Abstract
Long noncoding RNAs (lncRNAs) have been indicated as important regulators in various human cancers. However, the overall biological roles and clinical significance of most lncRNAs in colon carcinogenesis are not fully understood. Hence, we investigated the clinical significance, biological function and mechanism of LINC01234 in colon cancer. First, we analyzed LINC01234 alterations in colon cancer tissues and corresponding paracancerous tissues through the analysis of sequencing data obtained from The Cancer Genome Atlas and colon cancer patients. Next, we evaluated the effect of LINC01234 on colon cancer cell proliferation and its regulatory mechanism of serine hydroxymethyltransferase 2 (SHMT2) by acting as a competing endogenous RNA (ceRNA). We found that LINC01234 expression was significantly upregulated in colon cancer tissues and was associated with a shorter survival time. Furthermore, the knockdown of LINC01234 induced proliferation arrest via suppressing serine/glycine metabolism. Mechanistic investigations have indicated that LINC01234 functions as a ceRNA for miR-642a-5p, thereby leading to the derepression of its endogenous target serine hydroxymethyltransferase 2 (SHMT2). LINC01234 is significantly overexpressed in colon cancer, and the LINC01234–miR642a-5p–SHMT2 axis plays a critical role in colon cancer proliferation. Our findings may provide a potential new target for colon cancer diagnosis and therapy.
Subject terms: Colon cancer, Cell growth
Introduction
Colon cancer is the third most common cancer and the fourth leading cause of cancer death in the world1. By the year 2020, colon cancer is expected to increase by more than 2.2 million new cases and 1.1 million cancer deaths2. Despite recent progresses in the clinical diagnosis and treatment for colon cancer, the overall survival rate of colon cancer patients has not substantially increased3,4. In most patients, colon cancer is diagnosed at an advanced stage and is accompanied by malignant proliferation. Therefore, it is vital to identify further molecular mechanisms underlying colon cancer proliferation5,6.
Long noncoding RNAs (lncRNAs), a set of RNAs that are generally longer than 200 nucleotides in length, are emerging as novel important regulators in the tumorigenesis of cancer7,8. The mechanism underlying the function of lncRNA in cancer is very complicated. Generally, lncRNAs exert their function through regulating underlying target gene expression at the epigenetic, transcriptional, and posttranscriptional levels9–11, and then impact tumor proliferation12, apoptosis13, and metastasis14. Recently, a new regulatory mechanism has been identified in which crosstalk between lncRNAs and mRNAs occurs by competing for shared microRNA (miRNA) response elements (MREs), termed competing endogenous RNAs (ceRNAs)15. Increasing evidence has verified that ceRNA is a very important pathway in cancer progress regulation. For example, Lu et al. demonstrated that lncRNA BC032469 could function as a ceRNA to impair miR-1207–5p-dependent hTERT downregulation in gastric cancer16. The long noncoding RNA GAS5 could negatively regulate the adipogenic differentiation of MSCs by modulating the miR-18a/CTGF axis as a ceRNA17. Previously, our preliminary lncRNA microarray data showed that many lncRNA were dysregulated in colon cancer tissues18. Therefore, we propose that some lncRNAs may also play roles as ceRNAs, linking miRNAs and the posttranscriptional network in colon cancer.
LINC01234 (ENSG00000249550) is a highly abundant, conserved mammalian noncoding RNA, located at 12q24.13. As a novel molecule in the field of tumor biology, LINC01234 initially became well known for its involvement in predicting breast cancer survival19. Furthermore, LINC01234 was proven to be positively correlated with malignant processes and a poor outcome in gastric cancer20 and ovarian cancer21. Although Chen X et al. proved that the overexpression of LINC01234 promotes gastric cancer apoptosis and growth22, the overall biological role and underlying molecular mechanism of LINC01234 in cancer proliferation remain unclear. Tumors have high energetic and anabolic needs for rapid cell growth and proliferation23, and the serine/glycine metabolism pathway was recently identified as an important source of metabolic intermediates for these processes24. Serine hydroxymethyl transferase 2 (SHMT2)25, phosphoserine aminotransferase 1 (PSAT1)26, phosphoglycerate dehydrogenase (PHGDH)27 have been reported as key regulators in the serine/glycine metabolism pathway and are involved in cancer proliferation28. However, there is no study concerning the effect of lncRNA regulation on the serine/glycine metabolism pathway.
In the present study, we determined that LINC01234 upregulation is a characteristic molecular change in colon cancer and investigated the biological roles of LINC01234 on serine/glycine metabolism and proliferation for the first time. Moreover, mechanistic analysis has revealed that LINC01234 may function as a ceRNA to regulate the expression of SHMT2 through competition for miR-642a-5p, thus playing an important role in colon cancer progression29,30. The present work will provide new light on colon cancer diagnosis and therapy.
Materials and Methods
Tissue collection
Twenty pairs of colon cancer tissues and adjacent noncancer tissues were collected from 20 patients who underwent surgical resection of colon cancer at the Third XiangYa Hospital of Central South University (Changsha, China) after informed consent was obtained. The study was approved by the ethics committee of the Third XiangYa Hospital of Central South University. Patient consent was obtained from both the patients and patients’ families. The noncancer tissue samples were 5 cm from the edge of the tumors, and all samples were identified by a pathologist. Before the surgical resections, no preoperative treatment has been administered. After surgical resection, all tissue samples were immediately frozen in liquid nitrogen and were stored at −80 °C.
Immunohistochemistry
Total SHMT2 proteins in colon cancer tissue were detected by immunohistochemistry using rabbit SHMT2 antibody (GTX125939; GeneTex, USA). Sections from CRC tissues were cut to 5 μm in thickness. The presence of total SHMT2 protein was detected using the DAB staining kit (Auragene, China) according to the manufacturer’s instructions.
Cell lines and culture conditions
Human colonic epithelial cell lines NCM460 and colon cancer cell lines HT29, LoVo, SW480 and HCT116 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). NCM460 was cultured with McCoy’s 5a supplemented with 10% fetal bovine serum (Gibco, USA). HT-29, LoVo, SW480 and HCT116 were cultured in Dulbecco’s Modified Eagle Medium (HyClone, USA) supplemented with 10% fetal bovine serum.
RNA extraction and qRT-PCR analyses
Total RNA was extracted from cells using the TRIZOL reagent (Invitrogen, China), and the RNA was reverse transcribed using the Reverse Transcription Kit (Takara, China). The amount and quality of RNA were analyzed using the NanoDrop Lite system (Thermo, USA). The quantitative reverse transcriptase PCR (qRT-PCR) analyses were performed using SYBR Green qPCR Mix (TOYOBO, China) with an ABI 7300 instrument (Life Technology, USA). The ΔCt values of target genes were normalized to GAPDH. All the primers for qRT-PCR were purchased from RiboBio Co. Ltd. (Guangzhou, China), and the primer sequences are shown in Supplemental Table 1.
Western blot analysis
Proteins were extracted from colon cancer cells and tissues with RIPA lysis buffer (Auragene Bioscience, China) supplemented with a protease inhibitor cocktail (Auragene Bioscience, China) and phenylmethanesulfonyl fluoride (PMSF; Auragene Bioscience, China). Equal amounts (10 μg) of proteins were subjected to SDS-PAGE, and then they were transferred to a PVDF Immobilon-P membrane (Millipore, USA). The membrane was blocked with 3% BSA-TBST at room temperature for 90 min. Additionally, the membrane was continuously probed with primary antibodies at 4 °C overnight. Next, the membranes were washed and incubated with specific secondary antibodies. A GAPDH antibody was used as a control, and SHMT2 (1:1000; Cell Signaling) antibodies were used for each group.
Transfection
To knockdown the expression of LINC01234 and SHMT2, short hairpin RNA interference vectors were constructed by Auragene Bioscience of China. The nucleotide sequences of shRNAs for LINC0123422 and SHMT231 were synthesized according to a previously described sequence, and the sequences are listed in Supplemental Table 1. Human LINC01234 and SHMT2 transcript cDNA and short hairpin RNA directed against LINC01234 and SHMT2 were inserted into the pCDNA3.1 and pRNAT-U6.1/Neo vectors. The miR-642a-5p mimic (miR10003312–1–5) and inhibitors (miR20003312–1–5) were purchased from RiboBio Co. Ltd. (Guangzhou, China). Plasmid vectors (pCDNA-LINC01234, sh-LINC01234, pCDNA-SHMT2, sh-SHMT2, and empty vectors) for transfection were prepared using DNA Midiprep or Midiprep kits (Qiagen, Hilden, Germany) and were transfected into LoVo and HCT116 cells, according to the manufacturer’s instructions. The transfected cells expressing each vector were selected with 5 μg/mL of puromycin (BioFROXX, German) for ten constitutive days. The LINC01234 expression levels were examined by qRT-PCR, and the SHMT2 expression levels were examined by qRT-PCR and Western blotting.
Cell proliferation assay
The cell proliferation assay was conducted using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) solution (Sangon Biotech, China). Cells were seeded into 96-well plates at an initial density of 5000 cells/well at 48 h after transfection. At the beginning of culture, as well as at 24 h, 48 h and 72 h after starting the culture, the cells were treated with 10 μl of MTT solution. After 4 h of treatment, the medium was removed carefully, and 150 μl of DMSO solution (MP Biomedicals, USA) was added to lyse the cells. Finally, after 10 min of lysis, the absorbance was measured at 570 nm using a microplate reader (Multiskan MK; Thermo Scientific, USA).
Plate colony formation assay
Five hundred LoVo and HCT116 cells were counted and seeded into six-well plates and then were cultured at 37 °C in a humidified incubator with 5% CO2 for 10–14 d until the colonies were visible. The cells were washed with PBS and fixed with methanol for 30 min. The colonies were stained with crystal violet solution for 30 min and were washed with PBS. After air drying, the colonies with more than 50 cells were counted. For each treatment group, the wells were assessed in triplicate, and the experiments were independently repeated three times.
Gas Chromatography/Time-of-flight Mass Spectrometry analysis
Gas chromatography/time-of-flight mass spectrometry (GC/TOFMS) analysis was performed as previously described32,33. Generally, metabolic measurements of LoVo and HCT116 cells were carried out by GC/TOFMS analysis using an Agilent 7890 gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer. The system utilized a DB-5MS capillary column coated with 5% diphenyl cross-linked with 95% dimethylpolysiloxane. A 1-µL aliquot of the analyte was injected into a splitless injector. Helium was used as the carrier gas, the front inlet purge flow was 3 mL min−1, and the gas flow rate through the column was 20 mL min−1. The initial temperature was kept at 50 °C for 1 min, and then was raised to 330 °C at a rate of 10 °C min−1, followed by maintenance for 5 min at 330 °C. The injection, transfer line, and ion source temperatures were 280, 280, and 250 °C, respectively. The energy was −70 eV in the electron impact mode. The mass spectrometry data were acquired in the full-scan mode with an m/z range of 30–600 at a rate of 20 spectra per second after a solvent delay of 360 sec.
RNA immunoprecipitation
RNA immunoprecipitation (RIP) was used to investigate whether LINC01234 and miR-642a-5p could interact or bind with the potential binding protein Ago2 in LoVo and HCT116 cells. We used the EZMagna RIP kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. LoVo and HCT116 cells were lysed and incubated with protein A magnetic beads that were conjugated with antibodies at 4 °C. After 6 h, the beads were washed with wash buffer and then were incubated with 0.1% SDS/0.5 mg/ml Proteinase K for 30 min at 55 °C to remove proteins. Finally, immunoprecipitated RNA was subjected to qRT-PCR analysis to demonstrate the presence of LINC01234 and miR-642a-5p using specific primers.
Dual luciferase reporter assay
The complementary DNA fragment containing the wild-type (LINC01234-WT) or mutant LINC01234 (LINC01234-MUT) fragment were subcloned downstream of the luciferase gene within the psi-CHECK2 luciferase reporter vector. The 3ʹ untranslated region (UTR) of SHMT2 (SHMT2-WT) and corresponding mutant (SHMT2-MUT) were subcloned downstream of the luciferase gene within the psi-CHECK2 luciferase reporter vector. The miR-642a-5p mimic or miR-642a-5p inhibitor was cotransfected with LINC01234-WT or LINC01234-MUT reporter vectors using transfection reagent (Invitrogen, USA). Forty-eight hours after transfection, firefly and renilla luciferase activities in cell lysates were consecutively measured using the Dual-Luciferase Reporter Assay Kit (Promega, USA). Similarly, SHMT2-WT or SHMT2-MUT was cotransfected with the miR-642a-5p mimic or miR-642a-5p inhibitor for analysis.
Animals
For the tumor proliferation experiment, BALB/C nude mice (N = 40), aged 4–6 weeks, were purchased from the Hunan SJA Laboratory Animal Co., Ltd. Forty mice were randomly allocated into the following eight groups, containing 5 mice each: LoVo and HCT116 clones (negative control cells, sh-LINC01234 cells, sh-SHMT2 cells and miR-642a-5p cells). During the experiments, the animals were observed for any clinically relevant abnormalities daily. Tumors were first generated in BALB/C mice by subcutaneously implanting 1 × 106 LoVo or HCT116 cells into the right axillae of the mice. When the tumor volume reached 50 mm3, the tumors were measured twice a week, and the volume was calculated using the following formula: volume = width2 × length × 0.5234. All mice were monitored daily and euthanized after 4 weeks, followed by harvesting of the primary tumors.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM, USA) and GraphPad Prism 5 (GraphPad Software, USA). The data were expressed as means ± SD. Paired t test was used for statistical analyses between the groups. Differences between multiple groups were determined by one-way ANOVA. Spearman’s correlation analysis was used to calculate the correlation among LINC01234, miR-642a-5p and SHMT2, as appropriate, and P < 0.05 was statistically significant.
Results
LINC01234 is identified as an upregulated lncRNA and is associated with a poor prognosis in colon cancer
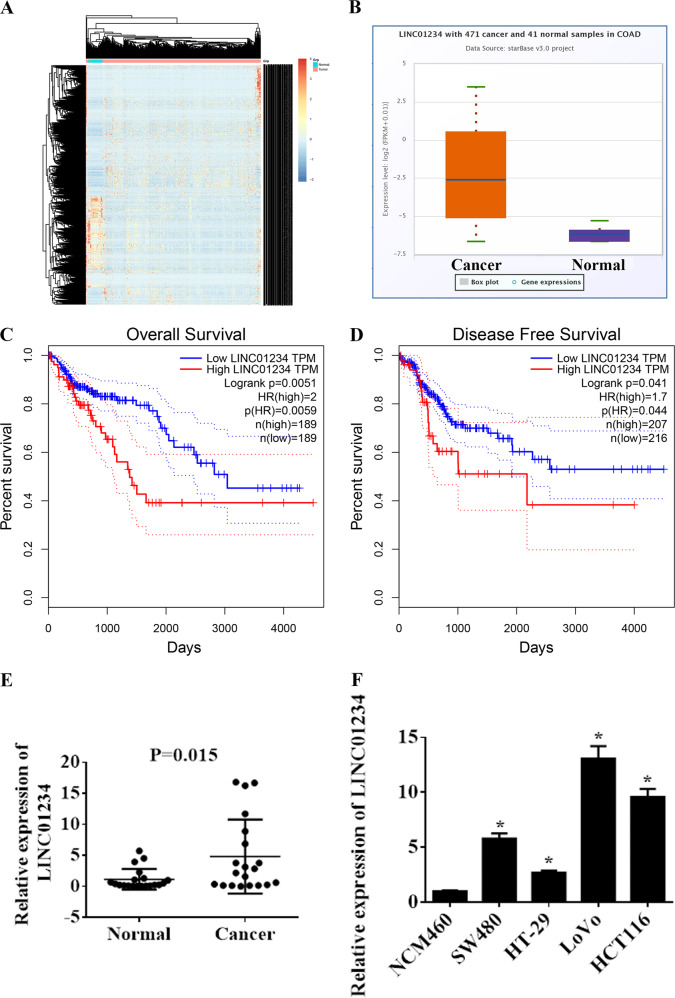
To identify dysregulated lncRNAs in colon cancer, we first searched for dysregulated lncRNAs in colon cancer by bioinformatics analysis of TCGA Colon Adenocarcinoma Datasets (COAD). According to the criteria for selecting DEGs, 1583 dysregulated lncRNAs were identified (Fig. 1a, Supplemental Table 2) (P < 0.01 and fold change > 1). Among them, LINC01234 was significantly upregulated in tumor tissues compare with that in nontumor tissues (Fig. 1b). We also examined the correlation between the LINC01234 expression level and the prognosis of colon cancer patients. Kaplan–Meier survival analysis showed that patients with higher LINC01234 levels had shorter overall survival and disease-free survival rates than those with lower levels of LINC01234 (Fig. 1c, d). Next, we measured the expression level of LINC01234 in 20 paired colon cancer tissues and adjacent noncancer tissues. Our result indicated that LINC01234 expression was significantly elevated in colon cancer tissues compared with that in adjacent noncancer tissues (P = 0.015) (Fig. 1e). Meanwhile, the expression level of LINC01234 was detected in 4 human colon cancer cell lines (HCT116, HT-29, LoVo, and SW480) and normal human colonic epithelial NCM460 cells via qRT-PCR analysis (Fig. 1f). LoVo and HCT116 cells with relatively higher LINC01234 expression was selected for subsequent functional assays.
Fig. 1.
LINC01234 expression is upregulated in colon cancer and is associated with poor prognosis. a Hierarchically clustered heatmap of upregulated and downregulated lncRNAs in TCGA COAD. b The relative expression of LINC01234 in colon cancer tissues compared with normal tissue was analyzed using TCGA COAD. c, d Kaplan–Meier overall survival and disease-free survival curves according to LINC01234 expression levels. e LINC01234 expression was analyzed by qRT-PCR in colon cancer tissues and corresponding adjacent nontumor tissues, and the data were presented as 2-△CT values (n = 20). f LINC01234 expression was analyzed by qRT-PCR in 4 human colon cancer cell lines (HCT116, HT-29, LoVo, and SW480) and normal human colonic epithelial NCM460 cells. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
Knockdown of LINC01234 inhibits colon cancer cell proliferation via suppressing serine/glycine metabolism
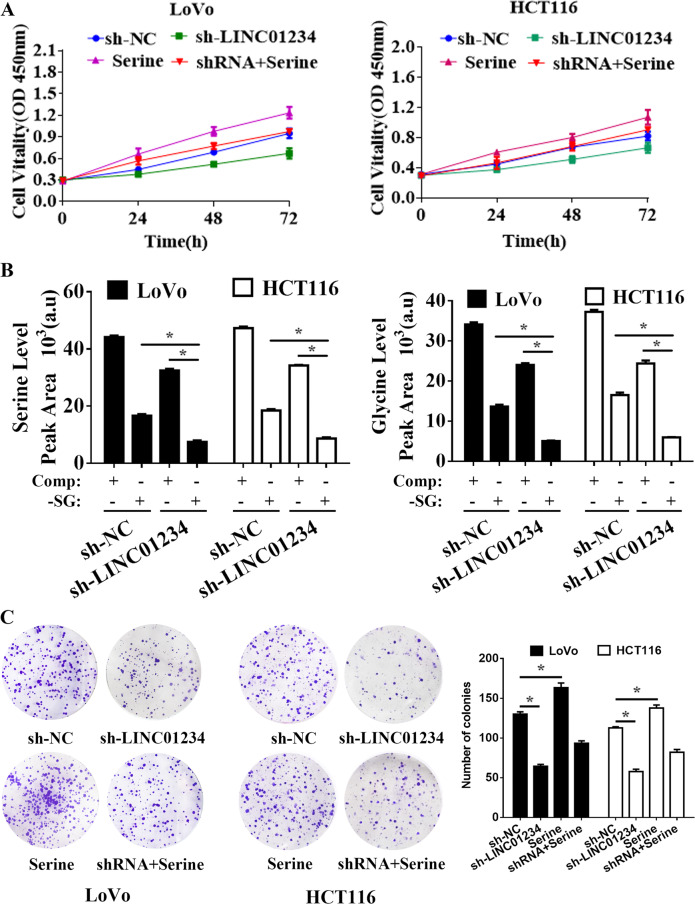
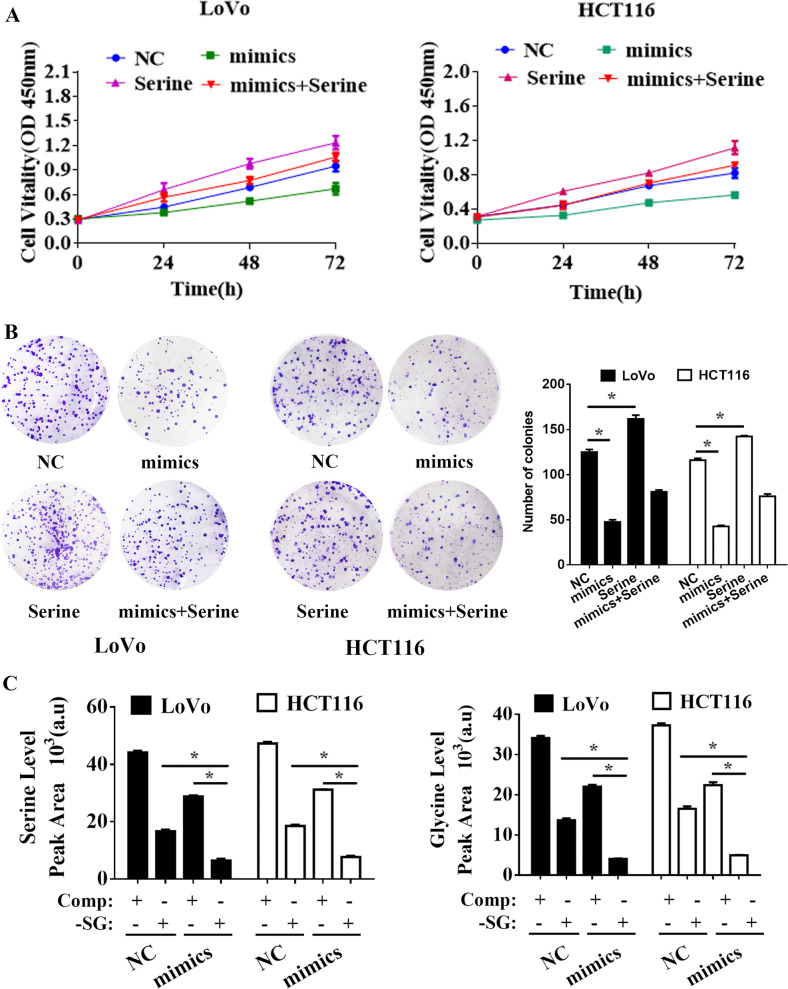
LINC01234 short hairpin RNA interference vectors were transfected into LoVo and HCT116 cells, and qRT-PCR was used to demonstrate LINC01234 silencing (Supplemental Fig. 1). Downregulation of LINC01234 substantially inhibited the cell proliferation of colon cancer cells, as indicated by MTT assay and colony formation assays (Fig. 2a, c). Because the serine/glycine biosynthetic pathway had already been proven to play an important role in cancer cell proliferation35, we applied additional cell samples (cultured in serine/glycine-deprived medium, same as follows) for GC-TOFMS examination to confirm whether LINC01234 silencing affects serine/glycine biosynthesis. The results showed that colon cancer cells serine and glycine levels were further reduced when LINC01234-depleted colon cancer cells were cultured in serine/glycine-deprived medium (Fig. 2b). Additionally, serine treatment could significantly promote colon cancer cell proliferation and reversed the effect of LINC01234 silencing (Fig. 2a and c). Collectively, these results demonstrated that the knockdown of LINC01234 exerted a critical effect on inhibiting colon cancer cell proliferation via suppressing serine/glycine metabolism.
Fig. 2.
Knockdown of LINC01234 inhibits colon cancer cell proliferation via suppressing serine/glycine metabolism. a MTT assays were used to determine the viability of negative control (NC), sh-LINC01234, serine treatment or serine treatment sh-LINC01234-transfected LoVo cells and HCT116 cells. b Stable isotope tracing experiments. LoVo cells and HCT116 cells expressing sh-NC or sh-LINC01234 were cultured in complete medium (Comp) or serine/glycine-deprived medium (-SG) for 24 h. GC-MS was used to detect the relative intracellular levels of serine (left) or glycine (right). Histobars represent the mean value of the peak area ± SD (arbitrary unit) corresponding to serine and glycine peaks on the MS chromatogram. c Colony formation assays were performed to determine the proliferation of negative control (NC), sh-LINC01234, serine treatment or serine treatment sh-LINC01234-transfected LoVo cells and HCT116 cells. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
LINC01234 promotes SHMT2 expression in colon cancer
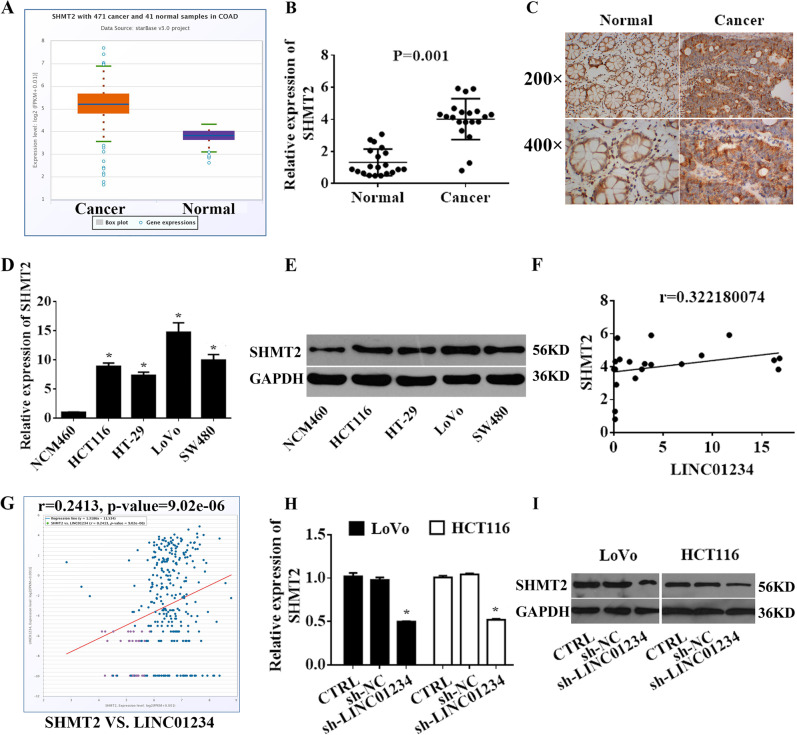
We further explored the mechanism of LINC01234 suppression of serine/glycine metabolism by first analyzing the mRNA sequencing data from TCGA Colon Adenocarcinoma Datasets (COAD). Among them, 14,144 coding gene mRNAs were significantly upregulated in colon cancer tissue (Supplemental Fig. 2A). Next, aberrant mRNA with potential roles in the serine/glycine pathway (hsa00260) were chosen for further study. We found that the expression levels SHMT2, PSAT1, PHGDH, PSPH, CBS, DAO, MAOA, AOC2, GNMT, PIPOX, ALAS2, AOC3 and MAOB were significantly increased in colon cancer tissues compared with those in normal tissues (Supplemental Fig. 2B). Among them, SHMT2 was significantly upregulated in tumor tissues compared with that in nontumor tissues (Fig. 3a–c). Additionally, the expression of SHMT2 was also upregulated in colon cancer cell lines (Fig. 3d, e). Furthermore, we found that LINC01234 expression was positively correlated with SHMT2 (Fig. 3f, g). Next, we hypothesized that LINC01234 may function through SHMT2. To test this hypothesis, we knocked down LINC01234 in colon cancer cells and found that the colon cancer cell expression level of SHMT2 was reduced by LINC01234 silencing treatment (Fig. 3h, i).
Fig. 3.
LINC01234 promotes SHMT2 expression in colon cancer. a Relative expression of SHMT2 in colon cancer tissues compared with that of normal tissue was analyzed using TCGA COAD. b SHMT2 expression was analyzed by qRT-PCR in colon cancer tissues and corresponding adjacent nontumor tissues, and the data were presented as 2−△CT values (n = 20). c SHMT2 expression was analyzed by IHC in colon cancer tissues and corresponding adjacent nontumor tissues. d, e. SHMT2 expression was analyzed by qRT-PCR and Western blotting in 4 human colon cancer cell lines (HCT116, HT-29, LoVo, and SW480) and normal human colonic epithelial NCM460 cells. f Association analysis of the relationship between LINC01234 and SHMT2 expression levels in 20 paired colon cancer tissues. g Association analysis of the relationship between LINC01234 and SHMT2 expression levels in TCGA COAD. h, i Relative mRNA and protein levels of SHMT2 in LoVo cells and HCT116 cells transfected with control, sh-NC or sh-LINC01234. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
LINC01234 activity is partially mediated by the positive regulation of SHMT2
To further determine the potential biological function of SHMT2 in colon cancer cells, short hairpin RNA interference vectors were constructed and transfected into LoVo and HCT116 cells. The knockdown efficiency of SHMT2 was confirmed by qRT-PCR and Western blotting (Supplemental Fig. 3A-B). Because SHMT2 is a key enzyme in serine/glycine metabolism to promote cell proliferation in various cancers35,36, MTT and colony formation assays were performed to evaluate SHMT2 function in colon cancer cell proliferation. As shown in Fig. 4a, b, knockdown of SHMT2 significantly inhibited colon cancer cell proliferation, and serine dramatically increased cell proliferation and significantly reversed the effect of SHMT2 silencing. Moreover, we applied additional cell samples for GC-TOFMS examination. The results showed that colon cancer cells serine and glycine levels were further reduced when SHMT2-depleted colon cancer cells were cultured in serine/glycine-deprived medium (Fig. 4c). Together, these data indicated that SHMT2, as a key enzyme in serine/glycine metabolism, plays a crucial role in maintaining the proliferation of colon cancer cells.
Fig. 4.
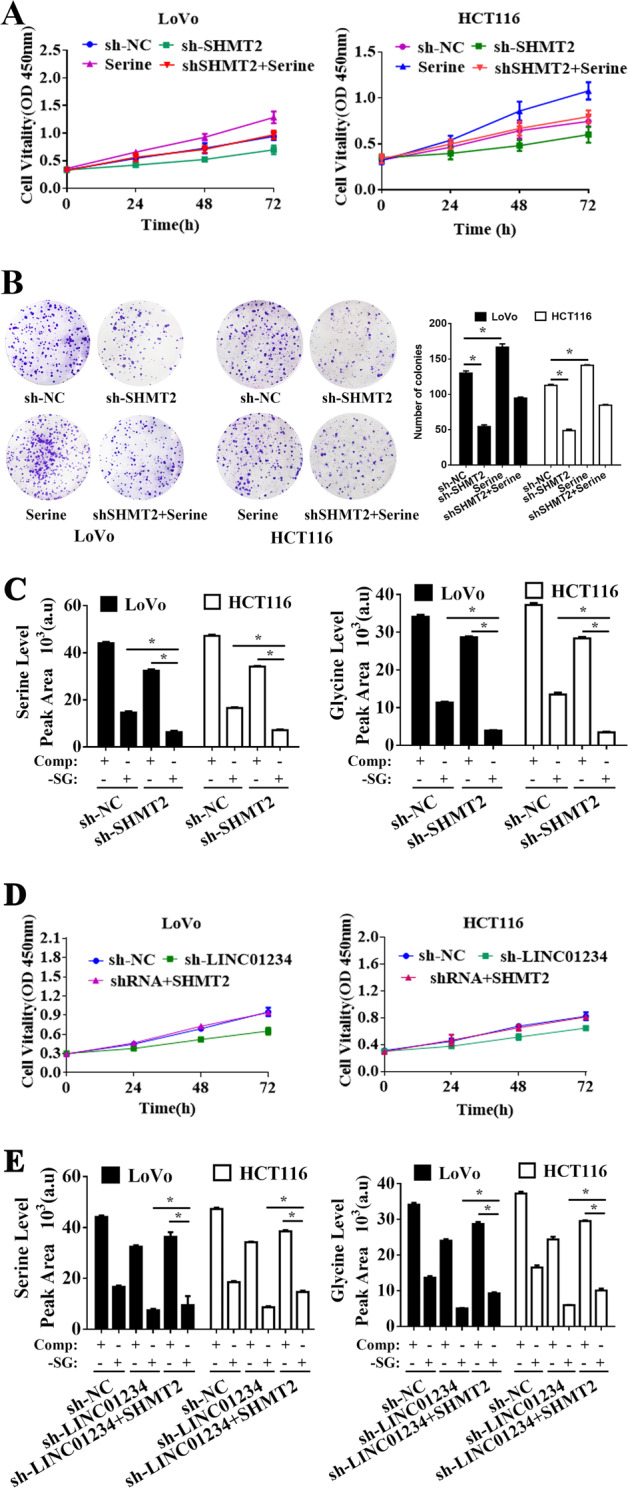
LINC01234 activity is partially mediated by the positive regulation of SHMT2. a MTT assays were used to determine the viability of sh-SHMT2-transfected LoVo cells and HCT116 cells. b Colony formation assays were performed to determine the proliferation of sh-NC, sh-SHMT2, serine treatment or serine treatment sh-SHMT2-transfected LoVo cells and HCT116 cells. c Stable isotope tracing experiments. LoVo cells and HCT116 cells expressing sh-NC or sh-SHMT2 were cultured in complete medium (Comp) or serine/glycine-deprived medium (-SG) for 24 h. GC-MS was used to detect the relative intracellular levels of serine (left) or glycine (right). Histobars represent the mean value of the peak area ± SD (arbitrary unit) corresponding to the serine and glycine peaks on the MS chromatogram. d MTT assays were used to determine the viability of LoVo cells and HCT116 cells transfected with NC, sh-SHMT2 or cotransfected sh-LINC01234 and SHMT2-expressing vector. e Stable isotope-tracing experiments. LoVo cells and HCT116 cells transfected with NC, sh-SHMT2 or cotransfected sh-LINC01234 and SHMT2-expressing vector were cultured in complete medium (Comp) or serine/glycine-deprived medium (-SG) for 24 h. GC-MS was used to detect the relative intracellular levels of serine (left) or glycine (right). Histobars represent the mean value of the peak area ± SD (arbitrary unit) corresponding to the serine and glycine peaks on the MS chromatogram. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
To further validate whether LINC01234 and SHMT2 were in the same axis, SHMT2-overexpressed plasmid was transfected into colon cancer cells with LINC01234 knocked down, revealing that SHMT2 overexpression reversed the effect of LINC01234 silencing on the decreased cell proliferation and activity of the serine/glycine biosynthetic pathway (Fig. 4d, e), suggesting that LINC01234 promotes cell proliferation, at least in part, through promoting SHMT2 expression.
miR-642a-5p may mediate a ceRNA network with LINC01234 and SHMT2
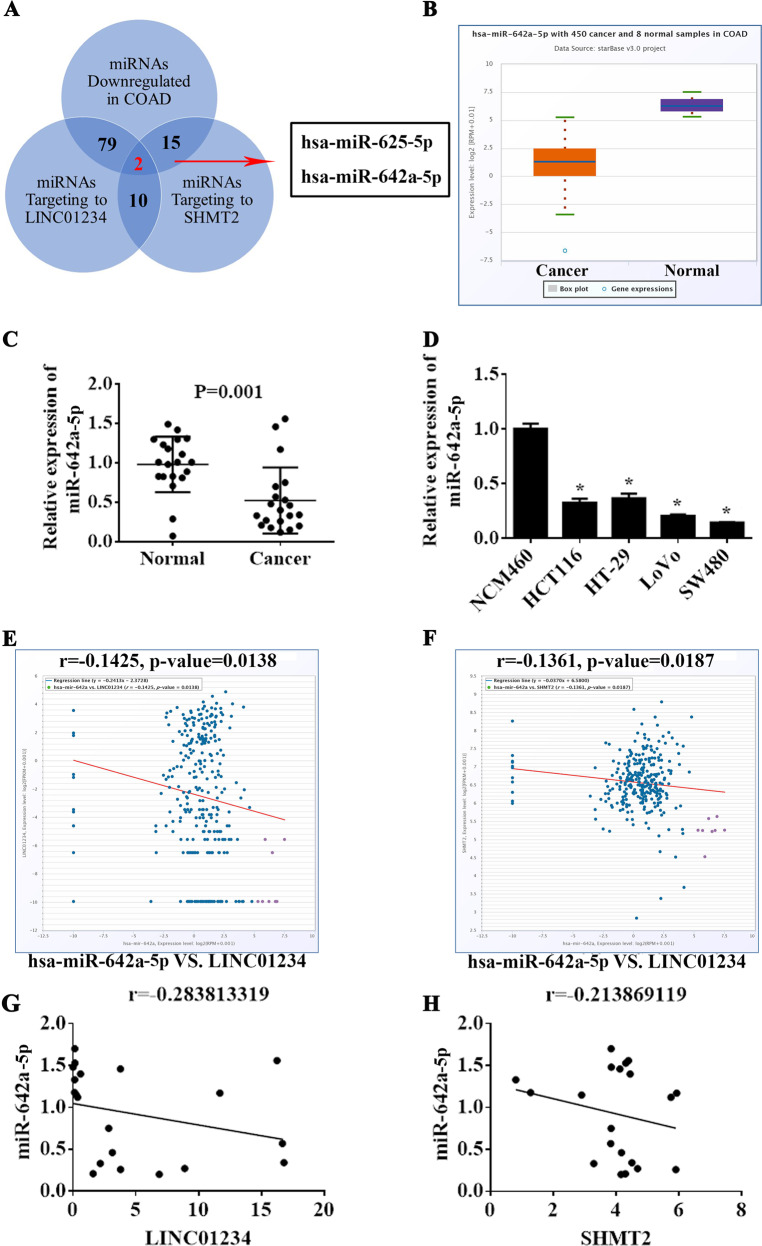
Accumulating evidence has suggested that lncRNAs might function as ceRNAs by binding to miRNAs and functionally liberating other RNA transcripts37. We speculated that LINC01234 may also function as a ceRNA of miRNAs in regulating SHMT2 expression and colon cancer cell proliferation. To determine this hypothesis, we used online bioinformatics databases (DIANA Tools LncBase V.2) and observed that the LINC01234 sequence contains potential binding sites with 332 miRNAs (the threshold is 0.7) (Supplemental Table 3). Additionally, DIANA microCLIP Tools also showed that 60 miRNAs experimentally supported the interaction with SHMT2 (Supplemental Table 4). Next, we analyzed the TCGA Colon Adenocarcinoma Datasets and found that 290 miRNAs were significantly downregulated in colon cancer tissues (Supplemental Fig. 4, Supplemental Table 5) (P < 0.01 and fold change > 1). As shown in Fig. 5a, LINC01234 and SHMT2 share 2 common significantly downregulated microRNAs (miR-625–5p and miR-642a-5p) in colon cancer, and these microRNAs can directly target LINC01234 and SHMT2. Among them, miR-642a-5p was significantly downregulated in tumor tissues compared with that in nontumor tissues and showed the highest binding score (Fig. 5b, c, Supplemental Figs. 5–6). Additionally, the expression of miR-642a-5p was downregulated in colon cancer cell lines (Fig. 5d). Furthermore, we found that both the expression of LINC01234 and SHMT2 were negatively associated with the expression of miR-642a-5p in ChipBase Datasets (Fig. 5e). These results were also confirmed in 20 paired colon cancer tissues (Fig. 5f). Hence, we chose miR-642a-5p as the candidate for further investigation.
Fig. 5.
miR-642a-5p may mediate a ceRNA network with LINC01234 and SHMT2. a The number of significantly downregulated miRNAs in TCGA COAD is 290. The numbers of predicted miRNAs of LINC01234 is 332 according to LncBase Predicted v.2. The number of experimentally validated miRNAs of SHMT2 is 120 according to TarBase v7.0. The number of overlapped miRNAs in the above three intersections is 2, indicating that LINC01234 and SHMT2 may share 2 common microRNAs with experimental evidence. b The relative expression of miR-642a-5p in colon cancer tissues compared with normal tissue was analyzed using TCGA COAD. c miR-642a-5p expression was analyzed by qRT-PCR in colon cancer tissues and corresponding adjacent nontumor tissues, and the data are presented as 2-△CT values (n = 20). d LINC01234 expression was analyzed by qRT-PCR in 4 human colon cancer cell lines (HCT116, HT-29, LoVo, and SW480) and normal human colonic epithelial NCM460 cells. e Association analysis of the relationship between miR-642a-5p and LINC01234 expression levels in TCGA COAD. f Association analysis of the relationship between miR-642a-5p and SHMT2 expression levels in TCGA COAD. g Association analysis of the relationship between miR-642a-5p and LINC01234 expression levels in 20 paired colon cancer tissues. h Association analysis of the relationship between miR-642a-5p and SHMT2 expression levels in 20 paired colon cancer tissues. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
Effects of miR-642a-5p on colon cancer cell proliferation in vitro
To investigate the role of miR-642a-5p in colon cancer, MTT and colony formation assays were performed. The results showed that the miR-642a-5p mimic could dramatically inhibit colon cancer cell proliferation, and serine treatment significantly reversed the effect of the miR-642a-5p mimic (Fig. 6a and b). Moreover, we applied additional cell samples for GC-TOFMS examination. The results showed that the serine and glycine levels in colon cancer cells were further reduced when colon cancer cells with the miR-642a-5p mimic were cultured in serine/glycine-deprived medium (Fig. 6c).
Fig. 6.
Effects of miR-642a-5p on colon cancer cell proliferation in vitro. a MTT assays were used to determine the viability of negative control (NC), miR-642a-5p, serine treatment or serine treatment miR-642a-5p-transfected LoVo cells and HCT116 cells. b Colony formation assays were performed to determine the proliferation of negative control (NC), miR-642a-5p, serine treatment or serine treatment miR-642a-5p-transfected LoVo cells and HCT116 cells. c Stable isotope tracing experiments. LoVo cells and HCT116 cells transfected with NC or miR-642a-5p were cultured in complete medium (Comp) or serine/glycine-deprived medium (-SG) for 24 h. GC-MS was used to detect the relative intracellular levels of serine (left) or glycine (right). Histobars represent the mean value of the peak area ± SD (arbitrary unit) corresponding to the serine and glycine peaks on the MS chromatogram. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
LINC01234 upregulates SHMT2 by competitively binding miR-642a-5p in colon cancer
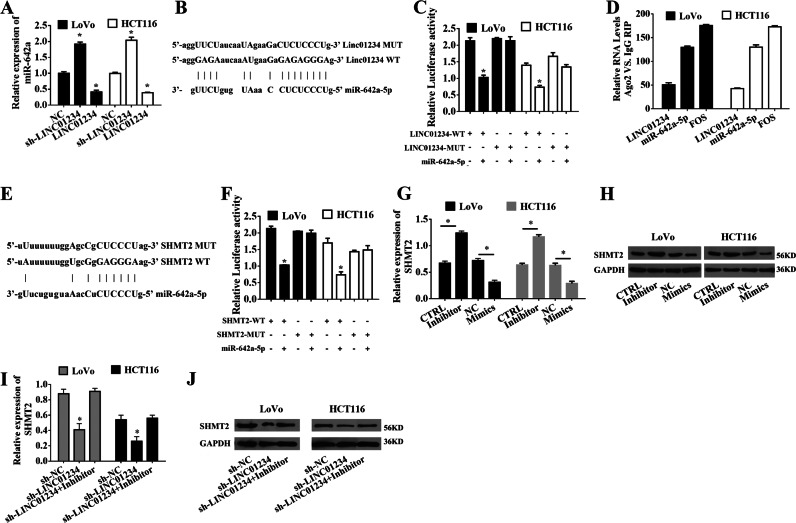
Next, we overexpressed LINC01234 and found that the levels of miR-642a-5p were decreased significantly, whereas LINC01234 knockdown displayed an opposite effect (Fig. 7a). To examine the potential lncRNA-miRNA interaction, we subcloned full-length LINC01234 or LINC01234 harboring a site-directed mutation in the miR-642a-5p binding site into the luciferase reporter vector (referred to as LINC01234-WT or LINC01234-MUT, respectively; Fig. 7b). The miR-642a-5p-mediated suppression of luciferase activity was abolished in this mutated LINC01234 construct compared with that in the wild-type vector (Fig. 7c). Additionally, RIP experiments showed that LINC01234 and miR-642a-5p were enriched in immunoprecipitated Ago2 compared with that in the control IgG (Fig. 7d). Subsequently, we found that the 3′-UTR of SHMT2 contains a potential miR-204–5p binding site through silico analysis (Fig. 7e). Next, to verify the interaction between miR-642a and SHMT2, we performed luciferase reporter assays driven by the wild-type 3′ UTR sequence of SHMT2, which contains the predicted miR-642a-5p binding site (WT-SHMT2), or mutant constructs containing a mutation in the miR-642a-5p binding site (MUT-SHMT2). These plasmids were cotransfected into colon cancer cells together with the miR-642a-5p mimic. The results showed that overexpression of miR-642a-5p resulted in a significant decrease in the luciferase activity of SHMT2-WT but not SHMT2-MUT (Fig. 7f). To determine whether SHMT2 is regulated by miR-642a-5p in colon cancer, we measured SHMT2 mRNA and protein levels when miR-642a-5p was overexpressed or inhibited in LoVo and HCT116 cells. We found that the SHMT2 mRNA and protein levels were significantly decreased or increased by miR-642a-5p overexpression or inhibition, respectively (Fig. 7g, h). Because LINC01234 can sponge miR-642a-5p, we next determined whether miR-642a-5p plays a role in the relationship between LINC01234 and SHMT2. We examined cells cotransfected with sh-LINC01234 and the miR-642a-5p inhibitor. Indeed, the suppression of SHMT2 expression levels induced by sh-LINC01234 was effectively reversed by the miR-642a-5p inhibitor (Fig. 7i, j).
Fig. 7.
LINC01234 upregulates SHMT2 expression by competitively binding to miR-642a-5p. a qRT-PCR analysis of miR-642a-5p expression in LoVo cells and HCT116 cells transfected with negative control (NC), sh-LINC01234 or LINC01234-expressing vectors. b Schematic view of the miR-642a-5p putative targeting site in the WT and MUT of LINC01234. c The luciferase reporter plasmid containing wild-type (WT) or mutant (MUT) LINC01234 was cotransfected into LoVo cells and HCT116 cells with miR-642a-5p. d The RNA levels in RNA immunoprecipitates are presented as the fold enrichment in Ago2 relative to IgG immunoprecipitates. e Schematic view of the miR-642a-5p putative targeting site in the WT and MUT 3’-UTR of SHMT2. f The luciferase reporter plasmid containing wild-type (WT) or mutant (MUT) 3’-UTR of SHMT2 was cotransfected into LoVo cells and HCT116 cells with miR-642a-5p. g qRT-PCR analysis of SHMT2 expression in LoVo cells and HCT116 cells transfected with control, miR-642a-5p inhibitor, negative control (NC) or miR-642a-5p mimic. h Western blot analysis of SHMT2 expression in LoVo cells and HCT116 cells transfected with control, miR-642a-5p inhibitor, negative control (NC) or miR-642a-5p mimic. i qRT-PCR analysis of SHMT2 expression in LoVo cells and HCT116 cells transfected with sh-NC, sh-LINC01234, or cotransfected sh-LINC01234 and miR-642a-5p inhibitor. j Western blot analysis of SHMT2 expression in LoVo cells and HCT116 cells transfected with sh-NC, sh-LINC01234, or cotransfected sh-LINC01234 and miR-642a-5p inhibitor. The experiments were performed in triplicate. The data are represented as means ± SD from three independent experiments. *P < 0.05
Collectively, these data suggest that LINC01234 modulates SHMT2 expression and colon cancer proliferation by competitively binding miR-642a-5p.
LINC01234-SHMT2-miR-642a-5p regulates proliferation in vitro
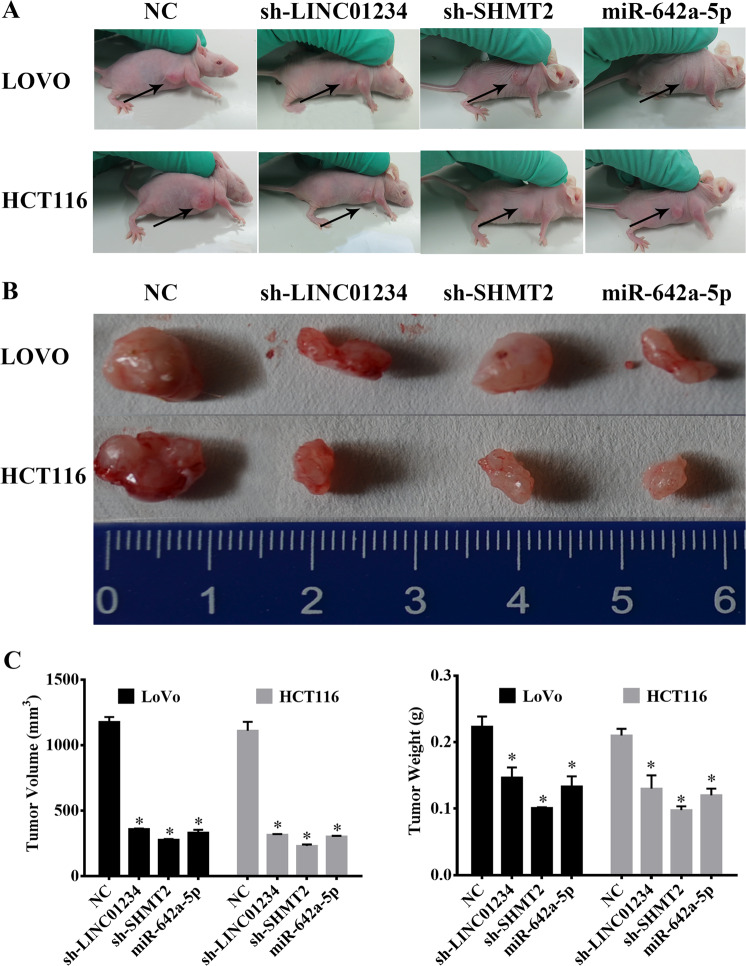
We previously demonstrated that LINC01234, SHMT2 and miR-642a-5p regulated colon cancer proliferation in vivo. Thus, we next explored the role of LINC01234, SHMT2 and miR-642a-5p in regulating colon cancer proliferation in vitro. We obtained pooled LoVo and HCT116 clones (NC cells, sh-LINC01234 cells, sh-SHMT2 cells and miR-642a-5p cells) that stably expressed different levels of LINC01234, SHMT2 and miR-642a-5p through G418 screening. Next, these cells were subcutaneously injected into nude mice. After 4 weeks, the subcutaneous tumors were harvested. In the various groups of five nude mice each, local cancers developed in all of the mice (Fig. 8a). The sh-LINC01234, sh-SHMT2 and miR-642a-5p groups showed significantly smaller tumors than the group treated with the scrambled sequence, indicating that LINC01234 and SHMT2 promoted tumor growth, and miR-642a-5p suppressed tumor growth (Fig. 8b, c, *P < 0.05).
Fig. 8.
Effect of LINC01234, SHMT2 and miR-642a-5p on tumor growth in vivo. a Tumors formed in nude mice. LoVo cells and HCT116 cells stably transfected with negative control (NC), sh-LINC01234, sh-SHMT2 or miR-642a-5p-expressing vectors were injected into the flanks of nude mice (n = 5), and the mice were sacrificed after 4 weeks. b Images of whole tumors from the nude mice injected with LoVo cells and HCT116 cells stably transfected with negative controls (NC), sh-LINC01234, sh-SHMT2 or miR-642a-5p-expressing vector. c The average volume and weight of the tumors from the mice injected with LoVo cells and HCT116 cells stably transfected with sh-LINC01234, sh-SHMT2 or miR-642a-5p-expressing vector were significantly lower than those injected with negative control cells. The data are expressed as the mean ± sd. *P < 0.05
Discussion
In recent years, lncRNAs have emerged as important regulators in tumor initiation and progression38,39, especially in tumor proliferation40,41. Here, we identified, for the first time, that LINC01234 expression was significantly elevated in colon cancer, and patients with higher LINC01234 levels had shorter overall survival and disease-free survival rates than those with lower levels of LINC01234. Furthermore, inhibition of LINC01234 expression significantly reduced tumor proliferation. Notably, rapid tumor proliferation requires the rapid construction of cellular components, including proteins42. Regarding amino acids, studies have confirmed that one-carbon amino acids (e.g., serine and glycine) may promote tumor cell proliferation43. Existing studies have largely focused on how these lncRNAs promote cancer proliferation. However, it is not clearly defined how cancer amino acid metabolism is regulated by lncRNA. Our data, further showing that LINC01234 can regulate serine/glycine metabolism, underline a potential underestimated function of LINC01234 in metabolism.
SHMT2, which plays a key role in cancer44, is a crucial enzyme in the serine/glycine metabolic pathway. Serine and glycine are well-known classic metabolites of glycolysis, and altered characterization of serine/glycine metabolism by SHMT2 is thought to be involved in maintaining the proliferation of cancer cells45–47. For example, Lee GY et al. mapped regions of recurrent amplification in a large collection of primary human cancers and identified SHMT2 as necessary for tumor cell survival36. Wang B et al. further proved that overexpressed SHMT2 promoted glioma growth48. In our study, we revealed for the first time that SHMT2 was upregulated in colon cancer tissues. Additionally, knockdown of SHMT2 inhibited colon cancer cell proliferation through inhibiting serine/glycine metabolism, which was regulated by LINC01234.
Generally, lncRNAs can function as ceRNAs, serving as miRNA sponges to block the tumor suppressor role of specific miRNAs and relieve the suppression of oncogenes caused by miRNAs to promote tumorigenesis49,50. For example, Yan et al. revealed that lncRNA Snhg1, acting as a nondegradable sponge for miR-338, promoted the expression of proto-oncogene CST3 and cell proliferation in primary esophageal cancer cells51. LINC00152 has been reported to act as an endogenous sponge of miR-193a-3a to confer oxaliplatin resistance in colon cancer52. These studies uncovered a new approach to identify the regulatory mechanism between LINC01234 and SHMT2. Based on the online bioinformatic prediction, we proposed that LINC01234 and SHMT2-mediated serine/glycine metabolism may be regulated by miR-642a-5p. Next, functional studies, such as luciferase reporter assays, validated that miR-642a-5p can bind to LINC01234 and the 3′ UTR of SHMT2, and LINC01234 can reverse the posttranscriptional suppression of SHMT2 caused by miR-642a-5p regulation.
In conclusion, our study revealed that LINC01234, which is significantly upregulated in colon cancer, could be a molecular sponge for miR-642a-5p, relieving the posttranscriptional suppression of SHMT2 caused by miR-642a-5p and consequently promoting cell proliferation via regulating serine/glycine metabolism (Supplement Fig. 7). Taken together, our findings suggest that LINC01234 may be a potential biomarker and target for colon cancer therapy.
Supplementary information
Acknowledgements
This work was supported by The New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (No. JY201611).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by G. Calin
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41419-023-05943-5
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors have contributed equally: Changwei Lin, Yi Zhang
Change history
7/11/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-023-05943-5
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41419-019-1352-4).
References
- 1.Siegel RL, et al. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, M. etal. Global patterns and trends in colorectal cancer incidence and mortality. Gut66, 683–391 (2016). [DOI] [PubMed]
- 3.Chen HY, et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy - Analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis. Colon & Rectum. 2003;46:22–31. doi: 10.1097/01.DCR.0000089106.71914.00. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, et al. Long Noncoding RNA BC032913 as a novel therapeutic target for colorectal cancer that suppresses metastasis by upregulating TIMP3. Mol. Ther. Nucleic Acids. 2017;8:469–481. doi: 10.1016/j.omtn.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rychahou PG, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc. Natl Acad. Sci. USA. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua, J. T. etal. Risk SNP-mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19. Cell, 10.1016/j.cell.2018.06.014 (2018). [DOI] [PubMed]
- 8.Deng, S. J. etal. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene10.1038/s41388-018-0382-1 (2018). [DOI] [PubMed]
- 9.Bueno MJ, Castro IPD, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 10.Tsai MC, et al. Long Noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesana M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian, Z. etal. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin. Cancer Res. 10.1158/1078-0432.CCR-17-2967 (2018). [DOI] [PubMed]
- 13.Hu P, Shangguan J, Zhang L. Downregulation of NUF2 inhibits tumor growth and induces apoptosis by regulating lncRNA AF339813. Int. J. Clin. Exp. Pathol. 2015;8:2638–2648. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Xiang B, Liu Y, Wang Y, Kan H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018;437:56–66. doi: 10.1016/j.canlet.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Ding, J. etal. Estrogen receptor beta promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene, 10.1038/s41388-018-0175-6 (2018). [DOI] [PubMed]
- 16.Lü MH, et al. Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene. 2016;35:3524. doi: 10.1038/onc.2015.413. [DOI] [PubMed] [Google Scholar]
- 17.Li M, et al. The long noncoding RNA GAS5 negatively regulates the adipogenic differentiation of MSCs by modulating the miR-18a/CTGF axis as a ceRNA. Cell Death Dis. 2018;9:554. doi: 10.1038/s41419-018-0627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, et al. Comprehensive analysis of differentially expressed profiles of lncRNAs and construction of miR-133b mediated ceRNA network in colorectal cancer. Oncotarget. 2017;8:21095–21105. doi: 10.18632/oncotarget.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, et al. Transcriptome sequencing uncovers a three-long noncoding RNA signature in predicting breast cancer survival. Sci. Rep. 2016;6:27931. doi: 10.1038/srep27931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, et al. Identification of aberrantly expressed long non-coding RNAs in stomach adenocarcinoma. Oncotarget. 2017;8:49201–49216. doi: 10.18632/oncotarget.17329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, et al. Expression profiles analysis reveals an integrated miRNA-lncRNA signature to predict survival in ovarian cancer patients with wild-type BRCA1/2. Oncotarget. 2017;8:68483–68492. doi: 10.18632/oncotarget.19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, et al. Long Noncoding RNA LINC01234 functions as a competing endogenous rna to regulate CBFB expression by sponging miR-204-5p in gastric cancer. Clin. Cancer Res. 2018;24:2002–2014. doi: 10.1158/1078-0432.CCR-17-2376. [DOI] [PubMed] [Google Scholar]
- 23.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, et al. SHMT2 desuccinylation by SIRT5 drives cancer cell proliferation. Cancer Res. 2018;78:372–386. doi: 10.1158/0008-5472.CAN-17-1912. [DOI] [PubMed] [Google Scholar]
- 26.Vie N, et al. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol. Cancer. 2008;7:14. doi: 10.1186/1476-4598-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samanta D, et al. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016;76:4430–4442. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- 28.Kim SK, Jung WH, Koo JS. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One. 2014;9:e101004. doi: 10.1371/journal.pone.0101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leivonen SK, et al. Identification of miR-193b targets in breast cancer cells and systems biological analysis of their functional impact. Mol. Cell. Proteom. 2011;10:M110 005322. doi: 10.1074/mcp.M110.005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, et al. miR-193b directly targets STMN1 and inhibits the malignant phenotype in colorectal cancer. Am. J. Cancer Res. 2016;6:2463–2475. [PMC free article] [PubMed] [Google Scholar]
- 31.Jain M, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji J, et al. GC-TOF/MS-based metabolomics approach to study the cellular immunotoxicity of deoxynivalenol on murine macrophage ANA-1 cells. Chem. Biol. Interact. 2016;256:94–101. doi: 10.1016/j.cbi.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Wang DW, et al. A novel mechanism of mTORC1-mediated serine/glycine metabolism in osteosarcoma development. Cell. Signal. 2017;29:107–114. doi: 10.1016/j.cellsig.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, et al. Differential proteome analysis identifies TGF-beta-related pro-metastatic proteins in a 4T1 murine breast cancer model. PLoS One. 2015;10:e0126483. doi: 10.1371/journal.pone.0126483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo CC, Chen WC, Teo XQ, Radda GK, Lee PT. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget. 2016;7:53005–53017. doi: 10.18632/oncotarget.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee GY, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74:3114–3126. doi: 10.1158/0008-5472.CAN-13-2683. [DOI] [PubMed] [Google Scholar]
- 37.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui, M. etal. Long non-coding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through miRNA-9/PPARA/ACSL1/cholesterol/RXRA/HULC signaling. Cancer Res.75, 846–857 (2015). [DOI] [PubMed]
- 39.Ruan X, Li P, Cangelosi A, Yang L, Cao H. A long non-coding RNA, lncLGR, regulates hepatic glucokinase expression and glycogen storage during fasting. Cell Rep. 2016;14:1867. doi: 10.1016/j.celrep.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, et al. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9:742. doi: 10.1038/s41419-018-0793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, et al. LncRNA MIR100HG promotes cell proliferation in triple-negative breast cancer through triplex formation with p27 loci. Cell Death Dis. 2018;9:805. doi: 10.1038/s41419-018-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palm W, et al. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keibler MA, et al. Metabolic requirements for cancer cell proliferation. Cancer Metab. 2016;4:16. doi: 10.1186/s40170-016-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 46.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michelsen TM, Pripp AH, Tonstad S, Tropé CG, Dørum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur. J. Cancer. 2009;45:82. doi: 10.1016/j.ejca.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Wang B, et al. Mitochondrial serine hydroxymethyltransferase 2 is a potential diagnostic and prognostic biomarker for human glioma. Clin. Neurol. & Neurosurg. 2017;154:28. doi: 10.1016/j.clineuro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Shao Y, et al. LncRNA-RMRP promotes carcinogenesis by acting as a miR-206 sponge and is used as a novel biomarker for gastric cancer. Oncotarget. 2016;7:37812. doi: 10.18632/oncotarget.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang WC, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan, Y. etal. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget8, 35750–35760 (2017). [DOI] [PMC free article] [PubMed]
- 52.Yue B, Cai DL, Liu CC, Fang CY, Yan DW. Linc00152 functions as a competing endogenous rna to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol. Ther. 2016;24:2064–2077. doi: 10.1038/mt.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.