM Benosman).Ovarian measurements were recorded on three orthogonal dimensions, using electronic calipers on the PACS system monitor. The ovarian volume was calculated with the formula D1 x D2 x D3 x 0.523 (in which D1 was the longitudinal diameter, D2 the anteroposterior diameter, and D3 the transverse diameter of the ovary [22]). The ovarian follicles number and localization within the ovary were defined using both T1-weighted gradient echo (GE), and T2-weighted turbo spin echo (TSE) images [22].
The criteria retained for the diagnosis were those from Rotterdam PCOS consensus Workshop [16]. We considered patients as having PCOS when their MRI images revealed more than 12 peripherally located follicles, with increased central stroma, and an ovarian volume >10 ml, as previously reported [23]. Informed consent was obtained from each patient's parents, and the study received the approval from the Local Ethic Committee.
2.2.2. Hormonal assays
Plasma LH, FSH, SDHEA, and insulin assays were performed by ECLIA method (COBAS; Roche-Diagnosis, Meylan, France). The inter- and intra-assay coefficients of variation (CVs) were 8% and 7% for LH and FSH respectively. Testosterone was measured by RIA assay after extraction with Ether (Immunotech, Beckman Coulter, Marseille, France), inter- and intra-assay CVs were between 2.9 and 9.7% at mid-range concentration.
3. Results
Eight adolescent girls with a mean age of 14.6 ± 1.47 years met our inclusion criteria. Their mean BMI was 36.04 ± 6.34 kg/m2, seven girls were obese with BMI Z-scores above 2 SDS (cases 1, 2, 4, 5, 6, 7, 8), one was overweight (>1 SDS BMI Z-score < 2 SDS) (case 3). The mean age at menarche was 11.6 ± 1.11 years. All, except case 6, presented with a history of menstrual irregularities that were persisting two years after menarche and had lasted at least six months before the diagnosis was made. Patients were diagnosed as having PCOS on the clinical criteria of hyperandrogenism (as detailed in material & methods section above), all had high total plasma testosterone levels (Table 1).
Table 1.
Patients'clinical characteristics, presenting signs and symptoms and biochemical parameters.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) | 15.1 | 12.1 | 14.9 | 15.0 | 16.0 | 16.5 | 13.0 | 14.0 |
| BMI z-score (SDS) | >2.0 | >2.0 | >1.0 | >2.0 | >2.0 | >2.0 | >2.0 | >2.0 |
| Age at menarche (yrs) | 12.6 | 10.0 | 13.0 | 12.5 | 11.0 | NS | 11.0 | 11.0 |
| Irregular menses (yes/no) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| Acanthosis nigricans (yes/no) | (+) | (+) | (−) | (+) | (+) | (+) | (+) | (+) |
| Acne/greasy hair (yes/no) | (+) | (+) | (+) | (−) | (−) | (+) | (+) | (+) |
| Hirsutism (yes/no) | (−) | (+) | (−) | (−) | (+) | (+) | (−) | (+) |
| Ferriman Gallwey score | 0 | 13.0 | 2.0 | 0 | 11.0 | 9.0 | 0 | 28.0 |
| Total testosterone (nmol/L) | 2.3 | 3.1 | 3.0 | 3.3 | 3.0 | 1.6 | 1.18 | 1.35 |
| DHEAS (ng/ml) | NA | 5347 | 10 378 | 4930 | 1732 | 4122 | 2740 | NA |
| Δ4 Androstenedione (nmol/l) | 11.2 | NA | 16.06 | 7.5 | 12.5 | 11.1 | 9.2 | 11.6 |
| Basal plasma insulin (pmol/l) | 138 | 86 | 49 | 358 | 157 | 356 | 123.3 | 233 |
| HOMA IR (N = 1) | 3.92 | NA | NA | 10.17 | 4.22 | 11.1 | NP | 19.6 |
| HOMA B (N < 100) | 290 | NA | NA 1187.73 | 704.61 800 | 434 | 279 | ||
| Glucose/insulin ratio (n > 4.5) | 8.44 | NA | 13.3 | 1.51 | 3.20 | 1.46 | 4.38 | 3.40 |
| LH/FSH | 1.36 | 1.53 | 1.20 | 1.45 | 1.34 | 0.93 | 2.40 | 1.61 |
NS: not started; NA: not available;NP:not performed; N:normal.
Clinical characteristics, age at diagnosis, age at menarche and presenting features are summarized in Table 1. Patient 1 was under followed-up for autoimmune thyroiditis. Another (case 3) had a positive history of central precocious puberty that had been managed with GnRH agonist from the age of 8.5 years to the age of 10.6 years; her plasma DHEAS being very high (Table 1), a long Synacthen test and adrenal glands CT scan were performed. Results were unremarkable, excluding any adrenal pathology. Acanthosis nigricans was present in 6/8 girls. Patient 6 had not entered menarche, her clinical and biochemical profile were compatible with PCOS, other causes of primary amenorrhea were excluded.
Biochemically, total plasma testosterone level was raised in all eight patients, with mean ± SDS value of 2.35 ± 0.93 nmol/L (N 0.11–0.57) (Free/total testosterone ratio could not be calculated as we did not measure free testosterone levels in most patients), plasma DHEAS results were available for six patients, with mean ± SDS value of 4875 ± 3017 ng/ml (N 651–3680) and mean ± SDS delta-4 Androstenedione of 11.31 ± 2.68 nmol/L (N 2.1–24.9).
Plasma markers of insulin resistance were abnormal with high mean ± SDS basal insulin of 187.54 ± 117.42 pmol/L (N 2.6–24.9), mean HOMA-IR 6.97 ± 3.46 SDS (N = 1), and mean HOMA-B of 615.9 ± 352.6 SDS (N < 100).
Oral Glucose Tolerance Test (OGTT) was performed in six patients, and results were normal in three (cases 1, 2 and 7), but frankly abnormal in three others (cases 4, 5 and 8), with plasma glucose at time 120 minutes of 1.58 g/L, 1.59 g/L and 1.32 g/L respectively (Table 1).
Plasma Prolactin was within normal ranges in all patients (results not shown). Mean plasma LH and FSH values were 7.57 ± 1.63 UI/L and 5.42 ± 1.73 UI/L respectively, with a mean LH/FSH ratio of 1.40.
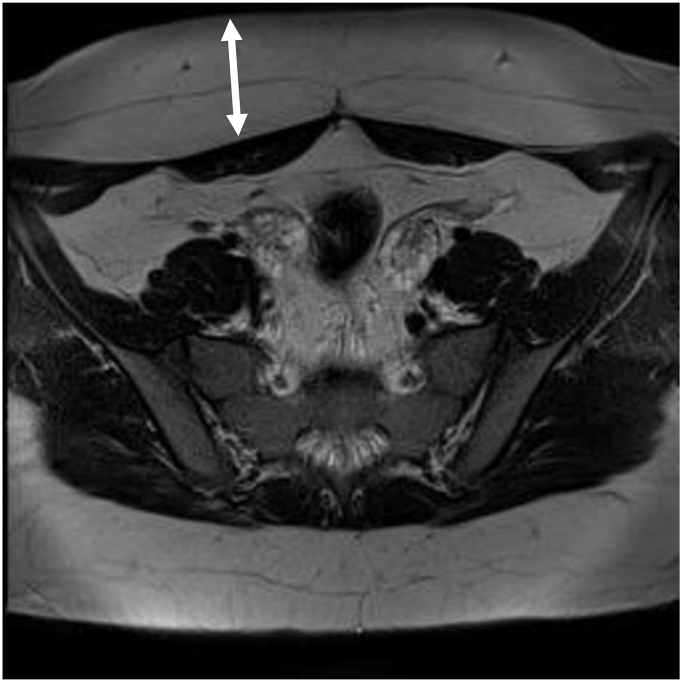
MRI Imaging Results: Attempts to perform transabdominal US being inconclusive with of poor yield, we decided to go for pelvic MRI in our overweight and obese virginal adolescent girls systematically. Abdominal US failure was mainly due to limitations in available US probes to allow better definition of ovarian structures, as this was hampered by excess subcutaneous abdominal fat as illustrated in Fig. 1.
Fig. 1.
T2-weighted image showing excess in subcutaneous abdominal fat.
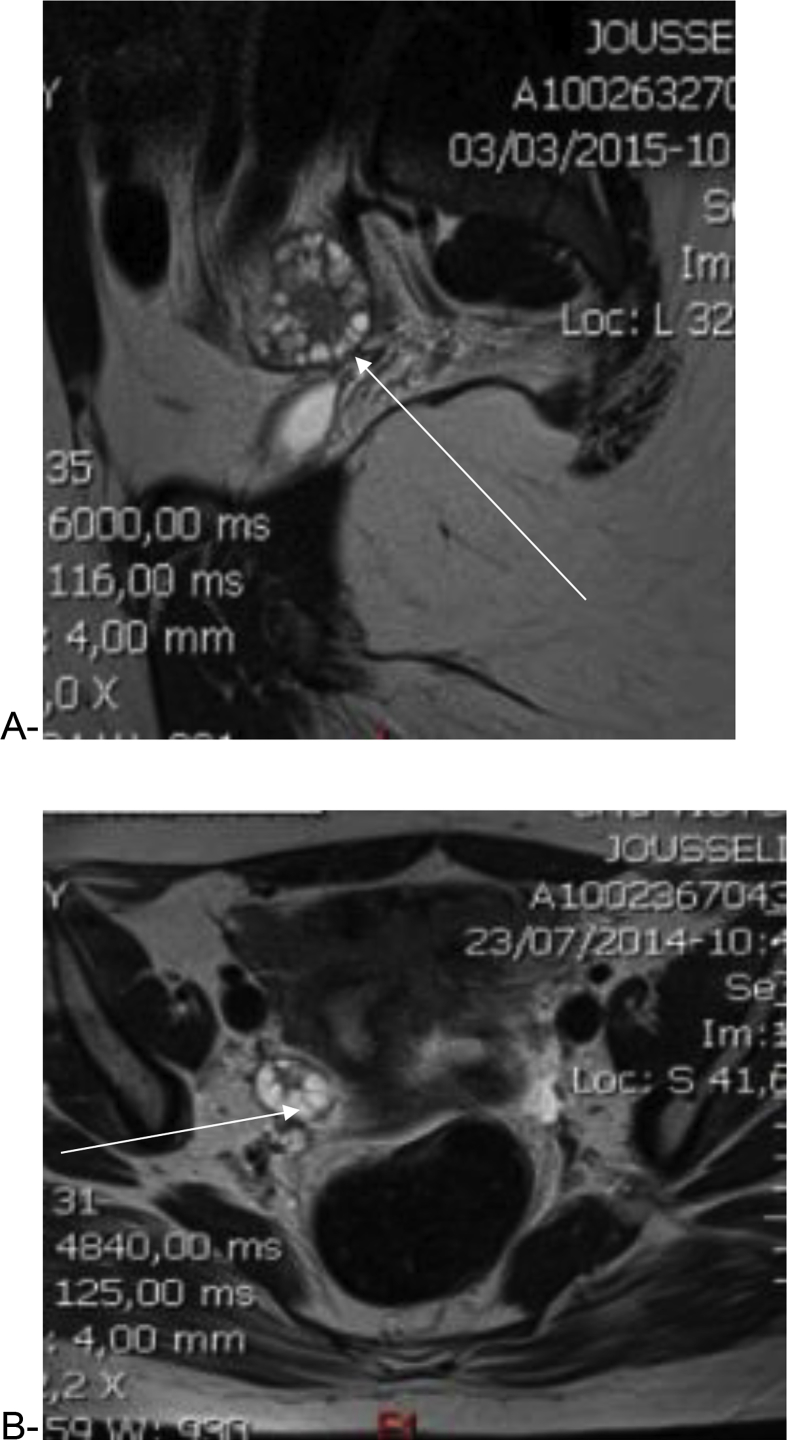
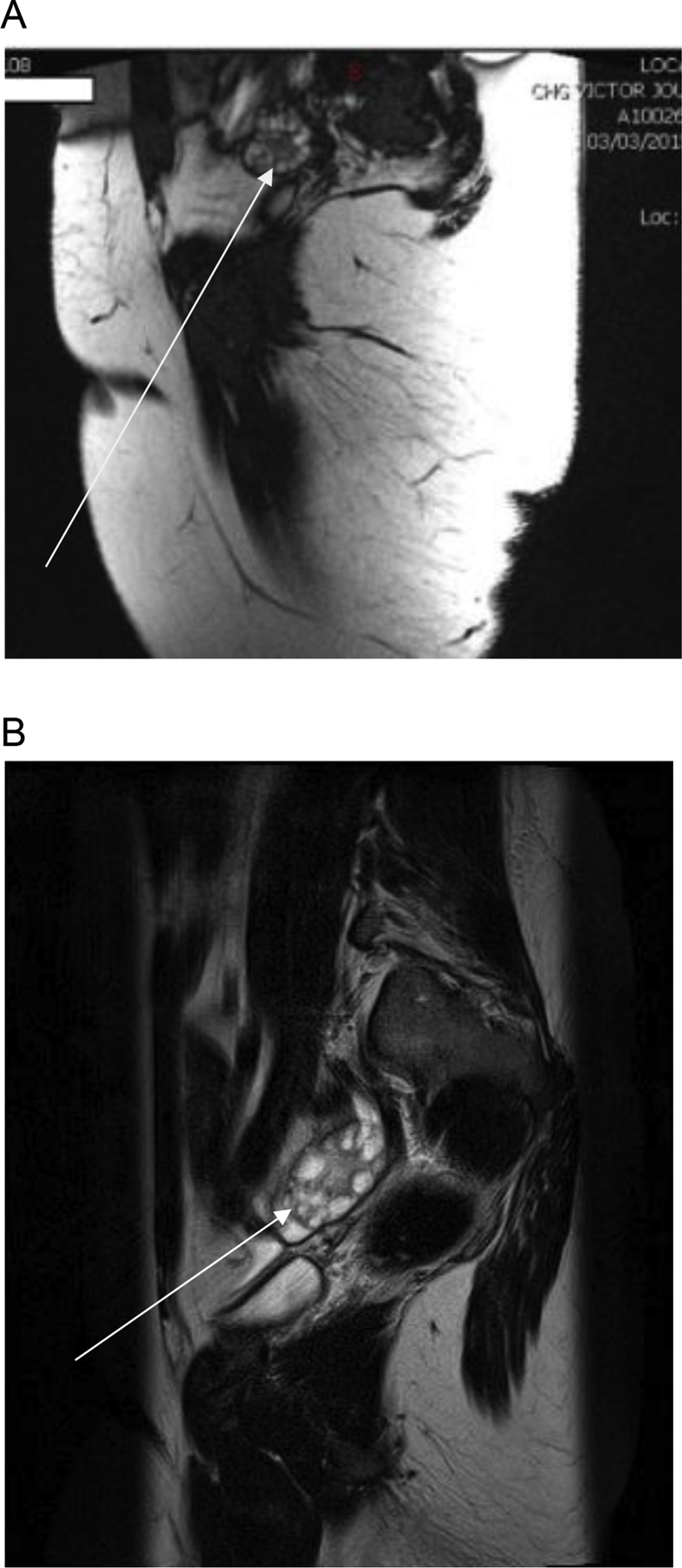
Mean ovarian volumes were 13.23 ± 1.96 SDS ml (right ovary) and 13.66 ± 1.26 SDS ml (left ovary) in our study population. The follicles distribution and their peripheral localization were clearly delineated in T2-wieghted images (Fig. 2, Fig. 3). Follicles number per ovary was >12 (22.0 versus 10.0 in Cappa et al. in normal control; Z = 2.12, P < 0.05). We were, unfortunately, not able to measure the follicular volume. Overall, pelvic MRI allowed better delineation of the ovarian morphology with a clear illustration of the peripheral distribution of the follicles along with increased stromal components as expected in PCOS (Fig. 3).
Fig. 2.
T2-weighted image showing multiple peripherally located ovarian cysts with increased stromal volume, axial section (A), and sagittal section (B).
Fig. 3.
T2 weighted axial section image showing peripherally located ovarian cysts in an obese adolescent girl (A & B).
4. Discussion
This study reports our short experience with pelvic MRI in the diagnosis of PCOS in eight overweight and obese virginal adolescent girls who had clinical and biochemical features of hyperandrogenism. Our findings corroborate those reported previously [22], [23], [24] and add to the existing body of evidence that has suggested pelvic MRI as a good alternative to pelvic Ultrasound in diagnosing PCOS, in a specific category of pediatric population (overweight and obese adolescents). PCOS is a common heterogeneous disorder, the diagnosis of which is hampered by the lack of universally accepted consensus in the pediatric population.
In adult patients, the diagnosis is based on the guidelines derived from different international conferences, the most known being those from the National Institute of Health conference criteria (1990), now abandoned, and those from the Rotterdam consensus (2003), and also those from the Androgen Excess PCOS Society consensus criteria (2006) [17], [27].
All our patients met the diagnostic criteria, and presented, as defined by the Rotterdam Consensus Workshop, clinical features associating hyperandrogenism and menstrual irregularities, to which ovarian findings of PCOS on the pelvic MRI are added. Although it is well recognized that diagnostic criteria for this disorder are essentially clinical and biochemical (the NIH criteria), it is admitted that performing pelvic imaging brings additional evidence of PCOS by showing its characteristic radiological features. In this respect, trans-abdominal PUS remains the first choice imaging modality by which ovarian morphology can be studied in patients, despite the fact that results are still interpreted on the basis of criteria derived from adult studies.
On the practical standpoint, PUS is carried out in virginal adolescent girls trans-abdominally rather than trans-vaginally as performed in adults. The latter route would normally improve the scan resolution, but its utilization is not feasible in virginal adolescent girls. Pelvic US performance and assessment of the ovarian morphology is both problematic and limited in adolescent girls for a number of reasons: firstly, the trans-abdominal approach is largely preferred to the transvaginal route, not practical in virginal girls, in addition to the discomfort induced by vaginal probes. Secondly, pelvic US yield is more or less dependent on the operator's experience and on the equipment quality. This being likely to introduce an inherent level of subjectivity to the diagnosis. Lastly, the quality of images may be impeded by the excess in abdominal and pelvic fat common in this age group. In a recent attempt to offer alternative radiological investigation, Korean authors have suggested trans-rectal US as a possible route in virginal PCOS patients, results seem interesting but need to be confirmed before it can be extended to the adolescent girls population [28].
Insulin testing and HOMA measures, intended to evaluate insulin resistance common in PCOS patients, suggest that most patients were, effectively, insulin resistant and likely to benefit from specific care (physical exercice and/or metformin), this being beyond the scope of this paper.
Our findings strengthen those reported by others and show that pelvic MRI allows better characterization of the ovarian morphology in overweight and obese adolescent girls with clinical and biochemical features of PCOS [22], [23], [24].
This study was not intended to compare pelvic MRI with the pelvic US; this has been done earlier by others, with results that support pelvic MRI superiority to the pelvic US, in a particular pediatric adolescent population [23], [24].
The absence of a control group in our study may be considered as limit, but our results clearly show that MRI gave clearer visualization of the ovarian anatomy with both mean volume of 23 ± 1.96 ml (right ovary) and 23 ± 1.26 ml (left ovary), and follicle numbers (mean 19 ± 3.28) that met the criteria for Polycystic ovaries as defined in the Rotterdam PCOS consensus Workshop [17]. The mean follicular number in our study population was well above that found in normal control subjects (<12 per ovary) as reported by Cappa et al. [23]. In spite of its yield and contribution to the diagnosis of PCOS in a defined pediatric population, there still are number of issues that need to be settled before the generalization of pelvic MRI: consensus on the ovarian volume, follicle number, stromal volume, technical modalities such as slice size (2, 4 or 6 mm) need to be established.
We have, like others, based the definition and diagnosis of PCOS on criteria derived from the pelvic US. Brown et al. have, however, shown that pelvic MRI would identify a larger number of follicles when 2 mm slices are captured [24]. It is, therefore, likely that pelvic US criteria which have served as the basis for early pelvic MRI studies may not be appropriate [22], [23], [24]. This observation calls, therefore, for prospective studies intended to lay down pelvic MRI imaging definition of criteria that should guide the diagnosis of PCOS in adolescent girls' population.
As suggested earlier by Yoo et al. [22], we also believe that pelvic MRI could be helpful in understanding the early changes which occur in the ovarian anatomy in obese girls developing PCOS. Here again, larger prospective studies are awaited. We have studied the ovarian morphology using 4 mm thickness slices in this study, whereas Brown et al. used 2 mm and reported a higher follicle count when they compared the yield with that obtained with 6 mm [24].
It is therefore questionable whether studying the ovarian anatomy with the 4 mm slices thickness as we have empirically done here is sufficient and/or perfectible.
This study has other limitations such as the small sample size, in addition to the fact that despite finding an increased stromal volume, we did not measure it precisely.
5. Conclusion
PCOS is a common disorder with long-term consequences which can be prevented if the diagnosis is made early enough during adolescence. Our findings support those previously reported by others and confirm that pelvic MRI is a reliable alternative to the pelvic US in overweight and obese adolescent girls in whom vaginal probes can not be utilized. The Pelvic US, using high definition probes remains the first imaging choice in lean girls. Large prospective studies are needed to set criteria for PCOS diagnosis by pelvic MRI in this age group.
Funding
None.
Conflict of interest
No conflict of interest to declare.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Fauser B.C., Tarlatzis R.W., Rebar R.S. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97 doi: 10.1016/j.fertnstert.2011.09.024. 28–38.e25. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R., Sanchez E.S., Knochenhauer C. Androgen excess in women: experience with over 1,000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 3.Carmina E., Rosato F., Janni A., Rizzo M., Longo R.A. Extensive clinical experience: relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91:2–6. doi: 10.1210/jc.2005-1457. [DOI] [PubMed] [Google Scholar]
- 4.Hashemipour M., Faghihimani S., Zolfaghary B., Hovsepian S., Ahamadi F., Haghighi S. Prevalence of polycystic ovary syndrome in girls aged 14–18 years in Isfahan Iran. Horm Res. 2004;62:278–282. doi: 10.1159/000081842. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll D.A. Polycystic ovary syndrome in adolescence. Ann N. Y Acad Sci. 2003;997:49–55. doi: 10.1196/annals.1290.006. [DOI] [PubMed] [Google Scholar]
- 6.Franks S. Adult polycystic ovary syndrome begins in childhood. Clin Endocrinol Metab. 2002;16:263–272. doi: 10.1053/beem.2002.0203. Best Practice and Research. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfield R.L. Identifying children at risk of polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 8.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 9.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 10.Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015 Oct;36(5):487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman R.J., Dewailly D., Legro R.S., Hickey T.E. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 12.Moran L.J., Misso M.L., Wild R.A., Norman R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 13.Elting M.W., Korsen T.J.M., Bezemer P.D., Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16:556–560. doi: 10.1093/humrep/16.3.556. [DOI] [PubMed] [Google Scholar]
- 14.Carmina E., Oberfield S.E., Lobo R.A. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:e201–e205. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Witchel S.F., Oberfield S., Rosenfield R.L., Codner E., Bonny A., Ibáñez L. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83:376–389. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]
- 16.Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004:41–77. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 17.Balen A.H., Laven J.S., Tan S.L., Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003 Nov-Dec;9(6):505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 18.Dewailly D., Lujan M.E., Carmina E., Cedars M.I., Laven J., Norman R.J. Definition and significance of polycystic ovarian morphology: a task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update. 2014;20(3):334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 19.Venturoli S., Porcu E., Fabbri R., Pluchinotta V., Ruggeri S., Macrelli S. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res. 1995;38:974–980. doi: 10.1203/00006450-199512000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Stanhope R., Adams J., Brook C.G. Evolution of polycystic ovaries in a girl with delayed menarche. A case report. J Reprod Med. 1988;33:482–484. [PubMed] [Google Scholar]
- 21.van Hooff M.H., Voorhorst F.J., Kaptein M.B., Hirasing R.A., Koppenaal C., Schoemaker J. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil Steril. 2000;74:49–58. doi: 10.1016/s0015-0282(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 22.Yoo R.Y., Sirlin C.B., Gottschalk M., Chang R.J. Ovarian imaging by magnetic resonance in obese adolescent girls with polycystic ovary syndrome: a pilot study. Fertil Steril. 2005;84:985–995. doi: 10.1016/j.fertnstert.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Cappa M., Scire G., Orazi C., Cambiaso P., Fiaschetti V., Fintini D. Pediatr Child Health. 2008;185(S1):S8–S13. [Google Scholar]
- 24.Brown M., Park A.S., Shayya R.F., Wolson T., Su H.I., Chang R.J. Ovarian imaging by magnetic resonance in adolescent girls with polycystic ovary syndrome and age-matched controls. J Magn Reson Imaging. 2013;38(3):689–693. doi: 10.1002/jmri.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenigsberg L.E., Agarwal C., Sin S., Shifteh K., Isasi C.R., Crespi R. Clinical utility of magnetic resonance imaging and ultrasonography for diagnosis of polycystic ovary syndrome in adolescent girls. Fertil Steril. 2015 Nov;104(5):1302–1309. doi: 10.1016/j.fertnstert.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferriman D., Gallwey J.D. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 27.Zawadski J.K., Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A., Givens J.R., Haseltine F., editors. Polycystic ovary syndrome. Blackwell Scientific; Boston: 1992. pp. 377–384. [Google Scholar]
- 28.Eun Lee D., Yun Park S., Ra Lee S., Jeong K., Won Chung H. Diagnostic usefulness of transrectal ultrasound compared with transvaginal ultrasound assessment in young Korean women with PCOS. J Menopausal Med. 2015;21:149–154. doi: 10.6118/jmm.2015.21.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]