Abstract
Objective
The aim of this study was to review the literature about the relevance of the whole body vibration (WBV) in decreasing the number of fractures in osteoporotic women.
Methods
Searches were performed by three independent researchers through the PubMed and PEDro databases.
Results
Only 0.1% of the publications with “Fracture and osteoporosis” have a relation with WBV exercise. The achievements have revealed a positive effect of this exercise in patients with risk factors for fractures like osteoporosis. Protocols were performed two to three times a week, from 6 up to 18 months, and with 12.6 up to 40 Hz as frequencies. Different tools were used to evaluate the effects of the WBV exercise in conditions that could cause fractures in postmenopausal women.
Conclusions
Although the paucity of research regarding direct effects of WBV in decreasing fractures, WBV could be a feasible and effective way to modify well-recognized risk factors for falls and fractures, improvements in some aspects of neuromuscular function and balance. More studies have to be performed establish protocols with well controlled parameters.
Keywords: Vibration, Fractures, Osteoporosis
1. Introduction
Fractures have a multifactorial etiology. Among the conditions that contribute to increase of fracture are the risk of bone fragility, the tendency of falls and presence of metabolic bone diseases [1], [2]. Some of these conditions for remain underdiagnosed and undertreated. In almost all patients with incident fractures, the absolute risk of subsequent fracture and mortality is highest immediately after the occurrence of the fracture. This risk is markedly increased in frail elderly patients [1]. The incidence of fragility fractures is increasing rapidly and has become a major public health concern due to they result in increased mortality and persistent physical morbidity. Osteoporosis (OP) and falls are the most important risk factors on fragility fractures [3]. The hip fracture, as a common consequence of OP, remains a major challenge to public health and represents 92% of the total cost caused by OP and has a high mortality [4]. OP is characterized by low bone mass, and affects many millions of people around the world [5], [4], [6], [7], it is mostly observed in female population, where a significant increase in incidence is recorded after menopause.
Several mechanisms are cited to be involved in decreasing the rate of fracture healing due to decreased quality of bone, like altered cell recruitment and angiogenesis [8]. The mechanical stimulation may also not be adequately absorbed due to delayed osteogenic capacity in these osteoporotic bones. But although the time of healing may be different, the regenerative process is considered to be the same, independently of the quality of the bone [8]. It was also demonstrated effects on estrogen receptors that are directly related to fracture healing in ovariectomized osteoporotic rats [9].
Fractures are associated with several social and economical issues. It is a major public health concern contributing annually to an estimated cost of $17 billion to the American health care system [5], [6], [10].
Various strategies of management, pharmacological and nonpharmacological therapies to OP, aiming to reduce fractures, have been suggested [11]. Weber-Rajek et al. [7] have considered that the postmenopausal OP (PMO) treatment has evolved focusing on prevention, screening, diagnosis and early and specified therapy. Nevertheless, the treatment of OP would be not only pharmacotherapy [12], [13]. Calcium and vitamin D intake, a healthy lifestyle, measures to prevent falls, increase balance and muscle strength through exercises [10], [12] are strongly recommended and are part of guidelines developed for PMO.
The effects of exercise on the prevention of the postmenopausal symptoms have been discussed and accepted. Different studies [14], [13], [15] have reported that postmenopausal symptoms can be prevented significantly by encouraging the women over middle age to gain the habit of exercising regularly. Moreover, it has been pointed out that in the treatment of OP, physical therapy could also improve the quality of life of patients [16], [17], [18], [19]. Sinaki [16] has considered that the exercise, whether for prevention or treatment, is one of the major tools for the management of bone loss. Exercises in the treatment of OP would be relevant to improve the axial stability and the locomotion through safe strengthening of muscles. Sinaki et al. [18] have reported that the spinal extensor resistive exercises can decrease the risk of vertebral fractures.
An important consideration is that in the management of the patients with OP, due to the increased risk of falls, it is that the physical activity must be safe [20]. Mechanical vibrations produced in oscillating/vibratory can be transmitted to the body of the patient generating WBV exercises. These WBV exercises, in appropriated biomechanical conditions, are a safe form of physical activity [21]. Following the piezoelectric theory, the interaction of mechanical vibration controlled by biomechanical parameters with the body would induce the process of bone formation [7], [22], [23]. Moreover, WBV may increase the level of growth hormone [24], parathyroid hormone (PTH) [25] and testosterone [26] in plasma, that might be associated with the prevention of sarcopenia and OP. WBV exercise may also increase muscular strength and power [27], [28] that would lead to a better neuromuscular function. Studies [29], [30] have also described that WBV exercise could improve cognitive functions in children and young adults; all of these benefits could help reduce the risk of falls fragility and fractures.
In studies with rats, it was demonstrated that low-magnitude high-frequency vibration (LMHFV) is an intervention that may act as bone quality enhancer in ovariectomized rats, including positive effects in bone biomarkers like osteocalcin and alkaline phosphatase and improvement in callus formation [8].
The aim of this study was to review the literature to verify the findings about the relevance of the WBV exercise in decreasing the number of fractures in women with OP or improving the healing process.
2. Material and methods
2.1. Search strategy
EMM, CFD, LLPD independently accessed bibliographical databases (PubMed and PEDro) through the Universidade do Estado do Rio de Janeiro on November, 2015.
As the searches for publications were carried out independently by the three reviewers, they then decided which publications would be excluded from the search results. Full papers were included for this narrative review if they met the search criteria and described a study using WBV generated by an oscillating/vibratory platform used to manage postmenopausal women independently on the year of the publication. Data were independently abstracted by three of the authors and disagreements were resolved by consensus.
After searching for OP and fractures, in order to acknowledge more completely the literature, the keywords – “Fracture” and “Osteoporosis” and “whole body vibration” – were searched together in the PubMed and PEDro databases. In this search all these publications were screened following exclusion and inclusion criteria.
2.2. Exclusion criteria to select the publications
Exclusion criteria allowed the elimination of unnecessary publications identified in the search. Papers were excluded if they were (i) published in a language different of the English; (ii) review articles; (iii) with combined treatments, (iv) case reports and findings no related to the bone.
2.3. Inclusion criteria to select the publications
To be included in this review, all studies investigating effect of WBV exercise on fracture healing and its effects on fractures risk factors in postmenopausal needed to comply with the following criteria: be a randomized controlled trial (RCT); in the absence of RCTs, single group experimental studies were also considered (cross-over designs); published in English. Studies were included if the postmenopausal women were taking supplementation of vitamin D and performed static or dynamic exercises on an oscillating/vibratory platform.
2.4. PRISMA flowchart involving the steps in selecting full papers
A flowchart, based in the PRISMA analysis [31], was done to show the steps in the selection of the full papers analyzed in this review.
2.5. Level of evidence of the selected papers
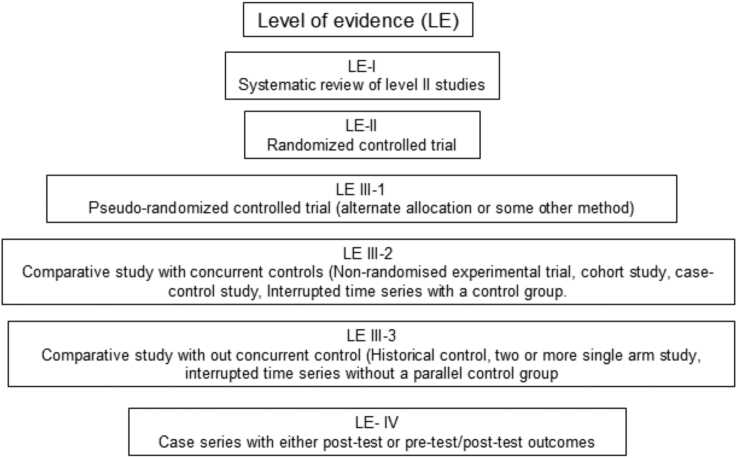
The included studies were classified according to the National Health and Medical Research Council hierarchy of evidence (NHMRC, 2009) [32] (Fig. 1). Each article was assigned to one reviewer, cross-checked by another reviewer and where there was disagreement, a third party was consulted and the issue discussed until consensus was reached.
Fig. 1.
Designation of levels of evidence (LE) according to the intervention research question (National Health and Medical Research Council (NHMRC - 2009).
2.6. Data extraction
Data was not comparable and therefore statistical pooling not appropriate with the result that the findings of this review were summarized in a narrative form.
3. Results
Table 1 shows the search performed with some keywords related to “OP” and “fracture” in the databases PubMed and PEDro. Considering PubMed, the NP involving “fracture and OP” is about 12% of the total NP with the keyword “fracture”. The percentage of the publications with the keywords - Fracture and OP and exercise – was only about 6% of the articles with “Fracture and OP”. Moreover, the percentage of articles resulted with the search “Fracture” and “OP” and “whole body vibration” was only about 2% of the publications with “Fracture” and “OP” and “exercise”.
Table 1.
Number of publications searched of with keyword related to “OP” and “fracture” in the databases PubMed and PEDro.
| Keywords | NP (PubMed) | NP (PEDro) |
|---|---|---|
| Fracture | 221,410 | 549 |
| Fracture and men | 11,126 | No items found |
| Fracture and women | 17,889 | 3 |
| Fracture and “postmenopausal woman” | 66 | 1 |
| Fracture and “postmenopausal woman” and “whole body vibration” | No items found | These keywords in this presentation were not recognized. |
| Fracture and OP | 24,914 | 101 |
| Fracture and OP and exercise | 1394 | 62 |
| Fracture and OP and “whole body vibration” | 26 | 4 |
NP – number of publications, OP – Osteoporosis.
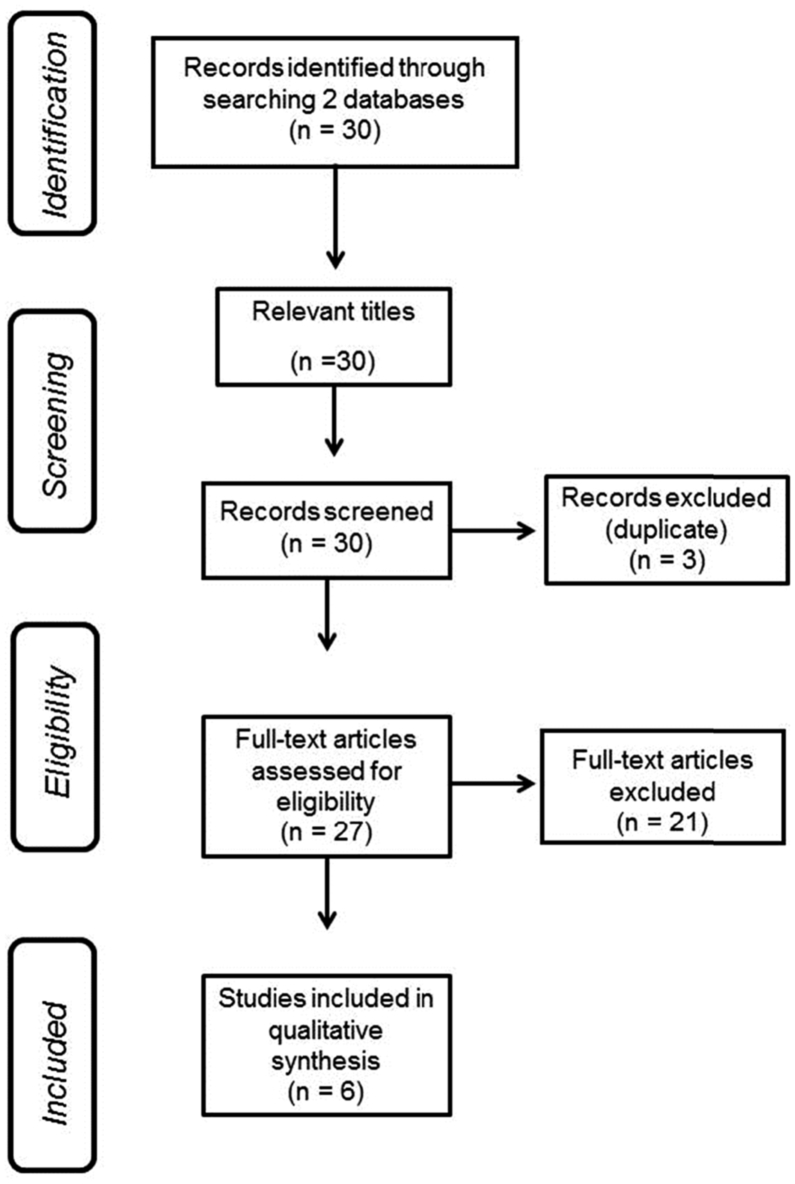
In Fig. 2, it is shown the flowchart with the steps used to select the papers that were screened. Considering the inclusion and exclusion criteria, of the thirty publications found in the PubMed and the PEDro databases with the keywords – “Fracture” and “Osteoporosis” and “whole body vibration” - only six papers fulfilled all the criteria to be analyzed in this review. Three were in duplicate, two were not written in English, ten were reviews, in five the investigations were performed with animals, and two were with young women. The paper published by Rohlmann et al., [33] has been deleted since it was a study to measure the effect of various vibration frequencies, amplitudes, device types and body positions on the loads acting on a lumbar vertebral body replacement. The article published by Rubin et al. [34] has not been considered because it was a study to establish if extremely low-level (<1 g, where 1 g = earth's gravitational field, or 9.8 ms−2) mechanical stimuli could be efficiently delivered to the axial skeleton of a human.
Fig. 2.
Flowchart indicating the steps to select the full papers analyzed in this review.
The level of evidence of the screened and select papers are LE-II (RCT) [6], [35], [36], [37], LE-III-1 [38], LE-III-2 [39] according to the NHMRC.
Table 2 shows the descriptions of each study and information about the protocols. The aims of the selected papers were to verify whether WBV exercises were effective for the management of PMO considering the parameters related to the bone improvement, neuromuscular function and risk of falls, but only one [38] was directly related to access its action on fractures reduction.
Table 2.
Information about the protocols, aim of the study and anthropometric characteristics of the participants used to the management of the PMO women with WBV exercises.
| References | Aim of the study | Subjects/age/time of the study | Protocols |
|---|---|---|---|
| Lai et al., 2013 [6] | To investigate the effect of high-frequency and high-magnitude WBV on the BMD of the lumbar spine in PMW. | 28 PMW (46–69 yr) were randomized into either the WBV group or the CG for a 6 mos trial. | The WBV group received an intervention of high-frequency (30 Hz) and high-magnitude (3.2 g) WBV in a natural full-standing posture for 5 min, 3xwk. |
| Stolzenberg et al., 2013 [39] | To investigate the effect of resistive exercise with either WBV training (VIB) or coordination/balance training (BAL) on neuromuscular function (countermovement jump, multiple 1-leg hopping, STST). | 68 PMW (61.2–71.3 yr) with osteopenia or OP were recruited for the study. 57 subjects completed the 9 mos. | The interventions were 2xwk, intervention period. All subjects conducted 30 min of resistance exercise each training day. The VIB-group performed additional training on the Galileo vibration exercise device. The BAL-group performed balance training. |
| von Stengel et al., 2011 [35] | To determine whether the effect of exercise on BMD and falls can be enhanced by WBV. | 151 PMW (68.5 ± 3.1 yr) were randomized in conventional TG; conventional training group including vibration (TGV); and wellness CG for 18 months. | TG conducted an exercise program consisting of 20 min dancing aerobics, 5 min balance training, 20 min functional gymnastics, and 15 min dynamic leg-strength training on vibration plates (without vibration) 2xwk. TGV performed an identical exercise regimen with vibration (25–35 Hz) during the leg-strengthening sequence. CG performed a low-intensity wellness program. |
| Beck and Norling, 2010 [38] | To observe the effect of low- and higher intensity WBV on risk factors for hip fracture in PMW. | 47 women (71.5 ± 9.0 yr)were randomized (8 mos) controlled trial design | Interventions to examine the influence of 2xwk low-intensity WBV (15 min, 30 Hz, 0.3 g) or higher intensity WBV (2 × 3 min, 12.5 Hz, 1 g). |
| Gusi et al., 2006 [36] | To compare the effects of WBV using a reciprocating platform at frequencies lower than 20 Hz and a walking-based exercise programme on BMD and balance in PMW. | 28 physically untrained PMW (60–76 yr) were assigned at random to a WBV group or a Walking group for 8 mos. | Both experimental programmes consisted of 3xwk. Vibratory session included 6 bouts of 1 min (12.6 Hz in frequency and 3 cm in amplitude with 60 degrees of knee flexion) with 1 min rest between bouts. Walking session was 55 min of walking and 5 min of stretching. Hip and lumbar BMD were measured using DEXA and balance was assessed by the blind flamingo test. |
| Verschueren et al., 2004 [37] | In this randomized controlled trial, hip BMD was measured in PMW after a 24-week WBV training program. | 70 PMW (58–74 yr) were randomly assigned to a WBVT, a RES, or a CG for 6 mos. | The WBV group and the RES group trained 3xwk. The WBV group performed static and dynamic knee-extensor exercises on a vibration platform (35–40 Hz), which mechanically loaded the bone and evoked reflexive muscle contractions. The RES group trained knee extensors by dynamic leg press and leg extension exercises, increasing from low (20 RM) to high (8 RM) resistance. The CG did not participate in any training. |
BMD – bone mineral density, STST – sit-to-stand test, CG – control group, min-minute, mos – months, PMO – postmenopausal osteoporosis, PMW – postmenopausal women, RES – resistance training group, TG – training group, wk-week, WBV – Whole body vibration, WBVT – Whole body vibration training, yr – year, BAL – balance, TGV – training group including vibration, DEXA – Dual energy X-ray absorptiometry, VIB – vibration.
The total number of participants was 392 and it varied from 28 [6], [36] up to 151 [35]. The ages varied from 46 [6] up to 80.5 [38] years old. The time used in the protocols varied from 6 [6], [37] up to 18 months [35]. The frequency of the mechanical vibration used in the protocols has varied from 12.6 [36] up to 40 Hz [37]. Interventions with WBV exercise were twice [35], [38], [39] or three times a week [6], [36], [37].
Table 3 shows the tools used for the authors in the studies, the outcomes and the conclusion of the selected articles used in this revision to verify the consequences of the use of WBV exercise in parameters related to the fracture of bone of postmenopausal women. Different outcome measures were used to evaluate the effect of the WBV exercises in parameters related to conditions that could promote fractures in the postmenopausal women, but no direct effect of WBV exercise on fracture healing in humans was found.
Table 3.
Tools, results and conclusions of the selected articles used in this revision related to the use of WBV exercise in the bone of postmenopausal women.
| Reference | Tools | Results | Conclusion |
|---|---|---|---|
| Lai et al., 2013 [6] | DEXA was used to measure the lumbar BMD before and after the intervention. | Six mos later, the BMD in the WBV group increased, while in the CG decreased. The lumbar BMD of the WBV group increased significantly. | This study with WBV yielded benefits to the BMD of the lumbar spine in PMW, and could therefore be provided as an alternative exercise. |
| Stolzenberg et al., 2013 [39] | Neuromuscular testing, Countermovement jump testing, MLH to examine the force development at the ankle joint and STST were performed. | An “intent-to-treat” analysis showed greater improvement in the VIB-group for peak countermovement power. The mean effect size for this parameter was greater change in VIB than BAL. In MLH, a better performance in the VIB-group after the intervention period was seen on a “per-protocol” analysis only. Both groups improved in the STST. | The current study provides evidence that short-duration WBV on exercise can have a greater impact on some aspects of neuromuscular function in PMW with low bone density than proprioceptive training. |
| von Stengel et al., 2011 [35] | BMD was measured at the hip and lumbar spine at baseline and follow-up using the DEXA method. Falls were recorded daily via the calendar method. | A multifunctional training program had a positive impact on lumbar BMD. The difference between the TG and the CG was significant. At the hip no changes were determined in either group. The fall frequency was significantly lower in TGV compared with CG, whereas the difference between TG and CG was not significant. | The application of vibration did not enhance these effects. However, only the training including WBV affected the number of falls significantly. |
| Beck and Norling, 2010 [38] | Anthropometrics, bone (whole body, hip, spine, forearm, and heel), muscle (wall squat and chair rise), and balance (tandem walk and single leg stance) were determined. | There were no between-group differences in any measure, but within-group effects were evident. Controls lost bone at the trochanter and lumbar spine, whereas WBV groups did not. WBV subjects improved wall squat and chair rise performance. | Eight mos of twice-weekly WBV may improve lower limb muscle function. These changes may translate to a decreased risk of falls and hip fracture. |
| Gusi et al., 2006 [36] | Hip and lumbar BMD were measured using DEXA and balance was assessed by the blind flamingo test. |
BMD at the femoral neck in the WBV group was increased compared to the Walking group. In contrast, the BMD at the lumbar spine was unaltered in both groups. Balance was improved in the WBV group but not in the Walking group. | The 8-mos course of vibratory exercise using a reciprocating plate is feasible and is more effective than walking to improve two major determinants of bone fractures (BMD at the femoral neck and Balance) |
| Verschueren et al., 2004 [37] | Hip BMD was measured using DEXA. Isometric and dynamic strength were measured by means of a motor-driven dynamometer. | Vibration training improved isometric and dynamic muscle strength and also increased BMD of the hip. No changes in hip BMD were observed in women participating in resistance training or age-matched controls. | WBV training increased BMD of the hip and it may be a feasible and effective way to modify well-recognized risk factors for falls and fractures in older women and support the need for further human studies. |
BMD – bone mineral density, Bone ALP – bone alkaline phosphatase; BUA – calcaneal broadband attenuation, CG – control group, DEXA – dual energy X-ray absorptiometry, min-minute, MLH – Multiple 1-leg hopping, mos – months, NTx/Cr – N-telopeptide X adjusted to creatinine, PMW – postmenopausal women, STST – Sit-to-Stand test, TG – training group, wk – week, WBV – whole body vibration, WBVT – whole body vibration training, year – yr, VIB – vibration, TGV – training group including vibration, BAL – balance.
To verify the bone quality of the bone, Dual energy X-ray absorptiometry (DEXA or DXA) in four [35], [6], [36], [37] of the six publications were analyzed. The Flamingo test [36], tandem walk and single leg stance [38] were used to verify the balance [36], countermovement jump testing, Multiple 1-leg development at the ankle joint and Sit-to-Stand test [39], the number of falls [35], wall squat and chair rise [38] were utilized to assess general approaches of the neuromuscular conditions, and isometric and dynamic strength were measured by means of a motor-driven dynamometer [37]. In all the four studies that have evaluated the BMD [35], [6], [36], [37], an improvement in the patients of the WBV intervention of this parameter was shown. Considering the neuromuscular evaluations, the fall frequency was significantly diminished due to the WBV intervention [35]; the balance has improved [36], as well as the wall squat and chair rise performance [38] and in isometric and dynamic muscle strength [37]. Several investigations concluded that WBV exercise could be a feasible and effective way to improve two major determinants of bone fractures, the BMD and the neuromuscular responses.
4. Discussion
The costs to the health care system [5] could justify the strong increase in the number of publications in fractures, its prevention and management. It has been very well studied about the non-pharmacological intervention for PMO with exercises, as they must be strongly recommended. However, it has been observed that few studies on “Fracture and OP” were related to exercise. Although WBV exercise is considered a modality of treatment, only 0.1% of the publications with “Fracture and OP” have a relation to it, even been considered a simple and convenient type of physical activity.
WBV exercises could lead to (a) the bone formation, preventing sarcopenia and OP [40], (b) increase the muscular strength and power that would lead to a better neuromuscular function and (c) improve cognitive functions. In the case of patients with OP, these findings could reduce the risk of falls and fractures. These considerations could justify the investigations about the importance of the WBV exercise in postmenopausal women in relation to the fractures. Considering the level of the evidence of the publications, four of the six selected papers are LE-II, RCT [35], [6], [36], [37], which may demonstrate the need to increase interest in this cheap and safe treatment strategy. Only one [38] was directly related to the measurement of fractures. In general, the achievements have revealed a positive effect of the WBV exercise in patients with OP according to Beck et al. [23] and Rauch et al. [41]. Vibration intensity may be best described in terms of acceleration, in g-forces. There are now many WBV devices commercially available, producing a wide range of accelerations; from 0.3 g to more than 10 g. Depending on devices, frequency and amplitude can be fixed by the manufacturer, or varied by adjusting a dial and/or foot placement (if the plate oscillates around a central fulcrum)”.
WBV exercise could be a feasible and effective way (i) to modify well-recognized risk factors for falls and fractures in older women [36], [37], [38], (ii) to reduce the number of falls significantly [35], (iii) to have a greater impact on some aspects of neuromuscular function in postmenopausal women [39] and to improve two major determinants of bone fractures, the BMD and the balance [36].
As a definite approach to risk factors for fractures, further investigations are needed to understand the dose–response of effects of the WBV exercise in patients with fractures and the possible reduction of time for fracture healing, since in rats it was demonstrated positive effects in from the genes to hormones profiles, as well as callus formation [8], [9].
A high heterogeneity in methods was observed and caution should be taken when generalizing the results, such as biomechanical parameters, type of the oscillating/vibratory platform, or the variability of the protocols used; those are potential limitations. Therefore, the increase of the number of studies with a higher methodological quality focusing specifically on a population without a great variability of the age would be desirable. The optimal target population for the therapy and long-term effects was not yet defined.
In conclusion, the interest in investigations related to fracture is increasing in the world, but still must be an enhancement in the evaluation of exercises effects in adequate and controlled parameters.
Authors' contributions
EMM, CD, DSM, CRSG and MBF participated in the conception and design of the study, as well, preparing the manuscript. CD, CRSG, DSM, PJM, BPC and MBF coordinated the clinical approaches of the study. DSC, CRG, EOGA, and LLPD did the searches in the databases and aided in the selection of the papers to be discussed in the manuscript. EMM, DSC, LLPD, PJM, BPC and EOGA aided in the corrections of the Tables. CD and BPC have corrected the English grammar. MBF have done the final version of the manuscript. MBF conceived the protocol, obtained funding and oversaw the study. All the authors read and approved the final manuscript.
Conflicts of interest
The authors declare that there are not financial conflict of interest (political, personal, religious, ideological, academic, intellectual, commercial or any other) in relation to this manuscript because this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank the Brazilian Government agencies (CNPq, FAPERJ) and UERJ for the support.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Martineau P., Bazarjani S., Zuckier L.S. Artifacts and incidental findings encountered on dual-energy X-ray absorptiometry: atlas and analysis. Semin Nucl Med. 2015;45:458–469. doi: 10.1053/j.semnuclmed.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Van den Bergh J.P., van Geel T.A., Geusens P.P. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8:163–172. doi: 10.1038/nrrheum.2011.217. [DOI] [PubMed] [Google Scholar]
- 3.Hong W., Cheng Q., Zhu X., Zhu H., Li H., Zhang X. Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly chinese men and women. PloS One. 2015;10:e0138102. doi: 10.1371/journal.pone.0138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis J.A., McCloskey E.V., Johansson H., Cooper C., Rizzoli R., Reginster J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Lai C.L., Tseng S.Y., Chen C.N., Liao W.C., Wang C.H., Lee M.C. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging. 2013;8:1603–1609. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber-Rajek M., Mieszkowski J., Niespodziński B., Ciechanowska K. Whole-body vibration exercise in postmenopausal osteoporosis. Prz Menopauzalny. 2015;14:41–47. doi: 10.5114/pm.2015.48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komrakova M., Hoffmann D.B., Nuehnen V., Stueber H., Wassmann M., Wicke M. The effect of vibration treatments combined with teriparatide or strontium ranelate on bone healing and muscle in ovariectomized rats. Calcif Tissue Int. 2016 doi: 10.1007/s00223-016-0156-0. [DOI] [PubMed] [Google Scholar]
- 9.Chow S.K., Leung K.S., Qin J., Guo A., Sun M., Qin L. Mechanical stimulation enhanced estrogen receptor expression and callus formation in diaphyseal long bone fracture healing in ovariectomy-induced osteoporotic rats. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3619-2. [DOI] [PubMed] [Google Scholar]
- 10.Geusens P. New insights into treatment of osteoporosis in postmenopausal women. RMD Open. 2015;1(Suppl. 1):e000051. doi: 10.1136/rmdopen-2015-000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud K.E. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–1242. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 12.Lin J.T., Lane J.M. Nonpharmacologic management of osteoporosis to minimize fracture risk. Nat Clin Pract Rheumatol. 2008;4:20–25. doi: 10.1038/ncprheum0702. [DOI] [PubMed] [Google Scholar]
- 13.Shangold M.M., Sherman C. Exercise and menopause: a time for positive changes. Phys Sportsmed. 1998;26:45–50. doi: 10.3810/psm.1998.12.1215. [DOI] [PubMed] [Google Scholar]
- 14.Teoman N., Ozcan A., Acar B. The effect of exercise on physical fitness and quality of life in postmenopausal women. Maturitas. 2004;47:71–77. doi: 10.1016/s0378-5122(03)00241-x. [DOI] [PubMed] [Google Scholar]
- 15.Howe T.E., Shea B., Dawson L.J., Downie F., Murray A., Ross C. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Sinaki M. Exercise for patients with osteoporosis: management of vertebral compression fractures and trunk strengthening for fall prevention. PM R. 2012;4:882–888. doi: 10.1016/j.pmrj.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Sinaki M. Critical appraisal of physical rehabilitation measures after osteoporotic vertebral fracture. Osteoporos Int. 2003;14:773–779. doi: 10.1007/s00198-003-1446-8. [DOI] [PubMed] [Google Scholar]
- 18.Sinaki M., Itoi E., Wahner H.W., Wollan P., Gelzcer R., Mullan B.P. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–841. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- 19.Delecluse C., Roelants M., Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35:1033–1041. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Cabello A., Gonzalez-Aguero A., Ara I., Casajus J.A., Vicente-Rodriguez G. Effects of a short-term whole body vibration intervention on physical fitness in elderly people. Maturitas. 2013;74:276–278. doi: 10.1016/j.maturitas.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Runge M., Rehfeld G., Resnicek E. Balance training and exercise in geriatric patients. J Musculoskelet Neuronal Interact. 2000;1:61–65. [PubMed] [Google Scholar]
- 22.Mavcic B., Antolic V. Optimal mechanical environment of the healing bone fracture/osteotomy. Int Orthop. 2012;36:689–695. doi: 10.1007/s00264-012-1487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck B.R. Vibration therapy to prevent bone loss and falls: mechanisms and efficacy. Curr Osteoporos Rep. 2015;13:381–389. doi: 10.1007/s11914-015-0294-8. [DOI] [PubMed] [Google Scholar]
- 24.Kvorning T., Bagger M., Caserotti P., Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur J Appl Physiol. 2006;96:615–625. doi: 10.1007/s00421-006-0139-3. [DOI] [PubMed] [Google Scholar]
- 25.Martín G., de Saa Y., Da Silva-Grigoletto M.E., Vaamonde D., Sarmiento S., García-Manso J.M. Effect of whole body vibration (WBV) on PTH in elderly subjects. Rev Andal Med Deporte. 2009;2:1–6. http://www.elsevier.es/es-revista-revista-andaluza-medicina-del-deporte-284-articulo-effect-whole-body-vibration-wbv--13134193. [Google Scholar]
- 26.Di Giminiani R., Fabiani L., Baldini G., Cardelli G., Giovannelli A., Tihanyi J. Hormonal and neuromuscular responses to mechanical vibration applied to upper extremity muscles. PloS One. 2014;9:e111521. doi: 10.1371/journal.pone.0111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallas G., Paradisis G., Kirialanis P., Mellos V., Argitaki P., Smirniotou A. The acute effects of different training loads of whole body vibration on flexibility and explosive strength of lower limbs in divers. Biol Sport. 2015;32:235–241. doi: 10.5604/20831862.1163373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji T., Yoon J., Aiba T., Kanamori A., Okura T., Tanaka K. Effects of whole-body vibration exercise on muscular strength and power, functional mobility and self-reported knee function in middle-aged and older Japanese women with knee pain. Knee. 2014;21:1088–1095. doi: 10.1016/j.knee.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Regterschot G.R., Van Heuvelen M.J., Zeinstra E.B., Fuermaier A.B., Tucha L., Koerts J. Whole body vibration improves cognition in healthy young adults. PloS One. 2014;9:e100506. doi: 10.1371/journal.pone.0100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Den Heijer A.E., Groen Y., Fuermaier A.B., van Heuvelen M.J., van der Zee E.A., Tucha L. Acute effects of whole body vibration on inhibition in healthy children. PloS One. 2015;10:e0140665. doi: 10.1371/journal.pone.0140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Merlin T., Weston A., Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian 'levels of evidence'. BMC Med Res Methodol. 2009;9:34. doi: 10.1186/1471-2288-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohlmann A., Schmidt H., Gast U., Kutzner I., Damm P., Bergmann G. In vivo measurements of the effect of whole body vibration on spinal loads. Eur Spine J. 2014;23:666–672. doi: 10.1007/s00586-013-3087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin C., Pope M., Fritton J.C., Magnusson M., Hansson T., McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 35.Von Stengel S., Kemmler W., Engelke K., Kalender W.A. Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22:317–325. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 36.Gusi N., Raimundo A., Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verschueren S.M., Roelants M., Delecluse C., Swinnen S., Vanderschueren D., Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Min Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 38.Beck B.R., Norling T.L. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil. 2010;89:997–1009. doi: 10.1097/PHM.0b013e3181f71063. [DOI] [PubMed] [Google Scholar]
- 39.Stolzenberg N., Belavy D.L., Rawer R., Felsenberg D. Vibration or balance training on neuromuscular performance in osteopenic women. Int J Sports Med. 2013;34:956–962. doi: 10.1055/s-0033-1334870. [DOI] [PubMed] [Google Scholar]
- 40.Cardinale M., Pope M.H. The effects of whole body vibration on humans: dangerous or advantageous? Acta Physiol Hung. 2003;90:195–206. doi: 10.1556/APhysiol.90.2003.3.2. [DOI] [PubMed] [Google Scholar]
- 41.Rauch F., Sievanen H., Boonen S., Cardinale M., Degens H., Felsenberg D. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10:193–198. [PubMed] [Google Scholar]