Abstract
Acute exposure to a salient stressor, such as in post-traumatic stress disorder, can have lasting impacts upon an individual and society. To study stress in rodents, some naturalistic methods have included acute exposure to a predator odor, such as the synthetic fox odor 2,4,5, trimethyl-3-thiazoline (TMT). These experiments explore the stress-related behaviors and cortical activity induced by TMT exposure in adult male C57BL/6J mice and the influence of the stress neuropeptide corticotropin-releasing factor (CRF) on these responses. Compared to H2O and a novel odorant, vanilla, mice exposed to TMT in the home cage showed increased avoidance and defensive burying indicative of evident stress responses. Consistent with stress-induced activation of the medial prefrontal cortex (mPFC), we found that the prelimbic (PL) and infralimbic (IL) subregions of the mPFC had elevated c-Fos immunolabeling after TMT and vanilla compared to H2O. Slice physiology recordings were performed in layers 2/3 and 5 of the PL and IL, following TMT, vanilla, or H2O exposure. In TMT mice, but not vanilla or H2O mice, PL layers 2/3 showed heightened spontaneous excitatory post-synaptic currents and synaptic drive, suggesting TMT enhanced excitatory transmission. Synaptic drive in PL was increased in both TMT and H2O mice following bath application of 300 nM CRF, but only H2O mice increased excitatory currents with 100 nM CRF, suggesting dose-effect curve shifts in TMT mice. Further, systemic pretreatment with the CRF-R1 antagonist CP154526 and bath application with the CRF-R1 antagonist NBI27914 reduced excitatory transmission in TMT mice, but not H2O mice. CP154526 also reduced stress-reactive behaviors induced by TMT. Taken together, these findings suggest that exposure to TMT leads to CRF-R1 driven changes in behavior and changes in synaptic function in layer 2/3 neurons in the PL, which are consistent with previous findings that CRF-R1 in the mPFC plays an important role in predator odor-related behaviors.
Subject terms: Stress and resilience, Synaptic transmission
Introduction
Not only can cognitive and behavioral perturbations occur after chronic stress, but also acute exposure to an extremely stressful or traumatic event to which an individual responds with fear, helplessness, or horror can develop into post-traumatic stress disorder (PTSD; [1]). Therefore, it is imperative to understand how acute stress can cause immediate neuroadaptations in vulnerable brain areas by studying preclinical models.
There are several proposed rodent models of PTSD, most involving a single exposure to a stressor leading to prolonged symptomatology [2]. The predator odor, 2,3,5-trimethyl-3-thiazoline (TMT), is a synthetic single-molecule component of fox feces with robust effects on avoidance [3]. TMT is thought to be an unconditioned threatening stimulus because naïve, laboratory bred, and raised rats and mice display fear-like responses on their first exposure to TMT [4] as well as corticosterone secretion [5]. Unsurprisingly, TMT increases c-Fos expression in specific brain regions involved in stress, anxiety, and fear, including the medial prefrontal cortex (mPFC) [6]. While numerous mPFC neuromodulators are likely involved in predator odorant driven behavioral responses, one of note is the stress neuropeptide corticotropin-releasing factor (CRF). CRF acts mainly through stimulation of the CRF type-1 receptor (CRF-R1), which is expressed throughout cortical areas in the brain, including the mPFC [7]. A recent study from the Gilpin laboratory found that avoidance of stimuli paired with bobcat urine is modulated by mPFC CRF-R1 signaling in rats [8]. Despite the behavioral relevance of CRF-R1 signaling in the mPFC in aversive behaviors, little is known about how acute exposure to an aversive predator odor can impact synaptic function, and its relation to CRF-R1 function.
This set of experiments builds upon existing literature by exploring the role of specific cortical layers in the CRF/CRF-R1 system in response to an acute stressor in mice, exposure to TMT predator odor. First, we observed prototypical stress and avoidance responses to TMT in the home cage compared to the novel odorant vanilla and H2O. After determining which subregions of the mPFC had the highest levels of c-Fos activation following TMT exposure, we measured synaptic transmission in layers 2/3 and 5 in the PL and IL of H2O-exposed, vanilla-exposed, and TMT-exposed mice. Lastly, we determined a modulatory role of CRF and CRF-R1 using a CRF-R1 antagonist to probe TMT driven behaviors and physiological alterations. These experiments demonstrate that a single exposure to predator odor can result in an increase in acute excitatory transmission to specific cortical subregions that are related to CRF-R1 activation, and further support TMT as a potent and effective stressor for mice.
Materials and methods
Animals
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) arrived at 6 weeks of age and were group-housed with littermates in a temperature-controlled vivarium maintained on a reversed light/dark cycle with lights off at 07:00 h. After one week in the vivarium, mice were single housed in fresh cages lined with corn cob pellet bedding and a nestlet. They had an additional week of single-housing, so mice were 8 weeks old during the tests. Mice had access to standard rodent chow and water at all times except during testing. A total of 82 mice were used [Suppl Table 1].
Predator odor exposure
During the 10-min pre-test, mice were habituated to a shortened cotton tip applicator held vertically with a plastic stand, referred to as the ‘object,’ which was placed in one corner of the rectangular home cage. For the post-test period, 2.5 µl of TMT (Part 300000368, Scotts Canada Ltd.), vanilla (VNL), or distilled water (H2O) was applied to the cotton tip, and mice were monitored for an additional 10 min. All trials were video recorded for hand-scoring by a blind observer or for locomotor analysis in Ethovision XT13 (Noldus, The Netherlands). The following behaviors were assessed in both pre-tests (baseline) and post-tests (trial): time spent contacting the object, time spent in the far corners of the cage, duration of defensive burying, and distance traveled. Object contact includes sniffing, biting, touching, or physically exploring the cotton tip apparatus. Defensive burying is the vigorous treading of bedding with the forepaws or nose. Of the first cohort of mice that were tested for behavioral analyses (n = 40), approximately half were used for c-Fos immunohistochemistry (n = 18), and half were used for electrophysiological recordings (n = 22) [see Suppl Table 1]. A small second cohort of mice (n = 10) were used for recordings but not included in behavioral analysis. For experiments exploring the role of CRF-R1 on behavior and physiology, a third cohort of mice (n = 32) were first habituated to handling and i.p. injections. On the test day, mice received a 10 ml/kg injection of saline or 10 mg/kg CP154526 (Tocris, Minneapolis, MN) 20 min before the pre-test trial. Saline was the vehicle for CP154526. This dose was chosen based on previous studies demonstrating it reduced anxiety-like and stress-related behaviors in mice [9]. There is empirical evidence that CP154526 is still present in cortical structures by the time of recordings [10].
c-Fos immunohistochemistry
Ninety minutes after TMT, VNL, or H2O exposure in the home cage, mice were anesthetized with a mixture of 2,2,2-tribromoethanol and 2-methyl-2-butanol in saline, then intracardially perfused with a chilled 0.01 mM phosphate buffer solution (PBS), followed by 4% paraformaldehyde (PFA) in PBS. Brain tissue was collected, post-fixed in 4% PFA overnight, then transferred to 30% sucrose/PBS and stored at 4 °C before coronal sections were obtained on a vibratome (Leica VT1000S). Free-floating brain sections (45 um thick) were washed in PBS, and then incubated in 50% methanol for 30 min followed by 3% H2O2 for 5 min. Tissue was then blocked in 0.3% Triton X-100 and 1% bovine serum albumin for 60 min before a 48 h incubation at 4 °C in the blocking buffer containing a rabbit anti-c-Fos antibody (1:3000, ABE457 Millipore). After the primary step, slices were washed in TNT buffer for 10 min then TNB buffer for 30 min. Slices were next incubated in a goat anti-rabbit horse radish peroxidase-conjugated IgG (1:200, NEF812001EA, PerkinElmer, Waltham, MA) for 30 min then washed in TNT for four 5-min washes. For amplification of the signal, tissue was then processed using a tyramine signal amplification (TSA) kit with a Cy3-tyramide (1:50, PerkinElmer, Waltham, MA) for 10 min. Four serial sections per animal were mounted on slides, sealed with a mounting medium containing DAPI (VectaShield), then coverslipped. Slides were imaged on a confocal laser-scanning microscope (LSM 800, Zeiss Microscopy, Thornwood, NY) and c-Fos immunoreactive cells were quantified using ZEN imaging software (Zeiss Microscopy, Thornwood, NY). Each subregion was analyzed over four serial slices, and the single mean was used per animal (n = 6 H2O, n = 6 VNL, n = 6 TMT). PL and IL regions and layers 2/3 and 5 were classified according to Franklin and Paxinos [11], Pittaras et al. [12], and Stewart and Plenz [13].
Slice electrophysiology
Ninety minutes following TMT, VNL, or H2O exposure in the home cage, mice were sacrificed via deep isoflurane anesthesia, and acutely-prepared coronal brain slices containing the mPFC were collected according to standard laboratory protocols [14]. Whole-cell voltage-clamp electrophysiological recordings were performed in pyramidal neurons in layers 2/3 and 5 in the PL and IL regions of the mPFC based on landmarks in the Allen Mouse Brain Atlas (Fig. 2a.) The effect of TMT on basal synaptic transmission was assessed in voltage clamp by adjusting the membrane potential and using a cesium methanesulfonate-based intracellular solution (135 mM cesium methanesulfonate, 10 mM KCl, 10 mM HEPES, 1 mM MgCl2, 0.2 mM EGTA, 4 mM MgATP, 0.3 mM GTP, 20 mM phosphocreatine, pH 7.3, 285-290 mOsmol). Lidocaine n-ethyl bromide (1 mg/ml) was included in the intracellular solution to block postsynaptic sodium currents. Neurons were held at -55 mV, the reversal potential for chloride, to assess glutamatergic synaptic transmission. In the same cell, neurons were held at +10 mV, the reversal potential for sodium, to assess GABAergic synaptic transmission. Fluctuations in current were used to determine spontaneous post-synaptic current (sEPSC or sIPSC) frequency and amplitude, as well as to calculate sEPSC/sIPSC ratios and synaptic drive (sEPSC frequency × amplitude / sIPSC frequency × amplitude). Synaptic transmission experiments in PL layer 2/3 were also performed in animals that received either 10 mg/kg CP154526 or saline prior to TMT or H2O exposure. CP154526 was used in the behavioral studies, as this prototypical CRF-R1 antagonist has been used more frequently in the behavioral literature. In contrast, NBI27914 is a more water soluble CRF-R1 antagonist used most frequently in electrophysiological recordings. In H2O and TMT mice, 500 nM NBI27914 was pre-applied in the ACSF for at least 10 min to verify subregion and layer specificity of CRF-R1 effects on synaptic transmission. To look at CRF-gated plasticity in the PL, 100 or 300 nM CRF was bath applied at a rate of 2 ml/min for 10 min after a stable baseline [15] in voltage clamp. For all experiments, (n = 2–4) cells were collected for each animal, given the four different subregions. Each condition includes at least (n = 4) mice/group. As mentioned previously, a small subset of mice (n = 10) were used for recordings but not included in behavioral analysis [see Suppl Table 1]. During CRF bath application experiments, baseline synaptic transmission was also included as cells for region-specific recordings. Electrophysiological recordings were then analyzed using Clampfit 10.7 software (Molecular Devices, Sunnyvale, CA).
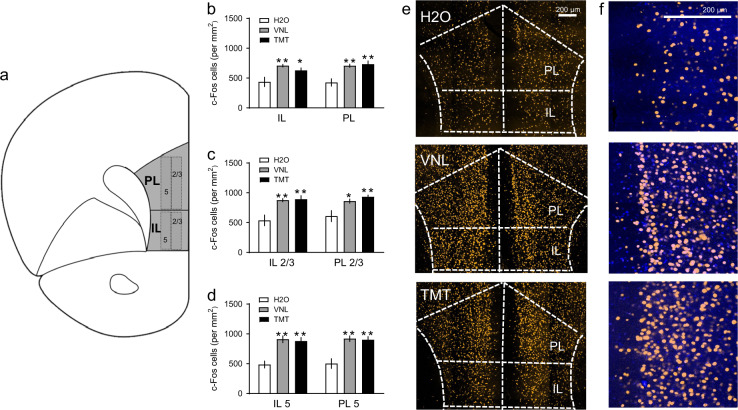
Fig. 2.
a Coronal section of the mPFC subregions, the prelimbic (PL) and infralimbic (IL) regions with layers 2/3 and 5, where c-Fos was quantified based on the mouse Allen Brain Atlas. b Mean c-Fos positive nuclei (per mm2) in the IL and PL after H2O (white), vanilla (VNL, gray) and TMT (black), then further counted within c IL 2/3 vs. PL 2/3, and d IL 5 vs. PL 5. e Representative images of c-Fos immunostaining (pseudocolored yellow) after H2O, VNL, and TMT with PL and IL regions outlined. The scale bar indicates 200 µm. f Close-up of the PL region after H2O, VNL, and TMT with c-Fos immunostaining (yellow) and DAPI (blue). The scale bar indicates 200 µm. *p < 0.05 vs. H2O, **p < 0.01 vs. H2O. Mean c-Fos immunoreactive cells for each subregion were counted across four serial sections for n = 6/group
Statistical analyses
Behavioral data including time spent in contact with the object, time spent in the far corners, defensive burying, and distance traveled were analyzed using mixed factor ANOVA to assess within-subjects pre-test versus post-test phases and between-group stress exposure (TMT vs. VNL vs. H2O). For drug effects, behavioral data were analyzed with two-way ANOVAs for between stress group and drug effect (saline vs. CP154526) differences. Separate two-way ANOVAs were run for the pre-test phase for assessing drug effects on baseline behavior. C-Fos data were analyzed by repeated measures two-way ANOVA, and mean c-Fos positive nuclei were compared between mPFC subregion (PL vs. IL) and stress exposure group. Further two-way ANOVAs were run between PL 2/3 and IL 2/3 as well as PL 5 and IL 5 layers. Electrophysiological data were analyzed using two-way ANOVA for mPFC subregion and stress group. The effects of 100 or 300 nM CRF bath application were also analyzed with a repeated measures two-way ANOVA for baseline versus drug application in TMT and H2O mice. Two-way ANOVAs also compared synaptic transmission of untreated (baseline) versus NBI27914-treated PL 2/3 cells. All significant main effects were then followed up by multiple comparisons using Bonferroni post hoc tests. We used Prism 7 (GraphPad Software, Inc.) for figure presentation and SigmaPlot 14.0 (Systat Software Inc.) for statistical analyses. All values presented are mean ± standard error of the mean (SEM), and α = 0.05.
Results
TMT elicits avoidance and stress behaviors in C57BL/6J mice
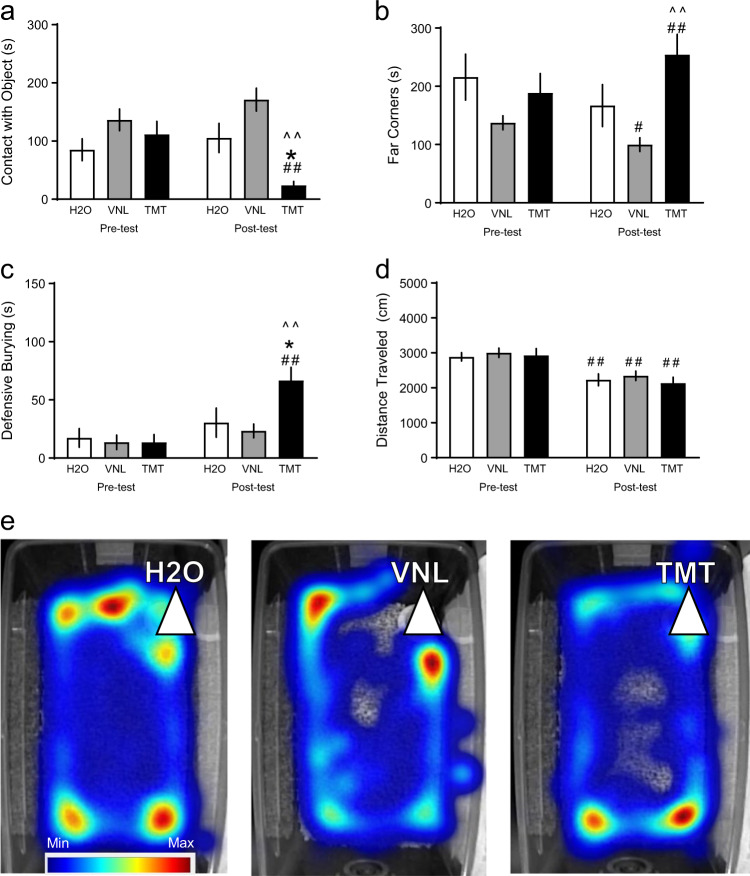
An initial cohort of adult male C57BL/6J mice were exposed to H2O, VNL, or TMT in the home cage (n = 12 H2O, n = 15 VNL, n = 13 TMT), and contact with the object (Fig. 1a,) time spent in the far corners of the cage (Fig. 1b), and defensive burying were observed (Fig. 1c). Two-way repeated measures ANOVA found an interaction between testing phase and stress exposure for contact with object [F(2,37) = 8.83, p < 0.001] and a main effect of test phase [F(2,37) = 8.33, p < 0.01]. TMT-exposed mice had less contact with the object in the post-test compared to both H2O-exposed mice [t(37) = 2.89, p < 0.05] and VNL-exposed mice [t(37) = 5.52, p < 0.001], which was also less than the pre-test [t(37) = 3.84, p < 0.001]. There was also an interaction between test phase and stress for time spent in the far corners of the cage [F(2,37) = 15.01, p < 0.001] and a main effect of test phase [F(2,37) = 3.99, p < 0.05]. Compared to the pre-test baseline, VNL mice spent less time in the far corners [t(37) = 2.47, p < 0.05], but TMT mice spent more time in the far corners [t(37) = 4.03, p < 0.001]. TMT mice differed from VNL mice in the post-test [t(37) = 3.93, p < 0.001]. We also found an interaction for total time defensive burying [F(2,37) = 7.16, p < 0.01] and a main effect on test phase [F(2,37) = 23.47, p < 0.001]. TMT-exposed mice buried significantly more than both H2O-exposed mice [t(37) = 2.92, p < 0.05] and VNL mice [t(37) = 3.70, p = 0.001], which was also greater than their pre-test baseline burying [t(37) = 5.82, p < 0.001]. Finally, distance traveled was quantified (Fig. 1d), where there was a main effect of time [F(1,37) = 39.72, p < 0.001]. All groups of mice, H2O [t(37) = 3.24, p < 0.01], VNL [t(37) = 3.63, p < 0.001], and TMT [t(37) = 4.07, p < 0.001], decreased distance traveled in the post-test compared to their pre-test, but there was no effect of TMT. Representative heat maps of total time spent in the home cage are shown after H2O, VNL, or TMT exposure (Fig. 1e).
Fig. 1.
After a 10-minute pre-test with a cotton tip apparatus in the home cage, mice were exposed to either 2.5 μl of water (H2O, white, n = 12), vanilla (VNL, gray, n = 15), or the fox-derived predator odor, TMT (black, n = 13), for 10 min post-test. Displayed bars are mean and SEM of a duration (s) of contact with the apparatus/object, b time spent in the far corners, c defensive burying, and d Distance traveled (cm) in the home cage. e Representative heat maps of time and location of mice after H2O, VNL, and TMT. The white triangle indicates the location of the stimulus. The legend indicates blue for minimum and red for maximum. *p < 0.05 vs. H2O, ^^p < 0.01 vs VNL, #p < 0.05 vs. pre-test, ##p < 0.01 vs. pre-test
TMT and vanilla produce neuronal activation in the medial prefrontal cortex
As a previous study from the Gilpin lab suggested the PFC as a critical site of action for predator odor-induced learning in rats [8], we first examined how acute TMT exposure could increase expression of the immediate early gene c-Fos in the mPFC (n = 6/group). The areas of interest in the mPFC, the PL and IL, are illustrated in Fig. 2a. Active neuronal populations were quantified (Fig. 2b), where we found an main effect of stress [F(2,15) = 10.01, p < 0.01]. TMT increased c-Fos cell counts in both the IL compared to H2O [t(15) = 2.63, p < 0.05], and in the PL compared to H2O [t(15) = 4.21, p = 0.001]. VNL also increased c-Fos cell counts in both the IL compared to H2O [t(15) = 3.72, p < 0.01], and in the PL compared to H2O [t(15) = 3.83, p < 0.01]. To further distinguish cortical layer specificity, layers 2/3 in the PL and IL were compared (Fig. 2c). There was a main effect of stress on c-Fos in mPFC layers 2/3 [F(2,15) = 10.23, p < 0.01]. Within IL 2/3, TMT [t(15) = 4.05, p < 0.01] and VNL [t(15) = 3.86, p < 0.01] had more c-Fos than H2O. Within PL 2/3, TMT [t(15) = 3.69, p < 0.01] and VNL [t(15) = 2.86, p < 0.05] had more c-Fos than H2O. As for mPFC layer 5, there was a main effect of stress [F(2,15) = 20.57, p < 0.001, Fig. 2d]. TMT had greater c-Fos compared to H2O in both IL 5 [t(15) = 4.65, p < 0.001] and PL 5 [t(15) = 4.70, p < 0.01]. VNL had greater c-Fos compared to H2O in both IL 5 [t(15) = 4.97, p < 0.001] and PL 5 [t(15) = 4.90, p < 0.01]. We show representative confocal images of fluorescent c-Fos staining after H2O, VNL, or TMT in the home cage with overlaid regions of interest (Fig. 2e). Further, close-up representative images of c-Fos immunohistochemistry overlaid with DAPI in the PL are displayed in Fig. 2f.
Increased synaptic drive of mPFC PL 2/3 after TMT exposure
We next used slice electrophysiology following acute exposure to TMT to determine if there was a change in neuronal function in the PL and IL. Synaptic transmission was recorded in PL and IL, in both layers 2/3 and 5. Of the total n = 32 mice used for physiology across two cohorts [see Suppl Table 1], sample sizes were n = 6–14 total cells/region, totaling n = 111 cells, with n = 11 mice for H2O, n = 10 for VNL, and n = 11 for TMT. Representative traces for sEPSC in PL 2/3 after H2O, VNL, and TMT are shown in Fig. 3a and sIPSC in Fig. 3b. A two-way ANOVA revealed a main effect of stress on sEPSC frequency [Fig. 3c, F(2,99) = 20.45, p < 0.001], specifically in PL layer 2/3, where TMT exposed mice had higher sEPSC frequency than H2O mice [t(99) = 5.32, p < 0.001] and VNL mice [t(95) = 4.05, p < 0.001]. PL layer 5 also exhibited greater sEPSC in TMT mice compared to H2O [t(99) = 3.09, p < 0.05] and VNL [t(99) = 3.29, p < 0.05]. There were no subregion differences or stress effect on sIPSC frequency (Fig. 3d). Consequently, we found an effect of TMT on the E/I ratio [Fig. 3e, F(2,99) = 8.83, p < 0.001], which was greater than H2O [t(99) = 4.98, p < 0.001] and VNL [t(99) = 4.06, p < 0.01] located in PL 2/3. There was a main effect of stress on sEPSC amplitude [Fig. 3f, F(2,99) = 21.14, p < 0.001] where TMT reduced sEPSC amplitude compared to VNL in PL 5 [t(99) = 3.21, p < 0.05], and IL 2/3 [t(99) = 3.70, p < 0.01] but not compared to H2O. TMT affected sIPSC amplitude [Fig. 3g, F(2,99) = 12.86, p < 0.001], compared to VNL specifically in PL layer 2/3 [t(99) = 3.24, p < 0.05] but not compared to H2O. VNL was also greater than H2O in PL 2/3 [t(99) = 3.88, p < 0.01]. Similarly, TMT increased synaptic drive [Fig. 3h, F(2,99) = 7.068, p < 0.01], specifically in the PL 2/3 cells versus H2O [t(99) = 4.65, p < 0.001] and VNL [t(99) = 3.99, p < 0.01].
Fig. 3.
Synaptic transmission recordings in PL layer 2/3, PL layer 5, IL layer 2/3, and IL layer 5 in mice exposed to H2O (white), VNL (gray), or TMT (black) featuring: a representative PL 2/3 spontaneous excitatory postsynaptic current (sEPSC) traces in H2O (left), VNL (center), and TMT (right), b representative PL 2/3 spontaneous inhibitory postsynaptic current (sIPSC) traces, c sEPSC frequency (Hz), d sIPSC frequency (Hz), e sEPSC/sIPSC (E/I) frequency ratio, f sEPSC amplitude (pA), g sIPSC amplitude (pA), and h synaptic drive defined as sEPSC frequency × amplitude divided by sIPSC frequency × amplitude. *p < 0.05 vs. H2O, **p < 0.01 vs. H2O. ^p < 0.05 vs. VNL, ^^p < 0.01 vs. VNL. Of the total n = 32 mice, sample sizes were n = 6-14 total cells/region, and with n = 11 mice for H2O, n = 10 for VNL, and n = 11 for TMT
CRF-R1 antagonist alters behavioral responses to TMT
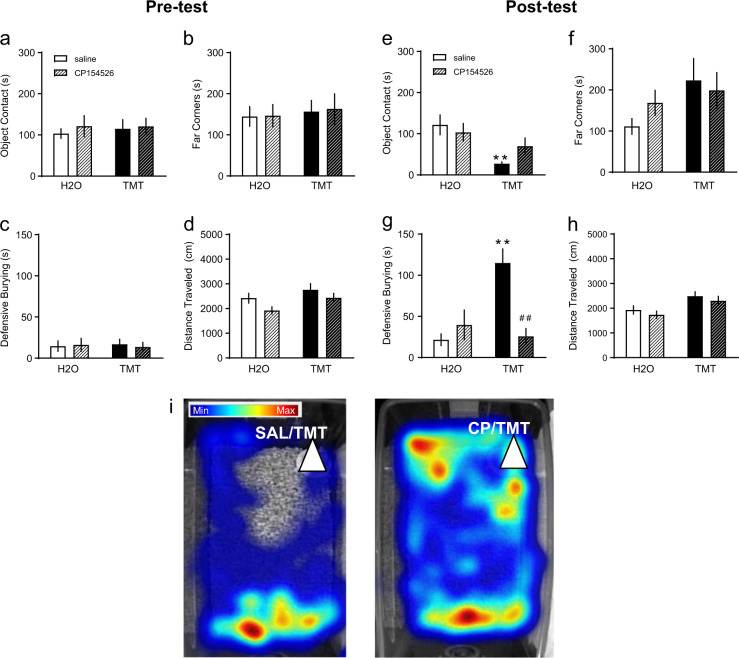
As previous studies have suggested that CRF-R1 signaling in the mPFC can alter behavioral responses to a predator odor [8], we next examined the role of CRF-R1 signaling in the behavioral response to TMT. The CRF-R1 antagonist CP154526 or saline was administered before H2O or TMT exposure in a third cohort of mice (n = 8/group, 32 total). During the pre-test trials, there were no differences between saline and CP154526 treatment for contact with the object, time in far corners, or defensive burying (Fig. 4a–c). There was a group effect of TMT on distance traveled [Fig. 4d, F(1,28) = 4.60, p < 0.05], but no main effect of drug or significant post-hoc differences. During the post-test, TMT affected contact with object [Fig. 4e, F(1,28) = 10.96, p < 0.01] where TMT decreased contact with object during saline trials [t(28) = 3.45, p < 0.01] but not after CP154526. There was no significant effect of drug or TMT on time spent in the far corners (Fig. 4f). A two-way ANOVA revealed an interaction for defensive burying [Fig. 4g, F(1,28) = 14.67, p < 0.001], a drug effect [F(1,28) = 8.061, p < 0.01], and stress effect [F(1,28) = 6.49, p < 0.05]. Like previous behavioral testing, TMT increased defensive burying in the saline condition [t(28) = 4.72, p < 0.001]. However, pretreatment with CP154526 decreased TMT-induced defensive burying [t(28) = 4.51, p < 0.001]. Overall there was a stress effect on distance traveled [Fig. 4h, F(1,28) = 10.74, p < 0.01], in which TMT increased distance traveled, but no main effect of drug. Representative heat maps of total time spent in the home cage after saline or CP154526 are shown after TMT exposure (Fig. 4i).
Fig. 4.
Mice were given i.p. 10 mg/kg CP154526, a CRF-R1 antagonist (striped), or saline (non-striped) before exposure to TMT (black) or H2O (white, n = 8/group). Behavioral measures assessed during the pre-test phase were: a contact with the object (s), b time spent in the far corners (s), c defensive burying (s), and d distance traveled (cm). These same variables were measured during the post-test: e contact with object, f time spent in the far corners, g defensive burying, and h distance traveled. i Representative heat maps of time and location of mice after saline and 10 mg/kg CP154526 pretreatment and TMT exposure. The white triangle indicates the location of the stimulus. The legend indicates blue for minimum and red for maximum. **p < 0.01 vs. H2O, ##p < 0.01 vs. saline
CRF-R1 antagonism blocks TMT effects and CRF mimics TMT in slice
Exposure to TMT had the most robust effects on synaptic function on layer 2/3 neurons in the PL, and CRF-R1 antagonism reduced stress-like behavior in vivo, so we next explored how CRF/CRF-R1 signaling could alter function in the mPFC. To test that CRF-R1 is engaged to alter PL 2/3 function following TMT exposure, we tested synaptic transmission in mice pretreated with a CRF-R1 antagonist or saline. Representative sEPSC traces are illustrated in Fig. 5a. In the third cohort of mice (n = 32), half were used for physiology (n = 16), and n = 10-12 total cells/group were collected from n = 4 mice/group, totaling n = 45 cells. Two-way ANOVA showed a stress effect on sEPSC frequency [Fig. 5b, F(1,41) = 4.60, p < 0.05], where TMT increased sEPSC compared to H2O in the saline group [t(41) = 2.88, p < 0.05]. In contrast, CP154526 decreased sEPSC frequency in the TMT group [t(41) = 2.63, p < 0.05]. There were no significant drug effects on sIPSC frequency (Fig. 5c). Comparing E/I frequency, there was a subsequent significant interaction for E/I ratio [not shown, F(1,41) = 4.80, p < 0.05]. TMT increased E/I ratio after saline injection [t(41) = 2.50, p < 0.05], and CP154526 reduced E/I ratio in TMT-exposed mice [t(41) = 2.70, p < 0.05]. There were no drug effects on sEPSC or sIPSC amplitude [Suppl Figs. 1a, 1b]. Overall, analysis of synaptic drive also showed a significant interaction [Fig. 5d, F(1,41) = 4.95, p < 0.05]. TMT increased synaptic drive after saline injection [t(41) = 2.95, p < 0.05], and CP154526 reduced synaptic drive in TMT-exposed mice [t(41) = 2.46, p < 0.05].
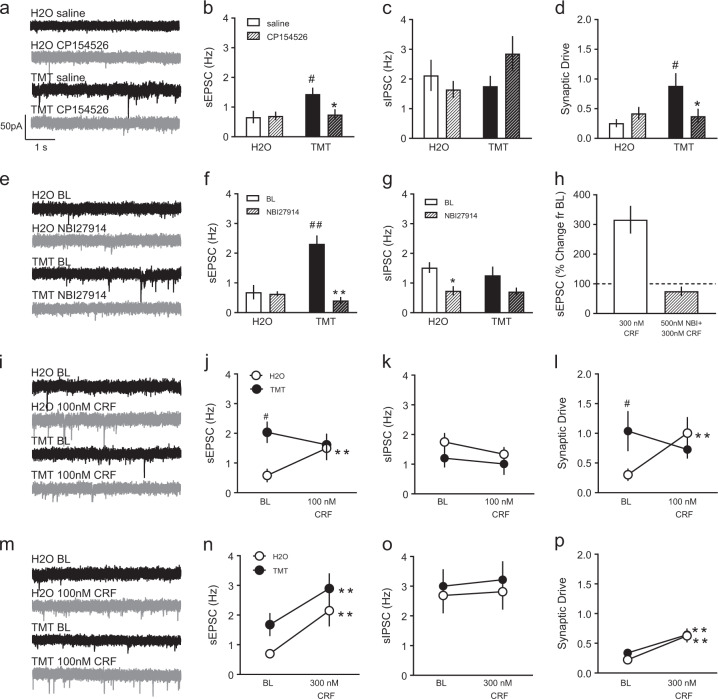
Fig. 5.
Separate mice were pretreated with 10 mg/kg CP154526 (striped) or saline (non-striped) before H2O or TMT. Representative sEPSC traces after saline (black) or CP154526 (gray) injections are shown in a. Electrophysiological recordings of b sEPSC frequency (Hz), c sIPSC frequency (Hz), and d synaptic drive. *p < 0.05 vs. saline. #p < 0.05 vs. H2O. Of the n = 16 mice used in cohort 3, n = 10–12 total cells/group were collected from n = 4 mice/group. To address PL 2/3-specificity of CRF-R1 effects, synaptic transmission was assessed after baseline (black) and NBI27914 (gray) bath application, depicted are sPESC in e. NBI27914 (striped) was applied to H2O (white) and TMT (black) slices compared to baseline (solid) f sEPSC frequency and g sIPSC frequency. *p < 0.05 vs. BL. **p < 0.01 vs. BL. ##p < 0.01 vs. H2O. h In H2O mice, the effects of 300 nM CRF on sEPSC frequency (percent change from baseline) was blocked by 500 nM NBI27914 bath application. Bath application of 100 nM CRF affected synaptic transmission in H2O- (white) and TMT-exposed (black) PL 2/3 cells as indicated by i) sEPSC traces after baseline (black) or CRF (gray), j sEPSC frequency (Hz), k sIPSC frequency (Hz), and l) synaptic drive. Bath application of 300 nM CRF affected synaptic transmission in PL 2/3 of the same groups shown in panel m after baseline (black) and CRF (gray) and as measured by n sEPSC frequency, o sIPSC frequency, and p synaptic drive. **p < 0.01 vs. BL. #p < 0.05 vs. H2O. Of the n = 32 mice from the region-specific experiments in cohorts 1 and 2, n = 8–10 total cells/group were collected from n = 4 mice/group
While this result supports the idea that TMT can evoke CRF-R1 dependent effects on synaptic function in layer 2/3 of the PL, the next step was to confirm if CRF-R1 antagonist effects were similar when bath applied directly onto brain slices containing PL 2/3 cells. Separate mice were prepared for NBI27914 treatment in slice. A subset of mice from the region-specific experiments were used with n = 8–11 cells/group, totaling n = 36 cells. sEPSC traces are shown in Fig. 5e. There was an interaction between stress and drug [Fig. 5f, F(1,32) = 23.02, p < 0.001], a main effect of stress [F(1,32) = 13.22, p < 0.01], and a main effect of drug [F(1,32) = 25.79, p < 0.001] for sEPSC frequency. Untreated baseline TMT cells had higher sEPSC frequency than H2O cells [t(32) = 5.84, p < 0.001], and NBI27914 decreased sEPSC frequency in TMT cells [t(32) = 7.15, p < 0.001] but not H2O cells. For inhibitory transmission, there was an effect of drug [Fig. 5g. F(1,32) = 11.91, p < 0.01]. In H2O cells, NBI27914 decreased sIPSC frequency compared to no drug [t(32) = 3.96, p < 0.05]. There were no drug effects on sEPSC or sIPSC amplitude [Suppl Figs. 1c, 1d]. NBI27914 also affected E/I ratio with in an interaction [F(1,32) = 11.23, p < 0.01], and a main effect of stress [not shown, F(1,32) = 4.93, p < 0.05]. Specifically, untreated TMT cells had higher E/I ratio compared to untreated H2O cells [t(32) = 3.86, p < 0.01], which was reduced with NBI27914 treatment [t(32) = 2.52, p < 0.01]. Also, synaptic drive was altered by the CRF-R1 antagonist in slice revealed by an interaction between stress and drug treatment [not shown, F(1,32) = 16.27, p < 0.01]. Again, TMT had higher baseline synaptic drive than H2O [t(32) = 3.98, p < 0.01, but NBI27914 suppressed synaptic drive in TMT mice [t(32) = 3.93, p < 0.01]. In H2O mice, the percent change of 300 nM CRF can be abolished by pre-application 500 nM NBI27914 in slice (Fig. 5h).
Next, we examined how bath application of 100 nM CRF altered synaptic function in these neurons in both control and TMT-exposed mice. Mice from the second cohort of region-specific experiments were used (n = 5 H2O, n = 4 TMT), totaling n = 11 cells for 100 nM CRF bath application and n = 18 total cells for 300 nM CRF bath application. Representative sEPSC are illustrated in Fig. 5i. There was an interaction between drug application and stress condition after 100 nM CRF on sEPSC frequency [Fig. 5j, F(1,9) = 8.41, p < 0.05]. TMT mice had greater baseline sEPSC than H2O mice [t(9) = 2.83, p < 0.05]. 100 nM CRF increased sEPSC only in H2O mice [t(9) = 3.30, p < 0.01]. There were no differences in sIPSC frequency after 100 nM CRF (Fig. 5k). There was a stress effect on sEPSC amplitude [Suppl Fig. 1e, F(1,9) = 8.70, p < 0.05] where H2O cells were greater than TMT at baseline [t(9) = 3.19, p < 0.01] and 100 nM CRF reduced H2O amplitude compared to baseline [t(9) = 2.35, p < 0.05]. sIPSC amplitude was not different within or between conditions [Suppl Fig. 1f]. 100 nM CRF also affected E/I ratio as revealed by an interaction [F(1,9) = 10.79, p < 0.01]. Again, TMT cells had larger E/I ratios at baseline versus H2O [t(9) = 3.23, p < 0.01], and 100 nM CRF increased E/I ratio in just the H2O condition [t(9) = 3.18, p < 0.05]. Similarly, an interaction was present for synaptic drive [Fig. 5l, F(1,9) = 11.09, p < 0.01] where 100 nM CRF increased synaptic drive in H2O cells compared to baseline [t(9) = 3.83, p < 0.01], which was lower than TMT synaptic drive at baseline [t(9) = 2.19, p < 0.05].
Since we observed a lower dose of 100 nM CRF affected synaptic transmission in control mice, we next applied a higher 300 nM CRF dose. Representative sEPSC are shown in Fig. 5m. Two-way repeated measures ANOVA revealed a significant drug effect [Fig. 5n, F(1,16) = 24.84, p < 0.0001], where 300 nM CRF increased sEPSC frequency in both H2O [t(16) = 3.64, p < 0.01] and TMT mice [t(16) = 3.41, p < 0.01]. There was no effect on sIPSC frequency (Fig. 5o). In line with these data, there was a drug effect on E/I ratio [F(1,16) = 41.26, p < 0.001], where 300 nM CRF increased the E/I ratio in both H2O [t(16) = 5.27, p < 0.001] and TMT-exposed mice [t(16) = 3.74, p < 0.01]. Here we also saw the baseline E/I difference between untreated H2O and TMT cells [t(16) = 2.08, p < 0.05]. There was an effect of TMT on sEPSC amplitude [Suppl Fig. 1g, F(1,16) = 10.32, p < 0.01], where TMT had lower baseline amplitude than H2O mice [t(16) = 2.76, p < 0.05], and CRF bath application did not affect this [t(16) = 3.17, p < 0.01]. We also found an interaction between 300 nM CRF and stress for sIPSC amplitude [Suppl Fig. 1h, F(1,16) = 9.27, p < 0.01] and a CRF effect [F(1,16) = 18.98, p < 0.001]. In the TMT mice, sIPSC amplitude decreased with CRF application [t(16) = 5.55, p < 0.001], which was less than the H2O mice [t(16) = 2.35, p < 0.05]. These total changes were reflected in a drug effect on synaptic drive [Fig. 5p, F(1,16) = 30.84, p < 0.001], where CRF increased synaptic drive in both H2O [t(16) = 4.23, p < 0.001] and TMT-exposed mice [t(16) = 3.60, p < 0.01].
Discussion
The current set of studies demonstrate a role for CRF-R1 as a modulator of predator odor-driven behavioral and physiological phenotypes. We exposed mice to a fox feces-derived predator odor, TMT, and observed robust increases in stress-related behaviors including avoidance and defensive burying compared to a novel odor, vanilla, and control stimulus, H2O. Acute TMT and vanilla exposure caused increased c-Fos activation in both the PL and IL subregions of the mPFC. However, we saw increased sEPSC and synaptic drive in PL layer 2/3 of TMT mice compared to H2O and vanilla. When we pretreated mice with a systemic CRF-R1 antagonist, mice spent greater time in contact with the TMT and less time defensive burying. We also found that i.p. CRF-R1 antagonist can block the TMT effect on synaptic function, normalizing PL 2/3 cells to non-stressed transmission. This reduction of sEPSC is similarly observed when a CRF-R1 antagonist is washed onto PL 2/3 cells in TMT mice. Further, bath application of two doses of CRF demonstrate that TMT shifts the potency of the dose-response curve. Taken together these data suggest that TMT exposure leads to CRF-R1 dependent changes in both behavior and synaptic function in layer 2/3 PL neurons.
In our studies, TMT increased avoidance behavior and defensive burying, suggestive of stress reactions to the aversive olfactory stimulus. Others have similarly reported decreases in time spent in the object zone, increases in far corners, and robust defensive burying in response to TMT, but not other predator odors like cat fur odor and β-phenylethalamine, in the home cage in C57BL/6J mice [16]. TMT can alter the spatial movement of specific behavioral modules like avoidance and freezing, as characterized by a 3D autoregressive mixed model [17]. Like fleeing and freezing, defensive burying is in the behavioral repertoire of innate unconditioned, species-specific defensive actions [4, 18]. This defensive behavior was originally observed forty years ago in rats using the shock prod in the home cage [19] but has also been observed with noxious smells [20] and predator odor [21]. Interestingly, we did not observe freezing behavior in the C57BL/6J mice, as other groups have reported [22]. It may be that freezing occurs in an unfamiliar open field while the active burying response occurs when substantial bedding is present, such as in the home cage. The 2.5 µl dose of TMT is also a low dose of the predator odor [3]. In some species, burying versus freezing strategies can also be present in combination and represent different adaptive strategies with individual differences, which is an avenue for future study.
Despite robust avoidance and defensive burying, there exists some inconsistencies between the behavioral descriptions, as shown in Fig. 1, versus the later saline/CRF-R1 antagonist trials, in Fig. 4, such as in locomotor activity. The initial experiments demonstrate a reduction in activity between pre-test and post-test across both H2O and TMT groups while the later drug testing shows TMT-induced increase in activity. It is possible that the reduction of locomotor behavior in non-handled mice occurs over time because of initial novelty and exploration during the pre-test, and further habituation during the post-test. In the drug experiments, TMT-exposed mice, regardless of drug injection, showed an increase in distance traveled. We attribute this increase in activity to a possible interaction between a potential handling confound and TMT exposure that was not revealed during the former unhandled cohort. In this manner, the time in the far corners of the home cage was trending towards an increase in the saline-TMT group, the same direction as the non-injected group, but this may be directly related to the increased locomotor activity in the home cage. These minor irregularities between cohorts convey that handling stress could be a confound despite habituation to i.p. injections. Even so, TMT consistently produced robust decreases in contact with the TMT and increased defensive burying across experiments.
We demonstrate that TMT exposure generates stress behaviors and increased synaptic transmission, both of which were mediated via CRF-R1 signaling. CRF similarly increases excitatory synaptic transmission, and CP154526 treatment suppresses defensive burying and reduces synaptic transmission. Central CRF infusions can potentiate autonomic and behavioral responses during a shock-prod defensive burying test in rats and defensive behaviors in mice [23, 24]. Our experiments confirm previous studies examining the CRF/CRF-R1 system in the mPFC after acute stressors. Specifically, recent investigations show that CRF in the vmPFC, consisting of the lower PL and IL, mediates conditioned avoidance to bobcat urine in rats [8]. In addition, a single episode of social defeat causes mPFC deficits in working memory through CRF/CRF-R1 microcircuits [25]. In both these studies, CRF infusions into the mPFC had similar effects to the stressor, and either intra-mPFC CRF-R1 deletion or CRF-R1 antagonist infusions ameliorated the stress-induced behavioral impairments. An important caveat of the present study is that we did not perform a local CRF-R1 manipulation in the PL. As such, it is possible that CRF-R1 signaling could drive network dependent effects that results in changes in glutamatergic transmission in the PFC. This is unlikely, as we were able to identify CRF-R1-dependent changes in neural transmission in PL 2/3 after systemic CP154526 treatment and washed on NBI27914 treatment, suggestive of TMT driving local CRF-R1 activation. NBI27914 completely blocked the 300 nM CRF enhancement of excitatory transmission, further implicating a local effect. We did not test if NBI27914 blocked the effect of CRF in the TMT mice, but we would expect that CRF would not have an effect with the CRF-R1 antagonist. Since these experiments were not conducted with tetrodotoxin in the bath to block action potentials, it is not entirely known if PL 2/3 also receives glutamatergic input from other regions. However, it does remain possible that the synaptic effects we are measuring in layer 2/3 of the PL do not mediate the TMT-driven behavioral phenotypes. An important limitation of this study is that we did not do microinjections of CP154526 into the mPFC, which would directly confirm causality. Future studies, using genetic approaches to remove CRF or CRF-R1 from a discrete cell population, combined with pharmacological approaches will provide insight in to this.
Additionally, we show increased c-Fos activation in the PL and IL after TMT like others ([26], but see [27]), which could very well be in Crhr1-containing cells, as PL and IL cells have increased Crhr1 mRNA after acute social defeat [25]. It is unlikely that these effects are CRF-R2-mediated since there is minimal CRF-R2 in the vmPFC [28]. Although, it is possible that CRF-R2 may be recruited to the plasma membrane in other brain sites after chronic, repeated TMT, as repeated social defeat promotes CRF-R1 internalization in the dorsal raphe [29]. These effects of CRF, or stress, acting on CRF-R1 can affect downstream signaling pathways such as protein kinase A [25, 30]. CRF-R1 activity may also act by modulating other neurotransmitters in the mPFC such as 5-HT [31]. Exposure to rat odor substantially increases extracellular 5-HT, dopamine, and acetylcholine in the PFC of mice [32]. It would be an interesting future direction to examine how CRF-R1 and 5-HT interact in response to TMT.
Our slice recordings found that TMT exposure produced the most robust effects in PL 2/3 compared to PL 5, IL 2/3, and IL 5. This relates to the original Wellman [33] study and others showing that pyramidal neurons in layer 2/3 of the PL and anterior cingulate have reorganized dendrites in response to stress [34]. More recently, others have shown that stress exposure during the first postnatal week hinders dendritic development in layers 2/3 and 5 pyramidal neurons in the PL and cingulate of neonatal mice, an effect reversed by antalarmin, a CRF-R1 antagonist [35]. Specifically, we saw an increase in sEPSC frequency, sEPSC/sIPSC ratio, and synaptic drive after TMT stress, with no effect on sIPSC frequency, indicating escalated glutamatergic activity in the PL 2/3. As we also confirm, it is well known that application of CRF excites the frontal cortex [36]. In the current study, the higher 300 nM dose of CRF increased excitatory transmission in control and stressed mice, but the lower 100 nM dose only affected the controls. This dose-dependent effect suggests TMT stress shifts the dose-effect curve of CRF. In the same vein, the decrease in excitatory synaptic transmission we observed after CRF-R1 antagonist pretreatment in vivo and ex vivo implies that the CRF-R1 antagonist may suppress glutamate signaling in the PL of TMT-exposed mice. Others have also blocked stress-enhanced excitatory mPFC transmission and dendritic remodeling with ionotropic glutamate receptor antagonists [37, 38]. Again, it is important to note that while prefrontal reorganization can occur after chronic stress, a single episode of inescapable footshock can enhance glutamate transmission in the PFC for up to 24 h [39], which may also occur with TMT exposure. This acute stressor induces rapid enhancement of depolarization-evoked glutamate overflow by increasing the readily releasable pool of glutamate vesicles in synaptic terminals of the PFC, which then increases corticosterone levels downstream [40, 41]. In our study of PL 2/3 synaptic transmission, we observed significant reductions in sEPSC and sIPSC amplitude after TMT exposure and with CRF bath application. Some have shown increases in NMDAR-EPSC and AMPAR-EPSC amplitude after acute forced swim in mPFC layer 5 [42], but others have also reported decreased sEPSC amplitude after adolescent stress in mPFC layer 2/3 [43, 44]. The effects on sEPSC/sIPSC amplitude appear secondary since the stress effect on sEPSC/sIPSC frequency dominates the increased synaptic drive. Notably, Liu and Aghajanian [45] find changes in layer 5 sEPSC frequency after repeated restraint stress, suggesting that acute stress could affect PL 2/3 initially but then later develop into changes throughout other mPFC layers with repeated stress.
In the present home cage tests, vanilla was used as a control for a novel odorant. Mice tended to increase contact with the vanilla compared to H2O and decreased the time spent in the far corners compared to the pre-test. Importantly, vanilla did not generate more defensive burying compared to the baseline. Insomuch, mice exposed to vanilla showed similar behaviors to those of H2O. Others have also demonstrated that mice show neutral to mild preference for vanilla scent in pairing with contexts or for olfactory tests of novel odorants [46–48]. Vanilla also increased c-Fos cell counts in both the IL and PL; however neither stress behaviors nor sEPSC frequency were altered by this novel odor. This is an example in which c-Fos induction represents multiple processes of neuronal activation since excitatory synaptic transmission was unchanged. We found an effect of vanilla on sIPSC amplitude compared to H2O, and sEPSC amplitude after vanilla was greater than TMT, but not significant vs. to H2O. There is precedence for olfactory learning affecting synaptic connectivity in the cortex, perhaps through a postsynaptic mechanism [49]. Although there are evident alterations in amplitude, the effects appear secondary, as synaptic drive is not affected by vanilla exposure in any layer or subregion. Future experiments could assess the roles of CRF or a CRF-R1 antagonist after vanilla exposure; however, this extends the scope of the current series of experiments. Nevertheless, we would expect to see similar effects as H2O and CRF manipulation. Overall, these data suggest that vanilla, as a novel odor, did not produce stress or anxiety-like behaviors, and amplitude changes in synaptic events may be related to novelty instead of vanilla scent in particular.
In future studies, it will be important to characterize both the upstream and downstream nuclei impacted by TMT stress exposure. The current study focused on mPFC layers and TMT stress, but there is a large body of literature studying the diverse olfactory systems that respond to TMT [50, 51]. Known circuits for the detection of threatening chemical cues start with the olfactory subsystem, leading to several amygdala subnuclei and the lateral hypothalamus [52] with involvement of the laterodorsal tegmentum and lateral habenula [22], interpeduncular nucleus and periaqueductal gray (PAG). One potentially important downstream site of action for TMT is the extended amygdala. Neurons in the extended amygdala, including in the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA), are involved in innate fear, anxiety, and stress. TMT induces c-Fos expression in the BNST of rats and C57BL/6J mice [5, 6, 27], and inactivation of the BNST blocks TMT-induced freezing [53]. However, to date there has not been an investigation of the cell-types or signaling mechanisms in the BNST that drive these TMT-related behaviors. A recent paper demonstrated that 5-HT2A receptors in the central amygdala can have opposing effects on innate fear, via TMT, and learned fear, via footshock, with separate processing through the dorsal PAG and ventral PAG [54]. Altogether, we will be interested in how the PL connects to these extended amygdala circuits to regulate TMT processing of stress, threat, and innate fear.
In conclusion, TMT exposure in the homecage may be a suitable model for a simple ethological stressor, as we are able to observe robust stress and defensive behaviors and mPFC plasticity in C57BL/6J mice. Additionally, this study confirms the important role of the CRF/CRF-R1 system in regulating stress behaviors and excitatory signaling in the mPFC. We identify that PL 2/3, among other mPFC layers, receives enhanced excitatory transmission after acute TMT via CRF-R1 signaling. In the future, it will be important to compare the acute neuroadaptations as in the current study with long-term adaptations after chronic stress .
Electronic supplementary material
Funding and disclosure
Research experiments were funded by NIH grants P60 AA011605 (TLK), R01 AA019454 (TLK), U01 AA020911 (TLK), T32 AA007573 (LSH), and F32 AA026485 (MMP). The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41386-024-01804-7
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/22/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41386-024-01804-7
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0279-2).
References
- 1.Yehuda R. Post-traumatic stress disorder. New Engl J Med. 2002;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–7. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 3.Fendt M, Endres T. 2, 3, 5-Trimethyl-3-thiazoline (TMT), a component of fox odor–just repugnant or really fear-inducing? Neurosci Biobehav Rev. 2008;32:1259–66. doi: 10.1016/j.neubiorev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Rosen JB, Asok A, Chakraborty T. The smell of fear: innate threat of 2, 5-dihydro-2, 4, 5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci. 2015;9:292. doi: 10.3389/fnins.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–51. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 6.Janitzky K, Kröber A, Schwegler H. TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress. Brain Res. 2015;1599:1–8. doi: 10.1016/j.brainres.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–508. doi: 10.1016/S0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber AL, Lu YL, Baynes BB, Richardson HN, Gilpin NW. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology. 2017;113:323–30. doi: 10.1016/j.neuropharm.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents Comparison with diazepam and buspirone. Psychopharmacology. 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- 10.Keller C, Bruelisauer A, Lemaire M, Enz A. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002;30:173–6. [DOI] [PubMed]
- 11.Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates, compact. The coronal plates and diagrams. Amsterdam: Elsevier Academic Press. 2008.
- 12.Pittaras EC, Faure A, Leray X, Moraitopoulou E, Cressant A, Rabat AA, et al. Neuronal nicotinic receptors are crucial for tuning of E/I balance in prelimbic cortex and for decision-making processes. Frontiers in Psychiatry. 2016;7:171. [DOI] [PMC free article] [PubMed]
- 13.Stewart CV, Plenz D. Inverted-U profile of dopamine–NMDA-mediated spontaneous avalanche recurrence in superficial layers of rat prefrontal cortex. J Neurosci. 2006;26:8148–59. [DOI] [PMC free article] [PubMed]
- 14.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1 dependent process. J Neurosci. 2008;28:13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Gómez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger SD, et al. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol. 2015;25:1340–6. doi: 10.1016/j.cub.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, et al. Mapping sub-second structure in mouse behavior. Neuron. 2015;88:1121–35. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–61. doi: 10.1016/S0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 19.Pinel JP, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978;92:708. doi: 10.1037/h0077494. [DOI] [Google Scholar]
- 20.Silverman AP. Rodents’ defence against cigarette smoke. Anim Behav. 1978;26:1279–81. doi: 10.1016/0003-3472(78)90124-0. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci. 2002;116:160. doi: 10.1037/0735-7044.116.1.160. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Yang J, Xi W, Hao S, Luo B, He X, et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci. 2016;19:283. doi: 10.1038/nn.4208. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Farrokhi C, Vasconcellos A, Blanchard RJ, Blanchard DC. Central infusion of ovine CRF (oCRF) potentiates defensive behaviors in CD-1 mice in the Mouse Defense Test Battery (MDTB) Behav Brain Res. 2006;171:1–8. doi: 10.1016/j.bbr.2006.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamant M, Croiset G, de Wied D. The effect of corticotropin-releasing factor (CRF) on autonomic and behavioral responses during shock-prod burying test in rats. Peptides. 1992;13:1149–58. doi: 10.1016/0196-9781(92)90022-U. [DOI] [PubMed] [Google Scholar]
- 25.Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, et al. Prefrontal cortex corticotropin-releasing factor receptor 1 conveys acute stress-induced executive dysfunction. Biol Psychiatry. 2016;80:743–53. doi: 10.1016/j.biopsych.2016.03.2106. [DOI] [PubMed] [Google Scholar]
- 26.Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD‐1 mice. Eur J Neurosci. 2004;20:2415–29. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- 27.Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT) Behav Brain Res. 2013;248:85–93. doi: 10.1016/j.bbr.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–94. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miguel TT, Gomes KS, Nunes-de-Souza RL. Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: role of protein kinase A (PKA) Horm Behav. 2014;66:247–56. doi: 10.1016/j.yhbeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, et al. Dissociation of μ‐opioid receptor and CRF‐R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addict Biol. 2016;21:111–24. doi: 10.1111/adb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DG, Davis RJ, Gehlert DR, Nomikos GG. Exposure to predator odor stress increases efflux of frontal cortex acetylcholine and monoamines in mice: comparisons with immobilization stress and reversal by chlordiazepoxide. Brain Res. 2006;1114:24–30. doi: 10.1016/j.brainres.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 33.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Dev Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 34.Radley JJ, Sisti HM, Hao J, Rocher A, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Yang XD, Liao XM, Uribe-Mariño A, Liu R, Xie XM, Jia J, et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF 1. Neuropsychopharmacology. 2015;40:1203. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberly LB, Dudley CA, Moss RL. Iontophoretic mapping of corticotropin-releasing factor (CRF) sensitive neurons in the rat forebrain. Peptides. 1983;4:837–41. doi: 10.1016/0196-9781(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–73. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musazzi L, Tornese P, Sala N, Popoli M. Acute stress is not acute: sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol Psychiatry. 2017;22:1226. doi: 10.1038/mp.2016.175. [DOI] [PubMed] [Google Scholar]
- 40.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- 42.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, & Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci. 2009;106:14075–9. [DOI] [PMC free article] [PubMed]
- 43.Caruso MJ, Crowley NA, Reiss DE, Caulfield JI, Luscher B, Cavigelli SA, et al. Adolescent social stress increases anxiety-like behavior and alters synaptic transmission, without influencing nicotine responses, in a sex-dependent manner. Neuroscience. 2018;373:182–98. [DOI] [PMC free article] [PubMed]
- 44.Urban KR, Valentino RJ. Age-and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cereb Cortex. 2017;27:244–53. doi: 10.1093/cercor/bhw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu RJ, Aghajanian GK. Stress blunts serotonin-and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci. 2008;105:359–64. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 47.Malkova NV, Collin ZY, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witt RM, Galligan MM, Despinoy JR, Segal R. Olfactory behavioral testing in the adult mouse. JoVE. 2009;23:949. [DOI] [PMC free article] [PubMed]
- 49.Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28:6664–9. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira AG, Moita MA. Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr Opin Neurobiol. 2016;41:179–87. doi: 10.1016/j.conb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72. doi: 10.3389/fnbeh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blomeley C, Garau C, Burdakov D. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat Neurosci. 2018;21:29. doi: 10.1038/s41593-017-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, et al. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell. 2015;163:1153–64. doi: 10.1016/j.cell.2015.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.