Abstract
Psychiatric symptoms of schizophrenia suggest alteration of cerebral neurons. However, the physical basis of the schizophrenia symptoms has not been delineated at the cellular level. Here, we report nanometer-scale three-dimensional analysis of brain tissues of schizophrenia and control cases. Structures of cerebral tissues of the anterior cingulate cortex were visualized with synchrotron radiation nanotomography. Tissue constituents visualized in the three-dimensional images were traced to build Cartesian coordinate models of tissue constituents, such as neurons and blood vessels. The obtained Cartesian coordinates were used for calculating curvature and torsion of neurites in order to analyze their geometry. Results of the geometric analyses indicated that the curvature of neurites is significantly different between schizophrenia and control cases. The mean curvature of distal neurites of the schizophrenia cases was ~1.5 times higher than that of the controls. The schizophrenia case with the highest neurite curvature carried a frame shift mutation in the GLO1 gene, suggesting that oxidative stress due to the GLO1 mutation caused the structural alteration of the neurites. The differences in the neurite curvature result in differences in the spatial trajectory and hence alter neuronal circuits. It has been shown that the anterior cingulate cortex analyzed in this study has emotional and cognitive functions. We suggest that the structural alteration of neurons in the schizophrenia cases should reflect psychiatric symptoms of schizophrenia.
Introduction
Schizophrenia is a chronic mental disorder that affects ~1% of the population1. Clinical symptoms of schizophrenia include hallucinations, delusions, emotional disorders, and cognitive dysfunction. The development of these symptoms suggests alterations in the connectivity between cerebral neurons. It has been reported that dendritic spines of neurons are significantly decreased in the external pyramidal layer of the cerebral cortex of schizophrenic brains2–4. Since dendritic spines form the majority of excitatory synapses, the loss of spines can directly impair neuronal connectivity. The reduced neuropil hypothesis5 posits that reductions in neuron size and arborization are the explanation for the reduced brain volume observed in schizophrenia6–9. The reductions in neuron size and arborization can perturb the neuronal structures, resulting in changes to the neuronal circuits. However, studies of brain tissues of schizophrenia patients have mainly been performed using two-dimensional images of tissue sections, whereas the neurons themselves are three-dimensional in nature.
It has been reported that three-dimensional structures of brain tissues can be analyzed with electron microscopy by reconstructing them from serially sectioned images10–12. Since soft tissues are deformed by sectioning, the deformations are artificially corrected in the three-dimensional reconstruction13,14. Therefore, three-dimensional image reconstructed from serial sections does not exactly reproduce the three-dimensional structure of the tissue. Another method to visualize the three-dimensional structure of biological tissue is confocal light microscopy. However, light microscopy cannot visualize structures behind opaque objects. Its resolution is three-dimensionally anisotropic and depends on the direction of the optical axis15. Although neuronal structures have been deposited in the NeuroMorpho.Org database16 including those of human cerebral cortex17, three-dimensional coordinates estimated from light microscopy images show irregular displacements especially along the optical axis and are not applicable to geometric analyses. Thus, resolution anisotropy, tissue opacity, and sectioning deformation can degrade the three-dimensional features that may be relevant to schizophrenia.
In this study, we analyzed brain tissue structures of schizophrenia patients and control cases with synchrotron radiation nanotomography18,19. X-ray microtomography and nanotomography20–25 can visualize three-dimensional opaque objects with nearly isotropic resolution26. Its reconstruction process does not involve any deformation correction, and hence, the obtained image reproduces the actual three-dimensional structures. Although the low X-ray contrast of the brain tissue itself initially limited visualizations to those of large-scale structures27, the use of high-Z element staining has since allowed neuronal networks in brain tissue to be observed28,29. Tissue constituents visualized in this study were traced to build three-dimensional Cartesian coordinate models of tissue structures, such as neurons and blood vessels. The three-dimensional tissue structures were reproduced as Cartesian coordinates through this process. The resultant neuronal coordinates can be used for analyzing the geometry of neurons in schizophrenia and control cases.
Cerebral tissues analyzed in this study are those of the anterior cingulate cortex (Brodmann area 24). It has been shown that this brain area has emotional and cognitive function30,31. It has also been reported that the anterior cingulate cortex is related to schizophrenia32,33, attention-deficit/hyperactivity disorder34, and obsessive–compulsive disorder35. Therefore, the structural character of neurons of the anterior cingulate cortex can affect the mental capabilities or the psychiatric symptoms. In this study, we examined differences of neuronal structures between schizophrenia and control cases and also between control individuals.
Materials and methods
Cerebral tissue samples
All post-mortem human cerebral tissues were collected with informed consent from the legal next of kin using protocols approved by the clinical study reviewing board of Tokai University School of Medicine (application no. 07R-018) and the ethics committee of Tokyo Metropolitan Institute of Medical Science (approval no. 17-18). This study was conducted under the approval of the ethics committee for the human subject study of Tokai University (approval nos. 11060, 11114, 12114, 13105, 14128, 15129, 16157, and 18012). Schizophrenia patients S1–S4 (Supplementary Table S1) were diagnosed according to the DSM-IV codes with the consensus of at least two experienced psychiatrists. Control patients (Supplementary Table S1) had been hospitalized because of a traffic injury (N1) or non-psychiatric lethal diseases (N2–N4) and were not psychiatrically evaluated. Since the cause of death of the N1 case was damage to the heart, histological changes in brain tissue specific to the injury can be excluded. The number of cases was determined in consideration of the available beamtime at the synchrotron radiation facilities. The control cases were selected so as to match the gender and age of the schizophrenia cases. Cases in which hemorrhage, infarction, or neoplasm were observed in the histological assessment of cerebral tissues were excluded. No previous records of schizophrenia were found for the control cases. The cerebral tissues of the anterior cingulate cortex (Brodmann area 24) were collected from the left hemispheres of the biopsied brains and subjected to Golgi impregnation, as described previously29, in order to visualize neurons in X-ray images.
The Golgi-stained tissues were first soaked in neat ethanol, then in n-butyl-glycidyl ether, and finally in Petropoxy 154 (Burnham Petrographics, USA) epoxy resin, as described previously36. The resin-soaked tissues were cut into rod shapes with approximate widths of 0.5 mm and lengths of 3–5 mm under a stereomicroscope and then transferred to borosilicate glass capillaries (W. Müller, Germany) filled with resin. The capillary diameter was ~0.8 mm. The capillaries were incubated at 90 °C for 70–90 h for curing the resin.
Nanotomography
The distal end of the capillary sample was sleeved with a brass tube using epoxy glue and secured with a setscrew to a brass or invar adapter specially designed for nanotomography. The mounted samples were placed in the experiment hutch as soon as possible in order to equilibrate their temperature with that of the sample stage. Nanotomography experiments using Fresnel zone plate optics were performed at the BL37XU18 and BL47XU37 beamlines of the SPring-8 synchrotron radiation facility and at the 32-ID beamline19 of the Advanced Photon Source (APS) of the Argonne National Laboratory. Since the nanotomography experiments were performed in the manner of local computed tomography (CT), only the region of interest within the viewing field (64–122 μm diameter; Supplementary Table S2) was visualized.
In the experiments at the SPring-8 beamlines, the tissue samples were mounted on a slide-guide rotation stage specially built for nanotomography (SPU-1A, Kohzu Precision, Japan). Transmission images were recorded with a CMOS-based imaging detector (ORCA-Flash4.0, Hamamatsu Photonics, Japan) using monochromatic radiation at 8 keV. Examples of raw images and their reconstructed slices are shown in Supplementary Figure S1. Octagonal sector condenser zone plates37 were used as beam condensers. The photon flux at the sample position of the BL37XU optics of the 2017.4 setup was estimated to be 1.0 × 1014 photons/mm2/s using Al2O3:C dosimeters (Nagase-Landauer, Japan). Since the specific gravity of Petropoxy 154 is 1.18 and its attenuation coefficient was estimated to be 6.1 cm−1 at 8 keV, this X-ray flux corresponds to an absorbed radiation dose by the resin of 7.0 × 104 Gy/s. In the nanotomography experiments at the APS beamline, the tissue samples were mounted on an air-bearing rotation stage (UPR-160AIR, PI miCos, Germany) during the 2016.6 beamtime, or on a motorized model 4R Block-Head air bearing spindle (Professional Instruments Company, USA) during the 2017.10 beamtime. Zernike phase-contrast images were recorded with a CMOS-based imaging detector (GS3-U3-51S5M-C, FLIR, USA) using monochromatic radiation at 8 keV. A polygonal beam shaping condenser and compound refractive lenses were used as beam condensers19. Spatial resolutions were estimated using three-dimensional square-wave patterns38 or from the Fourier domain plot39. The experimental conditions are summarized in Supplementary Table S2. The data collection procedure is described in the Supplementary Materials and Methods.
While absorption contrast was sufficient for visualization of the neurons in the SPring-8 BL37XU experiments, the Zernike phase-contrast method was used in order to enhance the sample contrast in the experiments at the BL47XU beamline of SPring-8 and at the 32-ID beamline of APS. Although a number of contrast-enhancing methods have been reported20,27,40, methodological prerequisites, such as the strict requirement of X-ray coherence in the holography, pose limitations in real applications. In this study, ~4 × 105 images of human brain tissues were collected in order to examine the statistical significance of their structural differences. Hence, practical aspects including the time efficiency and the methodological tolerance against the beam fluctuation should be taken into account. For these reasons, we used the Zernike method to enhance the sample contrast.
Tomographic reconstruction
Tomographic slices perpendicular to the sample rotation axis were reconstructed with the convolution-back-projection method using the RecView software36. The reconstruction calculation was performed by R.S. Image pixels of APS datasets were averaged by 2 × 2 binning prior to the tomographic reconstruction, since the original pixel size of 26 nm was approximately half the pixel sizes of the SPring-8 experiments (40.2, 48.3, and 59.6 nm, respectively; Supplementary Table S2) and the binned pixel size of 52 nm was sufficiently fine compared to the spatial resolution of the APS datasets (300 nm). Since the reconstruction of the SPring-8 datasets was performed without binning, the reconstructed voxel size was the same as the original pixel size. Multiple image sets taken by shifting the sample were aligned and stacked to obtain the entire three-dimensional image. The entire reconstructed volume of each dataset was subjected to further analysis.
Data blinding
Nanotomography datasets were analyzed with the role allotment of data management to R.S. and data analysis to R.M. The data manager reconstructed the tomographic slices of all datasets and coded the dataset name. The data manager managed the information regarding the case assignment of each dataset but had no access to the analysis results except for an indicator of the analysis amount. The data analyst built Cartesian coordinate models from the datasets but had no access to the case information. The whole model-building procedure was performed by R.M. in order to keep the modeling quality constant. The detailed protocol is described in the Supplementary Materials and Methods.
Cartesian coordinate models and geometric analysis
Cartesian coordinate models were built in four steps: (1) manual assignment of large structural constituents such as somata and blood capillary vessels, (2) automatic tracing to build a computer-generated model and its subsequent refinement, (3) examination of the entire three-dimensional image and manual intervention to modify the working model, and (4) final structural refinement. The geometric analysis was performed in three steps: (1) cell typing, (2) structure annotation, and (3) geometric parameter calculation. Detailed protocols are described in Supplementary Materials and Methods.
Results
Three-dimensional tissue structure
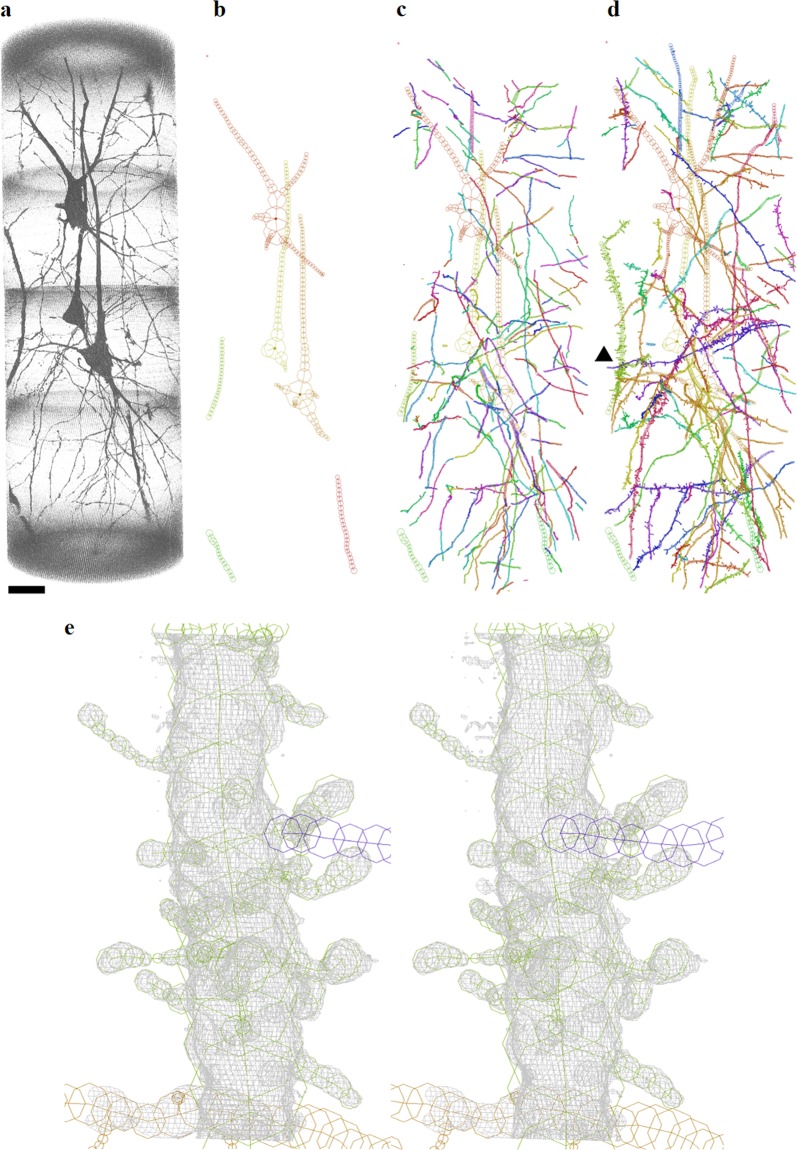
The cerebral cortex tissues analyzed in this study were taken from the left hemispheres of autopsied brains of four schizophrenia cases S1–S4 (age: mean 65 ± standard deviation 6 years; two males and two females) and four age/gender-matched control cases N1–N4 (64 ± 5 years; two males and two females). The N1 tissue may have suffered from a prolonged post-mortem time (85 h; Supplementary Table S1) compared with the other cases. Three-dimensional structures of these cerebral tissues were visualized with nanotomography18,19 (Fig. 1a and Supplementary Figure S2) at spatial resolutions of 180–300 nm (Supplementary Table S2). Tissue constituents, such as neurites and blood vessels visualized in the 55 obtained datasets, were traced to build three-dimensional Cartesian coordinate models of the tissues (Fig. 1b–d and Supplementary Figures S3 and S4). An example of a spiny dendrite superposed on a three-dimensional map is shown in Fig. 1e. Representative images and models of all datasets are shown in Supplementary Figures S2–S4. The statistics of the datasets and models are summarized in Supplementary Table S3. Coordinate files are available from https://mizutanilab.github.io (RRID:SCR_016529).
Fig. 1. Three-dimensional visualization of cerebral cortex neurons and their models represented with Cartesian coordinates.
The pial surface is toward the top. The three-dimensional image was rendered with VGStudio (Volume Graphics, Germany). The models were drawn using MCTrace60. Structural constituents of the model are color-coded. Nodes composing each constituent are indicated with circles. Dots indicate soma nodes. a Rendering of dataset N2C of the control N2 tissue. Voxel values of 500–1600 were rendered with the scatter HQ algorithm. Scale bar: 20 μm. b Initial model. Structures of somata and thick neurites were built manually in order to mask them in the subsequent automatic model generation. c Automatically generated model of the tissue structure. Neurites were searched by calculating the gradient vector flow61 throughout the image. The neurites found in the search were then traced using a three-dimensional Sobel filter62. d The computer-generated model was manually examined and edited according to the method used in protein crystallography. The obtained working model was refined with conjugate gradient minimization. The geometric parameters were calculated from the three-dimensional Cartesian coordinates of the refined model. e Stereo drawing of a spiny dendrite indicated with the arrow head in d. The structure is superposed on a three-dimensional map of the observed image. The map drawn in gray is contoured at 2.5 times the standard deviation (2.5 σ) of the image intensity with a grid size of 96.6 nm
Neurites in three-dimensional tissue can be regarded as three-dimensional curves. A three-dimensional curve can be represented with two parameters: curvature and torsion. Curvature corresponds to the reciprocal of the radius of the curve. Torsion represents the deviation of the curve from a plane. The neurites were divided into segments at each ramification point. The geometries of these neurite segments were analyzed by evaluating their curvature and torsion (Table 1). Spine structures were analyzed using the parameters of length, minimum radius, and maximum radius in addition to the curvature and torsion (Supplementary Table S4), since dendritic spines have been classified into several categories in terms of their neck width and length41,42. Spine density was defined as the number of spines per total length of spiny dendrites (Supplementary Tables S1 and S3).
Table 1.
Geometric parameters of neurites
| Case | Curvature (μm−1) | Torsion (μm−1) | |
|---|---|---|---|
| Total | Orphan neurite | ||
| S1 | 0.46 (0.28)/523 | 0.58 (0.30)/288 | −0.03 (0.35)/513 |
| S2 | 0.47 (0.32)/754 | 0.59 (0.34)/450 | −0.02 (0.37)/742 |
| S3 | 0.60 (0.34)/435 | 0.78 (0.32)/238 | 0.01 (0.41)/426 |
| S4 | 0.71 (0.36)/880 | 0.79 (0.36)/700 | 0.00 (0.33)/873 |
| N1 | 0.33 (0.22)/415 | 0.38 (0.21)/154 | 0.02 (0.44)/389 |
| N2 | 0.44 (0.21)/731 | 0.49 (0.21)/422 | 0.01 (0.32)/721 |
| N3 | 0.37 (0.21)/491 | 0.46 (0.21)/289 | 0.01 (0.27)/484 |
| N4 | 0.41 (0.23)/432 | 0.48 (0.24)/252 | −0.03 (0.34)/426 |
Values represent mean (sample standard deviation)/number of observations. S1–S4 are the schizophrenia cases, and N1–N4 are the control cases. The orphan neurite column represents statistics of neurites whose somata were outside of the viewing field
Neurite structures
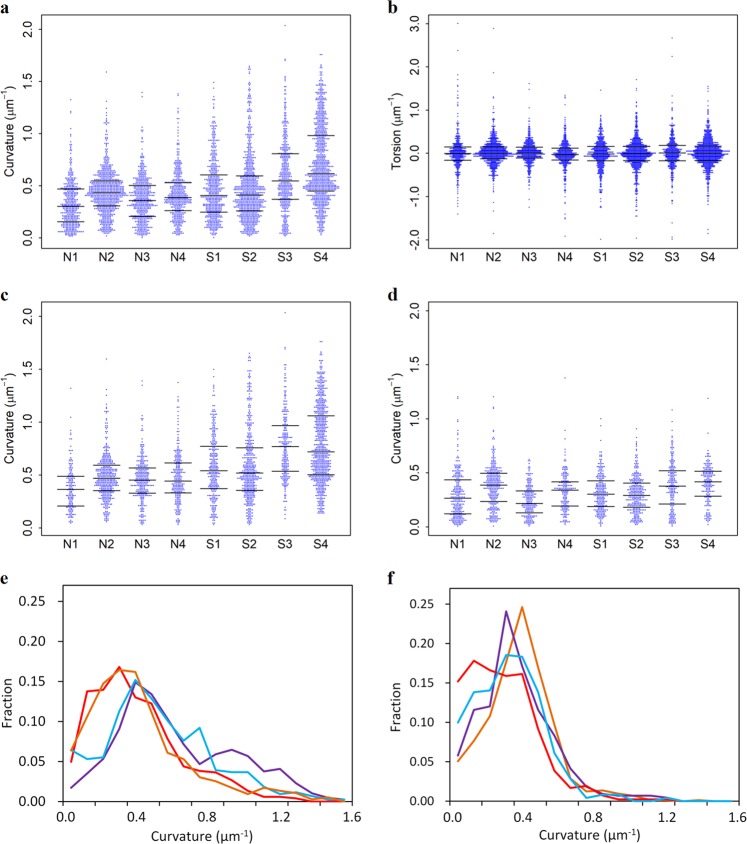
The curvatures and torsions of the neurite segments are summarized in Table 1. A total of 2737 neurite segments from the schizophrenia cases and 2254 segments from the control cases were analyzed. Figure 2a, b shows the distributions of the curvature and torsion of the segments. The curvature distribution of the schizophrenia cases exhibited long upper tails (Fig. 2a), showing a 45% increase on average. This resulted in a larger standard deviation in the curvature of the neurite segments of the schizophrenia cases (Table 1; 0.28–0.36 μm−1) in comparison with that of the control cases (0.21–0.23 μm−1). The curvature median exhibited a significant difference even between the four schizophrenia cases (p < 2.2 × 10−16 with the Kruskal–Wallis test) and between the four control cases (p < 2.2 × 10−16). In contrast, the torsion showed no apparent difference between the schizophrenia and control cases (Fig. 2b). There was no significant difference in torsion median between all the cases (p = 0.44 with the Kruskal–Wallis test). The torsion distribution of every case has a peak at the origin, indicating that the neurites have no chiral bias (Fig. 2b).
Fig. 2. Geometric analysis of neurites.
a Distribution of neurite curvature. Quartiles are indicated with bars. b Distribution of neurite torsion. c Distribution of curvature of orphan neurites without soma in the viewing field. d Distribution of curvature of neurites whose somata were visualized within the image. e Relative frequency of neurite in each 0.1 μm−1 bin of curvature. The schizophrenia S1 case is plotted in red, S2 in orange, S3 in cyan, and S4 in purple. f Neurite curvature of the control N1 case is plotted in red, N2 in orange, N3 in cyan, and N4 in purple
The neurites whose somata were visualized in the image are proximal ones within a viewing field width of 64–122 μm (Supplementary Table S2). Besides those laterally proximal structures, neurites without soma were also visualized. These orphan neurites should be distal parts of neuronal arbors whose somata were out of the viewing field. These two categories of neurites are separately plotted in Fig. 2c, d. The orphan neurites of the schizophrenia cases showed a wide curvature distribution, a 51% increase on average compared with the controls (Table 1). The mean curvatures of the orphan neurites were significantly different between the schizophrenia and control cases (Welch’s t-test, p = 0.020, four schizophrenia and four controls). These results indicate that distal neurites have high curvature in the case of schizophrenia.
The profiles shown in Fig. 2a–d vary between cases, even within the control cases, indicating that neuronal structures vary between individuals. The curvature median showed significant differences between the four control cases and also between the four schizophrenia cases, as described above. Figure 2e–f plots the relative frequency of the neurite curvatures. The frequency profiles were not identical between cases (Fig. 2e, f). The structures of the S2, S3, N1, and N3 cases were analyzed using multiple samples. The relative frequencies of the curvature in these multiple samples are separately plotted in Supplementary Figure S5. Multiple samples from the same individual have similar profiles, except that two samples of the N1 case show differences probably due to its long post-mortem time. These results suggest that the neuronal structures have features characteristic of each individual. A difference in neurite curvature results in a difference in the spatial trajectory and hence alters the neuronal circuits. The tissues analyzed in this study were taken from the anterior cingulate cortex. It has been shown that the anterior cingulate cortex has emotional and cognitive functions30,31. Therefore, the structural differences of the neurons of the anterior cingulate cortex should represent mental individuality or the psychiatric states of individuals.
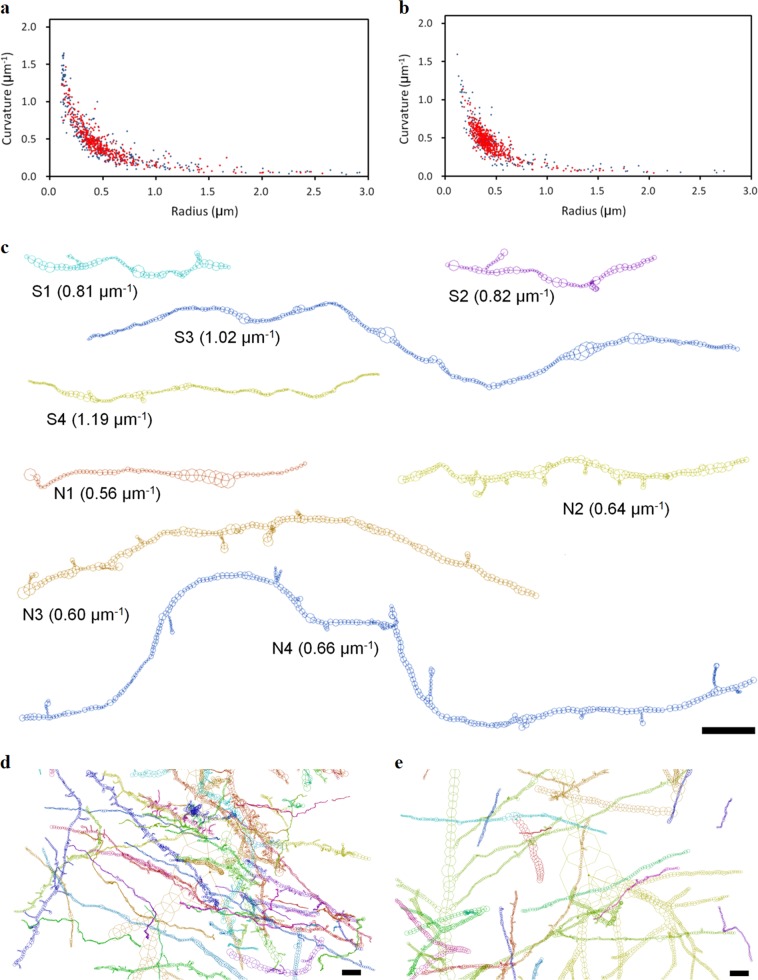
Figure 3a, b, along with Supplementary Figure S6, shows scatter plots of the mean curvature of the neurite trajectory and the mean radius of the neurite. The plots indicate reciprocal relationships between the trajectory curvature and the neurite radius. The schizophrenia cases show a wide distribution (Fig. 3a) for both spiny dendrites and smooth neurites, while the control cases show a narrow distribution (Fig. 3b). These plots indicate that the high-curvature neurites of the schizophrenia cases have a short radius.
Fig. 3. Neurites in schizophrenia and control cases.
a Scatter plot of curvature and radius of neurites in the schizophrenia S2 case. b Scatter plot of control N2 case. The horizontal axis represents the mean radius of the neurite as a fiber. Thin neurites are on the left, and thick ones are on the right. The vertical axis represents the mean curvature of the neurite trajectory. Spiny dendrites are indicated with red dots and smooth neurites with blue. Neurites with mean radii larger than 3 μm are omitted. c Neurite segments showing median curvature in the top quartile of each case. Mean curvature of each neurite is shown in parenthesis. The neurite of N1 is a branch on an apical dendrite of a pyramidal neuron. Others are orphan neurites whose somata are not visualized within the image. d Schizophrenia S4A structure. e Control N4A structure. Panels d and e were produced by placing the soma node of the largest pyramidal neuron at the figure center. The pial surface is toward the top. Structures are color-coded. Scale bars: 5 μm
Figure 3c shows representative structures of the neurites of the schizophrenia and control cases. The neurites of the schizophrenia cases exhibit frequent changes in direction (Fig. 3c), resulting in tortuous structures. The S4 neurite is thinner than the other neurites. In contrast, the neurites of the control cases show gradual and broad curves. The tissue structures of the S4 case and its age/gender-matched control N4 case are shown in Fig. 3d, e, respectively (also in Supplementary Videos S1 and S2). The structure of the S4 case is frizzy, whereas that of the N4 case is mostly straight. The S4 patient showed severe schizophrenic symptoms and bore a frame shift mutation in the GLO1 gene43,44. It has been shown that the GLO1 mutation can cause oxidative stress45. Therefore, the structural alteration of the neurites of the S4 case is ascribable to oxidative stress due to the GLO1 mutation.
Dendritic spine structures
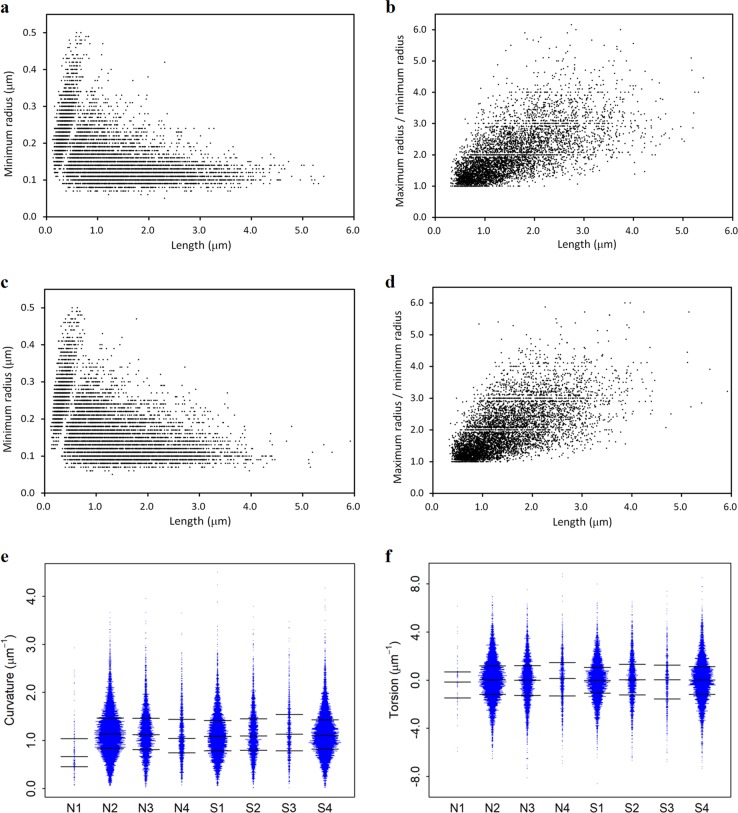
The geometric analyses of the dendritic spines are summarized in Supplementary Table S4. A total of 15,116 spines of schizophrenia cases and 12,885 spines of control cases were analyzed. It has been reported that dendritic spines can be categorized into several groups, such as mushroom spine and stubby spine41. Thin-necked and long spines can be characterized with a small minimum radius and long length, while stubby spines can be characterized with a large minimum radius and short length. Scatter plots of the spine length and minimum radius are shown in Fig. 4 and Supplementary Figure S7. The plots of the schizophrenia and control cases have similar profiles composed of a wedge-shaped domain and a triangle domain (Fig. 4a, c, Supplementary Figure S7). This indicates that dendritic spines can be categorized into two groups. Spines in the wedge domain exhibit short lengths and hence correspond to stubby spines. Their radius exhibits a nearly linear correlation to the length. Spines of the triangle domain exhibit long lengths and comparably short minimum radii and hence correspond to necked mushroom spines. The triangle distribution indicates that a longer spine has a thinner neck (Fig. 4a, c). The presence of a neck affects the ratio between maximum and minimum radii. However, the scatter plots of the radius ratio and spine length (Fig. 4b, d, Supplementary Figure S7) show no distinctive domains. This indicates that the neck morphology is continuous42. The schizophrenia and control cases show similar profiles in all the plots, suggesting that structures of dendritic spines in the schizophrenia cases analyzed in this study were the same as those of the controls.
Fig. 4. Geometric analysis of dendritic spines.
a Scatter plot of spine parameters in the schizophrenia S4 case. Minimum node radius is plotted against length. Three outliers (length/radius = 7.51/0.13, 6.24/0.10, and 6.23/0.10) were omitted. b Radius ratio between maximum/minimum radii of S4 is plotted against length. Four outliers (length/ratio = 7.51/2.77, 6.24/2.90, 6.23/3.70, and 3.55/7.43) were omitted. c Minimum radius of N2. An outlier (length/radius = 7.88/0.14) was omitted. d Radius ratio of N2. An outlier (length/ratio = 7.88/3.14) was omitted. e Distribution of spine curvature. Quartiles are indicated with bars. f Distribution of spine torsion
It has been reported that spine density in the external pyramidal layer (layer III) of the frontal cortex2 and the temporal cortex3 is significantly lower in schizophrenia cases than in control cases. It has also been reported that the difference in spine density is not significant in the internal pyramidal layer of the frontal cortex46 or in the external pyramidal layer of the occipital cortex2. In this study, we analyzed the spine structures mainly in the internal pyramidal layer of the anterior cingulate cortex. The obtained spine density per dendritic length is summarized in Supplementary Table S1. The spine density was comparable to those observed in the Golgi-stained frontal cortex47. No significant difference in mean spine density was found between the four schizophrenia and four control cases (Welch’s t-test, p = 0.56), though the sample size of this study is small. The spine density of the N1 case was lower than in the other cases, presumably due to the long post-mortem time. Except for this case, the curvatures and torsions of the spines showed similar distribution profiles (Fig. 4e, f), suggesting that the schizophrenia and control cases analyzed in this study share common spine structures.
Discussion
In the S4 schizophrenia case, the high curvature and short radius of the neurites should stem from the GLO1 frameshift mutation44. Although similar structural alterations were also observed in the S1, S2, and S3 schizophrenia cases, their causes are not clear at present. Some adverse effects similar to oxidative stress of the GLO1 mutation should have degraded the neuronal structures. Tortuous neurites have been observed in the cerebral cortex of schizophrenic brain48. It has been reported that the schizophrenia-susceptible DISC1 protein49 interacts with a number of factors associated with neuronal functions50. Apical dendrites of dentate gyrus neurons showed morphological alterations in mice carrying a DISC1 mutation51. A significant decrease in dendritic diameter was reported for a Shn2 knockout mouse with schizophrenia-like symptoms52. The disruption of any susceptible genes related to the neuronal structure or environmental risk factors that affect brain development can alter the geometry of neurites.
N-methyl-d-aspartate (NMDA) receptor antagonists including phencyclidine cause psychiatric symptoms similar to those of schizophrenia53. A corkscrew deformity of dendrites was reported in an animal model of the NMDA receptor hypofunction54. These reports suggest that the high-curvature neurites observed in this study are related to schizophrenia symptoms. However, we cannot exclude the possibility that antipsychotics affected the neuronal structures. Such drug effects can be elucidated by using nanotomography to analyze brain tissues of drug-treated animals.
The neurite of the schizophrenia cases showed higher curvature and shorter radius compared with the controls. The radius of the neurite affects its conductivity, resulting in altered connections between neurons. This neurite thinning should have a relationship to the tissue volume reduction observed in schizophrenia6–8. It has been suggested that a neurodevelopmental defect in the neuropil can explain the loss of cortical volume without loss of neurons55. The curvature of a neurite determines its spatial trajectory. A curve with a higher curvature reaches more positions in its three-dimensional vicinity, but needs to be longer to reach a distal position compared with a straight line. This can alter the connectivity of the neuronal circuit. It would be difficult to relieve or restore these nanometer-scale structural alterations of the tissue. Therefore, the deteriorative outcome of structurally altered neurons should be prevented before their incorporation into the neuronal circuit. The results obtained in this study hence support the consensus that early diagnosis and treatment is important for a better prognosis of schizophrenia.
A disadvantage of X-ray visualization of brain tissue is that the neurons show little contrast in X-ray images, since they are composed of light elements. In this study, cerebral tissues were stained with Golgi impregnation in order to label their neurons with silver. Therefore, the obtained results are tempered by the limitations of the staining method. Since only a small number of neurons are stochastically visualized in Golgi impregnation, the labeled neurons are limited representatives of the neuronal population56. The viewing field width and number of cases are also limitations. Although hundreds of neurites and thousands of spine structures were analyzed for each case, the results reported in this paper are of millimeter-sized tissues of the anterior cingulate cortex of four schizophrenia and four control cases. Another limitation of our case-control study is that the controls were not psychiatrically evaluated. There is a possibility that the control cases had latent mental diseases, although no geometric hallmark of schizophrenia was observed in them.
Biological individuality is encoded in the genome. At the macroscopic level, individuals are identified from the body structure, such as face, fingerprints, or overall brain connectivity57. However, at the cellular level, little evidence of mental personality has been delineated on the basis of neuronal structures, though histological studies suggested differences of cellular structures between individuals58,59. The results reported in this paper reveal differences in the tissue structure of the anterior cingulate cortex between schizophrenia and control cases, and also between control individuals. This suggests that geometric profiles of brain tissue are different between individuals. Structural differences of cerebral neurons can result in differences in brain circuits, and hence affect the mental individuality.
Human mental activities are performed through coordination of many diverse areas of the brain and cannot be explained from one study of a single area. The temporal lobe has been reported to show a tissue volume reduction in schizophrenia9, and hence, it should be analyzed with the same strategy. The results of this study also suggest that humans have nanometer- to micrometer-scale structural diversity in the cerebral cortex. Although the present sample size was sufficient for evaluating the statistical significance of the structural difference, schizophrenia is a complex and heterogeneous psychiatric disorder1,9. Therefore, the differences observed in this study should be re-examined by analyzing more three-dimensional structures of cerebral tissues of schizophrenia and control cases. Such further analyses will lead to a better understanding of our mental individuality and to better diagnosis and treatment of schizophrenia.
Supplementary information
Acknowledgements
We are grateful to Prof. Motoki Osawa and Akio Tsuboi (Tokai University School of Medicine) for their generous support of this study. We are also grateful to Prof. Yasuo Ohashi (Chuo University; Statcom Co., Ltd.) for his helpful advice regarding the statistical tests. We appreciate Prof. Yoshiro Yamamoto (Tokai University) for his helpful advice regarding the data analysis. We also appreciate Dr. Jun Horiuchi (Tokyo Metropolitan Institute of Medical Science) for his suggestions regarding the manuscript. We thank Noboru Kawabe (Support Center for Medical Research and Education, Tokai University) for assistance in preparing the histology sections. We also thank the Technical Service Coordination Office of Tokai University for assistance in preparing adapters for nanotomography. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (nos. 21611009, 25282250, and 25610126). The synchrotron radiation experiments at SPring-8 were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) given to R.M. (proposal nos. 2011B0034, 2012B0034, 2013A0034, 2013B0034, 2013B0041, 2014A1057, 2015A1160, 2015B1101, 2017A1143, 2018A1164, and 2018B1187). The synchrotron radiation experiments at the Advanced Photon Source of Argonne National Laboratory were performed during the 2016-2 and 2017-3 runs under General User Proposal GUP-45781 by R.M. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Code availability
The RecView software36 that was used for the tomographic reconstruction is available from https://mizutanilab.github.io under the BSD 2-Clause License. The model building and geometric analysis procedures were implemented in the MCTrace software60 available from https://mizutanilab.github.io under the BSD 2-Clause License.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-019-0427-4).
References
- 1.Carpenter WT, Jr., Buchanan RW. Schizophrenia. N. Engl. J. Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 2.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci. Lett. 2015;601:46–53. doi: 10.1016/j.neulet.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/S0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 6.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Olabi B, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Haijma SV, et al. Brain volumes in schizophrenia: a meta-analysis in over 18,000 subjects. Schizophr. Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakhshi K, Chance SA. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. doi: 10.1016/j.neuroscience.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Helmstaedter M. Cellular-resolution connectomics: challenges of dense neural circuit reconstruction. Nat. Methods. 2013;10:501–507. doi: 10.1038/nmeth.2476. [DOI] [PubMed] [Google Scholar]
- 11.Peddie CJ, Collinson LM. Exploring the third dimension: volume electron microscopy comes of age. Micron. 2014;61:9–19. doi: 10.1016/j.micron.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand DGC, et al. Whole-brain serial-section electron microscopy in larval zebrafish. Nature. 2017;545:345–349. doi: 10.1038/nature22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- 14.Hanslovsky P, Bogovic JA, Saalfeld S. Image-based correction of continuous and discontinuous non-planar axial distortion in serial section microscopy. Bioinformatics. 2017;33:1379–1386. doi: 10.1093/bioinformatics/btw794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson T. Resolution and optical sectioning in the confocal microscope. J. Microsc. 2011;244:113–121. doi: 10.1111/j.1365-2818.2011.03549.x. [DOI] [PubMed] [Google Scholar]
- 16.Ascoli GA. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 2006;7:318–324. doi: 10.1038/nrn1885. [DOI] [PubMed] [Google Scholar]
- 17.Watson KK, Jones TK, Allman JM. Dendritic architecture of the von Economo neurons. Neuroscience. 2006;141:1107–1112. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Takeuchi A, Terada Y, Uesugi K, Mizutani R. Recent progress of hard x-ray imaging microscopy and microtomography at BL37XU of SPring-8. AIP Conf. Proc. 2016;1696:020013. doi: 10.1063/1.4937507. [DOI] [Google Scholar]
- 19.De Andrade, V. et al. Nanoscale 3D imaging at the Advanced Photon Source. SPIE Newsroom10.1117/2.1201604.006461 (2016).
- 20.Takeuchi A, Uesugi K, Takano H, Suzuki Y. Submicrometer-resolution three-dimensional imaging with hard x-ray imaging microtomography. Rev. Sci. Instrum. 2002;73:4246–4249. doi: 10.1063/1.1515385. [DOI] [Google Scholar]
- 21.Schroer CG, et al. Nanotomography based on hard x-ray microscopy with refractive lenses. Appl. Phys. Lett. 2002;81:1527–1529. doi: 10.1063/1.1501451. [DOI] [Google Scholar]
- 22.Chu YS, et al. Hard-x-ray microscopy with Fresnel zone plates reaches 40nm Rayleigh resolution. Appl. Phys. Lett. 2008;92:103119. doi: 10.1063/1.2857476. [DOI] [Google Scholar]
- 23.Stampanoni M, et al. Broadband X-ray full field microscopy at a superbend. J. Phys. Conf. Ser. 2009;186:012018. doi: 10.1088/1742-6596/186/1/012018. [DOI] [Google Scholar]
- 24.Kaira CS, et al. Probing novel microstructural evolution mechanisms in aluminum alloys using 4D nanoscale characterization. Adv. Mater. 2017;29:1703482. doi: 10.1002/adma.201703482. [DOI] [PubMed] [Google Scholar]
- 25.Müller S, et al. Quantification and modeling of mechanical degradation in lithium-ion batteries based on nanoscale imaging. Nat. Commun. 2018;9:2340. doi: 10.1038/s41467-018-04477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizutani R, et al. Submicrometer tomographic resolution examined using a micro-fabricated test object. Micron. 2010;41:90–95. doi: 10.1016/j.micron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Beckmann F, Bonse U, Busch F, Günnewig O. X-ray microtomography (microCT) using phase contrast for the investigation of organic matter. J. Comput. Assist. Tomogr. 1997;21:539–553. doi: 10.1097/00004728-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani R, Takeuchi A, Hara T, Uesugi K, Suzuki Y. Computed tomography imaging of the neuronal structure of Drosophila brain. J. Synchrotron Radiat. 2007;14:282–287. doi: 10.1107/S0909049507009004. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani R, et al. Three-dimensional microtomographic imaging of human brain cortex. Brain Res. 2008;1199:53–61. doi: 10.1016/j.brainres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 32.Bouras C, Kövari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 33.Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol. Psychiatry. 1999;45:1542–1552. doi: 10.1016/S0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg DR, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- 36.Mizutani R, et al. Microtomographic analysis of neuronal circuits of human brain. Cereb. Cortex. 2010;20:1739–1748. doi: 10.1093/cercor/bhp237. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi A, Uesugi K, Suzuki Y. Zernike phase-contrast x-ray microscope with pseudo-Kohler illumination generated by sectored (polygon) condenser plate. J. Phys. Conf. Ser. 2009;186:012020. doi: 10.1088/1742-6596/186/1/012020. [DOI] [Google Scholar]
- 38.Mizutani R, Taguchi K, Takeuchi A, Uesugi K, Suzuki Y. Estimation of presampling modulation transfer function in synchrotron radiation microtomography. Nucl. Instrum. Meth A. 2010;621:615–619. doi: 10.1016/j.nima.2010.03.111. [DOI] [Google Scholar]
- 39.Mizutani R, et al. A method for estimating spatial resolution of real image in the Fourier domain. J. Microsc. 2016;261:57–66. doi: 10.1111/jmi.12315. [DOI] [PubMed] [Google Scholar]
- 40.Cloetens P, et al. Holotomography: quantitative phase tomography with micrometer resolution using hard synchrotron radiation x rays. Appl. Phys. Lett. 1999;75:2912–2914. doi: 10.1063/1.125225. [DOI] [Google Scholar]
- 41.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 42.Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai M, et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch. Gen. Psychiatry. 2010;67:589–597. doi: 10.1001/archgenpsychiatry.2010.62. [DOI] [PubMed] [Google Scholar]
- 44.Arai M, et al. Pentosidine accumulation in the pathophysiology of schizophrenia: overview of schizophrenia with carbonyl stress. IMARS Highlights. 2014;9:9–16. [Google Scholar]
- 45.Arai M, et al. Carbonyl stress and schizophrenia. Psychiatry Clin. Neurosci. 2014;68:655–665. doi: 10.1111/pcn.12216. [DOI] [PubMed] [Google Scholar]
- 46.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb. Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 48.Tatetsu S. A histopathological study of schizophrenic brains: findings in telencephalon. Psychiatr. Et. Neurol. Jpn. 1960;62:20–43. [Google Scholar]
- 49.Blackwood DH, et al. Schizophrenia and affective disorders -- cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandon NJ, et al. Understanding the role of DISC1 in psychiatric disease and during normal development. J. Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc. Natl Acad. Sci. USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakao A, et al. Immature morphological properties in subcellular-scale structures in the dentate gyrus of Schnurri-2 knockout mice: a model for schizophrenia and intellectual disability. Mol. Brain. 2017;10:60. doi: 10.1186/s13041-017-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr. Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wozniak DF, et al. Disseminated corticolimbic neuronal degeneration induced in rat brain by MK-801: potential relevance to Alzheimer’s disease. Neurobiol. Dis. 1998;5:305–322. doi: 10.1006/nbdi.1998.0206. [DOI] [PubMed] [Google Scholar]
- 55.Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J. Anat. 2010;217:324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finn ES, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerdes MJ, et al. Emerging understanding of multiscale tumor heterogeneity. Front. Oncol. 2014;4:366. doi: 10.3389/fonc.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natrajan R, et al. Microenvironmental heterogeneity parallels breast cancer progression: a histology-genomic integration analysis. PLoS Med. 2016;13:e1001961. doi: 10.1371/journal.pmed.1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizutani R, Saiga R, Takeuchi A, Uesugi K, Suzuki Y. Three-dimensional network of Drosophila brain hemisphere. J. Struct. Biol. 2013;184:271–279. doi: 10.1016/j.jsb.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Xu C, Prince JL. Snakes, shapes, and gradient vector flow. IEEE Trans. Image Process. 1998;7:359–369. doi: 10.1109/83.661186. [DOI] [PubMed] [Google Scholar]
- 62.Al-Kofahi KA, et al. Rapid automated three-dimensional tracing of neurons from confocal image stacks. IEEE Trans. Inf. Technol. Biomed. 2002;6:171–187. doi: 10.1109/TITB.2002.1006304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.