Abstract
Sarcopenia is the degenerative loss of muscle mass and function with aging. Recently sarcopenia was recognized as a clinical disease by the International Classification of Disease, 10th revision, Clinical Modification. An imbalance between protein synthesis and degradation causes a gradual loss of muscle mass, resulting in a decline of muscle function as a progress of sarcopenia. Many mechanisms involved in the onset of sarcopenia include age-related factors as well as activity-, disease-, and nutrition-related factors. The stage of sarcopenia reflecting the severity of conditions assists clinical management of sarcopenia. It is important that systemic descriptions of the disease conditions include age, sex, and other environmental risk factors as well as levels of physical function. To develop a new therapeutic intervention needed is the detailed understanding of molecular and cellular mechanisms by which apoptosis, autophagy, atrophy, and hypertrophy occur in the muscle stem cells, myotubes, and/or neuromuscular junction. The new strategy to managing sarcopenia will be signal-modulating small molecules, natural compounds, repurposing of old drugs, and muscle-specific microRNAs.
Keywords: Mechanisms, Therapy, Skeletal, Aging, Sarcopenia
1. Introduction to sarcopenia
The term sarcopenia was first described as an age-related decline in muscle mass using the cutting point of two standard deviations below the young adult mean [1]. The aging process represents the composition change of body components including skeletal muscle, fat, and bone mass. The osteoporosis in bone is strongly associated with the sarcopenia in skeletal muscle [2]. Moreover, according to the conditions related to musculoskeletal composition, the diseases have been precisely defined in terms such as sarcopenia, sarcopenic obesity, and osteosarcopenic obesity [3]. However, sarcopenia was considered as a description rather than a definition [4]. Sarcopenia was a reduced muscle mass with limited mobility, which excluded secondary conditions originated from a specific disease such as cancer [5]. It is an important step for the study and therapy of sarcopenia, since sarcopenia has been accepted as an independent disease by an International Classification of Disease, 10th revision, Clinical Modification code (M62.84) [6].
Although several definitions of clinical diagnosis of sarcopenia have been proposed [7], [8], [9], [10], [11], [12], [13], they still need to have worldwide consensus. The European Working Group on Sarcopenia in Older People (EWGSOP) has established a new clinical definition and developed consensus criteria for sarcopenia diagnosis [14]. The diagnostic method by EWGSOP uses three parameters including physical performance, muscle mass, and muscle strength. Recommended assessment methods for physical performance are 6-m usual gait speed (m/s). Muscle mass is measured by bioelectrical impedance analysis and dual energy X-ray absorptiometry, and is represented with relative appendicular skeletal mass/height (kg/m2). Muscle strength is measured by handgrip strength (kg). The harmful outcomes including mortality, the rate of falls, and incidence of hospitalization were the consequence of sarcopenia defined by EWGSOP [15].
Currently, sarcopenia biomarkers include interleukin-6, C-terminal agrin fragment, follistatin, and transforming growth factor beta (TGFβ) family members such as myostatin, activin A, growth and differentiation factor (GDF)-15, bone morphologic proteins, brain-derived neurotrophic factor, and irisin [16], [17], [18], [19], [20], [21], [22].
2. Signaling pathways and molecular targets for pharmacological intervention
Understanding the mechanisms in sarcopenia could help in designing intervention trials. The onset of sarcopenia may be the consequence of an imbalance between muscle protein synthesis and degradation, resulting in the skeletal muscle loss. The molecular and cellular mechanisms in sarcopenia include extrinsic changes in systemic environments and intrinsic changes within skeletal muscles [23]. The key molecules of signaling pathway in sarcopenia include Akt and Smad [24], [25] (Fig. 1). Insulin-like growth factor 1 (IGF-1)-PtdIns-3-OH kinase (PI3K)-Akt signaling is responsible for muscle protein synthesis [26]. Muscle loss can be indicated by the decreased level of positive regulators of muscle growth such as follistatin and irisin [18], [22], and/or increased level of negative regulators of muscle growth such as myostatin, activin A, and TGFβ [18], [20]. There are myriad factors involved in the symptoms of sarcopenia. Sarcopenia can be categorized into primary and secondary sarcopenia [14]. Primary sarcopenia has no other evident cause except aging, which include reduced sex hormones, apoptosis, and mitochondrial dysfunction. Secondary sarcopenia is related to activity (disuse, bed rest, and zero-gravity conditions), disease (failures or diseases in heart, lung, liver, kidney, brain, inflammatory, and endocrine), and nutrition (inadequate dietary intake of protein and energy).
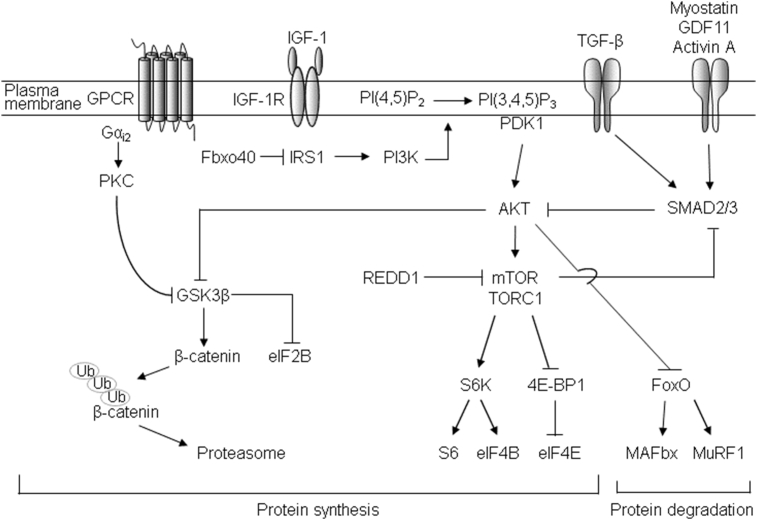
Fig. 1.
The signaling pathways involved in sarcopenia [24], [25]. Insulin/IGF-1 signaling positively regulates muscle mass by activation of Akt. IGF-1 activates Akt, and subsequently mTORC1 via IRS1-PI3K axis. mTORC1 induces protein synthesis by phosphorylating S6K and 4E-BP. Akt inhibits GSK3β, which in turn relieves inhibition onto the translation initiation factor eIF2B, resulting in increased protein synthesis. Akt also inhibits FOXO transcription factors, and thereby decreases the expression of MAFbx and MuRF1. Another E3 ligase, Fbxo40, ubiquitinates IRS1. Galpha-i2 pathway induces muscle hypertrophy in a PKC-dependent manner. The myostatin/GDF11/activin/TGFβ are negative muscle regulators. Activation of Smad proteins inhibits the Akt signal to FOXO-mediated expression of MAFbx and MuRF1. Akt-mTORC1 inhibits Smad 2,3 in a reciprocal manner. 4E-BP, eIF4E-binding protein; FOXO, Forkhead box O; GDF11, growth differentiation factor 11; GSK3β, glycogen synthase kinase 3 beta; IGF-1, insulin-like growth factor 1; IRS1, insulin receptor substrate 1; MAFbx, muscle atrophy Fbox; mTORC1, mammalian target of rapamycin complex 1; MuRF1, muscle ring finger 1; PI3K, PtdIns-3-OH kinase; S6K, S6 kinase 1.
IGF-PI3K-Akt signaling promotes skeletal myotube hypertrophy by activating mammalian target of rapamycin (mTOR) and by inactivating glycogen synthase kinase 3 (GSK3) [26]. mTOR subsequently phosphorylates the 70-kDa ribosomal protein S6 kinase, resulting in activating protein synthesis. Additionally, Akt inactivates GSK3β, which in turn enhances protein translation via the eIF-2B, while Akt inhibits protein degradation through Forkhead box O (FOXO)-mediated proteasome activity. The activation of mTOR complex 1 (mTORC1) in response to growth factors, feeding, and increased mechanical loading is a key step in inducing muscle hypertrophy by increasing protein synthesis [27].
Apoptosis is an indispensable process for maintaining tissue homeostasis in multicellular organisms. Apoptosis of satellite cells have contributed to the decline in muscle mass and function with aging [28]. Ablation of Nrf2, a redox-dependent transcription factor, leads to the activation of apoptosis pathways and decreased stem cell population, which result in impaired muscle regeneration in an oxidative stress condition [29]. The genetic deletion of peroxiredoxin 3, a major mitochondrial antioxidant enzyme, induces reactive oxygen species-mediated mitochondrial fragmentation and impaired mitochondrial membrane potential, leading to fatigue of muscle contraction in mice [30]. Denervation induces muscle atrophy through greater mitochondrial apoptotic susceptibility [31].
Proteolytic systems including calpain, proteasome, and lysosome are responsible for the majority of protein degradation in muscle cells. Among the muscle-specific proteins, desmin and dystrophin are susceptible to these protease activities, but alpha-actinin, tropomyosin, and filamin are relatively insensitive to these protease activities [32]. E3 ubiquitin ligases such as atrogin-1 and muscle ring finger-1 (MuRF-1) have been known to promote protein degradation in sarcopenia. Myostatin-linked molecules have also demonstrated to be abundant in sarcopenic muscles [33]. Increased protein degradation and decreased protein synthesis in sarcopenia is attributed to the activity of the ubiquitin-proteasome system interconnected with autophagy [34].
Autophagy is a self-destructive mechanism by which cells remove unnecessary components from themselves in order to promote survival. Muscle-specific deletion of a major autophagy gene, Atg7, shows sarcopenia phenotype, suggesting autophagy plays a role in the maintenance of muscle mass and strength by removing abnormal mitochondria and inclusions [35]. A recent study has shown that autophagy maintains muscle mass as well as its function during muscle aging. Boosting autophagy has prevented from age-related muscle dysfunction by enhancing the selective degradation of misfolded proteins and dysfunctional organelles [36]. The presence of insoluble protein aggregates in aged muscle may be due to the autophagic changes or defective autophagy signaling in aged skeletal muscles [37]. Sustained activation of mTORC1 in skeletal muscle cells reduces autophagy activity and leads to the accumulation of protein aggregates and myopathy [38]. Defective autophagy in aged satellite cells causes the cell cycle to exit into senescence, which consequently decreases the number and function of satellite cells. The cell cycle re-entering by re-establishment of autophagic activity can rejuvenate the aged satellite cells to some extent [39].
The function of neuromuscular system gradually deteriorates with age. Aged neuromuscular junctions exhibit elevated branches in presynaptic nerve terminals and increased distribution of receptor sites for neurotransmitters in the postsynaptic terminals [40]. Neuromuscular junctions deteriorate morphologically and show altered features in functional components therein, such as nicotinic acetylcholine receptor and agrin upon sarcopenia [41]. Low-density lipoprotein receptor-related protein 4 has acted bidirectionally and regulates synapse formation by forming a complex with muscle and skeletal receptor tyrosine-protein kinase (MuSK), binding agrin, and activating MuSK activity, thus leading to postsynaptic differentiation, while by functioning as a muscle-derived retrograde signals for the differentiation and stabilization of motor nerve terminals [42]. Loss of muscle strength is more relevant than loss of muscle mass in sarcopenia. Although loss of muscle mass contributes to the loss of muscle strength in older people, this loss of muscle strength precedes the associated loss of muscle mass. There are therapeutic concerns about maintaining or increasing muscle mass regardless of improvement in muscle strength [43].
Ca2+ signaling molecules have been associated with age-dependent muscle degeneration. In aged muscle, decreased expression of mitsugumin-29 induces abnormal interaction of dihydropyridine receptor with ryanodine receptor 1 (RyR1), which leads to compromised Ca2+ spark signaling [44]. Among the typical Ca2+ channels in muscles, RyR1 from aged mice is oxidized and cysteine-nitrosylated, resulting in leaky channels with increased open probability, which causes muscle weakness [45]. In addition, inositol 1,4,5-trisphosphate receptor expression was dramatically repressed in aged myoblasts, resulting in undetectable Ca2+ oscillation, which in turn modulated myogenic transcription factor such as myogenin. Thus, perturbation of Ca2+ homeostasis in aged muscle deteriorates not only excitation-contraction coupling but also myogenic potential, resulting in sarcopenia [46].
Satellite cells, skeletal muscle stem cells, are quiescent myogenic precursors found in the adult muscle between the basal lamina and the sarcolemma. Sarcopenia is developed by unbalanced protein synthesis and degradation as well as dysfunction of satellite cells. Studies investigating an age-related content of satellite cell contents in soleus muscle have revealed that the quality rather than quantity of satellite cells may be responsible for sarcopenia [47]. With aging, skeletal muscles lose their regenerative potential, in part due to deficiencies in satellite cells. The level of Smad4 proteins in satellite cells increases with aging, and suggestively restricts satellite cell amplification to enhance satellite cell differentiation during muscle regeneration [48]. Satellite cells lose their regenerative potential and self-renewal capacity by loss of their normal quiescent state with age. Derepression of p16(INK4) in aged satellite cells causes the conversion from the reversible quiescence state to a senescence state. Silencing of p16(INK4) restores the satellite cell self-renewal and muscle regenerative potential in aged muscle [49]. IGF-I could enhance aged muscle regrowth by decreasing the cell cycle inhibitor, p27Kip1, in satellite cells through the PI3K/Akt signaling axis [50]. The changes in the environment of satellite cells (niche), attributed by the neighboring diverse cell types such as immune cells, fibroblasts, capillary cells, during disuse and exercise play an important role in self-renewal and regenerative potential of satellite cells [51].
3. Possible therapeutic intervention strategies
Sex hormones are required to maintain the muscle mass and strength [52], [53]. Transdermal testosterone replacement has improved muscle strength and body compositions in hypogonadal men [54]. Meta-analyses of clinical trials provide evidence that testosterone treatment increases the skeletal muscle mass and also muscle strength to some degree [55]. Although the lack of significant changes in serum levels of prostate specific antigen has been demonstrated in testosterone administration, testosterone therapy has the concern of side effects involved in prostate [56]. An efficacious administration paradigm has proposed that testosterone replacement effects are fiber-type dependent, restricted to increases in cell size, and dependent on the treatment schedule [57]. In vitro studies have revealed that androgen increases local expression of IGF-1 levels [58] and inhibits FOXO, and activates both p38 mitogen-activated protein kinases and peroxisome proliferator-activated receptor-gamma coactivator 1 alpha [59]. Testosterone supplementation has reversed sarcopenia through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways [60].
Androgen has protective effects in skeletal muscle catabolism by inhibition of nuclear factor-κB-inducing kinase (NIK) accumulation [61]. In this report, the increase of NIK expression induced by glucocorticoid in skeletal muscle cells was prevented in the presence of testosterone. Selective androgen receptor modulators have been claimed to be beneficial for the treatment of sarcopenia [62]. Several reviews have described the therapeutic potential of selective androgen receptor modulators for skeletal muscle wasting [63], [64], [65], [66].
Myostatin (growth differentiation factor-8, GDF8), a member of TGFβ superfamily, is a negative regulator of myogenesis, because the absence of myostatin increased muscle size in mice and cattle [67]. GDF11 is highly homologous to myostatin, both mediate downstream signaling via activin receptor and Smad complex [68]. It is known that follistatin binds and neutralizes activin, myostatin, and GDF11 [69]. However, the function of GDF11 in skeletal muscle is controversial. GDF11 was identified as a circulating rejuvenating factor from heterochronic parabiosis mice [70]. Recently the observation has revealed that GDF11 and myostatin both inhibit myoblast differentiation, GDF11 significantly inhibits muscle regeneration and decreased satellite cell expansion in mice [71]. GDF11 treatment has resulted in a significant increase in tissue fibrosis, accompanied by attenuated functional recovery in a complex rat model of skeletal muscle injury that mimics physiological injuries seen in human patients [72]. The considerable attention focused on the blockade of activin receptor signaling for the suppression of skeletal muscle loss by soluble receptors and monoclonal antibodies [73], [74], [75]. Small-molecule screening methods have been developed for inhibitors of cellular response to myostatin and activin A [76].
Satellite cells isolated from old human and rodents have more apoptotic signals than youngers [77]. The reduced antioxidant activity of catalase and glutathione transferase in satellite cells isolated from the elderly individuals has been observed compared to those in cells derived from young people [78]. It is suggested that this decrease in the antioxidant capacity may reduce the regenerative ability of aged satellite cells. Green tea extract, a nutraceutical, increased satellite cell proliferation and differentiation, and decreased oxidative stress in aged rat [79]. Epigallocatechin-3-gallate supplementation reduced the apoptotic index and proapoptotic proteins, and improved muscle recovery after the atrophic stimulus [80]. However it is unlikely that nutraceuticals impact of green tea are restricted in satellite cell function including apoptosis. Epigallocatechin-3-gallate has protected sarcopenic muscles, in part through suppressing protein degradation and the ubiquitin-proteasome pathway, together with increased expression of anabolic factors [81]. Many mammalian tissues, especially in skeletal muscle contain taurine as a natural amino acid [82]. The involvement and therapeutic potential of taurine have been discussed in pathophysiological conditions and skeletal muscle myopathy [83]. The screening systems to discover new therapeutic molecules and to evaluate compounds involved in satellite cell proliferation and fusion using human and mouse primary myoblast as well as mouse C2C12 cells are summarized in Table 1 [84], [85], [86], [87], [88], [89], [90].
Table 1.
The screening systems to discover and evaluate enhancing compounds in myogenesis.
| Gene & reporter | Cells | Reference |
|---|---|---|
| Viability/ATPlite (luciferase) | Mouse, myoblast | Kang et al. [84] |
| Myogenesis (phenotype evaluation) | Human, satellite | Nierobisz et al. [85] |
| Myogenesis (state-selective fluorophore) | Mouse, C2C12 | Wagner et al. [86] |
| Mitochodrial biogenesis (Tfam-luciferase) | Mouse, C2C12 | Yoshino et al. [87] |
| Myofusion index | Mouse, C2C12 | Yang et al. [88] |
| Myotube fusion rate (2 fragment of GFP) | Mouse, C2C12 | Kodaka et al. [89] |
| E-box and MCK (luciferase, GFP) | Mouse, C2C12 | Ozturk-Kaloglu et al. [90] |
| Myogenesis (eMHC, InCell Elisa) | Mouse, myoblast/C2C12 | Park SS unpublished |
The results from a randomized controlled trial of angiotensin-converting enzyme (ACE) inhibitors on physical function involving 130 older patients with impairment of daily activities suggest a beneficial effect of ACE inhibitors in sarcopenia [91]. In a recent study, leprosy survivors who had taken 4,4′-diamino-diphenyl sulfone showed greater skeletal muscle mass and strength than those who had not taken the drug [92]. This result suggests that drug repurposing is a new strategy for the therapeutic approach of sarcopenia.
The finding that the elimination of Dicer activity in the myogenic compartment during embryogenesis display decreased skeletal muscle mass accompanied by abnormal myofiber morphology has demonstrated the crucial roles for microRNAs (miRNAs) as critical components required for myogenesis [93]. Expression profiling analysis of miRNAs and messenger RNAs revealed the contribution of miRNAs to muscle aging through various fields such as transcription, metabolic process, and kinase activity [94]. The differential expression with aging in mouse skeletal muscle has shown that 15 miRNAs are up-regulated and 19 miRNAs are down-regulated in total 34 miRNAs including miR-206 and miR-434 [94]. In myoblasts, 118 miRNAs were differentially expressed (47 up- and 71 down-regulated) [95]. Comparative analysis and validation studies have revealed that miR-455-3p was significantly decreased in muscle of atrophy model, whereas miR-434-3p was decreased in serum [96]. In the comparative expression analysis of miRNAs between young and aged mouse muscles showed that miR-431, a novel age-associated miRNA, modulates the skeletal myogenesis via regulation of Smad4 expression [95]. The finding that the overexpression of miR-206 in Duchenne muscular dystrophy mouse muscle increased the levels of several muscle-specific proteins has expected to provide a therapeutic potential of miRNAs for Duchenne muscular dystrophy [97]. Chemicals, antibodies, and food supplements are also in clinical trials (Table 2).
Table 2.
Trends in drug development for sarcopenia.
| Company or institute | Brand name | Component | Clinical trial |
|---|---|---|---|
| Abbott Nutrition | AN777 | Medical food mixture | Phase III |
| Merck Sharp & Dohme | MK-677 | GH releasing peptide | Phase III |
| Merck Sharp & Dohme | MK-0773 | Anabolic steroid | Phase II |
| Novartis | BYM338 (Bimagrumab) | Antibody (ActRIIB) | Phase II |
| Sanofi | REGN1033 (SAR391786) | Antibody (myostatin) | Phase II |
| Takeda Pharmaceuticals | Pioglitazone (Actos) | PPAR-γ agonist | Phase IV |
| Johns Hopkins University | Losartan | AT2R antagonist | Phase II |
| Mayo Clinic | Omega-3 | Unsaturated fatty acids | Phase I |
| National Institute on Aging | Anastrozole | Estrogen synthesis inhibition | Phase II |
| Seoul National University | Cetylpyridinium chloride | Cationic ammonium compound | Investigator trials |
| University of Colorado | Acetaminophen | NSAID | Investigator trials |
| University of Pennsylvania | Ghrelin | Hunger hormone | Phase II |
| Washington University | Dehydroepiandrosterone | Androgen precursor | Phase III |
ActRIIB, active receptor type IIB; AT2R, angiotensin II receptor; GH, growth hormone; NSAID, nonsteroidal anti-inflammatory drug; PPAR-γ, peroxisome proliferator-activated receptor-gamma.
Adapted from https://clinicaltrials.gov/.
The lifecourse approach to sarcopenia is an interesting new strategy. This approach proposed that the conditions and environments in early life influences on the muscle mass and function in later life at a molecular or cellular level [98]. A recent report based on the epidemiology of the 3 distinct physiological components of sarcopenia focuses on the similarities and differences between their patterns of variation with age, sex, geography, time, and the individual risk factors that cluster selectively with muscle mass, strength, and physical function [99].
4. Conclusions
Sarcopenia is well-defined as the gradual loss of muscle mass with aging due to unbalanced protein synthesis and degradation, which leads to a decline in muscle function. Multifactorial consequence of aging including chronic inflammation, neuromuscular junction dysfunction, and degenerative diseases contributes to the onset of sarcopenia. The detailed understanding of the molecular and cellular mechanisms in sarcopenia from the cell-based analysis and human/animal studies will shed lights on the developing the new therapeutic interventions. The new strategy to understanding and management of sarcopenia includes systemic approach to sarcopenia, signal modulating small molecules, drug repositioning (drug repurposing, new tricks for old drugs), and new finding of muscle-specific miRNAs.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by Bio & Medical Technology Development Program (2013M3A9B6076413, Ki-Sun Kwon) of the NRF granted by MSIP and KRIBB Research Initiative Program of South Korea.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Rosenberg I.H. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 2.Sjoblom S., Suuronen J., Rikkonen T., Honkanen R., Kroger H., Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–180. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Ilich J.Z., Kelly O.J., Inglis J.E., Panton L.B., Duque G., Ormsbee M.J. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15:51–60. doi: 10.1016/j.arr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Alchin D.R. Sarcopenia: describing rather than defining a condition. J Cachexia Sarcopenia Muscle. 2014;5:265–268. doi: 10.1007/s13539-014-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morley J.E., Abbatecola A.M., Argiles J.M., Baracos V., Bauer J., Bhasin S. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao L., Morley J.E. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc. 2016;17:675–677. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Muscaritoli M., Anker S.D., Argiles J., Aversa Z., Bauer J.M., Biolo G. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dam T.T., Peters K.W., Fragala M., Cawthon P.M., Harris T.B., McLean R. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 12.Cooper C., Dere W., Evans W., Kanis J.A., Rizzoli R., Sayer A.A. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–1848. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaudart C., Zaaria M., Pasleau F., Reginster J.Y., Bruyere O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srdic D., Plestina S., Sverko-Peternac A., Nikolac N., Simundic A.M., Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24:4495–4502. doi: 10.1007/s00520-016-3287-y. [DOI] [PubMed] [Google Scholar]
- 17.Landi F., Calvani R., Lorenzi M., Martone A.M., Tosato M., Drey M. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol. 2016;79:31–36. doi: 10.1016/j.exger.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann M., Halper B., Oesen S., Franzke B., Stuparits P., Tschan H. Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol. 2015;64:35–45. doi: 10.1016/j.exger.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Parise G., Snijders T. Myostatin inhibition for treatment of sarcopenia. Lancet Diabetes Endocrinol. 2015;3:917–918. doi: 10.1016/S2213-8587(15)00324-1. [DOI] [PubMed] [Google Scholar]
- 20.Scimeca M., Piccirilli E., Mastrangeli F., Rao C., Feola M., Orlandi A. Bone morphogenetic proteins and myostatin pathways: key mediator of human sarcopenia. J Transl Med. 2017;15:34. doi: 10.1186/s12967-017-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greising S.M., Ermilov L.G., Sieck G.C., Mantilla C.B. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015;593:431–440. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang J.S., Kim T.H., Nguyen T.T., Park K.S., Kim N., Kong I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int. 2017 doi: 10.1111/ggi.13030. [DOI] [PubMed] [Google Scholar]
- 23.Ryall J.G., Schertzer J.D., Lynch G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 24.Hanaoka B.Y., Peterson C.A., Horbinski C., Crofford L.J. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat Rev Rheumatol. 2012;8:448–457. doi: 10.1038/nrrheum.2012.85. [DOI] [PubMed] [Google Scholar]
- 25.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 27.Drummond M.J., Fry C.S., Glynn E.L., Dreyer H.C., Dhanani S., Timmerman K.L. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., Listrat A., Meunier B., Gueugneau M., Coudy-Gandilhon C., Combaret L. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell. 2014;13:254–262. doi: 10.1111/acel.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasimhan M., Hong J., Atieno N., Muthusamy V.R., Davidson C.J., Abu-Rmaileh N. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic Biol Med. 2014;71:402–414. doi: 10.1016/j.freeradbiomed.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K.P., Shin Y.J., Cho S.C., Lee S.M., Bahn Y.J., Kim J.Y. Peroxiredoxin 3 has a crucial role in the contractile function of skeletal muscle by regulating mitochondrial homeostasis. Free Radic Biol Med. 2014;77:298–306. doi: 10.1016/j.freeradbiomed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Adhihetty P.J., O'Leary M.F., Chabi B., Wicks K.L., Hood D.A. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- 32.Purintrapiban J., Wang M.C., Forsberg N.E. Degradation of sarcomeric and cytoskeletal proteins in cultured skeletal muscle cells. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:393–401. doi: 10.1016/s1096-4959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 33.Sakuma K., Aoi W., Yamaguchi A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. 2017;469:573–591. doi: 10.1007/s00424-016-1933-3. [DOI] [PubMed] [Google Scholar]
- 34.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Jiao J., Demontis F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol. 2017;34:1–6. doi: 10.1016/j.coph.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Wohlgemuth S.E., Seo A.Y., Marzetti E., Lees H.A., Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castets P., Lin S., Rion N., Di Fulvio S., Romanino K., Guridi M. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013;17:731–744. doi: 10.1016/j.cmet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Prat L., Martinez-Vicente M., Perdiguero E., Ortet L., Rodriguez-Ubreva J., Rebollo E. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 40.Deschenes M.R. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4:209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- 41.Rudolf R., Khan M.M., Labeit S., Deschenes M.R. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yumoto N., Kim N., Burden S.J. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489:438–442. doi: 10.1038/nature11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 44.Weisleder N., Brotto M., Komazaki S., Pan Z., Zhao X., Nosek T. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. 2006;174:639–645. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson D.C., Betzenhauser M.J., Reiken S., Meli A.C., Umanskaya A., Xie W. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi J.Y., Hwang C.Y., Lee B., Lee S.M., Bahn Y.J., Lee K.P. Age-associated repression of type 1 inositol 1, 4, 5-triphosphate receptor impairs muscle regeneration. Aging. 2016;8:2062–2080. doi: 10.18632/aging.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks N.E., Schuenke M.D., Hikida R.S. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J Physiol Sci. 2009;59:465–471. doi: 10.1007/s12576-009-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paris N.D., Soroka A., Klose A., Liu W., Chakkalakal J.V. Smad4 restricts differentiation to promote expansion of satellite cell derived progenitors during skeletal muscle regeneration. Elife. 2016;5 doi: 10.7554/eLife.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sousa-Victor P., Gutarra S., Garcia-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 50.Chakravarthy M.V., Booth F.W., Spangenburg E.E. The molecular responses of skeletal muscle satellite cells to continuous expression of IGF-1: implications for the rescue of induced muscular atrophy in aged rats. Int J Sport Nutr Exerc Metab. 2001;11(S44–8) doi: 10.1123/ijsnem.11.s1.s44. [DOI] [PubMed] [Google Scholar]
- 51.Bentzinger C.F., Wang Y.X., Dumont N.A., Rudnicki M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14:1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.La Colla A., Pronsato L., Milanesi L., Vasconsuelo A. 17beta-Estradiol and testosterone in sarcopenia: role of satellite cells. Ageing Res Rev. 2015;24:166–177. doi: 10.1016/j.arr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein J.S., Lee H., Burnett-Bowie S.A., Pallais J.C., Yu E.W., Borges L.F. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Swerdloff R.S., Iranmanesh A., Dobs A., Snyder P.J., Cunningham G. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 55.Starka L. Testosterone treatment of sarcopenia. Vnitr Lek. 2006;52:909–911. [PubMed] [Google Scholar]
- 56.Bhasin S., Tenover J.S. Age-associated sarcopenia–issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab. 1997;82:1659–1660. doi: 10.1210/jcem.82.6.4061. [DOI] [PubMed] [Google Scholar]
- 57.Fitts R.H., Peters J.R., Dillon E.L., Durham W.J., Sheffield-Moore M., Urban R.J. Weekly versus monthly testosterone administration on fast and slow skeletal muscle fibers in older adult males. J Clin Endocrinol Metab. 2015;100:E223–E231. doi: 10.1210/jc.2014-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sculthorpe N., Solomon A.M., Sinanan A.C., Bouloux P.M., Grace F., Lewis M.P. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med Sci Sports Exerc. 2012;44:610–615. doi: 10.1249/MSS.0b013e318237c5c0. [DOI] [PubMed] [Google Scholar]
- 59.Qin W., Pan J., Wu Y., Bauman W.A., Cardozo C. Protection against dexamethasone-induced muscle atrophy is related to modulation by testosterone of FOXO1 and PGC-1alpha. Biochem Biophys Res Commun. 2010;403:473–478. doi: 10.1016/j.bbrc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 60.Kovacheva E.L., Hikim A.P., Shen R., Sinha I., Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urban R.J., Dillon E.L., Choudhary S., Zhao Y., Horstman A.M., Tilton R.G. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc. 2014;125:27–42. [PMC free article] [PubMed] [Google Scholar]
- 62.Page S.T., Marck B.T., Tolliver J.M., Matsumoto A.M. Tissue selectivity of the anabolic steroid, 19-nor-4-androstenediol-3beta,17beta-diol in male Sprague Dawley rats: selective stimulation of muscle mass and bone mineral density relative to prostate mass. Endocrinology. 2008;149:1987–1993. doi: 10.1210/en.2007-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi S.M., Lee B.M. Comparative safety evaluation of selective androgen receptor modulators and anabolic androgenic steroids. Expert Opin Drug Saf. 2015;14:1773–1785. doi: 10.1517/14740338.2015.1094052. [DOI] [PubMed] [Google Scholar]
- 64.Dalton J.T., Taylor R.P., Mohler M.L., Steiner M.S. Selective androgen receptor modulators for the prevention and treatment of muscle wasting associated with cancer. Curr Opin Support Palliat Care. 2013;7:345–351. doi: 10.1097/SPC.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 65.Narayanan R., Mohler M.L., Bohl C.E., Miller D.D., Dalton J.T. Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal. 2008;6 doi: 10.1621/nrs.06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toth M. Myoanabolic steroids and selective androgen receptor modulators: mechanism of action and perspectives. Orv Hetil. 2009;150:2051–2059. doi: 10.1556/OH.2009.28739. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.J. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh S.P., Yeo C.Y., Lee Y., Schrewe H., Whitman M., Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749–2754. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneyer A.L., Sidis Y., Gulati A., Sun J.L., Keutmann H., Krasney P.A. Differential antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology. 2008;149:4589–4595. doi: 10.1210/en.2008-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loffredo F.S., Steinhauser M.L., Jay S.M., Gannon J., Pancoast J.R., Yalamanchi P. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egerman M.A., Cadena S.M., Gilbert J.A., Meyer A., Nelson H.N., Swalley S.E. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y., Sharma N., Dukes D., Myzithras M.B., Gupta P., Khalil A. GDF11 treatment attenuates the recovery of skeletal muscle function after injury in older rats. AAPS J. 2017;19:431–437. doi: 10.1208/s12248-016-0024-x. [DOI] [PubMed] [Google Scholar]
- 73.Cadena S.M., Tomkinson K.N., Monnell T.E., Spaits M.S., Kumar R., Underwood K.W. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J Appl Physiol. 2010;109:635–642. doi: 10.1152/japplphysiol.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fakhfakh R., Lee S.J., Tremblay J.P. Administration of a soluble activin type IIB receptor promotes the transplantation of human myoblasts in dystrophic mice. Cell Transpl. 2012;21:1419–1430. doi: 10.3727/096368911X627480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner K.R., Fleckenstein J.L., Amato A.A., Barohn R.J., Bushby K., Escolar D.M. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 76.Cash J.N., Angerman E.B., Kirby R.J., Merck L., Seibel W.L., Wortman M.D. Development of a small-molecule screening method for inhibitors of cellular response to myostatin and activin A. J Biomol Screen. 2013;18:837–844. doi: 10.1177/1087057113482585. [DOI] [PubMed] [Google Scholar]
- 77.Fulle S., Centurione L., Mancinelli R., Sancilio S., Manzoli F.A., Di Pietro R. Stem cell ageing and apoptosis. Curr Pharm Des. 2012;18:1694–1717. doi: 10.2174/138161212799859657. [DOI] [PubMed] [Google Scholar]
- 78.Fulle S., Di Donna S., Puglielli C., Pietrangelo T., Beccafico S., Bellomo R. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40:189–197. doi: 10.1016/j.exger.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Alway S.E., Bennett B.T., Wilson J.C., Sperringer J., Mohamed J.S., Edens N.K. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J Appl Physiol. 2015;118:319–330. doi: 10.1152/japplphysiol.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alway S.E., Bennett B.T., Wilson J.C., Edens N.K., Pereira S.L. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp Gerontol. 2014;50:82–94. doi: 10.1016/j.exger.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meador B.M., Mirza K.A., Tian M., Skelding M.B., Reaves L.A., Edens N.K. The green tea polyphenol epigallocatechin-3-gallate (EGCg) attenuates skeletal muscle atrophy in a rat model of sarcopenia. J Frailty Aging. 2015;4:209–215. doi: 10.14283/jfa.2015.58. [DOI] [PubMed] [Google Scholar]
- 82.Schaffer S.W., Jong C.J., Ramila K.C., Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17(1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Luca A., Pierno S., Camerino D.C. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang Y., Tierney M., Ong E., Zhang L., Piermarocchi C., Sacco A. Combinations of kinase inhibitors protecting myoblasts against hypoxia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nierobisz L.S., Cheatham B., Buehrer B.M., Sexton J.Z. High-content screening of human primary muscle satellite cells for new therapies for muscular atrophy/dystrophy. Curr Chem Genom Transl Med. 2013;7:21–29. doi: 10.2174/2213988501307010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner B.K., Carrinski H.A., Ahn Y.H., Kim Y.K., Gilbert T.J., Fomina D.A. Small-molecule fluorophores to detect cell-state switching in the context of high-throughput screening. J Am Chem Soc. 2008;130:4208–4209. doi: 10.1021/ja077656d. [DOI] [PubMed] [Google Scholar]
- 87.Yoshino M., Naka A., Sakamoto Y., Shibasaki A., Toh M., Tsukamoto S. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J Nutr Biochem. 2015;26:1193–1199. doi: 10.1016/j.jnutbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Yang Z., Nakagawa K., Sarkar A., Maruyama J., Iwasa H., Bao Y. Screening with a novel cell-based assay for TAZ activators identifies a compound that enhances myogenesis in C2C12 cells and facilitates muscle repair in a muscle injury model. Mol Cell Biol. 2014;34:1607–1621. doi: 10.1128/MCB.01346-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kodaka M., Yang Z., Nakagawa K., Maruyama J., Xu X., Sarkar A. A new cell-based assay to evaluate myogenesis in mouse myoblast C2C12 cells. Exp Cell Res. 2015;336:171–181. doi: 10.1016/j.yexcr.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Ozturk-Kaloglu D., Hercher D., Heher P., Posa-Markaryan K., Sperger S., Zimmermann A. A Noninvasive in vitro monitoring system reporting skeletal muscle differentiation. Tissue Eng Part C Methods. 2017;23:1–11. doi: 10.1089/ten.TEC.2016.0366. [DOI] [PubMed] [Google Scholar]
- 91.Sumukadas D., Witham M.D., Struthers A.D., McMurdo M.E. Ace inhibitors as a therapy for sarcopenia - evidence and possible mechanisms. J Nutr Health Aging. 2008;12:480–485. doi: 10.1007/BF02982709. [DOI] [PubMed] [Google Scholar]
- 92.Lee S.Y., Kim W., Park H.W., Park S.C., Kim I.K., Chung S.G. Anti-sarcopenic effects of diamino-diphenyl sulfone observed in elderly female leprosy survivors: a cross-sectional study. J Cachexia Sarcopenia Muscle. 2016;7:322–329. doi: 10.1002/jcsm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Rourke J.R., Georges S.A., Seay H.R., Tapscott S.J., McManus M.T., Goldhamer D.J. Essential role for Dicer during skeletal muscle development. Dev Biol. 2007;311:359–368. doi: 10.1016/j.ydbio.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J.Y., Park Y.K., Lee K.P., Lee S.M., Kang T.W., Kim H.J. Genome-wide profiling of the microRNA-mRNA regulatory network in skeletal muscle with aging. Aging. 2014;6:524–544. doi: 10.18632/aging.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee K.P., Shin Y.J., Panda A.C., Abdelmohsen K., Kim J.Y., Lee S.M. miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4. Genes Dev. 2015;29:1605–1617. doi: 10.1101/gad.263574.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jung H.J., Lee K.P., Milholland B., Shin Y.J., Kang J.S., Kwon K.S. Comprehensive miRNA profiling of skeletal muscle and serum in induced and normal mouse muscle atrophy during aging. J Gerontol A Biol Sci Med Sci. 2017 Mar 10 doi: 10.1093/gerona/glx025. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amirouche A., Jahnke V.E., Lunde J.A., Koulmann N., Freyssenet D.G., Jasmin B.J. Muscle-specific microRNA-206 targets multiple components in dystrophic skeletal muscle representing beneficial adaptations. Am J Physiol Cell Physiol. 2017;312:C209–C221. doi: 10.1152/ajpcell.00185.2016. [DOI] [PubMed] [Google Scholar]
- 98.Sayer A.A., Syddall H., Martin H., Patel H., Baylis D., Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–432. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shaw S.C., Dennison E.M., Cooper C. Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcif Tissue Int. 2017;101:229–247. doi: 10.1007/s00223-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]