Abstract
Sex steroids influence the maintenance and growth of muscles. Decline in androgens, estrogens and progesterone by aging leads to the loss of muscular function and mass, sarcopenia. These steroid hormones can interact with different signaling pathways through their receptors. To date, sex steroid hormone receptors and their exact roles are not completely defined in skeletal and smooth muscles. Although numerous studies focused on the effects of sex steroid hormones on different types of cells, still many unexplained molecular mechanisms in both skeletal and smooth muscle cells remain to be investigated. In this paper, many different molecular mechanisms that are activated or inhibited by sex steroids and those that influence the growth, proliferation, and differentiation of skeletal and smooth muscle cells are reviewed. Also, the similarities of cellular and molecular pathways of androgens, estrogens and progesterone in both skeletal and smooth muscle cells are highlighted. The reviewed signaling pathways and participating molecules can be targeted in the future development of novel therapeutics.
Keywords: Sarcopenia, Androgen, Estrogen, Progesterone, Skeletal muscle, Smooth muscle
1. Introduction
Muscles form 45 and 35 percent of lean body mass in men and women, respectively. In the third decade of human life, a slow and progressive decrease of muscle mass and function in a large proportion of older individuals has been called sarcopenia [1]. Sarcopenia accompanied by decreased mobility, physical disability, slow gait, and loss of independence which all lead to poor quality of life in older people. At the age of 65 years, nearly 5% of people and after 80 years approximately 50% of people may suffer sarcopenia [2], [3], [4]. Rapid aging of Korea population in 2005, 2010 and 2015 which the people with age 65 and older respectively included 9.1%, 11% and 13.1% of the Korean population can show the importance of attention to sarcopenia [5]. Age-related sarcopenia included skeletal muscle [6], smooth muscle [7] and cardiac muscle [8]. Although sarcopenia definition is evolving [9] but generally it is characterized by age-related muscle wasting. Apart from the aging, other factors such as autoimmune disorders, inflammatory disease, and endocrine dysfunction lead to sarcopenia [10]. Myofiber atrophy, intramuscular fat accumulation, loss of motor unit, and fibrosis are several sarcopenia pathologies which are introduced as responsible for deficits of muscle function and mass [11], [12]. In addition, suppression of anabolic signaling, abnormal function of mitochondria in muscle cells, and apoptosis play role in pathogenesis of sarcopenia [13], [14].
Loss in the function and mass of muscular tissue can be caused by gradual decrease of circulating concentration of estrogens and androgens [15]. For instance, men who received androgen deprivation therapy for prostate cancer showed declines in upper body muscle strength and function [16]. On the other hand, post-pubertal growth and sexual dimorphism of muscles are influenced by sex steroid hormones including estrogens and androgens [17]. Delineation of the current knowledge on how sex steroid hormones signals interact with controlling of the intracellular molecular pathways, may be useful for management of sarcopenia. This article reviews available information on the interaction between sex steroid hormones and muscles with a focus on the pathophysiology of sarcopenia from pre-clinical and clinical perspectives.
2. Sex steroid hormones effects on muscles
Systemic hormones and load bearing have important roles in regeneration and hypertrophy after muscle injury [18]. Anti- and pro-inflammatory cytokines, growth factors and sex steroid hormones are the major systemic hormones which influence muscle tissue function and mass [19]. Role of sex steroid hormones on skeletal muscles includes i) regulation of muscle metabolic function, ii) increase of muscle strength, iii) maintenance and growth of muscle mass [20], and iv) promotion of muscle repair after injury [21]. Therefore, men and women muscle health can be implicated by decline of sex steroid hormones [22] including decrease in mass and size of striated muscles [22]. Estrogens and androgens have different effects on the function of muscle cells regarding the sex, muscle cell types and/or muscle anatomical position [15].
Androgenic steroids including testosterone and mechanical loading increase skeletal muscle mass and growth in the normal males [23], [24], [25] and females [26]. Decrease in muscle size and strength is reported in the cancer patients [27], [28], individuals receiving androgen deprivation therapy such as prostate cancer patients as well as women in post-menopausal conditions [22] and men suffered hypogonadism [29]. In addition, premenopausal women with increased age experience decreases in androgens [30]. Endogenous suppression of testosterone in aged men and women accounts for sarcopenia, muscle fat infiltration and decreased muscular quality in men more than the women [11]. Low bioavailable testosterone associated with worse frailty status in the aged men [31]. In the men with androgen deficiency [25], [32], [33] and the castrated animal models [34], [35], it was reported that testosterone replacement therapy increased the strength and mass of muscle. Regarding to the effect of androgen and anatomical positions of skeletal muscle, muscles associated with copulation are more responsiveness to androgen than the weight bearing locomotive ones in male rat models [35]. Furthermore, in androgen receptor knockout mice, the peripheral muscle mass and size was not different with wild type mice but the weight of levator ani was reduced in comparison with the wild type mice [36].
Positive effects of estrogen replacement therapy including increase of muscle contractile function and post-exercise damage protection are shown in the ovariectomized rat models [37], [38], [39], [40]. In post-menopausal women conflicting findings after estrogen replacement therapy on muscular quality are demonstrated [41], [42], [43]. Regarding to the effect of estrogen and locomotion function of skeletal muscle, in female mice, higher weight bearing muscles have more responsiveness to estrogens [39]. Except skeletal muscles, the cardiac muscle [44] and smooth muscles [45] are more affected by estrogens. Furthermore, increase in body fat in males with estrogen deficiency is reported [46].
A few studies evaluated the progesterone effects on skeletal muscle function and growth. Progesterone receptor presence is reported in skeletal muscle cells [47] and some functions of this steroid hormone in skeletal muscle are evaluated. Skeletal muscle contractile characteristics were not affected by the fluctuations in progesterone levels throughout the menstrual cycle [48]. Furthermore, treatment with progesterone has no effect on the capacity of skeletal muscle to oxidize lipids. However, by physiological concentrations' treatment of ovariectomized rats with both estradiol and progesterone, progesterone inhibited the lipolytic effect of estradiol, and this was restored with pharmacological concentrations of estradiol [49]. In addition, progestins may reduce proliferation of satellite cells in skeletal muscle of cow [50]. Although the number of studies in comparison with the previous explained sex steroid hormones are low but some mechanisms are evaluated related to progesterone and skeletal muscle cell proliferation. In contrast, in smooth muscle cells, progesterone effects on cell proliferation, growth and function are more investigated. Both medroxyprogesterone acetate and etonogestrel reduce proliferation and migration of vascular smooth muscle cells [51].
3. Sex steroid hormone receptors' action in muscles
Presence and activity of androgen receptor, estrogen receptor-α and –β and progesterone receptor are shown in different cell types of skeletal muscles (Table 1). Mesenchymal stem cells, satellite cells, myoblasts and myocytes express androgen receptors [52], [53], [54]. Muscles are associated with reproduction express more androgen receptors than the weight bearing muscles and the receptors are more sensitive to androgens [35]. Up-regulation of expression of androgen receptors in rodents and human muscle cells is done by both increase in androgen concentration [21], [35], [55] and resistance trainings [56], [57], [58], [59]. On the other hand, in male mice with knocked out androgen receptor but not in females, muscle mass decreased [60]. In specific androgen receptor knockout satellite cells in male mice [61] and myocyte-specific androgen receptor knockout male mice [36], mass reduction of highly androgen sensitive muscles has been reported whereas other peripheral muscles were not affected by specific androgen receptor delectation. Contrary to those findings, over expression of androgen receptor in transgenic rats increased muscle mass, hypertrophied myofibers, decreased adipocytes and increased oxidative metabolism [62], [63]. Furthermore, androgen receptors expression is detected in different types of smooth muscle tissues including vessels [64], penis [65], and myometrium [66].
Table 1.
Detected receptors of sex steroids on different cell types of skeletal muscle.
| Cell types | Androgen receptor | Estrogen receptor-α | Estrogen receptor-β | Progesterone receptor | References |
|---|---|---|---|---|---|
| Mesenchymal stem cells | + | ND | ND | ND | [53] |
| Satellite cells | + | + | + | ND | [54], [258] |
| Myoblasts | + | + | + | ND | [52], [258] |
| Myocytes | + | + | + | + | [47], [52], [73], [258], [259] |
| Fibroblasts | + | + | + | ND | [53], [258] |
ND: there is no available data.
Satellite cells, myoblasts and myocytes express both estrogen receptor-α and -β in male and females [67]. Expression pattern of estrogen receptors differ between sexes [68]. However, deletion of estrogen receptor-β in female mice could not affect the muscle mass, but estrogen receptor-α knockout mice showed increase in muscle mass and decreased contractile properties [39]. In comparison with wildtype in both sexes, knocking out of estrogen receptor-β improved the contractile properties and post damage recovery in male mice but not in females [69]. In addition, expression of both types of estrogen receptors in smooth muscle cells have regulatory role in proliferation and differentiation of these cells [70], [71].
Although the presence progesterone receptors is detected [72] in skeletal muscles, their role is not as clear as the other sex steroid hormone receptors. On the other hand, although the presence of progesterone receptor in myoblast cells are reported [73], more investigations concerning to progesterone is necessary to confirm presence and absence of this type of sex steroid hormone receptor in other type of skeletal muscle cells. This despite the fact that numerous study clarified role of progesterone receptors in proliferation of smooth muscle cells of various tissues with inhibitory effects in vessels [74] and stimulatory effects in myometrium [75].
4. Sex steroid hormones and intracellular pathways in muscle cells
The role of sex steroid hormones in age-related changes in sarcopenia and alterations of intracellular signaling pathways in muscles is not well understood. Revealing the role of androgens and related receptors in increase of protein synthesis and skeletal muscle mass, understanding the molecular mechanisms that mediate these effects may help to develop supportive or even preventive therapy for the individuals suffering from sarcopenia. Muscle synthesis or degradation is regulated by several signaling pathways positively or negatively. Signaling pathways, paracrine mediators, and gene targets in the skeletal and smooth muscles which are regulated by androgens, estrogen and progesterone are summarized in Table 2.
Table 2.
Recognized physiological effects of sex steroids on signaling pathways, paracrine mediators, and gene targets in the skeletal and smooth muscles.
| Signaling pathways/molecules | Skeletal muscle |
Smooth muscle |
||||
|---|---|---|---|---|---|---|
| Androgen | Estrogen | Progesterone | Androgen | Estrogen | Progesterone | |
| Akt/mTOR | Stimulation [94]; inhibition [88] | Stimulation [103], [106] | ND | Stimulation [95] | Stimulation [108], [260] | Inhibition [110], [143] |
| FoxO3 | Inhibition [88], [119]; no effect [120] | No effect [120]; stimulation [106] | No effect [120] | NDa | ND | ND |
| MAPK1/3 (ERK1/2) | Stimulation [135] | No effect [103]; stimulation [139] | ND | Stimulation [137] | stimulation [140], [142] | Stimulation [114], [142], [143] |
| Wnt | Stimulation [149] | ND | ND | ND | Stimulation [157] | Stimulation [157] |
| Notch | Stimulation [152]; inhibition [149] | ND | ND | No effect [261] | ND | ND |
| NF-κB | Inhibition [166], [169] | ND | ND | Inhibition [170] | Inhibition [171], [262] | Inhibition [175]; stimulation [143] |
| TNF-α | ND | Inhibition [188] | ND | Inhibition [170] | Inhibition [190] | Inhibition [176], [192] |
| Interleukins | Inhibition [88] | Stimulation [188]; no effect [263] | Inhibition [192] | Inhibition [186] | No effect [264]; inhibition [265] | Inhibition [193]; no effect [175] |
| MRF (MyoD and myogenin) | Inhibition [119]; no effect [266] | Stimulation [203]; Inhibition [202] | Stimulation [50], [120] | NDb | ND | ND |
| CDK | Stimulation [154] | ND | ND | Inhibition [212] | Inhibition [217] | Inhibition [112], [143] |
| Myostatin/AcIIb/Activin | Inhibition [224], [267]; no effect [120] | No effect [120] | No effect [120] | NDc | Inhibition [227] | Inhibition [227] |
ND: there is no available data, although aFoxO3 [121], bCRFs [201] and cmyostatin [227] are detected in smooth muscles.
Akt, serine/threonine-specific protein kinase; CDK, cyclin-dependent kinase; ERK1/2, extracellular-signal-regulated kinases 1/2; FoxO3, Forkhead box O3; MAPK1/3, mitogen-activated protein kinase 1/3; MRF, myogenic regulating factors; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor-κB; TNF-α, Tumor necrosis factor.
4.1. IGF-I/Akt/mTOR pathway
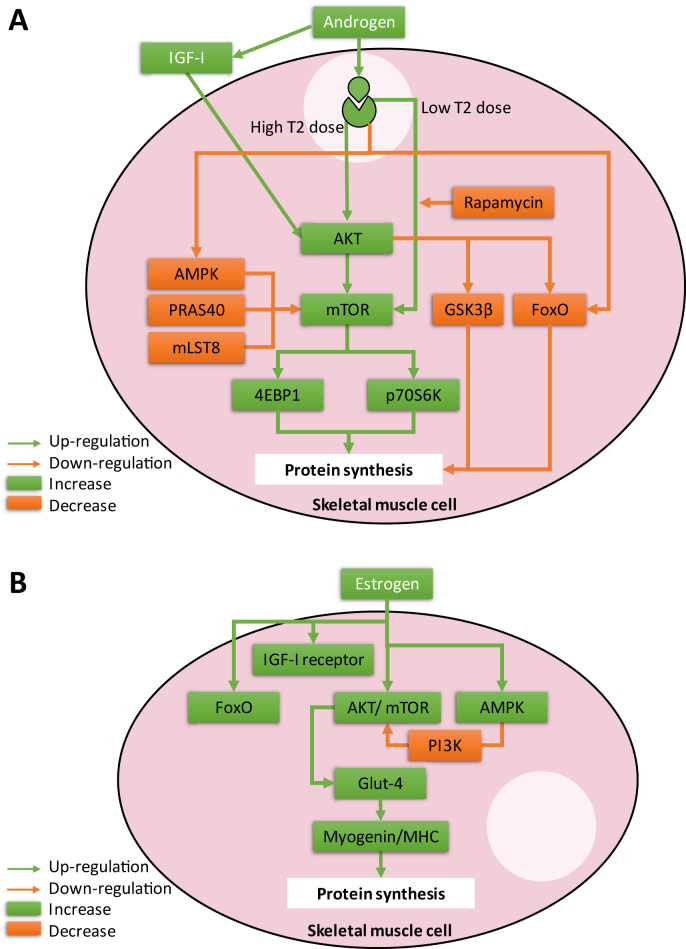
One of the pathways which is controlled by androgenic signals and has critical role in survival and growth of muscles via protein synthesis is the insulin growth factor-1 (IGF-I)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway (Fig. 1A) [76], [77]. Aging and inflammation can impede activation of muscle mTOR signaling [78]. Protein synthesis enhanced by phosphorylation of 4E-BP1 and p70S6K which are subsequent of activated mTOR by Akt [79]. Also, Akt can inhibit glycogen synthase kinase-3 beta (GSK3β) which is the inhibitor of protein synthesis [80]. On the other hand, two isoforms of IGF-I which synthesized in muscle tissue, mechano growth factor (MGF or IGF-IEc) and the IGF-I which is similar to the ones which is synthesized by liver, have auto- and paracrine effects [81]. IGF-I is increased by mechanical loading and testosterone by the activation of Akt and androgen receptors have critical role in IGF-I synthesis in myocytes [82]. Reduction in intramuscular IGF-I synthesis and circulating IGF-I is associated with deficiency of androgens in men [29], [83] or castrated rodents [84], [85]. On the other hand, androgen replacement in castrated rodents increased muscle performance without increase in muscle fiber growth or activation of the Akt/mTOR pathway [86]. Additionally, testosterone induced IGF-I/Akt pathway by increasing expression of IGF-I mRNA and glycogen synthase kinase 3β (GSK3β) phosphorylation in female rats [87]. Ambiguous findings are present on the effect of testosterone supplementation or castration on Akt activation in the skeletal muscles [85], [86], [88]. Differences in the type of muscles were examined as well as dose of androgen, type and duration of testosterone deficiency may exhibit the variability in the experimental findings on sensitivity of androgens on IGF-I/Akt/mTOR pathway in skeletal muscle.
Fig. 1.
Schematic diagram of (A) androgen and (B) estrogen effects on AKT/mTOR and FoxO signaling in skeletal muscle cells. Abbreviations: Akt, serine/threonine-specific protein kinase; AMPK, 5′-adenosine monophosphate-activated protein kinase FoxO, Forkhead box O; GSK3β, glycogen synthase kinase 3β; IGF-I, insulin growth factor-I; MHC, myosin heavy chain; mTOR, mechanistic target of rapamycin; T2, testosterone.
The other pathway can occur independent of activation of upstream Akt is the mammalian target of rapamycin complex 1 (mTORC1) which its activation regulates several cellular process such as protein synthesis [89], [90]. Testosterone induced increases in protein synthesis can be blocked by rapamycin, the mTORC1 inhibitor, in myotubes [91] (Fig. 1A). The mTORC1 repressors includes proline-rich Akt substrate-40 (PRAS40), mammalian lethal with SEC13 protein 8 (mLST8), and 5'-adenosine monophosphate-activated protein kinase (AMPK) [92], [93]. Synthesis of muscle myofibrillar protein decreased by androgen withdrawal through Akt/mTORC1 pathway and was independent of activation of AMPK and reversed by testosterone administration [94]. Low dose of testosterone activate mTOR pathway independent of Akt; while, high dose testosterone activated Akt pathway in skeletal muscle fibers [94]. In smooth muscles, same evidences of stimulatory effect of androgen on Akt pathway are reported [95], [96].
Estradiol induces the Akt phosphorylation in myoblasts (Fig. 1B) [97]. Decrease in muscle Akt phosphorylation which is induced by estrogen deficiency in ovariectomized rats caused decrease in muscle mass [98]. On the other hand, expression of IGF-I receptor in skeletal muscle cells increased in post-menopausal women after estrogen replacement while expressions of miRNAs such as miR-182, miR-223 and miR-142-3p decreased [99]. Considering the skeletal muscle glucose metabolism, activation of AMPK was suppressed in ovariectomized mice, but estradiol injection reverse it [100]. In addition, in skeletal muscles, Akt/mTOR pathway is inhibited by AMPK activation [101], [102]. Estradiol also increases translocation of glucose transporter, Glut-4 to the plasma membrane through Akt pathway and cause increase of myogenin and myosin heavy chain (MHC) which are important in morphological appearance of skeletal muscles [103], [104], [105]. Estradiol administration in postmenopausal women induced up-regulation of expression of mTOR genes [106]. Furthermore, in smooth muscle cells, IGF-I-stimulated phosphoinosotide-3-kinase (PI3K) pathway up-regulation is inhibited by increase in AMPK activation [107]. Estrogen induce smooth muscle acute response through the PI3-kinase/Akt signaling axis [108]. In uterine smooth muscles, estradiol up-regulates of the amount of p-mTOR in rat [109].
A limited number of investigations studying the effects of progesterone on the skeletal muscle cells, to the best of our knowledge, none evaluating this steroid effect on the IGF-I/Akt/mTOR pathway in the skeletal muscle cells. On the other hand, progesterone down-regulates key components of the mTOR pathway in myometrium [110]. Also, progesterone inhibits proliferation and migration of smooth muscle cells [74], [111], [112] by induction of p27 up-regulation through the progesterone/cSrc/AKT/ERK 2/p38-mediated signaling pathway [113], [114].
4.2. FoxO family
Akt inhibits protein degradation by the phosphorylation of transcription factors in forkhead box O (FoxO) family [115] (Fig. 1A). FoxO1 and 3 activated in muscle atrophy conditions in skeletal muscles [19], [116]. Testosterone down-regulates transcription of FoxO-related genes and expression of FoxO in skeletal muscle to inhibit muscle catabolism [85], [88], [117], while castration increased them [85]. In addition, testosterone can prevent dexamethasone-induced atrophy of skeletal muscle by blocking the expression of FoxO1 gene [118]. Suppression of FoxO family by androgen resulted in myogenin/ubiquitin ligase-mediated atrophy pathways and preserve muscle mass [119]. In postmenopausal women skeletal muscle, expression of FoxO3 mRNA is greater than premenopausal [120]. However, testosterone replacement therapy had no effects on the FoxO3 mRNA expression in postmenopausal women [120]. This different finding in postmenopausal women with previous investigations in rodent may related to aging effect on the inhibitory effects of androgen and its receptor with FoxO family and needs more investigation. On the other hand, although FoxO3 is detected in smooth muscle cells [121] but the effect of androgens on this molecular pathway has not been investigated yet.
The effect of estradiol on the FoxO family in skeletal muscle is controversial. However, it is shown that estradiol treatment had no effect on mRNA expression of FoxO3 in skeletal muscle [120], but it is also shown that estradiol treatment of postmenopausal women caused up-regulation of both FoxO3 and FoxO1 in the skeletal muscles (Fig. 1B) [106]. On the other hand, although FoxO3 is detected in smooth muscle [121] but there is no information on the effects of estrogens on the FoxO3.
Progesterone treatment had no effect on FOXO3 mRNA expression in skeletal muscle of premenopausal and postmenopausal women [120]. Although FoxO3 is detected in smooth muscles [121], but there is no data on the effect of progesterone on FoxO3.
4.3. MAPK pathway
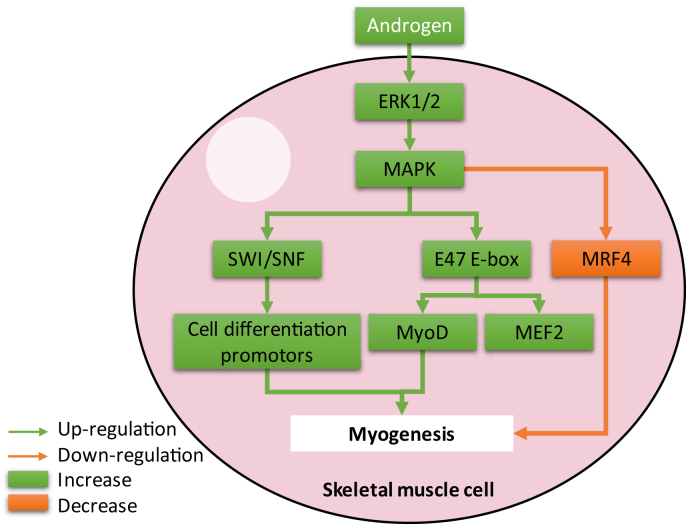
In the intracellular signal pathway, mitogen-activated protein kinases (MAPK) regulate muscle growth through i) SWItch/sucrose non-fermentable (SWI/SNF) complex recruitment for activation of muscle cell differentiation promoters [122], ii) by E47 E-box protein phosphorylation to enhance transcriptional activity of MyoD and myocyte enhancer factor-2 (MEF2)A, -C, and -D factors [104], [123], [124], and iii) phosphorylation of myogenic regulating factor 4 (MRF4) to inhibit it to facilitate myogenesis [125], [126] (Fig. 2). Extracellular-signal-regulated kinases 1/2 (ERK1/2), which are classical MAPK, have role in differentiation, division, growth in skeletal muscle cells. Their activation is enhanced by growth factors [127], exercises [128], diverse stress stimuli [129], and hormones [130], [131]. Chronic inflammation induces p38 and ERK1/2 signaling which leads to muscle wasting [132], [133]. Steroid hormones increased skeletal muscle cell calcium release from their reservoirs and simultaneously activate MAPK pathways [134]. It is also shown that testosterone could activate ERK1/2 pathway in skeletal muscle [135]. In smooth muscle cells testosterone had biphasic stimulatory and inhibitory effects in low and high concentration, respectively [136]. It is also shown in the both dose dependent effects of testosterone, stimulation or inhibition of MAPK pathway had role in regulation of smooth muscle cell proliferation [137]. Therefore, more investigations are necessary to clarify the MAPK pathway role in the androgen deficient sarcopenia patients or androgen supplementation in sarcopenia animal models.
Fig. 2.
Schematic diagram of androgen effects on mitogen-activated protein kinase (MAPK) signaling in skeletal muscle cells. Abbreviations: ERK1/2, extracellular-signal-regulated kinases 1/2; MAPK1/3, mitogen-activated protein kinase 1/3; MEF2, myocyte enhancer factor-2; MRF4, myogenic regulating factor 4; SWI/SNF, SWItch/sucrose non-fermentable.
The effects of estrogen on ERK/MAPK signaling in muscle cell differentiation is controversial [131], [138]. It is shown that ERK1/2 does not have role in muscle differentiation by estradiol [103]. In contrast, it is reported that ERK1/2 is activated by estradiol in skeletal muscle cells under differentiation conditions [139]. Estradiol induces the expression of myogenin and MHC in skeletal muscle by phosphorylation p38 [103]. Therefore, to clarify the role of MAPK pathway in skeletal muscle differentiation further investigation can be performed. On the other hand in smooth muscle, estradiol inhibits proliferation and migration of the cells via reversing phosphorylation of p42/44 and p38 MAPK and through estradiol receptors [140], [141]. In contrast to reduce myogenic tone of smooth muscle in pregnant uterus, estradiol has a direct chronic effect in the up-regulation of ERK1/2 expression [142].
No investigations evaluated the progesterone effects on the MAPK pathway in the skeletal muscle cells. On the other hand, progesterone increased ERK1/2 protein in smooth muscle cells [142]. It is also shown that in smooth muscle cells, progesterone inhibited cell proliferation by activating the cSrc/Kras/Raf-1/AKT/ERK/p38/IκBα/NFκB pathway up-regulates the expression of p21cip1 and p27kip1 through increasing the level of p53 protein [114], [143].
4.4. Wnt and Notch signaling
Wnt signaling stimulates the formation and differentiation of skeletal muscle in embryo [144], [145]. Activation of Myf5 (for Wnt1), MyoD (in the case of Wnt7a), or receptor Fzd7 (by Wnt7a) are the regenerative effects of Wnt signaling pathway in skeletal muscles [146], [147]. In addition, Wnt signaling decrease the inhibitory effect of Notch signaling to increase the differentiation of muscles via promoting the myogenic progenitors [148]. Androgen increased Wnt signaling via up-regulation of Numb in myoblasts [149]. Also, nandrolone, an anabolic androgen, activated Wnt-dependent up-regulation of Numb and resultant reduced Notch signaling during differentiation of muscle progenitor cells [149]. Furthermore, it is also shown that nandrolone diminished mdm2 levels to stabilize Numb protein in myoblasts [150]. Also, up-regulation of Numb by nandrolone in denervated muscle reduced Notch signaling [151]. Although some studies showed that indirectly and via the Wnt pathway androgens decreased the Notch signaling, but testosterone in murine muscles increased expression of Notch 1, Notch 2 and Delta 1 [152]. In addition, in older man satellite cells, testosterone increased Notch expression [153]. Furthermore, in aged skeletal muscle of mice, testosterone induced expression of Notch 1 receptor [154]. All these findings demonstrate the up-regulatory effect of testosterone on Notch signaling and skeletal muscle cell proliferation. Thus, the Wnt signaling and the Notch signaling are the other pathways which is stimulated by androgens to promote proliferation and then differentiation of skeletal muscle cells. On the other hand, although Wnt signaling pathway is described in smooth muscle cells [155] but the effect of androgens on this molecular pathway has not been investigated yet.
There is no information regarding the effects of estradiol on Wnt and Notch signaling in skeletal muscle, but it is shown that estradiol in combination with trenbolone acetate increase the expression of WISP2 (Wnt-1 inducible signaling pathway protein 2) in sheep skeletal muscle [156]. In smooth muscle, Estradiol administration induces secretion of WNT11 and WNT16, WNT ligands, from mouse myometrial cells which express estrogen receptor α [157]. In both smooth and skeletal muscle, the effect of estradiol on notch signaling is not clarified.
Studies on the progesterone effects via Wnt and Notch signaling in skeletal and smooth muscles are not available. There is only a study in smooth muscle myometrial cells which showed progesterone in combination with estrogen induces WNT ligands secretion [157].
4.4.1. NF-κB pathway
Skeletal myogenesis inhibition is enhanced by nuclear factor-κB (NF-κB) pathway activation [158]. The TNF-α and proteolysis-inducing factor (PIF) are the cytokines which activate NF-κB to induce proteolysis and apoptosis in skeletal muscles [159], [160], [161], [162], [163]. Stimulation of inflammatory pathways which activate NF-κB in sarcopenia leads to acute or gradual muscle wasting [164], [165]. NF-κB induces skeletal muscle atrophy by pro-inflammatory cytokines through downstream signaling requiring RelA/p65 [164]. In a normal skeletal muscle an upstream NF-κB pathway activating kinase, NF-κB–inducing kinase (NIK), maintains at low basal levels [166]. Stimulation of NF-κB noncanonical pathway leads to cIAP1/2 degrades and ubiquitinates TRAF3. TRAF3 releases and stabilizes NIK and causes NIK accumulation in cells [167], [168]. There is an association between aging and accumulation of NIK in skeletal muscles in older men [166]. Activation of NF-κB pathway by increase of NIK levels leads to catabolism of muscle proteins and sarcopenia progression. Testosterone supplementation can decrease NIK content in skeletal muscle of older men [166]. In addition, simultaneous testosterone and protein supplementation suppressed p52 and RelB expression in skeletal muscle of women [169]. In older women, suppressive effect of androgen administration on NF-κB pathway for prevention of women sarcopenia is not clarified. In contrast to the describe catabolic role of NF-κB in skeletal muscles, a canonical p50/RelA pathway and non-canonical p52/RelB pathway in the other model of NF-κB signaling in skeletal muscle suggests these pathway effects in myogenesis [158]. On the other hand, same inhibitory effect of androgens on smooth muscle degradation through suppression of NF-κB is also reported. IκB-α degradation and the LPS-induced NF-κB nuclear translocation were prevented by testosterone in rat smooth muscle of prostate [170].
However, NF-κB pathway is described in skeletal muscle cells [158] but there is no data on the effects of estrogens on the NF-κB pathway in these cells. In contrast, in smooth muscle cells, gene transfer of estrogen receptor-α and estradiol treatment inhibited NF-κB activation and induced cell proliferation in aged rat [171]. In the rat uterus, estradiol treatment also inhibited NOS II induction, which its promoter region contains NF-κB binding sites [172], [173]. Thus it can be postulated that in postmenopausal women, decrease in expression of functional estrogen receptor-α number and/or decrease in estradiol levels activate NF-κB, but experiments will be needed to clarify this subject both in smooth and skeletal muscles.
Such as the previous mentioned pathways, progesterone effect on this pathway in skeletal muscle also is not investigated. However, investigations on this subject in smooth muscle cells are available. It is shown that myometrium beside the infiltrated inflammatory cells is a major source of inflammatory cytokines [174]. Functional progesterone withdrawal during parturition activates NF-κB in myometrium through progesterone receptors [175]. Also, expression of progesterone receptors is down-regulated by IL-1β and TNF-α in myometrial smooth muscle cells [175], [176]. Therefore, these both mechanisms can activate NF-κB in smooth muscles by decreasing the progesterone effect in myometrium. In contrast, it is shown that in vascular smooth muscle cells progesterone increases both the formation of progesterone receptor-NFκB complex in the nucleus and the binding of progesterone receptor onto the NFκB binding fragment of the p53 promoter [143]. Therefore, in vascular smooth muscle cells activation of NF-κB is involved in the progesterone-induced up-regulation of p27 [143]. These controversies may be related to the cell types or species which are evaluated in these studies and need to be more investigated.
4.4.2. Interleukins and tumor necrosis factor
Proinflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 increase by androgen deficiency in both experimental and clinical studies [177], [178]; while, decrease after androgen replacement therapy [179]. Muscle mass and strength in elderly peoples inversely related to interlukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) concentrations [180]. Furthermore, protein breakdown of rat skeletal muscle is increased by the administration of IL-6 [181] or TNF-α [182]. Production of IL-6 has been increased by activation of p50/RelA [183]. In cancer cachexia, gradual chronic increase of IL-6 and absence of increased TNF-α in this catabolic diseases are reported [184]. It is also shown that following exercise, IL-6 is up-regulated in skeletal muscle [185]. Castration of mice induced increasing of IL-1α and decreasing of interferon-γ (IFNγ) and simultaneously levator ani muscle atrophy, however testosterone could prevent these side effects by down-regulation of IL-1α and up-regulation of IFNγ expressions [88]. On the other hand, although effect of TNF-α is described in skeletal muscle cells but the effect of androgens on this cytokine has not been clarified. In the smooth muscle, at subphysiological testosterone, levels TNF-α, IL-2, IL-6, IL-10, IL-12, and IL-13 were elevated [186].
After muscle injury, TNF-α expression increased within 24 h and the increase was higher in ovariectomized rats [187], [188]. Estradiol reduced TNF-α expression during regeneration of skeletal muscle via estrogen receptor-β but not estrogen receptor-α and also promote expression of IL-10 [188].
It is also shown that estrogen treatment of male subjects before acute eccentric exercise decreased neutrophil infiltration in skeletal muscle [189]. In smooth muscle cells, expression of cytokine-induced neutrophil chemoattractant-2β is inhibited by treatment with selective agonist of estrogen receptor-β and also TNF-α-treated smooth muscle cells chemotactic activity was reduced [190].
Probable role of progesterone in regulation of these factors in skeletal muscles are unclear. Although it is demonstrated that megestrol acetate, a synthetic progestin, reduced both skeletal muscle size and serum IL-6 [191], [192]. In smooth muscles this role is partially defined. Although, progesterone receptor does not play a role in down-regulation of pro-inflammatory gene networks induced by IL-1β [175], but IL-1α is decreased by progesterone through decreasing the stability of IL-1α mRNA by stimulating the production of an intracellular intermediate [193]. Therefore, it can be claimed that in both skeletal and smooth muscles, progesterone inhibit production of inflammatory cytokines.
4.4.3. Myogenic regulating factors
Muscle development and growth is controlled by the myogenic regulatory factors (MRFs) including myogenin, MyoD1, Myf5, and Myf6 [194]. Myogenesis can be inhibited by notch signaling through the repressing the MyoD [195], as well as by biding directly to MEF-2C [196]. Androgens repressed myogenin which regulates the myoblasts transition from the proliferative state to the terminally differentiated myocytes [119], [197]. The androgen maintains proliferation of myoblasts by delaying myotube formation [119]. In contrast with these findings, it is shown that testosterone via enhancement of androgen receptors may increase myogenin in mouse myoblast cell line [198]. While, dihydrotestosterone had no effects on expression of myogenin in human skeletal muscle cells (SkMC) as well as in in-vivo conditions in orchiectomy and androgen receptor knockout mice and also in other MRF including MyoD1, Myf5, and Myf [119]. On the other hand, in in-vivo conditions myogenin repressed by androgen/androgen receptor pathway [119]. Regarding, SkMC may not be an appropriate model for in-vitro evaluation of proliferation of myocytes, despite the fact that expression level of androgen receptors in SkMC is similar to in-vivo muscle cells [199]. Expression levels of myogenin decreased concomitant with increase in age and testosterone level in male mice [200]. The variation of in-vitro methods or even cell lines may result difference findings and evaluation of other kinds of in-vitro and in-vivo models will be necessary to clarify the effects of androgens on MRF. Furthermore, although MRF are detected in smooth muscle cells [201] but the effect of androgens on this molecular pathway has not been investigated yet.
The effects of estrogens on myogenic regulating factors are controversies. For instance the overexpression of exogenous ERα in the presence of E2 decreased the expressions of myogenin [202]. While, knockdown of ERβ reduced myogenin level in the presence or absence of estradiol [202]. It is shown that estradiol can induce expression of USP19 and with the above mentioned mechanism can repress myotube formation [202]. On the other hand, estradiol induced expression of MyoD mRNA in males to the levels found in females [203]. Therefore, further studies may clarify the role of estrogens in regulation of myogenic regulating factors in male and female skeletal muscles. In the smooth muscles, although myogenic regulating factors are detected [201] but the effect of estrogens on them are not available.
MYOD1 expression is increased by progesterone which resulted in satellite cell activation in postmenopausal women skeletal muscle cells [120]. In addition, progesterone and megestrol acetate increased myogenin mRNA expression 2.5 times in bovine satellite cells and C2C12 cultures [50]. In smooth muscle cells no data is available related to the effect of progesterone on myogenic regulating factors.
4.5. Cyclin-dependent kinase
Cyclin-dependent kinases (CDKs) are protein kinases family and are involved in regulating the cell cycle, mRNA processing, transcription, and the differentiation of cells. Increasing age is related to the induction of CDK inhibitors through Notch pathway [204]. It is shown that p21, a CDK inhibitor [205], interferes regeneration of satellite cells [206], [207], [208]. p21 expression increase is observed in mice concomitant with the increase of age [154]. Furthermore, this age-associated increase in expression of p21 was reversed by testosterone administration [154]. On the other hand, inhibition of c-jun NH2-terminal kinase (JNK) by testosterone could cause apoptosis suppression of muscle cells and increasing of muscle fiber growth [154]. Suppression of JNK and up-regulation of p21 could induce muscle cell proliferation [209], [210]. In androgen receptor knockout mice, expression of the Cdkn1c (p57Kip2), a cell cycle regulatory gene, increased [60] which has role in decreasing myoblast proliferation and increasing myoblast cell cycle exit and differentiation [211]. Therefore, in part via the androgen receptors/CDK pathway, the proliferative effect of androgens in skeletal muscle may be performed. On the other hand, in smooth muscles, testosterone inhibited activity of CDK2 and CDK6 [212]. CDK2 activity has role in transition of cells from G1 to S [213]. It is also shown that testosterone can block CDK2 in smooth muscles through activation of CDK inhibitors p21cip11 and p27kip1 [214], [215], [216].
However, no data was found that directly show the effect of estrogens on cyclin-dependent kinase in skeletal muscle cells but this phenomenon is defined to a certain extent. A metabolite of estradiol with no affinity for estrogen receptors, 2-Methoxyestradiol, arrests proliferating smooth muscle cells by inhibition of cyclin D1 and B1 expression and cdk1 and cdk4 activity and up-regulation of the expression of the cdk inhibitor p27 [217]. It is also shown that estradiol exert an antiproliferative effect in smooth muscle cells through the inhibition of the cyclin D1 promoter activity in transfected with cDNA for estrogen receptor-α [218]. In contrast, in uterine smooth muscle cells, estradiol-induced overproliferation was accompanied by and up-regulation of cyclin-dependent kinases and cyclin D1, cyclin E1, CDK2, CDK4, and CDK6 and down-regulation of cyclin-dependent kinases inhibitors [219]. Therefore, the effect of estrogens on cyclin-dependent kinase depends on the origin of smooth muscle cells.
Based on our knowledge, role of progesterone in cyclin-dependent kinase regulation in skeletal muscles is not explained. However, in smooth muscle cells, cyclin-dependent kinase system is inhibited by progesterone via increase of cyclin-dependent kinase-inhibitors, p21cip1 and p27kip1 [112], [143].
4.6. Myostatin
Myocytes produce myostatin or growth differentiation factor 8 which has an inhibitory autocrine action on muscle cell differentiation and growth [220]. By suppressing the MyoD activity, myostatin induces double muscled phenotype [221], [222], [223]. Myostatin mRNA expression was suppressed by testosterone in adult skeletal muscle of castrated rats [224]. It is shown that there is androgen response element (ARE) in the myostatin promoter [225]. The myostatin Knockout mice failed to produce myotubes which is related to silencing of Actc1, Acta1, and MyoD [226]. In contrast in women, myostatin mRNA expression after testosterone administration was not different in premenopausal and postmenopausal women [120]. Therefore, to clarify the effect of testosterone on expression of mysostatin in female skeletal muscles, further investigations are necessary. Furthermore, although myostatin is detected in smooth muscle cells of uterus [227] but the effect of androgens on this protein in myometrial cells has not been clarified.
Estradiol treatment had no effect on myostatin mRNA expression in skeletal muscle of premenopausal and postmenopausal women [120]. In contrast, in uterine smooth muscle cells of ovariectomized rats, myostatin expression increased estrogen treatment decreased myostatin expression [227].
In skeletal muscle cells, progesterone treatment had no effect on mRNA expression of myostatin in postmenopausal women [120]. On the other hand, in smooth muscle cells of myometrium of rat uterus, myostatin expression is weakly decreased by progesterone [227].
5. Sex steroid hormones therapy for sarcopenia
It is remains unknown whether the several mechanisms responsible for sarcopenia discussed above can be controlled by a single therapeutic intervention which leads to rescue of muscle function and mass in old age. Furthermore, for the treatment or prevention of sarcopenia a FDA approved modalities is not still present. On the other hand, limited efficiency of current pharmacologic interventions and lack of standardized primary outcome of anti-sarcopenia drugs are some difficulties in developing therapeutic interventions [228]. By the way, based on the mechanisms of the effects of sex steroid hormones, androgens, estrogens and progestogens several therapeutic agents for treatment of sarcopenia can be discussed.
5.1. Androgens-related therapeutics
In castrated aged mice, early indices of muscle regeneration are improved by testosterone supplementation [229]. In men, 300 to 1000 ng/dl (10.4 to 34.7 nmol/L) testosterone in serum is considered as normal and low concentrations is ≤ 300 ng/dl (10.4 nmol/L) testosterone and frank hypogonadism will be ≤ 250 ng/dl (8.7 nmol/L) testosterone [230]. Testosterone supplementation with low or low-normal levels in men aged older than 45 years increase muscle mass and strength [231], [232]. In adult man, testosterone therapy has no effect on prostate, cardiovascular outcomes and even mortality, but its adverse effect including decrease in high-density lipoprotein cholesterol and increase in hemoglobin and hematocrit is reported [233]. In addition, increased risk of cardiovascular adverse events, edema and worsening of sleep apnea were observed in older men who were administrated testosterone gel [230], [234]. Sarcopenic elderly men most frequently administered by intramuscular injection of long-acting testosterone esters such as testosterone enanthate or testosterone cypionate at a dose between 50 and 400 mg every 2–4 weeks [235]. Also, transdermal administration via patch or gel (1%, once daily between 50 and 100 mg) preparations and infrequently via oral administration are reported [230].
Dehydroepiandrosterone (DHEA) displays androgenic signaling in muscle cells by binding androgen receptors. Benefit of DHEA supplementation in muscle strength or body composition in older men and women remains inconclusive [236], [237]. On the other hand, DHEA in combination with exercise improved physical strength in frailer adults [179], [238]. Nandrolone (19-nortestosterone) is an anabolic steroid which through activation of Notch signaling reduces muscle atrophy in sarcopenia rat model [151]. Furthermore, administration oxandrolone in combination with progressive resistance training improved body composition in elderly women [239]. In addition, non-steroidal selective androgen receptor modulators (SARMs) represent therapeutics for the treatment of sarcopenia. Different SARMs including GLPG0492 [240], andarine [241], NEP28 [242], enobosarm [243], [244], [245], MK-0773 [246], and LGD2941 [247] are showed to have beneficial effects on improvement of muscle mass. Therefore, further clinical trials are necessary to evaluate effects of supplementation with testosterones, DHEA, nandrolone, oxandrolone, and SARMs on elderly sarcopenic patients.
5.2. Estrogens-related therapeutics
A meta-analysis showed that hormone therapy of postmenopausal women with estrogen approximately 5% increase strength of muscle [248]. Furthermore, risks of stroke, coronary heart disease, breast and skin cancer, and pulmonary embolism increased in postmenopausal women after use of combination of estrogen and progestin [249], [250], [251]. Therefore, for preventing or treating of sarcopenia in postmenopausal women estrogen therapy is not recommended.
5.3. Progestogens-related therapeutics
Study shows directly the therapeutic effect of progestogens on sarcopenia is not available. In menopausal women, progesterone administration (100 mg/d by using a vaginal insert) improved muscle protein synthesis [120]. In elderly persons, progestin such as megestrol acetate increases weight gain [252], [253]. On the other hand, oral bioidentical progesterone is safer and more efficient than synthetic progestins. Bioidentical progesterone cardiovascular safety [252], [254] even in combination with transdermal estradiol [255] and also lower risk of breast cancer [256], [257] is better than synthetic progestins.
6. Conclusions
Further studies are needed to clarify the exact effects of sex steroid hormones on the different types of muscle cells and their interaction with the molecular pathways controlling processes of apoptosis, proliferation and differentiation; but the current knowledge on these signaling pathways may help develop new therapeutics targeting the specifically related receptors on skeletal or muscle cells. As have been discussed, management of sarcopenia and improvement of muscle mass condition or control of this tissue wasting needs a wide range of studies that cover basic to clinical investigations.
Conflicts of interest
There is no conflict of interest.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2064077).
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Muller M.J., Geisler C., Pourhassan M., Gluer C.C., Bosy-Westphal A. Assessment and definition of lean body mass deficiency in the elderly. Eur J Clin Nutr. 2014;68:1220–1227. doi: 10.1038/ejcn.2014.169. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 3.Morley J.E. Sarcopenia in the elderly. Fam Pract. 2012;29(Suppl. 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 4.Morley J. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 5.Korea National Statistical Office . 2015. Statistics on the aged.http://kostat.go.kr/portal/english/news/1/23/2/index.board?bmode=list&bSeq=&aSeq=349205&pageNo=2&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt= [cited 2010 Oct 7]. Available from: [Google Scholar]
- 6.Lindle R., Metter E., Lynch N., Fleg J., Fozard J., Tobin J. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 7.Kunieda T., Minamino T., Nishi J-i, Tateno K., Oyama T., Katsuno T. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 8.Lin J., Lopez E.F., Jin Y., Van Remmen H., Bauch T., Han H.-C. Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cederholm T., Morley J.E. Sarcopenia: the new definitions. Curr Opin Clin Nutr Metab Care. 2015;18:1–4. doi: 10.1097/MCO.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 10.He W.A., Berardi E., Cardillo V.M., Acharyya S., Aulino P., Thomas-Ahner J. NF-κB–mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123:4821. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmonico M.J., Harris T.B., Visser M., Park S.W., Conroy M.B., Velasquez-Mieyer P. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Freire M., de Cabo R., Studenski S.A., Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584:1411–1416. doi: 10.1016/j.febslet.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 14.Narsale A.A., Carson J.A. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8:321–327. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson J.A., Manolagas S.C. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67–78. doi: 10.1016/j.bone.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez B.D., Jim H.S., Small B.J., Sutton S.K., Fishman M.N., Zachariah B. Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2015:1–7. doi: 10.1007/s00520-015-3016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells J.C.K. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Carlson B.M. Muscle regeneration and aging. Monogr Dev Biol. 1992;23:189–195. [PubMed] [Google Scholar]
- 19.Lecker S.H., Jagoe R.T., Gilbert A., Gomes M., Baracos V., Bailey J. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 20.McClung J., Davis J., Wilson M., Goldsmith E., Carson J. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol. 2006;100:2012–2023. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 21.White J.P., Baltgalvis K.A., Sato S., Wilson L.B., Carson J.A. Effect of nandrolone decanoate administration on recovery from bupivacaine-induced muscle injury. J Appl Physiol. 2009;107:1420–1430. doi: 10.1152/japplphysiol.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangenburg E.E., Geiger P.C., Leinwand L.A., Lowe D.A. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc. 2012;44:1653. doi: 10.1249/MSS.0b013e31825871fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhasin S., Storer T.W., Berman N., Callegari C., Clevenger B., Phillips J. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 24.Ferrando A.A., Tipton K.D., Doyle D., Phillips S.M., Cortiella J., Wolfe R.R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S., Storer T.W., Berman N., Yarasheski K.E., Clevenger B., Phillips J. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 26.Elbers J., Asscheman H., Seidell J., Gooren L. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol Endocrinol Metab. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- 27.Garcia J.M., Li H., Mann D., Epner D., Hayes T.G., Marcelli M. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–2591. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 28.Burney B.O., Hayes T.G., Smiechowska J., Cardwell G., Papusha V., Bhargava P. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700–E709. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 29.Grinspoon S., Corcoran C., Lee K., Burrows B., Hubbard J., Katznelson L. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–4058. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 30.Davison S., Bell R., Donath S., Montalto J., Davis S. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 31.Cawthon P.M., Ensrud K.E., Laughlin G.A., Cauley J.A., Dam T.-T.L., Barrett-Connor E. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94:3806–3815. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhasin S., Woodhouse L., Storer T.W. Androgen effects on body composition. Growth Horm IGF Res. 2003;13(Supplement):S63–S71. doi: 10.1016/s1096-6374(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 33.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 34.Axell A.-M., MacLean H.E., Plant D.R., Harcourt L.J., Davis J.A., Jimenez M. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab. 2006;291:E506–E516. doi: 10.1152/ajpendo.00058.2006. [DOI] [PubMed] [Google Scholar]
- 35.Antonio J., Wilson J.D., George F.W. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol. 1999;87:2016–2019. doi: 10.1152/jappl.1999.87.6.2016. [DOI] [PubMed] [Google Scholar]
- 36.Ophoff J., Proeyen K.V., Callewaert F., Gendt K.D., Bock K.D., Bosch A.V. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150:3558–3566. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- 37.Tanideh N., Sheikhani H.S., Salesi M., Tamadon A., Rostamzad K., Kardeh A. Effects of endurance exercise and estrogen supplementation on the proliferation of satellite cells. Comp Clin Pathol. 2014;23:1645–1649. [Google Scholar]
- 38.Enns D.L., Tiidus P.M. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104:347–353. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- 39.Brown M., Ning J., Ferreira J.A., Bogener J.L., Lubahn D.B. Estrogen receptor-α and-β and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab. 2009;296:E854–E861. doi: 10.1152/ajpendo.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClung J., Davis J., Carson J. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92:219–232. doi: 10.1113/expphysiol.2006.035071. [DOI] [PubMed] [Google Scholar]
- 41.Tiidus P.M., Lowe D.A., Brown M. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol. 2013;115:569–578. doi: 10.1152/japplphysiol.00629.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onambele-Pearson G.L. HRT affects skeletal muscle contractile characteristics: a definitive answer? J Appl Physiol. 2009;107:4–5. doi: 10.1152/japplphysiol.00448.2009. [DOI] [PubMed] [Google Scholar]
- 43.Enns D.L., Tiidus P.M. The influence of estrogen on skeletal muscle. Sports Med. 2010;40:41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Crandall C.J., Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am. 2013;42:227–253. doi: 10.1016/j.ecl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn M. Estrogen actions in the cardiovascular system. Climacteric. 2009;12:18–21. doi: 10.1080/13697130903020291. [DOI] [PubMed] [Google Scholar]
- 46.Finkelstein J.S., Lee H., Burnett-Bowie S.-A.M., Pallais J.C., Yu E.W., Borges L.F. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uotinen N., Puustinen R., Pasanen S., Manninen T., Kivineva M., Syvälä H. Distribution of progesterone receptor in female mouse tissues. Gen Comp Endocrinol. 1999;115:429–441. doi: 10.1006/gcen.1999.7333. [DOI] [PubMed] [Google Scholar]
- 48.Jonge X., Boot C., Thom J., Ruell P., Thompson M. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530:161–166. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell S., Febbraio M. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E803–E808. doi: 10.1152/ajpendo.2001.281.4.E803. [DOI] [PubMed] [Google Scholar]
- 50.Sissom E., Reinhardt C., Johnson B. Melengestrol acetate alters muscle cell proliferation in heifers and steers. J Anim Sci. 2006;84:2950–2958. doi: 10.2527/jas.2005-726. [DOI] [PubMed] [Google Scholar]
- 51.Kayisli U.A., Basar M., Guzeloglu-Kayisli O., Semerci N., Atkinson H.C., Shapiro J. Long-acting progestin-only contraceptives impair endometrial vasculature by inhibiting uterine vascular smooth muscle cell survival. Proc Natl Acad Sci U. S. A. 2015;112:5153–5158. doi: 10.1073/pnas.1424814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wannenes F., Caprio M., Gatta L., Fabbri A., Bonini S., Moretti C. Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol Cell Endocrinol. 2008;292:11–19. doi: 10.1016/j.mce.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Sinha-Hikim I., Taylor W.E., Gonzalez-Cadavid N.F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 54.Doumit M.E., Cook D.R., Merkel R.A. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- 55.Carson J.A., Lee W.J., McClung J., Hand G.A. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol. 2002;93:242–250. doi: 10.1152/japplphysiol.01212.2001. [DOI] [PubMed] [Google Scholar]
- 56.Bamman M.M., Shipp J.R., Jiang J., Gower B.A., Hunter G.R., Goodman A. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–E390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell C.J., Churchward-Venne T.A., Bellamy L., Parise G., Baker S.K., Phillips S.M. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8:e78636. doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee W.J., McClung J., Hand G., Carson J.A. Overload-induced androgen receptor expression in the aged rat hindlimb receiving nandrolone decanoate. J Appl Physiol. 2003;94:1153–1161. doi: 10.1152/japplphysiol.00822.2002. [DOI] [PubMed] [Google Scholar]
- 59.Lee W.J., Thompson R.W., McClung J.M., Carson J.A. Regulation of androgen receptor expression at the onset of functional overload in rat plantaris muscle. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1076–R1085. doi: 10.1152/ajpregu.00202.2003. [DOI] [PubMed] [Google Scholar]
- 60.MacLean H.E., Chiu W.M., Notini A.J., Axell A.-M., Davey R.A., McManus J.F. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 61.Dubois V., Laurent M.R., Sinnesael M., Cielen N., Helsen C., Clinckemalie L. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 2014;28:2979–2994. doi: 10.1096/fj.14-249748. [DOI] [PubMed] [Google Scholar]
- 62.Fernando S.M., Rao P., Niel L., Chatterjee D., Stagljar M., Monks D.A. Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology. 2010;151:3125–3132. doi: 10.1210/en.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niel L., Shah A.H., Lewis G.A., Mo K., Chatterjee D., Fernando S.M. Sexual differentiation of the spinal nucleus of the bulbocavernosus is not mediated solely by androgen receptors in muscle fibers. Endocrinology. 2009;150:3207–3213. doi: 10.1210/en.2008-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujimoto R., Morimoto I., Morita E., Sugimoto H., Ito Y., Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–174. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 65.Kim J.-W., Oh M.-M., Yoon C.-Y., Bae J.-H., Kim J.-J., Moon D.-G. The effect of diet-induced insulin resistance on DNA methylation of the androgen receptor promoter in the penile cavernosal smooth muscle of mice. Asian J Androl. 2013;15:487–491. doi: 10.1038/aja.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L., Li Y., Xie N., Shynlova O., Challis J.R., Slater D. Proliferative action of the androgen receptor in human uterine myometrial cells-a key regulator for myometrium phenotype programming. J Clin Endocrinol Metab. 2013;98:218–227. doi: 10.1210/jc.2012-2451. [DOI] [PubMed] [Google Scholar]
- 67.Wiik A., Ekman M., Johansson O., Jansson E., Esbjörnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009;131:181–189. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 68.Foryst-Ludwig A., Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Glenmark B., Nilsson M., Gao H., Gustafsson J.-Å., Dahlman-Wright K., Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-β. Am J Physiol Endocrinol Metab. 2004;287:E1125–E1131. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- 70.Ueda K., Lu Q., Baur W., Aronovitz M.J., Karas R.H. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2013;33:1837–1843. doi: 10.1161/ATVBAHA.112.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hogg M.E., Vavra A.K., Banerjee M.N., Martinez J., Jiang Q., Keefer L.K. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res. 2012;173:e1–e10. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feder D., Rodrigues Barros Godoy I., Guimarães Pereira M.L., Silva C.S., Nogueira Silvestre D., Fonseca F.L.A. Hormonal receptors in skeletal muscles of dystrophic Mdx mice. Biomed Res Int. 2013;2013:604635. doi: 10.1155/2013/604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gras F., Brunmair B., Quarré L., Szöcs Z., Waldhäusl W., Fürnsinn C. Progesterone impairs cell respiration and suppresses a compensatory increase in glucose transport in isolated rat skeletal muscle: a non-genomic mechanism contributing to metabolic adaptation to late pregnancy? Diabetologia. 2007;50:2544–2552. doi: 10.1007/s00125-007-0836-4. [DOI] [PubMed] [Google Scholar]
- 74.Lee W.-S., Harder J.A., YoshizuMi M., Lee M.-E., Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- 75.Nisolle M., Gillerot S., Casanas-Roux F., Squifflet J., Berliere M., Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod. 1999;14:2844–2850. doi: 10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- 76.Glass D. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. In: Rommel C., Vanhaesebroeck B., Vogt P.K., editors. Phosphoinositide 3-kinase in health and disease. Vol. 346. Springer Berlin Heidelberg; 2011. pp. 267–278. (Current topics in microbiology and immunology). [Google Scholar]
- 77.Frost R.A., Lang C.H. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 78.Frost R.A., Lang C.H. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology. 2011;26:83–96. doi: 10.1152/physiol.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 80.Verhees K.J., Schols A.M., Kelders M.C., den Kamp C.M.O., van der Velden J.L., Langen R.C. Glycogen synthase kinase-3β is required for the induction of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2011;301:C995–C1007. doi: 10.1152/ajpcell.00520.2010. [DOI] [PubMed] [Google Scholar]
- 81.Barton E.R. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 82.Chambon C., Duteil D., Vignaud A., Ferry A., Messaddeq N., Malivindi R. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U. S. A. 2010;107:14327–14332. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mauras N., Hayes V., Welch S., Rini A., Helgeson K., Dokler M. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 84.Öner J., Öner H., Sahin Z., Demir R., Üstünel İ. Melatonin is as effective as testosterone in the prevention of soleus muscle atrophy induced by castration in rats. Anat Rec. 2008;291:448–455. doi: 10.1002/ar.20659. [DOI] [PubMed] [Google Scholar]
- 85.Ibebunjo C., Eash J.K., Li C., Ma Q., Glass D.J. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab. 2011;300:E327–E340. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- 86.Hourdé C., Jagerschmidt C., Clément-Lacroix P., Vignaud A., Ammann P., Butler-Browne G.S. Androgen replacement therapy improves function in male rat muscles independently of hypertrophy and activation of the Akt/mTOR pathway. Acta Physiol. 2009;195:471–482. doi: 10.1111/j.1748-1716.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 87.Yin H.-N., Chai J.-K., Yu Y.-M., Wu C.-A.S., Yao Y.-M., Liu H. Regulation of signaling pathways downstream of IGF-I/insulin by androgen in skeletal muscle of glucocorticoid-treated rats. J Trauma. 2009;66:1083–1090. doi: 10.1097/TA.0b013e31817e7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haren M.T., Siddiqui A.M., Armbrecht H.J., Kevorkian R.T., Kim M.J., Haas M.J. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl. 2011;34:55–68. doi: 10.1111/j.1365-2605.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 89.Sarbassov dD., Ali S.M., Sabatini D.M. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Potier M., Darcel N., Tomé D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:54–58. doi: 10.1097/MCO.0b013e32831b9e01. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y., Bauman W.A., Blitzer R.D., Cardozo C. Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 2010;400:679–683. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- 92.Chan A.Y., Soltys C.-L.M., Young M.E., Proud C.G., Dyck J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 93.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 94.White J.P., Gao S., Puppa M.J., Sato S., Welle S.L., Carson J.A. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puttabyatappa Y., Stallone J.N., Ergul A., El-Remessy A.B., Kumar S., Black S. Peroxynitrite mediates testosterone-induced vasodilation of microvascular resistance vessels. J Pharmacol Exp Ther. 2013;345:7–14. doi: 10.1124/jpet.112.201947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J., Akishita M., Eto M., Ogawa S., Son B.-K., Kato S. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010;151:1822–1828. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 97.Vasconsuelo A., Milanesi L., Boland R. 17β-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2008;196:385–397. doi: 10.1677/JOE-07-0250. [DOI] [PubMed] [Google Scholar]
- 98.Sitnick M., Foley A.M., Brown M., Spangenburg E.E. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100:286–293. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- 99.Olivieri F., Ahtiainen M., Lazzarini R., Pöllänen E., Capri M., Lorenzi M. Hormone replacement therapy enhances IGF-1 signaling in skeletal muscle by diminishing miR-182 and miR-223 expressions: a study on postmenopausal monozygotic twin pairs. Aging Cell. 2014;13:850–861. doi: 10.1111/acel.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J.-Y., Jo K.-J., Kim O.-S., Kim B.-J., Kang D.-W., Lee K.-H. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010;87:358–366. doi: 10.1016/j.lfs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Thomson D.M., Fick C.A., Gordon S.E. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol. 2008;104:625–632. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- 102.Saha A.K., Xu X.J., Lawson E., Deoliveira R., Brandon A.E., Kraegen E.W. Downregulation of AMPK accompanies leucine-and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galluzzo P., Rastelli C., Bulzomi P., Acconcia F., Pallottini V., Marino M. 17β-estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. Am J Physiol Cell Physiol. 2009;297:C1249–C1262. doi: 10.1152/ajpcell.00188.2009. [DOI] [PubMed] [Google Scholar]
- 104.Lluís F., Perdiguero E., Nebreda A.R., Muñoz-Cánoves P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006;16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Vandromme M., Rochat A., Meier R., Carnac G., Besser D., Hemmings B.A. Protein kinase B β/Akt2 plays a specific role in muscle differentiation. J Biol Chem. 2001;276:8173–8179. doi: 10.1074/jbc.M005587200. [DOI] [PubMed] [Google Scholar]
- 106.Pöllänen E., Ronkainen P.H.A., Horttanainen M., Takala T., Puolakka J., Suominen H. Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Horm IGF Res. 2010;20:372–379. doi: 10.1016/j.ghir.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Ning J., Clemmons D.R. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol. 2010;24:1218–1229. doi: 10.1210/me.2009-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han G., Ma H., Chintala R., Miyake K., Fulton D.J., Barman S.A. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007;293:H314–H321. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- 109.Jaffer S., Shynlova O., Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150:4672–4680. doi: 10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- 110.Foster H.A., Davies J., Pink R.C., Turkcigdem S., Goumenou A., Carter D.R. The human myometrium differentially expresses mTOR signalling components before and during pregnancy: evidence for regulation by progesterone. J Steroid Biochem Mol Biol. 2014;139:166–172. doi: 10.1016/j.jsbmb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu S.-P., Chen T.-H., Chou Y.-P., Chen L.-C., Kuo C.-T., Lee T.-S. Extra-nuclear activation of progesterone receptor in regulating arterial smooth muscle cell migration. Atherosclerosis. 2011;217:83–89. doi: 10.1016/j.atherosclerosis.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 112.Lee W.-S., Liu C.-W., Juan S.-H., Liang Y.-C., Ho P.-Y., Lee Y.-H. Molecular mechanism of progesterone-induced antiproliferation in rat aortic smooth muscle cells. Endocrinology. 2003;144:2785–2790. doi: 10.1210/en.2003-0045. [DOI] [PubMed] [Google Scholar]
- 113.Wang H.-C., Lee W.-S. Progesterone induces RhoA inactivation in male rat aortic smooth muscle cells through up-regulation of p27kip1. Endocrinology. 2014;155:4473–4482. doi: 10.1210/en.2014-1344. [DOI] [PubMed] [Google Scholar]
- 114.Wang H.-C., Lee W.-S. Progesterone-induced migration inhibition in male rat aortic smooth muscle cells through the cSrc/AKT/ERK 2/p38 pathway-mediated up-regulation of p27. Endocrinology. 2014;155:1428–1435. doi: 10.1210/en.2013-1838. [DOI] [PubMed] [Google Scholar]
- 115.Latres E., Amini A.R., Amini A.A., Griffiths J., Martin F.J., Wei Y. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- 116.Lee S.W., Dai G., Hu Z., Wang X., Du J., Mitch W.E. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 117.Pires-Oliveira M., Maragno A.L.G., Parreiras-e-Silva L.T., Chiavegatti T., Gomes M.D., Godinho R.O. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. J Appl Physiol. 2010;108:266–273. doi: 10.1152/japplphysiol.00490.2009. [DOI] [PubMed] [Google Scholar]
- 118.Qin W., Pan J., Wu Y., Bauman W.A., Cardozo C. Protection against dexamethasone-induced muscle atrophy is related to modulation by testosterone of FOXO1 and PGC-1α. Biochem Biophys Res Commun. 2010;403:473–478. doi: 10.1016/j.bbrc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 119.Rana K., Lee N.K.L., Zajac J.D., MacLean H.E. Expression of androgen receptor target genes in skeletal muscle. Asian J Androl. 2014;16:675–683. doi: 10.4103/1008-682X.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith G.I., Yoshino J., Reeds D.N., Bradley D., Burrows R.E., Heisey H.D. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab. 2014;99:256–265. doi: 10.1210/jc.2013-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee H.-Y., Chung J.-W., Youn S.-W., Kim J.-Y., Park K.-W., Koo B.-K. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 122.Simone C., Forcales S.V., Hill D.A., Imbalzano A.N., Latella L., Puri P.L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 123.Wu Z., Woodring P.J., Bhakta K.S., Tamura K., Wen F., Feramisco J.R. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keren A., Tamir Y., Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 125.Perdiguero E., Ruiz-Bonilla V., Gresh L., Hui L., Ballestar E., Sousa-Victor P. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38α in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suelves M., Lluís F., Ruiz V., Nebreda A.R., Muñoz-Cánoves P. Phosphorylation of MRF4 transactivation domain by p38 mediates repression of specific myogenic genes. EMBO J. 2004;23:365–375. doi: 10.1038/sj.emboj.7600056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garrington T.P., Johnson G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 128.Aronson D., Violan M.A., Dufresne S.D., Zangen D., Fielding R.A., Goodyear L.J. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aronson D., Wojtaszewski J.F., Thorell A., Nygren J., Zangen D., Richter E.A. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol Cell Physiol. 1998;275:C555–C561. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- 130.Napoli R., Gibson L., Hirshman M.F., Boppart M.D., Dufresne S.D., Horton E.S. Epinephrine and insulin stimulate different mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Diabetes. 1998;47:1549–1554. doi: 10.2337/diabetes.47.10.1549. [DOI] [PubMed] [Google Scholar]
- 131.Gredinger E., Gerber A.N., Tamir Y., Tapscott S.J., Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J Biol Chem. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- 132.Penna F., Costamagna D., Fanzani A., Bonelli G., Baccino F.M., Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One. 2010;5:e13604. doi: 10.1371/journal.pone.0013604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H., Mittal A., Paul P.K., Kumar M., Srivastava D.S., Tyagi S.C. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase 9 (MMP-9) production in skeletal muscle through the activation of nuclear factor-kappaB-inducing kinase and p38 mitogen-activated protein kinase: a potential role of MMP-9 in myopathy. J Biol Chem. 2009;284:4439–4450. doi: 10.1074/jbc.M805546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morelli S., Buitrago C., Vazquez G., De Boland A.R., Boland R. Involvement of tyrosine kinase activity in 1α, 25 (OH) 2-vitamin D3 signal transduction in skeletal muscle cells. J Biol Chem. 2000;275:36021–36028. doi: 10.1074/jbc.M002025200. [DOI] [PubMed] [Google Scholar]
- 135.Estrada M., Espinosa A., Müller M., Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 136.Somjen D., Kohen F., Jaffe A., Amir-Zaltsman Y., Knoll E., Stern N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension. 1998;32:39–45. doi: 10.1161/01.hyp.32.1.39. [DOI] [PubMed] [Google Scholar]
- 137.Somjen D., Kohen F., Gayer B., Kulik T., Knoll E., Stern N. Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol. 2004;180:97–106. doi: 10.1677/joe.0.1800097. [DOI] [PubMed] [Google Scholar]
- 138.Bennett A.M., Tonks N.K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 139.Ronda A.C., Buitrago C., Colicheo A., de Boland A.R., Roldán E., Boland R. Activation of MAPKs by 1α,25(OH)2-Vitamin D3 and 17β-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J Steroid Biochem Mol Biol. 2007;103:462–466. doi: 10.1016/j.jsbmb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 140.Geraldes P., Sirois M.G., Bernatchez P.N., Tanguay J.-F. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2002;22:1585–1590. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- 141.Dubey R.K., Jackson E.K., Gillespie D.G., Zacharia L.C., Imthurn B., Keller P.J. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen-activated protein kinase activity. Arterioscler Thromb Vasc Biol. 2000;20:964–972. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- 142.Xiao D., Huang X., Yang S., Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang H.-C., Hsu S.-P., Lee W.-S. Extra-nuclear signaling pathway involved in progesterone-induced up-regulations of p21(cip1) and p27(kip1) in male rat aortic smooth muscle cells. PLoS One. 2015;10:e0125903. doi: 10.1371/journal.pone.0125903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cossu G., Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anakwe K., Robson L., Hadley J., Buxton P., Church V., Allen S. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. doi: 10.1242/dev.00538. [DOI] [PubMed] [Google Scholar]
- 146.Tajbakhsh S., Borello U., Vivarelli E., Kelly R., Papkoff J., Duprez D. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- 147.Le Grand F., Jones A.E., Seale V., Scimè A., Rudnicki M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brack A.S., Conboy I.M., Conboy M.J., Shen J., Rando T.A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 149.Liu X.-H., Wu Y., Yao S., Levine A.C., Kirschenbaum A., Collier L. Androgens up-regulate transcription of the Notch inhibitor Numb in C2C12 myoblasts via Wnt/β-catenin signaling to T cell factor elements in the Numb promoter. J Biol Chem. 2013;288:17990–17998. doi: 10.1074/jbc.M113.478487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X.H., Yao S., Levine A.C., Kirschenbaum A., Pan J., Wu Y. Nandrolone, an anabolic steroid, stabilizes Numb protein through inhibition of Mdm2 in C2C12 myoblasts. J Androl. 2012;33(6):1216–1223. doi: 10.2164/jandrol.112.016428. [DOI] [PubMed] [Google Scholar]
- 151.Liu X.-H., Yao S., Qiao R.-F., Levine A.C., Kirschenbaum A., Pan J. Nandrolone reduces activation of Notch signaling in denervated muscle associated with increased Numb expression. Biochem Biophys Res Commun. 2011;414:165–169. doi: 10.1016/j.bbrc.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 152.Brown D., Hikim A.P.S., Kovacheva E.L., Sinha-Hikim I. Mouse model of testosterone-induced muscle fiber hypertrophy: involvement of p38 mitogen-activated protein kinase-mediated Notch signaling. J Endocrinol. 2009;201:129–139. doi: 10.1677/JOE-08-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Griggs R.C., Kingston W., Jozefowicz R.F., Herr B.E., Forbes G., Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66:498–503. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- 154.Kovacheva E.L., Sinha Hikim A.P., Shen R., Sinha I., Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mill C., George S.J. Wnt signalling in smooth muscle cells and it's role in cardiovascular disorders. Cardiovasc Res. 2012;95:233–240. doi: 10.1093/cvr/cvs141. [DOI] [PubMed] [Google Scholar]
- 156.Kongsuwan K., Knox M.R., Allingham P.G., Pearson R., Dalrymple B.P. The effect of combination treatment with trenbolone acetate and estradiol-17β on skeletal muscle expression and plasma concentrations of oxytocin in sheep. Domest Anim Endocrinol. 2012;43:67–73. doi: 10.1016/j.domaniend.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 157.Ono M., Yin P., Navarro A., Moravek M.B., Coon J.S., Druschitz S.A. Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U. S. A. 2013;110:17053–17058. doi: 10.1073/pnas.1313650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bakkar N., Guttridge D.C. NF-κB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev. 2010;90:495–511. doi: 10.1152/physrev.00040.2009. [DOI] [PubMed] [Google Scholar]
- 159.Wyke S.M., Tisdale M.J. NF-κB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin–proteasome system in skeletal muscle. Br J Cancer. 2005;92:711–721. doi: 10.1038/sj.bjc.6602402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Burckart K., Beca S., Urban R.J., Sheffield-Moore M. Pathogenesis of muscle wasting in cancer cachexia: targeted anabolic and anticatabolic therapies. Curr Opin Clin Nutr Metab Care. 2010;13:410–416. doi: 10.1097/MCO.0b013e328339fdd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saini A., Faulkner S., Al-Shanti N., Stewart C. Powerful signals for weak muscles. Ageing Res Rev. 2009;8:251–267. doi: 10.1016/j.arr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 162.Zhang P., Chen X., Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69:310–321. doi: 10.1016/j.mehy.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 163.Ladner K.J., Caligiuri M.A., Guttridge D.C. Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 164.Yamaki T., Wu C.-L., Gustin M., Lim J., Jackman R.W., Kandarian S.C. Rel A/p65 is required for cytokine-induced myotube atrophy. Am J Physiol Cell Physiol. 2012;303:C135–C142. doi: 10.1152/ajpcell.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar A., Bhatnagar S., Paul P.K. TWEAK and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2012;15:233–239. doi: 10.1097/MCO.0b013e328351c3fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Urban R.J., Dillon E.L., Choudhary S., Zhao Y., Horstman A.M., Tilton R.G. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc. 2014;125:27–44. [PMC free article] [PubMed] [Google Scholar]
- 167.Zarnegar B.J., Wang Y., Mahoney D.J., Dempsey P.W., Cheung H.H., He J. Activation of noncanonical NF-κB requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2, TRAF3 and the kinase NIK. Nat Immunol. 2008:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vallabhapurapu S., Matsuzawa A., Zhang W., Tseng P.-H., Keats J.J., Wang H. Non-redundant and complementary functions of adaptor proteins TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dillon E.L., Basra G., Horstman A.M., Casperson S.L., Randolph K.M., Durham W.J. Cancer cachexia and anabolic interventions: a case report. J Cachexia Sarcopenia Muscle. 2012;3:253–263. doi: 10.1007/s13539-012-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leimgruber C., Quintar A.A., García L.N., Petiti J.P., De Paul A.L., Maldonado C.A. Testosterone abrogates TLR4 activation in prostate smooth muscle cells contributing to the preservation of a differentiated phenotype. J Cell Physiol. 2013;228:1551–1560. doi: 10.1002/jcp.24314. [DOI] [PubMed] [Google Scholar]
- 171.Sharma R.V., Gurjar M.V., Bhalla R.C. Estrogen receptor-α gene transfer inhibits proliferation and NF-κB activation in VSM cells from female rats. J Appl Physiol. 2001;91:2400–2406. doi: 10.1152/jappl.2001.91.5.2400. [DOI] [PubMed] [Google Scholar]
- 172.Yallampalli C., Dong Y.-L. Estradiol-17β inhibits nitric oxide synthase (NOS)-II and stimulates NOS-III gene expression in the rat uterus. Biol Reprod. 2000;63:34–41. doi: 10.1095/biolreprod63.1.34. [DOI] [PubMed] [Google Scholar]
- 173.Xie Q.W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khanjani S., Kandola M.K., Lindstrom T.M., Sooranna S.R., Melchionda M., Lee Y.S. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med. 2011;15:809–824. doi: 10.1111/j.1582-4934.2010.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee Y., Sooranna S.R., Terzidou V., Christian M., Brosens J., Huhtinen K. Interactions between inflammatory signals and the progesterone receptor in regulating gene expression in pregnant human uterine myocytes. J Cell Mol Med. 2012;16:2487–2503. doi: 10.1111/j.1582-4934.2012.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Leite R.S., Brown A.G., Strauss J.F. Tumor necrosis factor-α suppresses the expression of steroid receptor coactivator-1 and-2: a possible mechanism contributing to changes in steroid hormone responsiveness. FASEB J. 2004;18:1418–1420. doi: 10.1096/fj.04-1684fje. [DOI] [PubMed] [Google Scholar]
- 177.Kelly D.M., Sellers D.J., Woodroofe M.N., Jones T.H., Channer K.S. Effect of testosterone on inflammatory markers in the development of early atherogenesis in the testicular-feminized mouse model. Endocr Res. 2013;38:125–138. doi: 10.3109/07435800.2012.735307. [DOI] [PubMed] [Google Scholar]
- 178.Saylor P.J., Kozak K.R., Smith M.R., Ancukiewicz M.A., Efstathiou J.A., Zietman A.L. Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. Oncologist. 2012;17:212–219. doi: 10.1634/theoncologist.2011-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Norata G.D., Tibolla G., Seccomandi P.M., Poletti A., Catapano A.L. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–554. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- 180.Visser M., Pahor M., Taaffe D.R., Goodpaster B.H., Simonsick E.M., Newman A.B. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 181.Goodman M.N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 182.Goodman M.N. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol Endocrinol Metab. 1991;260:E727–E730. doi: 10.1152/ajpendo.1991.260.5.E727. [DOI] [PubMed] [Google Scholar]
- 183.Baeza-Raja B., Muñoz-Cánoves P. p38 MAPK-induced nuclear factor-κB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell. 2004;15:2013–2026. doi: 10.1091/mbc.E03-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Iwase S., Murakami T., Saito Y., Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patients. Eur Cytokine Netw. 2004;15:312–316. [PubMed] [Google Scholar]
- 185.Nielsen A.R., Pedersen B.K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–839. doi: 10.1139/H07-054. [DOI] [PubMed] [Google Scholar]
- 186.Freeman B.M., Mountain D.J., Brock T.C., Chapman J.R., Kirkpatrick S.S., Freeman M.B. Low testosterone elevates interleukin family cytokines in a rodent model: a possible mechanism for the potentiation of vascular disease in androgen-deficient males. J Surg Res. 2014;190:319–327. doi: 10.1016/j.jss.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 187.Warren G.L., Hulderman T., Jensen N., McKinstry M., Mishra M., Luster M.I. Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J. 2002;16:1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- 188.Velders M., Schleipen B., Fritzemeier K.H., Zierau O., Diel P. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 189.MacNeil L.G., Baker S.K., Stevic I., Tarnopolsky M.A. 17β-estradiol attenuates exercise-induced neutrophil infiltration in men. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1443–R1451. doi: 10.1152/ajpregu.00689.2009. [DOI] [PubMed] [Google Scholar]
- 190.Xing D., Feng W., Miller A.P., Weathington N.M., Chen Y.-F., Novak L. Estrogen modulates TNF-α-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-β activation. Am J Physiol Heart Circ Physiol. 2007;292:H2607–H2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 191.Lambert C.P., Sullivan D.H., Freeling S.A., Lindquist D.M., Evans W.J. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2002;87:2100–2106. doi: 10.1210/jcem.87.5.8505. [DOI] [PubMed] [Google Scholar]
- 192.Lambert C.P., Sullivan D.H., Evans W.J. Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2003;58:M165–M170. doi: 10.1093/gerona/58.2.m165. [DOI] [PubMed] [Google Scholar]
- 193.Lan L., Vinci J.M., Melendez J.A., Jeffrey J.J., Wilcox B.D. Progesterone mediates decreases in uterine smooth muscle cell interleukin-1α by a mechanism involving decreased stability of IL-1α mRNA. Mol Cell Endocrinol. 1999;155:123–133. doi: 10.1016/s0303-7207(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 194.Chandra S., Terragni J., Zhang G., Pradhan S., Haushka S., Johnston D. Tissue-specific epigenetics in gene neighborhoods: myogenic transcription factor genes. Hum Mol Genet. 2015;24(16):4660–4673. doi: 10.1093/hmg/ddv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vasyutina E., Lenhard D.C., Birchmeier C. Notch function in myogenesis. Cell Cycle. 2007;6:1450–1453. [PubMed] [Google Scholar]
- 196.Wilson-Rawls J., Molkentin J.D., Black B.L., Olson E.N. Activated notch inhibits myogenic activity of the MADS-Box transcription factor myocyte enhancer factor 2C. Mol Cell Biol. 1999;19:2853–2862. doi: 10.1128/mcb.19.4.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hasty P., Bradley A., Morris J.H., Edmondson D.G., Venuti J.M., Olson E.N. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 198.Lee D.K. Androgen receptor enhances myogenin expression and accelerates differentiation. Biochem Biophys Res Commun. 2002;294:408–413. doi: 10.1016/S0006-291X(02)00504-1. [DOI] [PubMed] [Google Scholar]
- 199.Chen Y., Lee N., Zajac J., MacLean H. Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J Endocrinol Invest. 2008;31:910–918. doi: 10.1007/BF03346441. [DOI] [PubMed] [Google Scholar]
- 200.Barkley M.S., Goldman B.D. A quantitative study of serum testosterone, sex accessory organ growth, and the development of intermale aggression in the mouse. Horm Behav. 1977;8:208–218. doi: 10.1016/0018-506x(77)90038-1. [DOI] [PubMed] [Google Scholar]
- 201.Graves D.C., Yablonka–Reuveni Z. Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: a unique Myf5(-)/MyoD(+) myogenic program. J Histochem Cytochem. 2000;48:1173–1193. doi: 10.1177/002215540004800902. [DOI] [PubMed] [Google Scholar]
- 202.Ogawa M., Yamaji R., Higashimura Y., Harada N., Ashida H., Nakano Y. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem. 2011;286:41455–41465. doi: 10.1074/jbc.M111.276824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pedraza-Alva G., Zingg J.M., Donda A., Pérez-Martínez L. Estrogen receptor regulates MyoD gene expression by preventing AP-1-mediated repression. Biochem Biophys Res Commun. 2009;389:360–365. doi: 10.1016/j.bbrc.2009.08.153. [DOI] [PubMed] [Google Scholar]
- 204.Carlson M.E., Hsu M., Conboy I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Child E.S., Mann D.J. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 206.Li Y., Li J., Zhu J., Sun B., Branca M., Tang Y. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15:1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 207.McCroskery S., Thomas M., Maxwell L., Sharma M., Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dasarathy S., Dodig M., Muc S.M., Kalhan S.C., McCullough A.J. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1124–G1130. doi: 10.1152/ajpgi.00202.2004. [DOI] [PubMed] [Google Scholar]
- 209.Thomas M., Langley B., Berry C., Sharma M., Kirk S., Bass J. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 210.Huang Z., Chen D., Zhang K., Yu B., Chen X., Meng J. Regulation of myostatin signaling by c-Jun N-terminal kinase in C2C12 cells. Cell Signal. 2007;19:2286–2295. doi: 10.1016/j.cellsig.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 211.Zhang P., Wong C., Liu D., Finegold M., Harper J.W., Elledge S.J. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bowles D.K., Maddali K.K., Dhulipala V., Korzick D.H. PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. Am J Physiol Cell Physiol. 2007;293:C805–C813. doi: 10.1152/ajpcell.00127.2007. [DOI] [PubMed] [Google Scholar]
- 213.Brooks E.E., Gray N.S., Joly A., Kerwar S.S., Lum R., Mackman R.L. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J Biol Chem. 1997;272:29207–29211. doi: 10.1074/jbc.272.46.29207. [DOI] [PubMed] [Google Scholar]
- 214.Braun-Dullaeus R.C., Mann M.J., Ziegler A., von der Leyen H.E., Dzau V.J. A novel role for the cyclin-dependent kinase inhibitor p27Kip1 in angiotensin II-stimulated vascular smooth muscle cell hypertrophy. J Clin Invest. 1999;104:815–823. doi: 10.1172/JCI5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen D., Krasinski K., Sylvester A., Chen J., Nisen P.D., Andrés V. Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997;99:2334–2341. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tanner F.C., Boehm M., Akyürek L.M., San H., Yang Z.-Y., Tashiro J. Differential effects of the cyclin-dependent kinase inhibitors p27Kip1, p21Cip1, and p16Ink4 on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 217.Barchiesi F., Jackson E.K., Fingerle J., Gillespie D.G., Odermatt B., Dubey R.K. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 218.Takahashi K., Ohmichi M., Yoshida M., Hisamoto K., Mabuchi S., Arimoto-Ishida E. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J Endocrinol. 2003;178:319–329. doi: 10.1677/joe.0.1780319. [DOI] [PubMed] [Google Scholar]
- 219.Sun F.-Q., Duan H., Wang S., Li J.-J. 17β-estradiol induces overproliferation in adenomyotic human uterine smooth muscle cells of the junctional zone through hyperactivation of the estrogen receptor-enhanced RhoA/ROCK signaling pathway. Reprod Sci. 2015;22:1436–1444. doi: 10.1177/1933719115584447. [DOI] [PubMed] [Google Scholar]
- 220.Ríos R., Carneiro I., Arce V.M., Devesa J. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol. 2002;282:C993–C999. doi: 10.1152/ajpcell.00372.2001. [DOI] [PubMed] [Google Scholar]
- 221.Guttridge D.C. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:443–450. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- 222.Kollias H.D., McDermott J.C. Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 223.Lee S.-J. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 224.Mendler L., Baka Z., Kovács-Simon A., Dux L. Androgens negatively regulate myostatin expression in an androgen-dependent skeletal muscle. Biochem Biophys Res Commun. 2007;361:237–242. doi: 10.1016/j.bbrc.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 225.Ma K., Mallidis C., Artaza J., Taylor W., Gonzalez-Cadavid N., Bhasin S. Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281:E1128–E1136. doi: 10.1152/ajpendo.2001.281.6.E1128. [DOI] [PubMed] [Google Scholar]
- 226.Tsao J., Vernet D.A., Gelfand R., Kovanecz I., Nolazco G., Bruhn K.W. Myostatin genetic inactivation inhibits myogenesis by muscle-derived stem cells in vitro but not when implanted in the mdx mouse muscle. Stem Cell Res Ther. 2013;4:4. doi: 10.1186/scrt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ciarmela P., Wiater E., Smith S.M., Vale W. Presence, actions, and regulation of myostatin in rat uterus and myometrial cells. Endocrinology. 2009;150:906–914. doi: 10.1210/en.2008-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cruz-Jentoft A.J., Baeyens J.P., Bauer M., Jr., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Serra C., Tangherlini F., Rudy S., Lee D., Toraldo G., Sandor N.L. Testosterone improves the regeneration of old and young mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2013;68:17–26. doi: 10.1093/gerona/gls083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Borst S.E., Yarrow J.F. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am J Physiol Endocrinol Metab. 2015;308:E1035–E1042. doi: 10.1152/ajpendo.00111.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bhasin S., Calof O.M., Storer T.W., Lee M.L., Mazer N.A., Jasuja R. Drug Insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ottenbacher K.J., Ottenbacher M.E., Ottenbacher A.J., Acha A.A., Ostir G.V. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54:1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fernandez-Balsells M.M., Murad M.H., Lane M., Lampropulos J.F., Albuquerque F., Mullan R.J. Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 234.Basaria S., Coviello A.D., Travison T.G., Storer T.W., Farwell W.R., Jette A.M. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yassin A.A., Haffejee M. Testosterone depot injection in male hypogonadism: a critical appraisal. Clin Interv Aging. 2007;2:577–590. [PMC free article] [PubMed] [Google Scholar]
- 236.Percheron G., Hogrel J.-Y., Denot-Ledunois S., Fayet G., Forette F., Baulieu E.-E. Effect of 1-year oral administration of dehydroepiandrosterone to 60-to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163:720–727. doi: 10.1001/archinte.163.6.720. [DOI] [PubMed] [Google Scholar]
- 237.Baker W.L., Karan S., Kenny A.M. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011;59:997–1002. doi: 10.1111/j.1532-5415.2011.03410.x. [DOI] [PubMed] [Google Scholar]
- 238.Villareal D.T., Holloszy J.O. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 239.Mavros Y., O'Neill E., Connerty M., Bean J.F., Broe K., Kiel D.P. Oxandrolone augmentation of resistance training in older women: a randomized trial. Med Sci Sports Exerc. 2015;47:2257–2267. doi: 10.1249/MSS.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 240.Cozzoli A., Capogrosso R.F., Sblendorio V.T., Dinardo M.M., Jagerschmidt C., Namour F. GLPG0492, a novel selective androgen receptor modulator, improves muscle performance in the exercised-mdx mouse model of muscular dystrophy. Pharmacol Res. 2013;72:9–24. doi: 10.1016/j.phrs.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 241.Thevis M., Geyer H., Kamber M., Schänzer W. Detection of the arylpropionamide-derived selective androgen receptor modulator (SARM) S-4 (Andarine) in a black-market product. Drug Test Analysis. 2009;1:387–392. doi: 10.1002/dta.91. [DOI] [PubMed] [Google Scholar]
- 242.Akita K., Harada K., Ichihara J., Takata N., Takahashi Y., Saito K. A novel selective androgen receptor modulator, NEP28, is efficacious in muscle and brain without serious side effects on prostate. Eur J Pharmacol. 2013;720:107–114. doi: 10.1016/j.ejphar.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 243.Srinath R., Dobs A. Enobosarm (GTx-024, S-22): a potential treatment for cachexia. Future Oncol. 2014;10:187–194. doi: 10.2217/fon.13.273. [DOI] [PubMed] [Google Scholar]
- 244.Dalton J.T., Barnette K.G., Bohl C.E., Hancock M.L., Rodriguez D., Dodson S.T. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yanase T., Tanabe M., Nomiyama T. Transdisciplinary Approach for Sarcopenia. Appication of selective androgen receptor modulator to the therapy of sarcopenia. Clin Calcium. 2014;24:1501–1508. [PubMed] [Google Scholar]
- 246.Papanicolaou D.A., Ather S., Zhu H., Zhou Y., Lutkiewicz J., Scott B. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17:533–543. doi: 10.1007/s12603-013-0335-x. [DOI] [PubMed] [Google Scholar]
- 247.Riedmaier I., Tichopad A., Reiter M., Pfaffl M.W., Meyer H.H. Influence of testosterone and a novel SARM on gene expression in whole blood of Macaca fascicularis. J Steroid Biochem Mol Biol. 2009;114:167–173. doi: 10.1016/j.jsbmb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 248.Greising S.M., Baltgalvis K.A., Lowe D.A., Warren G.L. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Marjoribanks J., Farquhar C., Roberts H., Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD004143.pub4. P. 7. CD004143. [DOI] [PubMed] [Google Scholar]
- 250.Cody J.D., Jacobs M.L., Richardson K., Moehrer B., Hextall A. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2012;10:CD001405. doi: 10.1002/14651858.CD001405.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Investigators WGftWsHI Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 252.Wen H.-S., Li X., Cao Y.-Z., Zhang C.-C., Yang F., Shi Y.-M. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy. 2012;58:461–467. doi: 10.1159/000346446. [DOI] [PubMed] [Google Scholar]
- 253.Karcic E., Philpot C., Morley J. Treating malnutrition with megestrol acetate: literature review and review of our experience. J Nutr Health Aging. 2002;6:191–200. [PubMed] [Google Scholar]
- 254.Fragala M.S., Jajtner A.R., Beyer K.S., Townsend J.R., Emerson N.S., Scanlon T.C. Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle. 2014;5:139–148. doi: 10.1007/s13539-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Canonico M., Oger E., Plu-Bureau G., Conard J., Meyer G., Lévesque H. Hormone therapy and venous thromboembolism among postmenopausal women impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 256.Fournier A., Berrino F., Riboli E., Avenel V., Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448–454. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 257.Fournier A., Berrino F., Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kalbe C., Mau M., Wollenhaupt K., Rehfeldt C. Evidence for estrogen receptor α and β expression in skeletal muscle of pigs. Histochem Cell Biol. 2007;127:95–107. doi: 10.1007/s00418-006-0224-z. [DOI] [PubMed] [Google Scholar]
- 259.Copas P., Bukovsky A., Asbury B., Elder R.F., Caudle M.R. Estrogen, progesterone, and androgen receptor expression in levator ani muscle and fascia. J Womens Health Gend Based Med. 2001;10:785–795. doi: 10.1089/15246090152636541. [DOI] [PubMed] [Google Scholar]
- 260.Yin X.J., Wang G., Khan-Dawood F.S. Requirements of phosphatidylinositol-3 kinase and mammalian target of rapamycin for estrogen-induced proliferation in uterine leiomyoma- and myometrium-derived cell lines. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2006.09.037. 176.e1–5. [DOI] [PubMed] [Google Scholar]
- 261.Orr B., Grace O.C., Vanpoucke G., Ashley G.R., Thomson A.A. A role for notch signaling in stromal survival and differentiation during prostate development. Endocrinology. 2009;150:463–472. doi: 10.1210/en.2008-0383. [DOI] [PubMed] [Google Scholar]
- 262.Speir E., Yu Z.-X., Takeda K., Ferrans V.J., Cannon R.O. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation. 2000;102:2990–2996. doi: 10.1161/01.cir.102.24.2990. [DOI] [PubMed] [Google Scholar]
- 263.Pingel J., Langberg H., Skovgaard D., Koskinen S., Flyvbjerg A., Frystyk J. Effects of transdermal estrogen on collagen turnover at rest and in response to exercise in postmenopausal women. J Appl Physiol. 2012;113:1040–1047. doi: 10.1152/japplphysiol.01463.2011. [DOI] [PubMed] [Google Scholar]
- 264.Maret A., Clamens S., Delrieu I., Elhage R., Arnal J-Fo, Bayard F. Expression of the interleukin-6 gene is constitutive and not regulated by estrogen in rat vascular smooth muscle cells in culture. Endocrinology. 1999;140:2876–2882. doi: 10.1210/endo.140.6.6763. [DOI] [PubMed] [Google Scholar]
- 265.Kikuchi N., Urabe M., Iwasa K., Okubo T., Tsuchiya H., Hosoda T. Atheroprotective effect of estriol and estrone sulfate on human vascular smooth muscle cells. J Steroid Biochem Mol Biol. 2000;72:71–78. doi: 10.1016/s0960-0760(99)00149-1. [DOI] [PubMed] [Google Scholar]
- 266.Kvorning T., Andersen M., Brixen K., Schjerling P., Suetta C., Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol. 2007;578:579–593. doi: 10.1113/jphysiol.2006.122671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kawada S., Okuno M., Ishii N. Testosterone causes decrease in the content of skeletal muscle myostatin. Int J Sport Health Sci. 2006;4:44–48. [Google Scholar]