Abstract
Reduced social motivation is a hallmark of individuals with autism spectrum disorders (ASDs). Although the exact neural mechanisms are unclear, oxytocin has been shown to enhance motivation and attention to social stimuli, suggesting a potential to augment social reinforcement learning as the central mechanism of behavioral interventions in ASD. We tested how reinforcement learning in social contexts and associated reward prediction error (RPE) signals in the nucleus accumbens (NAcc) were modulated by intranasal oxytocin. Male adults with a childhood diagnosis of ASD (n = 15) and healthy controls (n = 24; aged 18–26 years) performed a probabilistic reinforcement learning task during functional magnetic resonance imaging in a single-center (research center in Germany), randomized double-blind, placebo-controlled cross-over trial. The interventions were intranasal oxytocin (Syntocinon®, Novartis; 10 puffs = 20 international units (IUs) per treatment) and placebo spray. Using computational modeling of behavioral data, trial-by-trial RPE signals were assessed and related to brain activation in NAcc during reinforcing feedback in social and non-social contexts. The order of oxytocin/placebo was randomized for 60 participants. Twenty-one participants were excluded from analyses, leaving 39 for the final analysis. Behaviorally, individuals with ASD showed enhanced learning under oxytocin when the learning target as well as feedback was social as compared to non-social (social vs. non-social target: 87.09% vs. 71.29%, 95% confidence interval (CI): 7.28–24.33, p = .003; social vs. non-social feedback: 81.00% vs. 71.29%, 95% CI: 2.81–16.61, p = .027). Correspondingly, oxytocin enhanced the correlation of the RPE signal with NAcc activation during social (vs. non-social) feedback in ASD (3.48 vs. −1.12, respectively, 95% CI: 2.98–6.22, p = .000), whereas in controls, this effect was found in the placebo condition (2.90 vs. −1.14, respectively, 95% CI: 1.07–7.01, p = .010). In ASD, a similar pattern emerged when the learning target was social (3.00 vs. −0.64, respectively, 95% CI: −0.13 to 7.41, p = .057), whereas controls showed a reduced correlation for social learning targets under oxytocin (−0.70 vs. 2.72, respectively, 95% CI: −5.86 to 0.98, p = .008). The current data suggest that intranasal oxytocin has the potential to enhance social reinforcement learning in ASD. Future studies are warranted that investigate whether oxytocin can potentiate social learning when combined with behavioral therapies, resulting in greater treatment benefits than traditional behavior-only approaches.
Subject terms: Reward, Autism spectrum disorders
Introduction
To date, there is still no approved pharmacological treatment for the core social symptoms of autism spectrum disorders (ASDs). The neuropeptide oxytocin (OXT) has been proposed as a promising candidate for treating ASD-related social deficits, as it has been shown to enhance motivation and attention to social stimuli by making them more salient, thereby facilitating social learning and memory [1]. At present, the most effective treatment for improving social functioning in ASD are behavioral therapies, which build on the principles of reward-based operant reinforcement learning, such as applied behavior analysis or social skills training [2, 3]. Such interventions are costly and time consuming, but often fail to benefit a substantial number of affected individuals [2]. Thus, a better understanding of the mechanisms for social learning is urgently needed to improve current treatments.
The dopaminergic (DA) system, including the striatum, plays an essential role for reinforcement learning. The ventral striatum, specifically the nucleus accumbens (NAcc), signals reward and the expectation thereof, in order to initiate changes in behavior [4]. Brain activation of the NAcc is closely associated with the processing of reward prediction errors (RPEs), that is, the difference between an actual and an expected reward [5]. RPE signals in the NAcc are generated by phasic activity of DA neurons in the ventral tegmental area, reflecting the basic neural mechanism underlying reinforcement learning [4]. In animal studies it could be demonstrated that OXT closely interacts with the DA reward system [6]. For example, OXT modulates social learning, such as establishing social preference and bonding, and acts specifically as a social reinforcement signal within the NAcc [6, 7]. In humans, Hu et al. [8] demonstrated increased learning selectively for social feedback under OXT along with changes in striatal brain activation.
Several lines of research have indicated that the OXT system is altered in ASD. Genetic variation of the OXT receptor is significantly associated with ASD [9, 10], and baseline plasma OXT may relate to (social) functioning in affected individuals [11]. Animal models of ASD-associated behavior suggest dysfunctions of the OXT system, which could be ameliorated with OXT administration (e.g., [12]). Single dosage studies in humans suggest improved prosocial functioning after OXT administration (e.g., [13, 14]) and increased brain activation and connectivity in striatal brain regions [15, 16]; however, clinical trials with repeated OXT administration have produced mixed findings [17]. Given the proposed link between OXT- and DA-mediated social learning, surprisingly few studies have addressed combined effects of behavioral and pharmacological interventions (e.g., [18]), and no study has yet investigated the influence of OXT on social reinforcement learning and the associated neural mechanisms in ASD.
Thus, in the present study we used a probabilistic social reinforcement learning task in young adults with and without ASD during functional magnetic resonance imaging (fMRI) with intranasal OXT and placebo (PLC) administration in a randomized double-blind within-subjects cross-over design. We expected OXT to improve learning in social contexts in ASD, as well as an association with enhanced RPE signals in the NAcc, indicating that OXT in ASD may alleviate social learning deficits through an influence on brain mechanisms mediating reinforcement learning.
Materials and methods
Study design
A single-center, double-blind, PLC-controlled cross-over trial was performed between April 2013 and August 2016. All participants received both OXT and PLC treatments in randomized order to compare the modulatory effect of OXT on social reinforcement learning and associated RPE signals in the NAcc vs. PLC. The study was approved by the ethical committee of the University Hospital RWTH Aachen, Germany, and registered at the US National Institutes of Health (ClinicalTrials.gov) #NCT01712464 before the beginning of recruitment (https://clinicaltrials.gov/ct2/show/NCT01712464).
Participants
Thirty-five healthy male healthy control (HC) participants (aged between 18 and 25 years) and 25 male individuals with ASD (aged between 18 and 26 years) were included into the randomization procedure (i.e., allocation to treatment of OXT or PLC on the first visit). Several participants had to be excluded from the analysis for various reasons (dropout on the second visit, HC n = 1; anatomical brain abnormality, ASD n = 1, HC n = 1; technical and data quality problems, ASD n = 3, HC n = 6; correct guess of the administered pharmacological substance, HC n = 1; poor task performance, i.e., acquisition of a “wrong” association (significant preference (>60%) of the not reinforced option) either within the second half or across all trials of at least one condition, ASD n = 2, HC n = 2; medication status, ASD n = 4). In total, 24 HC (mean age = 22.09, SD = 1.88, mean IQ = 119.10, SD = 9.35) and 15 ASD (mean age = 21.79, SD = 2.60, mean IQ = 113.53, SD = 11.61) participants were included in the final analysis. They all had normal language function and were not taking any psychotropic medications at the time of scanning. Because of menstrual cycle-related changes in plasma OXT [19], no women were included in this study. See Table 1 for demographic information of the final participant samples. A more detailed description of the trial protocol (including a CONSORT Flow Diagram) and details about exclusion criteria, screening procedure, and other behavioral measures is provided in the supplement. Individuals with ASD were recruited from a database of participants with ASD from previous studies at the Departments of Child and Adolescent Psychiatry in Aachen (RWTH Aachen University) or Frankfurt am Main (Goethe University). All participants with ASD had received a childhood diagnosis by experienced clinicians and reached cut-offs on the Autism Diagnostic Observation Schedule- Generic (ADOS-G) and/or the Autism Diagnostic Interview-Revised (ADI-R). They were screened for other current psychiatric and neurological disorders with a brief clinical interview during the screening procedure. Two participants reported a diagnosis of attention-deficit/hyperactivity disorder (ADHD) and respective medication during childhood, but no current medication or symptoms. Four participants reported a depressive episode in the past, one reported vocal tics during childhood, but no current symptoms. HC participants were recruited from databases of previous studies at the Department of Child and Adolescent Psychiatry in Aachen or via local advertisements. They had no indication of developmental delay or history of any neurological or psychiatric disorder, as assessed by a brief clinical interview. The Beck Depression Inventory-Second Edition was used to screen for depressive symptoms (ASD: M = 5.20, SD = 4.75 vs. HC: M = 3.13, SD = 3.03; p = .103). For a dimensional measurement of reciprocal social behavior, participants filled in the Social Responsiveness Scale (self rated). The ASD group showed on average moderate deficiencies in social behavior (T = 68; M = 92.2, SD = 21.7, missing data n = 2), whereas the HC group displayed no deficits (T = 47; M = 31.6, SD = 26.9, missing data n = 8) (see Supplement for further details). The mean score in the ASD group is comparable to reports from other studies investigating adults (e.g., [20–22]). All experimental procedures were conducted at the Research Center Jülich, Germany, with written informed consent of all participants after they had received a complete description of the study.
Table 1.
Demographic characteristics of the final participant samples
| Characteristic | HC participants (N = 24) | ASD participants (N = 15) | df | Analysis by Student’s t test (two-tailed) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | ||
| % Male | 100 | 100 | |||||
| Age (years) | 22.1 | 1.88 | 21.79 | 2.60 | 23.20 | −0.39 | .700 |
| IQ (estimated) | 119.1 | 9.35 | 113.53 | 11.61 | 37.00 | −1.66 | .106 |
| TCI-140 | 42.99 | 4.93 | 39.39 | 5.57 | 37.00 | −2.11 | .041 |
| TCI-140 RD | 43.21 | 9.93 | 32.27 | 14.21 | 37.00 | −2.83 | .007 |
HC healthy control, ASD autism spectrum disorder, TCI-140 Temperament and Character Inventory-140, TCI-140 RD Reward Dependence Scale of the TCI-140
Procedure
Participants took part in two sessions on two consecutive days. Each session consisted of (1) intranasal administration of OXT (Syntocinon®, Novartis; 10 puffs = 20 IUs per treatment) or PLC spray, (2) two blood draws, (3) an (f)MRI scan, and (4) neuropsychological assessment and questionnaires. OXT/PLC was administered ~45 min (mean = 48 min, SD = 5.61) before the beginning of the fMRI scan to ensure maximum availability of OXT in the central nervous system [23]. One blood sample was drawn before OXT/PLC administration for baseline, and a second before the beginning of the fMRI measurement for post hoc validation of OXT-plasma levels during the fMRI measurement. Please refer to the Supplement for the OXT-plasma analysis.
Probabilistic reinforcement learning task
We employed a modified probabilistic social reinforcement learning task (similar to [24]). Participants were asked to indicate by button press with their left and right index finger whether a learning target would belong to category A or B, followed by probabilistic feedback. They were informed that the categories were arbitrary and had to be learned by means of probabilistic feedback with no underlying rule defining the category. The feedback was either rewarding upon correct choice or neutral upon incorrect choice, both with a probability of 75% (accordingly, a probability of 25% for incongruent, “false” feedback). Three different conditions were used, that is, SN (social target–non-social feedback), NN (non-social target–non-social feedback), and NS (non-social target–social feedback). Social learning targets were video clips of a male or female person looking at the participant with a neutral facial expression (SN conditions). Non-social learning targets were video clips of colored fractals (NN, NS conditions). Social feedbacks were video clips of a male or female person smiling at the participant and giving him “thumbs up”, or neutral video clips of this person with eyes closed and snipping fingers as if listening to music [25] (NS condition). Non-social feedback was provided by videos of a colored fractal where a green checkmark or a blue cross appeared (SN, NN conditions). To be able to identify potentially differential contributions of targets or feedback being social during reinforcement learning, we focused on NS and SN conditions and their comparisons to the NN condition. Due to time constraints, we were not able to include a condition with social feedback following a social target (SS condition) (Fig. 1).
Fig. 1.
Illustration and timing of the (a) SN, (b) NN, and (c) NS condition with rewarding feedback conditions were presented in three separate runs (SN, NN, NS) of approximately 15 min, with 64 trials each (including two stimuli of each category A or B) resulting in 16 repetitions for each stimulus throughout each run. Each stimulus appeared twice in a learning block of eight trials with no immediate repetition. Trials were presented in a pseudo-random order and the order of the three runs was counterbalanced between subjects. During each target presentation (max 2000 ms), participants selected via button press whether the learning target belonged to category A or B. Upon choice, a fixation cross (3000–5000 ms) appeared, followed by a feedback screen (2000 ms) with either positive or neutral probabilistic feedback, depending on correct or incorrect category choice, respectively. A complete trial took 13 s, resulting in an inter-trial interval of (4000–6000 ms)
Analysis of behavioral data
Behavioral data were analyzed using the SPSS 21 software (IBM Corporation, Armonk, NY, USA). For each experimental condition and subject, the percentage of correct responses was calculated. To assess learning effects over time and to account for effects during the initial performance and later stages of the learning phase (i.e., potential floor and ceiling effects), behavioral data were subdivided for each task condition into three intervals, with the first two consisting of three blocks (eight trials each) and the last one consisting of two blocks. General linear model repeated-measure analyses (mixed analysis of variance (ANOVA)) were used to assess main effects and interactions with treatment condition (OXT/PLC), task condition (NN/NS/SN), and interval (1/2/3) as within-subjects factors and group (ASD/HC) as between-subjects factor. For post hoc tests, Bonferroni’s adjustment procedure was used.
Computational model
Importantly, behavioral choice data were further analyzed using computational modeling of reinforcement learning, according to a basic Q-learning algorithm and a softmax decision function [26]. Learning parameter α was estimated using maximum-likelihood estimation and RPE and Q-values for each trial were calculated (see Supplement for more details).
Functional magnetic resonance imaging
The fMRI protocol and analysis are described in detail in the Supplement. In short, scans were acquired on a 3-Tesla head-dedicated MRI system (MagnetomTrioTim, Siemens, Erlangen, Germany) using a T1-weighted 3D magnetization prepared rapid acquisition gradient echo sequence and T2*-weighted echo planar imaging scans during task performance.
Image preprocessing and analysis were performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Functional images were realigned to the mean image, and anatomical scans co-registered to the mean image, segmented and normalized to the Montreal Neurological Institute template. Functional volumes were normalized and smoothed at 6 mm full-width at half-maximum isotropic Gaussian kernel. The learning target phase (when subjects performed the category selection) and the feedback phase (when subjects received feedback in response to their choice) of the task were modeled separately using stick functions, convolved with the hemodynamic response function and its first-order temporal derivative. Motion parameters were included as regressors. Feedback events were parametrically modulated by trial-wise individual RPE values. In line with previous findings [8] and our focus on RPE processing in the brain [5], the second-level analysis focused on the parametric modulation of feedback events. β-Values representing this modulation were taken to the second level with all conditions modeled separately in a flexible factorial ANOVA, with the within-subjects factors treatment condition (OXT/PLC) and task condition (NN/NS/SN) and the between-subjects factor group (ASD/HC). For the whole brain analysis, only effects above a significance threshold of p < .05 (cluster-level corrected, p < .001 voxel level) are reported. Regions of interest (ROI) analyses were thresholded at p < .05 (voxel level), family-wise error-corrected for the ROI. Our analysis focused on brain activation within an anatomical ROI of the NAcc (defined as primary outcome measure before the beginning of recruitment; see https://clinicaltrials.gov/ct2/show/NCT01712464). Exploratory analyses were also performed for the amygdala (see Supplement).
Results
Behavioral results
For both participants with ASD (F (2, 28) = 34.74, p < .001, ηp2 = .71) and HC (F (1.28, 29.49) = 155.35, p < .001, ηp2 = .87), we observed a significant main effect of interval on the percentage of correct trials. This effect was evident across and separately for each experimental condition (all ps < .001; interval 1 vs. 2: 67.11% vs. 82.95%, respectively, 95% CI: −18.46 to −13.23, interval 1 vs. 3: 67.11% vs. 87.13%, respectively, 95% CI: −23.06 to −16.98, interval 2 vs. 3: 82.95 vs. 87.13, respectively, 95% CI: −5.67 to −2.70), suggesting successful learning of the stimulus–feedback association during the course of the experiment.
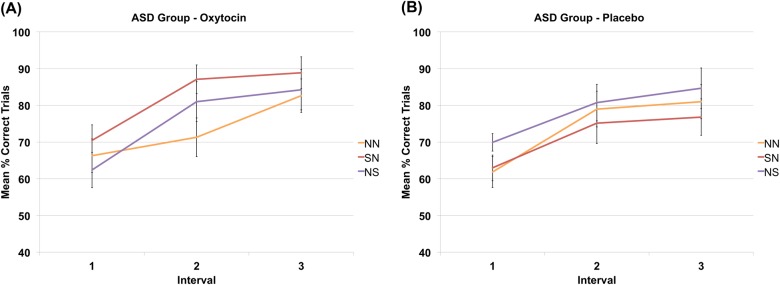
Testing for OXT-induced effects in participants with ASD (according to our a priori hypothesis) revealed a significant treatment × task interaction (F (2, 28) = 3.45, p = .046, ηp2 = .20), indicating a higher percentage of correct trials for social targets (i.e., SN vs. NN) in the OXT condition (SN: 81.20% vs. NN: 72.07%, 95% CI: −15.15 to −3.12, p = .006, pcorr = .018), but not in the PLC condition (SN: 70.87% vs. NN: 73.07%, 95% CI: −9.53 to 5.13, p > −.10). Also, a significant task × treatment × interval interaction (F (4, 56) = 2.72, p = .039, ηp2 = .16) revealed that in the second interval (F (2, 28) = 10.83, p < .001, pcorr < .001, ηp2 = .44), individuals with ASD had a higher mean percentage of correct trials for both social feedback (i.e., NS vs. NN, 81.00% vs. 71.29%, respectively, 95% CI: 2.81–16.61, p = .009, pcorr = .027) and the social learning target (i.e., SN vs. NN, 87.09% vs. 71.29%, respectively, 95% CI: 7.28–24.33, p = .001, pcorr = .003) in the OXT condition, but not in the PLC condition (all p > .10) (see Fig. 2). Similar analyses for the HC group revealed no main effects or interactions (see Supplement for further behavioral analyses).
Fig. 2.
Task × treatment × interval interaction in the ASD group. Participants with ASD showed better learning with social as compared to non-social targets and feedback in the OXT (a) but not PLC (b) condition during the second learning interval of the probabilistic reinforcement learning task during fMRI. ASD autism spectrum disorder, NN task condition with non-social learning target and non-social feedback, NS task condition with non-social learning target and social feedback, SN task condition with social learning target and non-social feedback
Imaging results
Whole-brain analysis
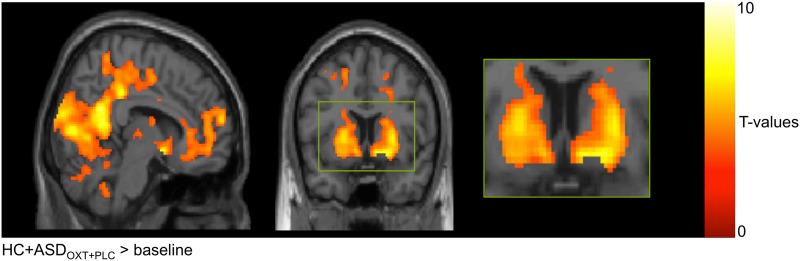
Across groups, tasks, and treatment conditions ((HC + ASDOXT_PLC) > baseline), the whole-brain analysis revealed a significant correlation of the RPE signal with a broad neural network (Fig. 3). Importantly, significant activation was observed within the striatum, including NAcc and putamen [27, 28]. See supplement Table S2 for an overview of feedback RPE signals within the brain across ASD and HC participants.
Fig. 3.
Regions in the fMRI task where activation was associated with learning from feedback across groups, treatment, and task conditions. Results were significant at p < .05 (cluster-level corrected, p < .001 voxel level, k = 164 voxels). For illustrative purposes, the uncorrected level is presented here, but results are reported for the cluster-level correction. HC healthy control, ASD autism spectrum disorder, OXT oxytocin, PLC placebo
ROI analysis
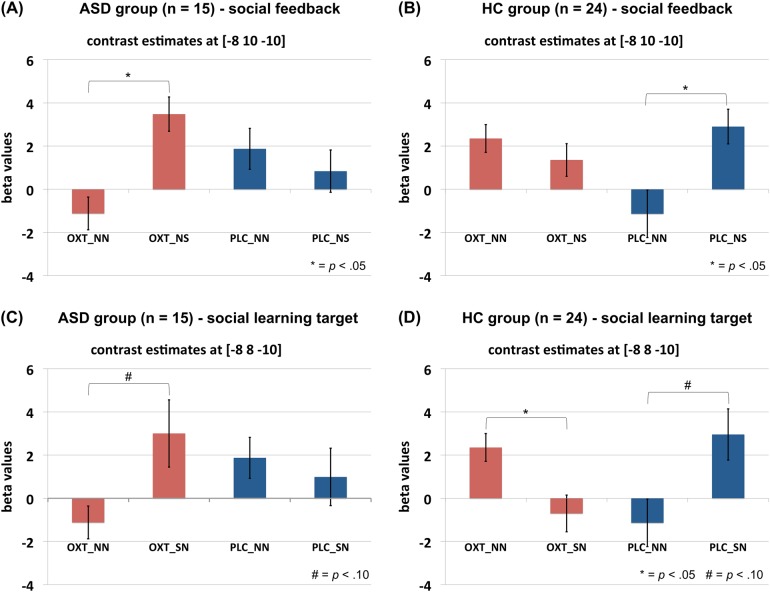
Using the NAcc as our a priori defined anatomical ROI, we observed a significant group × social feedback × treatment interaction ([−8 10 −10], Z = 3.40) (Fig. 4a, b). The interaction was due to the ASD group showing a higher correlation of the RPE signal with brain activation in the left NAcc for social feedback as compared to non-social feedback in the OXT (NS: 3.48 vs. NN: −1.12, 95% CI: 2.98–6.22, F (1, 14) = 37.02, p < .001, ηp2 = .73) but not PLC condition (NS: .84 vs. NN: 1.87, 95% CI: −3.80 to 1.73], F (1, 14) = .65, p = .435, ηp2 = .04), whereas the HC participants had a higher correlation for social feedback as compared to non-social feedback in the PLC (NS: 2.90 vs. NN: −1.14, 95% CI: 1.07 to 7.01, F (1, 23) = 7.93, p = .010, ηp2 = .26) but not OXT condition (NS: 1.36 vs. NN: 2.35, 95% CI: −3.25 to 1.26, F (1, 23) = .83, p = .371, ηp2 = .04). Also, a significant group × social learning target× treatment interaction was found ([−8 8 −10], Z = 4.02) (Fig. 4c, d). The interaction was due to the ASD group showing a marginally higher correlation of brain activation in the left NAcc for social as compared to non-social learning targets in the OXT (SN: 3.00 vs. NN: −.64, 95% CI: −.13 to −7.41, F (1, 14) = 4.29, p = .057, ηp2 = .24) but not PLC condition (SN: .99 vs. NN: 2.23, 95% CI: −5.39 to 2.90, p = .531), whereas HC had a marginally higher correlation for social as compared to non-social learning targets in the PLC (SN: 2.96 vs. NN: −1.35, 95% CI: −.42 to 9.02, F (1, 23) = 3.56, p = .072, ηp2 = .13) condition. Moreover, HC showed a higher correlation for non-social rather than social learning targets in the OXT condition (SN: −.70 vs. NN: 2.72, 95% CI: −5.86 to −.98, F (1, 23) = 8.44, p = .008, ηp2 = .27).
Fig. 4.
Neural correlates for the interaction between group × social feedback × treatment (a, b) and the interaction between group × social learning target × treatment (c, d). Contrast estimates at the corresponding peak voxel ([−8 10 −10] and [−8 8 −10], respectively) of the NAcc ROI are depicted. β-values (vertical axis) represent parameter estimates for the degree of the correlation of brain activation with the RPE. ASD: N = 15, HC: N = 24. *p < .05; #p < .10. RPE reward prediction error, ASD autism spectrum disorder, HC healthy control, OXT oxytocin, PLC placebo, NN task condition with non-social learning target and non-social feedback, NS task condition with non-social learning target and social feedback, SN task condition with social learning target and non-social feedback
Brain–behavior correlations
We also explored possible correlations between ASD symptom indices, individual characteristics related to reward processing and neural activity in the NAcc during social feedback in the OXT as compared to the PLC condition (OXTNS > PLCNS)], within the ASD group using a multiple regression analysis. We observed a negative correlation between brain activation in the left NAcc ([−12 12 −6], Z = 3.27) and the Reward Dependence (RD) Subscale of the Temperament and Character Inventory-140 (TCI-140), implying that individuals with ASD with lower RD showed more activation in the NAcc during social feedback in the OXT as compared to the PLC condition (see Supplement).
Discussion
To our knowledge, the present study is the first to demonstrate OXT-induced enhancement of social learning in high-functioning ASD and an associated modulation of the RPE signal in the NAcc, a central neural hub for reinforcement-based learning. Our results suggest that the beneficial effect of OXT on social processing in ASD [13, 14] is mediated by an enhancement of the brain’s motivational system, selectively in response to social stimuli, eventually boosting reinforcement learning in social situations. Thus, future studies investigating long-term efficacy of OXT as pharmacotherapy in ASD should consider that OXT might be particularly efficient in concert with behavioral interventions with an emphasis on socially reinforcing context to promote learning.
Effects of OXT on task performance
Following a single intranasal OXT challenge, we observed enhanced social learning in ASD, but no equivalent effect in HC. Similarly, a previous study demonstrated task performance increases under OXT selectively for participants with low social proficiency using an incentive delay task with socially rewarding feedback [29]. At first sight, our results seem at odds with other reports demonstrating that OXT facilitates learning from social feedback in HC [8, 24]. However, we focused exclusively on reinforcing feedback, whereas these studies included also aversive feedback (i.e., angry faces) upon incorrect choices. Facilitated learning in such contexts might be mediated by other effects of OXT, for example, reduced threat sensitivity [30], or a decrease in aversiveness of negative stimuli [31]. Future studies should directly test the differential contributions of reinforcing and aversive feedback on OXT-induced effects.
Modulatory influences of OXT on RPE signals in the NAcc
Using RPE modeling, our task engaged a network of areas typically involved in reinforcement learning. In particular, NAcc activity is assumed to reflect RPEs, that is, differences between expectation and receipt of reward in order to adjust behavior [5, 32]. We did not find group differences within the general learning network, suggesting no overall functional disruption of reinforcement learning in ASD. This is consistent with the observation that behavioral interventions in ASD rely heavily on reinforcement-based learning to successfully modify behavior (e.g., [2]). At the same time, we could demonstrate a particular sensitivity of NAcc activity for social feedback in HC (but not ASD) for the PLC condition, supporting prior findings of impaired social reward processing in ASD (e.g., [33, 34], in line with the social motivation theory of autism [35]). Our finding of an enhancing effect of OXT for social vs. non-social feedback in ASD suggests that OXT has the potential to restore the typical preference for social rewards in HC. Thus, learning from reinforcing feedback during a social situation appears to be important for the effect of OXT in ASD. The most parsimonious explanation for these results is that this effect is mediated by an OXT-induced increase of DA signaling during social situations, resulting in a targeted enhancement of social approach motivation. This mechanism may also drive improvements in social processing (e.g., [13, 14]) and modulation in cortico-striatal activation and connectivity [15, 16] as reported in earlier OXT-challenged studies in ASD. These findings are well in accordance with the social motivation theory of autism [35], suggesting that a deficient OXT–DA interaction within the NAcc could be an important mechanism to account for reduced social motivation and, ultimately, impaired sociability in ASD.
Exploratory analyses also revealed that individual differences in RD (as measured by the TCI-140) was associated with the increase of RPE-correlated activation in the NAcc under OXT in ASD, suggesting stronger OXT effects in individuals with lower sociability and a tendency to learn less from rewarding interpersonal feedback (see Supplemental data). This result is also in line with treatment outcomes of social skills trainings, showing that individuals with lowest skills usually benefit most [3].
Involvement of OXT in DA modulation of learning
According to numerous animal studies, OXT and DA interact within the NAcc (see, e.g., [6], for a review) to promote learning from social encounters as a prerequisite for establishing and maintaining social affiliations [36, 37]. Similarly, the “social salience hypothesis of OXT” [1] suggests that OXT plays an overarching role for regulating the salience of social cues through its interaction with the DA system. Our findings critically add to a growing body of evidence for links between OXT and the DA reward circuitry, including the striatum (e.g., [29]), by providing a plausible mechanistic explanation in the context of social reinforcement learning, that is, the amplification of striatal RPE signals as one potential mechanism of increased saliency. This notion is also compatible with the view that OXT may primarily amplify approach-related behaviors [38]. Here, we found this effect only in individuals with ASD, suggesting that OXT effects may be dependent on individual differences in social functioning [1, 29] and that OXT–DA interaction might constitute a central mechanism of reduced social motivation in ASD [39]. Importantly, we observed the effects during exclusively reward-based reinforcement learning (i.e., in the absence of aversive stimuli), suggesting an independent contribution of OXT on DA-mediated approach behavior for social functioning.
Accordingly, we did not observe effects of OXT in the amygdala. The amygdala has been associated with salience signaling [1], probably mediating dampening effects on stress and anxiety (e.g., [40]). The occurrence of such effects may be confined to or more pronounced for negative social stimuli (such as threatening or aversive faces). Thus, anxiolytic properties of OXT and their interactions with DA within the amygdala [39, 41] do not seem to be essential for a beneficial effect on social reward-based reinforcement learning.
Further studies with a focus on stress and anxiety in combination with reinforcement learning are warranted to elucidate this issue.
Limitations
Although the ASD sample was comparable to typical adult ASD populations in previous studies, we would like to emphasize that the average severity of deficits in reciprocal social behavior was moderate, all participants were male and very high functioning with respect to their cognitive abilities. Thus, the present findings only apply to this subgroup of high-functioning ASD. Given the high heterogeneity within the autism spectrum, generalization to the broader ASD population should be tested in future studies. A replication of our data with larger samples including women and individuals with lower functioning ASD, as well as a focus on children and adolescents is warranted. Furthermore, more research into comorbid conditions is necessary (e.g., ADHD, social anxiety) which also show impaired social reward processing, albeit in a different direction (e.g., hypersensitivity to social rewards in ADHD [42]). Future studies using a similar experimental design probably should include fully social conditions (i.e., social feedback and target). These may yield even stronger effects and are more comparable to real-life situations (e.g., feedback of the therapist during a social skills training, where both the learning target and the feedback are typically social).
Conclusions and future directions
We provide clear evidence for a neurobiological plausible mechanism of OXT-induced behavioral enhancement of social reinforcement learning in high-functioning ASD, that is, the modulation of RPE signals in the NAcc. Our results implicate that OXT may unfold its therapeutic potential most efficiently in concert with targeted behavioral interventions, which provide opportunities for learning within social contexts along with immediate reinforcement, which is positive and explicitly rewarding (e.g., praise). Single-dose administration studies have generally shown positive effects in ASD [13–16], which might be related to an overall enhancing effect on the motivational system in social contexts as demonstrated specifically for reinforcement learning here. In contrast, longer-term treatment studies using multiple dosing per day often failed to demonstrate treatment effects [17] and one important reason might be that these were not designed to provide specific social learning contexts around the times of administration [20], or may have interfered with psychotropic (e.g., in particular DA) medication [43]. Well-controlled studies which systematically combine social learning opportunities with OXT administration are lacking (but see ref. [18]), but are urgently needed to further elucidate this issue.
Taken together, our findings suggest that it is crucial to further investigate the promising potential of combining OXT with behavioral interventions to inform modifications that might improve current treatment approaches.
Funding and disclosure
Dr. Freitag has been consultant for Desitin and Roche within the past 3 years. She receives royalties for books and psychotherapy intervention manuals on ASD, ADHD as well as Major Depressive Disorder. Dr. Cholemkery receives royalties for books and psychotherapy intervention manuals on ASD. Dr. Konrad has received speaking fees from Shire Pharmaceuticals. The other authors declare no competing interests. This work was primarily supported by grants to Dr. Schulte-Rüther (German Research Foundation (DFG, SCHU 2493/2-1), Excellence Initiative of the German federal and state governments). Dr. Freitag was supported by the grant FR2069/2-1 from the German Research Foundation (DFG).
Electronic supplementary material
Acknowledgements
We would like to thank Alexander Firk, Marlen Mildebrandt, Hannah Schopf, and Saskia Theune for assisting with participant recruitment, data collection, and data entry. For the discussion of the statistical analyses, we are very grateful to Dr. Wolfgang Scharke. We would further like to thank all participants who took part in this study and made an indispensable contribution. We also would like to thank our colleagues at the INM3, INM4, and the Child Neuropsychology Section of the University Hospital RWTH Aachen for their continued support during data acquisition and discussions of the data.
Footnotes
Trial Registration: The trial is registered at the US National Institutes of Health (ClinicalTrails.gov): #NCT01712464.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0258-7).
References
- 1.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Vismara LA, Rogers SJ. Behavioral treatments in autism spectrum disorder: what do we know? Annu Rev Clin Psychol. 2010;6:447–68. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- 3.Freitag CM, Jensen K, Elsuni L, Sachse M, Herpertz-Dahlmann B, Schulte-Rüther M, et al. Group-based cognitive behavioural psychotherapy for children and adolescents with ASD: the randomized, multicentre, controlled SOSTA—Net Trial. J Child Psychol Psychiatry. 2016;57:596–605. doi: 10.1111/jcpp.12509. [DOI] [PubMed] [Google Scholar]
- 4.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 5.Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–95. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–84. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Qi S, Becker B, Luo L, Gao S, Gong Q, et al. Oxytocin selectively facilitates learning with social feedback and increases activity and functional connectivity in emotional memory and reward processing regions. Hum Brain Mapp. 2015;36:2132–46. doi: 10.1002/hbm.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranz TM, Kopp M, Waltes R, Sachse M, Duketis E, Jarczok TA, et al. Meta-analysis and association of two common polymorphisms of the human oxytocin receptor gene in autism spectrum disorder. Autism Res. 2016;9:1036–45. doi: 10.1002/aur.1597. [DOI] [PubMed] [Google Scholar]
- 10.Di Napoli A, Warrier V, Baron-Cohen S, Chakrabarti B. Genetic variation in the oxytocin receptor (OXTR) gene is associated with Asperger Syndrome. Mol Autism. 2014;5:48. doi: 10.1186/2040-2392-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alabdali A, Al-Ayadhi L, El-Ansary A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J Neuroinflamm. 2014;11:4. doi: 10.1186/1742-2094-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harony-Nicolas H, Kay M, Hoffmann J, du Klein ME, Bozdagi-Gunal O, Riad M, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife. 2017;6:e18904. doi: 10.7554/eLife.18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–71. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Gordon I, Vander Wyk BC, Bennett RH, Cordeaus C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110:20953–8. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon I, Jack A, Pretzsch CM, Vander Wyk B, Leckman JF, Feldman R, et al. Intranasal oxytocin enhances connectivity in the neural circuitry supporting social motivation and social perception in children with autism. Sci Rep. 2016;6:35054. doi: 10.1038/srep35054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: a critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 2016;79:234–42. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44:521–31. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 19.Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–9. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, et al. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain. 2014;137:3073–86. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- 22.South M, Carr ALW, Stephenson KG, Maisel ME, Cox JC. Symptom overlap on the SRS-2 adult self-report between adults with ASD and adults with high anxiety. Autism Res. 2017;10:1215–20. doi: 10.1002/aur.1764. [DOI] [PubMed] [Google Scholar]
- 23.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurlemann R, Patin A, Onur O, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51:2062–9. doi: 10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daw N. Trial-by-trial data analysis using computational models. In: Delgado MR, Phelps EA, Robbins TW, editors. Decision making, affect, and learning: attention and performance XXIII. UK: Oxford University Press; 2011. p. 3–38.
- 27.Averbeck BB, Costa VD. Motivational neural circuits underlying reinforcement learning. Nat Neurosci. 2017;20:505–12. doi: 10.1038/nn.4506. [DOI] [PubMed] [Google Scholar]
- 28.Kunisato Y, Okada G, Okamoto Y. Reinforcement learning by striatum. Brain Nerve. 2009;61:405–11. [PubMed] [Google Scholar]
- 29.Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–9. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Domes G, Kumbier E, Heinrichs M, Herpertz SC. Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with Asperger Syndrome. Neuropsychopharmacology. 2014;39:698–706. doi: 10.1038/npp.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology. 2010;35:2502–9. doi: 10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohls G, Schulte-Rüther M, Nehrkorn B, Müller K, Fink GR, Kamp-Becker I, et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. 2013;8:565–72. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The Social Motivation Theory of autism. Trends Cogn Sci. 2012;16:231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav Brain Sci. 2005;28:313–50. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- 37.Skuse DH, Gallagher L. Dopaminergic–neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Kemp AH, Guastella AJ. The role of oxytocin in human affect: a novel hypothesis. Curr Dir Psychol Sci. 2011;20:222–31. doi: 10.1177/0963721411417547. [DOI] [Google Scholar]
- 39.Gorka SM, Fitzgerald DA, de Wit H, Phan KL. Cannabinoid modulation of amygdala subregion functional connectivity to social signals of threat. Int J Neuropsychopharmacol. 2014;18:1–6. doi: 10.1093/ijnp/pyu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanat M, Heinrichs M, Mader I, Tebartz van Elst L, Domes G. Oxytocin modulates amygdala reactivity to masked fearful eyes. Neuropsychopharmacology. 2015;40:2632–8. doi: 10.1038/npp.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. 2011;37:1077–87. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohls G, Herpertz-Dahlmann B, Konrad K. Hyperresponsiveness to social rewards in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) Behav Brain Funct. 2009;5:20. doi: 10.1186/1744-9081-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21:1225–31. doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.