Abstract
Magnetic insulators have wide-ranging applications, including microwave devices, permanent magnets and future spintronic devices. However, the record Curie temperature (TC), which determines the temperature range in which any ferri/ferromagnetic system remains stable, has stood still for over eight decades. Here we report that a highly B-site ordered cubic double-perovskite insulator, Sr3OsO6, has the highest TC (of ~1060 K) among all insulators and oxides; also, this is the highest magnetic ordering temperature in any compound without 3d transition elements. The cubic B-site ordering is confirmed by atomic-resolution scanning transmission electron microscopy. The electronic structure calculations elucidate a ferromagnetic insulating state with Jeff = 3/2 driven by the large spin-orbit coupling of Os6+ 5d2 orbitals. Moreover, the Sr3OsO6 films are epitaxially grown on SrTiO3 substrates, suggesting that they are compatible with device fabrication processes and thus promising for spintronic applications.
Pursuing high Curie temperature magnetic insulators has been one of the extensively studied subjects due to their wide appeal for spintronic applications. Here the authors experimentally and theoretically demonstrate a record high Curie temperature over 1000 K in B-site ordered double-perovskite, Sr3OsO6.
Introduction
Magnetic insulators have been intensively studied for over 100 years, and they, in particular ferrites, are considered to be the cradle of magnetic exchange interactions in solids. Their wide range of applications include microwave devices1 and permanent magnets2. They are also suitable for spintronic devices owing to their high resistivity3, low magnetic damping4, and spin-dependent tunneling probabilities5. The Curie temperature (TC) is the crucial factor determining the temperature range in which any ferri/ferromagnetic system remains stable. However, the record TC has stood still for over eight decades in insulators and oxides (943 K for spinel ferrite LiFe5O8)6.
The B-site ordered double-perovskite A2BB′O6 family includes lots of fascinating magnetic materials such as half-metals7–9, multiferroic materials10, antiferromagnetic (AFM) materials11, and magnetic insulators12–14. The A site is usually occupied by an alkaline-earth or rare-earth element, and B and B′ are transition metal elements. Explorations of magnetism have mainly focused on varying the combination of transition metal elements at B and B′ sites, and it has been believed that having them occupied by two different transition metal elements is a prerequisite for a magnetic order at high temperatures8. Some 4d or 5d element-containing double-perovskites, e.g., Sr2FeMoO67 (TC = 415 K), Sr2CrReO69 (TC = 634 K), and Sr2CrOsO613 (TC = 725 K), reach a point of ferromagnetic (FM) instability at high temperatures, although the majority of double-perovskites show an AFM order or weak spin-glass behavior15.
In this work, we show that a highly B-site ordered double-perovskite insulator, Sr3OsO6, surpasses the long-standing TC record by more than 100 K. In contrast to other 4d or 5d double-perovskites that follow the above-mentioned criteria, we discovered FM ordering above 1000 K in Sr3OsO6, in which only one 5d transition metal element occupies the B sites. Remarkably, the TC of Sr3OsO6 (~1060 K) is about ten times higher than the previous highest magnetic transition temperature in double-perovskites including only one transition element (Sr2MgOsO6, AFM, TN = 110 K)16. We revealed this B-site ordering using atomic-resolution scanning transmission electron microscopy. The density functional theory (DFT) calculations suggest that the large spin–orbit coupling (SOC) of Os6+ 5d2 orbitals drives the system toward a Jeff = 3/2 FM insulating state17–19. Moreover, the Sr3OsO6 is the epitaxially grown osmate, which connotes that it is compatible with device fabrication processes and thus promising for spintronic applications.
Results
Epitaxial growth and crystallographic analyses
High-quality single-crystalline B-site-ordered double-perovskite Sr3OsO6 films (300-nm thick) were epitaxially grown on (001) SrTiO3 substrates with an abrupt substrate/film interface in a custom-designed molecular beam epitaxy (MBE) setup capable of precisely controlling elemental fluxes even for elements with high melting points, e.g., Os (3033 °C) (Methods). Maintaining a precise flux rate for each constituent cation (Os and Sr) with a simultaneous supply of O3 is essential for avoiding deterioration of the magnetic properties as a deviation of only 2% from the optimal Os/Sr ratio is fatal (Methods).
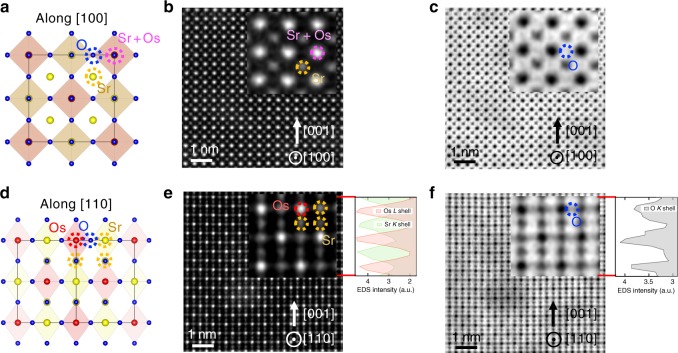
High-resolution scanning transmission electron microscopy (STEM) and transmission electron diffraction (TED), combined with high-resolution reciprocal space mapping (HRRSM) and reflection high-energy electron diffraction (RHEED), ascertained a cubic double-perovskite structure8,12,20 (Methods). As schematically shown in Fig. 1a, d (viewed along [100] and [110] directions), Sr- or Os-occupied, fully Sr-occupied, fully Os-occupied, and fully oxygen-occupied columns exist. The STEM images overtly demonstrate that these columns are arranged in a spatially ordered sequence. Since the intensity in the high-angle annular dark-field (HAADF)-STEM image is proportional to ~Zn (n ~ 1.7–2.0, and Z is the atomic number), in Fig. 1b, the white spheres and gray ones are assigned to Sr- (Z = 38) or Os- (Z = 76) occupied and fully Sr-occupied columns, respectively. In Fig. 1e, the white spheres and gray ones are assigned to fully Os-occupied and fully Sr-occupied columns, respectively. In contrast to HAADF-STEM, Oxygen is emphasized in annular bright-field (ABF)-STEM images. Accordingly, Oxygen ions occupying the expected positions are clearly visible (labeled O (insets of Fig. 1c, f)). The energy dispersive X-ray spectroscopy (EDS)-STEM intensity profiles along the [001] direction shown in Fig. 1e and f complementarily confirm the above elemental assignments. The peak positions in the EDS profile of the Os L shell, Sr K shell, and oxygen K shell agree well with the Os, Sr, and oxygen positions, respectively, determined by STEM. The STEM observation revealed the rock-salt type order of Os6+, whereas the hexavalent state of Os is confirmed by X-ray photoemission spectroscopy (XPS) measurements (METHODS), to an excellent extent, and this ordering is driven by the large difference in the electronic charges and ionic radii between Sr2+ and Os6+21. Consequently, the experimentally derived crystal structure does not allow for Os–O–Os paths. Therefore, advanced mechanisms need to be considered, since the Goodenough–Kanamori rules22, which well predict magnetic interactions between two next-nearest-neighbor magnetic cations through a nonmagnetic anion, do not cover the theoretical framework for the FM order in Sr3OsO6.
Fig. 1.
Atomic-resolution STEM images of a Sr3OsO6 film. a Schematic diagram of the Sr3OsO6 viewed along the [100] direction. b, c HAADF-STEM (b) and ABF-STEM (c) images near the center of the Sr3OsO6 layer along the [100] direction. d Schematic diagram of the Sr3OsO6 viewed along the [110] direction. e, f HAADF-STEM (e) and ABF-STEM (f) images near the center of the Sr3OsO6 layer along the [110] direction. The scale bars are 1 nm. The insets in b, c, e, and f show enlarged views together with EDS-STEM intensity profiles along the [001] direction. In all figures, purple, yellow, red, and blue dotted circles indicate Sr- or Os-occupied, fully Sr-occupied, fully Os-occupied, and fully oxygen-occupied columns, respectively
Magnetic properties
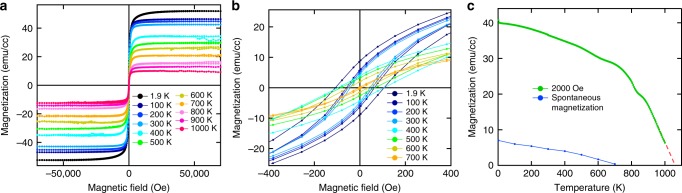
Figure 2a, b shows the temperature dependence of magnetization versus the magnetic field (M–H) of a Sr3OsO6 film. The hysteretic response of the Sr3OsO6 film shows a soft magnetic behavior with the small coercive field of ~100 Oe at 1.9 K (Fig. 2b), and the saturation magnetization at 70,000 Oe (Fig. 2a) decreases with increasing temperature. The saturation magnetization persists up to 1000 K [limit of measurement range (Methods)], indicating TC > 1000 K. Figure 2c shows the magnetization versus temperature (M−T) curve with H = 2000 Oe. In Fig. 2c, we also plot the spontaneous magnetization as a function of temperature. The TC value, estimated from the extrapolation of the M−T curve to the zero magnetization axis, is about 1060 K (Fig. 2c). This is the highest TC among all insulators and oxides, and the highest magnetic ordering temperature in any compound without 3d transition elements23.
Fig. 2.
Magnetic properties of a Sr3OsO6 film. a In-plane M–H curves at 1.9 up to 1000 K for a Sr3OsO6 film grown on (001) SrTiO3. Here, H was applied to the [100] direction. b Close-up near the zero magnetic field in a. c M–T curve with H = 2000 Oe applied to the [100] direction for a Sr3OsO6 film grown on (001) SrTiO3. Spontaneous magnetization deduced from Fig. 2b as a function of temperature is also shown
Noteworthy, the saturation magnetization of Sr3OsO6 (~49 emu/cc at 1.9 K) is significantly smaller than that for typical magnetic metals; e.g., Nd2Fe14B (~1280 emu/cc), SmCo5 (~860 emu/cc) and Alnico 5 (~1120 emu/cc)24, and typical ferrites; e.g., CoFe2O4 (~430 emu/cc), Y3Fe5O12 (~170 emu/cc) and LiFe5O8 (~390 emu/cc)6. The small saturation magnetization, unique to Sr3OsO6, may encourage the development of spintronic devices utilizing small stray fields and low-energy spin-transfer-torque switching25, which are advantageous for high-density integration and low-power consumption. This small saturation magnetization is, most likely, associated with the low composition ratio of Os in Sr3OsO6. The saturation magnetic moment of Os at 1.9 K was estimated to be 0.77 μB/Os, which is smaller than the expected value of the spin-only magnetic moment of 2 μB for the Os6+ (5d2 ) state with S = 1. This apparent deviation requires that SOC has to be taken into account, which is often the case with 5d systems18,26,27.
Electrical properties
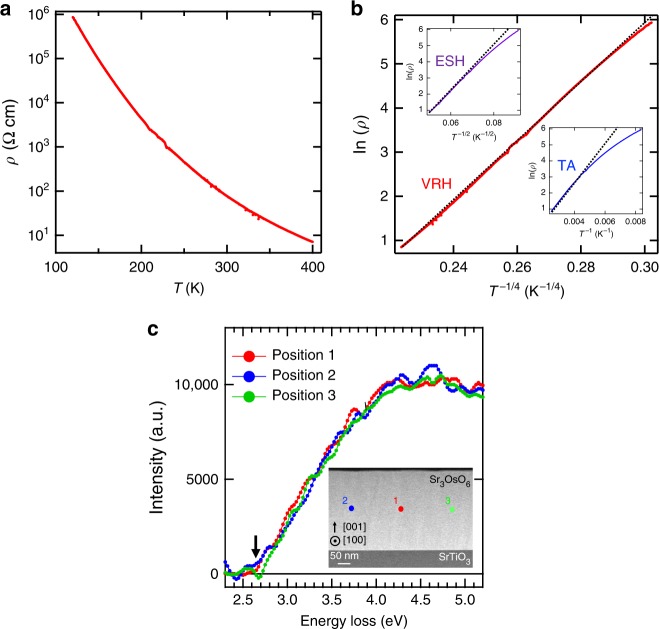
While such high TC is common for systems with free charge carriers, e.g., Fe3O4 and Co, their absence in Sr3OsO6 requires other exchange mechanisms. The temperature dependence of resistivity (ρ) for a Sr3OsO6 film is shown in Fig. 3a. It increases with decreasing temperature and it exceeds the measurable range below 120 K. The electronic charge carriers [5d electrons in the Os6+ state] move by hopping between localized electronic states, and this is supported by ln(ρ) ∝ T−1/4 [variable range hopping (VRH) model] (Fig. 3b) along with the high resistivity value [ρ(300 K) = 75 Ω cm]. Other mechanisms, e.g., ln(ρ) ∝ T−1/2 [Efros–Shklovskii Hopping (ESH) model] and ln(ρ) ∝ T−1 [thermal activation (TA) model], are not supported by the electronic transport measurements. Figure 3c shows electron energy loss spectroscopy (EELS) spectra of a Sr3OsO6 film measured at three different positions as indicated in the cross-sectional STEM image (inset of Fig. 3c) with a spot size of ~4 nm. The EELS spectrum of a material corresponds to the loss function , where is a complex dielectric function. The three EELS spectra (Fig. 3c) taken of Sr3OsO6 are almost identical, indicating that electronic states are uniform in the entire Sr3OsO6 film. The optical bandgap (indicated by the black arrow), at which EELS intensities start to increase28,29, is ~2.65 eV. Accordingly, models based on the double exchange interaction where itinerant electrons are driving the magnetic order, can be ruled out as the origin of ferromagnetic order in Sr3OsO6. In addition, the long distance between nearest Os atoms (5.81 Å) renders the possibility of direct exchange interactions unlikely30,31.
Fig. 3.
Resistivity and dielectric properties of a Sr3OsO6 film. a ρ–T curve for a Sr3OsO6 film. b Logarithm of ρ versus T−1/4 plot, corresponding to the VRH model. The insets of b show the logarithm of ρ versus T−1/2 and T−1 plots, corresponding to the ESH and TA models, respectively. The black dashed lines in b are guides to the eye. c The EELS spectra of a Sr3OsO6 film measured at the spots indicated in the cross-sectional STEM image (inset). The background was corrected with a power-law fit from 2 to 2.3 eV
Electronic-structure calculations
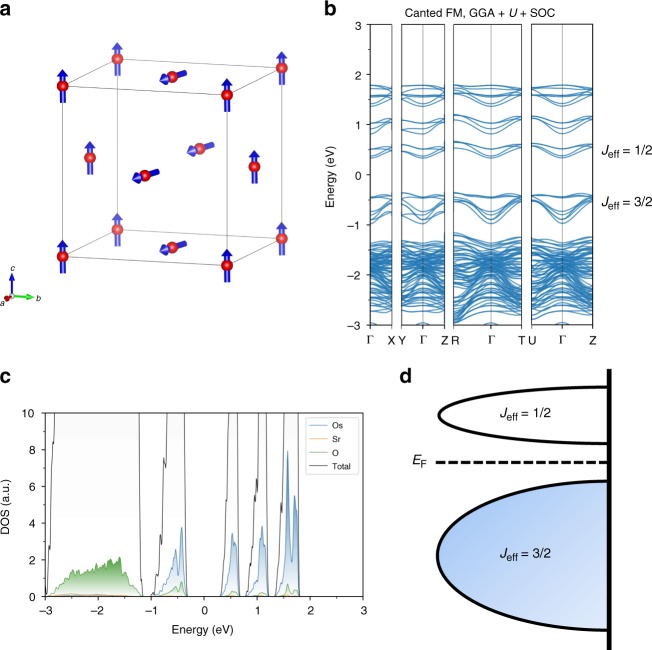
We analyzed the electronic and magnetic states of Sr3OsO6 by DFT with SOC (METHODS) using calculations with the Perdew–Burke–Ernzerhof (PBE) functional32. Canted FM order (Fig. 4a) was found to have the lowest total energy among many possible magnetic arrangements. However, the energy differences between the canted FM order and the collinear FM (Supplementary Fig. 10a), (001) AFM (Supplementary Fig. 10b), and (111) AFM orders (Supplementary Fig. 10c) are very small (~3.6 meV per atom, ~1.4 meV per atom and ~0.29 meV per atom, respectively), implying a competition among these orders. Note that canted magnetic orders have been reported in other Os containing double-perovskites11,33. The optical bandgap determined by GGA-PBE + U + SOC calculations of Sr3OsO6 with the canted FM order is ~0.69 eV, and this corresponds to an insulating state (Fig. 4b, c). With U + SOC, the t2g↑ states are split into effective total angular momenta Jeff = 3/2 (doublet) and Jeff = 1/2 (singlet) states while opening a gap. The Jeff = 3/2 states are fully occupied with two 5d electrons per Os6+, resulting in the insulating state. Note that these calculations reveal a metallic ground state (i.e., no gap at the Fermi energy (EF)), if U = 0 eV. This is because the band dispersions of Jeff = 3/2 and Jeff = 1/2 states are greater than the spin–orbit splitting. The calculated magnetic moment of osmium is 1.56 μB/Os, which is closer to the experimentally determined value (0.77 μB/Os) as compared with the spin-only magnetic moment (2 μB/Os). We also calculated the element-specific partial density-of-state (PDOS) of Sr3OsO6 for the canted FM order by the Heyd–Scuseria–Ernzerhof (HSE) functional + SOC method (Supplementary Fig. 11) to properly implement the interaction length scale found in solids. Consequently, the DFT calculations with the HSE functional have superior prediction capabilities regarding band gaps of insulators34. The bandgap estimated by the HSE + SOC method (~1.41 eV) is closer to the experimentally obtained one (~2.65 eV) than that estimated by the GGA-PBE + U + SOC calculations (~0.69 eV). Although further work is required to reveal the underlying mechanisms driving the FM order in Sr3OsO6, our calculations provide information on the magnetic arrangement at the ground state and how the energy gap is opened by the interplay between Coulomb repulsion (U) and SOC.
Fig. 4.
Electronic-structure calculations with the PBE functional. a Schematic diagram of the magnetic ground state (canted FM order) of Sr3OsO6 obtained from the DFT calculation. In a, red spheres and blue arrows indicate Os atoms and magnetic moments of Os atoms, respectively, and the Sr and O atoms are omitted for simplicity. b The band structures for Sr3OsO6 with the canted FM order calculated by GGA + U + SOC. c The element-specific partial density-of-state (PDOS) for the canted FM order calculated by GGA + U + SOC. In c, blue, orange, and green curves indicate the density-of-states per one Os, Sr, and O atoms, respectively. In b and c, the origin of energy was taken at the center of the bandgap. d Schematic energy diagrams for the Os 5d2 configurations. In d, only PDOS for Os is taken into account and the contributions by Sr and O are omitted for simplicity
Discussion
The origin of the robust ferromagnetism in this magnetic insulator (Sr3OsO6) remains murky, and it appears to be a challenge to reveal the underlying electronic exchange mechanisms. The extended superexchange paths (Os–O–Sr–O–Os), which are well recognized to drive the magnetic order in Os containing double-perovskites30,31,35, are one possible origin of the ferromagnetism. Since a high magnetic ordering temperature through the extended superexchange paths requires a strong p–d hybridization35, further investigations into the electronic structures of Sr3OsO6, such as X-ray magnetic circular dichroism (XMCD), are required. Nevertheless, our DFT calculations with both the PBE (Fig. 4c) and HSE (Supplementary Fig. 11) functionals imply a non-negligible p–d hybridization.
Attention should be paid to the excellent Os order, since cationic disorder deteriorates magnetic ordering in magnetic insulators36–38. Besides, Ca3OsO6 shows an antiferromagnetic order below 50 K21, despite its high Os order and being isoelectronic to Sr3OsO6. A remarkable difference between Sr3OsO6 and Ca3OsO6 is their crystal structure (Ca3OsO6 is monoclinic with tilted OsO6 octahedrons). Such a difference in magnetic order despite the isoelectronic structures was also reported for the isoelectronic pair SrRuO3 (pseudo-cubic perovskite, ferromagnetic metal) and CaRuO3 (orthorhombic perovskite, paramagnetic metal)39, and the difference in the magnetic order in SrRuO3 and CaRuO3 is thought to be associated with the strength of the perovskite distortion. The network morphology hosting the mechanisms of exchange interactions is subject to such distortions, thus, likely driving Ca3OsO6 towards an antiferromagnetic instability. In addition, the cubic double-perovskite structure, in which only one 5d transition metal element occupies the B site, possesses 12 nearest neighbor magnetic ions in contrast to other 4d or 5d element-containing double-perovskites, in which both B and B′ sites are occupied by two different transition metal elements, e.g., Sr2FeMoO6 (TC = 415 K)7, Sr2CrReO6 (TC = 634 K)9 and Sr2CrOsO6 (TC = 725 K)13. This could be one of the reasons for the robust ferromagnetism in Sr3OsO6 as predicted for halide double-perovskites40. Therefore, we suggest that the high Os order with the cubic structure plays an essential role in the robust ferromagnetism achieved in Sr3OsO6.
Our current findings in epitaxial Sr3OsO6 films—an extraordinarily high TC of 1060 K, Jeff = 3/2 insulating state, rock-salt type Os6+ order and small magnetic moment—enrich the family of ferri/ferromagnetic insulators. Although the underlying electronic exchange mechanisms driving the robust FM order in Sr3OsO6 remain murky, applications of Sr3OsO6 to oxide-electronics41,42 beyond the current ferrite technology are feasible.
Methods
Growth of Sr3OsO6 on SrTiO3
We grew the high-quality epitaxial B-site ordered double-perovskite (001) Sr3OsO6 films (300- or 250-nm thick) on (001) SrTiO3 substrates (CrysTec GmbH) in a custom-designed molecular beam epitaxy (MBE) system43,44 (Supplementary Fig. 1a). After cleaning with CHCl3 (10 min, two times) and acetone (5 min) by an ultrasonic cleaner, the SrTiO3 substrate was introduced in the MBE growth chamber. After degassing the substrate at 400 °C for 30 min and successive thermal cleaning at 650 °C for 30 min, we grew a Sr3OsO6 film. The growth temperature was 650 °C. The oxidation during the growth was carried out with O3 gas (non-distilled, ~10% concentration) from a commercial ozone generator as a flow rate of 2 sccm. After the growth, films were cooled to room temperature under ultra-high vacuum (UHV). The MBE system is equipped with multiple e-beam evaporators (Hydra, Thermionics) for Sr and Os. Note that to avoid a formation of OsO4, which is highly toxic, we used Os chunks with a radius of about 3 mm (not Os powder), which are stable in air at room temperature. The electron impact emission spectroscopy (EIES) sensor (Guardian, Inficon) is located next to the sample heater in the MBE in the same horizontal plane. The sensor head is equipped with a filament, which generates thermal electrons for the excitation of Sr and Os atoms. Optical band-pass filters are used for element-specific detection of the excited optical signals, since the emitted light spectra are characteristic for Sr and Os. The EIES sensor is equipped with photomultipliers (PMTs) located outside of the vacuum chamber that convert optical signals into electrical signals. The Sr and Os fluxes measured by EIES were kept constant (Supplementary Fig. 1b) by the proportional-integral-derivative (PID) control of the evaporation source power supply. We optimized the flux ratio of Sr and Os to ascertain Sr3OsO6 films with a high saturation magnetic moment. Supplementary Fig. 2 shows the in-plane M–H curves at 300 K for Sr3OsO6 films grown with different flux ratios of Sr and Os. The saturation magnetic moment of the film grown with the flux ratio of Sr:Os = 2.05:1 is ten or more times larger than those for the films grown with the flux ratio of Sr:Os = 2.05:1.02 and 2.05:0.98. This means that the magnetic properties of Sr3OsO6 films are very sensitive to the Sr/Os ratio and that well-controlled Sr and Os fluxes during the growth are important for the high saturation magnetic moment. Therefore, in this study, we set the flux ratio of Sr:Os at 2.05:1.
The cubic crystal structure of Sr3OsO6 is illustrated in Supplementary Fig. 3a. Supplementary Fig. 3b,c show reflection high-energy electron diffraction (RHEED) patterns of a Sr3OsO6 thin film surface, where the sharp streaky patterns with clear surface reconstruction indicate the growth of the Sr3OsO6 film in a layer-by-layer manner, leading to the high crystalline quality of the film. Notably, [01l] diffractions are not seen (Supplementary Fig. 3b) due to extinction rules, indicating the formation of a cubic B-site ordered double-perovskite8,12,20. The cubic structure model is further supported by high-resolution X-ray reciprocal space mapping (HRRSM) (Supplementary Fig. 3d): the in-plane and out-of-plane lattice constants of Sr3OsO6 are identical within the resolution limits (8.24 ± 0.03 and 8.22 ± 0.03 Å, respectively). It is therefore reasonable that the Sr3OsO6 films are epitaxially but not coherently grown on the SrTiO3 (3.905 Å) substrate.
Transmission electron microscopy and transmission electron diffraction
High-angle annular dark-field (HAADF), annular bright-field (ABF) scanning transmission electron microscopy (STEM) images, and transmission electron diffraction (TED) patterns were taken with a JEOL JEM-ARM 200F microscope. Electron energy loss spectroscopy (EELS) spectra of a Sr3OsO6 film were recorded from three spots with a ~4-nm diameter also with a JEOL JEM-ARM 200F microscope.
Supplementary Figs. 3e–g show cross-sectional HAADF-STEM images of a Sr3OsO6 film taken along the [100] direction. At a glance, one can recognize that a single-crystalline Sr3OsO6 film with an abrupt substrate/film interface has been grown epitaxially on a (001) SrTiO3 substrate, as expected from the RHEED. Misfit dislocations at the Sr3OsO6/SrTiO3 interface (Supplementary Fig. 3g) are due to the ~5% larger lattice constant of the perovskite Sr3OsO6 lattice (8.23 Å/2 = 4.115 Å) than that of SrTiO3 (3.905 Å). The cubic crystal structure of Sr3OsO6 was also confirmed by the STEM analysis. In addition to the [100] direction (Supplementary Figs. 3e–g), the epitaxial growth of the Sr3OsO6 layer on the SrTiO3 substrate was also confirmed by STEM images taken along the [110] direction (Supplementary Figs 4a–c). The rock-salt type order of Os6+ (Fig. 1) is observed to an excellent extent (Supplementary Figs 4d,e).
Supplementary Fig. 5 shows the TED pattern for a Sr3OsO6 film taken along the [110] direction. The diffraction pattern agrees very well with the calculated diffraction pattern for the ideal cubic B-site ordered double-perovskite structure shown in Supplementary Fig. 3a. The extinction rules for a fully B-site ordered double-perovskite demand that either even (hkl) or odd (hkl) peaks are permitted20—and that is exactly what is seen here, confirming the cubic B-site ordered double-perovskite structure.
Chemical composition of a Sr3OsO6 film
Supplementary Fig. 6a shows the depth profile of the chemical composition of a Sr3OsO6 film (250-nm thick) estimated from Rutherford backscattering spectroscopy (RBS). The chemical composition of the Sr3OsO6 layer is uniform (Sr:Os:O = 2.7 ± 0.1:1.15 ± 0.05:6.15 ± 0.4). The concentrations of Os and Oxygen are slightly larger than those for an ideal composition (Sr:Os:O = 3:1:6). This difference may originate from the non-stoichiometry and existence of a small amount of paramagnetic metallic OsO245–47, which was observed in the X-ray diffraction (XRD) measurements, as described below. To exclude the possibility of the contamination by magnetic impurities, we performed EDS measurement for a Sr3OsO6 film (Supplementary Fig. 6b). There are no other peaks except for Sr, Os, Ti, O, and C, which confirms the absence of magnetic impurities.
X-ray diffraction
We performed θ–2θ and reciprocal space map XRD measurements of the Sr3OsO6 films with a Bruker D8 diffractometer using monochromatic Cu Kα1 radiation at room temperature. In Supplementary Fig. 6c, we show the θ–2θ XRD pattern for a Sr3OsO6 film on (001) SrTiO3. In addition to the diffraction peaks of the SrTiO3 substrates, (002) and (004) diffractions from Sr3OsO6 are clearly observed. No (001) and (003) diffractions from Sr3OsO6 are seen due to the extinction rules. Note that traces of OsO2, which is known as a paramagnetic metal45–47, are detected as indicated by *. The XRD intensities of OsO2 are about 700 times smaller than those of Sr3OsO6, and segregation of OsO2 is not discernible in the STEM images, indicating that the volume fraction of OsO2 (paramagnetic metal45–47) is negligible. Therefore, Sr3OsO6 dominates the magnetic response of the film.
X-ray photoemission measurements
X-ray photoemission spectroscopy (XPS) is one of the most powerful methods to determine the valence of Os in compounds48,49, since the 4f7/2 core level binding energies in Os compounds with well-defined oxidation states are known. An ULVAC-PHI Model XPS5700 with a monochromatized Al Kα (1486.6 eV) source operated at 200 W was used for the experiments. The scale of binding energy was calibrated against the C 1s line (284.6 eV). Supplementary Fig. 7 shows the Os 4f spectrum of a Sr3OsO6 film at 300 K. The observed 4f7/2 binding energy (54.1 eV) is close to the reported values for those of Os6+ states (53.2–53.8 eV) and far from those for Os2+ states (49.7 eV), Os3+ states (50.4–51.0 eV), Os4+ states (51.7–52.3 eV), and Os8+ states (55.9–56.3 eV)48,49. Accordingly, the hexavalent state of Os (Os6+) is supported. Note that a shoulder structure at ~53 eV may originate from a surface layer formed due to the slightly hygroscopic nature of Sr3OsO6 because the sample was transferred to the XPS apparatus in atmosphere.
Resistivity measurements
Resistivity was measured using the four-probe method in a Physical Property Measurement System (PPMS) Dynacool sample chamber. The Ag electrodes were deposited on a Sr3OsO6 surface and connected to an Agilent 3458A Multimeter.
Magnetic measurements
The magnetization measurements for Sr3OsO6 films were performed with a Quantum Design MPMS3 SQUID-VSM magnetometer. Using a quartz sample holder (oven sample holder), we measured the M–T curves while increasing the temperature from 1.9 (300) to 300 (1000) K with H = 2000 Oe applied along the [100] or [110] direction. In the M–T measurements, M was measured with increasing temperature after the sample was cooled to 1.9 (300) K from 300 (1000) K without a magnetic field. We also measured M–H curves at 1.9–300 K (400–1000 K) using the quartz sample holder (oven sample holder).
To check the accuracy of the measurement temperature in the MPMS SQUID-VSM magnetometer, we measured the magnetic properties of a pure Ni reference plate (Quantum Design Part Number: 4505–155). The M–H curves at 300 and 1000 K show FM and paramagnetic response, respectively (Supplementary Fig. 8a), and the magnetization of Ni rapidly increases between 623 and 629 K (Supplementary Fig. 8b). These results indicate that the TC of Ni is between 623 and 629 K. This is consistent with the TC value in the literature (627 K)50. Thus, the error in the measurement temperature in the MPMS SQUID-VSM magnetometer is less than ±4 K.
Supplementary Fig. 8c shows the in-plane M−H curves at 1.9, 300, and 1000 K of a SrTiO3 substrate. They show only a linear diamagnetic response at 300 and 1000 K. The nonlinear magnetic response near the zero magnetic field at 1.9 K indicates the existence of paramagnetic impurities in the SrTiO3 substrates. In Fig. 2, the linear diamagnetic response of the magnetic moment for the SrTiO3 substrate was subtracted from the raw M(H) and M(T).
Supplementary Fig. 8d shows the M–T curve with H = 2000 Oe for the oven sample holder without a sample. The curve shows a dip structure at around 800 K. This means that the dip structure in the M–T curve at around 800 K for the Sr3OsO6 film (Fig. 2c) is an unavoidable experimental artifact.
The magnetic properties of Sr3OsO6 at 300 K did not change much after it was heated to 1000 K as shown in Supplementary Fig. 9a. This means that heating to 1000 K does not affect much its magnetic properties.
Although the Sr3OsO6 films were epitaxially grown on the SrTiO3 substrates, the shapes of the in-plane M−H curves measured with H applied to the [100] or [110] direction are identical (Supplementary Fig. 9b). This indicates that the in-plane magnetic anisotropy of the Sr3OsO6 film is negligibly small. This small magnetic anisotropy might be related to the misfit dislocations (Supplementary Fig 3e–g), which often decrease the magnetic anisotropy of magnetic insulators36–38.
The electronic-structure calculations
The electronic-structure calculations were based on density functional theory (DFT). The calculations were performed by using the Vienna Ab initio Simulation Package (VASP)51,52 with the projector augmented-wave (PAW)53,54 method and the Perdew–Burke–Ernzerhof (PBE) functional32 within the generalized gradient approximation (GGA)55. To describe the localization of Os 5d electrons accurately, we used the DFT + U calculations56. The value of the screened Coulomb interaction U = 3 eV was used for the Os atoms. This value is comparable to reported values for Os containing double-perovskites (2–4 eV)14,18,19,31. The contribution of the spin–orbit coupling (SOC) was also included in our calculations. The crystal structure was optimized for the conventional unit cell (40 atoms) of Sr3OsO6 whose lattice constant was fixed to the experimental value 8.23 Å. We performed the optimization until all forces on the atoms become smaller than 10−5 eV/Å with a Γ-centered 2 × 2 × 2 k-point grid and cut-off energy of 800 eV. The total energies and electronic structures were calculated with the optimized crystal structures resulting in the canted FM ground state (Fig. 4a).
By comparing the total energy of the magnetic ground state (canted FM order) (Fig. 4a) with those of the collinear FM order (Supplementary Fig. 10a), the (001) AFM order (Supplementary Fig. 10b) and the (111) AFM order (Supplementary Fig. 10c), we found that the energy differences between the canted FM order and the other orders are very small (~3.6, ~1.4, and ~0.29 meV per atom, respectively), implying a competition among these orders.
We also calculated the element-specific partial density-of-state (PDOS) of Sr3OsO6 for the canted FM order by the Heyd–Scuseria–Ernzerhof (HSE) + SOC method (Supplementary Fig. 11).
Supplementary information
Acknowledgements
We thank Ken-ichi Sasaki for a valuable discussion, Ai Ikeda for her help with the X-ray diffraction and resistivity measurements, Hiroshi Irie for his help with the resistivity measurements, and Kazuhide Kumakura for his help with the MPMS3 SQUID-VSM oven option.
Author contributions
Y.K. and Y.K.W. prepared the samples. Y.K.W. performed experimental measurements and data analysis. N.T. and S.T. carried out the electronic-structure calculations. Y.K.W. wrote the paper. Y.K.W., Y.K., N.T., T.B., S.T., Y.T., and H.Y. contributed to the manuscript and the interpretation of the data.
Data availability
The data that support the plots in this paper and other findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-08440-6.
References
- 1.Naito Y, Suetake K. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw. Theory Tech. 1973;19:65. doi: 10.1109/TMTT.1971.1127446. [DOI] [Google Scholar]
- 2.Tenaud P, Morel A, Kools F, Le Breton JM, Lechevallier L. Recent improvement of hard ferrite permanent magnets based on La-Co substitution. J. Alloy. Comp. 2004;370:331. doi: 10.1016/j.jallcom.2003.09.106. [DOI] [Google Scholar]
- 3.Avci CO, et al. Current-induced switching in a magnetic insulator. Nat. Mater. 2017;16:309. doi: 10.1038/nmat4812. [DOI] [PubMed] [Google Scholar]
- 4.Kagiwara Y, et al. Transmission of electrical signals by spin-wave interconversion in a magnetic insulator. Nature. 2010;464:262. doi: 10.1038/nature08876. [DOI] [PubMed] [Google Scholar]
- 5.Moodera JS, Hao X, Gibson GA, Meservey R. Electron-spin polarization in tunnel junctions in zero applied field with ferromagnetic EuS barriers. Phys. Rev. Lett. 1988;61:637. doi: 10.1103/PhysRevLett.61.637. [DOI] [PubMed] [Google Scholar]
- 6.Posnjak E, Barth TFW. A new type of crystal fine-structure: lithium ferrite (Li2O·e2O3) Phys. Rev. 1931;38:2234. doi: 10.1103/PhysRev.38.2234. [DOI] [Google Scholar]
- 7.Kobayashi KI, Kimura T, Sawada H, Terakura K, Tokura Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature. 1998;395:677. doi: 10.1038/27167. [DOI] [Google Scholar]
- 8.Chen Wt, et al. A half-metallic A- and B-site-ordered quadruple perovskite oxide CaCu3Fe2Re2O12 with large magnetization and a high transition temperature. Nat. Commun. 2014;5:3909. doi: 10.1038/ncomms4909. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, et al. Metallic ordered double-perovskite Sr2CrReO6 with maximum Curie temperature of 635 K. Appl. Phys. Lett. 2002;81:328. doi: 10.1063/1.1493646. [DOI] [Google Scholar]
- 10.Sakai M, et al. Multiferroic thin film of Bi2NiMnO6 with ordered double-perovskite structure. Appl. Phys. Lett. 2007;90:072903. doi: 10.1063/1.2539575. [DOI] [Google Scholar]
- 11.Yan B, et al. Lattice-site-specific spin dynamics in double perovskite Sr2CoOsO6. Phys. Rev. Lett. 2014;112:147202. doi: 10.1103/PhysRevLett.112.147202. [DOI] [PubMed] [Google Scholar]
- 12.Iwasawa H, et al. Strong correlation effects of the Re 5d electrons on the metal-insulator transition in Ca2FeReO6. Phys. Rev. B. 2005;71:075106. doi: 10.1103/PhysRevB.71.075106. [DOI] [Google Scholar]
- 13.Krockenberger Y, et al. Sr2CrOsO6: End point of a spin-polarized metal-insulator transition by 5d band filling. Phys. Rev. B. 2007;75:020404(R). doi: 10.1103/PhysRevB.75.020404. [DOI] [Google Scholar]
- 14.Feng HL, et al. Ba2NiOsO6: a Dirac-Mott insulator with ferromagnetism near 100 K. Phys. Rev. B. 2016;94:235158. doi: 10.1103/PhysRevB.94.235158. [DOI] [Google Scholar]
- 15.Battle PD, Gibb TC, Herod AJ, Kim SH, Munns PH. Investigation of magnetic frustration in A2FeMO6 (A = Ca, Sr, Ba; M = Nb, Ta, Sb) by magnetometry and mossbauer spectroscopy. J. Mater. Chem. 1995;5:865. doi: 10.1039/JM9950500865. [DOI] [Google Scholar]
- 16.Yuan Y, et al. High-pressure synthesis, crystal structures, and magnetic properties of 5d double-perovskite oxides Ca2MgOsO6 and Sr2MgOsO6. Inorg. Chem. 2015;54:3422. doi: 10.1021/ic503086a. [DOI] [PubMed] [Google Scholar]
- 17.Erickson AS, et al. Ferromagnetism in the Mott insulator Ba2NaOsO6. Phys. Rev. Lett. 2007;99:016404. doi: 10.1103/PhysRevLett.99.016404. [DOI] [PubMed] [Google Scholar]
- 18.Xiang HJ, Whangbo MH. Cooperative effect of electron correlation and spin-orbit coupling on the electronic and magnetic properties of Ba2NaOsO6. Phys. Rev. B. 2007;75:052407. doi: 10.1103/PhysRevB.75.052407. [DOI] [Google Scholar]
- 19.Gangopadhyay S, Pickett WE. Spin-orbit coupling, strong correlation, and insulator-metal transitions: The Jeff = 3/2 ferromagnetic Dirac-Mott insulator Ba2NaOsO6. Phys. Rev. B. 2015;91:045133. doi: 10.1103/PhysRevB.91.045133. [DOI] [Google Scholar]
- 20.Manako T, et al. Epitaxial thin films of ordered double perovskite Sr2FeMoO6. Appl. Phys. Lett. 1999;74:2215. doi: 10.1063/1.123805. [DOI] [Google Scholar]
- 21.Feng HL, et al. High-pressure crystal growth and electromagnetic properties of 5d double-perovskite Ca3OsO6. J. Solid State Chem. 2013;201:186. doi: 10.1016/j.jssc.2013.02.037. [DOI] [Google Scholar]
- 22.Goodenough JB. Magnetism and the Chemical Bond. New York: Wiley; 1963. [Google Scholar]
- 23.Rodriguez EE, et al. High temperature magnetic ordering in the 4d perovskite SrTcO3. Phys. Rev. Lett. 2011;106:067201. doi: 10.1103/PhysRevLett.106.067201. [DOI] [PubMed] [Google Scholar]
- 24.Coey JMD. Hard magnetic materials: a perspective. IEEE Trans. Magn. 2011;47:4671. doi: 10.1109/TMAG.2011.2166975. [DOI] [Google Scholar]
- 25.Stiles MD, Zangwill A. Anatomy of spin-transfer torque. Phys. Rev. B. 2002;66:014407. doi: 10.1103/PhysRevB.66.014407. [DOI] [Google Scholar]
- 26.Calder S, et al. Spin-orbit-driven magnetic structure and excitation in the 5d pyrochlore Cd2Os2 O7. Nat. Commun. 2016;7:11651. doi: 10.1038/ncomms11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BJ, et al. Phase-sensitive observation of a spin-orbital Mott state in Sr2IrO4. Science. 2009;323:1329. doi: 10.1126/science.1167106. [DOI] [PubMed] [Google Scholar]
- 28.Oleshko V, Amkreutz M, Overhof H. Electronic, dielectric, and optical properties of individual composite silver halide microcrystals using the EELS and LMTO-ASA techniques. Phys. Rev. B. 2003;67:115409. doi: 10.1103/PhysRevB.67.115409. [DOI] [Google Scholar]
- 29.Stoughton S, et al. Adsorption-controlled growth of BiVO4 by molecular-beam epitaxy. Appl. Phys. Lett. Mater. 2013;1:42112. [Google Scholar]
- 30.Shi Y, et al. Crystal growth and structure and magnetic properties of the 5d oxide Ca3LiOsO6: extended superexchange magnetic interaction in oxide. J. Am. Chem. Soc. 2010;132:8474–8483. doi: 10.1021/ja102606q. [DOI] [PubMed] [Google Scholar]
- 31.Kanungo S, Yan B, Felser C, Jansen M. Active role of nonmagnetic cations in magnetic interactions for double-perovskite Sr2BOsO6 (B = Y,In,Sc) Phys. Rev. B. 2016;93:161116. doi: 10.1103/PhysRevB.93.161116. [DOI] [Google Scholar]
- 32.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 33.Feng HL, et al. Canted ferrimagnetism and giant coercivity in the nonstoichiometric double perovskite La2Ni1.19Os0.81O6. Phys. Rev. B. 2018;97:184407. doi: 10.1103/PhysRevB.97.184407. [DOI] [Google Scholar]
- 34.Tran F, Blaha P. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 2009;102:226401. doi: 10.1103/PhysRevLett.102.226401. [DOI] [PubMed] [Google Scholar]
- 35.Taylor AE, et al. Magnetic order and electronic structure of the 5d3 double perovskite Sr2ScOsO6. Phys. Rev. B. 2015;91:100406. doi: 10.1103/PhysRevB.91.100406. [DOI] [Google Scholar]
- 36.Margulies DT, et al. Origin of the anomalous magnetic behavior in single crystal Fe3O4 films. Phys. Rev. Lett. 1997;79:5162. doi: 10.1103/PhysRevLett.79.5162. [DOI] [Google Scholar]
- 37.Wakabayashi YK, et al. Electronic structure and magnetic properties of magnetically dead layers in epitaxial CoFe2O4/Al2O3/Si(111) films studied by X-ray magnetic circular dichroism. Phys. Rev. B. 2017;96:104410. doi: 10.1103/PhysRevB.96.104410. [DOI] [Google Scholar]
- 38.Wakabayashi YK, et al. Cation distribution and magnetic properties in ultrathin (Ni1-xCox)Fe2O4 (x = 0–1) layers on Si(111) studied by soft x-ray magnetic circular dichroism. Phys. Rev. Mater. 2018;2:104416. doi: 10.1103/PhysRevMaterials.2.104416. [DOI] [Google Scholar]
- 39.Koster G, et al. Structure, physical properties, and applications of SrRuO3 thin films. Rev. Mod. Phys. 2012;84:253. doi: 10.1103/RevModPhys.84.253. [DOI] [Google Scholar]
- 40.Cai B, et al. A class of Pb-free double perovskite halide semiconductors with intrinsic ferromagnetism, large spin splitting and high Curie temperature. Mater. Horiz. 2018;5:961. doi: 10.1039/C8MH00590G. [DOI] [Google Scholar]
- 41.Mannhart J, Schlom DG. Oxide interfaces—an opportunity for electronics. Science. 2010;327:1607. doi: 10.1126/science.1181862. [DOI] [PubMed] [Google Scholar]
- 42.Hwang HY, et al. Emergent phenomena at oxide interfaces. Nat. Mater. 2012;11:103. doi: 10.1038/nmat3223. [DOI] [PubMed] [Google Scholar]
- 43.Naito M, Sato H. Stoichiometry control of atomic beam fluxes by precipitated impurity phase detection in growth of (Pr,Ce)2CuO4 and (La,Sr)2CuO4 films. Appl. Phys. Lett. 1995;67:2557. doi: 10.1063/1.114431. [DOI] [Google Scholar]
- 44.Yamamoto H, Krockenberger Y, Naito M. Multi-source MBE with high-precision rate control system as a synthesis method sui generis for multi-cation metal oxides. J. Cryst. Growth. 2013;378:184. doi: 10.1016/j.jcrysgro.2012.12.057. [DOI] [Google Scholar]
- 45.Graebner JE, Greiner ES, Ryden WD. Magnetothermal oscillations in RuO2, OsO2, and IrO2. Phys. Rev. B. 1976;13:2426. doi: 10.1103/PhysRevB.13.2426. [DOI] [Google Scholar]
- 46.Greedan JE, Willson DB, Haas TE. The metallic nature of osmium dioxide. Inorg. Chem. 1968;7:2461. doi: 10.1021/ic50069a059. [DOI] [Google Scholar]
- 47.Yen PC, Chen RS, Chen CC, Huang YS, Tiong KK. Growth and characterization of OsO2 single crystals. J. Cryst. Growth. 2004;262:271. doi: 10.1016/j.jcrysgro.2003.10.021. [DOI] [Google Scholar]
- 48.White DL, Andrews SB, Faller JW, Barrnett RJ. The chemical nature of osmium tetroxide fixation and staining of membranes by X-ray photoelectron spectroscopy. Biochim. Biophys. Acta. 1976;436:577–592. doi: 10.1016/0005-2736(76)90442-9. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa Y, et al. X-ray photoelectron spectroscopy of highly conducting and amorphous osmium dioxide thin films. Thin. Solid. Films. 1999;347:56–59. doi: 10.1016/S0040-6090(98)01612-5. [DOI] [Google Scholar]
- 50.Kouvel JS, Fisher ME. Detailed magnetic behavior of nickel near its Curie temperature. Phys. Rev. 1964;136:1626. doi: 10.1103/PhysRev.136.A1626. [DOI] [Google Scholar]
- 51.Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B. 1993;47:558. doi: 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- 52.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 53.Blochl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 54.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 55.Perdew JP. Accurate density functional for the energy: real-space cutoff of the gradient expansion for the exchange hole. Phys. Rev. Lett. 1985;55:1665. doi: 10.1103/PhysRevLett.55.1665. [DOI] [PubMed] [Google Scholar]
- 56.Dudarev SL, Savrasov SY, Humphreys CJ, Sutton AP. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B. 1998;57:1505. doi: 10.1103/PhysRevB.57.1505. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots in this paper and other findings of this study are available from the corresponding author upon request.