Abstract
The majority of CA-MRSA infections present as skin and soft tissue infections such as abscesses or cellulitis. However, CA-MRSA can cause invasive infections such as joint infections, necrotizing pneumonia or septicemia. Here we describe five cases with CA-MRSA bacteremia complicated with osteoarticular infection, necrotizing pneumonia, and infective endocarditis. We report these case series to outline the spectrum of invasive CA-MRSA diseases and to demonstrate clinical outcome. Early proper intervention with regular revisiting the empirical treatment based on local susceptibility data is crucial. More data on the risk factors for acquiring and spread of CA-MRSA in children are required.
Keywords: CA-MRSA, Invasive, Endocarditis, Children
1. Introduction
Community acquired methicillin resistant Staphylococcus aureus (CA-MRSA) infection in pediatrics is a growing issue global [1], [2]. In Saudi Arabia, CA-MRSA assessed in one retrospective study in Outpatient children at a university hospital from 2005 to 2008; they observed that 29.8% of clinical S. aureus isolates were CA-MRSA, of these cases, 64.7% not associated with known risk factors [3]. A recent prospective study in 2015 done by F. Alaklobi et al. reported that the rate of MRSA carriage between children in Riyadh province was within the range reported internationally (23.2%) [4]. The data from the United States report that MRSA represents up to 76% of all group obtained Staphylococcus aureus separates in some pediatric focuses [5]. Current reports depict progressively serious infection on account of CA-MRSA with proposals that CA-MRSA may bring about more extreme disease than group gained methicillin sensitive Staphylococcus aureus (CA-MSSA) [1], [2], [6]. Most CA-MRSA strains contain the harmfulness factor consider Panton-Valentine leukocidin (PVL) [7]. Rates of invasive MRSA infections in children remain remarkably lower than what has reported in adults. However, rates among the children increased about 10% per year since 2005 [8]. As of late, there have been reports of obtrusive CA-MRSA tainting pediatrics without hazard variables, with confined instances of life-debilitating illness (1). MRSA bacteremia can associate with metastatic sites of infection such as endocarditis, osteomyelitis, epidural or psoas abscesses with associated mortality reach 30% [9], (2); also CA-MRSA can cause severe necrotizing pneumonia in young, immunocompetent patients [10]. We report these case series to outline the spectrum of invasive CA-MRSA diseases and to demonstrate clinical outcome in five cases had invasive disease was described Table 1.
Table 1.
Clinical characteristics of cases has invasive infection with CA-MRSA strains.
| Gender | Origin | Infection site | Risk factor | Treatment | Complication | Outcome | |
|---|---|---|---|---|---|---|---|
| 5 years | Male | Saudi | Blood stream infection/Left femur osteomyelitis/gluteal abscess | No | IV piperacillin-tazobactam + IV cloxacillin x 4 days then Teicoplanin x 10 days. | No | Good |
| IV Clindamycin + IV Rifampicin x 4 weeks + Irrigation and debridement | |||||||
| PO Clindamycin x 2 weeks | |||||||
| 3 years | Male | Saudi | Blood stream infection/infective endocarditis | No | IV Vancomycin x 6 weeks | No | Good |
| IV Gentamicin + IV Rifampicin x 2 weeks | |||||||
| 10 years | Male | Saudi | Blood stream infection/Right hip septic arthritis | No | IV Vancomycin x PO Clindamycin | No | Good |
| 4 years | Female | Saudi | Blood stream infection/Right hip septic arthritis/lung abscess/infective endocarditis | CVC for TPN | Piperacillin - tazobactam and vancomycin (Empirical) | No | Good |
| IV Gentamicin | |||||||
| IV Clindamycin | |||||||
| Enoxaparin sodium | |||||||
| Aspiration plus arthrotomy and debridement | |||||||
| 2 years | Male | Saudi | Blood stream infection/left knee septic arthritis/Left femur osteomyelitis |
No | IV Vancomycin x 6 weeks | No | Good |
2. Cases
2.1. Case 1
A five-year-old boy, presented with a history of fever and left hip pain with inability to bear weight for two weeks, grew CA-MRSA based on susceptibility. MRI showed bone and joint changes suggestive of osteoarticular infection and gluteal abscess. Treated initially with Teicoplanin where blood is sterilized?, then shifted to clindamycin and rifampin to enhance local improvement and incision and drainage were done twice to evacuate the re-collected pus at the gluteal region. Initial workup revealed blood white cell count was 10 × 109/L, the erythrocyte sedimentation rate (ESR) 120 mm/h. Clinical improvement was lacking for two weeks, and repeated MRI showed soft tissue thickening with enhancement in keeping with partially treated osteomyelitis, anterolateral of the femoral head and subgluteal region collection with mild lateral subluxation of the femoral head was noted (Fig. 1A and B). After one month of continuous intravenous therapy, the patient showed marked clinical improvement. Moreover, inflammatory markers were going down accordingly. Medications were step-down to oral clindamycin, and complete clinical improvement and radiological resolution were noted at three months follow-up.
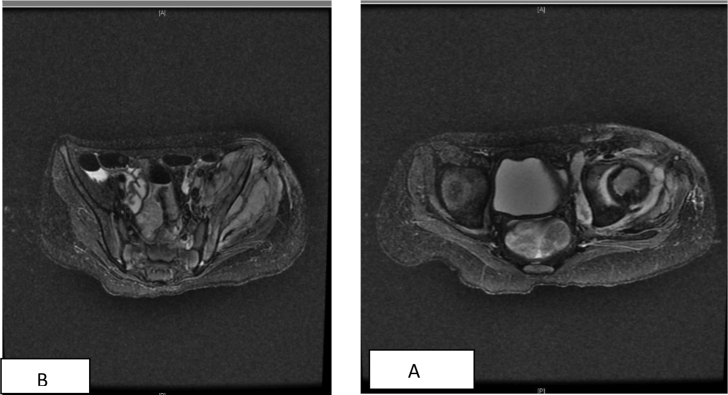
Fig. 1.
Bilateral hip MRI showed Soft tissue thickening and edema with mild enhancement at the left hip joint extending to the right hemi-pelvic muscle in keeping with residual infection/partially treated osteomyelitis A. Small collections seen at antro-lateral aspect of left femoral head measuring 1 × 0.7 cm. B. Small collection at left sub gluteus region measuring 1.3 × 6 cm.
2.2. Case 2
A three-year-old boy, presented with prolonged fever and decreased activity, with no clear focus, blood culture revealed MRSA which only resistant to clindamycin. Vancomycin initiated initially, but with no level adjustment. Fever continued with continuing bacteremia, laboratory workup showed that a white blood cell count of 8.3 × 109/L, hemoglobin 7.5 g/dL, and a platelet count of 226 × 109/L, erythrocyte sedimentation rate (ESR) 42 mm/h, The serum C-reactive protein (CRP) level was 3.2 mg/dL (normal, <0.5 mg/dL). A chest radiograph was unremarkable, while Transthoracic echocardiography disclosed mild tricuspid regurgitation, normal LV function, and vegetation found on the right ventricle: at the tricuspid valve with a size of 11.1 × 8.2 mm (Fig. 2). So MRSA native valve infective endocarditis verified, where vancomycin level adjusted as treating continues bacteremia and gentamicin, and rifampin added. Fever and bacteremia resolved after 13 days from starting the combined therapy, repeated investigations did not show extend focus. The patient was stepped down to monotherapy (vancomycin) after clearance of bacteremia, where therapy discontinued after a course of 8 weeks with gradual resolution of heart vegetation on echocardiography follow-up.
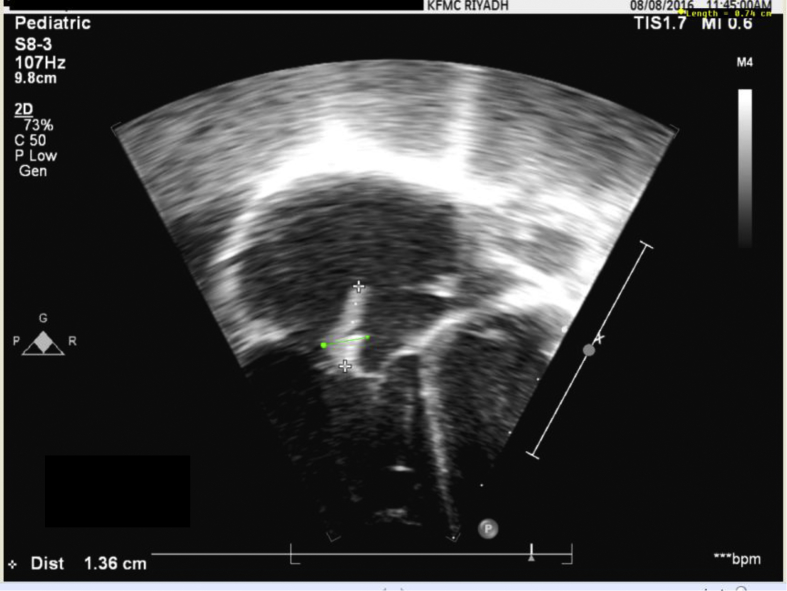
Fig. 2.
Transthoracic echocardiography showing tricuspid valve vegetations with a size of 11.1 × 8.2 mm.
2.3. Case 3
A 10-year-old boy, known to has a nephritic syndrome. Presented with a history of right thigh pain with inability to bear weight for a one-month duration, with a one-week history of fever. Initial workup showed WBC 7.4 x109/L with 72% Neutrophils, ESR of 74 mm/h and serum C-reactive protein 182 mg/dl and Blood culture grew MRSA with full sensitivity, MRI demonstrated right hip septic arthritis with effusion and right femur osteomyelitis (Fig. 3). Patient treated with intravenous vancomycin then soon shifted to oral clindamycin with smooth clinical improvement.
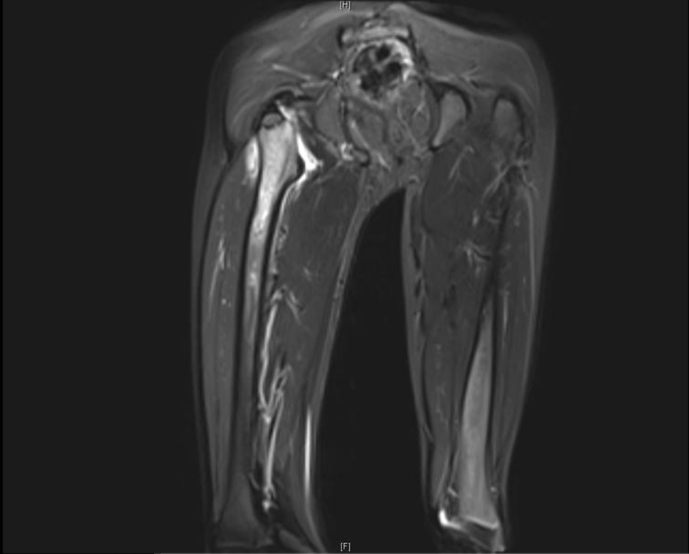
Fig. 3.
MRI right thigh showed Abnormal T2 signal intensity of the upper right femur associated with right hip joint effusion and abnormal deep muscular signal intensity. These findings correspond to acute osteomyelitis.
2.4. Case 4
A four-year-old girl, a case of enteropathy on total parenteral nutrition through a central venous catheter (CVC) was presented with a history of fever along with an inability to bear weight with marked movement limitation at the right hip joint for three days. Initial workup demonstrated: white blood cells count 8.5 x 109/L with 90% neutrophils, hemoglobin 8 g/dl, platelets 95 x 109/L. ESR 84 mm/h and serum C-reactive protein 214 mg/dl, and blood culture centrally and peripherally revealed MRSA which was fully sensitive, septic arthritis with effusion on the right hip was approved radiologically, where the patient underwent debridement and drainage procedure with negative fluid and tissue culture. Vancomycin and gentamicin initiated. Clinical improvement with a clearance of bacteremia documented, but after two weeks on treatment patient developed respiratory symptoms where CT chest demonstrated right side lung abscess (Fig. 4). Transthoracic echocardiography at day seventeen of admission showed vegetation at the inferior vena cava and right atrium junction for which central line removed, then after the patient showed stable clinical condition till he finished the treatment course.
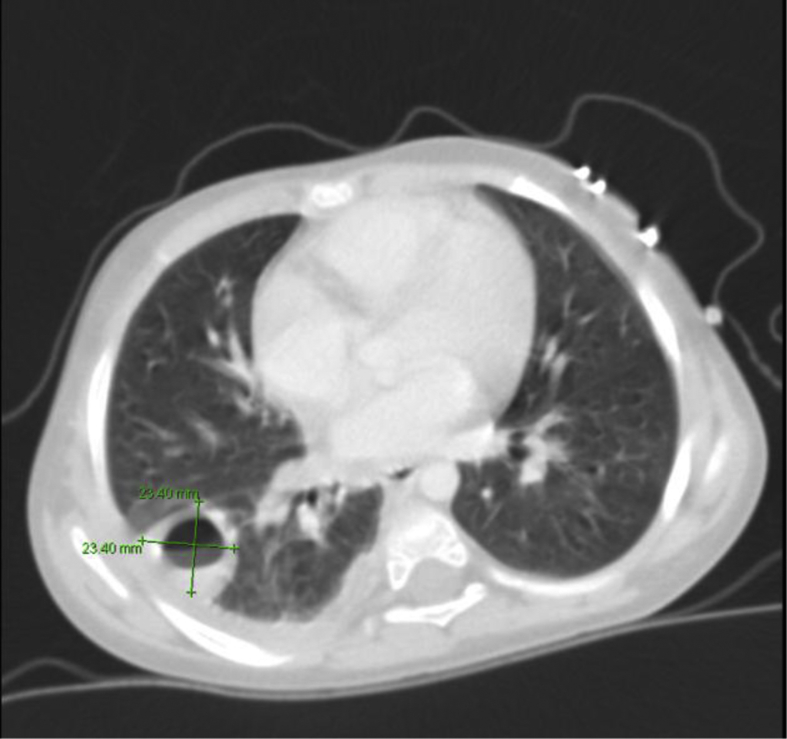
Fig. 4.
CT scan of the chest: abscess formation in the right lower lobe associated with airspace infiltration and mild right pleural effusion.
2.5. Case 5
A 2-year-old boy, who has a speech delayed and behavioral disorder is presented with a history of left knee pain with inability to bear weight for three days duration, proceeded by a history of trauma with a small abrasion on the same site one day before presentation. Initial workup showed WBC 13 x109/L with 71% Neutrophils, ESR of 120 mm/h and serum C-reactive protein 186 mg/dl and Blood culture grew MRSA with full sensitivity, MRI demonstrated severe septic arthritis and osteomyelitis involving the lower femoral epiphysis and metaphysis with surrounding widespread soft tissue inflammation. The patient started on intravenous vancomycin then joint aspiration was done where synovial fluid culture revealed no growth. Patient completed six weeks duration of intravenous vancomycin with a complete clinical resolution without any noted sequelae.
2.6. Microbiological data
Methicillin resistance was defined by minimum inhibitory concentration using the PHOENIX system (Becton Dickinson Company) and confirmed with the E-test methods and interpretation guidelines of the Clinical and Laboratory Standards Institute (CLSI). The four MRSA isolates had similar antibiotic susceptibility patterns, with resistance to oxacillin and susceptibility to erythromycin, clindamycin, ciprofloxacin, rifampin, vancomycin, trimethoprim-sulfamethoxazole, and linezolid. Deviation from this sensitivity pattern observed in one isolate with resistance to clindamycin with oxacillin. The isolates were not obtainable for molecular characterization.
3. Discussion
We have portrayed clinical signs of five invasive instances of CA-MRSA disease in pediatrics. Common clinical disease entities included osteomyelitis/arthritis; deep-seated abscess, necrotizing pneumonia, and invasive endocardium involvement also demonstrated. No known risk factors noted except in one patient where she has a central venous catheter for total parenteral nutrition. All cases did not end with mortality or disabilities. In one review of a United States incidence of CA-MRSA infection increased in children between 2005 and 2010. The incidence rate of invasive CA-MRSA infection per 100,000 children grew from 1.1 in 2005 to 1.7 in 2010 [8]. The five cases portrayed here, delineate the potential destructiveness of CA-MRSA. Four patients gave components of osteomyelitis with quick progression in one patient and development of pyomyositis. Deep venous thrombosis has progressively seen in youngsters in relationship with osteomyelitis because of S. aureus containing the PVL genes [11], the things that not seen in our reported cases. There are negligible information on molecular characteristics and kind of normal MRSA strains in Saudi people group, however as of late noted there is an expansion in the quantity of patients with CA-MRSA. Bukhari-Huda et al. reported that CA-MRSA accounted for 33% (15/45) of the total MRSA cases from King Fahad Hospital, 40% of them were younger than 18 years of age [12]. EE Bukhari et al. did a retrospective review. King Khaled University Hospital in Riyadh reported that over three years, only five cases (5/80) of invasive CA-MRSA infections identified without reported deaths [6]. Among the reported pediatric invasive CA-MRSA infections in Taiwanese children, 18/31 (58.1%) children had bone/joint infections. The lower limbs were the most affected sites and included the hip joint in 10 (55.6%), femur in 5 (27.8%), tibia in 3 (16.7%), and fibula in one (5.6%). Deep-seated soft tissue infections including pyomyositis and necrotizing fasciitis involving predominantly the lower limbs and abdomen identified in 45.2% in the same study [13]. In this report, same findings were there where hip involvement was there in three cases, and the outcome was uneventful in all. Pneumonia caused by CA-MRSA is uncommon but potentially severe, S. aureus necrotizing pneumonia is associated with young, immunocompetent patients [10]. We identify pulmonary involvement with musculoskeletal infections in one case demonstrated to have a lung abscess (patient 4). There have been increasing cases of infective endocarditis (IE) caused by CA-MRSA in adult patients; however, just a few clinical cases have described in children [7], [11], [14]. In one prospective study done in Northwestern Ontario detected cases of positive CA-MRSA bacteremia in 2012 and 2013, 23 cases of CA-MRSA bacteremia managed through a 2-year study period, intravenous drug use accounted for only 17% of cases. One death and 2 cases of endocarditis occurred [15]. Although pre-existing congenital heart diseases is the dominant risk factor for childhood IE, there were growing indications of a continuous shift of IE amongst children without cardiac anomaly [16], [17]. In the presented cases, we identify two cases with IE, (Patient 2) who had no pre-existing cardiac anomaly, no skin lesions were detected. (Patient 4) Has a risk factor representing in a central venous catheter for total parenteral nutrition. The deferred utilization of compelling anti-infection agents may add to genuine entanglements and demise [18]. Vancomycin prescribed forever undermining infections suspected to be MRSA [19]. Four of our announced patients got vancomycin treatment as an initial treatment. Clindamycin is dynamic against MSSA and most strains of CA-MRSA. Success reported in the treatment of invasive CA-MRSA in children, including osteomyelitis, septic arthritis, pneumonia, and lymphadenitis [19]. Be that as it may, Clindamycin is bacteriostatic agents and not prescribed as monotherapy of extreme staphylococcal sepsis. Additionally, D-test ought to be performed before clindamycin treatment for erythromycin-resistant isolates to recognize clindamycin inducible resistance with potential treatment disappointment. [20] Open debridement may be necessary in cases of suspected hip sepsis osteoarticular infection, particularly in children. [21] The ideal term of treatment relies on upon seriousness and site of infection, individual reaction to treatment and specific host components, for example, immunocompromised status. Patients like the detailed cases who have extreme sepsis, osteomyelitis, and pneumonic empyema/septic emboli may require a month and a half or a greater amount of treatment. The emergence of CA-MRSA strains in Saudi Arabia implies that the likelihood of methicillin resistance must consider while choosing the empiric treatment for serious assumed S. aureus infections.
4. Conclusion
Invasive CA-MRSA infections can arise in otherwise healthy individuals who do not have any risk factors which make it one of the virulent organisms. Invasive disease can include bacteremia associated with metastatic sites of infection such as endocarditis, osteomyelitis and necrotizing pneumonia. Prevalence of CA-MRSA appears to be variable, however, with this invasive set of conditions, we believe that awareness of the broad spectrum of CA-MRSA disease among the health care provider is essential where early identify and starting effective empirical therapy is crucial. The outcome with early proper conduct as reported in this case series was favorable. More data on the risk factors for acquisition and spread of CA-MRSA in children are required.
Ethical approval
An institutional review board (IRB) approved a study.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Stankovic C., Mahajan P.V. Healthy children with invasive community-acquired methicillin-resistant staphylococcus aureus infections. Pediatr Emerg Care. 2006;22(5):361–363. doi: 10.1097/01.pec.0000215652.27137.c7. [DOI] [PubMed] [Google Scholar]
- 2.Mermel L.A., Allon M., Bouza E., Craven D.E., Flynn P., O'grady N.P. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of america. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Otaibi F.E., Bukhari E.E. Community-acquired methicillin-resistant staphylococcus aureus in outpatient children assisted at a university hospital in Saudi Arabia: a 3-year study (2005–2008) J Pediatr Infect Dis. 2010;5(4):369–376. [Google Scholar]
- 4.Alaklobi F., Aljobair F., Alrashod A., Alhababi R., Alshamrani M., Alamin W. The prevalence of community-associated methicillin-resistant staphylococcus aureus among outpatient children in a tertiary hospital: a prospective observational study in riyadh, Saudi Arabia. Int J Pediatr Adolesc Med. 2015;2(3):136–140. doi: 10.1016/j.ijpam.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nourse C., Starr M., Munckhof W. Community-acquired methicillin-resistant staphylococcus aureus causes severe disseminated infection and deep venous thrombosis in children: literature review and recommendations for management. J Paediatr Child Health. 2007;43(10):656–661. doi: 10.1111/j.1440-1754.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 6.Bukhari E.E., Al-Otaibi F.E. Severe community-acquired infection caused by methicillin-resistant staphylococcus aureus in saudi arabian children. Saudi Med J. 2009;30(12):1595–1600. [PubMed] [Google Scholar]
- 7.Bahrain M., Vasiliades M., Wolff M., Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant staphylococcus aureus infections. Scand J Infect Dis. 2006;38(8):702–707. doi: 10.1080/00365540500447150. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto M., Mu Y., Lynfield R., Bulens S.N., Nadle J., Aragon D. Trends in invasive methicillin-resistant staphylococcus aureus infections. Pediatrics. 2013;132(4):e824. doi: 10.1542/peds.2013-1112. [DOI] [PubMed] [Google Scholar]
- 9.Thwaites G.E., Edgeworth J.D., Gkrania-Klotsas E., Kirby A., Tilley R., Trk M.E. Clinical management of staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11(3):208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 10.Tsai Y., Ku Y. Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med. 2012;18(3):246–252. doi: 10.1097/MCP.0b013e3283521022. [DOI] [PubMed] [Google Scholar]
- 11.Millar B.C., Prendergast B.D., Moore J.E. Community-associated MRSA (CA-MRSA): an emerging pathogen in infective endocarditis. J Antimicrob Chemother. 2008;61(1):1–7. doi: 10.1093/jac/dkm410. [DOI] [PubMed] [Google Scholar]
- 12.Bukharie H.A., Abdelhadi M.S., Saeed I.A., Rubaish A.M., Larbi E.B. Emergence of methicillin-resistant staphylococcus aureus as a community pathogen. Diagn Microbiol Infect Dis. 2001;40(1):1–4. doi: 10.1016/s0732-8893(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen C., Su L., Chiu C., Lin T., Wong K., Chen Y.M. Clinical features and molecular characteristics of invasive community-acquired methicillin-resistant staphylococcus aureus infections in taiwanese children. Diagn Microbiol Infect Dis. 2007;59(3):287–293. doi: 10.1016/j.diagmicrobio.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Tsai H., Chao P., Sy C., Lee S.S., Chen Y., Wann S. Community-associated methicillin-resistant staphylococcus aureus infective endocarditis with panton-valentine leukocidin gene in an injection drug user with HIV infection. Intern Med. 2008;47(16):1485–1489. doi: 10.2169/internalmedicine.47.0878. [DOI] [PubMed] [Google Scholar]
- 15.Kirlew M., Rea S., Schroeter A. Article original invasive CA-MRSA in northwestern. Can J Rural Med. 2014;19(3) [PubMed] [Google Scholar]
- 16.Alshammary A., Hervas-Malo M., Robinson J.L. Pediatric infective endocarditis: has staphylococcus aureus overtaken viridans group streptococci as the predominant etiological agent? Can J Infect Dis Med Microbiol. 2008;19(1):63–68. doi: 10.1155/2008/867342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day M.D., Gauvreau K., Shulman S., Newburger J.W. Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119(6):865–870. doi: 10.1161/CIRCULATIONAHA.108.798751. [DOI] [PubMed] [Google Scholar]
- 18.Miles F., Voss L., Segedin E., Anderson B.J. Review of staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch Dis Child. 2005;90(12):1274–1278. doi: 10.1136/adc.2005.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb 1;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 20.Munckhof W.J., Kleinschmidt S.L., Schooneveldt J.M. Inducible clindamycin resistance in erythromycin-resistant, non-multiresistant, methicillin-resistant staphylococcus aureus. Pathology. 2004;36(4):373–374. doi: 10.1080/00313020410001721573. [DOI] [PubMed] [Google Scholar]
- 21.Kang S., Sanghera T., Mangwani J., Paterson J., Ramachandran M. The management of septic arthritis in children. Bone Joint J. 2009;91(9):1127–1133. doi: 10.1302/0301-620X.91B9.22530. [DOI] [PubMed] [Google Scholar]