Abstract
During exposure to high altitude, hypoxia develops because of reductions in barometric pressure and partial pressure of O2. Although several studies have examined the effects of hypoxia on exercise performance and physiological responses, such as maximal minute ventilation ( Emax) and maximal oxygen uptake (O2max), how barometric pressure reduction (hypobaria) modulates them remains largely unknown. In this study, 11 young men performed incremental treadmill running tests to exhaustion under three conditions chosen at random: normobaric normoxia (NN; 763 ± 5 mmHg of barometric pressure, equivalent to sea level), hypobaric hypoxia (HH; 492 ± 1 mmHg of barometric pressure, equivalent to 3500 m above sea level (m a.s.l.)), and hypobaric normoxia (HN; 492 ± 1 mmHg of barometric pressure while breathing 32.2 ± 0.1% O2 to match the inspiratory O2 content under NN). Emax was higher in HN than in NN (160.9 ± 10.7 vs. 150.7 ± 10.0 L min−1, P < 0.05). However, no differences in O2max and arterial oxyhemoglobin saturation were observed between NN and HN (all P > 0.05). Time to exhaustion was longer in HN than in NN (932 ± 83 vs. 910 ± 79 s, P < 0.05). These results suggest that reduced air density during exposure to an altitude of 3500 m a.s.l. increases maximal ventilation and extends time to exhaustion without affecting oxygen consumption or arterial oxygen saturation.
Keywords: Airflow resistance, altitude, hypobaric condition, ventilation, O2max
Introduction
Maximal oxygen uptake (O2max) and endurance exercise performance decline with elevations in altitude because of reduced ambient partial O2 pressure (Fulco et al. 1998; Derchak et al. 2000). Pulmonary ventilation increases exponentially with decreases in ambient partial O2 pressure. This response partly counteracts reduced alveolar partial pressures of oxygen (PAO2) and thus, arterial oxyhemoglobin saturation (SaO2) (Calbet et al. 2003; Ogawa et al. 2007). Previous studies have demonstrated that individuals with greater increases in maximal minute ventilation (Emax) under acute hypobaric hypoxia (HH) relative to normobaric normoxia (NN) showed smaller reductions in O2max (Marconi et al. 2004; Ogawa et al. 2007). Therefore, greater increases in ventilation during hypoxic exercise appear to be beneficial for minimizing reductions in O2max.
In most acute hypoxia studies, normobaric hypoxia (NH) condition is employed to investigate the influences of exposure to high altitude on physiological responses and exercise performance. However, whether HH and NH are physiologically equivalent remains debatable (Millet et al. 2012). For example, resting E and SaO2 tend to be lower under HH conditions than under NH conditions (Coppel et al. 2015). Furthermore, Saugy et al. (2016) showed that the magnitude of the reduction in cycling performance was greater during exposures to HH compared to that with NH exposure (Coppel et al. 2015), which implied that the HH condition might be more detrimental to exercise performance and physiological responses.
Under HH condition (e.g., high‐altitude exposure), air density, and therefore, air resistance, are lower than they are at sea level (Gautier et al. 1997). Thus, reductions in barometric pressure that are associated with acute high‐altitude exposures could affect the physiological responses. Studies have demonstrated that breathing a helium–oxygen (He–O2) gas mixture, which could greatly reduce airflow resistance (Mink and Wood 1980; Papamoschou 1995), increases Emax during maximal exercise under hypoxic conditions relative to breathing non‐He–O2 under controlled conditions. Moreover, increases in O2max and Emax were observed by breathing He–O2 compared to that with non‐He–O2 (Esposito and Ferretti 1997; Ogawa et al. 2010), even under the NN condition (Powers et al. 1986). Furthermore, the effect of hypobaric normoxia (HN) was explored in early studies (Cerretelli 1976; Marconi et al. 2004) of chronic high‐altitude conditions with pure enriched O2 gas mixture breathing. Those studies showed that O2max was higher in HN than in NN. Whether reduced air density in acute hypobaric conditions increases Emax and O2max in a similar manner to that observed with He–O2 breathing and chronic HN remains to be determined.
Therefore, this study tested the hypothesis that acute hypobaria associated with exposure to the HH condition increases E and O2max, thereby improving endurance exercise performance. Further, as a secondary purpose, we estimated whether hypobaria would lower the oxygen consumption of respiratory muscles. If reduced air density under hypobaric conditions could lower O2 in the respiratory muscles due to the decreased work of breathing, this might improve exercise performance. Similarly, Harms et al. (1997, 1998, 2000) reported that unloading the respiratory muscles’ work during intensive exercise resulted in a greater distribution of the available cardiac output to the active locomotor muscles, thereby improving exercise tolerance with no change in O2.
Materials and Methods
Ethical approval
This study was approved by the Human Subjects Committees of the University of Tsukuba in accordance with the guidelines set forth in the Declaration of Helsinki. All participants provided verbal and written informed consent before participating in this study.
Participants
Eleven healthy young men (age, 24 ± 4 years; height, 1.73 ± 0.07 m; body mass, 63.3 ± 4.8 kg) including three physically active students and eight long‐ or middle‐distance runners on the university track and field team participated in this study. All participants lived at low altitudes and had not been exposed to altitudes >1000 m within the 6 months prior to the study.
Incremental running test
Each participant performed an incremental running test to exhaustion in an environmental chamber (Shimazu Co. Ltd., Kyoto, Japan) under three conditions (performed randomly and on separate days): NN (20.9 ± 0.1% O2 at 763 ± 5 mmHg of barometric pressure, equivalent to sea level), HH (20.9 ± 0.1% O2 at 492 ± 1 mmHg of barometric pressure, equivalent to 3500 meters above sea level (m a.s.l.)), and hypobaric normoxia (HN; 32.2 ± 0.1% O2 at 492 ± 1 mmHg of barometric pressure). The partial pressure of O2 in NN and HN were matched (159 mmHg in both conditions), which enabled an assessment of the effects of reducing the barometric pressure without stimulating a hypoxic effect. The study room temperature was maintained at 20.2 ± 0.4°C and was continuously ventilated to minimize increases in the CO2 concentration in the air. Each participant performed self‐selected warm‐up exercises (stretching and jogging) outside the laboratory. The structure of the warm‐up was similar in all three conditions. Thereafter, participants entered the environmental chamber. For the hypobaric conditions (i.e., HH and HN), the chamber was gradually decompressed to achieve a barometric pressure equivalent to that at 3500 m a.s.l. in 20 min. For safety reasons, we avoided rapid decompression of the chamber. Each running test began within 20 min after completing the decompression. Under all conditions, the participants breathed through a face mask that covered the nose and mouth. The mask was connected via low‐resistance silicon pipes to a large reservoir bag. The incremental running test was performed on a treadmill at an inclination of 0°, which was maintained throughout the experiment. The initial running speed was set at 160 to 220 m/min, depending on the participant's running ability and was subsequently increased by 20 m/min every 2 min, such that 240 or 280 m/min was achieved within 15 min. Thereafter, the running speed was increased by 10 m/min every 1 min until exhaustion (Ogawa et al. 2007, 2010). When nearing O2max, the expired gas was collected in Douglas reservoir bags every 1 min.
Mimic ventilation trial
As a secondary test, 10 of the 11 participants who completed the incremental running test subsequently participated in a mimic ventilation trial performed under NN and HH conditions (in random order) to determine the oxygen consumption of the respiratory muscles during the incremental running test. After obtaining 5‐min baseline resting measurements in either NN or HH, the participants started a voluntary hyperventilation process while in the standing position. Since SaO2 was 100% under HH conditions during the voluntary hyperventilation process, HH under the mimic ventilation trial was assumed to be the same as HN. The participants were instructed to reproduce the tidal volume (VT) and respiratory frequency (f R) observed at O2max under each condition for 7 min. VT and f R were adjusted to the target level using a computer that showed breath‐by‐breath measurements of VT and f R. During the mimic ventilation, 100% CO2 was added to the inspiratory gas to maintain the end tidal pressure of CO2 (PETCO2) at normocapnic levels.
Measurements
Incremental running test
O2, CO2, and E were calculated using the Douglas bag method. O2 and CO2 concentrations were measured using a mass spectrometer (ARCO1000; ARCO; Chiba, Japan), which was calibrated with a standardized gas of known composition (O2, 15.00%; CO2, 5.00%; and N2, balanced). The volume inside the bag was determined using a dry gas meter (DC‐5A; Shinagawa; Tokyo, Japan), which was carefully calibrated with a 2‐L syringe before the experiment. All participants accomplished two of the following three criteria for O2max: constant O2 despite increases in running speed (increase in <2.0 mL kg−1 min−1); the respiratory quotient >1.1; maximal heart rate (HRmax) achieved was >90% of the age‐predicted value. Moreover, all participants reported a Borg scale of 20 and were not able to maintain the last‐stage running speed despite strong verbal encouragement. We also measured expiratory O2 and CO2 fractions (FEO2 and FECO2) breath‐by‐breath using a mass spectrometer (ARCO1000). We estimated PAO2 as:
where PIO2 is the partial pressure of inspiratory O2, PETO2 is the end tidal O2 pressure, and R is the respiratory quotient.
Alveolar ventilation ( A) was calculated as:
where PETCO2 is the end tidal CO2 pressure. SaO2 and heart rate (HR) were measured using a forehead pulse oximeter (N‐595; Nellcor, Hayward, CA) and an HR monitor (Vantage NV; POLAR, Finland), respectively. In this study, time to exhaustion during incremental testing was used as an index of exercise performance.
Mimic ventilatory test
Breath‐by‐breath FEO2 and FECO2 and flow volume were determined using a mass spectrometer (ARCO1000) and a spirometer (MINATO AS300i; Minato Medical; Osaka, Japan), respectively. During voluntary hyperventilation, O2, E, VT, and f R were calculated. The O2 of respiratory muscles (O2rm) was calculated by subtracting resting O2 from O2 recorded during the last 30 s of voluntary hyperventilation (O2vent). The mouth pressure was measured using a pressure transducer probe inserted into a mouthpiece and was reported at a sampling rate of 200 Hz. The peak inspiratory and expiratory mouth pressures (PImax and PEmax) were determined during each respiratory cycle.
Statistical analysis
Data are expressed as means ± standard deviations (SD). Variables obtained during the incremental exercise tests were analyzed using one‐way repeated‐measures analyses of variance with an experimental condition factor (NN, HH, and HN). After detecting the main effects, Fisher's least significant difference tests were performed as post hoc tests. Variables obtained during the mimic ventilatory test were analyzed using paired t‐tests (NN vs. HN). P values < 0.05 were considered statistically significant. SPSS 24 (IBM Corp., Armonk, NY) was used for all statistical analyses.
Results
Incremental running test
Resting SaO2 was similar for HN versus NN (98 ± 2% vs. 98 ± 2%). Emax was 6.8% higher in HN than in NN (Table 1). As hypothesized, Emax was higher (4.3%) in HN than in HH (Table 1). Similarly, f R was higher in HN than in HH (Table 1). Greater ventilation was not paralleled by greater O2max such that O2max was similar between HN and NN (Table 1). However, the time to exhaustion was longer in HN than in NN (Table 1). No difference in CO2max between NN and HN was noted. SaO2 at the point of exhaustion did not differ between HH and HN and no difference in maximal HRmax between NN and HN was observed (Table 1).
Table 1.
Variables measured at O2max
| NN | HH | HN | |
|---|---|---|---|
| O2max [mL min−1] | 3974 ± 338 | 2860 ± 241a | 4011 ± 327b |
| O2max [mL kg−1 min−1] | 63.0 ± 4.7 | 46.0 ± 5.6a | 63.6 ± 5.6b |
| CO2max [mL min−1] | 4580 ± 282 | 3506 ± 215a | 4531 ± 327b |
| Emax [L min−1] | 150.7 ± 10.0 | 154.2 ± 11.8 | 160.9 ± 10.6a, b |
| f R [breaths min−1] | 68 ± 10 | 70 ± 10 | 73 ± 10a, b |
| VT [L] | 2.2 ± 1.03 | 2.25 ± 0.36 | 2.25 ± 0.3 |
| E O2 −1 [ml ml−1] | 38.1 ± 3.6 | 54.3 ± 6.6a | 40.4 ± 4.9a, b |
| E CO2 −1 [ml ml−1] | 33.0 ± 3.1 | 44.0 ± 3.1a | 35.6 ± 3.5a, b |
| A [L min−1] | 101.8 ± 6.6 | 95.3 ± 10.0a | 106.8 ± 8.6b |
| PETCO2 [mmHg] | 39.2 ± 3.3 | 31.6 ± 3.9a | 36.8 ± 3.5b |
| PAO2 [mmHg] | 128.5 ± 10.6 | 76.9 ± 3.5a | 126.2 ± 4.2b |
| SaO2 [%] | 91 ± 3 | 69 ± 4a | 90 ± 5b |
| HRmax [beats min−1] | 195 ± 3 | 181 ± 8a | 192 ± 8b |
| Time to exhaustion [s] | 910 ± 79 | 614 ± 73a | 932 ± 83a, b |
Values are mean ± standard deviation (n = 11).
NN: normobaric normoxia; HH: hypobaric hypoxia; HN: hypobaric normoxia; O2max: maximal oxygen uptake; CO2max: maximal carbon dioxide output; f R: respiratory frequency; VT: tidal volume; VA: alveolar ventilation; PETCO2: end tidal CO2 pressure; PAO2: partial pressure of alveolar O2; SaO2: arterial oxyhemoglobin saturation; HRmax: maximal heart rate.
P < 0.05 versus NN.
P < 0.05 versus HH.
Mimic ventilation trial
Table 2 and Figure 1 show the results of the mimic ventilation trials. The participants controlled their VT and f R to achieve the level of Emax in NN and HN. SaO2 was 100 ± 0% in both HN and NN conditions. O2vent was lower in HN than in NN. O2rm was 23.1% lower in HN than in NN (5.7 ± 1.8 vs. 7.7 ± 2.0 mL kg−1 min−1, respectively; P < 0.05). Thus, the calculated percentage of O2rm against whole‐body O2max was lower in HN than in NN (9.1 ± 3.4 vs. 12.4 ± 3.6%, P < 0.05). PImax was 27.6% lower in HN than in NN. PEmax was 23.2% lower in HH than in NN (Table 2).
Table 2.
Variables analyzed during the last 30 s of voluntary hyperventilation at rest
| NN | HN | % change | |
|---|---|---|---|
| E [L min−1] | 147.9 ± 11.9 | 158.1 ± 12.7a | 6.9 |
| f R [breaths min−1] | 68 ± 10 | 73 ± 11a | 7.4 |
| VT [L] | 2.23 ± 0.28 | 2.24 ± 0.3 | −0.2 |
| O2mimc [mL kg−1 min−1] | 12.19 ± 2.11 | 10.15 ± 1.66a | −15.5 |
| O2rest [mL kg−1 min−1] | 4.46 ± 0.84 | 4.48 ± 1.02 | 1.11 |
| O2vent [mL kg−1 min−1] | 7.73 ± 2.04 | 5.67 ± 1.80a | −23.1 |
| %O2max | 12.4 ± 3.6 | 9.1 ± 3.4a | −23.4 |
| SaO2 [%] | 100 ± 0 | 100 ± 0 | 0 |
| PImax [cmH2O] | 8.98 ± 2.80 | 6.20 ± 2.00a | −27.6 |
| PEmax [cmH2O] | −9.15 ± 2.11 | −6.87 ± 1.59a | −23.2 |
Values are mean ± standard deviation (n = 10).
NN: normobaric normoxia; HN: hypobaric normoxia; E: minute ventilation; f R: respiratory frequency; VT: tidal volume; O2mimc: oxygen uptake during mimic ventilation; O2rest: oxygen uptake at rest; O2vent: calculated O2 at respiratory muscles; %O2max: percentage occupation of O2vent to O2max; PI max: peak inspiratory mouth pressure; PE max: peak expiratory mouth pressure.
P < 0.05 versus NN.
Figure 1.
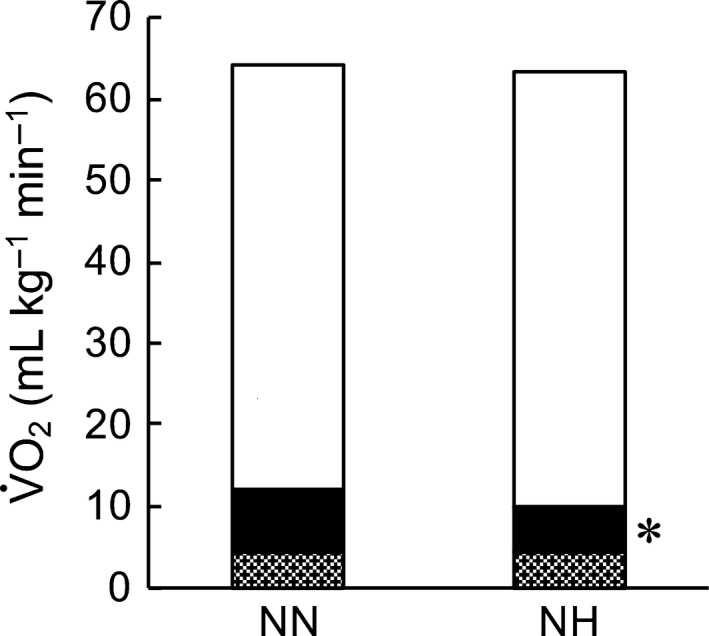
Estimated oxygen consumption at O2max. Grey area shows O2 at rest, the black area shows the O2 of the respiratory muscles, and the white area shows other tissues. NN, normobaric normoxia; HN, hypobaric normoxia. *P < 0.05 versus NN.
Discussion
To the best of our knowledge, this is the first study to assess the effects of reduced barometric pressure during acute hypobaric conditions on ventilatory and metabolic responses, as well as the effects on endurance exercise performance during maximal running exercise. The incremental running exercise was performed on separate days under NN (20.9 ± 0.1% O2 at 763 ± 5 mmHg), HH (20.9 ± 0.1% O2 at 492 ± 1.1 mmHg), and HN (32.2 ± 0.1% O2 at 492 ± 1 mmHg) conditions. Emax was higher in HN than in NN, although O2max did not differ between HN and NN. However, time to exhaustion was longer in HN than in NN. These results suggest that reduced air density associated with acute exposure to 3500 m a.s.l. increases ventilation and improves exercise performance without affecting whole‐body aerobic metabolism.
Our results demonstrate that Emax during maximal running is higher in HN than that in NN (Table 1), which could be attributed to reduced air resistance. Higher flow rates through the airways and alveolar branches occurring during maximal exercise often induce turbulent airflow, which is a factor that contributes to increased flow resistance (West 2005). Theoretically, air density would be 0.83 kg m−3 at 3500 m a.s.l. and 1.20 kg m−3 at sea level, indicating that flow resistance in the airways would be lower in HN than in NN. This ultimately may represent the underlying reason for the higher Emax in HN. Our results also demonstrated that E/O2 and E/CO2 under HN were greater in comparison to those under NN, implying that air‐flow resistance was altered by hypobaria.
Pulmonary ventilation exponentially increases with decreases in ambient partial pressures of O2. As previously discussed, our results suggest that air decompression associated with exposure to HH could increase ventilation during exercise. The Emax in HH was expected to be higher than that in the other two conditions as a consequence of hypoxia and air decompression. However, we observed that Emax in HH was not different from that in NN and HN. The precise reason for this finding remains to be established. However, it may be attributable to the reduced absolute exercise intensity (running speed) at O2max in HH relative to the other two conditions. Hence, greater ventilatory drive associated with the combination of hypoxia and air decompression is offset by a lower ventilatory drive associated with lower exercise intensity.
SaO2 at maximal running in NN was 91% (Table 1), indicating that our participants developed exercise‐induced arterial hypoxemia. Under the NN condition, any increase in oxygen supply due to increased ventilation appears to have increased SaO2 and O2max. Although Emax during maximal running was higher in HN than in NN, neither SaO2 nor O2max increased (Table 1). In contrast, Powers et al. (1986) reported that among individuals with exercise‐induced arterial hypoxemia under NN, breathing He–O2 resulted in increased O2max and a 29% increase in Emax during intense exercise. We also previously reported that in HH at 2500 m a.s.l., breathing He‐O2 increased O2max and resulted in a 15.1% increase in Emax (Ogawa et al. 2010). In the present study, the lack of effect from increased E on SaO2 and O2max under the HN condition could be due to a relatively smaller increase in Emax (6.8%) relative to that experienced under the NN condition (previous studies utilizing He–O2 gas showed a greater increase in E of 15–29%). Moreover, this study demonstrated that the increase in Emax was mainly caused by an increase in f R without a measurable increase in VT. This result implies that a large portion of the increase in Emax in HN relative to that in NN may have resulted from increased dead space with a minimal increase in alveolar ventilation. In fact, A and PAO2 were not different between HN and NN (Table 1). One might think that the reduced airway resistance associated with hypobaria would reduce turbulent airflow, thereby minimizing physiological dead space; however, this effect, if present, may have been overpowered by the rapid shallow breathing that occurred in HN.
The lack of effect of increased ventilation on O2max in HN is in line with the estimations reported in previous studies. Regarding reduced air density, Esposito and Ferretti (1997) demonstrated that when E increased with He‐O2 breathing, O2max increased during He‐O2 breathing under hypoxic conditions, while O2max did not increase under normoxic conditions. Although the air density in He‐O2 is greatly reduced compared to that in 3500 m a.s.l. hypobaria, our results under HN were consistent with the results of their normoxic He‐O2 breathing results. Further, as a limitation of O2max, the ventilatory resistance that limits the flow of O2 from the atmosphere to the alveolar sacs could be analyzed using the multifactorial model proposed by di Prampero (2003). According to this model, the resistance imposed on O2 flow because of ventilatory resistance decreased by 13% under HN compared to that under NN (data not shown). If the resistance to O2 flow is altered, thereby changing O2max, the fractional limitation to O2max imposed by ventilatory resistance to O2 flow (Fv) can be calculated. Ferretti and di Prampero (1995) reported that Fv was 5% under NN. The calculated Fv in the present study was 4%, which agrees closely with the estimations made by Ferretti and di Prampero (1995), implying that the contribution of E was not a limiting factor for O2max in NN among the participants in the current study.
Exercise performance based on the time to exhaustion was extended in HN compared with that in NN (Table 1). This implies that reduced airway resistance associated with hypobaric exposure could improve endurance exercise performance. This may be counterintuitive, as O2max (aerobic energy supply) did not differ between HN and NN conditions in this study (Table 1). However, similar results were also reported by Marconi et al. (2004) with chronic hypobaric hypoxic exposure (5050 m a.s.l.). We do not know the exact mechanism by which reduced airway resistance under HH conditions improves endurance exercise performance without affecting O2, but some insights could be gleaned from a previous work. Diaphragmatic fatigue during strenuous ventilation has been shown to increase the activity of sympathetic nerves that innervate muscles (Derchak et al. 2000). This results in reduced active muscle blood flow (Sheel et al. 2001). O2rm comprises a significant portion of whole‐body O2 because of hyperventilation that occurs during intense exercise (Aaron et al. 1992; Vella et al. 2006). Along these lines, Harms et al. reported that unloading the work of respiratory muscles because of inspiratory assistance during intensive exercise results in improved exercise tolerance with a greater distribution of the available cardiac output to active locomotor muscles with no increase in whole‐body O2 (Harms et al. 1997, 1998, 2000). In our study, PImax and PEmax were lower and E was higher in HN compared with that in NN during the mimic ventilatory tests (Table 2). This suggests that air flow resistance during maximal exercise may be reduced because of reductions in air density associated with hypobaric exposure (3500 m a.s.l.). Further, decreased work during respiration was indirectly supported by our results. We demonstrated that the estimated O2rm was lower under HN than under NN and that the estimated distribution of O2rm was lower under HN than under NN (Table 2 and Fig. 1). These results suggest that the oxygen supply to active muscles was increased in exchange for reducing the oxygen consumption of the respiratory muscles. Therefore, this may improve exercise performance in HN compared with that in NN.
Limitations
A limitation of this study was that participants knew the conditions under which they were exercising. We do not know if this might have affected our results and if so, to what extent. We assessed the influence of hypobaria by comparing responses between NN and HN conditions in the absence of hypoxia. Additional studies are required to elucidate whether hypobaria can modulate responses under hypoxic conditions. Our results also may have been different if a different exercise protocol had been employed. Finally, we did not directly assess airway resistance. However, in the present study, we observed lower oral pressure and respiratory muscle O2 despite the fact that a higher Emax was observed under HN compared with NN. Therefore, we believe that airway resistance was substantially reduced with exposure to HH. Moreover, had we employed a cycling model, we might have been able to assess the relationships between E and O2 at a given work rate. This information would be helpful to evaluate whether respiratory efficiency would be altered under hypobaric conditions.
Conclusion
We found that Emax was higher and the time to exhaustion during incremental running was extended under HN compared with that in NN and there was no difference in O2max. This suggests that reduced air density under the hypobaric condition of 3500 m a.s.l. improved exercise performance without increasing aerobic energy supply, possibly because of a reduced oxygen supply to respiratory muscles and a concomitant increase in oxygen supply to active muscles.
Conflict of Interest
None.
Acknowledgment
We are grateful to the University of Tsukuba track and field team for their participation in this study.
Ogawa T., Fujii N., Kurimoto Y., Nishiyasu T.. Effect of hypobaria on maximal ventilation, oxygen uptake, and exercise performance during running under hypobaric normoxic conditions. Physiol Rep, 7 (3), 2019, e14002, 10.14814/phy2.14002
Funding information
This study was supported by Grant‐in‐Aid for Young Scientists (B; 22700666) and by the Japan Society for the Promotion of Science.
References
- Aaron, E. A. , Seow K. C., Johnson B. D., and Dempsey J. A.. 1992. Oxygen cost of exercise hyperpnea: implications for performance. J. Appl. Physiol. 72:1818–1825. [DOI] [PubMed] [Google Scholar]
- Calbet, J. A. L. , Boushel R., Radegran G., Sondergaard H., Wagner P. D., and Saltin B.. 2003. Determinant of maximal oxygen uptake in severe acute hypoxia. Am. J. Appl. Physiol. Regul. Integr. Comp. Physiol. 284:R291–R303. [DOI] [PubMed] [Google Scholar]
- Cerretelli, P. 1976. Limiting factors to oxygen transport on Mount Everest. J. Appl. Physiol. 40:658–667. [DOI] [PubMed] [Google Scholar]
- Coppel, J. , Hennis P., Gilbert‐Kawai E., and Grocott M. P.. 2015. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extrem. Physiol. Med. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derchak, P. A. , Stager J. M., Tanner D. A., and Chapman R. F.. 2000. Expiratory flow limitation confounds ventilatory response during exercise in athletes. Med. Sci. Sports Exerc. 32:1873–1879. [DOI] [PubMed] [Google Scholar]
- Esposito, F. , and Ferretti G.. 1997. The effects of breathing He‐O2 mixtures on maximal oxygen consumption in normoxic and hypoxic men. J. Physiol. 503:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti, G. , and di Prampero P.. 1995. Factors limiting maximal O2 consumption: effects of acute changes in ventilation. Respir. Physiol. 99:259–271. [DOI] [PubMed] [Google Scholar]
- Fulco, C. S. , Rock P. B., and Cymerman A.. 1998. Maximal and submaximal exercise performance at altitude. Aviat. Space Environ. Med. 69:793–801. [PubMed] [Google Scholar]
- Gautier, H. , Peslin R., Grassino A., Milic‐Emili J., Hannhart B., Powell E., et al. 1997. Mechanical properties of the lungs during acclimatization to altitude. J. Appl. Physiol. 52:1573–1583. [DOI] [PubMed] [Google Scholar]
- Harms, C. A. , Babrock M. A., McClaran S. R., Pegelow D. F., Nickele G. A., Nelson W. B., et al. 1997. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 82:1573–1583. [DOI] [PubMed] [Google Scholar]
- Harms, C. A. , Wetter T. J., McClaran S. R., Pegelow D. F., Nickele G. A., Nelson W., et al. 1998. Effect of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 85:609–618. [DOI] [PubMed] [Google Scholar]
- Harms, C. A. , Wetter T. J., St. Croix C. M., Pegelow D. F., and Dempsey J. A.. 2000. Effect of respiratory muscle work on exercise performance. J. Appl. Physiol. 89: 131–138. [DOI] [PubMed] [Google Scholar]
- Marconi, C. , Marzorati M., Grassi B., Basnyat B., Colombini A., Kayser B., et al. 2004. Second generation Tibetan lowlanders acclimatize to high altitude more quickly than Caucasians. J. Physiol. 556:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet, G. P. , Faiss R., and Pialoux V.. 2012. Point: Hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J. Appl. Physiol. 112:1783–1784. [DOI] [PubMed] [Google Scholar]
- Mink, S. N. , and Wood D. H.. 1980. How does HeO2 increase maximum expiratory flow in human lungs? J. Clin. Invest. 66:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, T. , Hayashi K., Ichinose M., and Nishiyasu T.. 2007. Relationship between rest ventilatory chemosensitivity and maximal oxygen uptake in moderate hypobaric hypoxia. J. Appl. Physiol. 103:1221–1222. [DOI] [PubMed] [Google Scholar]
- Ogawa, T. , Calbet J. A. L., Honda Y., Fujii N., and Nishiyasu T.. 2010. The effects of breathing a helium–oxygen gas mixture on maximal pulmonary ventilation and maximal oxygen consumption during exercise in acute moderate hypobaric hypoxia. Eur. J. Appl. Physiol. 110:853–861. [DOI] [PubMed] [Google Scholar]
- Papamoschou, D. 1995. Theoretical validation of the respiratory benefits of helium‐oxygen mixtures. Respir. Physiol. 99:183–190. [DOI] [PubMed] [Google Scholar]
- Powers, S. K. , Jacques M., Richard R., and Beadle R. E.. 1986. Effects of breathing a normoxic He‐O2 gas mixture on exercise tolerance and VO2max . Int. J. Sports Med. 7:217–220. [DOI] [PubMed] [Google Scholar]
- di Prampero, P. E. 2003. Factors limiting maximal performances in humans. Eur. J. Appl. Physiol. 90:420–429. [DOI] [PubMed] [Google Scholar]
- Saugy, J. J. , Rupp T., Faiss R., Lamon A., Bourdillon N., and Millet G. P.. 2016. Cycling time trial is more altered in hypobaric than normobaric hypoxia. Med. Sci. Sports Exerc. 48:680–688. [DOI] [PubMed] [Google Scholar]
- Sheel, A. W. , Derchak P. A., Morgan B. J., Pegelow D. F., Jacques A. J., and Dempsey J. A.. 2001. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J. Physiol. 537:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella, C. A. , Marks D., and Robergs R. A.. 2006. Oxygen cost of ventilation during incremental exercise to VO2max . Respirology 11:175–181. [DOI] [PubMed] [Google Scholar]
- West, J. B . 2005. Mechanics of breathing. How the lung is supported and moved Pp. 93–120 In West J. B, ed. Respiratory Physiology: The Essentials. 7th ed Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]


