Abstract
“Locomotive syndrome” is used to designate the condition of individuals with musculoskeletal disease who are highly likely to require nursing care. This article reviews screening, prevalence, causal and related factors, and the relationship between locomotive syndrome and falls and fractures in older adults with this syndrome. A few self-administered questionnaire tools are available to assess individuals for locomotive syndrome. Additionally, screening methods, including a physical functioning assessment, are appropriate for detailed discrimination of locomotive syndrome. The prevalence of locomotive syndrome is significantly higher in women than in men, and tends to increase markedly from 70 years of age. More severe locomotive syndrome is related to knee pain, osteoporosis, sarcopenia, and lumbar disease. The incidence of falling in locomotive syndrome is higher than the incidence for the older population in general. Locomotive training including squats and a unipedal standing exercise has been recommending to prevent locomotive syndrome. This training improves muscle strength and balance function for older people who have a risk for locomotive syndrome.
Keywords: Locomotive syndrome, Fall, Fractures
1. Introduction
The aging of the population is a major concern in Asian countries [1]. Increased life expectancy and the declining birth rate have rapidly increased the aging of the Japanese population. In 2015, the average life expectancy reached 80.5 in men and 86.8 years in women, and almost one-fourth (24.1%) of the population in Japan was aged 65 years or older [1]. Advancing aging has increased the prevalence of musculoskeletal disease [2], including osteoarthritis, osteoporosis, lumbar spondylosis, and spinal stenosis; and the number of patients with hip fracture was estimated at 190,000 in Japan in 2012 [3]. Because of this situation, in 25% of the cases claiming nursing care insurance the reasons were musculoskeletal disease, fractures, and falls [4].
Against this background, the Japanese Orthopaedic Association has proposed the term “locomotive syndrome (LS)” to designate the condition of individuals with musculoskeletal disease who are highly likely to require nursing care [5]. The purpose of this concept is to raise awareness of healthcare for the locomotive system in elderly Japanese. The locomotive system is important for maintaining quality of life because locomotor function, joints, bones, peripheral nerves and muscles directly affect the activities of daily living (ADLs) in the geriatric population. Thus, early screening and detection of individuals with LS is important to prevent falls and fractures as a result of progressive LS. Intervention and care for musculoskeletal disease are needed to maintain quality of life (QOL).
This article reviews the screening, prevalence, causes and related factors, and the relationship between LS and falls and fractures in older adults with this syndrome. Additionally, we have proposed strategies to avoid LS.
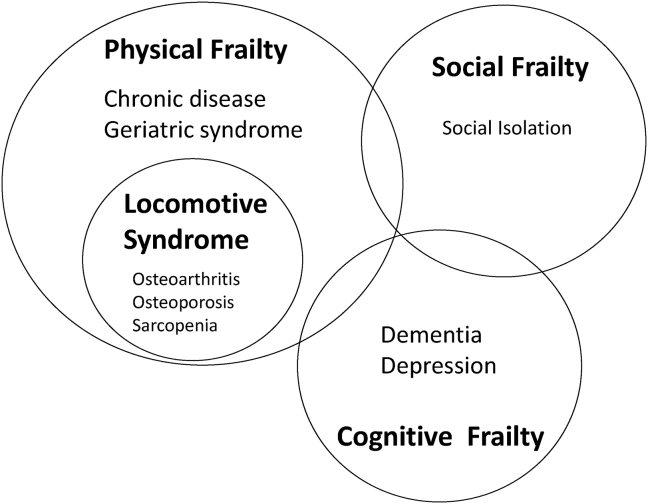
2. Concept of LS compared with frailty
LS is recognized as the condition of individuals with musculoskeletal disease who are highly likely to require nursing care. The concept of ‘Frailty’ is used to identify older adults at high risk of death, disability, and institutionalization. The terms are similar, but LS is specified as physical frailty with musculoskeletal disease without social or cognitive frailty (Fig. 1). Sarcopenia is a component of locomotive syndrome, and both have common themes of poor physical performance and slow gait. In older adults with frailty, LS and sarcopenia more likely represent the consequences of a permanent disruption of homeostasis.
Fig. 1.
Relationship between frailty and locomotive syndrome. Locomotive syndrome is included in the concept of frailty that it is composed of three components.
3. Screening tests for LS
A few screening methods for LS including self-administered questionnaires are known. Loco-check is a simple self-check method for awareness of LS (Table 1). An individual can check whether or not they have LS by examining their daily activities [3]. Seichi et al. [6] developed a 25-question Geriatric Locomotive Function Scale (GLFS-25) as a detailed screening tool to identify the population at high risk for LS. In addition, GLFS-5 is provided as a short version of the GLFS-25 [6]. Several physical function tests to assess LS are possible. Reference values to discriminate LS for a timed up-and-go test (TUG), one-leg standing time, back muscle strength, 10 m gait time, maximum stride and grip strength in men are 6.7 s, 21 s, 78 kg, 5.5 s and, 119 cm, and 34 kg, respectively, and for women are 7.5 s, 15 s, 40 kg, 6.2 s, 104 cm, and 22 kg, respectively [7]. Seichi et al. proposed using a GLFS-25 score of 16 and/or a cutoff for the one-leg standing time of 19 s for individuals aged ≤70, 10 s for individuals aged >70 and ≤75, and 6 s for individuals aged >75 when screening older adults [8]. Japanese women with LS had a shorter unipedal stance time and a longer normal and fast 6 min walking time than those without LS [9]. We reported that acceleration signals during gait seen using an accelerometer have the potential to become general indicators for LS in the elderly [10]. Several physical assessments have validity for screening for LS, and assessment of gait function or potential for one-leg standing have been important methods for the screening because gait dysfunction and decline of extremity muscle strength can indicate LS.
Table 1.
Loco-check.
| 1 | You cannot put on each sock of a pair while standing on one leg |
| 2 | You stumble or slip in your house |
| 3 | You need to use a handrail when going upstairs |
| 4 | You cannot get across the road at a crossing before the traffic light changes |
| 5 | You have difficulty walking continuously for 15 min |
| 6 | You find it difficult to walk home carrying a shopping bag weighing about 2 kg (e.g., two 1-L milk packs). |
| 7 | You find it difficult to do housework requiring physical strength (e.g., use of a vacuum cleaner to clean the rooms, putting futons into and taking them out of the closet) |
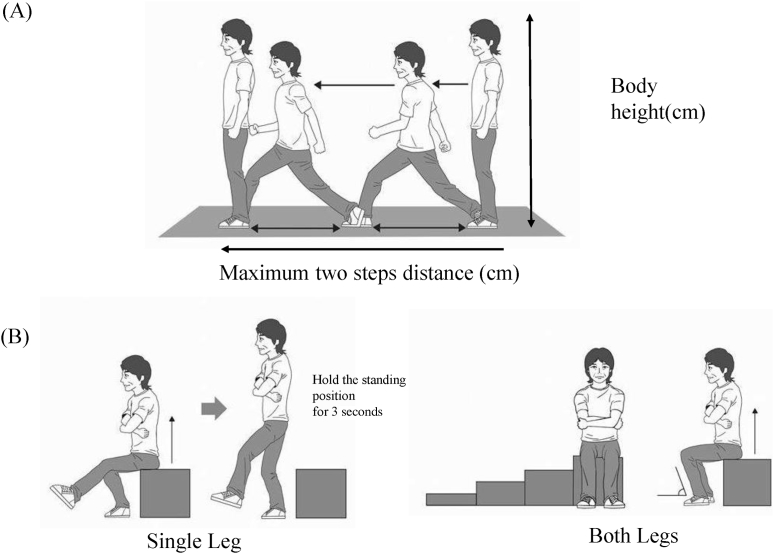
Recently, a new set of pre-existing scales, “Locomotive Syndrome Risk Test”, was developed; the two-step test, stand-up test, and GLFS-25 are proposed as screening tools to identify people at high risk for LS (Fig. 2) [11]. In stage 1, the prevalence of the indices in LS risk test stages 1 and 2 was highest for a two-step test score <1.3, followed by difficulty with one-leg standing from a 40 cm high seat in the stand-up test and 25-question GLFS score ≥7. In stage 2, the prevalence also was highest for a two-step test score <1.1, but the prevalence of a 25-question GLFS score ≥16 was higher than that for difficulty with standing from a 20 cm high seat using both legs in the stand-up test. If at least one of the 3 tests: the two-step test, stand-up test, or GLFS-25 is positive, the individual is defined as having LS (stage 1 or stage 2).
Fig. 2.
Two-step test and stand-up test. (A) Two-step test: subjects move two steps forward to the maximum extent possible. The maximum two steps distance divided by individual's body height and the value for the 2 steps is summed. (B) Stand-up test: first, subjects sit on a 40 cm stool and stand up using one leg. If the subjects cannot perform this trial, they are allowed to try to standing from a 20 cm stool using both legs.
It is easy to conduct a Loco-check in the older population and this test can facilitate assessment of the elderly at local medical check-ups. GLFS-25 is a more detailed screening questionnaire than Loco-check; however, it may be more difficult for older adults to complete and consume more time. GLFS-5 may substitute in this situation. Locomotive syndrome risk tests, included physical functioning tests, may be more appropriate for detailed discrimination of LS, although these methods are difficult for the older elderly. We consider each screening test can provide different information depending on the situation, subject or setting. Assessment methods for LS have been developed specifically for older Japanese adults; the English version has not been sufficiently validated, but this is warranted for future study.
4. Prevalence of LS
A previous study included 135 participants aged 70 years or older [12], and found 50.3% as having LS by using Loco-check. Another study [13] including 722 participants aged 56.6 years on average found 56 of 264 (21.2%) men and 165 of 463 (35.6%) women were classified with LS using criteria defined by Loco-check. By contrast, other studies using GLFS-25 or GLFS-5 for screening of LS found the prevalence of LS in subjects aged 70 years is about 16% [7], [14], [15], [16]. When GLFS-5 was used a similar prevalence of LS was found compared with the full-version GLFS-25 [10], [17], [18]; namely, about 17% in a group of similar age and sex were found using the GLFS-25.
A large cross-sectional internet survey was performed by using the GLFS-25 [19]. This was conducted to estimate the prevalence of LS in Japan. Of the 4500 participants who completed the survey, the mean value for the GLFS-25 was significantly higher in those aged in their 70s than it was in the other age groups. LS as defined by this test was significantly higher in women (12.3%) than in men (7.9%). Highlights of this study were that the prevalence of LS was 8.4% of those aged in their 40s, 9.2% for those in their 50s, 8.3% for those in their 60s, and 16.3% for those in their 70s. This study estimated that around 6.5 million individuals in Japan have LS.
The prevalence of LS was significantly higher in women than in men, and the prevalence of LS tended to increase sharply from the age of 70 years; the prevalence is about 16%–17% using GLFS-25 or GLFS-5 in the participants aged 70 years or more. In the future, using new screening methods including physical functioning tests will be more widespread, and we can expect to clarify the prevalence of LS using these tests. Clinicians should carefully select which screening tests are used because the prevalence may be different as a result of the screening test selected.
5. Causal and related factors of LS (Table 2)
Table 2.
Summary of studies on musculoskeletal disease associated with LS.
| Musculoskeletal condition | Reference | Participants (average age, sex) | LS screening | Findings |
|---|---|---|---|---|
| Knee pain | Matsumoto et al. [17] | 217 older adults (73.4 y, 80 men, 137 women) | GLFS-5 | Diagnosis of knee osteoarthritis is 39.5% in the LS. |
| Muramoto et al. [7] | 406 volunteers (68.8 y, 167 men, 239 women) | GLFS-25 | VAS score of knee pain between non-LS and LS is 7.4 vs 35.3 (in men) and 8.9 vs 33.5 (in women). | |
| Hirano et al. [20] | 364 participants (67.6 y, 131 men, 233 women) | GLFS-25 | VAS score of knee pain between non-LS and LS is 35.5 vs 9.0 (right) and 33.2 vs 8.0 (left). Correlation between GLFS-25 and knee pain is 0.506 (right) and 0.523 (left). | |
| Muramoto et al. [21] | 358 volunteers (66.0 y, 128 men, 230 women) | GLFS-25 | GLFS-25 score and knee pain in multiple regression analysis is 0.265 (β). | |
| Osteoporosis | Matsumoto et al. [17] | 217 older adults (73.4 y, 80 men, 137 women) | GLFS-5 | 57.9% of older adults identified as having osteoporosis using QUS device in the LS. |
| Izuka et al. [14] | 287 participants (64.7 y, 100 men, 187 women) | GLFS-25 | GLFS-25 score correlated with the %YAM of the SOS using QUS. | |
| Sarcopenia | Matsumoto et al. [17] | 217 older adults (73.4 y, 80 men, 137 women) | GLFS-5 | 15.8% of older adults with LS had sarcopenia. |
| Momoki et al. [27] | 186 women aged over 65 y (77.7 y) | Loco-check | Sarcopenia was identified in 21.0% of participants. LS was significantly associated with sarcopenia. | |
| Lumbar disease and dysfunction | Izuka et al. [14] | 287 participants (64.7 y, 100 men and 187 women) | GLFS-25 | GLFS-25 score correlated with low back pain. |
| Hirano et al. [16] | 135 participants (76.5 y, 54 men, 81 women) | Loco-check | Back muscle strength and an increase in spinal inclination angle were significantly associated with LS. | |
| Hirano et al. [27] | 315 participants (68.0 y, 115 men, 200 women) | Loco-check | Back muscle strength was significantly associated with LS. | |
| Hirano et al. [28] | 105 men (69.5 y) | Loco-check | A decrease in back muscle strength and an increase in spinal inclination may be the most important risk factors for LS. |
LS, locomotive syndrome; VAS, visual analogue scale; GLFS, geriatric locomotive function scale; SOS, speed of sound; QUS, quantitative ultrasound.
5.1. Knee osteoarthritis and knee pain
Individuals with LS have a greater likelihood of diagnosis (39.5%) with knee osteoarthritis (knee OA) than those without LS [17]. Knee OA is apparently a cause and related to LS because there is a relationship between knee pain (Visual Analogue Scale or VAS score) and LS [7], [17], [20], [21]. In patients with knee OA, there is less weight bearing to support the leg on the side with knee OA during walking; thus, this gait pattern compensates by using other joints to maintain balance while walking [22]. These patients are therefore more likely to fall. Similarly, elderly patients who have undergone total knee arthroplasty (TKA) also have a higher risk of falling [23], [24]. The probability of falling remains for those patients, although TKA improves knee pain, the range of motion, knee deformity, and gait function. We propose that older adults with TKA should also be defined as having LS. In general, knee pain and gait dysfunction because of knee OA reduces gait speed, endurance, and regularity. These variables are directly related to LS, which indicates locomotor dysfunction.
5.2. Osteoporosis
There is a relationship between LS and osteoporosis [14], [17], [21]. The percentage of young adult mean (%YAM) of the speed of sound using quantitative ultrasound (QUS) methods was significantly lower in 43 subjects with LS identified by the GLFS-25 than in 244 subjects without LS (68% vs 78%) [14]. The prevalence of osteoporosis identified using QUS methods increases as the severity of LS becomes higher (32.5% vs 57.9%) [17]. However, all studies evaluated bone mass using QUS, rather than dual-energy X-ray absorption (DXA), which is the criterion standard for diagnosis of osteoporosis. Thus, it is not sufficiently clear whether LS indicates low bone mass. Nevertheless, individuals who were identified as having LS also have possible low bone mass and an increased risk of fracture because higher QUS values reflect a higher risk of fracture [25].
5.3. Sarcopenia
Two studies found a relationship between LS and sarcopenia [17], [26]. One study showed subjects with sarcopenia were older, had a lower body mass index and calf circumference, and were more likely to have LS, as identified by Loco-check. In multivariate analysis, LS was significantly associated with sarcopenia [26]. Another study showed 15.8% older adults with LS have sarcopenia, but the multivariate analysis with adjusted age and sex did not show a relationship between LS and sarcopenia [17]. Sarcopenia therefore has no clear cause-and-effect relationship with LS because both studies were of a cross-sectional design, and sarcopenia has a strong relationship with age and sex.
5.4. Lumbar disease and dysfunction
Low back pain was significantly more frequently observed in 43 subjects with LS than in 244 subjects without LS when adjusted for age, gender, and body mass index (BMI) [14]. Low back pain decreases the QOL and ADL because of the difficulty it causes in standing work and walking in older adults [16]. A decrease in back muscle strength and an increase in spinal inclination angle are the most important risk factors for LS [12], [27], [28]. Lumbar kyphosis may be an important factor related to this lumbar dysfunction, because elderly people who have kyphosis and vertebral fracture with frail bone mass, are more likely to fall than those who do not [29], [30]. Several spinal dysfunctions may lead to gait disorder and low back pain to reduce ADL in these individuals.
5.5. Other disease
The GLFS-25 score showed a significant correlation with waist circumference (74 cm in non-LS and 80 cm in LS) [15]. Central obesity is significantly associated with LS, and waist circumference can be a useful variable with which to assess the risk of LS in elderly women. Lifestyle-related diseases, such as being overweight or having diabetes, may cause osteoarthritis or osteoporosis; therefore, a relationship between metabolic disease and musculoskeletal disease may exist [31]. Progressive LS may lead to both, but the relationship between them is not yet clear. Future study is needed to clarify the relationship between LS and metabolic diseases.
6. QOL
Iizuka et al. reported that a finding of LS on the basis of Loco-check is significantly associated with Euro Qol-5D utility value and Euro Qol-VAS score, and that a population identified as having LS by use of Loco-check also had reduced health related quality of life [32]. Worse spinal alignment such as trunk deformity is associated with a lower QOL score [33]. LS was defined as “locomotive dysfunction” because musculoskeletal disease, especially lower extremity and spine dysfunction, causes deteriorated gait function and directly decreases social activities in older adults.
7. Fall and fractures as a result of LS
There are insufficient data regarding the incidence of falling and fracture in the elderly population with LS. Only one study provided the incidence of falling among older people with LS (34.2%) [17]. This incidence is higher than for the older population in general whose rate is 15%–20% [34], suggesting that older people with LS or who have a musculoskeletal disease have a greater risk of falling.
It is well-known that osteoarthritis is a risk factor for falling [27]. Older adults who have knee pain have a higher prevalence of falling (30%) than those without knee pain [28]. Patients with a clinical diagnosis of knee OA have a greater risk of all nonvertebral and hip fractures than patients with knee pain alone. The risk of falling in patients who have a diagnosis of knee OA is 43%–63% [35], [36], [37]. Musculoskeletal pain is a risk factor for falling; individuals with knee OA have higher coefficients of variation in step length, step width, and double support time, proprioceptive impairment, and consistency of movement, because abnormal lower leg alignment increases the risk of falling [38].
A large cohort study in Japan showed radiographic lumbar spondylosis was significantly associated with multiple falls, and lower back pain and knee pain were independently associated with multiple falls in women after adjusted related factors, and that 39% of those with lumbar spondylosis were likely to have multiple falls [36]. Patients with lumbar spinal stenosis and patients with osteoarthritis were more likely to fall compared with the elderly in general because they had gait dysfunction with neurogenic claudication [39], [40], [41]. Additionally, 74.3% of patients with lumbar spinal stenosis had a vitamin D deficiency. Vitamin D deficiency is a high risk factor for falls and fractures [42].
About 30% of postmenopausal women with osteoporosis have had a fall [29], [43]. Surprisingly, 50% of women with osteoporosis had fallen at least once in the previous 12 months [44]. Our previous study [45] showed the prevalence of falling in these individuals in Japan is 17.6%, which is lower than the rate found in other studies. Nevertheless, these women have a poor standing balance because of spinal deformity, and force platform analyses showed that older women with osteoporosis had a decrease of postural control, rather than kyphotic postural alignment [46], [47], and a higher center of pressure displacement and velocity than older adults without osteoporosis. Older adults with osteoporosis are more likely to have a fracture as a result of falling.
There is wide support for a relationship between pain and falling. Older adults who have pain in various joints, including hip joints [48], [49], knee joints [36], [48], [50], ankle joints [49], [51], [52] and lower back pain [36], [49], [53] have a higher risk of falling. A meta-analysis showed that older adults who have pain in their lower extremities and chronic pain have an increased risk of falling [54]. Furthermore, chronic pain in more than two areas and greater severity of symptoms further increases the risk of falling [49]. More severe LS may result from increased pain or severity of musculoskeletal symptoms related to falling. Older adults who have more than one type of musculoskeletal disease are more likely to fall than older adults who only have one type of musculoskeletal disease. Thus, elderly persons with LS who have pain or severe musculoskeletal symptoms have a greater risk of falls and fractures.
8. Strategies to prevent LS
LS is a wide-ranging concept for older adults who had decreased locomotive dysfunction because of musculoskeletal problems. Medication, pain reduction, and maintenance of bone strength may be needed to treat the principal musculoskeletal disease suffered by individuals with LS.
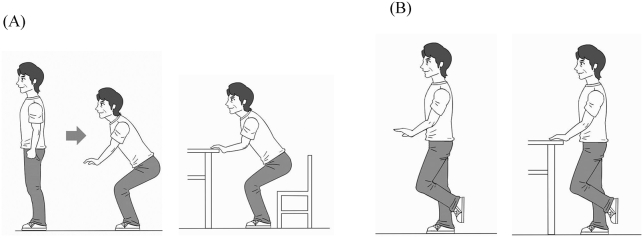
In general, exercise intervention had been recommended for prevention of LS. Locomotive-training (Loco-Tre) (squats and one-leg standing exercises) can be suggested to improve muscle strength and balance function in older people with LS [55] (Fig. 3). These exercises provide a greater percent maximum voluntary contraction (%MVC) for lower muscles. In older adults, the burden of muscle activity during exercise normalized by the muscle's MVC is 80% on hip abductor muscle during the one-leg standing exercise and 100% on the knee extensor muscle during the squat exercise [56]. This percentage is sufficient burden to impact muscle strength. Intervention with this training has been reported. A two-month intervention with Loco-Tre improved TUG and unipedal standing times in older adults with LS [57], [58]. The one-leg standing exercise is often performed for balance training to prevent falls [59]. Adherence to these exercises is as high as 69.3% over 2 months [60]. This exercise is simple and easy for older adults to understand and this may be useful for compliance.
Fig. 3.
Loco-Tre. (A) Squat exercise: Legs are opened with the feet a shoulder-width apart, and with toes spread a little, and then the person squats as if sitting. If subjects have knee pain, they should be allowed to stabilize themselves using both hands on a table. (B) One-leg standing exercise: subjects stand on each leg for 30 s with their arms resting. If subjects had balance dysfunction or history of falling, they may be allowed to stabilize themselves using one or both hands on a table.
For the middle aged with LS as a result of metabolic disease, aerobic exercise is recommended to reduce weight. Maruya et al. [58] reported Loco-Tre intervention did not improve physical parameters in individuals with higher BMI. By contrast, we found a higher prevalence of osteoporosis in people with LS than in those without [17]. We propose LS can be subdivided into two subclassifications, an osteoporotic type found in older people with lower BMI, and an overweight type found in obese individuals. In the osteoporotic type; older adults may become socially isolated because of deterioration of their locomotive system as a result of osteoporosis or sarcopenia [61]. The skeletal and the muscular systems are tightly intertwined because the strongest mechanical forces applied to bones are those created by muscle contractions that condition bone density, strength, and microarchitecture [62]. Therefore, a decrease in muscle strength with advancing age, geriatric syndrome, or chronic disease leads to lower bone strength. Older adults with osteoporosis or sarcopenia present gait variability or dysfunction [43], [63], which have a strong relationship with the incidence of falling [29], [64]. Deterioration of locomotive systems, osteopenia or lower muscle mass, leads to fragility fractures and severe LS. By contrast, in the obesity type, older adults who are overweight with related metabolic disease are more prevalent among patients with musculoskeletal disease and may develop LS. The incidence of knee osteoarthritis is significantly related to the number of metabolic syndrome components, such as being overweight, because the burden of being overweight can load joints during walking [65]. Moreover, persons with obesity and type-2 diabetes are subject to osteoarthritis and impaired glucose tolerance, which generally leads to a decrease in muscle strength [66]. Thus, we suggest metabolic syndrome also causes LS.
We recommend prescribing suitable exercise for each type of LS; muscle training for maintaining muscle and bone mass, and aerobic exercise to reduce and control body weight. Additionally, vitamin D may be needed to maintain bone strength and reduce the risk of falling. While vitamin D supplements and exercise are recommended to prevent falls in older people [67], we recommend that exercise is prescribed based on assessment of the type of LS, and that comprehensive care is needed to improve or prevent LS.
9. Conclusion
LS is a syndrome found in the geriatric population, and is associated with age, sex, and musculoskeletal conditions. The prevalence of LS is about 16% in people in their 70s as assessed by the GLFS 25. We recommend exercise intervention for LS based on assessment of the type of LS: osteoporotic type or obesity type. Comprehensive care should include medication, vitamin D supplements, and exercise to improve or prevent LS.
Conflict of interest
The authors report no conflict of interest.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Ageing in Asian. 3/17/2016. http://www8.cao.go.jp/kourei/whitepaper/w-2014/zenbun/s1_1_5.html [Google Scholar]
- 2.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Min Metab. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 3.Hagino H. Fragility fracture prevention: review from a Japanese perspective. Yonago Acta Med. 2012;55:21–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Outline of the result of National Livelihood Survey [Internet]. [cited 2016 Feb 2]. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa07/4-2.html.
- 5.Nakamura K.A. “Super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13:1–2. doi: 10.1007/s00776-007-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seichi A., Hoshino Y., Doi T., Akai M., Tobimatsu Y., Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: the 25-question geriatric locomotive function scale. J Orthop Sci. 2012;17:163–172. doi: 10.1007/s00776-011-0193-5. [DOI] [PubMed] [Google Scholar]
- 7.Muramoto A., Imagama S., Ito Z., Hirano K., Tauchi R., Ishiguro N. Threshold values of physical performance tests for locomotive syndrome. J Orthop Sci. 2013;18:618–626. doi: 10.1007/s00776-013-0382-5. [DOI] [PubMed] [Google Scholar]
- 8.Seichi A., Hoshino Y., Doi T., Akai M., Tobimatsu Y., Kita K. Determination of the optimal cutoff time to use when screening elderly people for locomotive syndrome using the one-leg standing test (with eyes open) J Orthop Sci. 2014;19:620–626. doi: 10.1007/s00776-014-0581-8. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M., Hashizume H., Oka H., Okada M., Takakura R., Hisari A. Physical performance measures associated with locomotive syndrome in middle-aged and older Japanese women. J Geriatr Phys Ther. 2015;38:202–207. doi: 10.1519/JPT.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H., Hagino H., Osaki M., Tanishima S., Tanimura C., Matsuura A. Gait variability analysed using an accelerometer is associated with locomotive syndrome among the general elderly population: the GAINA study. J Orthop Sci. 2016;21:354–360. doi: 10.1016/j.jos.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura N., Muraki S., Oka H., Tanaka S., Ogata T., Kawaguchi H. Association between new indices in the locomotive syndrome risk test and decline in mobility: third survey of the ROAD study. J Orthop Sci. 2015;20:896–905. doi: 10.1007/s00776-015-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Impact of spinal imbalance and back muscle strength on locomotive syndrome in community-living elderly people. J Orthop Sci. 2012;17:532–537. doi: 10.1007/s00776-012-0266-0. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki E., Ishibashi Y., Tsuda E., Ono A., Yamamoto Y., Inoue R. Evaluation of locomotive disability using loco-check: a cross-sectional study in the Japanese general population. J Orthop Sci. 2013;18:121–129. doi: 10.1007/s00776-012-0329-2. [DOI] [PubMed] [Google Scholar]
- 14.Iizuka Y., Iizuka H., Mieda T., Tajika T., Yamamoto A., Takagishi K. Population-based study of the association of osteoporosis and chronic musculoskeletal pain and locomotive syndrome: the Katashina study. J Orthop Sci. 2015;20:1085–1089. doi: 10.1007/s00776-015-0774-9. [DOI] [PubMed] [Google Scholar]
- 15.Muramoto A., Imagama S., Ito Z., Hirano K., Tauchi R., Ishiguro N. Waist circumference is associated with locomotive syndrome in elderly females. J Orthop Sci. 2014;19:612–619. doi: 10.1007/s00776-014-0559-6. [DOI] [PubMed] [Google Scholar]
- 16.Hirano K., Imagama S., Hasegawa Y., Ito Z., Muramoto A., Ishiguro N. Impact of low back pain, knee pain, and timed up-and-go test on quality of life in community-living people. J Orthop Sci. 2014;19:164–171. doi: 10.1007/s00776-013-0476-0. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto H., Nakaso N., Matsuura A., Akita T., Hagino H. Relationship between severity of locomotive syndrome and the incidence of falling, prevalence of low bone mass, and sarcopenia. Rigaku Ryohogaku. 2016;43:36–46. [in Japanese] [Google Scholar]
- 18.Sasaki K., Takada J., Hana K., Iba K., Koumi K., Yamashita T. The investigation of the relationship between locomotive syndrome and the risk of fragility fractures. Seikei Saigaigeka. 2014;57:571–576. [in Japanese] [Google Scholar]
- 19.Kimura A., Seichi A., Konno S., Yabuki S., Hayashi K. Prevalence of locomotive syndrome in Japan: a nationwide, cross-sectional internet survey. J Orthop Sci. 2014;19:792–797. doi: 10.1007/s00776-014-0606-3. [DOI] [PubMed] [Google Scholar]
- 20.Hirano K., Imagama S., Hasegawa Y., Ito Z., Muramoto A., Ishiguro N. The influence of locomotive syndrome on health-related quality of life in a community-living population. Mod Rheumatol. 2013;23:939–944. doi: 10.1007/s10165-012-0770-2. [DOI] [PubMed] [Google Scholar]
- 21.Muramoto A., Imagama S., Ito Z., Hirano K., Ishiguro N., Hasegawa Y. Physical performance tests are useful for evaluating and monitoring the severity of locomotive syndrome. J Orthop Sci. 2012;17:782–788. doi: 10.1007/s00776-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen H.L., Lu T.W., Wang T.M., Huang S.C. Biomechanical strategies for successful obstacle crossing with the trailing limb in older adults with medial compartment knee osteoarthritis. J Biomech. 2008;41:753–761. doi: 10.1016/j.jbiomech.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto H., Okuno M., Nakamura T., Yamamoto K., Osaki M., Hagino H. Incidence and risk factors for falling in patients after total knee arthroplasty compared to healthy elderly individuals. Yonago Acta Med. 2014;57:137–145. [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto H., Okuno M., Nakamura T., Yamamoto K., Hagino H. Fall incidence and risk factors in patients after total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132:555–563. doi: 10.1007/s00402-011-1418-y. [DOI] [PubMed] [Google Scholar]
- 25.Pisani P., Renna M.D., Conversano F., Casciaro E., Muratore M., Quarta E. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J Radiol. 2013;5:398–410. doi: 10.4329/wjr.v5.i11.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momoki C., Habu D., Ogura J., Tada A., Hasei A., Sakurai K. Relationships between sarcopenia and household status and locomotive syndrome in a community-dwelling elderly women in Japan. Geriatr Gerontol Int. 2016 Jan 21 doi: 10.1111/ggi.12674. doi: 10.1111/ggi.12674. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Impact of back muscle strength and aging on locomotive syndrome in community living Japanese women. Nagoya J Med Sci. 2013;75:47–55. [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Effect of back muscle strength and sagittal spinal imbalance on locomotive syndrome in Japanese men. Orthopedics. 2012;35:e1073–e1078. doi: 10.3928/01477447-20120621-25. [DOI] [PubMed] [Google Scholar]
- 29.Arnold C.M., Busch A.J., Schachter C.L., Harrison L., Olszynski W. The relationship of intrinsic fall risk factors to a recent history of falling in older women with osteoporosis. J Orthop Sports Phys Ther. 2005;35:452–460. doi: 10.2519/jospt.2005.35.7.452. [DOI] [PubMed] [Google Scholar]
- 30.Kado D.M., Huang M.H., Nguyen C.B., Barrett-Connor E., Greendale G.A. Hyperkyphotic posture and risk of injurious falls in older persons: the Rancho Bernardo Study. J Gerontol A Biol Sci Med Sci. 2007;62:652–657. doi: 10.1093/gerona/62.6.652. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura N., Muraki S., Oka H., Kawaguchi H., Nakamura K., Akune T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J Rheumatol. 2011;38:921–930. doi: 10.3899/jrheum.100569. [DOI] [PubMed] [Google Scholar]
- 32.Iizuka Y., Iizuka H., Mieda T., Tajika T., Yamamoto A., Takagishi K. Association between “loco-check” and EuroQol, a comprehensive instrument for assessing health-related quality of life: a study of the Japanese general population. J Orthop Sci. 2014;19(5):786–791. doi: 10.1007/s00776-014-0602-7. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T., Ishida K., Hirose D., Nagano Y., Okumiya K., Nishinaga M. Trunk deformity is associated with a reduction in outdoor activities of daily living and life satisfaction in community-dwelling older people. Osteoporos Int. 2005;16:273–279. doi: 10.1007/s00198-004-1669-3. [DOI] [PubMed] [Google Scholar]
- 34.Yasumura S., Haga H., Nagai H., Suzuki T., Amano H., Shibata H. Rate of falls and the correlates among elderly people living in an urban community in Japan. Age Ageing. 1994;23:323–327. doi: 10.1093/ageing/23.4.323. [DOI] [PubMed] [Google Scholar]
- 35.Williams S.B., Brand C.A., Hill K.D., Hunt S.B., Moran H. Feasibility and outcomes of a home-based exercise program on improving balance and gait stability in women with lower-limb osteoarthritis or rheumatoid arthritis: a pilot study. Arch Phys Med Rehabil. 2010;91:106–114. doi: 10.1016/j.apmr.2009.08.150. [DOI] [PubMed] [Google Scholar]
- 36.Muraki S., Akune T., Oka H., En-Yo Y., Yoshida M., Nakamura K. Prevalence of falls and the association with knee osteoarthritis and lumbar spondylosis as well as knee and lower back pain in Japanese men and women. Arthritis Care Res. 2011;63:1425–1431. doi: 10.1002/acr.20562. [DOI] [PubMed] [Google Scholar]
- 37.Levinger P., Menz H.B., Wee E., Feller J.A., Bartlett J.R., Bergman N.R. Physiological risk factors for falls in people with knee osteoarthritis before and early after knee replacement surgery. Knee Surg Sports Traumatol Arthrosc. 2011;19:1082–1089. doi: 10.1007/s00167-010-1325-8. [DOI] [PubMed] [Google Scholar]
- 38.Hoops M.L., Rosenblatt N.J., Hurt C.P., Crenshaw J., Grabiner M.D. Does lower extremity osteoarthritis exacerbate risk factors for falls in older adults? Womens Health. 2012;8:685–696. doi: 10.2217/whe.12.53. quiz 97–8. [DOI] [PubMed] [Google Scholar]
- 39.Papadakis N.C., Christakis D.G., Tzagarakis G.N., Chlouverakis G.I., Kampanis N.A., Stergiopoulos K.N. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30:1171–1186. doi: 10.1088/0967-3334/30/11/003. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.J., Chun H.J., Han C.D., Moon S.H., Kang K.T., Kim H.S. The risk assessment of a fall in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2011;36:E588–E592. doi: 10.1097/BRS.0b013e3181f92d8e. [DOI] [PubMed] [Google Scholar]
- 41.Conrad B.P., Shokat M.S., Abbasi A.Z., Vincent H.K., Seay A., Kennedy D.J. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987–992. doi: 10.1016/j.gaitpost.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim T.H., Lee B.H., Lee H.M., Lee S.H., Park J.O., Kim H.S. Prevalence of vitamin D deficiency in patients with lumbar spinal stenosis and its relationship with pain. Pain Physician. 2013;16:165–176. [PubMed] [Google Scholar]
- 43.Palombaro K.M., Hack L.M., Mangione K.K., Barr A.E., Newton R.A., Magri F. Gait variability detects women in early postmenopause with low bone mineral density. Phys Ther. 2009;89:1315–1326. doi: 10.2522/ptj.20080401. [DOI] [PubMed] [Google Scholar]
- 44.da Silva R.B., Costa-Paiva L., Morais S.S., Mezzalira R., Ferreira Nde O., Pinto-Neto A.M. Predictors of falls in women with and without osteoporosis. J Orthop Sports Phys Ther. 2010;40:582–588. doi: 10.2519/jospt.2010.3239. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto H., Hagino H., Sageshima H., Osaki M., Tanishima S., Tanimura C. Diagnosis of knee osteoarthritis and gait variability increases risk of falling for osteoporotic older adults: the GAINA study. Osteoporos Sarcopenia. 2015;1:46–52. [Google Scholar]
- 46.Brech G.C., Plapler P.G., de Souza Meirelles E., Marcolino F.M., Greve J.M. Evaluation of the association between osteoporosis and postural balance in postmenopausal women. Gait Posture. 2013;38:321–325. doi: 10.1016/j.gaitpost.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Burke T.N., Franca F.J., Meneses S.R., Cardoso V.I., Pereira R.M., Danilevicius C.F. Postural control among elderly women with and without osteoporosis: is there a difference? Sao Paulo Med J. 2010;128:219–224. doi: 10.1590/S1516-31802010000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecchi F., Molino-Lova R., Di Iorio A., Conti A.A., Mannoni A., Lauretani F. Measures of physical performance capture the excess disability associated with hip pain or knee pain in older persons. J Gerontol A Biol Sci Med Sci. 2009;64:1316–1324. doi: 10.1093/gerona/glp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leveille S.G., Jones R.N., Kiely D.K., Hausdorff J.M., Shmerling R.H., Guralnik J.M. Chronic musculoskeletal pain and the occurrence of falls in an older population. J Am Med Assoc. 2009;302:2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arden N.K., Crozier S., Smith H., Anderson F., Edwards C., Raphael H. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum. 2006;55:610–615. doi: 10.1002/art.22088. [DOI] [PubMed] [Google Scholar]
- 51.Chaiwanichsiri D., Janchai S., Tantisiriwat N. Foot disorders and falls in older persons. Gerontology. 2009;55:296–302. doi: 10.1159/000181149. [DOI] [PubMed] [Google Scholar]
- 52.Mickle K.J., Munro B.J., Lord S.R., Menz H.B., Steele J.R. Foot pain, plantar pressures, and falls in older people: a prospective study. J Am Geriatr Soc. 2010;58:1936–1940. doi: 10.1111/j.1532-5415.2010.03061.x. [DOI] [PubMed] [Google Scholar]
- 53.Bekibele C.O., Gureje O. Fall incidence in a population of elderly persons in Nigeria. Gerontology. 2010;56:278–283. doi: 10.1159/000236327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stubbs B., Binnekade T., Eggermont L., Sepehry A.A., Patchay S., Schofield P. Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:175–187. doi: 10.1016/j.apmr.2013.08.241. e9. [DOI] [PubMed] [Google Scholar]
- 55.Challenge for locomotive syndrome [Internet]. [cited 2016 Feb 2]. Available from: https://locomo-joa.jp/check/locotre/.
- 56.Matsumoto H., Hagino H. Electromyography analysis on lower extremity exercise in the elderly compared with those in younger subjects. J Phys Med. 2010;21:336–342. [in Japanese] [Google Scholar]
- 57.Sasaki K., Sugita T., Kikuchi Y., Ohta M., Hosokawa N., Higai S. The efficacy of locomotive training for the elderly population with locomotive syndrome. J East Jpn Assoc Orthop Traumatol. 2012;24:53–56. [Google Scholar]
- 58.Maruya K., Hiroaki F., Arai T., Hosoi T., Ishibashi H. Exercise interventions for improving motor function in community-dwelling middle-aged and elderly: effects due to differences in body mass index. Osteoporos Jpn. 2015;23:99–107. [in Japanese] [Google Scholar]
- 59.Sakamoto K., Endo N., Harada A., Sakada T., Tsushita K., Kita K. Why not use your own body weight to prevent falls? A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for </=15 s. J Orthop Sci. 2013;18:110–120. doi: 10.1007/s00776-012-0328-3. [DOI] [PubMed] [Google Scholar]
- 60.Hosoi T., Fujita H., Arai T., Ishibashi H. The functional characteristics of people who continued locomotion training. Rigakuryoho Kagaku. 2012;27:407–410. [in Japanese] [Google Scholar]
- 61.Cesari M., Landi F., Vellas B., Bernabei R., Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cederholm T., Cruz-Jentoft A.J., Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013;49:111–117. [PubMed] [Google Scholar]
- 63.Schwenk M., Howe C., Saleh A., Mohler J., Grewal G., Armstrong D. Frailty and technology: a systematic review of gait analysis in those with frailty. Gerontology. 2014;60:79–89. doi: 10.1159/000354211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada M., Nishiguchi S., Fukutani N., Tanigawa T., Yukutake T., Kayama H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14:911–915. doi: 10.1016/j.jamda.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Yoshimura N. Epidemiology of osteoarthritis in Japan : the ROAD study. Clin Calcium. 2011;21:821–825. [PubMed] [Google Scholar]
- 66.Williams M.F., London D.A., Husni E.M., Navaneethan S., Kashyap S.R. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complications. 2016 Jul;30(5):944–950. doi: 10.1016/j.jdiacomp.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 67.Uusi-Rasi K., Patil R., Karinkanta S., Kannus P., Tokola K., Lamberg-Allardt C. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015;175:703–711. doi: 10.1001/jamainternmed.2015.0225. [DOI] [PubMed] [Google Scholar]