Abstract
The use of cannabis for therapeutic and recreational purposes is growing exponentially. Nevertheless, substantial questions remain concerning the potential cognitive and affective side-effects associated with cannabis exposure. In particular, the effects of specific marijuana-derived phytocannabinoids on neural regions such as the prefrontal cortex (PFC) are of concern, given the role of the PFC in both executive cognitive function and affective processing. The main biologically active phytocannabinoids, ∆-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), interact with multiple neurotransmitter systems important for these processes directly within the PFC. Considerable evidence has demonstrated that acute or chronic THC exposure may induce psychotomimetic effects, whereas CBD has been shown to produce potentially therapeutic effects for both psychosis and/or anxiety-related symptoms. Using an integrative combination of cognitive and affective behavioral pharmacological assays in rats, we report that acute intra-PFC infusions of THC produce anxiogenic effects while producing no impairments in executive function. In contrast, acute infusions of intra-PFC CBD impaired attentional set-shifting and spatial working memory, without interfering with anxiety or sociability behaviors. In contrast, intra-PFC CBD reversed the cognitive impairments induced by acute glutamatergic antagonism within the PFC, and blocked the anxiogenic properties of THC, suggesting that the therapeutic properties of CBD within the PFC may be present only during pathologically aberrant states within the PFC. Interestingly, the effects of PFC THC vs. CBD were found to be mediated through dissociable CB1 vs. 5-HT1A-dependent receptor signaling mechanisms, directly in the PFC.
Subject terms: Cognitive control, Prefrontal cortex, Behavioural methods
INTRODUCTION
Cannabis is the most widely used illicit substance worldwide, with increasing numbers of jurisdictions moving towards full legalization. Concomitant with these trends, there is increasing interest in determining the potential therapeutic properties and possible deleterious side-effects of specific cannabis-derived phytocannabinoids for a variety of psychiatric and non-psychiatric health conditions. Cannabis contains > 100 distinct phytocannabinoids of which ∆-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) represent the most prevalent bioactive constituents. THC and CBD interact with numerous neurotransmitter pathways involved with the processing of cognitive and affective behaviors and have been shown to modulate these behavioral phenomena through interactions with the prefrontal cortex (PFC) [1–3]. Previous research has suggested that whereas THC exposure can induce pro-psychotic side-effects [4, 5], CBD may counteract these THC-induced effects and produce therapeutic benefits in the treatment of various neuropsychiatric symptoms [6]. For example, CBD was shown to reduce fear-related behaviors [2, 7], induce antidepressant-like effects [8], possess anticonvulsant properties [9] and potent anti-psychotic effects directly in the mesolimbic system [10, 11]. Nevertheless, the precise neuroanatomical and neuropharmacological mechanisms by which THC and CBD may modulate cognitive and affective processing are not entirely understood.
In addition to their differential effects on both cognitive and affective processing, THC and CBD exert differential pharmacological effects, with THC acting primarily as a partial agonist of the CB1 receptor (CB1R) [12]. In contrast, CBD possesses a more diverse pharmacological profile with known actions on the 5-HT1A receptor (5-HT1AR), vanilloid receptor type 1, negative allosteric modulation of CB1 receptors and weak antagonism of CB2 receptors, partial agonism of D2 receptors and inhibition of anandamide hydrolysis [8, 13, 14]. We have previously reported that THC and CBD produce opposing modulatory effects directly within the mesocorticolimbic circuitry, on several molecular signaling cascades linked to cognitive and affective function, including the glycogen-synthase-kinase-3 (GSK-3), mammalian target of rapamycin (mTOR), p-70-sS6-kinase and protein kinase B (akt) [11, 15]. Additionally, we have reported that CBD directly in the nucleus accumbens can potently block the formation of fear-related memory through functional interactions with the 5-HT1AR [7].
Executive functions, memory and affective processing are critical for adaptive behaviors. One critical PFC-dependent function is cognitive flexibility, which allows for the adjustment of behavioral reactions in response to changing environmental conditions and demands [16, 17]. Comorbid impairments in cognitive and affective processing have been described in many psychiatric diseases, including schizophrenia [18], as well as following acute or chronic cannabis exposure [19]. Working memory, decision making, flexibility, anxiety, fear and social behavior are regulated via complex neural circuits that include critical integrative connections with the PFC [17, 20]. In humans, PFC damage leads to the development of social isolation and apathy, behavioral inflexibility and impairments in decision making [21]. In rodents, damage to the PFC dysregulates social behaviors and causes deficits in attentional set-shifting [17, 22]. Similarly, acute or neurodevelopmental exposure to THC induce deficits in working memory [23], impairs cognitive flexibility [24] and increases anxiety [15] suggesting that THC-induced deficits in these domains are likely mediated through PFC-related pathology.
Here, we compared the potential effects of intra-PFC THC, CBD and THC/CBD combinations in behavioral tests of cognitive flexibility, working memory, anxiety and social interaction. We demonstrate that while acute intra-PFC THC produces anxiogenic effects that are blocked by CBD and a CB1R antagonist, THC produced no apparent cognitive deficits. In contrast, intra-PFC CBD produced deficits in cognitive flexibility and working memory in naïve rats but was effective in reversing the cognitive impairments induced by intra-PFC NMDA receptor blockade, through a 5-HT1AR-dependent mechanism. These findings demonstrate that intra-PFC THC and CBD can produce dissociable side-effects on cognition and affective processing, via differential pharmacological and state-dependent mechanisms.
MATERIALS AND METHODS
Rats
Adult male Sprague Dawley rats (n = 183; 350–400 g at arrival; Charles River, Quebec, Canada) were single-housed in temperature (24 ± 2 °C) and humidity controlled (55 ± 10%) animal facility rooms. The light:dark cycle was 12:12 h (light on at 7:00 a.m.). Food and water were available ad libitum unless stated otherwise. All procedures were approved by the Institutional Animal Care Committee and complied with the Canadian and National Institute of Health Guides for the Care and Use of Laboratory Rats (NIH Publication #20-23). All rats were behaviorally naïve prior to beginning the set-shift procedure. Following set-shifting testing, rats were allowed to return to their pre-set-shift body weights, prior to undergoing additional behavioral tests.
Surgery
Rats were anesthetized with a ketamine (80 mg/kg; Vetoquinol)/xylazine (6 mg/kg; Bayer) mixture (i.p.) and positioned in a stereotaxic apparatus (David Kopf Instruments). Post-surgical analgesia was ascertained with meloxicam (subcutaneous; 1 mg/kg; Boehringer Ingelheim). The scalp was incised, and burr holes were prepared above the PFC skull region. Two stainless steel guide cannulae (22 G, PlasticsOne) were implanted into the prelimbic PFC (coordinates: 3.1 mm anterior, 1.4 mm lateral from Bregma (10˚ angle), and 3.0 mm ventral to dura; [25]), and secured with anchoring screws and dental cement. Rats were allowed one-week recovery prior to commencement of experiments.
Drugs and injection procedure
(-)-Cannabidiol, NAD299 hydrochloride, AM251 and (+)-MK801 maleate were from Tocris and THC from Cayman Chemical (Ann Arbor, MI, USA). All drugs were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and diluted to final concentrations (final DMSO 5%) in saline containing 5% cremophor EL (Sigma-Aldrich). Before testing rats were handled for 5 min per day. For microinfusions, rats were gently restrained prior to insertion of the microinjectors. All microinfusions were 500 nl/hemisphere. Injectors were removed after 1 min and behavioral testing began 5 min later. The following drug concentrations (indicated later with subscript i.e. THC100) were used (in ng/500 nl): THC (10, 50, 100 or 500), CBD (10, 100 or 500), NAD299 (100), AM251 (100 or 200) and (in μg/500 nl) MK801 (3 or 6). When two drugs were tested simultaneously they were injected as a co-mixture. To avoid possible sub-chronic effects of infused substances, any given treatment was administered only once. A seven-day rest period was allowed between the different behavioral tests to allow proper drug washout between tests.
Attentional set-shifting
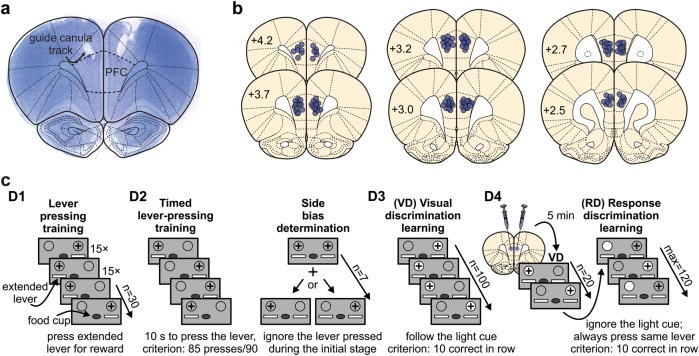
Prior to training, rats were food restricted to ~85% of their free feeding body weight and familiarized with the reward cue (45 mg sucrose pellets; banana flavor; BioServ, USA). Set-shifting was conducted in an operant chamber (Med-associates, St Albans VT, USA) enclosed in a sound-attenuating box. The test chamber (30.5 × 24 × 21 cm) was equipped with a house light, two retractable levers, pellet receptacle and two cue lights (Fig. 1c). The chamber was computer controlled with customized software procedures (MED-PC IV, Med-Associates) adapted from [26] and [16].
Fig. 1.
Histology and set-shift procedure. a Microphotograph of Cresyl violet stained coronal section containing guide canulae tracks for injections directly in the prefrontal cortex (PFC). b Schematic representation of the injection sites (circles) superimposed on the coronal plane of a rat brain (modified from [25]). Numbers on the schemes indicate distance from Bregma point. c Experimental timeline for the attentional set-shift procedure. The grey rectangles represent front panel of the operant box, circles correspond to the light cues (gray = light off; white = light on), white rectangles correspond to extended levers, the lever rewarded with sucrose pellet is indicated with plus sign above it; D1-D4 indicate different days of the procedure. Note that rats were injected with the drug solution on the last day of the procedure
Briefly, rats were placed in the chamber (house light on, one lever extended) and learned to associate each lever press with a food pellet reward. After 15 presses the lever was retracted, the alternate lever was inserted, and the rat needed to press it 15 times. Subsequently, the levers were randomly inserted into the chamber (15× each) until pressed by the rat. Next, the house light was switched off and a timed lever-pressing trial started (trial duration: 20 s). Trials began with illumination of the chamber and insertion of one lever for 10 s. Pressing the lever resulted in reward and a 4 s light cue. A failed trial (omission trial) resulted in lever retraction, no reward and turning off the house light. After 30 trials the rat was removed from the chamber. The next day the rat was exposed to the timed trials until a performance criterion of ≥85 presses/90 trials was achieved.
Following this, the rats’ preferred lever was determined based on seven sets of trials (set: initial trial + secondary trial/s). All trials began with chamber illumination and insertion of both levers for 10 s. During the initial trial, pressing any lever was rewarded while during the secondary trial/s only pressing the lever other than during initial trial. A set was considered completed when both levers were pressed. The lever with more presses on the initial trials was considered as ‘preferred’.
The next day rats were exposed to 100 visual-cue discrimination trials beginning with illumination of one cue light, which indicated the correct lever position. After 3 s the house light was switched on and both levers inserted for 10 s. Only a correct response was rewarded. The successful training criterion was 10 successive correct presses.
The next day the rat received a specific intra-PFC drug treatment and after 5 min 20 visual-cue discrimination trials were presented to test long-term memory retrieval and motoric functions. Immediately after, the response discrimination procedure began (set-shift = 10 successive correct presses; max 120 trials). Trials were similar to visual discrimination trials, except that the correct response was set to the non-preferred lever (see above) irrespectively of the cue light position.
The total number of trials to set-shift (excluding omissions) and the total number of errors were calculated. Perseverative (>5 errors/16 successive trials) and regressive errors (≤5 errors/16 successive trials) were counted when an error was made by following the light cue and never-reinforced errors when an error was made but the cue light indicated the correct lever.
Spontaneous alternation behavior
The Y-maze (black nonreflecting acrylic, 3 arms at 120˚ from each other; arm length: 50 cm; wall height: 40 cm) was located on the floor and dimly illuminated (40 lux). Following room acclimatization (1 h), intra-PFC-injected rats were placed at the end of one arm facing the wall, and allowed to explore the maze freely for 10 min. Alternation behavior was video recorded and the sequence and total number of arm entries (all paws in) was scored off-line. The alternation score was calculated as unique triplets/(total arm entries-2), where unique triplets = a consecutive entry to all three arms (i.e. ABC; Fig. 5a). Re-entries to the same arm (i.e. AA) and returns to previously visited arm (i.e. ABA) were scored separately.
Fig. 5.
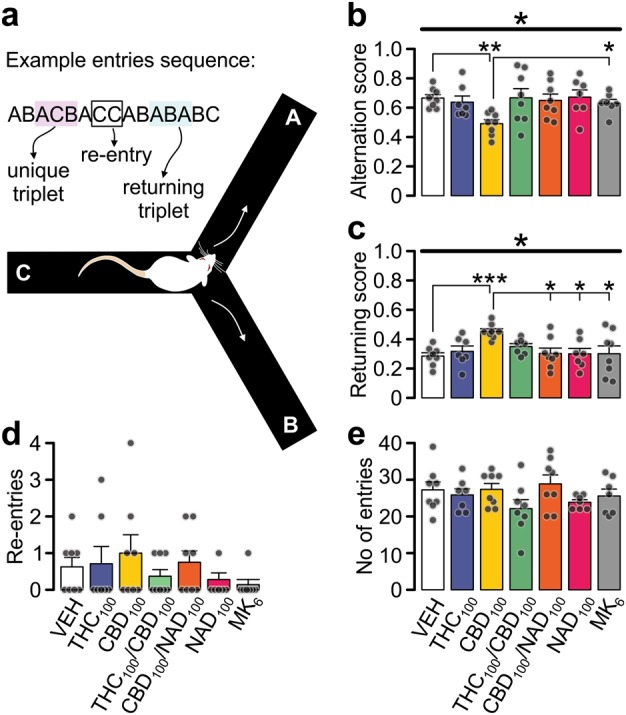
Intra-PFC Cannabidiol induces deficits in spontaneous alternation behavior. a Schematic representation of the Y-maze apparatus and the criteria for measurements. b Alternation score of rats injected intra-PFC with CBD was greatly reduced suggesting impairments of spatial working memory. c Returning scores were significantly increased in CBD-treated rats while (d) number of re-entries was not changed. e The total number of arm entries was not changed by the treatment indicating that the effect of CBD on alternation was not related to changes in locomotion. None of the measured parameters was changed upon blockade of NMDA receptors with MK801. Data represent mean ± s.e.m. Group sizes (n) and drug concentrations in ng/hemisphere, except of MK801 (in µg/hemisphere): VEH (8), THC100 (7), CBD100 (8), CBD100/THC100 (8), CBD100/NAD299100 (8), NAD299100 (7), MK8016 (7). Treatment groups were compared with one-way ANOVA (b, c, e) followed with Games-Howell post-hoc test; or Kruskal–Wallis test (d). *p < 0.05; **p < 0.01; ***p < 0.001
Elevated plus maze test
The elevated plus maze (EPM) apparatus (black acrylic, 4 arms (10 × 50 cm) stemming from a 10 × 10 cm platform and forming a plus shape) was raised above the floor by 50 cm and was dimly illuminated (40 lux). Two opposite arms were enclosed with 40 cm high walls while other two arms were opened (except for 1 cm high ledge). Intra-PFC-injected rats were placed on the central platform (facing closed arm) and explored the maze for 10 min. Behavior was video recorded and analyzed offline (Behaview software; www.pmbogusz.net). The number of entries (all paws in) and the time spent in closed and open arms was scored.
The three-chambered social approach test
The social interaction apparatus consisted of a transparent acrylic chamber divided into three equal compartments separated with guillotine doors [15]. One day before testing rats were room acclimated for 30 min and subsequently habituated to the apparatus (5 min center + 8 min entire apparatus). Social interaction testing consisted of 2 phases. In phase 1 (social motivation test), following a 30 min acclimatization, rats received assigned intra-PFC microinfusions and placed in the central compartment (5 min; guillotine doors in place). Subsequently, wire enclosures were placed in the side compartments (one contained a stranger male rat) and the tested rat could explore the entire apparatus for 8 min. In phase 2 (social memory test), a new, novel unfamiliar rat was placed in the previously empty enclosure cage and the test rat could explore both chambers (containing the previously encountered rat or the new stranger rat) for 8 min. Behaviors were video recorded and analyzed offline. The total duration of exploratory bouts that the rat spent with a stranger vs. the empty enclosure (phase 1) was calculated as a sociability score = tstranger/(tstranger + tempty). The time spent with the previously encountered vs. novel stranger rat in phase 2, was calculated as a social recognition score = tnovel/(tnovel + tfamiliar).
Histology
At the end of experiments rats were overdosed with pentobarbital (Euthanyl) and decapitated. Brains were removed, fixed in 10% formalin and cryoprotected in 30% sucrose solution. Coronal sections (50 μm thick) were cut, mounted on glass slides and stained with Cresyl violet. Sections with the cannula tip locations were microphotographed and referred to a rat brain atlas [25].
Statistical analysis
Statistical analyses were performed using SPSS Statistics version 24 (IBM). Sampled data were tested for outliers and normality distribution using Kolmogorov-Smirnov tests (significance: p = 0.05). In analyses of set-shift experiments the VEH group was compared separately to THC (THC10-500), CBD (CBD10–500), (CBD100/THC100, CBD100/NAD299100 and NAD299100), and MK801 (MK8013,6, MK8016/CBD100 and MK8016/CBD500) groups. Statistical comparisons between groups were assessed using one-way ANOVA (normal distributions) and appropriate post-hoc tests: Gabriel (non-equal sample sizes; homogenous variances) or Games-Howell (non-homogenous variances). Homogeneity of variances was tested using Levene statistics (significance: p = 0.05). Non-normally distributed data samples were compared statistically with Kruskal–Wallis (K–W) tests (significance: p = 0.05) followed with Mann–Whitney U tests between the relevant groups.
RESULTS
Intra-PFC infusion of CBD impairs cognitive flexibility in naïve rats
Given the known role of PFC cannabinoid signaling in the mediation of cognitive flexibility processing [27, 28], we first examined how intra-PFC THC or CBD may modulate set-shifting (see Methods). Histological analyses (Fig. 1a, b) confirmed that injections were within the anatomical boundaries of PFC [25].
Drug naïve rats were trained to press the lever paired with a light cue to obtain food reward. One day after successful training (10 consecutive correct responses) rats received intra-PFC VEH or THC (THC10,50,100,500ng/side) and 5 min later underwent testing (Fig. 1c). THC infusions (all doses) did not impair the retrieval of visual discrimination memory as all groups performed similarly to VEH controls. Subsequently, the rule was changed, and rats needed to ignore the light cue and always press the non-preferred lever (see Methods). This shift in task contingency was accompanied with an increased rate of incorrect responses, indicated by the prevalence of perseverative errors. Neither the number of trials to set-shift (Fig. 2a) nor the total number of errors (Fig. 2b) committed by THC rats differed significantly from the VEH group. There was a significant effect of treatment on the number of regressive errors (Fig. 2d; K–W test: Χ2(4) = 10.530, p = 0.032), with THC10-treated rats making significantly less regressive errors than VEH (p = 0.019). Perseverative (Fig. 2c) and never-reinforced (Fig. 2d) errors, were not affected. This data suggests that intra-PFC THC did not affect set-shifting abilities as perseverance levels in THC groups were similar to controls.
Fig. 2.
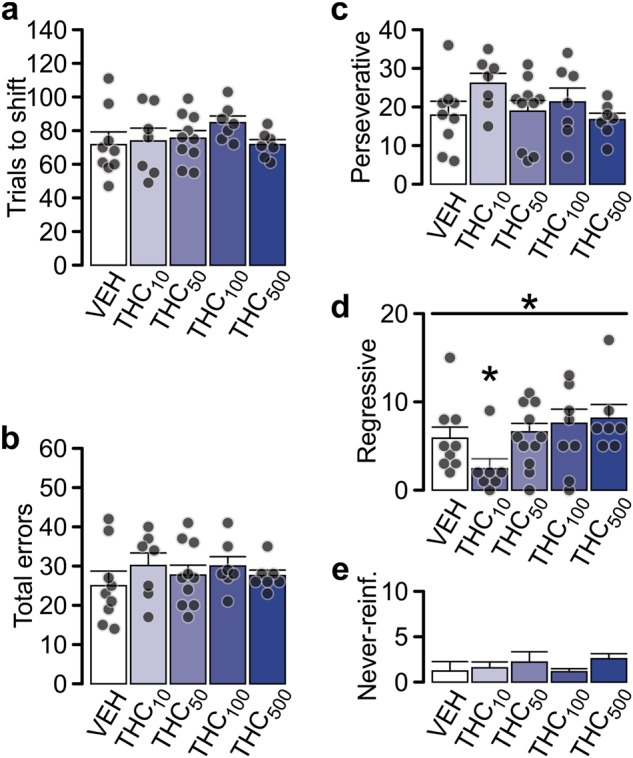
Intra-PFC THC treatment does not affect attentional set-shifting. a The number of trials needed to set-shift from visual to response strategy or the number of errors (b) was not affected with THC treatment. Subcategorization of errors revealed that the number of perseverative errors (c) or never-reinforced errors (e) was unchanged relative to VEH control. The number of regressive errors (d) in THC10 group was significantly lower than in the VEH control. Subscript numbering indicates drug concentrations in ng/hemisphere. Black circles indicate data points (not shown in e for clarity). Data represent mean ± s.e.m. Group sizes (n): VEH (9), THC10 (7), THC50 (10), THC100 (7), THC500 (7). Respective treatment groups were compared with one-way ANOVA followed with Gabriel post-hoc or Kruskal–Wallis test followed with Mann–Whitney U tests. *p < 0.05 vs. VEH group
Next, we tested the possible effects of intra-PFC CBD (CBD10,100,500ng/side) infusions. Retrieval of visual discrimination memory (light cue = reward) was not affected by CBD. However, upon the rule change, there was a significant effect of treatment on number of trials to set-shift (Fig. 3a; K–W test: Χ2(6) = 15.468; p = 0.017). Specifically, rats treated with CBD100 and CBD500 required significantly more trials to complete the task (p = 0.004 and p = 0.015, respectively), while CBD10 did not induce impairment. Consequently, the number of committed errors was also affected (Fig. 3b; one-way ANOVA: F(6,55) = 2.702, p = 0.024), however, only CBD100 displayed significantly more errors than VEH controls (p = 0.019). CBD treatment specifically increased the number of perseverant errors (Fig. 3c; one-way ANOVA: F(6,55) = 3.324, p = 0.008) with CBD100 being the effective dose (p = 0.014). Regressive and never-reinforced errors were not affected by CBD (Fig. 3d, e). Thus, intra-PFC CBD dose-dependently impaired attentional flexibility in rats.
Fig. 3.
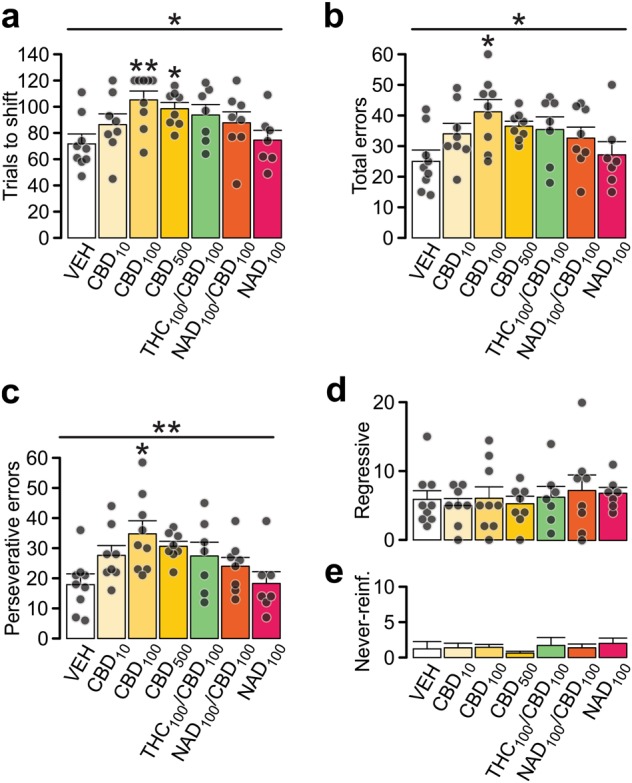
Intra-PFC CBD treatment impairs attentional set-shifting. a The number of trials needed to set-shift from visual to response strategy was increased with intra-PFC CBD treatment. Subscript numbering indicates drug concentrations in ng/hemisphere. Black circles indicate data points (not shown in (e) for clarity). The CBD-induced impairment was weakened by co-application of THC or the 5-HT1a receptor antagonist, NAD299. NAD299 alone did not affect set-shifting. b, Total number of errors performed during set-shifting task was increased in the CBD-treated rats. c–e, Errors were categorized into perseverative (c), regressive (d) or never-reinforced (e). Detailed analysis revealed that rats that needed more trials to complete the task showed a specific increase in perseverance suggesting impaired flexibility. Data represent mean ± s.e.m. Group sizes (n): VEH (9, same as in Fig. 2), CBD10 (8), CBD100(9), CBD500(8), CBD100/THC100 (7), CBD100/NAD299100 (8), NAD299100 (7). Respective treatment groups were compared with one-way ANOVA followed with Gabriel post-hoc; or Kruskal–Wallis test followed with Mann–Whitney U tests. *p < 0.05; **p < 0.01 vs. VEH group
CBD targets several types of receptors and channels including CB1 and 5-HT1A receptors [8]. Therefore, we next examined if co-application of ~equimolar concentrations of THC or a selective 5-HT1AR antagonist, NAD299, would block the effects of intra-PFC CBD100. Although the number of trials and errors remained elevated in CBD100/THC100 and CBD100/NAD299100 groups, they did not significantly differ from VEH or CBD100 (Fig. 3a, b). Similarly, the number of perseverative, regressive and never-reinforced errors did not significantly differ from VEH (Fig. 3c–e). Importantly, rats treated with NAD299100 alone were similar to VEH controls. Thus, THC co-administration or selective blockade of the 5-HT1AR is sufficient to prevent the deleterious effects of intra-PFC CBD on set-shifting behaviors.
Intra-PFC CBD blocks the cognitive impairments induced by acute NMDA-receptor antagonism
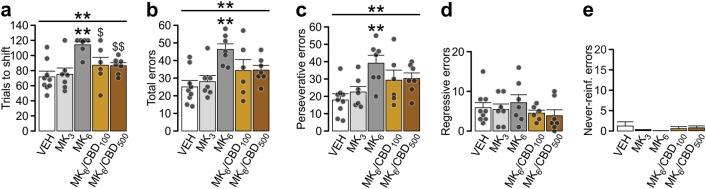
Considering the reported therapeutic properties of CBD in the treatment of numerous neuro-pathological conditions involving PFC dysfunction [29] we hypothesized that CBD’s acute effects on cognitive flexibility in naïve rats might be state dependent and that the cognitive enhancing effects of CBD may be only observable in induced states of prefrontal cortical pathology. Normal PFC-dependent flexibility requires NMDA receptor (NMDAR) signaling and blocking NMDARs with the antagonist MK801 induces profound behavioral flexibility deficiencies [30]. First, we identified an intra-PFC dose of MK801 capable of inducing robust flexibility impairments (6 μg/hemisphere) and co-infused it with the behaviorally effective doses of CBD100-500. There was a significant effect of treatment on number of trials to set-shift (Fig. 4a; K–W test: Χ2(4) = 14.796, p = 0.005), total error count (Fig. 4b; one-way ANOVA: F(4,35) = 4.938, p = 0.003) and perseverance (Fig. 4c; one-way ANOVA: F(4,35) = 4.621, p = 0.005). Post-hoc analyses revealed that MK8016 treated rats required significantly more trials to set-shift (p = 0.002), performed more errors (p = 0.002) and displayed increased perseverance (p = 0.003) relative to VEH controls. MK8013 treatment had no effect on the number of trials to set-shift, errors or perseverance. The deleterious effect of MK8016 was reversed by co-application of CBD100 or CBD500 and although the number of trials to set-shift, errors and perseverance values were still elevated, they did not differ statistically from VEH controls. Importantly, the number of trials to set-shift in the MK8016/CBD100 and MK8016/CBD500 group differed significantly from MK8016 alone (p = 0.035 and p = 0.004, respectively) and did not differ from VEH controls. Thus, in contrast to CBD-induced flexibility impairments in naïve rats, CBD was able to reverse the effects of MK801-induced flexibility impairments, directly in the PFC.
Fig. 4.
Intra-PFC CBD blocks MK801-induced impairment of set-shifting behavior. Blocking NMDA receptors within PFC using high dose of non-competitive antagonist MK801 (concentrations given in µg/hemisphere) induced impairment that was rescued with co-injection of CBD. a The number of trials to shift and (b) total number of errors performed during set-shifting task was increased in the MK801 treated rats. c–e Errors were categorized into perseverative (c), regressive (d) or never-reinforced (e). Detailed analysis revealed that rats that needed more trials to complete the task showed a specific increase in perseverance suggesting impaired flexibility. Data represent mean ± s.e.m. Group sizes (n): VEH (9, same as in Fig. 2), MK8013 (7), MK8016 (7), MK8016/CBD100 (6), MK8016/CBD500 (7). Respective treatment groups were compared with one-way ANOVA followed with Gabriel post-hoc; or Kruskal–Wallis test followed with Mann–Whitney U tests. *p < 0.05; **p < 0.01 vs. VEH group; $p < 0.05; $$p < 0.01 vs. MK8016
Intra-PFC CBD impairs spontaneous alternation behavior
We next examined the potential effects of our previously established effective doses of intra-PFC THC or CBD on PFC-dependent working memory processing using the spontaneous alternation Y-maze test (Fig. 5a).
There was a significant effect of drug treatment on spontaneous alternation behavior (Fig. 5b, one-way ANOVA: (F(6,52) = 2.432, p = 0.040). While THC100 rats alternated similarly to VEH controls, CBD100 rats displayed attenuated alternation (p = 0.004). The returning score was also significantly affected by drug treatment (Fig. 5c; one-way ANOVA: F(6,52) = 2.968, p = 0.016) with CBD100 rats displaying significantly higher score than controls (p = 0.001), whereas number of re-entries was not affected (Fig. 5d). This suggests that altered alternation scores were not resulting from increased re-entries to the same arm but rather increased return ratio to previously visited arm. Co-application of CBD100 with THC100 or NAD299100 restored alternation behavior to control values. Importantly, NAD299100 alone or MK8016 did not interfere with alternation behavior. There was also no significant effect of drug treatment on total numbers of arm entries (Fig. 5e), suggesting that the observed CBD100-induced alternation deficit was not due to decreased locomotion. Thus, intra-PFC CBD100, but not THC100, impaired spatial working memory. Moreover, CBD-induced deficits were reversed by co-application of NAD299100 or THC100, demonstrating the involvement of 5-HT1A transmission in these effects and suggesting that activation of PFC CB1R can prevent CBD-induced deficits.
Intra-PFC THC increases anxiety-like behaviors which are reversed by CBD co-administration
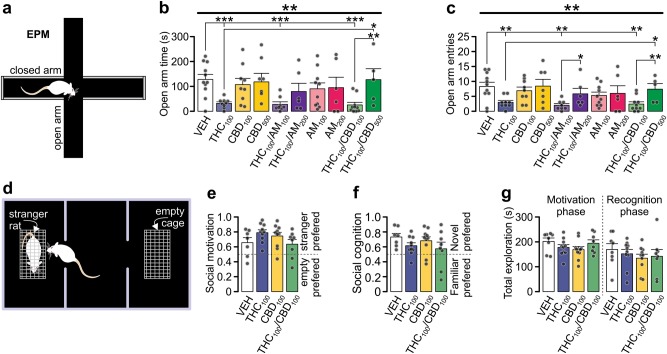
The endocannabinoid system is critically involved in the regulation of anxiety-related behaviors [31]. To examine the potential effects of intra-PFC THC or CBD on anxiety-like behaviors, we used elevated plus maze test (EPM; see methods; Fig. 6a).
Fig. 6.
Effects of THC and CBD on anxiety-like behaviors. a Schematic summary of the elevated plus maze test. Anxiety-like behaviors of rats treated with THC was increased as the time that animals spent in the open arms (b) and the number of entries to the open arms (c) were decreased. This effect was reversed by co-application of CB1 receptor antagonist AM251 or higher concentration of CBD. Data represent mean ± s.e.m. Group sizes (n): VEH (11), THC100 (9), CBD100(9), CBD500(7), THC100/AM251100 (8), THC100/AM251200 (6), AM251100 (10), AM251200 (6), THC100/CBD100 (10), THC100/CBD500 (5); subscripts indicate concentration in ng/hemisphere d Schematic of the three-chambered social approach apparatus. The test consisted of two phases. During phase I (sociability test), a stranger rat was enclosed in one of the cages as depicted on the scheme. During phase II (social memory test), a new rat was added in the previously empty cage. The time that the treated rat spent exploring enclosures during both phases was measured and corresponding scores were calculated. Social motivation (e) and social cognition (f) were not affected with the treatments. Also the total exploration times (g) did not differ between the groups. Data represent mean ± s.e.m. Group sizes: VEH (8), THC100 (10), CBD100 (10), THC100/CBD100 (8); subscripts indicate concentrations in ng/hemisphere. Respective treatment groups were compared with one-way ANOVA (e–g) or Kruskal–Wallis (b, c) test followed with Mann–Whitney U test. *p < 0.05; **p < 0.01; ***p < 0.001
There was a significant effect of drug treatment on times spent in the open arms (Fig. 6b; K–W test: Χ2(9) = 24.911, p = 0.003) and on the number of open arm entries (Fig. 6c; K–W test: Χ2(9) = 21.866, p = 0.009). Post-hoc analysis revealed that THC100 rats displayed significantly reduced open arm durations (p = 0.001) and entries (p = 0.008) relative to controls. CBD100 treated rats did not differ from controls. This data suggests that intra-PFC infusion of THC increases anxiety-like behaviors.
Since THC serves as a partial CB1R agonist [12], we next examined if co-administration with a CB1R antagonist, AM251, might block the anxiogenic effects of THC. While a lower dose of AM251100 did not block THC-induced anxiogenic effects, a higher dose (AM251200) was sufficient to block THC-induced anxiety-like behaviors (open arm time: p = 0.001 and p = 0.151; open arm entries: p = 0.141 and p = 0.168, respectively). AM251200 by itself did not produce any effects in the number of entries or open arm times (Fig. 6b, c).
We next tested whether CBD may mitigate THC-induced anxiety-like behaviors. Co-administration of CBD + THC dose-dependently blocked the anxiogenic effects of THC. Thus, unlike CBD100, CBD500 effectively blocked THC-induced anxiety-like behaviors (open arm entries p = 0.005 and p = 0.360; open arm times p = 0.001 and p = 0.457, respectively). Importantly application of CBD500 alone did not induce anxiolytic-like effects relative to controls.
Neither Intra-PFC THC nor CBD affect sociability or social memory behaviors
Cannabinoid exposure has been shown to strongly modulate sociability and social memory processing [32, 15]. To determine the potential effects of intra-PFC THC or CBD on social interaction and memory processing, we used a three-chambered social approach test (Fig. 6d). Statistical analysis shown that social motivation was not affected by THC100, CBD100 or THC100/CBD100 treatments (Fig. 6e), as all groups preferred a stranger rat vs. an empty enclosure. In addition, total exploration times did not differ between groups (Fig. 6g left panel). Similarly, social recognition scores were not affected by drug treatments as all groups displayed on average comparable levels of preference for the novel vs. familiar rat (Fig. 6f). Total exploration time did not differ between groups (Fig. 6g right panel), indicating that drugs were not interfering with locomotion.
DISCUSSION
Clinical and pre-clinical research has identified critical functional differences between the two primary phytocannabinoids in marijuana, THC and CBD [11, 15, 33]. Understanding these differences, particularly in terms of their potential impact on neuropsychiatric side-effects, has important implications for predicting the relative effects of CBD or THC exposure following recreational or therapeutic cannabis use. Here we specifically examined the potential effects of both phytocannabinoids directly in the PFC. We identified several cognitive domains whereby acute CBD may produce deleterious effects on cognitive flexibility and spatial working memory. In contrast, consistent with the emerging identification of CBD as a potential treatment option for various neuropsychiatric conditions [10, 11, 33], we found that intra-PFC CBD could reverse the cognitive impairments induced by pharmacological dysregulation of NMDA glutamatergic signaling in the PFC. Interestingly, the deleterious effects of CBD were blocked with a selective 5-HT1AR antagonist or with THC co-administration, suggesting the functional involvement of intra-PFC 5-HT1A and CB1 receptor signaling, as underlying mechanistic substrates responsible for the acute CBD effects on cognitive impairments.
In contrast to the effects of CBD, we found that acute intra-PFC THC administration did not affect cognitive processing. These results are at odds with previous reports showing that either acute [23] or chronic, neurodevelopmental exposure to high THC levels can impact cognitive function, by causing long-term psychotomimetic prefrontal neuroadaptations [15]. Interestingly, this may suggest that the deleterious effects of THC may require chronic exposure and/or may involve neural regions extrinsic to the PFC or require simultaneous actions across multiple neural regions. Alternatively, it is possible that doses tested in the present studies were below those required to induce acute cognitive disruption, directly in the PFC. We also found that intra-PFC THC produced anxiogenic effects in the EPM test. Interestingly, CBD was able to dose-dependently reverse THC-induced anxiety, whilst producing no direct effects on anxiety by itself, further demonstrating the divergent functional effects of intra-PFC THC vs. CBD.
Effects of THC and CBD on executive function and working memory
The PFC plays a crucial role in executive functioning, including cognitive flexibility and working memory [20]. In rats, the prelimbic PFC is explicitly involved in temporal switching between strategies related to spatial cues and in the integration of internal physiological states with salient environmental cues [17]. Previous studies have shown that elevated levels of anandamide, an endogenous agonist of CB1R, are associated with improved cognitive flexibility while increased levels of 2-arachidonoylglycerol, disrupt these functions [34]. Here, we found no effect of acute, intra-PFC THC exposure on cognitive flexibility or working memory. These results are generally consistent with the extant literature. For example, although higher doses of systemic THC disrupt working and short-term memory [35], direct intra-PFC THC impairs spatial working memory in a radial maze task only after one-hour delay, but not at short delays [23]. Accordingly, we show that zero-delay spatial working memory in the Y-maze is not influenced by intra-PFC THC. Systemic THC exposure or overexpression of CB1R within the PFC, interferes with set-shifting abilities by disrupting the reversal learning, but not the acquisition of new associations [24, 28]. Accordingly, we show that intra-PFC THC-treated rats can switch strategies from visual to spatial discrimination as quickly as controls. Nevertheless, given the differences between the present results and previous studies showing significant cognitive impairments associated with acute THC exposure [4, 23, 35], future studies are required to localize neural regions outside the PFC which may be responsible for the THC-induced cognitive deficits.
Unlike THC, acute intra-PFC CBD produced significant impairments in cognitive flexibility and working memory demonstrated by impairments in the rats’ ability to disengage from visually-guided actions, an increased number of trials needed to shift the strategy and increased perseveration (Fig. 3a–c). Behavioral set-shifting relies on the ability to reassign the attention from a stimulus set that was useful for predicting the response outcome (i.e. pressing the lever indicated with light = food reward) to a newly relevant stimulus set (i.e. pressing the lever on the left side = food reward while light no longer signals the correct lever). The set-shifting procedure used here is an analog of the Wisconsin Card Sorting Task (WCST) used in human research for testing frontal executive function [36]. Interestingly, marijuana users showing high CBD hair concentrations, although displaying improved attention and concentration, showed impaired performance in the WCST [27]. Accordingly, we show that intra-PFC CBD, did not affect memory recall but selectively impaired set-shifting (Fig. 3), suggesting that CBD might interfere with PFC circuitry responsible for inhibitory control over behavioral responding [37].
In agreement with this hypothesis is a report showing that systemic CBD application impairs pre-pulse inhibition (PPI) that can be reverted by THC co-administration [37]. However, CBD infused in the ventral striatum, can reverse amphetamine-induced PPI deficits in rats [11]. In terms of working memory, one previous report showed that systemic CBD did not interfere with working memory [35]. In contrast, we demonstrate that intra-PFC CBD impaired spontaneous alternations scores, suggesting that direct prefronto-cortical CBD exposure may modulate working memory performance, independently of non-localized, systemic CBD exposure.
CBD targets mainly the serotonin 5-HT1AR [7, 38] which is purportedly expressed in > 50% of PFC neurons [39]. Activation of postsynaptic 5-HT1AR results in cell hyperpolarization and spiking suppression [39], while activation of presynaptic 5-HT1A autoreceptors inhibits serotonin release leading to decreased PFC neuronal excitability [40]. This may suggest that, depending upon the localized effects of 5-HT1A signaling in the PFC, reduced levels of 5-HT may underlie CBD-related memory impairments observed in the present study. For example, depletion of 5-HT in the non-human primate PFC increases perseverance without affecting memory recall [41]. Additionally, systemic activation of 5-HT1AR impairs working memory [42] and reduces attentional tracking abilities [43]. This suggests that 5-HT1AR activation may promote response perseverance and consistently we show that a 5-HT1A antagonist partially blocks CBD-induced perseveration. Given the multi-target profile of CBD it is important to mention that other channels and receptors, like PPRγ, TRPV, GPR55 or CB2 might be also involved in the observed CBD effects. Interestingly, PFC 5-HT1AR decrease NMDA-mediated currents and thereby interfere with long-term memory formation [44, 45]. In contrast, activation of PFC 5-HT1AR was shown to diminish attentional impairments induced by an NMDA antagonist, suggesting that PFC 5-HT1AR may counteract cognitive deficits elicited by dysfunctional PFC glutamatergic transmission [46]. Accordingly, our data demonstrates that MK801-induced flexibility impairment can be reversed by co-application of CBD, implicating the therapeutic potential for CBD in treatment of PFC cognitive deficits in pathological states.
Effects of THC and CBD on anxiety-related and social behaviors
The reinforcing effects of marijuana in humans are usually accompanied by anxiolysis and increased social motivation. However, increased anxiety, panic and psychosis-like symptoms were also communicated [4, 5]. Similarly divergent effects of THC exposure on anxiety were reported in animal studies [3, 47]. For example, intra-PFC THC has been shown to produce anxiolytic effects at doses orders of magnitude higher than those used by us [e.g., 10 μg; [3]], suggesting dose-dependent bi-phasic effects on anxiety processing. Indeed, we observed anxiogenic effects of intra-PFC THC at 0.1 μg dose. While future studies are required to characterize dose-dependency of THC-induced bi-phasic behavioral effects on anxiety-related processing, one explanation may relate to how CB1R dose-dependently modulate sub-cortical DA activity states in the mesolimbic pathway. For example, lower concentrations of a synthetic CB1R agonist, WIN-55, increase DAergic activity states, fear-related behaviors and memory processing, whereas higher concentrations blunt sub-cortical DA activity states and dampen fear-related responding [48]. Thus, given the agonist effects of THC on CB1R, one possibility is that higher THC doses may serve to decrease DAergic activity states and dampen anxiety.
In addition, we found that CBD + THC co-administration was sufficient to block the anxiogenic effects of THC and this anxiolytic property of CBD might relate to 5-HT1AR activation by CBD [8]. Indeed, systemic stimulation of 5-HT1AR has been shown to exert anxiolytic-like effects [49]. While future studies are required to more fully examine how THC vs. CBD-related signaling mechanisms may differentially regulate anxiety-like behaviors in the PFC, the present study demonstrates that CBD can dose-dependently mitigate the anxiogenic properties of THC directly in the PFC.
Considerable evidence links cannabinoid exposure to modulation of social behaviors. For example, individuals with social anxiety are more likely to use cannabis to relieve anxiety symptoms [50]. On the other hand, heavy cannabis use is correlated with increased anxiety and poorer social functioning [51]. Animal studies have shown that deficits in social motivation and social cognition might result from neurodevelopmental THC exposure [15] or activation of ventral hippocampal CB1Rs [32]. Genetic deletion of CB1R gene leads to social withdrawal [52] and promotes anxiety-like behaviors toward novel conspecifics [53] . Here, we observed no effects of acute intra-PFC THC or CBD on either social motivation or social memory processing. While future studies are required to determine potential dose-dependency of intra-PFC THC or CBD on sociability, the present findings suggest that concentrations that are sufficient to produce anxiety or cognitive deficits, respectively, are not sufficient to concomitantly disrupt normal social behaviors.
Conclusions
The present findings add to clinical and pre-clinical evidence demonstrating dissociable roles for CBD and THC in modulating neuropsychiatric symptoms in both cognitive and affective domains. In terms of anxiety-related symptoms, our evidence underscores the importance of balancing THC/CBD ratios during marijuana exposure to mitigate potential anxiogenic THC effects. In terms of regulation of cognitive flexibility and working memory, our data suggests that the prefrontal effects of CBD may have differential outcomes for these cognitive domains as a consequence of existing pathology. Specifically, we found that CBD may produce PFC-dependent cognitive disruption in otherwise healthy subjects, but produce ameliorative effects during states of cortical disruption induced by NMDAR blockade. An important caveat to this conclusion is that the present studies exclusively used direct, intra-PFC microinfusions of CBD. It is certainly possible that the observed behavioral effects would not be observed following systemic exposure to CBD, or following CBD infusions to other mesocorticolimbic neural regions. Indeed, we have previously reported that intra-accumbens CBD infusions can robustly block neuropsychiatric-like cognitive impairments induced by neural states of pathology, such as psychotomimetic endophenotypes [11] and produce anti-psychotic-like modulation of mesolimbic dopaminergic activity states [7, 11]. Moreover, given that co-application of THC reversed CBD-induced cognitive impairments, these findings may have important implications for how THC/CBD ratios may differentially regulate specific neuropsychiatric symptom profiles, within distinct brain circuits.
Acknowledgments
Funding:
This work supported by the Canadian Institutes of Health Research (MOP 272999), the Ontario Mental Health Foundation, and the Natural Science and Engineering Research Council of Canada (N.S.E.R.C).
Author Contributions:
HJS, SRL designed the research study. HJS performed the behavioral experiments with help from SJD, JR, CN and CELJ. HJS analyzed the data. HJS and BP performed histology. SRL, BLA, NR, contributed essential reagents and equipment. HJS and SRL wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hanna J. Szkudlarek, Phone: +(519) 661-2111, Email: hszkudla@uwo.ca
Steven R. Laviolette, Phone: +(519) 661-2111, Email: steven.laviolette@schulich.uwo.ca
References
- 1.Fogaça MV, Reis FM, Campos AC, Guimarães FS. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur Neuropsychopharmacol. 2014;24:410–9. doi: 10.1016/j.euroneuro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207:105–11. doi: 10.1016/j.bbr.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 3.Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behavior. Neuropharmacology. 2008;54:151–60. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by delta9-tetrahydrocannabinol: a neural basis for the effects of cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–51. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 6.Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162:153–61. doi: 10.1016/j.schres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Norris C, Loureiro M, Kramar C, Zunder J, Renard J, Rushlow W, et al. Cannabidiol modulates fear memory formation through interactions with serotonergic transmission in the mesolimbic system. Neuropsychopharmacology. 2016;41:2839–50. doi: 10.1038/npp.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartim AG, Guimarães FS, Joca SR. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—possible involvement of 5-HT1A and CB1 receptors. Behav Brain Res. 2016;303:218–27. doi: 10.1016/j.bbr.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of dravet syndrome. Proc Natl Acad Sci USA. 2017;114:11229–34. doi: 10.1073/pnas.1711351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renard J, Loureiro M, Rosen LG, Zunder J, de Oliveira C, Schmid S, et al. Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J Neurosci. 2016;36:5160–9. doi: 10.1523/JNEUROSCI.3387-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, et al. Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cereb Cortex. 2017;27:1297–310. doi: 10.1093/cercor/bhv335. [DOI] [PubMed] [Google Scholar]
- 16.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–79. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat Rev Neurosci. 2017;18:335–46. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci. 2016;17:293–306. doi: 10.1038/nrn.2016.28. [DOI] [PubMed] [Google Scholar]
- 20.Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471–82. doi: 10.1016/j.jphysparis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Clark L, Manes F. Social and emotional decision-making following frontal lobe injury. Neurocase. 2004;10:398–403. doi: 10.1080/13554790490882799. [DOI] [PubMed] [Google Scholar]
- 22.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva de Melo LC, Cruz AP, Rios Valentim SJ, Jr, Marinho AR, Mendonça JB, Nakamura-Palacios EM. Delta(9)-THC administered into the medial prefrontal cortex disrupts the spatial working memory. Psychopharmacol (Berl) 2005;183:54–64. doi: 10.1007/s00213-005-0141-1. [DOI] [PubMed] [Google Scholar]
- 24.Egerton A, Brett RR, Pratt JA. Acute Δ9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–1905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos, G and C Watson. The Rat Brain in Stereotaxic Coordinates 6th edn, (Academic press, San Diego, 2007).
- 26.Desai SJ, Allman BL, Rajakumar N. Combination of behaviorally sub-effective doses of glutamate NMDA and dopamine D1 receptor antagonists impairs executive function. Behav Brain Res. 2017;323:24–31. doi: 10.1016/j.bbr.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, et al. Dorsolateral prefrontal cortex n-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–9. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Klugmann M, Goepfrich A, Friemel CM, Schneider M. AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Front Behav Neurosci. 2011;5:37. doi: 10.3389/fnbeh.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohleder C, Müller JK, Lange B, Leweke FM. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–37. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 31.Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 32.Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–47. doi: 10.1038/npp.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renard J, Norris C, Rushlow W, Laviolette SR. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;75:157–65. doi: 10.1016/j.neubiorev.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Fagundo AB, de la Torre R, Jiménez-Murcia S, Agüera Z, Pastor A, Casanueva FF, et al. Modulation of the endocannabinoids n-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) on executive functions in humans. PloS ONE. 2013;8:e66387. doi: 10.1371/journal.pone.0066387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropharmacology. 2004;47:1170–9. doi: 10.1016/j.neuropharm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Schmittmann VD, Visser I, Raijmakers ME. Multiple learning modes in the development of performance on a rule-based category-learning task. Neuropsychologia. 2006;44:2079–91. doi: 10.1016/j.neuropsychologia.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, et al. Pharmacokinetic and behavioral profile of THC, CBD, and THC + CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017;27:1223–37. doi: 10.1016/j.euroneuro.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacol (Berl) 2011;213:465–73. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- 39.Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–64. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–80. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 42.Herremans AH, Hijzen TH, Olivier B, Slangen JL. serotonergic drug effects on a delayed conditional discrimination task in the rat; involvement of the 5-HT1A receptor in working memory. J Psychopharmacol. 1995;9:242–50. doi: 10.1177/026988119500900307. [DOI] [PubMed] [Google Scholar]
- 43.Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory and the serotonin 5-ht1a and 5-HT2A receptors. J Cogn Neurosci. 2005;17:1497–508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 44.Edagawa Y, Saito H, Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur J Pharmacol. 1998;349:221–4. doi: 10.1016/S0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- 45.Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–67. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- 47.Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in plus maze. J Pharmacol Exp Ther. 1990;253(3):1002–9. [PubMed] [Google Scholar]
- 48.Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J Neurosci. 2014;34:13096–109. doi: 10.1523/JNEUROSCI.1297-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collinson N, Dawson GR. On the elevated plus-maze the anxiolytic-like effects of the 5-HT(1A) agonist, 8-OH-DPAT, but not the anxiogenic-like effects of the 5-HT(1A) partial agonist, buspirone, are blocked by the 5-HT1A antagonist, WAY 100635. Psychopharmacol (Berl) 1997;132:35–43. doi: 10.1007/s002130050317. [DOI] [PubMed] [Google Scholar]
- 50.Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res. 2008;42:230–9. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehndiratta SS, Wig NN. psychosocial effects of longterm cannabis use in india. a study of fifty heavy users and controls. Drug Alcohol Depend. 1975;1:71–81. doi: 10.1016/0376-8716(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 52.Haller J., Varga B., Ledent C., Barna I., Freund T. F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. European Journal of Neuroscience. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 53.Litvin Y., Phan A., Hill M. N., Pfaff D. W., McEwen B. S. CBreceptor signaling regulates social anxiety and memory. Genes, Brain and Behavior. 2013;12:479–489. doi: 10.1111/gbb.12045. [DOI] [PubMed] [Google Scholar]