Abstract
Background and objectives
Methotrexate (MTX) is a chemotherapeutic agent that functions as a folic acid antagonist. The frequency of high dose methotrexate (HDMTX)-associated toxicity is variable. In this study, we investigated the frequency of myelotoxicity and hepatotoxicity 7 days after HDMTX infusion.
Patients and methods
This study included children diagnosed with acute lymphoblastic leukemia (ALL) between January 2010 and April 2015. The patient blood counts and biochemical parameters measured before and after 7 days of HDMTX infusion were retrospectively recorded. We assessed HDMTX infusions for 48 children. The number of patients and drug doses included the following: 17 children receiving 1 g/m2 (68 infusions), 14 children receiving 2 g/m2 (56 infusions), and 17 children receiving 5 g/m2 (68 infusions). The classification of toxicity was made based on the Common Terminology Criteria for Adverse Events (CTCAE) 2010 criteria. Myelotoxicity was defined as a hemoglobin level <10 g/L and absolute neutrophil count <1 × 109/L or platelet count <75 × 109/L. The presence of transaminase levels ≥5 times the upper limit was considered to be hepatotoxicity grade ≥3. The MTX levels at 42 h in patients with and without toxicity were compared to evaluate the correlation between MTX levels, hematologic parameters, and transaminase levels.
Results
Myelotoxicity was observed in 35.2%, 37.5%, and 33.8% of the infusions, and hepatotoxicity grade ≥3 was detected in 13.2%, 12.5%, and 11.7% of the infusions in patients receiving 1, 2 and 5 g/m2 HDMTX after 7 days, respectively. There was no statistically significant difference between MTX levels at 42 h in patients with and without toxicity (P > .05, for all). There was no correlation between hematologic parameters and transaminase levels and MTX levels at 42 h.
Conclusion
Hematologic toxicity was the most common toxicity observed. The data indicate the hematologic toxicity increased after repeated cycles in patients receiving 5 g/m2. However, the hepatic toxicity decreased with additional cycles. Our results show the level of MTX at 42 h is not effective to identify toxicity.
Keywords: Children, Leukemia, High dose methotrexate, Myelotoxicity, Hepatotoxicity
1. Introduction
Methotrexate inhibits dihydrofolate reductase and was initially developed as an anti-cancer treatment in the 1940s [1]. The most commonly described side-effects of high dose methotrexate (HDMTX) therapy are myelosuppression, oral mucositis, and acute liver toxicity with transient elevation of transaminase levels [1]. These adverse effects increase the risk of infection which can delay the scheduled therapy. There is a difference between methotrexate (MTX) associated myelosuppression and toxicity. MTX-associated toxicity is related to several factors including drug dose, the duration of administration, patient risk factors, and genetic factors [2], [3]. In addition, the criteria used to assess toxicity are variable. For example, a study showed the most common toxicity was hematologic toxicity, and 64–87% of the patients developed grade 3 and higher toxicity [3], [4]. The incidence of transient high levels of transaminase was reported as 64% [5]. There are currently no data regarding the use of HDMTX pharmacokinetic and toxicity information to predict hepatic and hematologic toxicity in children with acute lymphoblastic leukemia. Csordas et al [6] determined there is no correlation between MTX level and hematologic toxicity. However, Rask et al [4] reported there is a relationship between elevated serum MTX levels and hematologic toxicity.
In this study, we aimed to determine the hepatic and hematologic toxicity frequency and assessed whether there is a significant relationship between treatment toxicity and MTX42 levels in children taking different doses of HDMTX.
2. Patients and methods
This study included 48 children diagnosed with ALL at Eskişehir Osmangazi University Faculty of Medicine, Pediatric Hematology/Oncology Department between January 2010 and April 2015. The age, gender, leukemia immune phenotype, and the administered HDMTX doses were retrospectively recorded from the file records. The patient hemoglobin (Hb), absolute neutrophil count (ANC), platelet (PLT) count, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were recorded before MTX administration and on the 7th day following drug infusion.
The patients were stratified into three risk groups. The first risk group was defined using the following criteria: (1) Standard risk (SR) patients have an initial leukocyte count < 20 × 109/L and age ≥1 year or <6 years and absolute blast count in the peripheral blood on day 8 after 7 days of prednisolone treatment <1 × 109/L and M1 (<5% blasts) or M2 (≥5% to <25% blasts) marrow on day 15, and M1 marrow on day 33 (all criteria must be fulfilled). The second group (2) was the high risk (HR) group and is defined as at least one of the following: absolute blast count in the peripheral blood on day 8 after 7 days of prednisolone treatment >1 × 109/L, M3 (>25% blasts) marrow on day 15, M2 or M3 marrow on day 33, t (9; 22) (BCR-ABL), t (4; 11) (MLL-AF4), or hypodiploidy ≤ 45. The third group (3) was the Intermediate risk (IR) grouped and was defined as the patients who were classified as neither SR nor HR. The HR patients were not included in the study because their consolidation treatment is different.
All patients received 4 HDMTX infusions at 2-week intervals on days 8, 22, 36, and 50 of the consolidation phase. The patients also received oral 6-mercaptopurine (6-MP) at a dose of 25 mg/m2/day continuously during the consolidation phase of chemotherapy in addition to HDMTX. Any concurrent treatment with trimoxazole was paused in all patients 2 days before and 3 days after the HDTMX infusion. We excluded patients whose absolute neutrophil count was <1 × 109/L with a platelet count <75 × 109/L or had liver function tests above the upper limits before the HDMTX infusion.
The patients received 3 different doses of HDMTX. The preB ALL SR patients received 2 g/m2 which is in accordance with the ALL-IC BFM 2009 protocol. The preB ALL IR and T ALL SR/IR patients received 5 g/m2 of HDTMX. According to BFM TRALL 2000 protocol, preB ALL SR/IR patients received 1 g/m2 of HDTMX. As per BFM TRALL 2000 protocol, the 1 g/m2 was given within 36 h and as per the ALLIC BFM 2009 protocol the doses of 2 and 5 g/m2 were administered via 24-h of infusion. Alkalization was used to maintain the urine pH ≥ 7 from −4 h through +72 h after the start of the MTX infusion. The urine was tested by dipstick. Briefly, a bolus infusion of 2 mmol/kg NaHCO3 with 2 ml/kg distilled water was delivered over 1 h. Thereafter, 500 ml 0.45% NaCl/5% dextrose +40 mmol NaHCO3 + 10 ml KCl 7.45% hydration fluid was administered during 4 h. The HDMTX infusion was started only when a urine pH was greater than 7. The bolus infusion was repeated if the urine pH was less than 7. All patients received a loading dose of methotrexate as 1/10 of the total MTX dose over 30 min. The remaining 9/10 dose was given over the following 23 h 30 min or 35 h 30 min for children treated with 2 g/m2, 5 g/m2, and 1 g/m2, respectively. We provided a total 3000 ml/m2 parallel hydration with 0.45% NaCl/5% dextrose containing NaHCO3 (180 mmol/m2/24 h) + KCl 7.45% (90 ml/m2/24 h) during the 72 h or 48 h to children treated with 2 g/m2, 5 g/m2, and 1 g/m2, respectively.
The patients also received standard calcium leucovorin administered via the intravenous route (IV) at a dose of 15 mg/m2 at 42, 48, and 54 h in patients receiving 1 g/m2, 2 g/m2, and 5 g/m2, respectively. A MTX level at 42 h > 1 μmol/L was defined as extended MTX elimination. These patients were treated with 30 mg/m2 of calcium leucovorin at 42 h. The MTX serum levels were monitored until they decreased below 0.25 μmol/L.
The blood toxicity and the liver enzyme levels were classified based on the Common Terminology Criteria for Adverse Events 2010 guideline (Table 1) [7]. Myelotoxicity was defined as hemoglobin (Hb) < 10 g/L and absolute neutrophil count (ANC) <1 × 109/L or platelet (PLT) count <75 × 109/L. Transaminase levels of ≥5 times the upper limit were assessed as ≥ grade 3 hepatotoxicity. The reference range of our hospital for transaminases is 40 IU/L.
Table 1.
Toxicity criteria according to the Common Terminology Criteria for Adverse Events (CTCAE) 2010 guideline.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Hb (g/L) | LLN-10 | 8–10 | <8 | Life-threatening anemia |
| ANC(×109/L) | LLN-1.5 × 1 | 1.5–1 | 1–0.5 | <0.5 |
| PLT (×109/L) | LLN-75 | 75–50 | 50–25 | <25 |
| AST and ALT (IU/L) | >ULN-3 × ULN | 3–5 × ULN | 5–20 × ULN | >20 × ULN |
Hb: Hemoglobin, ANC: Absolute neutrophil count, PLT: Platelet count, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, LLN: Lower limit of normal, ULN: Upper limit of normal.
The myelotoxicity and hepatotoxicity frequency was investigated after repeated cycles, then, the toxicity frequencies for the three groups were compared. The MTX42 levels are the drug concentration values when the first calcium leucovorin treatment is provided. The MTX42 levels in patients with and without hepatic toxicity were compared in the patients receiving 2 and 5 g/m2.
The correlation between MTX42h levels and Hb, AST, ALT, ANC, and PLT count were evaluated for each cycle. There was no evaluation conducted for the patients receiving 1 g/m2 as their MTX levels were not obtained.
3. Statistical analysis
The analyses were conducted using SPSS 21 software. The patients were grouped based on drug dose. The normality of distributions was evaluated with the Kolmogorov–Smirnov test. The mean pre- and post-infusion values were analyzed with the paired sample t-test and the Wilcoxon test. We used the Analysis of variance (ANOVA) for independent groups. The comparison of MTX42h levels was performed using the Mann Whitney U-test. The Pearson correlation test was used to evaluate the correlation between variables. All P < .05 values were considered statistically significant.
4. Results
In this study, there were 192 HDMTX infusions delivered to 48 leukemic children (1 g/m2: 17 children, 68 infusions; 2 g/m2: 14 children, 56 infusions; 5 g/m2: 17 children, 68 infusions) between 2 and 17 years of age. There were 25 females (52%) and 23 males (48%). The mean patient age of those who received 1 g/m2, 2 g/m2, and 5 g/m2 were 6.17 (2.94) years, 3.78 (3.06) years, and 8.23 (5.25) years, respectively. There were 46 cases with pre- B ALL and 2 cases with ALL. The clinical characteristics of the children are presented in Table 2.
Table 2.
Clinical characteristics of children.
| Dose | 1 g/m2 (n = 17) | 2 g/m2 (n = 14) | 5 g/m2 (n = 17) |
|---|---|---|---|
| Infusions | 68 | 56 | 68 |
| Gender (F/M) | 5/12 | 7/7 | 13/4 |
| SR/IR | 13/4 | 13/1 | –/17 |
| Age | 6.17 (2.94) | 3.78 (3.06) | 8.23 (5.25) |
| Immunophenotype | Pre B-ALL (17) | Pre B-ALL (13) T-ALL (1) |
Pre-B ALL (16) T-ALL (1) |
SR: Standard risk, IR: Intermediate risk.
We detected a statistically significant reduction in Hb level and ANC (P < .05, P < .001, respectively) by comparing the mean laboratory data obtained prior to drug infusion and at day 7. There was no statistically significant difference in the PLT count. However, there was a statistically significant increase in ALT and AST levels (P < .001, for both) (Table 3).
Table 3.
The mean values of Hb, AST, ALT with PLT and ANC before and after 7 days of HDMTX infusion.
| Before infusion | After 7 days of HDMTX infusion | P | |
|---|---|---|---|
| Hb (g/L) | 10.70 (1.07) | 10.47 (1.28) | <.05a |
| ANC(×109L) | 1663.22 (587.61) | 1280.26 (583.54) | <.001a |
| PLT (×109L) | 195875.00 (148812.50–237062.50) | 182750.00 (142750.00–226500.00) | >.05b |
| ALT (IU/L) | 24.25 (19.75–35.68) | 51.50 (32.62–123.37) | <.001b |
| AST (IU/L) | 26.50 (24.06–30.18) | 40.37 (29.62–62.00) | <.001b |
Hb: Hemoglobin, ANC: Absolute neutrophil count, PLT: Platelet count, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase.
Paired sample t test.
Wilcoxon test.
Neutropenia ≥ grade 3 developed in 23.5%, 19.6%, and 20.5% of the HDMTX infusions in the patients receiving 1, 2 and 5 g/m2, respectively. Myelotoxicity was observed in 12 (70.5%), 10 (71.4%), and 12 (70.5%) children following 24 (35.2%), 23 (41%), and 23 (33.8%) infusions in patients receiving 1 g/m2, 2 g/m2, and 5 g/m2, respectively. Hepatotoxicity ≥ grade 3 was detected in 5 (29.4%), 5 (35.7%), and 4 (23.5%) patients after 9 (13.2%), 7 (12.5%) and 8 (11.7%) infusions in children receiving 1 g/m2, 2 g/m2, and 5 g/m2, respectively (Table 4). There was no statistically difference in the frequency of myelotoxicity and hepatotoxicity grade ≥3 (Table 5).
Table 4.
Toxicity frequencies with regard to doses.
| 1 g/m2 |
2 g/m2 |
5 g/m2 |
||||
|---|---|---|---|---|---|---|
| İnfusion (n/%) | Patient (n/%) | İnfusion (n/%) | Patient (n/%) | İnfusion (n/%) | Patient (n/%) | |
| ANC<1 × 109/L | 16 (%23.5) | 13 (%76.4) | 11 (%19.6) | 9 (%64.2) | 14 (%20.5) | 10 (%58.8) |
| PLT<75 × 109/L | 3 (%4.4) | 3 (%17.6) | 6 (%10.7) | 2 (%14.2) | 1 (%1.4) | 1 (%5.8) |
| Hb < 10 g/L & ANC<1 × 109/L | 21 (%30.8) | 9 (%52.9) | 17 (%30.3) | 8 (%57.2) | 22 (%32.4) | 11 (%64.7) |
| Myelotoxicity | 24 (%35.2) | 12 (%70.5) | 23 (%41) | 10 (%71.4) | 23 (%33.8) | 12 (%70.5) |
| ALT 40–120 IU/L (Grade 1) | 21 (%25) | 13 (%76.4) | 19 (%33.9) | 11 (%78.5) | 16 (%23.5) | 9 (%52.9) |
| ALT 120–200 (Grade 2) | 5 (%7.3) | 5 (%29.4) | 5 (%8.9) | 4 (%28.5) | 3 (%4.4) | 3 (%17.6) |
| ALT 200–800 (Grade 3) | 8 (%11.7) | 4 (%23.5) | 5 (%8.9) | 4 (%28.5) | 5 (%7.3) | 4 (%23.5) |
| ALT>800 (Grade 4) | 1 | 1 | 1 | 1 | – | – |
| Hepatotoxicity grade≥ 3 | 9 (%13.2) | 5 (%29.4) | 6 (%10.7) | 5 (%35.7) | 5 (%7.3) | 4 (%23.5) |
Myelotoxicity: Hb level <10 g/L and ANC <1 × 109/L or PLT <75.000 × 109/L Hepatotoxicity grade ≥ 3: Transaminase levels ≥5 times higher than the upper limit of normal (≥200 IU/L).
Table 5.
Comparison of the frequency of myelotoxicity and hepatotoxicity grade ≥3.
| 1 g/m2 | 2 g/m2 | 5 g/m2 | Pa | |
|---|---|---|---|---|
| Myelotoxicity | 24 (%35.2) | 21 (%37.5) | 23 (%33.8) | >.05 |
| Hepatotoxicity grade≥ 3 | 9 (%13.2) | 7 (%12.5) | 8 (%11.7) | >.05 |
One way ANNOVA.
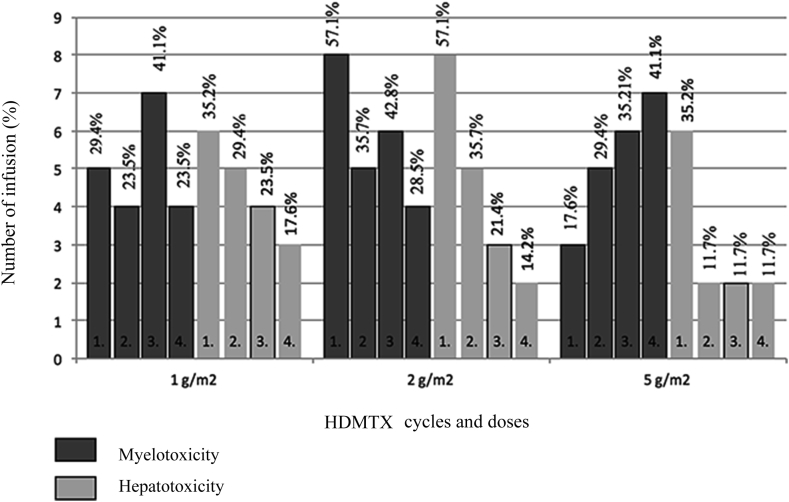
The frequency of grade ≥3 hepatotoxicity was 2-fold higher than myelotoxicity after the 1st cycle in cases receiving 5 g/m2. The frequency of myelotoxicity increased in repeated cycles in patients receiving 5 g/m2. The frequency of myelotoxicity showed variability among repeated cycles in the patients receiving 1 g/m2 and 2 g/m2. There was a reduction in the frequency of hepatotoxicity observed for all three dose groups after repeated cycles (Fig. 1).
Figure 1.
Myelotoxicity and hepatotoxicity grade ≥3 in HDMTX cycles.
The data revealed 13 of 22 patients (59%) (5 patients; 2 g/m2, 8 patients; 5 g/m2) who developed myelotoxicity and 5 of 9 patients (55%) (4 patients; 2 g/m2 and 1 patient; 5 g/m2) who developed hepatic toxicity had extended MTX elimination.
There was no difference in MTX42 levels between the patients with and without hematologic toxicity or the patients with and without hepatotoxicity (P > .05, all) (Table 6, Table 7). There was no correlation observed between MTX42 levels and the values of Hb, AST, ALT, ANC, and PLT count (P < .05, for all).
Table 6.
Comparison of MTX42h levels in patients with and without hematologic toxicity and distribution with reference to the cycles.
| 2 g/m2 |
Pa | 5 g/m2 |
Pa | |||
|---|---|---|---|---|---|---|
| Hematologic toxicity+ | Hematologic toxicity- | Hematologic toxicity+ | Hematologic toxicity- | |||
| Cycles 1 | 1.04 (0.29–2.02) (n = 8) | 0.53 (0.22–0.90) (n = 6) | >.05 | 0.64 (0.52–1.53) (n = 3) | 0.92 (0.52–1.17) (n = 14) | >.05 |
| Cycles 2 | 0.96 (0.39–1.08) (n = 5) | 0.70 (0.28–0.83) (n = 9) | >.05 | 0.97 (0.34–1.65) (n = 5) | 0.54 (0.41–1.01) (n = 12) | >.05 |
| Cycles 3 | 0.31 (0.22–1.22) (n = 6) | 0.50 (0.48–0.99) (n = 8) | >.05 | 0.95 (0.38–1.62) (n = 6) | 0.45 (0.28–1.06) (n = 11) | >.05 |
| Cycles 4 | 0.56 (0.16–0.95) (n = 4) | 0.58 (0.33–2.23) (n = 10) | >.05 | 0.68 (0.29–1.94) (n = 7) | 0.47 (0.28–0.97) (n = 10) | >.05 |
The first row shows MTX levels at 42 h and the second row shows number of patients.
Mann Whitney U- test.
Table 7.
Comparison of MTX42h levels in patients with and without hepatotoxicity and distribution with reference to the cycles.
| 2 g/m2 |
Pa | 5 g/m2 |
Pa | |||
|---|---|---|---|---|---|---|
| Hepatotoxicity+ | Hepatotoxicity- | Hepatotoxicity+ | Hepatotoxicity- | |||
| Cycles 1 | 0.29 (0.22–2.02) (n = 8) | 0.80 (0.38–1.06) (n = 6) | >.05 | 0.77 (0.444–1.92) (n = 6) | 0.88 (0.56–1.20) (n = 11) | >.05 |
| Cycles 2 | 0.86 (0.43–1.02) (n = 5) | 0.70 (0.25–0.99) (n = 9) | >.05 | 0.47 (0.28–0.99) (n = 2) | 0.70 (0.44–1,12) (n = 15) | >.05 |
| Cycles 3 | 0.88 (0.31–1.2) (n = 3) | 0.49 (0.26–0.86) (n = 11) | >.05 | 0.37 (0.24–0.90) (n = 2) | 0.68 (0.42–1,09) (n = 15) | >.05 |
| Cycles 4 | 0.94 (0.72–1.1) (n = 2) | 0.40 (0.17–1.06) (n = 12) | >.05 | 0.42 (0.26–1.48) (n = 2) | 0.60 (0.28–0.99) (n = 15) | >.05 |
The first row shows MTX levels at 42 nd h and the second row shows number of patients.
Mann Whitney U-test.
5. Discussion
The most commonly described side-effects of MTX therapy are myelosuppression, acute liver toxicity, nephrotoxicity, mucositis, and neurotoxicity [4], [8], [9], [10], [11], [12]. Shimasaki et al [3] reported that the only factor that affected HDMTX-associated toxicity development was high MTX levels. Additionally, genetic differences affecting the absorption, distribution, metabolism, and expression of the drug were also significantly involved. The chemotherapeutics used in combination with HDMTX may also affect toxicity. Previous studies reported that using HDMTX with increased doses of 6-MP may also increase hematologic and hepatic toxicity [13], [14].
Prior studies have reported that there are different ratios for the frequency of HDMTX toxicity. Kapoor et al [15] detected neutropenia ≥ grade 3 (ANC < 1 × 109/L) in 24.8% of the infusions in patients receiving HDMTX at 5 g/m2. In our study, neutropenia ≥ grade 3 developed in 23.5%, 19.6%, and 20.5% of the HDMTX infusions at doses of 1, 2 and 5 g/m2, respectively (Table 4). The toxicity rates in our study were similar to each other and were slightly lower than those reported in other studies. In the same study, transient elevation of transaminases occurred in 35% of cycles and most were grade 1 and 2 (32%), as defined by serum ALT levels up to 5 times above normal (grade 3 and 4 in 3 cycles) levels. In our study, hepatotoxicity grade ≥3 was detected in 13.2%, 12.5%, and 11.7% of the HDMTX infusions for patients 1, 2, and 5 g/m2, respectively (Table 4, Table 5). Our rates for hepatotoxicity ≥ grade 3 were higher. Tsurasawa et al [16] reported that the hepatic and gastrointestinal system toxicity was higher after first drug administration and was followed by a sharp reduction in toxicity incidence in the subsequent cycles. In contrast to non-hematologic toxicity, the incidence of hematologic toxicity varied slightly along with HDMTX cycles. Ridolfi et al [17] reported that there was no increase in hematologic and hepatic toxicity with repeated drug administrations. Rask et al [4] identified a significant correlation between leukopenia and cycle count. However, there is no significant correlation between leukopenia and clinical or pharmacokinetic parameters. Our study demonstrated the frequency of hematologic toxicity changed with repeated infusions. We found that the frequency of hematologic toxicity gradually increased in the group receiving 5 g/m2 (Fig. 1). It is thought that the increase in myelosuppression in subsequent cycles is related to the accumulation of cytotoxic metabolites of MTX and 6-MP [4].
The frequency of hepatotoxicity was higher with the first infusion in all three doses, then, showed a subsequent reduction (Fig. 1). The reduction in hepatotoxicity after the first infusion could be explained by the significantly increased plasma folate concentration resulting from repeated leucovorin administrations during HDMTX cycles [18].
Holmboe et al [19] reported that osteosarcoma patients receiving HDMTX showed a relationship between the levels of peak ALT and 7-OH MTX, which is a metabolite of MTX. According to the results of their study, there is no relationship between the levels of peak ALT and peak MTX levels. Additionally, Csordas et al [6] did not identify any correlation between bone marrow toxicity and the levels of serum MTX. In the same study, the authors determined that there was no correlation between 7-OH MTX levels and hepatotoxicity or bone marrow toxicity. However, according to Rask et al, [4], an important risk factor for temporary increases in ALT levels is the extended presence of high serum MTX levels.
In this study, the MTX42 levels in patients with myelotoxicity and hepatotoxicity were not different from patients without toxicity (Table 6, Table 7). However, MTX elimination was extended in 59% of the patients with hematologic toxicity and 55% of patients with hepatic toxicity. These results suggest the levels of MTX42 are not able to identify hematologic and hepatic toxicity risks.
Previous studies have shown using 6-MP in combination with HDMTX affects toxicity frequency. Frandsen et al [20] found patients receiving increased 6-MP (50–75 mg/m2) doses did not experience more toxicity than patients receiving 25 mg/m2 per day. Furthermore, Niekerk et al [13] reported that they compared patients receiving 5 g/m2 HDMTX with patients receiving different doses of 6-MP; the hepatic and hematologic toxicity were higher in the patients receiving 75 mg/m2 than those receiving 25 mg/m2. The authors speculated the cause of these results was the increasing cytotoxic effect because MTX increases both the bioavailability and enzymatic activation of 6-MP [14], [21]. The same researchers also found hepatotoxicity was higher in patients not receiving 6-MP than patients receiving 6-MP, and there was no logical explanation for this result. There is a strong correlation between the severity of myelosuppression, delayed treatment, and the dose of 6-MP [13], [22]. Levinsen et al [14] reported increased levels of 6-MP are related to both hematologic and hepatic toxicity.
The patients received standard 6-MP doses (25 mg/m2). Therefore, the dose of 6-MP was not an important factor affecting the frequency of toxicity. However, it is important to consider the individual differences in the metabolism of 6-MP.
6. Conclusion
Our results indicate that myelotoxicity occurred in approximately 35% of patients, and severe hepatic toxicity occurred in 10% of treated patients. There was no increase in toxicity due to dose administration. Although the frequency of myelotoxicity increased in patients receiving 5 g/m2, the hepatotoxicity frequency decreased. There is no difference between MTX42h levels in patients with and without toxicity. Furthermore, there is no correlation between blood count, AST-ALT levels, and MTX42h levels. These results suggest that MTX42h levels are not able to predict hematologic and hepatic toxicity. Thus, there is a need for further studies to confirm these results because our patient population is limited.
Ethical clearance
This study followed the Declaration of Helsinki on medical protocol and ethics and the regional Ethical Review Board of Eskişehir Osmangazi University Faculty of Medicine approved the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to report.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Contributor Information
Zeynep Canan Özdemir, Email: efecanan@yahoo.com.
Ayşe Bozkurt Turhan, Email: aysebturhan@hotmail.com.
Yeter Düzenli Kar, Email: yeterduzenli@yahoo.com.
Özcan Bör, Email: obor@ogu.edu.tr.
References
- 1.Khan Z.A., Tripathi R., Mishra B. Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv. 2012;9:151–169. doi: 10.1517/17425247.2012.642362. [DOI] [PubMed] [Google Scholar]
- 2.Neuman M.G., Cameron R.G., Haber J.A., Katz G.G., Malkiewicz I.M., Shear N.H. Inducers of cytochrome P450 2E1 enhance methotrexate-induced hepatocytoxicity. Clin Biochem. 1999;32:519–536. doi: 10.1016/s0009-9120(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 3.Shimasaki N., Mori T., Samejima H., Sato R., Shimada H., Yahagi N. Effects of methylenetetrahydrofolate reductase and reduced folate carrier 1 polymorphisms on high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol. 2006;28:64–68. doi: 10.1097/01.mph.0000198269.61948.90. [DOI] [PubMed] [Google Scholar]
- 4.Rask C., Albertioni F., Bentzen S.M., Schroeder H., Peterson C. Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia: a logistic regression analysis. Acta Oncol. 1998;37:277–284. doi: 10.1080/028418698429586. [DOI] [PubMed] [Google Scholar]
- 5.Exadaktylos P., Reiss T., Schobess R., Hommann M., Höhne S., Beck A. Acute hepatotoxicity with intermediate-dose methotrexate in children with leukemia and non-Hodgkin's lymphoma. Klin Paediatr. 1994;206:315–318. doi: 10.1055/s-2008-1046622. [DOI] [PubMed] [Google Scholar]
- 6.Csordas K., Hegyi M., Eipel O.T., Muller J., Erdelyi D.J., Kovacs G.T. Comparison of pharmacokinetics and toxicity after high-dose methotrexate treatments in children with acute lymphoblastic leukemia. Anticancer Drugs. 2013;24:189–197. doi: 10.1097/CAD.0b013e32835b8662. [DOI] [PubMed] [Google Scholar]
- 7.U.S. National Institutes of Health . 2011. National Cancer Institute CTEP CTCAE v3.0.http://ctep.cancer.gov/reporting/ctc.html Available at: [last accessed June 2011] [Google Scholar]
- 8.Fisgin T., Yarali N., Kara A., Bozkurt C., Birken D., Erten U. Hemostatic side effects of high-dose methotrexate in childhood acute lymphoblastic leukemia. Pediatr Haematol Oncol. 2004;21:77–83. [PubMed] [Google Scholar]
- 9.van Outryve S., Schrijvers D., van den Brande J., Wilmes P., Bogers J., van Marck E. Methotrexate-associated liver toxicity in a patient with breast cancer: case report and literature review. Neth J Med. 2002;60:216–222. [PubMed] [Google Scholar]
- 10.Inaba H., Khan R.B., Laningham F.H., Crews K.R., Pui C.H., Daw N.C. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008;19:178–184. doi: 10.1093/annonc/mdm466. [DOI] [PubMed] [Google Scholar]
- 11.Kaur I., Dogra S., De D., Kanwar A.J. Systemic methotrexate treatment in childhood psoriasis: further experience in 24 children from India. Pediatr Dermatol. 2008;25:184–188. doi: 10.1111/j.1525-1470.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- 12.Widemann B.C., Adamson P.C. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11 doi: 10.1634/theoncologist.11-6-694. 694–03. [DOI] [PubMed] [Google Scholar]
- 13.van Kooten Niekerk P.B., Schmiegelow K., Schroeder H. Influence of methylene tetrahydrofolate reductase polymorphisms and coadministration of antimetabolites on toxicity after high dose methotrexate. Eur J Haematol. 2008;81:391–398. doi: 10.1111/j.1600-0609.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 14.Levinsen M., Rosthøj S., Nygaard U., Heldrup J., Harila-Saari A., Jonsson O.G. Myelotoxicity after high-dose methotrexate in childhood acute leukemia is influenced by 6-mercaptopurine dosing but not by intermediate thiopurine methyltransferase activity. Cancer Chemother Pharmacol. 2015;75:59–66. doi: 10.1007/s00280-014-2613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor G., Sinha R., Abedin S. Experience with high dose methotrexate therapy in childhood acute lymphoblastic leukemia in a tertiary care cancer centre of a developing country. Pediatr Blood Cancer. 2012;59:448–453. doi: 10.1002/pbc.24081. [DOI] [PubMed] [Google Scholar]
- 16.Tsurusawa M., Gosho M., Mori T., Mitsui T., Sunami S., Kobayashi R. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood non Hodgkin lymphoma. Pediatr Blood Cancer. 2015;62:279–284. doi: 10.1002/pbc.25305. [DOI] [PubMed] [Google Scholar]
- 17.Ridolfi L., Barisone E., Vivalda M., Vivenza C., Brach Del Prever A., Leone L. Toxicity of high dose methotrexate repeated infusions in children treated for acute lymphoblastic leukemia and osteosarcoma. Minerva Pediatr. 1996;48:193–200. [PubMed] [Google Scholar]
- 18.Sterba J., Dusek L., Demlova R., Valik D. Pretreatment plasma folate modulates the pharmacodynamics effect of high-dose methotrexate in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma: ‘folate overrescue’ concept revisited. Clin Chem. 2006;52:692–700. doi: 10.1373/clinchem.2005.061150. [DOI] [PubMed] [Google Scholar]
- 19.Holmboe L., Andersen A.M., Mørkrid L., Slørdal L., Hall K.S. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73:106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frandsen T.L., Abrahamsson J., Lausen B., Vettenranta K., Heyman M., Behrentz M. Individualized toxicity-titrated 6 mercaptopurine increments during high-dose methotrexate consolidation treatment of lower risk childhood lymphoblastic leukemia. A Nordic Society of Paediatric Haematologyand Oncology (NOPHO) pilot study. Br J Haematol. 2011;155:244–247. doi: 10.1111/j.1365-2141.2011.08835.x. [DOI] [PubMed] [Google Scholar]
- 21.Giverhaug T., Loennechen T., Aarbakke J. The interaction of 6-mercaptopurine (6-MP) and methotrexate (MTX) Gen Pharmacol. 1999;33:341–346. doi: 10.1016/s0306-3623(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 22.Schmiegelow K., Bretton-Meyer U. 6-mercaptopurine dosage and pharmacokinetics influence the degree of bone marrow toxicity following high-dose methotrexate in children with acute lymphoblastic leukemia. Leukemia. 2001;15:74–79. doi: 10.1038/sj.leu.2401986. [DOI] [PubMed] [Google Scholar]