Abstract
Extracytoplasmic (ECF) σ factors, the largest class of alternative σ factors, are related to primary σ factors, but have simpler structures, comprising only two of six conserved functional modules in primary σ factors: region 2 (σR2) and region 4 (σR4). Here, we report crystal structures of transcription initiation complexes containing Mycobacterium tuberculosis RNA polymerase (RNAP), M. tuberculosis ECF σ factor σL, and promoter DNA. The structures show that σR2 and σR4 of the ECF σ factor occupy the same sites on RNAP as in primary σ factors, show that the connector between σR2 and σR4 of the ECF σ factor–although shorter and unrelated in sequence–follows the same path through RNAP as in primary σ factors, and show that the ECF σ factor uses the same strategy to bind and unwind promoter DNA as primary σ factors. The results define protein-protein and protein-DNA interactions involved in ECF-σ-factor-dependent transcription initiation.
No structural data have been available for RNA polymerase holoenzymes or transcription initiation complexes that contain extracytoplasmic σ factors. Here the authors report the crystal structures of transcription initiation complexes comprising Mycobacterium tuberculosis RNA polymerase, extracytoplasmic σ factor σL and promoter DNA.
Introduction
Bacterial transcription initiation is carried out by an RNA polymerase (RNAP) holoenzyme comprising RNAP core enzyme and a σ factor1. Bacteria contain a primary σ factor (group-1 σ factor; σ70 in Escherichia coli; σA in other bacteria) that mediates transcription initiation at most genes required for growth under most conditions and sets of alternative σ factors that mediate transcription initiation at sets of genes required in certain cell types, developmental states, or environmental conditions1.
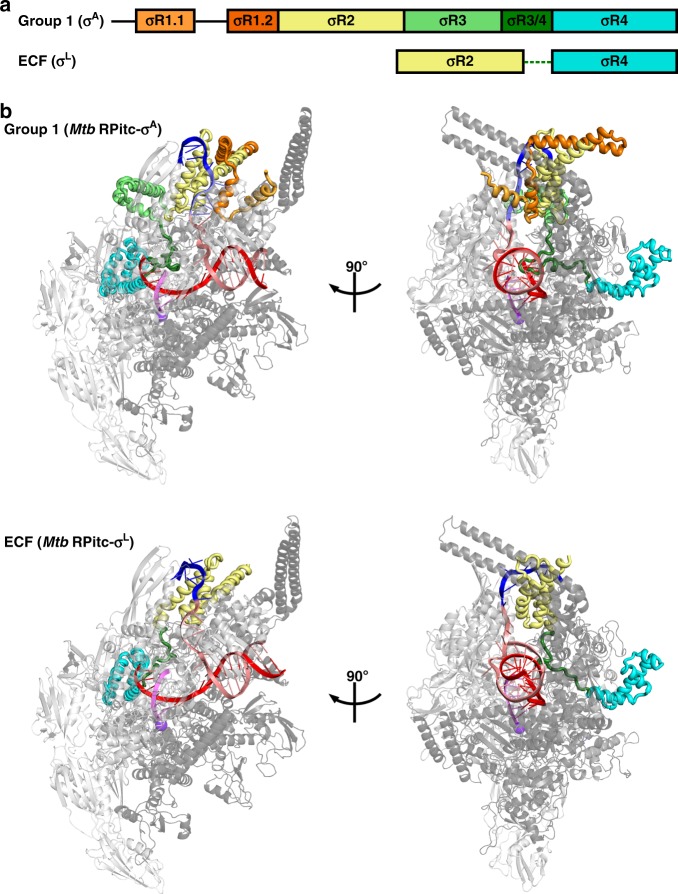
Group-1 σ factors contain six conserved functional modules: σ regions 1.1, 1.2, 2, 3, 3/4 linker, and 4 (σR1.1, σR1.2, σR2, σR3, σR3/4 linker, and σR4; Fig. 1a)1. σR1.1 plays a regulatory role, inhibiting interactions between free, non-RNAP-bound, σ and DNA. σR1.2, σR2, σR3, and σR4 play roles in promoter recognition. σR2 and σR4 recognize the promoter -10 element and the promoter -35 element, respectively, and σR1.2 and σR3 recognize sequences immediately downstream and immediately upstream, respectively of the promoter -10 element. The σR3/4 linker plays multiple crucial roles2–11. The σR3/4 linker connects σR2 to σR4; the σR3/4 linker enters the RNAP active-center cleft, where it interacts with template-strand ssDNA of the unwound transcription bubble, pre-organizing template-strand ssDNA to adopt a helical conformation and to engage the RNAP active center, thereby facilitating initiating-nucleotide binding and de novo transcription initiation; and the σR3/4 linker exits the RNAP active-center cleft by threading through the RNAP RNA exit channel. Before RNA synthesis takes place, the σR3/4 linker serves as a molecular mimic of RNA, or molecular placeholder for RNA, through its interactions with template-strand ssDNA and the RNAP RNA exit channel. As RNA synthesis takes place, the σR3/4 linker then is displaced—off of template-strand ssDNA and out of the RNAP RNA exit channel—driven by steric interactions with the 5′-end of the nascent RNA. The σR3/4 linker must be displaced from template-strand ssDNA during initial transcription; this requirement imposes energy barriers associated with initial-transcription pausing and abortive initiation. The σR3/4 linker must be displaced from the RNAP RNA exit channel during the transition between initial transcription and transcription elongation; this requirement imposes energy barriers that are exploited to trigger promoter escape and to transform the transcription initiation complex into the transcription elongation complex.
Fig. 1.
Structures of group-1 and ECF σ factors. a Structural organization of group-1 (σA) and ECF (σL) σ factors. Conserved regions σR1.1, σR1.2, σR2, σR3, σR3/4 linker, and σR4 are in light orange, dark orange, yellow, green, dark green, and cyan, respectively. Dashed line, non-conserved σR2/4 linker present in ECF σ factors. b Crystal structures of group-1 (Mtb RPitc-σA; PDB 5UH8) and ECF (Mtb RPitc-σL; PDB 6DVC) transcription initiation complexes (two orthogonal views of each). σ factors are shown in tube representations with conserved regions colored as in (a). Gray ribbon, RNAP core enzyme; blue, pink, red, and magenta ribbons, -10 element of DNA nontemplate strand, rest of DNA nontemplate strand, DNA template strand, and RNA product; violet sphere, RNAP active-center Mg2+. Other colors are as in (a). See Supplementary Figs. 1, 2, and 7
Crystal structures of RNAP holoenzyme and transcription initiation complexes containing group-1 σ factors define the protein–protein and protein–nucleic acid interactions involved in group-1-σ-factor-dependent transcription initiation, and extensive biochemical and biophysical characterization defines the protein–protein and protein–nucleic acid interactions and mechanisms involved in group-1-σ-factor-dependent transcription initiation2,3,6,7,12–19.
Alternative σ factors—with the exception of the alternative σ factor mediating the response to nitrogen starvation (σ54 in E. coli; σN in other bacteria)20,21—are members of the same protein family as group-1 σ factors1. Group-2 and group-3 alternative σ factors are closely related in structure to group-1 σ factors, lacking only functional modules σR1.1 (in group-2 σ factors) or σR1.1 and σR1.2 (in group-3 σ factors). The close structural similarity of group-2 and group-3 σ factors to group-1 σ factors, together with crystal structures of transcription initiation complexes containing group-2 σ factors22, facilitates an understanding of the mechanism of group-2- and group-3-σ-factor-dependent transcription initiation.
Group-4 alternative σ factors—also referred to as “extracytoplasmic σ factors” (ECF σ factors), based on functional roles in response to cell-surface and other extracytoplasmic stresses—are only distantly related to group-1 σ factors and are substantially smaller than group-1 σ factors, lacking four of the six functional modules present in group-1 σ factors (Fig. 1a)1,23–30. ECF σ factors comprise only a module related to σR2 (the module that recognizes promoter -10 elements in group-1 σ factors), a module related to σR4 (the module that recognizes promoter -35 elements in group-1 σ factors), and a short σR2/4 linker that has no detectable sequence similarity to the σR3/4 linker of group-1 σ factors. No structural information previously has been reported for RNAP holoenzymes or transcription initiation complexes containing ECF σ factors. In the absence of structural information for ECF σ factors, it has been unclear how ECF σ factors, despite lacking sequences homologous to the σR3/4 linker of group-1 σ factors, are able to connect σR2 and σR4 with an appropriate spacing to recognize promoter -10 and -35 elements, are able to pre-organize the DNA template strand to facilitate initiating-nucleotide binding and de novo transcription initiation; and are able to coordinate entry of RNA into the RNA-exit channel with promoter escape. In addition, in the absence of structural information, and with comparatively limited sequence similarity between σR2 of ECF σ factors and σR2 of group-1 σ factors1, it has been unclear whether σR2 of ECF σ factors adopts the same fold as σR2 of group-1 σ factors and uses the same strategy to bind and unwind the promoter -10 element as group-1 σ factors.
ECF σ factors are numerically the largest, and functionally the most diverse, alternative σ factors1,24–30. Fully 10 of the 13 σ factors in Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, are ECF σ factors: σC, σD, σE, σG, σH, σI, σJ, σK, σL, and σM, mediating responses to nutrition depletion, surface stress, temperature stress, oxidative stress, pH stress, growth in stationary phase, and growth in macrophages31–35. For example, the Mtb ECF σ factor σL (Supplementary Fig. 1A) mediates the response to oxidative stress and regulates its own synthesis, polyketide-synthase synthesis, cell-wall synthesis, lipid transport, the oxidative state of exported proteins, and virulence36–38.
In this work, we have determined crystal structures, at 3.3–3.8 Å resolution, of functional transcription initiation complexes comprising Mtb RNAP, the Mtb RNAP ECF σ factor σL, and nucleic-acid scaffolds corresponding to the transcription bubble and downstream dsDNA of an ECF-σ-factor-dependent RNAP-promoter open complex (Mtb RPo-σL) or an RNAP-promoter initial transcribing complex (Mtb RPitc-σL) (Table 1; Fig. 1; Supplementary Figs. 1, 2).
Table 1.
Structure data collection and refinement statistics
| Structure | Mtb RPitc5-σL_sp4 | Mtb RPitc5-σL_sp5 | Mtb RPitc5-σL_sp6 |
| PDB code | 6DV9 | 6DVB | 6DVC |
| Data collection a | |||
| Source | APS 19-ID | SSRL-9-2 | APS 19-ID |
| Space group | P212121 | P212121 | P212121 |
| Cell dimensions | |||
| a, b, c (Å) | 143.3, 161.4, 237.7 | 143.7, 160.6, 240.4 | 146.3, 161.5, 240.6 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 50.0–3.8 (3.9–3.8) | 50.0-3.8 (3.9–3.8) | 50.0-3.3 (3.4–3.3) |
| Number of unique reflections | 53,020 | 53,728 | 98,083 |
| R merge b | 0.162 (0.567) | 0.188 (0.706) | 0.175 (0.710) |
| R meas | 0.172 (0.613) | 0.200 (0.752) | 0.184 (0.764) |
| R pim | 0.056 (0.222) | 0.066 (0.246) | 0.055 (0.273) |
| CC1/2 (highest resolution shell) | 0.526 | 0.715 | 0.558 |
| I/σI | 8.6 (2.2) | 7.3 (1.9) | 13 (1.9) |
| Completeness (%) | 96.6 (90.5) | 97.1 (96.5) | 98.9 (99.3) |
| Redundancy | 10.7 (9.9) | 8.2 (8.1) | 10.4 (7.4) |
| Anomalous completeness (%) | N/A | N/A | N/A |
| Anomalous redundancy | N/A | N/A | N/A |
| Refinement a | |||
| Resolution (Å) | 50.0–3.8 | 50.0–3.8 | 50.0–3.3 |
| Number of unique reflections | 52,512 | 53,617 | 85,687 |
| Number of test reflections | 2625 | 2691 | 4267 |
| Rwork/Rfree | 0.18/0.23 (0.29/0.32) | 0.20/0.24 (0.32/0.35) | 0.19/0.23 (0.34/0.35) |
| Number of atoms | |||
| Protein | 24,956 | 24,974 | 24,982 |
| Ligand/ion | 3 | 2 | 2 |
| r.m.s. deviations | |||
| Bond lengths (Å) | 0.002 | 0.002 | 0.002 |
| Bond angles (°) | 0.489 | 0.457 | 0.468 |
| MolProbity statistics | |||
| Clash score | 7.2 | 6.3 | 6.0 |
| Rotamer outliers (%) | 1.6 | 2.4 | 2.6 |
| Cβ outliers (%) | 0 | 0 | 0 |
| Ramachandran plot | |||
| Favored (%) | 95.2 | 95.4 | 95.5 |
| Outliers (%) | 0.3 | 0.2 | 0.3 |
| Structure | Mtb [BrU]RPo-σL_sp6 | Mtb [SeMet15,76]RPo-σL_sp6 |
| PDB code | 6DVD | 6DVE |
| Data collection a | ||
| Source | APS 19-ID | APS 19-ID |
| Space group | P212121 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 142.1, 161.5, 239.4 | 142.8, 160.6, 240.2 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 50.0–3.9 (4.0–3.9) | 50.0–3.8 (3.9–3.8) |
| Number of unique reflections | 44,608 | 50,281 |
| R merge b | 0.110 (0.768) | 0.178 (>1.000) |
| R meas | 0.121 (0.847) | 0.187 (>1.000) |
| R pim | 0.047 (0.348) | 0.062 (0.608) |
| CC1/2 (highest resolution shell) | 0.797 | 0.589 |
| I/σI | 14.3 (1.6) | 10.2 (1.0) |
| Completeness (%) | 87.4 (68.5) | 92.1 (79.3) |
| Redundancy | 6.0 (4.8) | 9.3 (5.5) |
| Anomalous completeness (%) | 87.4 | 92.1 |
| Anomalous redundancy | 6.0 | 9.3 |
| Refinement a | ||
| Resolution (Å) | 45.9–3.9 | 46.7–3.8 |
| Number of unique reflections | 37,593 | 40,877 |
| Number of test reflections | 1996 | 1987 |
| Rwork/Rfree | 0.22/0.24 (0.25/0.26) | 0.20/0.24 (0.25/0.30) |
| Number of atoms | ||
| Protein | 24,743 | 24,782 |
| Ligand/ion | 2 | 4 |
| r.m.s. deviations | ||
| Bond lengths (Å) | 0.002 | 0.002 |
| Bond angles (°) | 0.491 | 0.492 |
| MolProbity statistics | ||
| Clash score | 6.6 | 6.8 |
| Rotamer outliers (%) | 2.6 | 1.7 |
| Cβ outliers (%) | 0 | 0 |
| Ramachandran plot | ||
| Favored (%) | 95.3 | 95.0 |
| Outliers (%) | 0.3 | 0.3 |
aData for the highest resolution shell are presented in parentheses
bRmerge values for 6DVB, 6DVC, 6DVD, and 6DVE reflect an anisotropic component
Results
Structures of Mtb RPo-σL and Mtb RPitc-σL
Structures were determined using recombinant Mtb RNAP core enzyme prepared by co-expression of Mtb RNAP subunit genes in E. coli, recombinant Mtb σL, and synthetic nucleic-acid scaffolds based on the sequence of the σL-dependent promoter P-sigL (the promoter responsible for expression of the gene encoding σL)36–38 (Supplementary Figs. 1, 2). Transcription experiments demonstrate that Mtb RNAP-σA holoenzyme (containing the group-1 σ factor σA) does not efficiently perform transcription initiation at the P-sigL promoter, whereas Mtb RNAP-σL holoenzyme (containing the ECF σ factor σL) does (Supplementary Fig. 1E). We prepared “downstream-fork-junction” nucleic-acid scaffolds containing P-sigL sequences, analogous to the downstream-fork-junction nucleic-acid scaffolds containing consensus group-1-σ-factor-dependent promoter sequences used previously for structural analysis of group-1-σ-factor-dependent transcription initiation (Supplementary Fig. 2, left panels). Because the P-sigL transcription start site (TSS) had been mapped only provisionally36–38, we prepared and analyzed a set of downstream-fork-junction nucleic-acid scaffolds having different lengths—4 nt, 5 nt, 6, or 7 nt—of the “spacer” between the P-sigL promoter -10 region and downstream dsDNA (Supplementary Fig. 2, left panels). Transcription experiments indicated that all analyzed nucleic-acid scaffolds were functional in σL-dependent de novo transcription initiation at the expected TSS (with the initiating nucleotide base-pairing to template-strand ssDNA 2 nt upstream of dsDNA), and σL-dependent primer-dependent transcription initiation at the expected TSS (with the primer 3′ nucleotide base-pairing to template-strand ssDNA 2 nt upstream of dsDNA), with highest levels of function observed for a spacer length of 6 nt (Supplementary Fig. 1F, G). Robotic crystallization trials identified crystallization conditions yielding high-quality crystals for spacer lengths of 4 nt, 5 nt, or 6 nt (Table 1; Supplementary Fig. 2, center panels). X-ray datasets were collected at synchrotron beam sources, and structures were solved by molecular replacement and refined to 3.3–3.8 Å resolution (Table 1; Supplementary Fig. 2, right panels). Experimental electron-density maps showed clear density for RNAP, σL, and nucleic acids (Supplementary Fig. 2, right panels). The resulting structures were essentially identical for nucleic-acid scaffolds having spacer lengths of 4 nt, 5 nt, or 6 nt (Supplementary Fig. 2, right panels). However, map quality was highest for the nucleic-acid scaffold having a spacer length of 6 nt, and therefore subsequent analysis focussed on structures with a spacer length of 6 nt (Mtb RPitc5-σL_sp6). For the nucleic-acid scaffold containing a 6 nt spacer, the translocational state of the transcription complex was experimentally verified by preparation of a scaffold having a single 5-bromo-dU substitution and collection of bromine anomalous diffraction data (Table 1; Supplementary Fig. 2D). The fit of σL separately was experimentally verified by preparation of a selenomethionine-labeled σL derivative and collection of selenium anomalous diffraction data (Table 1; Supplementary Fig. 2E).
Interactions between ECF σ factor and RNAP
The structural organization of the ECF σL-factor-dependent transcription initiation complex is unexpectedly similar to that of a group-1 σA-factor-dependent transcription initiation complex (Figs. 1b and 2). σR2 and σR4 of σL occupy the same positions on RNAP, and make the same interactions with RNAP, as σR2 and σR4 of σA factor (Fig. 2). Despite the smaller size of the connector between σR2 and σR4 in σL as compared to σA (20 residues vs. 84 residues if one includes σR3; 20 residues vs. 28 residues if one does not include σR3; Supplementary Fig. 1A), the connector in σL spans the full distance between the σR2 and σR4 binding positions on RNAP and follows a path through RNAP similar to that of the connector in σA (Fig. 2). Thus, the σL σR2/4 linker, like the σA σR3/4 linker2–4,6,7,12–19, first enters the RNAP active-center cleft and approaches the RNAP active center, and then makes a sharp turn and exits the RNAP active-center cleft through the RNAP RNA-exit channel.
Fig. 2.
Protein–protein interactions between group-1 and ECF σ factors and RNAP core enzyme. a Protein–protein interactions by group-1 (σA) σ factor. b Protein–protein interactions by ECF (σL) σ factor. Colors are as in Fig. 1. See Supplementary Fig. 7
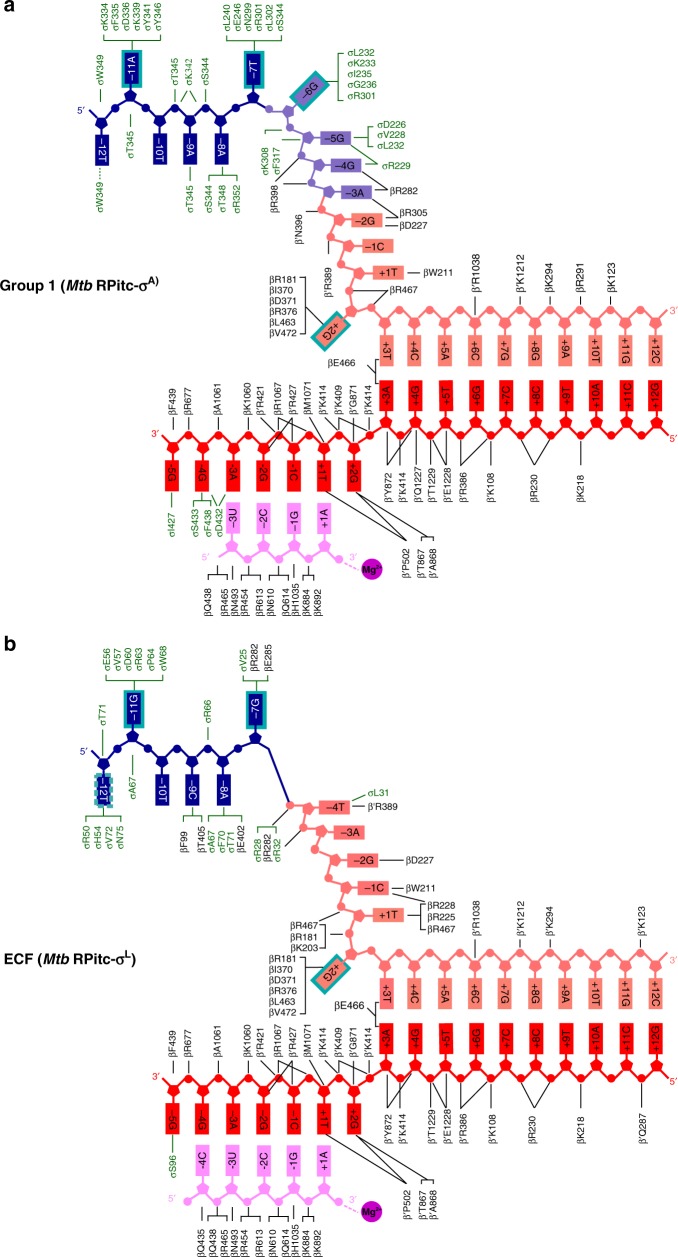
Inside the RNAP active-center cleft, the σL σR2/4 linker, like the σA σR3/4 linker6,7,14–19, makes direct interactions with template-strand ssDNA nucleotides of the unwound transcription bubble (Figs. 2–4, Supplementary Fig. 3A). The interactions of the σL σR2/4 linker with template-strand ssDNA include a direct H-bonded interaction of σL Ser96 with a Watson–Crick H-bonding atom of the template-strand nucleotide at promoter position -5 (Fig. 3b; Supplementary Fig. 3A, bottom). The interactions of the σL σR2/4 linker with template-strand ssDNA are similar to, but less extensive than, those of the σA σR3/4 linker with template-strand ssDNA, which include direct H-bonded interactions of σA Asp432 and Ser433 with Watson–Crick H-bonding atoms of template-strand ssDNA nucleotides at promoter positions -4 and -3 (Fig. 3a; Supplementary Fig. 3A).
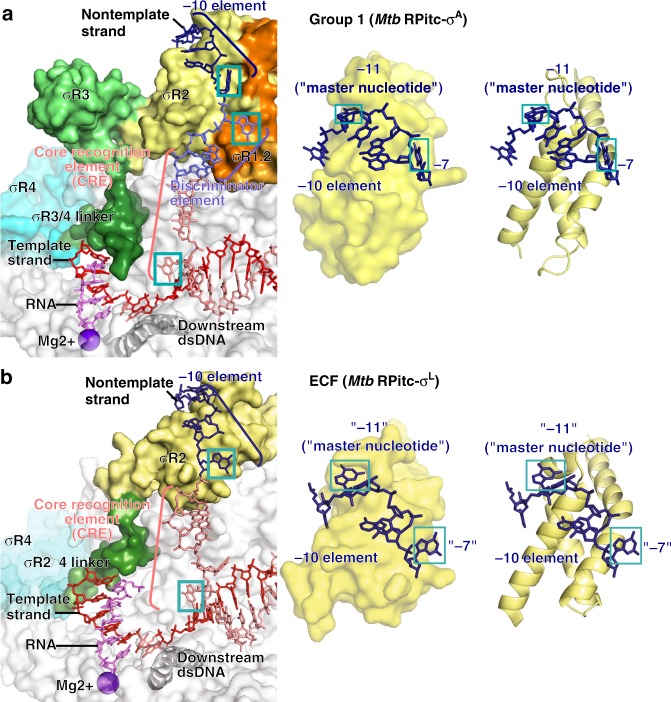
Fig. 4.
Protein–nucleic acid interactions by group-1 and ECF σ factors: interactions with transcription bubble. a Left: interactions of Mtb RNAP and σA with transcription-bubble nontemplate strand, transcription-bubble template strand, and downstream dsDNA. Right: interactions of σA σR2 with σA-dependent promoter -10 element. For promoter positions -11 (“master nucleotide”)40 and -7, bases are unstacked and inserted into pockets (cyan boxes). Colors are as in Figs. 1–3. b Left: interactions of Mtb RNAP and σL with transcription-bubble nontemplate strand, transcription-bubble template strand, and downstream dsDNA. Right: interactions of σL σR2 with σL-dependent promoter -10 element. For two promoter positions, here designated “-11” (“master nucleotide”) and “-7”, by analogy to corresponding nucleotides in group-1-σ-factor complex (panel a), bases are unstacked and inserted into pockets (cyan boxes). For one additional nucleotide, here designated “-12”, the base also appears to be unstacked and inserted into a pocket (dashed cyan box). See Supplementary Figs. 4, 5 and 7
Fig. 3.
Protein–nucleic acid interactions by group-1 and ECF σ factors: summary. a Summary of protein–nucleic acid interactions in Mtb RPitc-σA. Black residue numbers and lines, interactions by Mtb RNAP; green residue numbers and lines, interactions by Mtb σA; blue, -10 element of DNA nontemplate strand; light blue, discriminator element of DNA nontemplate strand; pink, rest of DNA nontemplate strand; red, DNA template strand; magenta, RNA product; violet circle, RNAP active-center Mg2+; cyan boxes, bases unstacked and inserted into protein pockets. Residues are numbered as in Mtb RNAP. b Summary of protein–nucleic acid interactions in Mtb RPitc-σL. Green residue numbers and lines, interactions by Mtb σL. Other colors are as in (a). See Supplementary Figs. 3–5 and 7
In the case of the group-1 σ factor, σA, the interactions between this segment of the σR3/4 linker and template-strand ssDNA pre-organize template-strand ssDNA to adopt a helical conformation and to engage the RNAP active-center nucleotide-addition site6, thereby facilitating initiating-nucleotide binding and de novo initiation2,5,6,8. The similarity of the interactions made by the ECF σ factor, σL, suggests that ECF σ factors likewise pre-organize template-strand ssDNA and facilitate initiating-nucleotide binding and de novo initiation.
In the case of the group-1 σ factor, σA, the interactions between this segment of the σR3/4 linker and template-strand ssDNA must be broken, and this segment of the σR3/4 linker must be displaced, when the nascent RNA reaches a length >4 nt during initial transcription, and this requirement for breakage of interactions and displacement is thought to impose an energy barrier that results in, or enhances, abortive initiation2,5,6,7,8 and initial-transcription pausing9–11. The similarity of the interactions made by the ECF σ factor, σL, suggests that ECF σ factors likewise have a similar requirement for displacement of a linker segment during initial transcription—in this case, when the nascent RNA reaches a length of >5 nt (Supplementary Fig. 3B)—and that this similar requirement imposes an energy barrier that results in, or enhances, abortive initiation and initial-transcription pausing. Consistent with this hypothesis, transcription and transcript-release experiments indicate that Mtb RNAP-σL holoenzyme efficiently performs abortive initiation, producing and releasing short abortive RNA products (Supplementary Fig. 3C).
The five C-terminal residues of the σL σR2/4 linker, like the ten C-terminal residues of the σA σR3/4 linker, exit the RNAP active-center cleft and connect to σR4 by threading through the RNAP RNA-exit channel (Fig. 2). In the case of the group-1 σ factor, σA, the C-terminal segment of the σR3/4 linker must be displaced from the RNA-exit channel when the nascent RNA reaches a length of 11 nt at the end of initial transcription and moves into the RNA-exit channel, and this displacement is thought to alter interactions between σR4 and RNAP, thereby triggering promoter escape and transforming the transcription initiation complex into a transcription elongation complex2–4. The similarity of the threading through the RNAP RNA-exit channel by the ECF σ factor, σL, suggests that ECF σ factors have a similar requirement for displacement of a linker C-terminal segment and have a similar mechanism of promoter escape and transformation from transcription initiation complexes into transcription elongation complexes.
In its interactions with template-strand ssDNA and the RNAP RNA-exit channel, the σL σR2/4 linker, like the σA σR3/4 linker2–4, appears to serve as a molecular mimic, or a molecular placeholder, for nascent RNA, making interactions with template-strand ssDNA and the RNAP RNA-exit channel in early stages of transcription initiation that subsequently, in late stages of transcription initiation and in transcription elongation, are made by nascent RNA. The σL σR2/4 linker and the σΑ σR3/4 linker, both have net negative charge (Supplementary Fig. 1A), and both employ extended conformations (fully extended for the σL σR2/4 linker; largely extended for the σΑ σR3/4 linker; Fig. 2) to interact with template-strand ssDNA and the RNAP RNA-exit channel, consistent with function as molecular mimics of a negatively charged, extended nascent RNA. Nevertheless, the σL σR2/4 linker and the σΑ σR3/4 linker exhibit no detectable sequence similarity (Supplementary Fig. 1A) and no detailed structural similarity (Fig. 2). We conclude that the σR2/4 linker of an ECF σ factor and the σR3/4 linker of a group-1 σ factor provide an example of functional analogy in the absence of structural homology.
Interactions between ECF σ factor and promoter -10 element
The structure reveals the interactions between the ECF σ factor, σL, and promoter DNA that mediate recognition of the promoter -10 element (Figs. 3–5; Supplementary Fig. 4). The σL conserved module σR2, like the σA conserved module σR2, mediates recognition of the promoter -10 element through interactions with nontemplate-strand ssDNA in the unwound transcription bubble (Figs. 3 and 4). In the case of the group-1 σ factor, σA, a crucial aspect of recognition of the promoter -10 element is unstacking of nucleotides, flipping of nucleotides, and insertion of nucleotides into protein pockets at two positions of the σA-dependent promoter6,39: i.e., position -11 (referred to as the “master nucleotide”, based on its especially important role in promoter recognition)40 and position -7 (Figs. 3a and 4a; Supplementary Fig. 4). The ECF σ factor, σL, unstacks nucleotides, flips nucleotides, and inserts nucleotides into protein pockets at the corresponding positions of the σL-dependent promoter (here designated positions “-11” and “-7”; Figs. 3b, 4b, and 5; Supplementary Fig. 4) and also unstacks and inserts a nucleotide into a protein pocket at one additional position of the σL-dependent promoter (position “-12”; Figs. 4b and 5).
Fig. 5.
Recognition by Mtb σL of σL-promoter -10 element: interactions with nontemplate strand positions “-12” through “-7”. For each promoter position, left subpanel shows DNA nucleotides in stick representation to highlight individual protein-nucleotide interactions, and right subpanel shows DNA nucleotide base moieties in space-filling representation to highlight protein-base steric complementarity. Yellow surfaces, solvent-accessible surfaces of Mtb σL σR2; gray surfaces, solvent-accessible surfaces of Mtb RNAP β subunit; dark blue surfaces, van der Waals surfaces of bases of σL-promoter -10 element; yellow ribbons, Mtb σL σR2 backbone; gray ribbons, Mtb RNAP β subunit backbone; yellow, yellow-blue, and yellow-red stick representations, σL carbon, nitrogen, and oxygen atoms, respectively; white, blue, red, and orange stick representations, DNA carbon, nitrogen, oxygen, and phosphorous atoms, respectively; blue dashed lines, H-bonds. Residues are numbered as in Mtb RNAP and σL, and, in parentheses, as in E. coli RNAP and σ70. See Supplementary Fig. 4
RNAP σL holoenzyme unstacks, flips, and inserts into a protein pocket a guanosine at position “-11” of the σL-dependent promoter, making extensive interactions with the base moiety of the guanosine, including multiple direct H-bonded interactions with Watson–Crick H-bonding atoms (Figs. 3, 4b, and 5; Supplementary Fig. 4A). The interactions between σL and guanosine at position “-11” of the σL-dependent promoter are similar to the interactions between RNAP σA holoenzyme and adenosine at position -11 of the σA-dependent promoter -10 element, including, in particular, similar stacking interactions of σL aromatic amino acid Trp68 with guanosine and of corresponding σA aromatic amino acid Tyr436 with adenosine (Supplementary Fig. 4A). The different specificities—guanosine at position “-11” for σL vs. adenosine at position -11 for σA—arise from differences in the H-bond-donor/H-bond-acceptor character of atoms forming the floors of the relevant protein pockets of σL and σA, with H-bonding complementarity to guanosine in σL and H-bonding complementarity to adenosine in σA (Supplementary Fig. 4A).
RNAP σL holoenzyme also unstacks, flips, and inserts into a protein pocket a guanosine at position “-7” of the σL-dependent promoter, making extensive interactions with the base moiety of the guanosine, including a direct H-bonded interaction with a Watson–Crick H-bonding atom (Figs. 3, 4b, and 5; Supplementary Fig. 4B). These interactions are similar in location to, but different in detail from, the interactions made by RNAP σA holoenzyme with thymidine at position -7 of the σA-dependent promoter (Fig. 4; Supplementary Fig. 4B). The differences in detail arise from the fact that σL does not contain conserved module σR1.2. In the case of σL, the interactions involve residues of σR2 and residues of RNAP β subunit, with the base moiety of the guanosine at position “-7” being inserted into a cleft between σR2 and β (Fig. 5; Supplementary Fig. 4B). In contrast, in the case of σA, the interactions involve residues of σR2 and residues of σR1.2, with the base moiety of the thymidine at position -7 being inserted into a cleft between σR2 and σR1.2 (Supplementary Fig. 4B).
RNAP σL holoenzyme also appears to unstack and insert into a protein pocket a thymidine at position “-12” of the σL-dependent promoter (Figs. 4b and 5), placing one face of the base moiety of the thymidine in a shallow surface pocket, in position to make a direct H-bonded interaction with a Watson–Crick atom (Fig. 5). The interaction with an unstacked nucleotide inserted into a protein pocket implies that position “-12” of the σL-dependent promoter must be ssDNA in RPo-σL and RPitc-σL, and thus that the transcription bubble must extend to position “-12” in RPo-σL and RPitc-σL. This interaction does not have a counterpart in the σA-dependent transcription initiation complex, in which position -12 of the σA-dependent promoter is dsDNA and in which the transcription bubble extends only to position -1115–19.
In addition to these potential specificity-determining interactions with unstacked nucleotides inserted into protein pockets, RNAP σL holoenzyme makes potentially specificity-determining interactions with positions “-9” and “-8” of the σL-dependent promoter (Fig. 5). RNAP σL holoenzyme makes a direct H-bonded interaction, through RNAP β subunit, with a Watson–Crick atom of the base moiety of cytidine at position “-9” (Fig. 5) and makes two direct H-bonded interactions, through σR2 and RNAP β subunit, with a Watson–Crick atom of the base moiety of adenosine at position “-8” (Fig. 5).
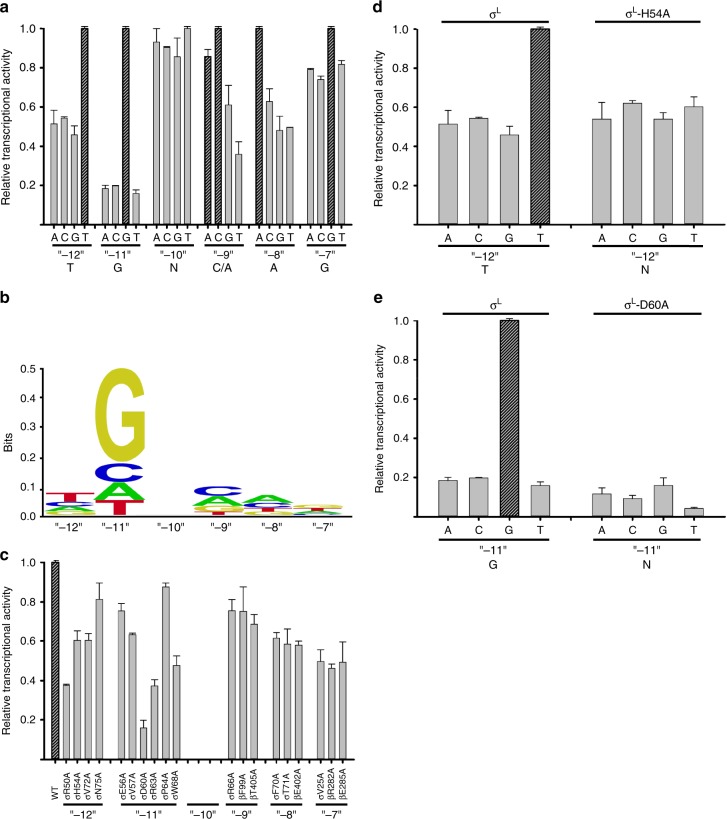
Biochemical experiments assessing effects of all possible single-base-pair substitutions at each position of the P-sigL promoter -10 region confirm the functional significance of the positions contacted in the crystal structure (positions “-12”, “-11”, “-9”, “-8”, and “-7”; Fig. 6a), confirm the sequence preferences at these positions inferred from the crystal structure (Fig. 6a), and yield a revised consensus sequence for the σL-dependent -10 element of T“-12”-G“-11”-N“-10”-C/A“-9”-A“-8”-G“-7” (Fig. 6b). The revised consensus sequence for the σL-dependent -10 element matches the literature consensus sequence33–36 in its first four positions (T“-12”-G“-11”-N“-10”-C/A“-9”) and extends the literature consensus sequence for two additional positions (A“-8”-G“-7”). Consistent with the observed extensive network of H-bonded interactions involving position “-11G” (Figs. 3 and 5; Supplementary Fig. 4A), specificity is observed to be strongest at position “-11” (Fig. 6a, b). Three of four characterized Mtb σL-dependent promoters36,37 match the consensus sequence of Fig. 6b at position “-11” (P-sigL, P-pks10, and P-Rv1139c); two of four match at newly defined position “-8” (P-sigL and P-pks10), and two of four match at newly defined position “-7” (P-sigL and P-Rv1139c).
Fig. 6.
Recognition by Mtb σL of σL-promoter -10 element: experimental data. a Systematic-substitution experiments defining σL-dependent promoter -10-element consensus sequence. Relative transcriptional activities of derivatives of σL-dependent promoter P-sigL having all possible single base-pair substitutions at each position of promoter -10 element, “-12” through “-7”. Inferred consensus nucleotides are shown at the bottom, and data for inferred consensus nucleotides are hatched. Error bars, SE (N = 3). b Sequence logo for σL-promoter -10-element consensus sequence [generated using transcription data from (a) and enoLOGOS71 (http://biodev.hgen.pitt.edu/enologos/); input setting “energy (2)” and weight-type setting “probabilities”]. c Alanine-scanning experiments41 demonstrating functional importance of observed amino acid-base interactions in recognition of σL-promoter -10 element. Effects on transcription of alanine substitutions of σL amino acids that contact σL-dependent promoter -10 element, positions “-12” through “-7” (identities of contacting amino acids from Figs. 3 and 5). d, e Loss-of-contact experiments42–45 indicating that σL residues His54 and Asp60 determine specificity at position “-12” and “-11”, respectively. Left: transcriptional activity with wild-type σL for all possible single base-pair-substitutions at indicated position (strong specificity for consensus base pair). Right: transcriptional activity of σL derivatives having alanine substitutions (no specificity for consensus base pair). Error bars, SE (N = 3). See Supplementary Fig. 6. Source data are provided as a Source Data file
“Alanine-scanning” experiments41, in which residues of σL that contact -10-element nucleotides in the crystal structure are substituted with alanine and effects on σL-dependent transcription are quantified, confirm the functional significance of the observed interactions (Fig. 6c).
“Loss-of-contact” experiments42–45, in which residues of σL that contact -10-element nucleotides in the crystal structure are substituted with alanine and effects on specificity at the contacted positions are quantified, confirm that σL His54 contributes to specificity for thymidine at position “-12” (Fig. 6d) and that σL Asp60 contributes to specificity for guanosine at position “-11” (Fig. 6e). In the crystal structure, σL His54 makes a van der Waals interaction with the 5′-methyl group of the base moiety of thymidine at position “-12” (Fig. 5); in loss-of-contact experiments, substitution of His54 by alanine eliminates specificity for thymidine at position “-12” (Fig. 6d). In the crystal structure, σL Asp60 makes an H-bonded interaction with Watson–Crick atoms of the base moiety of guanosine at position “-11” (Fig. 5; Supplementary Fig. 4A); in loss-of-contact experiments, substitution of Asp60 by alanine eliminates specificity for guanosine at position “-11” (Fig. 6e).
Interactions between ECF σ factor and promoter CRE
The structure reveals the interactions between RNAP σL holoenzyme and nontemplate-strand ssDNA downstream of the promoter -10 element in the ECF σL-dependent transcription initiation complex (Fig. 3b; Supplementary Fig. S5). In the case of group-1-σ-factor-dependent transcription initiation complexes, sequence-specific interactions occur between RNAP β subunit and a 6 nt segment of nontemplate-strand ssDNA downstream of the promoter -10 element referred to as the “core recognition element” (CRE; positions −6 through +2)6,19,46. These interactions include, most notably, (1) stacking of a tryptophan residue of RNAP β subunit on the base moiety of thymidine at nontemplate-strand position +1 (Supplementary Fig. 5A), and (2) unstacking, flipping, and insertion into a protein pocket, formed by the RNAP β subunit, of the guanosine at nontemplate-strand position +2 (Figs. 3 and 4a; Supplementary Fig. 5B). The identical interactions occur in the ECF σL-dependent transcription initiation complex (Figs. 3 and 4b; Supplementary Fig. 5).
Biochemical experiments assessing effects of all possible base-pair substitutions at positions downstream of the P-sigL promoter -10 element (positions −4 through +2) confirm the functional significance of the interactions in the crystal structure with thymidine at position +1 and guanosine at position +2 (Supplementary Fig. 6A) and yield a CRE consensus sequence for an ECF σL-dependent transcription initiation complex (Supplementary Fig. 6B) similar to the CRE consensus sequence for a group-1-σ-factor-dependent transcription initiation complex6,46. Three of four characterized Mtb σL-dependent promoters36,37 match the CRE consensus sequence of Supplementary Fig. 6B at position +2, the position at which a guanosine is unstacked, flipped, and inserted into a protein pocket (P-sigL, P-pks10, and P-Rv1139c).
Discussion
Our structural results show that: (1) σR2 and σR4 of an ECF σ factor σL adopt the same folds and interact with the same sites on RNAP as σR2 and σR4 of a group-1 σ factor (Figs. 1 and 2); (2) the connector between σR2 and σR4 of ECF σ factor σL enters the RNAP active-center cleft to interact with template-strand ssDNA and then exits the RNAP active-center cleft by threading through the RNAP RNA-exit channel in a manner functionally analogous—but not structurally homologous—to the connector between σR2 and σR4 of a group-1 σ factor (Figs. 1 and 2; Supplementary Fig. 3); (3) ECF σ factor σL recognizes the -10 element of a σL-dependent promoter by unstacking nucleotides and inserting nucleotides into protein pockets at three positions of the transcription-bubble nontemplate-strand ssDNA (positions “-12”, “-11”, and “-7”; Figs. 3–5; Supplementary Fig. 4), and (4) RNAP recognizes the CRE of a σL-dependent promoter by stacking a nucleotide on a tryptophan and by unstacking, flipping, and inserting a nucleotide into a protein pocket (positions +1 and +2; Figs. 3 and 4; Supplementary Fig. 5). Our biochemical results confirm the functional significance of the observed protein–DNA interactions with the -10 element and CRE of a σL-dependent promoter (Fig. 6a; Supplementary Fig. 6A), provide consensus sequences for the -10 element and CRE of a σL-dependent promoter (Fig. 6b; Supplementary Fig. 6B), and define individual specificity-determining amino-acid–base interactions for two positions of the -10 element of a σL-dependent promoter (positions “-12” and “-11”; Fig. 6c, d). The results provide an indispensable foundation for understanding the structural and mechanistic basis of ECF-σ-factor-dependent transcription initiation.
Our results regarding the connector between σR2 and σR4 of an ECF σ factor, in conjunction with previous results, indicate that all classes of bacterial σ factors contain structural modules that enter the RNAP active-center cleft to interact with template-strand ssDNA and then leave the RNAP active-center cleft by threading through the RNAP RNA-exit channel, providing mechanisms to facilitate de novo initiation, to coordinate extension of the nascent RNA with abortive initiation and initial-transcription pausing, and to coordinate entry of RNA into the RNA-exit channel with promoter escape. For ECF σ factors, as shown here, the relevant structural module is the σR2/4 linker (Figs. 3 and 4; Supplementary Fig. 3); for group-1, group-2, and group-3 σ factors, the module is the functionally analogous—but not structurally homologous—σR3/4 linker2,3,6,7,12–19,22; and for group-σ54/σN σ factors, the module is the functionally analogous—but not structurally homologous—region II.3 (RII.3)20,21.
More broadly, our results, in conjunction with previous results, indicate that cellular transcription initiation complexes in all organisms—bacteria, archaea, and eukaryotes—contain structural modules that enter the RNAP active-center cleft to interact with template-strand ssDNA and then leave the RNAP active-center cleft by threading through the RNAP RNA-exit channel. In different classes of bacterial transcription initiation complexes, as described in the preceding paragraph, these roles are performed by the functionally analogous—but not structurally homologous—σR2/4 linker, σR3/4 linker, and RII.32,3,6,7,12–22. In archaeal transcription initiation complexes, these roles are performed by the TFB zinc ribbon and CSB, which are unrelated to the σR2/4 linker, σR3/4 linker, and RII.347. In eukaryotic RNAP-I-, RNAP-II-, and RNAP-III-dependent transcription initiation complexes, these roles are performed by the Rrn7 zinc ribbon and B-reader, the TFIIB zinc ribbon and B-reader, and the Brf1 zinc ribbon, respectively, each of which is unrelated to the σR2/4 linker, σR3/4 linker, and RII.348–56. It is extraordinary that non-homologous, structurally and phylogenetically unrelated, structural modules are used to perform the same roles in different transcription initiation complexes, and is unknown how or why this occurs.
Our results define the protein–DNA interactions that ECF σ factor σL uses to recognize the -10 element of a σL-dependent promoter. The consensus sequence obtained in this work for the 10-element of a σL-dependent promoter, T“-12”-G“-11”-N“-10”-C/A“-9”-A“-8”-G“-7” (Fig. 6b), confirms and extends the literature-consensus sequence33–36, and the structural data of this work account for specificity at each specified position of the consensus sequence (Figs. 3–5; Supplementary Fig. 4).
Previous work indicates that RNAP-σL holoenzyme prefers a C-G sequence immediately upstream of the -10-element (C“-14”-G“-13” in our numbering system)33–36. Further previous work indicates that this preference may be shared by many RNAP-ECF-σ-factor holoenzymes25,29,32–35,38,57; for example, at least 8 of 10 Mtb RNAP-ECF-σ-factor holoenzymes—Mtb RNAP-σC, -σD, -σE, -σG, -σH, -σJ, -σL, and -σM holoenzymes—exhibit this preference32–36,38. In this work, we performed crystallization using nucleic-acid scaffolds that did not contain C“-14”-G“-13”, and therefore our crystal structures do not definitively account for the preference for C“-14”-G“-13”. However, with the assumption that template-strand nucleotides at positions “-14” and “-13” of a σL-dependent transcription initiation complex are positioned similarly to those in a group-1-σ-factor-dependent transcription initiation complex15–18, our crystal structures suggest that the C-terminal α-helix of σL σR2 (σL residues 78–82) potentially could make direct, specificity-determining contacts with template-strand nucleotides at these positions. A similar mechanism for recognition of C-G immediately upstream of the -10 element has been proposed for the group-3 σ factor E. coli σ2858.
Both our structural results and biochemical results point to the special importance of the nontemplate-strand nucleotide at position “-11” (“master nucleotide”; Figs. 3–6; Supplementary Fig. 4A). Our results regarding recognition of the “-11” “master nucleotide” by an ECF σ factor are consistent with the NMR structure of a complex comprising σR2 from the E. coli ECF σ factor σE and a 5 nt oligodeoxyribonucleotide corresponding to part of the nontemplate strand of the -10 element of a σE-dependent promoter28,59. The NMR structure showed unstacking, flipping, and insertion into a protein pocket of the “-11” “master nucleotide” (a cytidine, rather than a guanosine, reflecting the different specificities of E. coli σE vs. Mtb σL)28,59. The NMR structure did not show unstacking and flipping of the nucleotide at position “-7”, reflecting the fact that the oligodeoxyribonucleotide in the NMR structure did not extend to position “-7”28,59. The NMR structure also did not show unstacking of the nucleotide at position “-12”28,59, possibly reflecting an uncertainty in the NMR structure, or possibly reflecting a difference between E. coli σE and Mtb σL in recognition of position “-12”.
Based on the NMR structure, Campagne et al.28,59 hypothesized that the loop of σR2 that forms the protein pocket into which the “-11” “master nucleotide” is inserted—“loop L3” (residues 63-72 of E. coli σE, which correspond to residues 56-67 of Mtb σL)—serves as a functionally independent, modular determinant of specificity at the “master-nucleotide” position, such that different loop-L3 sequences confer different specificities at the “master-nucleotide” position, in each case, through interactions with an unstacked, flipped, and inserted “master nucleotide”. Campagne et al.28,59 supported this hypothesis by identifying examples of L3-loop sequences that conferred specificity for cytidine, thymidine, and adenosine at the “master-nucleotide” position, and by providing evidence that swapping L3-loop sequences swaps specificity at the “master-nucleotide” position. Our results provide further support for the hypothesis by identifying an example of an L3-loop sequence, the Mtb σL loop-L3 sequence, that confers specificity for guanosine at the “master-nucleotide” position, and by documenting that specificity for guanosine involves interactions with an unstacked, flipped, and inserted “master nucleotide” (Figs. 3–5; Supplementary Fig. 4A).
In the crystal form identified and analyzed in this work, σR2 of each molecule of transcription initiation complex makes no interactions with other molecules of transcription initiation complex in the crystal lattice (Supplementary Fig. 7A), and, therefore, with this crystal form, it may be possible to substitute σR2 without losing the ability to form crystals (Supplementary Fig. 7A). This potentially provides a platform for systematic structural analysis of σR2 and σR2-DNA interactions for the 13 Mtb σ factors, by determination of crystal structures of transcription initiation complexes containing chimeric σ factors57,60 comprising σR2 of a Mtb σ factor of interest fused to the σR2/4 linker through σR4 of Mtb σL (Supplementary Fig. 7B; left red arrow) and containing the promoter sequence for the Mtb σ factor of interest. In the crystal form identified and analyzed in this work, there also are no lattice interactions for the connector between σR2 and σR4, and it appears likely there would be no lattice interactions even if that connector were to contain σR3 and a σR3/4 linker, as in group-1, group-2, and group-3 σ factors (Supplementary Figure 7A). Accordingly, this crystal form potentially provides a platform for systematic structural analysis not only of σR2 and its protein-DNA interactions, but also of the connector between σR2 and σR4 and its protein-DNA interactions, for the 13 Mtb σ factors, by determination of crystal structures of transcription initiation complexes containing chimeric σ factors comprising σR2 and the connector of one Mtb σ factor fused to σR4 of Mtb σL (Supplementary Figure 7B, right red arrow) and containing the promoter sequence for the Mtb σ factor of interest.
Methods
M. tuberculosis RNAP core enzyme
Mtb RNAP core enzyme was prepared by co-expression of genes for Mtb RNAP β′ subunit, RNAP β subunit, N-terminally decahistidine-tagged RNAP α subunit, and RNAP ω subunit in E. coli [plasmids pACYC-rpoA, pCOLADuet-rpoB-rpoC, and pCDF-rpoZ61 (gift of J. Mukhopadhyay, Bose Institute, Kolkata, India); E. coli strain BL21(DE3) (Invitrogen)], followed by cell lysis, polyethylenimine precipitation, ammonium sulfate precipitation, immobilized-metal-ion affinity chromatography on Ni-NTA agarose (Qiagen), and anion-exchange chromatography on Mono Q (GE Healthcare), as described19.
M. tuberculosis RNAP σA
Mtb RNAP σA was prepared by expression of a gene for N-terminally hexahistidine-tagged Mtb σA in E. coli [plasmid pET30a-Mtb-σA 62 (gift of S. Rodrigue, Universitė de Sherbrooke, Canada); E. coli strain BL21(DE3) (Invitrogen)], followed by cell lysis, immobilized-metal-ion affinity chromatography on Ni-NTA agarose (Qiagen), and anion-exchange chromatography on Mono Q (GE Healthcare), as described19.
M. tuberculosis σL
Mtb RNAP σL was prepared by expression of a gene for N-terminally hexahistidine-tagged Mtb σL in E. coli, followed by cell lysis, immobilized-metal-ion affinity chromatography on Ni-NTA agarose (Qiagen), and anion-exchange chromatography on Mono Q (GE Healthcare), as follows. E. coli strain BL21(DE3) (Invitrogen) was transformed with plasmid pSR3262 (gift of S. Rodrigue, Universitė de Sherbrooke, Canada), encoding N-terminally hexahistidine-tagged Mtb σL under control of the bacteriophage T7 gene 10 promoter. Single colonies of the resulting transformants were used to inoculate 50 ml LB broth containing 50 μg/ml kanamycin, and cultures were incubated 16 h at 37 °C with shaking. Aliquots (10 ml) were used to inoculate 1 L LB broth containing 50 μg/ml kanamycin, cultures were incubated at 37 °C with shaking until OD600 = 0.8, cultures were induced by addition of isopropyl-β-D-thiogalactoside to 1 mM, and cultures were further incubated 16 h at 16 °C. Cells were harvested by centrifugation (4000×g; 15 min at 4 °C), re-suspended in buffer A (10 mM Tris–HCl, pH 7.9, 300 mM NaCl, 5 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, and 5% glycerol), and lysed using an EmulsiFlex-C5 cell disruptor (Avestin). The lysate was centrifuged (20,000×g; 30 min at 4 °C), the pellet was re-suspended in buffer B (8 M urea, 10 mM Tris–HCl, pH 7.9, 10 mM MgCl2, 10 mM ZnCl2, 1 mM EDTA, 10 mM DTT and 10% glycerol), and the suspension was further centrifuged (20,000×g; 30 min at 4 °C). The supernatant was loaded onto a 5 ml column of Ni2+-NTA-agarose (Qiagen) pre-equilibrated in buffer B, and the column was washed with 9 × 15 ml buffer B containing 5, 10, 20, 30, 40, 50, 60, 70, and 80 mM imidazole, and eluted with 50 ml buffer B containing 200 mM imidazole. The sample was subjected to step dialysis for renaturation [10 kDa MWCO Amicon Ultra-15 centrifugal ultrafilters (EMD Millipore); dialysis 4 h at 4 °C against 8 volumes 50% (v/v) buffer C (10 mM Tris–HCl, pH 7.9, 200 mM NaCl, 1 mM DTT, 0.1 mM EDTA, and 5% glycerol) in buffer B; dialysis 4 h at 4 °C against 8 volumes 75% (v/v) buffer C in buffer B; dialysis 4 h at 4°C against 8 volumes 87.5% (v/v) buffer C in buffer B; and dialysis 4 h at 4 °C against 8 volumes buffer C]; further purified by gel filtration chromatography on a HiLoad 16/60 Superdex 200 prep grade column (GE Healthcare) in 20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, and 1 mM 2-mercaptoethanol; concentrated to 10 mg/ml in the same buffer using 10 kDa MWCO Amicon Ultra-15 centrifugal ultrafilters (EMD Millipore); and stored in aliquots at −80 °C. Yields were ~5 mg/L, and purities were ~95%.
Alanine-substituted σL derivatives were prepared as described above for the preparation of σL, but using plasmid pSR32 derivatives constructed using site-directed mutagenesis (QuikChange Site-Directed Mutagenesis Kit; Agilent).
Selenomethionine-substituted σL was prepared as described above for the preparation of σL, but using production cultures in 2 L SelenoMethionine Medium Base plus Nutrient Mix63 (Molecular Dimensions) containing 50 μg/ml kanamycin, incubating at 37 °C with shaking until OD600 = 0.8, adding L-selenomethionine (Molecular Dimensions) to 0.3 mM and incubating 15 min at 37 °C with shaking, and adding isopropyl-β-D-thiogalactoside to 0.5 mM IPTG and incubating 16 h at 16 °C with shaking.
M. tuberculosis RNAP σA holoenzyme
Mtb RNAP σA holoenzyme was prepared by co-expression of genes for Mtb RNAP β′ subunit, RNAP β subunit, N-terminally decahistidine-tagged RNAP α subunit, RNAP ω subunit, and N-terminally hexahistidine-tagged σA in E. coli [plasmids pACYC-rpoA-sigA, pCOLADuet-rpoB-rpoC, and pCDF-rpoZ61 (gift of J. Mukhopadhyay, Bose Institute, Kolkata, India); E. coli strain BL21 (DE3) (Invitrogen, Inc.)], followed by use of cell lysis, polyethylenimine precipitation, ammonium sulfate precipitation, immobilized-metal-ion affinity chromatography on Ni-NTA agarose (Qiagen), and anion-exchange chromatography on Mono Q (GE Healthcare), as described19.
M. tuberculosis RNAP σL holoenzyme
Mtb RNAP core enzyme and Mtb σL or σL derivative were incubated in a 1:4 ratio in 20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, and 1 mM 2-mercaptoethanol for 12 h at 4 °C. The reaction mixture was applied to a HiLoad 16/60 Superdex S200 column (GE Healthcare) equilibrated in the same buffer, and the column was eluted with 120 ml of the same buffer. Fractions containing Mtb RNAP σL holoenzyme were pooled, concentrated to ~10 mg/ml using 30 kDa MWCO Amicon Ultra-15 centrifugal ultrafilters (EMD Millipore), and stored in aliquots at −80 °C.
Oligonucleotides
Oligodeoxyribonucleotides (Integrated DNA Technologies) and the pentaribonucleotide 5′-CpUpCpGpA-3′ (TriLink) were dissolved in nuclease-free water (Ambion) to 3 mM and were stored at −80 °C.
Nucleic-acid scaffolds
Nucleic-acid scaffolds RPitc5_sp4, RPitc5_sp5, RPitc5_sp6, and RPo_sp6 (sequences in Supplementary Fig. 2) were prepared as follows: nontemplate-strand oligodeoxyribonucleotide (0.5 mM), template-strand oligodeoxyribonucleotide (0.55 mM), and, where indicated, pentaribonucleotide (1 mM) in 40 µl 20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, and 1 mM 2-mercaptoethanol, were heated 5 min at 95 °C, cooled to 25 °C in 2 °C steps with 1 min per step using a thermal cycler (Applied Biosystems), and stored at −80 °C.
Transcription initiation complexes
Transcription initiation complexes were assembled by mixing 16 µl 50 µM Mtb RNAP σL holoenzyme (in 20 mM Tris–HCl, pH 8.0, 75 mM NaCl, 5 mM MgCl2, and 5 mM dithiothreitol) and 4 µl 0.4 mM nucleic-acid scaffold (previous section) in 5 mM Tris–HCl, pH 7.7, 0.2 M NaCl, and 10 mM MgCl2, and incubating 1 h at 25 °C.
Crystallization, cryo-cooling, and crystal soaking
Robotic crystallization trials were performed for Mtb RPitc5-σL_sp6 using a Gryphon liquid handling system (Art Robbins Instruments), commercial screening solutions (Emerald Biosystems, Hampton Research, and Qiagen), and the sitting-drop vapor-diffusion technique (drop: 0.2 µl transcription initiation complex (previous section) plus 0.2 µl screening solution; reservoir: 60 µl screening solution; 22 °C). 900 conditions were screened. Under several conditions, Mtb RPitc5-σL_sp6 crystals appeared within 2 weeks. Conditions were optimized using the hanging-drop vapor-diffusion technique at 22 °C. The optimized conditions for Mtb RPitc5-σL_sp6 (drop: 1 µl 40 µM Mtb RPitc5-σL_sp6 in 20 mM Tris–HCl, pH 8.0, 75 mM NaCl, 5 mM MgCl2, and 5 mM dithiothreitol plus 1 µl 100 mM sodium citrate, pH 5.5, 200 mM sodium acetate, and 10% PEG4000; reservoir: 400 µl 100 mM sodium citrate, pH 5.5, 200 mM sodium acetate, and 10% PEG4000; 22 °C) yielded high-quality, rod-like crystals with dimensions of 0.4 mm × 0.1 mm × 0.1 mm in 2 weeks (Supplementary Fig. 2). Crystals were transferred to reservoir solution containing 18% (v/v) (2 R,3 R)-(-)-2,3-butanediol (Sigma-Aldrich) and flash-cooled with liquid nitrogen. Analogous procedures were used for Mtb RPitc5-σL_sp4, RPitc5-σL_sp5, RPitc-σL_sp6, [BrU]RPo-σL_sp6, and [SeMet15,76] RPo-σL_sp6.
Diffraction data collection
Diffraction data were collected from cryo-cooled crystals at Argonne National Laboratory beamline 19ID-D and Stanford Synchrotron Radiation Lightsource SSRL-9-2. Data were processed using HKL200064. The resolution cut-off criteria were: (i) I/σ > = 1.0, (ii) CC1/2 (highest resolution shell) >0.5.
Structure determination and structure refinement
The structure of Mtb RPitc5-σL_sp6 was solved by molecular replacement with MOLREP65 using the structure of Mtb RPo (PDB 5UHA)19, omitting σA and nucleic acids, as the search model. One molecule of RNAP was present in the asymmetric unit. Early-stage refinement included rigid-body refinement of RNAP core enzyme, followed by rigid-body refinement of each subunit of RNAP core enzyme, followed by rigid-body refinement of 38 domains of RNAP core enzyme (methods as described6). Electron density for σL and nucleic acids was unambiguous, but was not included in models in early-stage refinement. Cycles of iterative model building with Coot66 and refinement with Phenix67 then were performed. Improvement of the coordinate model resulted in improvement of phasing, and electron density maps for σL and nucleic acids, which were not included in models at this stage, improved over successive cycles. σL and nucleic acids then were built into the model and refined in a stepwise fashion. The final model was generated by XYZ-coordinate refinement with secondary-structure restraints, followed by group B-factor and individual B-factor refinement. The final model, refined to Rwork and Rfree of 0.19 and 0.23, respectively, was deposited in the PDB with accession code 6DVC (Table 1).
Analogous procedures were used to solve and preliminarily refine structures of Mtb RPitc5-σL_sp4, RPitc5-σL_sp5, RPitc5-σL_sp6, and [BrU]RPo-σL_sp6; models of σL and nucleic acids then were built into mFo-DFc difference maps, and additional cycles of refinement and model building were performed. The final models were deposited in the PDB with accession codes 6DV9, 6DVB, and 6DVD (Table 1).
Analogous procedures were used to solve and preliminarily refine the structure of [SeMet15,76]RPo-σL_sp6; selenium anomalous signals then were used to determine positions of σL SeMet15 and σL SeMet76, and to confirm the register of σL protein residues. The final model was deposited in the PDB with accession code 6DVE (Table 1).
Distance cut-offs for assignment of H-bonds and van der Waals interactions were 3.5 and 4.5 Å, respectively.
Transcription assays
For transcription experiments in Fig. 6 and Supplementary Figs. 1E and 6, reaction mixtures contained (10 µl): 75 nM Mtb RNAP σL holoenzyme or Mtb RNAP σL holoenzyme derivative, 25 nM DNA fragment P-N25-lac [5′-GCCGCC-3′, followed by positions −100 to −1 of bacteriophage T5 N25 promoter68, followed by positions +1 to +9 of E. coli P-lac69, followed by 5′-AGGATCACAATTTCACACAG-3′; prepared by annealing synthetic oligodeoxyribonucleotides, followed by PCR amplification] or DNA fragment P-sigL-lac or single-base-pair-substituted derivative thereof [5′-GCCGCC-3′, followed by positions −100 to −1 of Mtb P-sigL36–38 or single-base-pair-substituted derivative thereof, followed by positions +1 to +9 of E. coli P-lac69, followed by 5′-AGGATCACAATTTCACACAG-3′; prepared by annealing synthetic oligodeoxyribonucleotides, followed by PCR amplification], 100 µM [α32P]-UTP (0.03 Bq/fmol), 100 µM ATP, and 100 µM GTP in transcription buffer (40 mM Tris–HCl, pH 8.0, 75 mM NaCl, 5 mM MgCl2, 2.5 mM DTT, and 12.5% glycerol). Reaction components other than DNA and nucleotides were pre-incubated 5 min at 22 °C; DNA was added and reaction mixtures were incubated 5 min at 37 °C; and nucleotides were added and reaction mixtures were further incubated 5 min at 37 °C. Reactions were terminated by addition of 2 µl loading buffer (80% formamide, 10 mM EDTA, 0.04% bromophenol blue, and 0.04% xylene cyanol). Products were heated 5 min at 95 °C, cooled 5 min on ice, and applied to 16% polyacrylamide (19:1 acrylamide:bisacrylamide, 7 M urea) slab gels (Bio-Rad), electrophoresed in TBE (90 mM Tris–borate, pH 8.0, and 0.2 mM EDTA), and analyzed by storage-phosphor scanning (Typhoon: GE Healthcare). Relative transcriptional activities were calculated from yields of full-length RNA products.
Transcription experiments in Supplementary Fig. 1F were performed in the same manner as transcription experiments in Fig. 6 and Supplementary Figs. 1E and 6, but using reaction mixtures containing (10 μl): 600 nM Mtb RNAP σL holoenzyme, 400 nM annealed nontemplate and template strands of nucleic-acid scaffolds RPitc5_sp4, RPitc5_sp5, RPitc_sp6, and RPitc5_sp7 (sequences in Supplementary Fig. 2 for RPitc5_sp4, RPitc5_sp5, RPitc_sp6; 5′-CGTGTCAGTAAGCTGTCACGGATGCAGG-3′ and 5′-CCTGCATCCGTGAGTCGAGGG-3′ for RPitc5_sp7), 1 mM [α32P]-UTP (0.003 Bq/fmol), 1 mM ATP, and 1 mM CTP in transcription buffer.
Transcription experiments in Supplementary Fig. 1G were performed in the same manner as transcription experiments in Fig. 6 and Supplementary Figs. 1E and 6, but using reaction mixtures containing (10 μl): 75 nM Mtb RNAP σL holoenzyme, 25 nM annealed nontemplate and template strands of nucleic-acid scaffolds RPitc5_sp4, RPitc5_sp5, RPitc_sp6, and RPitc5_sp7 (sequences as in preceding paragraph), 500 μM GpA (added together with nucleotides), 100 µM [α32P]-UTP (0.03 Bq/fmol), and 100 μM CTP in transcription buffer.
Transcription experiments in Supplementary Fig. 3C (left panel and lanes 1–2 in right panel) were performed in the same manner as transcription experiments in Fig. 6 and Supplementary Figs. 1E and 6, but including 500 μM ApA (TriLink) in reaction mixtures (added together with nucleotides).
Transcript-release assays
Transcript-release assays70 (Supplementary Fig. 3B, right panel, lanes 3–4) were performed by carrying out transcription experiments with transcription complexes immobilized on streptavidin-coated magnetic beads, dividing reaction mixtures into supernatants and pellets by magnetic partitioning, and analyzing transcripts in supernatants (released transcripts) and pellets (unreleased transcripts). Reaction mixtures contained (50 µl): 75 nM Mtb RNAP σL holoenzyme, 25 nM DNA fragment biotin-P-sigL-lac immobilized on streptavidin-coated magnetic beads [prepared by mixing 1.25 pmol biotinylated DNA fragment (biotin incorporated at 5′ end of nontemplate-strand oligodeoxyribonucleotide during synthesis) and 0.05 mg Streptavidin MagneSphere Paramagnetic Particles (Promega; pre-washed with 3 × 150 µl transcription buffer) in 100 µl transcription buffer 30 min at 22 °C, and performing three cycles of removal of supernatant by magnetic partitioning followed by re-suspension in 150 μl transcription buffer at 22 °C], 500 µM ApA, 100 μM [α32P]-UTP (0.03 Bq/fmol), 100 µM ATP, and 100 μM GTP in transcription buffer. Reaction components other than bead-immobilized DNA, ApA, and NTPs were pre-incubated 5 min at 22 °C; bead-immobilized DNA was added and reaction mixtures were incubated 5 min at 37 °C; and ApA and NTPs were added and incubated 5 min at 37 °C. Reaction mixtures were separated into supernatants and pellets by magnetic partitioning. Supernatants were mixed with 10 µl loading buffer, heated 5 min at 95 °C, cooled 5 min on ice, and analyzed by urea-PAGE and storage-phosphor imaging as in the preceding section. Pellets were washed with 3 × 200 µl transcription buffer at 22 °C; mixed with 50 µl loading buffer, heated 5 min at 95 °C, cooled 5 min on ice, and analyzed by urea-PAGE and storage-phosphor imaging as in the preceding section.
Data analysis
Data for transcription activities are means of at least three technical replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by NIH Grant GM041376 to R.H.E. The authors thank J. Mukhopadhyay and S. Rodrigue for plasmids and APS at Argonne National Laboratory and Stanford Synchrotron Radiation Lightsource for beamline access.
Author contributions
W.L. and M.S.C. prepared RNAP derivatives. W.L., Y.F. and K.D. performed structure determination. W.L., D.D. and S.M. performed sequence analyses and biochemical experiments. R.H.E. designed the study, analyzed data, and wrote the paper.
Data availability
Atomic coordinates and structure factors for the crystal structures of Mtb RPitc5-σL_sp4, RPitc5-σL_sp5, RPitc5-sL_sp6, [BrU]RPo-σL_sp6, and [SeMet15,76]RPo-σL_sp6 have been deposited in the PDB with accession codes PDB 6DV9, 6DVB, 6DVC, 6DVD, and 6DVE, respectively. The source data underlying Fig. 6a, c–e and Supplementary Figs. 1C, 1E–G, 3C, and 6A are provided as a Source Data File. Other data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08443-3.
References
- 1.Feklistov A, Sharon BD, Darst SA, Gross CA. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu. Rev. Microbiol. 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 2.Murakami K, Masuda S, Darst S. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 3.Vassylyev D, et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 4.Mekler V, et al. Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell. 2002;108:599–614. doi: 10.1016/S0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- 5.Kulbachinskiy A, Mustaev A. Region 3.2 of the sigma subunit contributes to the binding of the 3′-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation. J. Biol. Chem. 2006;281:18273–18276. doi: 10.1074/jbc.C600060200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Structural basis of transcription initiation. Science. 2012;338:1076–1080. doi: 10.1126/science.1227786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu R, et al. Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme. J. Biol. Chem. 2014;289:24549–24559. doi: 10.1074/jbc.M114.584037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pupov D, Kuzin I, Bass I, Kulbachinskiy A. Distinct functions of the RNA polymerase sigma subunit region 3.2 in RNA priming and promoter escape. Nucleic Acids Res. 2014;42:4494–4504. doi: 10.1093/nar/gkt1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchi D, et al. RNA polymerase pausing during initial transcription. Mol. Cell. 2016;63:939–950. doi: 10.1016/j.molcel.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner E, et al. Backtracked and paused transcription initiation intermediate of Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E6562–E6571. doi: 10.1073/pnas.1605038113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulin D, et al. Pausing controls branching between productive and non-productive pathways during initial transcription in bacteria. Nat. Commun. 2018;9:1478. doi: 10.1038/s41467-018-03902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami K. The x-ray crystal structure of Escherichia coli RNA polymerase sigma70 holoenzyme. J. Biol. Chem. 2013;288:9126–9134. doi: 10.1074/jbc.M112.430900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae B, et al. Phage T7 Gp2 inhibition of Escherichia coli RNA polymerase involves misappropriation of sigma70 domain 1.1. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19772–19777. doi: 10.1073/pnas.1314576110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. GE23077 binds to the RNA polymerase ‘i’ and ‘i+1′ sites and prevents the binding of initiating nucleotides. eLife. 2014;3:e02450. doi: 10.7554/eLife.02450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo Y, Steitz T. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol. Cell. 2015;58:534–540. doi: 10.1016/j.molcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae B, Feklistov A, Lass-Napiorkowska A, Landick R, Darst S. Structure of a bacterial RNA polymerase holoenzyme open promoter complex. eLife. 2015;4:e08504. doi: 10.7554/eLife.08504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Zhang Y, Ebright RH. Structural basis of transcription activation. Science. 2016;352:1330–1333. doi: 10.1126/science.aaf4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubin, E. et al. Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. eLife6, e22520 (2017). [DOI] [PMC free article] [PubMed]
- 19.Lin W, et al. Structural basis of Mycobacterium tuberculosis transcription and transcription inhibition. Mol. Cell. 2017;66:169–179. doi: 10.1016/j.molcel.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, et al. Structures of the RNA polymerase-sigma54 reveal new and conserved regulatory strategies. Science. 2015;349:882–885. doi: 10.1126/science.aab1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glyde R, et al. Structures of RNA polymerase closed and intermediate complexes reveal mechanisms of DNA opening and transcription initiation. Mol. Cell. 2017;67:106–116. doi: 10.1016/j.molcel.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Zuo Y, Steitz TA. Structures of E. coli sigmaS-transcription initiation complexes provide new insights into polymerase mechanism. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4051–4056. doi: 10.1073/pnas.1520555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonetto M, Brown K, Rudd KE, Buttner M. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 25.Helmann J. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 2002;46:47–110. doi: 10.1016/S0065-2911(02)46002-X. [DOI] [PubMed] [Google Scholar]
- 26.Staron A, et al. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol. Microbiol. 2009;74:557–581. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 27.Mascher T. Signaling diversity and evolution of extracytoplasmic function (ECF) sigma factors. Curr. Opin. Microbiol. 2013;16:148–155. doi: 10.1016/j.mib.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Campagne S, Allain F, Vorholt J. Extra cytoplasmic function sigma factors, recent structural insights into promoter recognition and regulation. Curr. Opin. Struct. Biol. 2015;30:71–78. doi: 10.1016/j.sbi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Helmann J. Bacillus subtilis extracytoplasmic function (ECF) sigma factors and defense of the cell envelope. Curr. Opin. Microbiol. 2016;30:122–132. doi: 10.1016/j.mib.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sineva E, Savkina M, Ades S. Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Curr. Opin. Microbiol. 2017;36:128–137. doi: 10.1016/j.mib.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manganelli R, et al. Sigma factors and global gene regulation in Mycobacterium tuberculosis. J. Bacteriol. 2004;186:895–902. doi: 10.1128/JB.186.4.895-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigue S, Provvedi R, Jacques P, Gaudreau L, Manganelli R. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 2006;30:926–941. doi: 10.1111/j.1574-6976.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 33.Sachdeva P, Misra R, Tyagi A, Singh Y. The sigma factors of Mycobacterium tuberculosis: regulation of the regulators. FEBS J. 2010;277:605–626. doi: 10.1111/j.1742-4658.2009.07479.x. [DOI] [PubMed] [Google Scholar]
- 34.Newton-Foot M, Gey van Pittius NC. The complex architecture of mycobacterial promoters. Tuberculosis. 2013;93:60–74. doi: 10.1016/j.tube.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Manganelli R. Sigma factors: key molecules in Mycobacterium tuberculosis physiology and virulence. Microbiol. Spectr. 2014;2:MGM2-0007-2013. doi: 10.1128/microbiolspec.MGM2-0007-2013. [DOI] [PubMed] [Google Scholar]
- 36.Hahn M, Raman S, Anaya M, Husson R. The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and is required for virulence. J. Bacteriol. 2005;187:7062–7071. doi: 10.1128/JB.187.20.7062-7071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dainese E, et al. Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor sigma L and roles in virulence and in global regulation of gene expression. Infect. Immun. 2006;74:2457–2461. doi: 10.1128/IAI.74.4.2457-2461.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigue S, et al. Identification of mycobacterial sigma factor binding sites by chromatin immunoprecipitation assays. J. Bacteriol. 2007;189:1505–1513. doi: 10.1128/JB.01371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feklistov A, Darst S. Structural basis for promoter-10 element recognition by the bacterial RNA polymerase sigma subunit. Cell. 2011;147:1257–1269. doi: 10.1016/j.cell.2011.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim H, Lee H, Roy S, Adhya S. A “master” in base unpairing during isomerization of a promoter upon RNA polymerase binding. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14849–14852. doi: 10.1073/pnas.261517398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham B, Wells J. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 42.Ebright R. Use of loss-of-contact substitutions to identify residues involved in an amino acid-base pair contact: effect of substitution of Gln18 of Lac repressor by Gly, Ser, and Leu. J. Biomol. Struct. Dyn. 1985;3:281–297. doi: 10.1080/07391102.1985.10508417. [DOI] [PubMed] [Google Scholar]
- 43.Ebright R. Evidence for a contact between glutamine-18 of lac repressor and base pair 7 of lac operator. Proc. Natl. Acad. Sci. U.S.A. 1986;83:303–307. doi: 10.1073/pnas.83.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebright R. Identification of amino acid–base pair contacts by genetic methods. Methods Enzymol. 1991;208:620–640. doi: 10.1016/0076-6879(91)08032-D. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Ebright R. Identification of a contact between arginine-180 of the catabolite gene activator protein (CAP) and base pair 5 of the DNA site in the CAP-DNA complex. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4717–4721. doi: 10.1073/pnas.87.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vahedian-Movahed, H. Sequence-specific Interactions Between RNA Polymerase and the Core Recognition Element. Ph.D. dissertation, Rutgers University, New Brunswick, NJ (2017).
- 47.Renfrow M, et al. Transcription factor B contacts promoter DNA near the transcription start site of the archaeal transcription initiation complex. J. Biol. Chem. 2004;279:2825–2531. doi: 10.1074/jbc.M311433200. [DOI] [PubMed] [Google Scholar]
- 48.Kostrewa D, et al. RNA polymerase II-TFIIB structure and mechanism of transcription initiation. Nature. 2009;462:323–330. doi: 10.1038/nature08548. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Bushnell D, Wang D, Calero G, Kornberg R. Structure of an RNA polymerase II-TFIIB complex and the transcription initiation mechanism. Science. 2010;327:206–209. doi: 10.1126/science.1182015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Y, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature. 2016;533:359–365. doi: 10.1038/nature17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plaschka C, et al. Transcription initiation complex structures elucidate DNA opening. Nature. 2016;533:353–358. doi: 10.1038/nature17990. [DOI] [PubMed] [Google Scholar]
- 52.Engel C, et al. Structural basis of RNA polymerase I transcription initiation. Cell. 2017;169:120–131. doi: 10.1016/j.cell.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Han Y, et al. Structural mechanism of ATP-independent transcription initiation by RNA polymerase I. eLife. 2017;6:e27414. doi: 10.7554/eLife.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadian Y, et al. Structural insights into transcription initiation by yeast RNA polymerase I. EMBO J. 2017;36:2698–2709. doi: 10.15252/embj.201796958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vorlander MK, Khatter H, Wetzel R, Hagen W, Muller C. Molecular mechanism of promoter opening by RNA polymerase III. Nature. 2018;553:295–300. doi: 10.1038/nature25440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abascal-Palacios G, Ramsay EP, Beuron F, Morris E, Vannini A. Structural basis of RNA polymerase III transcription initiation. Nature. 2018;553:301–306. doi: 10.1038/nature25441. [DOI] [PubMed] [Google Scholar]
- 57.Rhodius V, et al. Design of orthogonal genetic switches based on a crosstalk map of sigmas, anti-sigmas, and promoters. Mol. Syst. Biol. 2013;9:702. doi: 10.1038/msb.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koo B, Rhodius V, Campbell E, Gross C. Mutational analysis of Escherichia coli sigma28 and its target promoters reveals recognition of a composite -10 region, comprised of an ‘extended -10 motif’ and a core-10 element. Mol. Microbiol. 2009;72:830–843. doi: 10.1111/j.1365-2958.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campagne S, Marsh M, Capitani G, Vorholt JA, Allain F. Structural basis for -10 promoter element melting by environmentally induced sigma factors. Nat. Struct. Mol. Biol. 2014;21:269–276. doi: 10.1038/nsmb.2777. [DOI] [PubMed] [Google Scholar]
- 60.Kumar A, et al. A hybrid sigma subunit directs RNA polymerase to a hybrid promoter in Escherichia coli. J. Mol. Biol. 1995;246:563–571. doi: 10.1016/s0022-2836(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee R, Rudra P, Prajapati R, Sengupta S, Mukhopadhyay J. Optimization of recombinant Mycobacterium tuberculosis RNA polymerase expression and purification. Tuberculosis. 2014;94:397–404. doi: 10.1016/j.tube.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Jacques J, Rodrigue S, Brzezinski R, Gaudreau L. A recombinant Mycobacterium tuberculosis in vitro transcription system. FEMS Microbiol. Lett. 2006;255:140–147. doi: 10.1111/j.1574-6968.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 63.Stols L, Millard C, Dementieva I, Donnelly M. Production of selenomethionine-labeled proteins in two-liter plastic bottles for structure determination. J. Struct. Funct. Genomics. 2004;5:95–102. doi: 10.1023/B:JSFG.0000029196.87615.6e. [DOI] [PubMed] [Google Scholar]
- 64.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 65.Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D. 1994;54:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 66.Emsley P, Lohkamp B, Scott W, Cowtan K. Features and development of Coot. Acta Crystallogr. D. 2010;66:486. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams P, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kammerer W, Deuschle U, Gentz R, Bujard H. Functional dissection of Escherichia coli promoters: information in the transcribed region is involved in late steps of the overall process. EMBO J. 1986;5:2995–3000. doi: 10.1002/j.1460-2075.1986.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dickson R, Abelson J, Barnes W, Reznikoff W. Genetic regulation: the lac control region. Science. 1975;187:17–35. doi: 10.1126/science.187.4171.17. [DOI] [PubMed] [Google Scholar]
- 70.Yarnell W, Roberts J. Mechanism of intrinsic transcription termination and antitermination. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
- 71.Workman C, et al. enoLOGOS: a versatile web tool for energy normalized sequence logos. Nucleic Acids Res. 2005;33:W389–W392. doi: 10.1093/nar/gki439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and structure factors for the crystal structures of Mtb RPitc5-σL_sp4, RPitc5-σL_sp5, RPitc5-sL_sp6, [BrU]RPo-σL_sp6, and [SeMet15,76]RPo-σL_sp6 have been deposited in the PDB with accession codes PDB 6DV9, 6DVB, 6DVC, 6DVD, and 6DVE, respectively. The source data underlying Fig. 6a, c–e and Supplementary Figs. 1C, 1E–G, 3C, and 6A are provided as a Source Data File. Other data are available from the corresponding author upon reasonable request.