Abstract
The monoamine neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) exerts an inhibitory influence over motivation, but the circuits mediating this are unknown. Here, we used an optogenetic approach to isolate the contribution of dorsal raphe nucleus (DRN) 5-HT neurons and 5-HT innervation of the mesolimbic dopamine (DA) system to motivated behavior in mice. We found that optogenetic stimulation of DRN 5-HT neurons enhanced downstream 5-HT release, but this was not sufficient to inhibit operant responding for saccharin, a measure of motivated behavior. However, combining optogenetic stimulation of DRN 5-HT neurons with a low dose of the selective serotonin reuptake inhibitor (SSRI) citalopram synergistically reduced operant responding. We then examined whether these effects could be recapitulated if optogenetic stimulation specifically targeted 5-HT terminals in the ventral tegmental area (VTA) or nucleus accumbens (NAc) of the mesolimbic DA system. Optogenetic stimulation of 5-HT input to the VTA combined with citalopram treatment produced a synergistic decrease in responding for saccharin, resembling the changes produced by targeting 5-HT neurons in the DRN. However, this effect was not observed when optogenetic stimulation targeted 5-HT terminals in the NAc. Taken together, these results suggest that DRN 5-HT neurons exert an inhibitory influence over operant responding for reward through a direct interaction with the mesolimbic DA system at the level of the VTA. These studies support an oppositional interaction between 5-HT and DA systems in controlling motivation and goal-directed behavior, and have important implications for the development and refinement of treatment strategies for psychiatric disorders such as depression and addiction.
Subject terms: Motivation, Addiction, Reward, Depression, Transporters in the nervous system
Introduction
Acquisition of beneficial stimuli such as food, water, or sexual partners depends on the ability of such stimuli to elicit appetitive behaviors—a process known as incentive motivation. Incentive motivation is coordinated by interactions among several neurotransmitter systems in the brain, and many lines of evidence suggest that the serotonin (5-hydroxytryptamine; 5-HT) system plays a particularly important role. For example, enhancing whole-brain 5-HT activity reduces feeding behavior [1], operant responding for food [2, 3], brain stimulation reward [4], drug self-administration [5], and appetitive behavior elicited by reward-associated stimuli [6–8]. The effects of 5-HT on motivated behavior may be mediated by neurons originating in the brainstem dorsal raphe nucleus (DRN), given that lesions or pharmacological inhibition of these neurons induces feeding [9], increases the efficacy of brain stimulation reward [10], and supports the formation of a conditioned place preference [11]. Although these inactivation studies suggest that DRN 5-HT neurons have an inhibitory role in motivated behavior, the downstream targets mediating this effect have not been identified.
Serotonin may modulate incentive motivation through interactions with the mesolimbic dopamine (DA) system [12–14]. The mesolimbic DA system is comprised of DA neurons originating in the ventral tegmental area (VTA) which predominantly terminate in the nucleus accumbens (NAc). This circuit is critical for reward processing and motivation [15–17]; enhancing mesolimbic DA activity reinforces operant behavior [18, 19], while reducing DA activity inhibits reward-related behaviors [20–22]. Given that both the VTA and NAc receive extensive serotonergic innervation [23], 5-HT neurons are ideally positioned to modulate mesolimbic DA activity at the level of DA cell bodies and their terminals, respectively. In fact, serotonin has been shown to reduce DA-dependent behavior [24–27], presumably by suppressing mesolimbic DA system activity [28–32]. Thus, 5-HT may inhibit motivated behavior by opposing mesolimbic DA system function via interactions with the VTA and NAc.
Here, we use a combined optogenetic and pharmacological approach to identify a potential pathway through which 5-HT may inhibit incentive motivation. We first examined whether optogenetic stimulation of 5-HT output from the DRN altered operant responding for the primary reinforcer saccharin as a measure of incentive motivation. In contrast to the effects of acute, pharmacological enhancement of whole-brain 5-HT via blockade of 5-HT reuptake [8], we found that optogenetic stimulation of DRN 5-HT neurons had no effect on responding for saccharin. However, when combined with the selective serotonin reuptake inhibitor (SSRI) citalopram, optogenetic stimulation of DRN 5-HT neurons synergistically reduced responding for saccharin. This decrease in responding was recapitulated by targeting 5-HT terminals in the VTA, but not in the NAc. These findings suggest that DRN 5-HT neurons inhibit operant responding for reward through an interaction with the VTA.
Materials and methods
Subjects
To express the excitatory opsin channelrhodopsin2 (ChR2) in 5-HT neurons, Ai32+/+ mice (Jackson 012569; [33]) were crossed with ePet-Cre+/− mice (Jackson 012712; Fig. 1a). The ePet domain is specific to 5-HT neurons, and has been used to direct expression of ChR2 in 5-HT neurons [34–36]. Experimental mice were Ai32+/+ and either ePet-Cre+/− (ChR2+) or ePet-Cre negative (ChR2−). ChR2+ mice exhibited expression of ChR2-EYFP in raphe 5-HT neurons (Fig. 1b), as others have observed [37]; no EYFP-positive cells were observed in ChR2− mice (data not shown). Experiments were performed on adult (10+ weeks old) male mice, unless otherwise specified. All mice were group-housed in temperature and humidity controlled environments on a 12 h light/dark cycle (lights on at 7:00 am) with food available ad libitum. Following surgery, mice were single-housed. In tests of responding for saccharin, mice received 2 h of limited water access each day. In all other experiments, water was available ad libitum. Experiments adhered to the Canadian Council of Animal Care standards and followed protocols approved by the Centre for Addiction and Mental Health Animal Care Committee and the University of Toronto Faculty of Medicine Animal Care Committee.
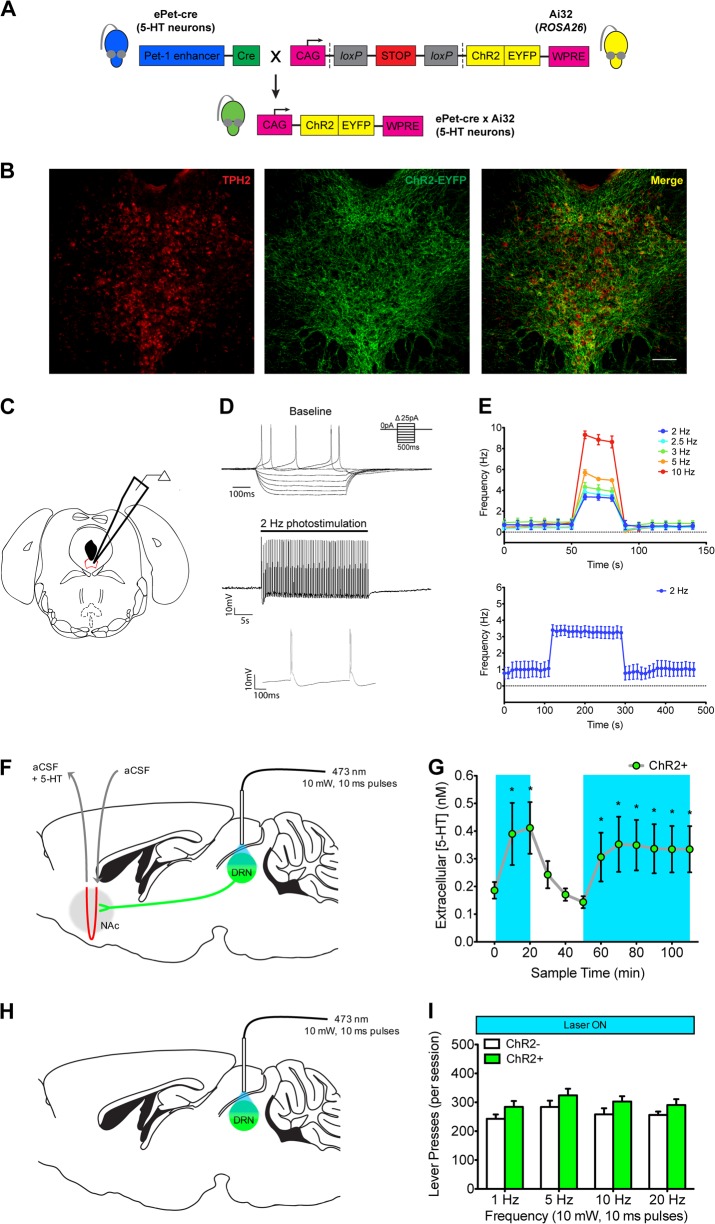
Fig. 1.
Optogenetic stimulation causes dorsal raphe nucleus (DRN) 5-HT neuron activation and enhances downstream 5-HT release, but does not alter operant responding for reward. a Expression of ChR2-EYFP specifically in 5-HT neurons was achieved by crossing Ai32+/+ mice and ePet-cre+/− mice. b Representative coronal section showing ChR2-EYFP expression in the DRN: left, 5-HT neurons immunostained for tryptophan hydroxylase-2, a marker of 5-HT neurons; middle, cells expressing ChR2-EYFP; right, merge of tryptophan hydroxylase-2 and ChR2-EYFP channels. Scale bar represents 100 μm. c Schematic for ex vivo patch-clamp recordings of ChR2-EYFP expressing DRN neurons. d Characteristic electrophysiological response of an EYFP-positive serotonergic DRN neuron to current steps and 2 Hz photostimulation trains. e Top: average (±SEM) firing frequencies of DRN 5-HT neurons (n = 13) elicited by 30 s trains of optogenetic stimulation (2–10 Hz). Bottom: Averaged trace illustrating that extended photostimulation (2 Hz, 200 s) can maintain a significantly elevated firing frequency compared to baseline (paired-samples t-test: t(7) = 5.52, p < 0.001), when tested in a subset of DRN 5-HT neurons. f Schematic for in vivo microdialysis with optogenetic stimulation of ChR2-EYFP expressing DRN 5-HT neurons. g Average (±SEM) extracellular 5-HT concentration within the NAc from anesthetized ChR2+ mice (n = 5) during periods of photostimulation (blue shaded areas) or no photostimulation (unshaded areas). *p < 0.05 vs. previous baseline sample from post hoc analysis. h Schematic for optogenetic stimulation (473 nm light, 10 mW, 10 ms pulses) of DRN 5-HT neurons throughout tests of operant responding for saccharin. i Optogenetic stimulation of DRN 5-HT neurons does not alter operant responding for saccharin: mean lever presses (±SEM) for saccharin was not different between ChR2− (n = 10; white bars) and ChR2+ mice (n = 11; green bars) at any photostimulation frequency tested (Group × Frequency: F(3,57) = 0.06, ns; Group: F(3,57) = 2.94, ns; independent-samples t-tests: all t(19) < 1.57, all p > 0.066)
Drugs
Citalopram HBr (Toronto Research Chemicals, Toronto, Canada) was dissolved in 0.9% saline, and injected intraperitoneally in a volume of 10 ml/kg. Citalopram was injected 20 min prior to behavioral testing with dose order determined by a Latin square and all test sessions separated by 72 h. Doses are expressed in terms of the free base, and were determined from previous work in our laboratory [8].
Ex vivo electrophysiology
Coronal brain slices (400 μm) containing the DRN were obtained for all experiments using a vibratome (Dosaka Pro-7 Linear Slicer; SciMedia) in ice-cold oxygenated sucrose-substituted artificial cerebrospinal fluid (aCSF). The slices were recovered at 30 °C for a minimum of 2 h in aCSF solution containing: 128 mM NaCl, 26 mM NaHCO3, 10 mM d-glucose, 2 mM CaCl2, 2 mM KCl, 2 mM MgSO4, 1.25 mM NaH2PO4, pH 7.4 and saturated with 95% O2/5% CO2. l-Tryptophan (2.5 μM; Sigma-Aldrich) was included during the recovery and recording period to maintain 5-HT synthesis [38]. Patch-clamp recording was performed in l-tryptophan aCSF oxygenated with 95% O2/5% CO2 at 30° C flowing at 3–4 mL/min. Patch pipettes (2–5 MΩ) contained solution with: 120 mM potassium gluconate, 5 mM KCl, 10 mM Na2-Phosphocreatine, 10 mM HEPES buffer, 2 mM MgCl2, 120 mM potassium gluconate, 4 mM K2-ATP, 0.4 mM Na2-GTP (adjusted to pH 7.3 with KOH). DRN neurons were visualized using a fixed-stage microscope (Olympus BX50WI), and 5-HT neurons were targeted based on EYFP expression. Whole-cell recordings were performed in current-clamp with a Multiclamp 700B amplifier using pClamp10.2 software (Molecular Devices). Optogenetic stimulation (10 ms pulses; 2–10 Hz) was performed using a microscope-mounted collimated LED (473 nm, Thorlabs; 2.5 mW at the slice). Citalopram (1 μM) was bath applied in l-tryptophan-containing aCSF to determine effects on membrane properties and optogenetic stimulation response. The citalopram concentration was chosen to be within the range of citalopram that reaches the brain following a 1–10 mg/kg systemic injection [39, 40].
In vivo microdialysis and high-performance liquid chromatography
Microdialysis procedures were carried out as previously described [41] and detailed in Supplementary Methods 1.1. Throughout the procedure, mice were maintained under inhaled isoflurane anesthesia (2%) with body temperature held at 37 °C. Mice were mounted on a stereotaxic frame, and burr holes were made in the skull above the DRN and left NAc. An optical fiber (200 µm core, 0.39 NA) connected to a 473 nm laser was positioned above the DRN (Interaural: A/P −0.8, M/L 0, D/V +3.0). Laser output was controlled by a waveform generator. A microdialysis probe (2 mm cuprophane membrane) was lowered into the NAc (Bregma: A/P + 1.5, M/L −0.7, D/V −5.0 from probe tip). The NAc was chosen as a target to sample 5-HT release following optogenetic stimulation because it receives dense input from the DRN, and is a large enough region in the mouse brain to easily accommodate a microdialysis probe. The probe was continuously perfused with aCSF at 1 µl/min. Sampling began 90 min following probe insertion. Samples were collected every 10 min, and immediately analyzed for 5-HT concentration via high-performance liquid chromatography. Baseline 5-HT concentration was considered stable when three consecutive samples varied less than 10%. Experimental procedures are detailed in Supplementary Methods 1.2.
Behavioral testing
Responding for saccharin was measured in operant conditioning boxes (22 × 18 × 13 cm; Med Associates, St Albans, Vermont). On the front wall of the chamber, a reinforcer magazine containing an infrared photodetector centered 2.5 cm above the floor. A motor-driven dipper could be raised to deliver 0.02 ml of liquid through a hole in the magazine floor. One retractable lever was located on the left of the magazine, which was extended for the duration of operant testing. Operant boxes were illuminated by a houselight, and enclosed in a sound-attenuating chamber equipped with a ventilation fan. Mice could be tethered to optical patch cables through a hole in the roof of the operant box, which was connected in series to an optical commutator and a 473 nm laser controlled by a waveform generator (See Supplementary Methods 2.1).
Responding for saccharin was carried out as previously described [8], and detailed in Supplementary Methods 2.2. Mice were maintained on water restriction throughout testing. In 40-min sessions, mice were first trained to lever press for 0.02 ml saccharin (0.2% w/v) presented in a dipper for 5 s on a fixed ratio 1 schedule of reinforcement followed by a random ratio 4 (RR4) schedule of reinforcement (1-in-4 chance of response being rewarded). After stabilization of responding on the RR4 schedule, mice underwent stereotaxic surgery (detailed in Supplementary Methods 2.3) to implant optical fibers above the DRN (midline), VTA (bilateral), or NAc (bilateral). One week following surgery, responding for saccharin was examined in 20-min sessions with mice tethered to the optical commutator. Mice first received 10 re-training sessions, wherein no photostimulation was applied, to learn to accommodate the tether inside the operant chamber. In subsequent tests, photostimulation was applied for the duration of testing, unless otherwise specified. Experimental procedures are detailed in Supplementary Methods 2.4–2.6.
Locomotor activity was assessed as previously described [42] under dim lighting using 50 × 50 × 35 cm plexiglass chambers with an array of 11 externally mounted photobeams (Med Associates). Activity counts were recorded as horizontal photobeam breaks. Serotonin syndrome measurements were carried out under bright lighting in a 25 × 35 × 25 cm plexiglass chamber with a side-mounted camera. Procedures are described in Supplementary Methods 2.5.
Histology
Immunohistochemistry was performed on brains collected from ChR2+ and ChR2− mice (see Supplementary Methods 3.1). Primary antibodies used were mouse monoclonal anti-TPH2 (1:500; Sigma-Aldrich) and chicken anti-GFP (1:1000; Abcam). Secondary antibodies used were donkey anti-mouse Alexa 594 (1:1000; Invitrogen) and donkey anti-chicken Alexa 488 (1:1000; Jackson Immunoresearch). Sections were imaged using a confocal microscope.
Statistics
Data were analyzed using Statistica v13 (TIBCO, Palo Alto, CA). Electrophysiology and serotonin syndrome data were analyzed using paired and independent-samples t-tests. Neurochemical data were analyzed using one-way ANOVAs with Sample as a within-subjects factor. Behavioral testing data with photostimulation alone were analyzed using independent-samples t-tests, or one-way ANOVAs comparing each photostimulation session with previous and subsequent “OFF” sessions. Behavioral testing data that combined photostimulation and citalopram treatment were analyzed using mixed-model two or three-way ANOVAs, depending on experimental design, with Group (ChR2− and ChR2+) as a between-subjects factor, and Laser (ON or OFF), Citalopram (vehicle, 5, or 10 mg/kg), and Time (across a session) as within-subjects factors. Post hoc analyses were performed using Fisher’s LSD test.
Results
Optogenetic stimulation increases DRN 5-HT neuron activity and causes downstream 5-HT release
Ex vivo patch-clamp electrophysiology experiments (Fig. 1c) confirmed that ChR2-EYFP-expressing 5-HT neurons (n = 13 from four ChR2+ mice) were reliably depolarized by 10 ms pulses of 473 nm blue light, which frequently elicited doublets (Fig. 1d; Table S1). These neurons easily followed 30-s stimulation trains at different frequencies (2–10 Hz; Fig. 1e, top panel). Moreover, labeled 5-HT neurons reliably sustained action potential firing in response to prolonged photostimulation trains delivered at 2 Hz (Fig. 1e, bottom panel; paired-samples t-test: t(7) = 5.52, p < 0.001) and 3 Hz (data not shown). In vivo microdialysis experiments in anesthetized female ChR2+ mice (n = 6) confirmed that optogenetic stimulation of DRN 5-HT neurons increased downstream 5-HT release (Fig. 1f). Photostimulation (2.5 Hz, 10 mW, 10 ms pulses) for 20 min enhanced extracellular 5-HT levels (Fig. 1g; Sample: F(7, 40) = 7.49, p < 0.001; Post hoc: p < 0.001 for samples 2 and 3 vs. sample 1). Thirty minutes later, photostimulation was reapplied for 60 min. During this time, 5-HT levels increased to a plateau within 20 min (Fig. 1g; Samples 6–12; Sample: F(6,28) = 5.24, p < 0.01; Post hoc: p < 0.01 for samples 7–12 vs. sample 6), consistent with previous reports that 5-HT release can be sustained for extended periods of time [43].
Optogenetic stimulation of DRN 5-HT neurons does not alter responding for a primary reinforcer
We next investigated the effects of optogenetic stimulation (10 mW, 10 ms pulses) of DRN 5-HT neurons on responding for saccharin in ChR2− (n = 10) and ChR2+ (n = 11) mice (Fig. 1h). We tested different photostimulation frequencies (1, 5, 10, and 20 Hz) chosen to represent different firing frequencies of DRN 5-HT neurons observed during reward-related behavior [44, 45]. However, optogenetic stimulation had no effect on responding at any frequency tested (Fig. 1i; Group × Frequency: F(3,57) = 0.06, ns; Group: F(3,57) = 2.94, ns; independent-samples t-tests: all t(19) < 1.57, all p > 0.066). It is possible that responding for saccharin was unaffected by optogenetic stimulation because rapid reuptake prevents the accumulation of 5-HT typically required to induce a behavioral change. Indeed, most studies examining the involvement of 5-HT in reward-related behavior employ manipulations that impair 5-HT reuptake rather than stimulate 5-HT release, and these two manipulations produce distinct patterns of brain activity [46]. Thus, we next tested whether combining optogenetic stimulation with a low dose of the SSRI citalopram could alter responding for saccharin, which would presumably enable accumulation of 5-HT within downstream targets of the DRN.
Combining optogenetic stimulation of DRN 5-HT neurons with citalopram treatment synergistically enhances extracellular 5-HT levels
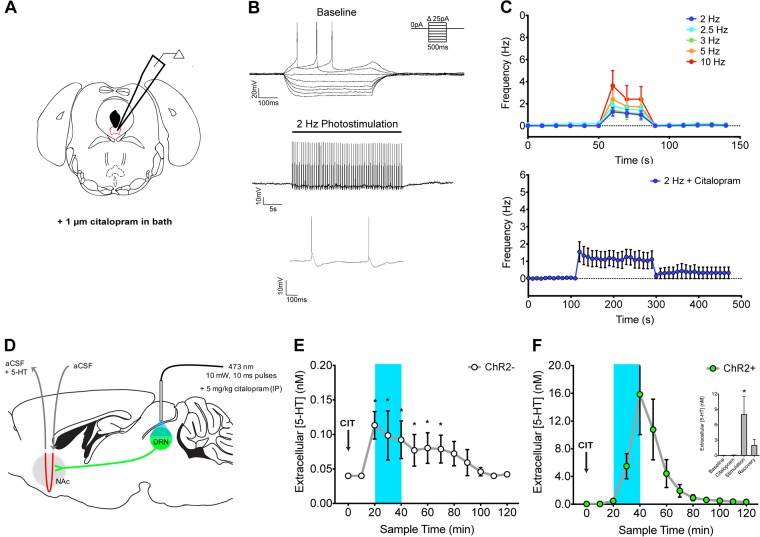
In DRN slices from ChR2+ mice, bath application of 1 µM citalopram resulted in a decrease in firing rate in EYFP-positive neurons (n = 8 from three ChR2+ mice; Fig. 2a) due to the loss of doublet firing that was observed under baseline conditions. This change likely resulted from citalopram’s effects on membrane potential (baseline: −68 ± 3 mV, n = 13; citalopram: -81 ± 4 mV, n = 8; unpaired t-test: t(19) = 2.6; p < 0.05) and input resistance (baseline: 521 ± 44 MΩ; citalopram: 340 ± 68 MΩ; t(19) = 2.3; p < 0.05) (Fig. 2b; Table S1). Despite these electrophysiological changes, optogenetic stimulation elicited firing of 5-HT neurons at multiple frequencies (Fig. 2c, top panel). Further, with citalopram on board, DRN 5-HT neurons could still be entrained to fire for prolonged periods in response to 2 Hz photostimulation (Fig. 3c, bottom panel; paired-samples t-test: t(5) = 4.2, p < 0.01) and 3 Hz photostimulation (data not shown). Changes in downstream 5-HT levels following combined DRN photostimulation (2.5 Hz, 10 mW, 10 ms pulses) and citalopram treatment were then measured in ChR2− (n = 4) and ChR2 + (n = 4) mice (Fig. 2d). In ChR2− mice, citalopram increased extracellular 5-HT concentration to a peak within 20 min which steadily declined over testing (Fig. 2e; Sample: F(12,36) = 3.758, p < 0.01; Post hoc: Samples 3–8 vs. Sample 1, all p < 0.05); DRN photostimulation had no further effect (Post hoc: Samples 4–5 vs. Sample 3, ns). In ChR2+ mice, combined DRN photostimulation and citalopram treatment produced a large increase in 5-HT concentration during the 20 min photostimulation period, although the magnitude of this effect was highly variable (Fig. 2f; Sample 3–5: F(12, 36) = 4.13, p < 0.05). Averaging data for the ChR2+ group across periods of testing (Baseline, Citalopram Treatment, Stimulation, Recovery) shows that photostimulation produced a significant increase in extracellular 5-HT (Fig. 2f inset; sampling period: F(3,12) = 5.15, p < 0.05; Post hoc: Stimulation vs. Baseline, p < 0.05).
Fig. 2.
Combining optogenetic stimulation of dorsal raphe nucleus (DRN) 5-HT neurons with the SSRI citalopram synergistically enhances downstream levels of extracellular 5-HT. a Schematic for ex vivo patch-clamp recordings combining optogenetic stimulation ChR2-EYFP expressing DRN neurons with bath application of 1 μm citalopram. b Electrophysiological response of an EYFP-positive 5-HT neuron to current steps in the presence of 1 µM citalopram, and combined with 2 Hz photostimulation. c Top: Average (±SEM) firing frequencies of DRN 5-HT neurons (n = 8) elicited by 30 s trains of optogenetic stimulation with 1 μm citalopram in bath (2–10 Hz). Bottom: Averaged trace illustrates that extended photostimulation (2 Hz, 200 s) can maintain a significantly elevated firing frequency compared to baseline (paired-samples t-test: t(5) = 4.4, p < 0.01), when tested in a subset of DRN 5-HT neurons. d Schematic for in vivo microdialysis experiments combining 2.5 Hz optogenetic stimulation of DRN 5-HT neurons with systemic 5 mg/kg citalopram treatment. e Extracellular 5-HT concentration within the NAc of anesthetized ChR2− mice (e; n = 4) and ChR2+ mice (f, n = 4) following intraperitoneal citalopram injection (“CIT”) and optogenetic stimulation of DRN 5-HT neurons (blue shaded area). Inset in f shows average 5-HT concentration within each phase of sampling for ChR2+ mice. *p < 0.05 vs. previous baseline sample
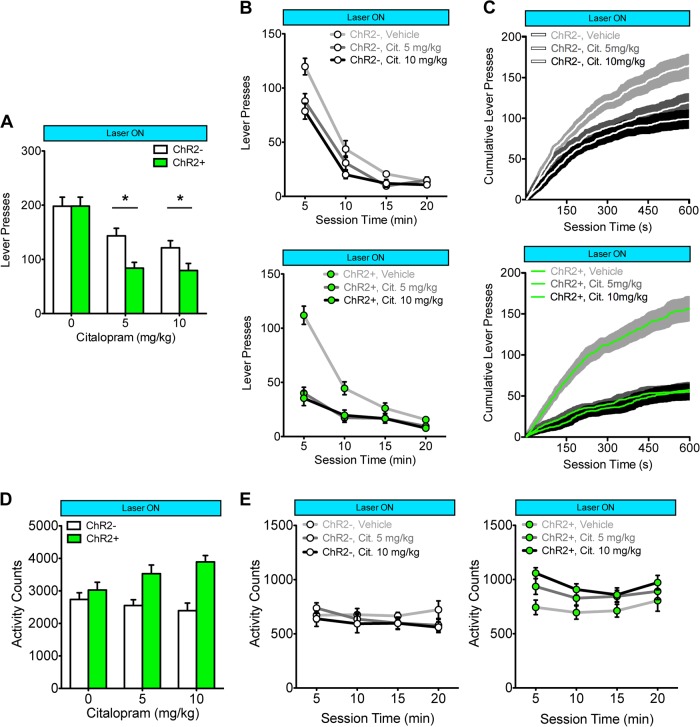
Fig. 3.
Combining optogenetic stimulation (10 mW, 10 ms pulses) of dorsal raphe nucleus (DRN) 5-HT neurons with the SSRI citalopram synergistically reduces operant responding for saccharin. a The combination of 2.5 Hz DRN photostimulation and citalopram treatment reduced responding to a greater extent in ChR2+ mice (n = 11, green bars) compared to ChR2− mice (n = 10, white bars; Group × Citalopram: F(2,38) = 4.66, p < 0.05; *p < 0.05 between ChR2− and ChR2+). b Within-session data from a; Compared to ChR2− mice (top, white symbols), ChR2+ mice (bottom, green symbols) showed a greater decline in responding early in test sessions (Group × Citalopram × Time: F(6,114) = 4.01, p < 0.001). c Average (±SEM, shaded area) cumulative response rate plots for the first 10 min of testing in ChR2− (top, white line) and ChR2+ mice (bottom, green line). d Combining DRN 5-HT neuron photostimulation with citalopram treatment increased locomotor activity (Group × Citalopram: F(2,38) = 19.18, p < 0.001). e Within-session data from d; the increase in activity produced by combined photostimulation and citalopram treatment was consistent across time (Group × Citalopram × Time: F(6,114) = 0.77, ns, Group × Citalopram: F(2,38) = 19.20, p < 0.001). Data are expressed as mean (±SEM)
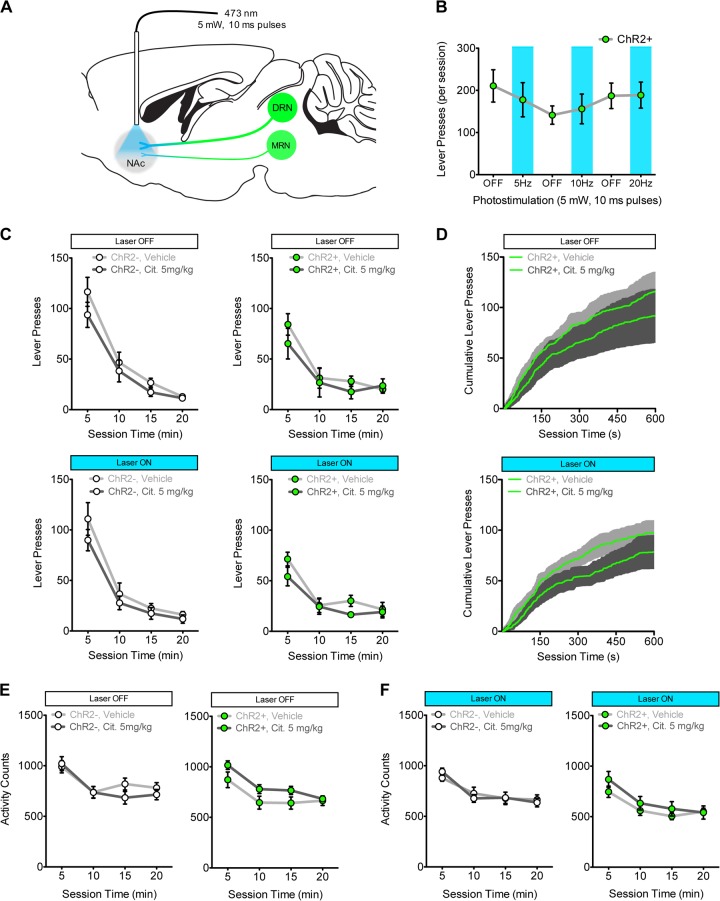
Optogenetic stimulation of DRN 5-HT neurons combined with citalopram treatment synergistically reduces responding for a primary reinforcer
Combining DRN photostimulation (2.5 Hz, 10 mW, 10 ms pulses) and citalopram treatment (5, 10 mg/kg, or vehicle) synergistically reduced responding for saccharin in ChR2+ mice relative to ChR2− mice (Fig. 3a; mice from Fig. 1i; Group × Citalopram: F(2,38) = 4.66, p < 0.05; post hoc: 5 and 10 mg/kg both p < 0.05, vehicle, ns). A within-session analysis found that this difference was particularly pronounced at the beginning of testing (Fig. 3b; Group × Citalopram × Time: F(6,114) = 4.01, p < 0.001). Average cumulative response plots (Fig. 3c) demonstrate that combined DRN photostimulation and citalopram treatment greatly reduced response rates from the beginning of the session in ChR2+ (bottom panel) mice compared to ChR2− mice (top panel). This experiment was repeated using higher photostimulation frequencies, and a similar effect was observed with 5 Hz (Figure S1-A; Group × Citalopram × Time: F(6,114) = 11.45, p < 0.0001) and 10 Hz (Figure S1-B; Group × Citalopram × Time: F(6,114) = 13.79, p < 0.0001). In tests of locomotor activity, the combination of photostimulation and citalopram increased activity (Fig. 3d; Group × Citalopram: F(2,38) = 19.18, p < 0.0001), which was consistent across time (Fig. 3e; Group × Citalopram × Time: F(6,114) = 0.77, ns; Group × Citalopram: F(2,38) = 19.20, p < 0.0001). DRN photostimulation combined with citalopram did not produce any observable signs of serotonin syndrome in ChR2+ mice (See Table S2; all t(19) < 1.51, all p > 0.148).
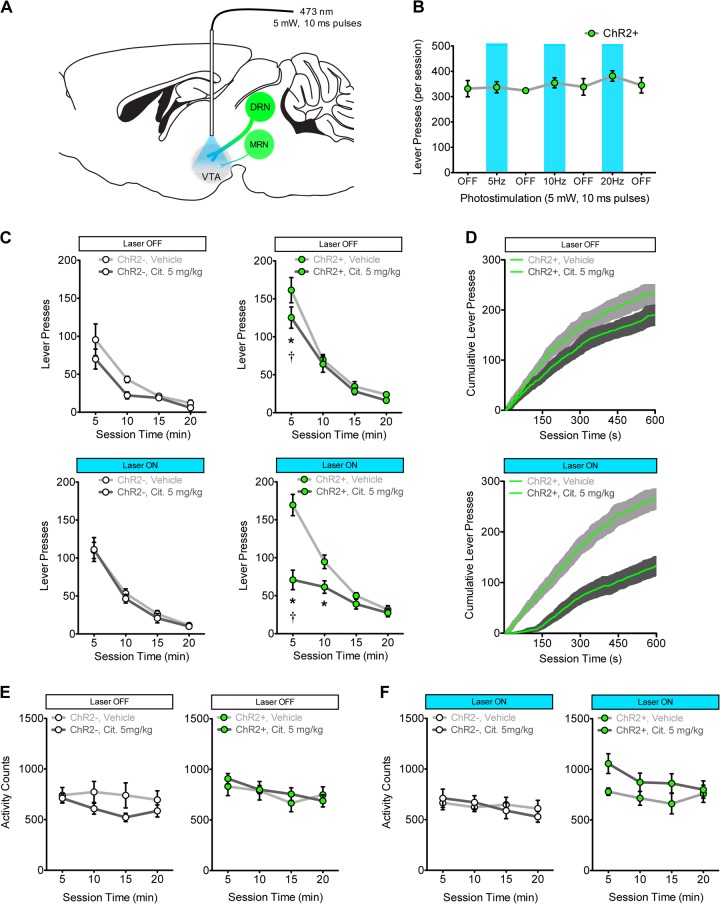
Optogenetic stimulation of 5-HT input to the VTA combined with citalopram treatment synergistically reduces responding for a primary reinforcer
Following acquisition of responding for saccharin on a RR4 schedule of reinforcement, ChR2− (n = 5) and ChR2+ (n = 7) mice received bilateral optical fiber implants targeting the VTA (Fig. 4a; Figure S2A). The effect of photostimulation alone (5 mW, 10 ms pulses) was first examined in ChR2+ mice. On alternating test days, mice received either photostimulation at different frequencies (5, 10, or 20 Hz), or no photostimulation (“OFF”). Photostimulation of 5-HT terminals in the VTA alone had no effect on responding for saccharin in ChR2+ mice (Fig. 4b; ANOVAs across all OFF–ON–OFF periods: all F(2,12) < 1.04, all p > 0.325). Next, both ChR2− and ChR2+ mice were tested on four occasions in a randomized order following injection of vehicle or 5 mg/kg citalopram with and without the application of 5 Hz VTA photostimulation. In ChR2+ mice (Fig. 4c, right panels), combining photostimulation of 5-HT terminals in the VTA with citalopram treatment reduced responding in a time-dependent manner (Laser × Citalopram × Time: F(3,18) = 5.09, p < 0.05). Tests of simple interactions at each time bin found that within the first 5 min of testing, photostimulation combined with citalopram reduced responding to a greater extent than citalopram alone (Laser × Citalopram interaction: F(1,6) = 32.13, p < 0.001; Post hoc: vehicle vs. citalopram at both photostimulation conditions p < 0.01, citalopram 5 mg/kg laser ON vs. OFF p < 0.001). Although no significant interactions were observed for the remaining time bins (Laser × Citalopram: all F < 2.80, all p > 0.14), post hoc analyses found a significant reduction in the second time bin only when photostimulation was combined with citalopram (p < 0.05). In ChR2− mice (Fig. 4c, left panels), no differential effects were observed between citalopram alone and citalopram combined with photostimulation (Laser × Citalopram × Time: F(3,12) = 1.43, ns). Average cumulative response plots for ChR2+ mice (Fig. 4d) demonstrate that the effect of citalopram combined with photostimulation was driven by a reduction in response rate early on in test sessions. In tests of locomotor activity, citalopram alone did not differentially affect ChR2− and ChR2+ mice (Fig. 4e; Group × Citalopram: F(1,10) = 3.219, ns; Citalopram: F(1,10) = 0.727, ns). When photostimulation was combined with citalopram treatment, activity was increased consistently across time (Fig. 4f; Group × Citalopram × Time: F(3,30) = 1.09, ns; Group × Citalopram: F(1,10) = 6.80, p < 0.05).
Fig. 4.
Optogenetic stimulation of 5-HT terminals in the ventral tegmental area (VTA) combined with 5 mg/kg citalopram treatment synergistically reduces operant responding for saccharin. a Schematic for optogenetic stimulation of 5-HT terminals in the VTA (5 mW, 10 ms pulses, 5 Hz) applied throughout tests of responding for saccharin in ChR2− mice (n = 5, white symbols) and ChR2+ mice (n = 7, green symbols). DRN, dorsal raphe nucleus; MRN, median raphe nucleus (MRN presented for completeness, weight of lines illustrates differential contribution of 5-HT inputs from DRN and MRN). b Photostimulation alone did not alter responding for saccharin in ChR2+ mice at any frequency tested; responding was not different between photostimulation sessions (blue shaded area) and sessions without photostimulation (“OFF”; ANOVAs across all OFF–ON–OFF periods: F(1,13) < 1.04, p > 0.325). c Combining citalopram treatment with optogenetic stimulation of 5-HT inputs to the VTA does not significantly alter responding in ChR2− mice (left panels; Laser × Citalopram × Time: F(3,12) = 1.43, ns), but reduced responding in a time-dependent manner in ChR2+ mice (right panels; Laser × Citalopram × Time: F(3,18) = 5.09, p < 0.05; two-way ANOVA in first 5 min: Laser × Citalopram: F(1,6) = 32.13, p < 0.001; †p < 0.001 between laser ON and OFF for 5 mg/kg citalopram; *p < 0.05 between vehicle and citalopram). d Average (±SEM, shaded area) cumulative response rate plots for the first 10 min of test sessions from ChR2+ mice treated with citalopram alone (top) or citalopram combined with photostimulation (bottom). e Citalopram alone did not differentially alter locomotor activity between ChR2− (left) and ChR2+ (right) mice (Group × Citalopram: F(1,10) = 3.219, ns; Citalopram: F(1,10) = 0.727, ns). f Combination of photostimulation with citalopram treatment produced an increase in activity that was consistent across time (Group × Citalopram × Time: F(3,30) = 1.09, ns; Group × Citalopram: F(1,10) = 6.80, p < 0.05). Data are expressed as mean (±SEM)
Optogenetic stimulation of 5-HT input to the NAc combined with citalopram treatment does not reduce responding for a primary reinforcer
Following acquisition of responding for saccharin, ChR2− (n = 8) and ChR2+ (n = 8) mice received bilateral optical fiber implants targeting the NAc (Fig. 5a; Figure S2B). These mice were trained and tested in an identical manner to mice implanted with fibers targeting the VTA (see previous section). Photostimulation of 5-HT terminals in the NAc alone had no effect on responding in ChR2+ mice (Fig. 5b; ANOVAs across all OFF–ON–OFF periods: F(2,14) < 0.25, ns). Combining photostimulation of 5-HT terminals in the NAc with citalopram treatment did not differentially affect responding across time for saccharin in ChR2+ mice (Fig. 5c, right panels; Laser × Citalopram × Time: F(3,21) = 0.68, ns) or ChR2− mice (Fig. 5c, left panels; Laser × Citalopram × Time: F(3,21) = 0.26, ns). Average cumulative response plots in ChR2+ mice (Fig. 5d) show that the combined photostimulation and citalopram condition produced a similar pattern of responding to the citalopram alone condition. Similarly, no differential effects on locomotor activity were observed between ChR2− or ChR2+ mice following citalopram alone (Fig. 5e; Group × Citalopram: F(1,14) = 0.34, ns; Citalopram: F(1,14) = 0.857, ns) or in combination with photostimulation (Fig. 5f; Group × Citalopram × Time: F(3,42) = 0.93, p = 0.4347; Group × Citalopram: F(1,14) = 0.02, ns).
Fig. 5.
Combining optogenetic stimulation of 5-HT terminals in the nucleus accumbens (NAc) with 5 mg/kg citalopram treatment does not alter operant responding for saccharin. a Schematic for optogenetic stimulation of 5-HT terminals in the NAc (5 mW, 10 ms pulses, 5 Hz) applied throughout tests of responding for saccharin in ChR2− mice (n = 8, white symbols) and ChR2+ mice (n = 8, green symbols). DRN, dorsal raphe nucleus; MRN, median raphe nucleus (MRN presented for completeness, weight of lines illustrates differential contribution of 5-HT inputs from DRN and MRN). b Photostimulation alone did not alter responding for saccharin in ChR2 + mice at any frequency tested. Responding was not different between photostimulation sessions (blue shaded area) and sessions without photostimulation (“OFF”; ANOVAs across all OFF–ON–OFF periods: F(1,15) < 0.25, p > 0.325). c Combining photostimulation of 5-HT terminals in the NAc with citalopram treatment did not differentially affect responding for saccharin across time in ChR2− mice (left panels; Laser × Citalopram × Time: F(3,21) = 0.26, ns) and ChR2+ mice (right panels; Laser × Citalopram × Time: F(3,21) = 0.68, ns). d Average (±SEM, shaded area) cumulative response rate plots for the first 10 min of test sessions from ChR2+ mice treated with citalopram alone (top) or citalopram combined with photostimulation (bottom). Locomotor activity was unaffected by citalopram alone (e; Group × Citalopram: F(1,14) = 0.34, ns; Citalopram: F(1,14) = 0.857, ns) or the combination of citalopram and photostimulation (f; Group × Citalopram × Time: F(3,42) = 0.93, ns; Group × Citalopram: F(1,14) = 0.02, ns). Data are expressed as mean (±SEM)
Discussion
The present work examines the role of 5-HT neurons originating in the DRN and their downstream inputs to the mesolimbic DA system in modulating incentive motivation. Using a combined optogenetic and pharmacological approach we demonstrate that enhancing DRN 5-HT output suppresses operant responding for the primary reinforcer saccharin, and that these effects are reproduced by selective enhancement of 5-HT input to the VTA, but not the NAc. These findings are consistent with an inhibitory role for DRN 5-HT neurons in modulating incentive motivation, and suggest that these effects may be mediated by a direct interaction with the mesolimbic DA system via inputs to the VTA.
The majority of ascending 5-HT neurons originate in the DRN [23, 47], and evidence suggests these neurons are important for modulating incentive motivation. Dorsal raphe 5-HT neurons appear to code some aspects of rewarding stimuli [44, 45, 48–50], and reducing DRN 5-HT output facilitates motivated behaviors [9–11]. However, previous studies have been unable to identify the effects of enhancing DRN 5-HT output on incentive motivation due to an inability to selectively target 5-HT neurons for activation. To overcome this, we used an optogenetic approach to stimulate genetically identified 5-HT neurons. Consistent with studies inhibiting DRN 5-HT output, optogenetic stimulation of DRN 5-HT neurons combined with a low dose of the SSRI citalopram reduced operant responding for a primary reinforcer. This effect also parallels findings that elevating whole-brain 5-HT reduces operant responding for reward, including work from our lab using similar operant testing procedures [8].
The ability of DRN 5-HT neurons to inhibit operant responding for reward may be mediated by a downstream interaction with the mesolimbic DA system. The DRN innervates both the VTA and the NAc, and 5-HT has been shown to inhibit mesolimbic DA activity. For example, global pharmacological inhibition or lesions of 5-HT neurons increases the firing rate of VTA DA neurons [28, 51], which may be mediated by 5-HT neurons originating in the DRN [29, 31]. Given the importance of the mesolimbic DA system in promoting reward-related behavior, inhibition of this system would be expected to reduce operant responding for rewards. Indeed, we show that enhancing 5-HT input to the VTA reduces operant responding for saccharin in a manner similar to enhancing DRN 5-HT output. These studies provide initial evidence that 5-HT neurons inhibit incentive motivation through a direct interaction with the mesolimbic DA system, consistent with the idea that 5-HT and DA act in opposition to coordinate goal-directed behavior [12, 13, 52, 53].
It is possible that photostimulation of 5-HT terminals in the VTA may also activate serotonergic fibers of passage within the medial forebrain bundle located ventral to the optical fiber. This may be unlikely considering that stimulation of 5-HT fibers in the NAc itself, a major terminal region of those ascending 5-HT neurons, did not alter responding for saccharin. However, this limitation applies to any optogenetic study targeting 5-HT fibers in the midbrain, and should be addressed in future studies.
Enhancing 5-HT input to the VTA reduced responding for saccharin, while enhancing 5-HT input to the NAc had no effect. Although this finding suggests that the VTA is important for mediating the effects of 5-HT on operant responding for reward, it does not preclude a modulatory role for 5-HT within the NAc. Previous studies have demonstrated that activation or blockade of 5-HT receptors in the NAc has various effects on NAc activity and reward-related behavior [54–56]. Several factors may explain the lack of effects observed here, including the specific reinforcer used, the behavior examined, or the complexity of 5-HT signaling within the NAc [57, 58]. Future studies should investigate how 5-HT signaling within the NAc, as well as the VTA, alters other behavioral measures of incentive motivation, and identify the contribution of different 5-HT receptors to these effects.
In order to confirm that photostimulation of the DRN activated 5-HT neurons, we measured extracellular levels of 5-HT in the NAc. The NAc was chosen for this purpose because it receives strong input from the DRN [47], and is a large enough structure to accommodate placement of a microdialysis probe in the mouse brain. Photostimulation of the DRN produced a small and sustained elevation of 5-HT levels, but this manipulation was not sufficient to alter responding for saccharin. It is possible that operant responding for a primary reinforcer such as saccharin may be an over-trained response, or may be a behavior that is controlled by multiple neurochemical systems which would be difficult to modify by small, naturally occurring changes in 5-HT release. Based on our previous work with citalopram, and serotonin transporter knockout mice, we suggested that 5-HT influences on incentive motivation may be more apparent with reinforcers that maintain lower levels of responding [8]. Accordingly, it would be of interest to examine whether photostimulation of 5-HT neurons, in the absence of citalopram, is sufficient to reduce responding for stimuli such as conditioned, or sensory, reinforcers.
Responding for saccharin was reduced by combining a low dose of citalopram with photostimulation of the DRN (and subsequently the VTA). This combination also increased levels of extracellular 5-HT in the NAc to a much higher degree than photostimulation alone. However, since stimulation of NAc terminals did not alter responding for saccharin, it seems unlikely that the behavioral effects of citalopram combined with DRN stimulation are mediated by this extraordinarily high level of 5-HT release. Although we did not measure 5-HT levels in the VTA due to technical limitations of sampling from this region, it is probable that 5-HT levels would also be elevated to a great extent here. The magnitude of increase in 5-HT release observed raises a concern about the physiological relevance of these neurochemical effects in relation to behavioral changes. However, reductions in responding were apparent within 5 min of testing, much earlier than the peak 5-HT release observed to be produced by this combined manipulation. Further, the differential impact of photostimulation (combined with citalopram) of the VTA vs. the NAc points to a functional difference between 5-HT inputs to these two brain areas.
The combination of citalopram treatment with optogenetic stimulation of DRN 5-HT neurons or inputs to the VTA reduced operant responding early on in test sessions. However, responding still declined further within the session, reaching similar rates of responding as observed under control conditions. This suggests that the reduction in responding is not attributable to some generalized non-specific impairment, which might be expected to induce a stable, low level of responding across the session. Consistent with this view, the combination of citalopram and optogenetic stimulation did not induce signs of the serotonin syndrome or reduce locomotor activity. In fact, a small increase in locomotor activity was observed. This effect may relate to the opposing effects of 5-HT2A and 5-HT2C receptors on locomotor activity in mice [59]. Given that citalopram has some 5-HT2C receptor antagonist properties [60], this mild increased locomotion may result from a shift towards 5-HT2A-mediated signaling, facilitating locomotor activity [59]. Thus, it is unlikely that the reductions in responding for reward under these conditions resulted from general disruptions to behavioral sequencing or compromised motor function. Examination of the effects of serotonergic manipulations on feeding behavior suggest that 5-HT alters feeding motivation by enhancing the onset of satiety [61]. If a similar process were operating here, we would expect changes in responding to occur late rather than early in the session. That pattern was not observed, suggesting that a satiety-like effect does not mediate the reductions in responding. One possible explanation for the pattern of behavior observed in these studies is a reduction in incentive motivation for the primary reinforcer. We have previously hypothesized that 5-HT inhibits operant responding for multiple types of reinforcers, including saccharin, by reducing incentive motivation [8]. Consistent with this idea, informal observation of the behavior of animals in the present study showed that under conditions of combined citalopram and photostimulation, mice often made a series of responses and collected the reward before disengaging for a brief period and subsequently resuming responding. However, further studies using different types of rewarding stimuli are needed to test the hypothesis that the effects observed here are indeed a result of a 5-HT-mediated reduction in incentive motivation.
An important observation from these experiments is that behavior was altered only when optogenetic stimulation was combined with citalopram treatment, and the effects were not frequency-dependent. These findings may highlight the importance of 5-HT transporter function in regulating 5-HT neurotransmission and its behavioral consequences [43]. The 5-HT transporter tightly regulates 5-HT levels in the extracellular space and rapidly terminates 5-HT signaling [43, 62]. We show that optogenetic stimulation of DRN 5-HT neurons increases extracellular 5-HT, but this reaches a plateau (Fig. 1g), likely due to rapid reuptake by the 5-HT transporter. When the 5-HT transporter is inhibited by citalopram, extracellular 5-HT is increased (Fig. 2e) concurrent with a compensatory decrease in the DRN 5-HT neuron firing rate (Figs. 2b, c), as shown by others as well [63]. Thus, combining optogenetic stimulation with citalopram likely enabled 5-HT to accumulate, which may be required to produce the behavioral effects observed.
The present findings may help explain why serotonin-based pharmacotherapies are ineffective in treating psychiatric disorders in certain patients. Drugs that block the serotonin transporter, including the SSRIs citalopram (Celexa) and fluoxetine (Prozac), are front-line treatments for depression, obsessive-compulsive disorder, and generalized anxiety disorder. However, these compounds typically have a delayed therapeutic onset—a period that can be marked by worsening psychiatric symptoms and elevated risk of suicide [64]. Compared to non-serotonergic antidepressants, SSRIs produce side effects of avolition and sexual dysfunction—possibly reflecting motivational impairments—at higher rates [65–68]. Further, antidepressant medications that are less selective for the 5-HT system seem to be more effective in treating motivational symptoms of depression [69, 70]. It is plausible that SSRI-mediated enhancement of 5-HT neurotransmission dampens behavioral responses to rewarding stimuli through an interaction with mesolimbic DA system activity, which may lead to exacerbation of pathological symptoms in the short term.
In conclusion, the present studies suggest that DRN 5-HT neurons exert an inhibitory influence over incentive motivation for a primary reward, and point towards a DRN–VTA pathway mediating this effect. Future studies should more extensively characterize this 5-HT–DA interaction in the context of other behavioral measures of incentive motivation and identify the receptor mechanisms involved. A better understanding of how 5-HT and DA interact to control incentive motivation will help uncover fundamental mechanisms of reward processing and motivation, which are critical for refining treatment strategies for psychiatric illness.
Funding and disclosure
This work was supported by an operating grant (MOP-13628) from the Canadian Institutes of Health Research (CIHR) to P.J.F., a Canada Research Chair in Developmental Cortical Physiology to E.K.L., a Discovery Grant from the Natural Science and Engineering Research Council of Canada (NSERC) to E.K.L., a CIHR postdoctoral fellowship A.R.A., and a Doctoral Scholarship to C.J.B. The authors declare no competing interests.
Electronic supplementary material
Acknowledgements
C.J.B. and P.J.F. conceptualized the study, designed behavioral and microdialysis experiments, and wrote the manuscript. D.C. and E.K.L. designed the electrophysiological experiments. C.J.B., A.R.A., D.C., X.L. and X.J. carried out experiments. A.R.A., D.C. and E.K.L. edited the manuscript. E.K.L. and P.J.F. supervised experiments. The authors would like to thank Dr. Junchul Kim and Dr. Suzanne Erb for helpful comments on this work, and Arthur Godino and Dr. Marine Salery for assistance with figure preparation.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0271-x).
References
- 1.Goudie AJ, Thornton EW, Wheeler TJ. Effects of Lilly 110140, a specific inhibitor of 5-hydroxytryptamine uptake, on food intake and on 5-hydroxytryptophan-induced anorexia. Evidence for serotoninergic inhibition of feeding. J Pharm Pharmacol. 1976;28:318–20. doi: 10.1111/j.2042-7158.1976.tb04165.x. [DOI] [PubMed] [Google Scholar]
- 2.Sanders AC, Hussain AJ, Hen R, Zhuang X. Chronic blockade or constitutive deletion of the serotonin transporter reduces operant responding for food reward. Neuropsychopharmacology. 2007;32:2321–9. doi: 10.1038/sj.npp.1301368. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DM. Effects of cocaine and fenfluramine on progressive-ratio performance. Pharmacol Biochem Behav. 1977;7:555–8. doi: 10.1016/0091-3057(77)90253-2. [DOI] [PubMed] [Google Scholar]
- 4.Katz RJ, Carroll BJ. Intracranial reward after Lilly 110140 (fluoxetine HCl): evidence for an inhibitory role for serotonin. Psychopharmacology (Berl) 1977;51:189–93. doi: 10.1007/BF00431739. [DOI] [PubMed] [Google Scholar]
- 5.Leccese AP, Lyness WH. The effects of putative 5-hydroxytryptamine receptor active agents on D-amphetamine self-administration in controls and rats with 5,7-dihydroxytryptamine median forebrain bundle lesions. Brain Res. 1984;303:153–62. doi: 10.1016/0006-8993(84)90223-3. [DOI] [PubMed] [Google Scholar]
- 6.Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:146–54. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher PJ. Effects of d-fenfluramine and metergoline on responding for conditioned reward and the response potentiating effect of nucleus accumbens d-amphetamine. Psychopharmacology (Berl) 1995;118:155–63. doi: 10.1007/BF02245834. [DOI] [PubMed] [Google Scholar]
- 8.Browne CJ, Fletcher PJ. Decreased incentive motivation following knockout or acute blockade of the serotonin transporter: role of the 5-HT2C receptor. Neuropsychopharmacology. 2016;41:2566–76. doi: 10.1038/npp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutson PH, Dourish CT, Curzon G. Neurochemical and behavioural evidence for mediation of the hyperphagic action of 8-OH-DPAT by 5-HT cell body autoreceptors. Eur J Pharmacol. 1986;129:347–52. doi: 10.1016/0014-2999(86)90445-0. [DOI] [PubMed] [Google Scholar]
- 10.Ahn KC, Pazderka-Robinson H, Clements R, Ashcroft R, Ali T, Morse C, et al. Differential effects of intra-midbrain raphe and systemic 8-OH-DPAT on VTA self-stimulation thresholds in rats. Psychopharmacology (Berl) 2005;178:381–8. doi: 10.1007/s00213-004-2031-3. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher PJ, Ming ZH, Higgins GA. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology (Berl) 1993;113:31–6. doi: 10.1007/BF02244330. [DOI] [PubMed] [Google Scholar]
- 12.Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–16. doi: 10.1016/S0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 13.Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–15. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 14.Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–82. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 15.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/S0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 16.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 18.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–33. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips AG, Fibiger HC. Long-term deficits in stimulation-induced behaviors and self-stimulation after 6-hydroxydopamine administration in rats. Behav Biol. 1976;16:127–43. doi: 10.1016/S0091-6773(76)91226-8. [DOI] [PubMed] [Google Scholar]
- 21.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–7. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 22.Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–66. doi: 10.1016/S0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- 23.Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–38. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher PJ, Davies M. A pharmacological analysis of the eating response induced by 8-OH-DPAT injected into the dorsal raphé nucleus reveals the involvement of a dopaminergic mechanism. Psychopharmacologia. 1990;100:188–94. doi: 10.1007/BF02244404. [DOI] [PubMed] [Google Scholar]
- 25.Invernizzi RW, Cervo L, Samanin R. 8-Hydroxy-2-(di-n-propylamino) tetralin, a selective serotonin1A receptor agonist, blocks haloperidol-induced catalepsy by an action on raphe nuclei medianus and dorsalis. Neuropharmacology. 1988;27:515–8. doi: 10.1016/0028-3908(88)90134-7. [DOI] [PubMed] [Google Scholar]
- 26.Muscat R, Montgomery AM, Willner P. Blockade of 8-OH-DPAT-induced feeding by dopamine antagonists. Psychopharmacology (Berl) 1989;99:402–8. doi: 10.1007/BF00445567. [DOI] [PubMed] [Google Scholar]
- 27.Szumlinski KK, Frys KA, Kalivas PW. Dissociable roles for the dorsal and median raphé in the facilitatory effect of 5-HT1A receptor stimulation upon cocaine-induced locomotion and sensitization. Neuropsychopharmacology. 2004;29:1675–87. doi: 10.1038/sj.npp.1300473. [DOI] [PubMed] [Google Scholar]
- 28.Arborelius L, Chergui K, Murase S, Nomikos GG, Höök BB, Chouvet G, et al. The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH-301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiede Arch Pharmacol. 1993;347:353–62. doi: 10.1007/BF00165384. [DOI] [PubMed] [Google Scholar]
- 29.Gervais J, Rouillard C. Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse. 2000;35:281–91. doi: 10.1002/(SICI)1098-2396(20000315)35:4<281::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Hervé D, Simon H, Blanc G, Lemoal M, Glowinski J, Tassin JP. Opposite changes in dopamine utilization in the nucleus accumbens and the frontal cortex after electrolytic lesion of the median raphe in the rat. Brain Res. 1981;216:422–8. doi: 10.1016/0006-8993(81)90144-X. [DOI] [PubMed] [Google Scholar]
- 31.Lejeune F, Millan MJ. Induction of burst firing in ventral tegmental area dopaminergic neurons by activation of serotonin (5-HT)1A receptors: WAY 100,635-reversible actions of the highly selective ligands, flesinoxan and S 15535. Synapse. 1998;30:172–80. doi: 10.1002/(SICI)1098-2396(199810)30:2<172::AID-SYN7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Prisco S, Esposito E. Differential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br J Pharmacol. 1995;116:1923–31. doi: 10.1111/j.1476-5381.1995.tb16684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–74. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RAM, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8:1857–69. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–7. [DOI] [PMC free article] [PubMed]
- 37.Teixeira CM, Rosen ZB, Suri D, Sun Q, Hersh M, Sargin D et al. Hippocampal 5-HT input regulates memory formation and Schaffer collateral excitation. Neuron. 2018;98:992–1004. [DOI] [PMC free article] [PubMed]
- 38.Liu RJ, Lambe EK, Aghajanian GK. Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur J Neurosci. 2005;21:945–58. doi: 10.1111/j.1460-9568.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson L, Carlsson B, Hiemke C, Ahlner J, Bengtsson F, Schmitt U, et al. Altered brain concentrations of citalopram and escitalopram in P-glycoprotein deficient mice after acute and chronic treatment. Eur Neuropsychopharmacol. 2013;23:1636–44. doi: 10.1016/j.euroneuro.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Uhr M, Grauer MT. abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res. 2003;37:179–85. doi: 10.1016/S0022-3956(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 41.Browne CJ, Ji X, Higgins GA, Fletcher PJ, Harvey-Lewis C. Pharmacological modulation of 5-HT2C receptor activity produces bidirectional changes in locomotor activity, responding for a conditioned reinforcer, and mesolimbic DA release in C57BL/6 mice. Neuropsychopharmacology. 2017;42:2178–87. doi: 10.1038/npp.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009;57:259–67. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Hashemi P, Dankoski EC, Lama R, Wood KM, Takmakov P, Wightman RM. Brain dopamine and serotonin differ in regulation and its consequences. Proc Natl Acad Sci USA. 2012;109:11510–5. doi: 10.1073/pnas.1201547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–43. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–72. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giorgi A, Migliarini S, Galbusera A, Maddaloni G, Mereu M, Margiani G, et al. Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 2017;21:910–8. doi: 10.1016/j.celrep.2017.09.087. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 48.Cohen JY, Amoroso MW, Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife. 2015;4:463. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Dalph N, Hyland BI. Association with reward negatively modulates short latency phasic conditioned responses of dorsal raphe nucleus neurons in freely moving rats. J Neurosci. 2013;33:5065–78. doi: 10.1523/JNEUROSCI.5679-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Neurophysiol. 2009;102:3026–37. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- 51.Guiard BP, Mansari ElM, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–39. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- 52.Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, Witcher MR, et al. The protective action encoding of serotonin transients in the human brain. Neuropsychopharmacology. 2018;43:1425–35. doi: 10.1038/npp.2017.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boureau YL, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res. 2005;29:1146–55. doi: 10.1097/01.ALC.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- 55.Fletcher PJ. Injection of 5-HT into the nucleus accumbens reduces the effects of d-amphetamine on responding for conditioned reward. Psychopharmacologia. 1996;126:62–69. doi: 10.1007/BF02246412. [DOI] [PubMed] [Google Scholar]
- 56.Redgrave P, Horrell RI. Potentiation of central reward by localised perfusion of acetylcholine and 5-hydroxytryptamine. Nature. 1976;262:305–7. doi: 10.1038/262305a0. [DOI] [PubMed] [Google Scholar]
- 57.Brown P, Molliver ME. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci. 2000;20:1952–63. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–17. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- 59.Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pälvimäki EP, Roth BL, Majasuo H, Laakso A, Kuoppamäki M, Syvälahti E, et al. Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor. Psychopharmacologia. 1996;126:234–40. doi: 10.1007/BF02246453. [DOI] [PubMed] [Google Scholar]
- 61.Blundell JE. Is there a role for serotonin (5-hydroxytryptamine) in feeding? Int J Obes. 1977;1:15–42. [PubMed] [Google Scholar]
- 62.Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–60. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci. 2001;21:2178–85. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–43. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 65.Clayton AH, Montejo AL. Major depressive disorder, antidepressants, and sexual dysfunction. J Clin Psychiatry. 2006;67:33–37. [PubMed] [Google Scholar]
- 66.Opbroek A, Delgado PL, Laukes C, McGahuey C, Katsanis J, Moreno FA, et al. Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses? Int J Neuropsychopharmacol. 2002;5:147–51. doi: 10.1017/S1461145702002870. [DOI] [PubMed] [Google Scholar]
- 67.Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195:211–7. doi: 10.1192/bjp.bp.108.051110. [DOI] [PubMed] [Google Scholar]
- 68.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259–66. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 69.Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2006;21:461–71. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- 70.Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52:1469–78. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.