Abstract
Objectives
Osteoporosis and fracture impose a significant health care burden on the contemporary populations in developing countries. The Vietnam Osteoporosis Study (VOS) sought to assess the burden of osteoporosis and its comorbidities in men and women.
Methods
The study was designed as a population-based family investigation in which families were randomly recruited from Ho Chi Minh City, Vietnam. Individuals were assessed for bone health, including bone mineral density (BMD) and body composition and trabecular and cortical bone properties by pQCT (peripheral quantitative computed tomography). Fasting blood samples were obtained for the analysis of plasma glucose, glycosylated hemoglobin, and bone turnover markers. Genomic DNA extraction from whole blood samples for further genetic and genomic analyses.
Results
We have recruited more than 4157 individuals from 817 families. The average age of participants was 51, with approximately 45% of the individuals aged 50 years and older. Approximately 3% of participants were obese (body mass index ≥ 30 kg/m2), and 21% were overweight. Notably, 11% of participants aged 40 years and older were diabetic. Among those aged 50 years and older, approximately 14% of women and 5% of men had osteoporosis (i.e., femoral neck BMD T-scores ≤ −2.5). There were modest correlations between volumetric BMD and areal BMD.
Conclusions
VOS is a major bone research project in Vietnam aimed at comprehensively documenting the burden osteoporosis, its co-occurrence of chronic diseases, and their underlying etiologies. The Study will make important contributions to the literature of bone health worldwide.
Keywords: Osteoporosis, Sarcopenia, Muscle strength, Bone density, Peripheral quantitative computed tomography, Comorbidity
1. Introduction
Osteoporosis and its consequence of fragility are increasingly recognized as a major public health burden in contemporary populations. It is highly prevalent among the elderly of both genders. In the Caucasian populations, the lifetime risk of fracture is about 50% for women and 30% for men [1]. In women, the lifetime risk of hip fracture is higher than the lifetime risk of having invasive breast cancer [2]. Moreover, for women aged 50 years, their risk of death related to a hip fracture is equivalent to their risk of death from breast cancer and 4 times greater than that from endometrial cancer [3]. Currently, due to lack of prospective studies, no data on the lifetime risk of fracture in Asian populations are available. However, it is expected that with the ongoing increase in ageing in Asian populations, the burden of osteoporosis is expected to increase in the near future.
Another important aspect of osteoporosis is that patients with a pre-existing fracture are at increased risk of refractures [4] and mortality [5]. Indeed, among individuals with an incident fracture, the risk of a refracture is increased by 30%–40% within 3 years [5]. However, more disturbingly, fracture is associated with reduced life expectancy or increased risk of premature mortality, with men having higher risk of death than women [6]. The 5-year postfracture cumulative survival probability for men and women were 48% and 59%, respectively. Approximately one-fifth of patients with hip fracture die within 12 months after the event [7], and the 5-year cumulative survival probability was 63%. These data collectively indicate that osteoporotic fracture, particularly hip fracture, is a serious condition because it is associated with increased risk of death, a fact that is not well known within the primary care community.
Vietnam, a developing country in Southeast Asia, is an ideal setting for studying osteoporosis. The country has a population of 92 million, with per capita gross domestic product being ∼2000 United States dollar. The population is undergone ageing, with increase in the proportion of people aged 60 years and above. The country is in the stage of rapid urbanization with significant changes in lifestyle factors. Thus, there is an increasing interest in the epidemiology of osteoporosis in Vietnam. In a recent study in Vietnam, we have estimated that approximately 29% of women aged 50 + years had osteoporosis [8] and about 28% of them have vertebral fractures [9]. These data show that the magnitude of osteoporosis in Vietnam is similar to that in other countries in Asia and in the West.
Osteoporosis has a complex pathophysiology. The susceptibility to osteoporosis and its consequence of fracture is determined by factors related to hormone, lifestyle, environmental exposures, and genetics. Osteoporotic patients usually have comorbidities that are mostly associated with osteoporosis such as obesity, diabetes, cardiovascular disease, metabolic syndrome and osteoarthritis. These complex noncommunicable diseases (NCDs) are characterized by deterioration in multiple dimensions also related to lifestyle, genetics and hormones. Thus, osteoporosis and these chronic diseases are linked in a networking manner through the genetic factors that can be referred to as “diseasome.” In the Wnt pathway alone, on average each disease shared ∼15 neighbors with each other disease, indicating high comorbidity within the network [10].
Vietnam has also undergone remarkable changes in disease burdens parallel with the economic development. Indeed, the prevalence of NCDs is rapidly increasing. According to a recent report, NCDs collectively account for 71% of total burden of disease in Vietnam, including 60% of all-cause deaths [11]. Compared with two decades ago, the disease burden attributable to NCDs represents a 30% increase. As a result, osteoporotic fracture, which is usually associated with comorbidity NCDs, has an increase in mortality risk. However, the burden of and risk factors for osteoporotic fractures and comorbidity NCDs have not been well documented in Vietnam and a complete map of interassociations between chronic morbidities and osteoporosis has not been developed.
The hypothesis underlying the present study is that osteoporosis and its multimorbidity NCDs share genes, their encoded proteins and pathways that stratify patients in subgroups and their tendency to co-occur together. The overall goal of VOS is to map genetic and environmental factors that underlie the risk of fragility fracture and the co-occurrence of related chronic diseases (i.e., the so-called “diseasome”) in the Vietnamese people.
We pursue the following specific aims: (1) to assess the skeletal burden and its associated morbidities in the general population. We aim to estimate the prevalence of osteoporosis, osteoarthritis and multimorbidity, the incidence of fragility fractures and osteoarthritis in men and women by age group; (2) to determine the extent to which variation in osteoporosis, fracture and osteoarthritis susceptibility is determined by genetic factors. We also aim to identify genetic variants that are associated with osteoporosis, fracture and osteoarthritis; (3) to understand the interplay between genes and environmental factors in the determination of the association between osteoporosis and other chronic diseases, NCDs including osteoarthritis, sarcopenia, obesity, diabetes, metabolic syndrome, and cardiovascular disease.
This project will contribute significant new information on the genetic and environmental bases of osteoporosis and related chronic diseases. The project will also contribute to the knowledge on the specific genes that underline the between subject variation in skeletal parameters.
2. Methods
2.1. Study design
The study is designed as a population based family study. Participants were drawn from multiple families who were living in Ho Chi Minh City and rural areas. The study's procedure and protocol were approved by the research and ethics committee of the People's Hospital 115 on August 6, 2015 (approval number: 297/BV-NCKH). The Study was conducted according to the ethical principles of the Declaration of Helsinki, and all participants gave written informed consent.
We used 2 approaches to recruit participants. In the first approach, we contacted community organizations to solicit a list of members, and from the list we ran a computer program to randomly selected individuals who met the age and sex criteria. A letter was then sent to the selected individuals to invite them and their family members to participate in the Study. In the second approach, we recruited participants via television, the Internet, and flyers in universities. The flyers described (in Vietnamese) the study's purposes, procedures, and benefits of participants. Individuals agreed to participate in the study were then transported to the Bone and Muscle Research Laboratory at the Ton Duc Thang University for clinical assessment and evaluation. The participants do not receive any financial incentive, but they received a free health check-up, and lipid analyses.
The inclusion criteria were broad: men and women aged between 18 years and older, who agreed to participate in the study. We excluded individuals deemed to have impaired cognitive function or are not willing to give informed consent or were physically unable to complete clinical tests. The participants will then be followed for 10 years to record the incidence of fractures and chronic diseases.
2.2. Measurements
Extensive data were collected at baseline. Each participant was administered with a structured questionnaire by a trained interviewer. The questionnaire solicits information concerning their demographic factors, clinical history, medication use, lifestyle factors, physical activity, dietary habits, history of falls and fractures, and anthropometric factors.
2.2.1. Anthropometry
Height and weight were measured by an electronic portable, wall-mounted stadiometer (Seca Model 769; Seca Corp., CA, USA) without shoes or ornaments or hats or heavy layers of clothing. Body mass index (BMI) was derived as the weight in kilograms divided by the square of the height in meters. We classified the BMI into 4 groups as follows: underweight (if BMI < 17 kg/m2); normal (BMI between 17 and 22 kg/m2); overweight (BMI between 23 and 27.4 kg/m2); and obese (BMI > 27.4 kg/m2).
Waist circumference (WC) and hip circumference (HC) were also measured in each participant by using the World Health Organization (WHO) protocol [12]. HC was measured around the widest portion of the buttocks (in standing position) by using a measuring tape. WC was measured at the midpoint between lower margin of the least palpable rib and the top of the iliac crest. Waist to hip ratio (WHR) was derived as the ratio of WC over HC. Central obesity was defined as WHR > 0.85 for women or >0.90 for men.
2.2.2. Lifestyle data
Participants were also asked to provide information on current and past smoking habits. Cigarette smoking was classified into 2 broad groups: past smoking and current smoking. In addition, the cumulative smoking exposure was assessed by the number of pack-years by multiplying the number of years smoked with the average number of packs per day.
Alcohol intake in average numbers of standard drinks per day, at present as well as within the last 5 years, was obtained. Drinks per interval was estimated by multiplying quantity by frequency for days of the week and more than usual and adding. The alcohol intake was quantified by the number of years alcohol consumed, number of drinks per day during the drinking years (total number of drinks/total number of days in drinking years), number of drinks per drinking day (total number of drinks/total number of days on which alcohol was consumed in drinking years). In addition, we also ascertain tea and soft drink intakes by using the same method of quantification as used in the ascertainment of alcohol intakes.
Physical activity was measured based on the WHO Stepwise Approach to Surveillance (Steps) of Non-Communicable Disease Risk Factors [13], the validated questionnaire is built with 15 questions to collect data of the time spending for work, traveling to and from places, recreational activities and sedentary behavior in a typical week.
Clinical data including blood pressure, pulse, and reproductive history (i.e., parity, age of menarche, and age of menopause), medical history (i.e., previous fracture, previous and current use of pharmacological therapies) were also obtained. Two blood pressure measurements were taken (5 min apart) in seated position, and the mean of 2 measurements was taken as the individual's blood pressure. Individuals were classified as having hypertension if their average systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
2.3. Skeletal assessments
2.3.1. Bone mineral density
Areal BMD was measured at the lumbar spine, femoral neck, total hip and whole body using a Hologic Horizon (Hologic Corp., Bedford, MA, USA). For the lumbar spine, we measured BMD from L2 to L4. The densitometer was standardized by phantom before each measurement. The measurement was done by a qualified radiology technologist. Based on 20 individuals, the coefficient of variation in BMD at our lab was 1.5% for the lumbar spine and 1.7% for the hip. Fat mass and lean mass were derived from the whole body scan.
2.3.2. Diagnosis of osteoporosis
Based on the femoral neck BMD measurement, we calculated the sex-specific T-score using our reference data on peak BMD that has been published previously [8]. Based on the T-score, we classify individuals into 3 groups according to the WHO's criteria: (1) osteoporosis, if the T-score is equal to or lower than −2.5; (2) osteopenia, if T-score is between −1 and −2.5; and (3) normal, if T-score is equal or greater than −1.
Trabecular bone score (TBS) was performed by the TBS iNsight Software, version 2.1 (Medimaps, Merignac, France). These analyses were performed blind to fracture status and any clinical parameters. The software uses the anterior-posterior spine raw image(s) from the densitometer, including the BMD region of interest and edge detection so that the TBS calculation is performed over exactly the same region of interest as the BMD measurement. In the current analysis, we used a research version of the commercialized TBS iNsight software, which allows for large batched analyses from a workstation. The short-term coefficient of variation in TBS determinations has been reported in several mono-center studies ranges from 1.1% to 1.9% [14].
Hip geometrical measures including hip axis length, were derived from the femoral neck scan. Calculation of geometry parameters for the neck, shaft, and intertrochanteric regions with narrow neck across the narrowest diameter of the (1) femoral neck, (2) intertrochanteric along the bisector of the neck-shaft angle, and (3) the shaft, 2 cm distal to the midpoint of the lesser trochanter. For each region the distribution of the bone mass across the bone is extracted then geometry properties are derived as follows:
-
•
Cross sectional area (mm2): equivalent to the amount of (cortical equivalent) bone surface area in the cross-section after excluding all trabecular and soft tissue spaces.
-
•
Cross sectional moment of inertia (mm4): for bending in the image plane from bone mass profile integral. Index of structural rigidity; reflects distribution of mass about the center of a structural element.
-
•
Section modulus (mm3): indicator of bending strength for maximum bending stress in the image plane.
-
•
Buckling ratio: relative thickness of narrow neck cortex as an estimate of cortical stability in buckling.
2.3.3. pQCT measurements
We used a peripheral quantitative computer tomography (pQCT) XCT3000 (Stratec Medizintechnik GmbH, Pforzheim, Germany) to measure bone volume and bone geometry including the cortical (outer shell) and trabecular (inner spongy bone) compartments of the tibia and radius. The following parameters were obtained from the pQCT:
-
•
Volumetric bone mineral density (vBMD)
-
•
Cross-sectional area
-
•
Cortical thickness, endocortical (inner side of the cortical shell)
-
•
Periosteal (outer side of the cortical shell) circumference
-
•
Section modulus (mm3)
-
•
Cross-sectional and polar moment of inertia (CSMI and PMI): CSMI is an estimation of the resistance of bone to bending, whereas PMI represents the ability of bone to resist torsion
-
•
Strength-strain index which relates to bending and torsional strength of the bone.
2.4. Radiographic evaluation
Anteroposterior and oblige digital X-ray (FCR Capsula XLII, Fujifilm Corp., Tokyo, Japan) of the hands, knee, hip and spine were obtained from each participant. The assessment of osteoarthritis is based on the Kellgren-Lawrence scoring system which is recommended by the WHO as a standard method for studying in large epidemiologic studies. The radiographs are read by 2 experienced rheumatologist who are unaware of the clinical conditions of participants. In each area (e.g., knee, wrist, and hip), the presence or absence of osteophytes, joint space narrowing, sclerosis and cysts is examined for each hand joint using the Kellgren-Lawrence system of scoring: 0, none; 1, possible osteophytes only; 2, definite osteophytes and possible joint space narrowing; 3, multiple osteophytes, definite joint space narrowing and possible bony deformity; and 4, large osteophytes, severe joint space narrowing, severe sclerosis and definite bony deformity. The presence of radiographic osteoarthritis is defined if the grade is 2 or more in at least one joint.
2.5. Muscle strength assessment
A hand dynamometer (Baseline Hand Dynamometer - HiRes Gauge - ER 300 lb. Capacity, 3B Scientific, Atlanta, GA, USA) was used to measure the strength of both right and left hands for all participants. Moreover, back and feet strengths were measured by back - leg dynamometer (Baseline Back-Leg-Chest Dynamometer - Oversize Platform - 660 lb. Capacity, 3B Scientific). Results of measurement were calculated by kg unit, with the smallest measuring value is 0.1 kg and 0.5 kg for hand and back, respectively. Each individual was measured twice, and the highest value was used for the analysis.
Muscle function motion analysis, performance analysis and fitness test were measured by Leonardo mechanograph GRFP (Stratec). The parameters include:
-
•
Individual performance (anearobic peak power, Esslinger Fitness Index, EFI: W/kg body weight normalized to gender and age from 2 to 99 years)
-
•
Individual maximum voluntary force per leg
-
•
Individual performance during chair rising test
-
•
Maximum isometric grip force
2.6. Carotid intima-media thickness
The intima media thickness were measured with Philips Ultrasound machine – model HD7XE with a 7 MHz linear transducer. These measurements were made at the common carotid artery, carotid bulb, and the proximal internal carotid artery of both side. All images were stored as DICOM format for analysis. Intima-media thickness values of more than 0.9 mm (European Society of Cardiology) or over the 75% (American Society of Echocardiography) were considered abnormal.
2.7. Laboratory analyses
Fasting venous blood sample (12 mL) was taken from each participant by venipuncture between 7 AM and 11 AM. The serum was immediately frozen to −20°C prior to biochemical analysis which was taken within 24 hours after the collection.
2.7.1. Glycemia measurements
Glycosylated hemoglobin (HbA1c) levels were measured with high pressure liquid chromatography (HPLC) analyzers ADAMS A1c HA-8160 (Arkray, Kyoto, Japan). The intra- and interassay coefficient of variation for this HPLC is less than 1%. Fasting plasma glucose (FPG) levels were determined by the hexokinase method (Advia 1800 Autoanalyzer; Bayer Diagnostics, Leverkusen, Germany) with an intra-measurement coefficient of variance of 0.98%–1.34%.
An individual was diagnosed to have diabetes if the individual's HbA1c value ≥ 6.5% (48 mmol/mol) [15]. Prediabetes was defined as HbA1c value between 5.7% and 6.4% (39–46 mmol/mol) [16]. In addition and for comparison, diabetes was also diagnosed as individuals with FPG ≥ 7.0 mmol/L, and prediabetes was classified as FPG between 5.6 and 6.9 mmol/L (i.e., impaired fasting glucose).
2.7.2. Lipid measurements
Total cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides were measured by an enzymatic calorimetric test (Advia 1800, Autoanalyzer; Bayer Diagnostics). The intraassay coefficient of variation was 0.6% for cholesterol and 1.6% for triglycerides. All specimens were analyzed at the MEDIC's Department of Biochemistry and Paraclinical Services (Ho Chi Minh City, Vietnam). An individual was classified as having “high cholesterol” if the individual's total cholesterol > 6.2, or LDL cholesterol > 4.0, or triglycerides >2.2.
2.7.3. Bone turnover markers
The concentration of beta-C-telopeptide (CTX) and procollagen type 1 N-propeptide (P1NP) were analyzed by immunological techniques electrochemical luminescence on the Roche Elecsys 1010/2010 automated analyzer (Roche Diagnostics GmbH, Mannheim, Germany). This analysis method can determine the concentration of P1NP within limit of 5–1200 ng/mL, and beta-CTX in limit of 10–6000 pg/mL. The interassay coefficient of variation was <20%.
2.7.4. DNA samples
Blood samples will be collected and stored at −80°C. DNA was extracted from leucocytes using either QIAamp DNA Mini Blood Kit (Qiagen Pty Ltd., Victoria, Australia) or phenol/chloroform.
3. Results
Until now (31/12/2016), 4157 individuals (65% are women) from more than 817 families have participated in VOS. The distribution of participants by gender and age group is shown in Table 1. The average age of both genders was 51, with approximately 53% of women and 45% of men aged 50 years and older. Key baseline characteristics are shown in Table 2. The mean BMI of the participants was 22.8 (standard deviation, 3.4) kg/m2 in women and 23.3 (3.3) kg/m2 in men. Approximately 3% of participants were obese (BMI ≥ 30 kg/m2), and 21% were overweight. A majority of participants were married (70% in women and 77% in men). A majority (54%) of the participants had high school education. About 29% of the women were housewives, and 12% of men were retirees. In men, 41% were self-reported current smokers, and 45% were regular alcohol drinkers.
Table 1.
Age and sex distribution in the Vietnam Osteoporosis Study.
| Age group, yr | Women (n = 2699) | Men (n = 1458) |
|---|---|---|
| 18–29 | 352 (13.0) | 294 (20.2) |
| 30–39 | 317 (11.7) | 228 (15.6) |
| 40–49 | 609 (22.6) | 284 (19.5) |
| 50–59 | 833 (30.9) | 396 (27.2) |
| 60–69 | 416 (15.4) | 186 (12.8) |
| 70–99 | 172 (6.4) | 70 (4.8) |
Values are presented as number (%).
Table 2.
Marital status, educational level, occupation, and lifestyle factors.
| Variable | Women (n = 2699) | Men (n = 1458) |
|---|---|---|
| Height, cm | 152.9 ± 5.5 | 164.1 ± 6.1 |
| Weight, kg | 53.2 ± 8.2 | 62.9 ± 10.1 |
| Body mass index, kg/m2 | 22.8 ± 3.4 | 23.3 ± 3.3 |
| BMI category | ||
| Underweight | 195 (7.2) | 82 (5.6) |
| Normal | 1910 (70.8) | 962 (66.0) |
| Overweight | 509 (18.9) | 370 (25.4) |
| Obese | 85 (3.1) | 44 (3.0) |
| Marital status | ||
| Married | 1876 (69.5) | 1114 (76.5) |
| Divorced | 80 (3.0) | 14 (1.0) |
| Single | 572 (21.2) | 315 (21.6) |
| Widowed | 170 (6.3) | 13 (0.9) |
| Educational attainment | ||
| Some primary schooling | 49 (1.8) | 7 (0.5) |
| Primary | 591 (21.9) | 188 (12.9) |
| Secondary | 1368 (50.7) | 800 (55.0) |
| Tertiary (college and university) | 689 (25.5) | 459 (31.6) |
| Occupation | ||
| Salespersons | 396 (14.7) | 208 (14.3) |
| Officer workers | 336 (12.4) | 189 (13.0) |
| Farmers | 140 (5.2) | 124 (8.5) |
| Manufacturing workers | 135 (5.0) | 116 (8.0) |
| Retirees | 237 (8.8) | 182 (12.5) |
| Housewives | 768 (28.5) | NA |
| Lifestyle factors | ||
| Regular alcohol use | 883 (32.7) | 653 (44.8) |
| Current smokers | 25 (0.9) | 600 (41.2) |
| Coffee drinking | 1077 (40.0) | 846 (58.0) |
| Tea drinking | 863 (32.0) | 727 (50.0) |
NA, not applicable.
Among those aged 50 years and older, approximately 14% of women and 5.3% of men had osteoporosis (i.e., femoral neck BMD T-scores ≤ −2.5). The prevalence of osteoporosis and osteopenia increased with advancing age, reaching the highest among those aged 70 years and older (Table 3). Using the cutoff value of HbA1c ≥ 6.5% (48 mmol/mol), the prevalence of diabetes in women and men combined was 11%. There was no significant difference in the prevalence between women and men (P = 0.22). Among the 330 individuals with diabetes, only 125 (38%) were clinically known to have the disease and were on diabetic treatment.
Table 3.
Prevalence of osteoporosis, hypertension, and diabetes among individuals aged 40 years and older.
| Variable | Age group, yr |
|||
|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–99 | |
| Women | ||||
| Number | 609 | 833 | 416 | 172 |
| Osteoporosis | 1 (0.2) | 56 (6.7) | 67 (16.1) | 75 (43.6) |
| Osteopenia | 159 (26.1) | 431 (51.7) | 267 (64.2) | 91 (52.9) |
| Hypertension | 106 (17.4) | 200 (24.0) | 107 (25.7) | 56 (32.6) |
| Diabetes | 21 (3.4) | 84 (10.1) | 75 (18.0) | 48 (28.0) |
| Prediabetes | 139 (22.8) | 355 (42.6) | 216 (51.9) | 74 (43.0) |
| High cholesterol | 65 (10.7) | 92 (11.0) | 49 (11.8) | 13 (7.6) |
| Men | ||||
| Number | 284 | 396 | 186 | 70 |
| Osteoporosis | 4 (1.4) | 13 (3.3) | 7 (3.8) | 15 (21.4) |
| Osteopenia | 84 (29.6) | 171 (43.2) | 111 (60.0) | 37 (52.9) |
| Hypertension | 79 (27.8) | 122 (34.4) | 64 (34.4) | 16 (77.1) |
| Diabetes | 14 (4.9) | 49 (12.4) | 30 (16.1) | 9 (12.9) |
| Prediabetes | 94 (33.0) | 147 (37.1) | 72 (38.7) | 38 (54.3) |
| High cholesterol | 40 (14.0) | 53 (13.4) | 19 (10.2) | 6 (8.6) |
Values are presented as number (%).
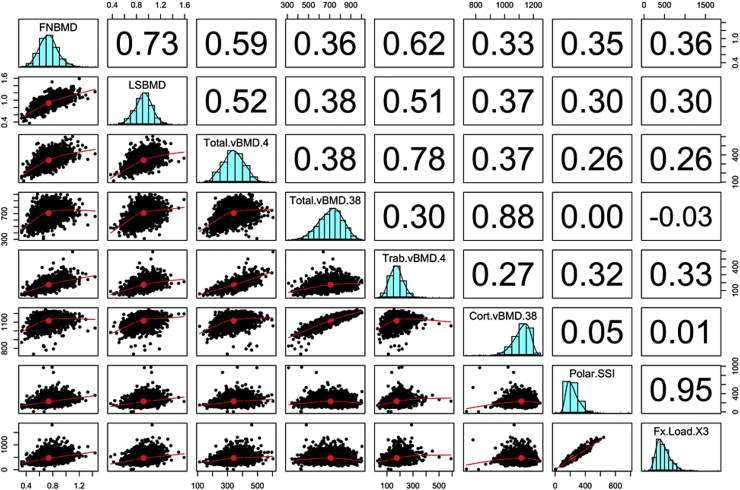
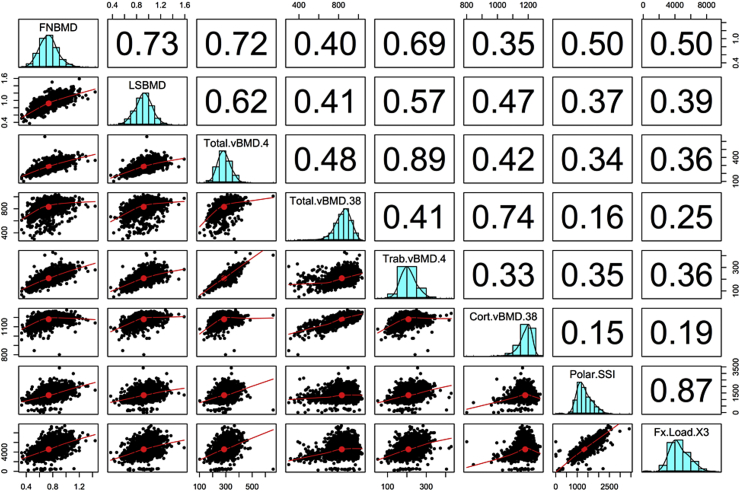
We measured bone properties (using pQCT) in a subset of 1383 women and 828 men in VOS. The median age of this subset was 47 years (interquartile range, 32–56 years). Descriptive statistics on pQCT bone parameters are shown in Table 4. As expected, trabecular and cortical bone area, volumetric BMD at the radius and tibia were greater in men than women. The relationship between age and vBMD largely followed a quadratic function, with peak level being reached at the age of 20–30 years. The decline of vBMD started from the age of 50 years, with women having higher rate of loss than men (data not shown). At the radius, the rate of loss in trabecular vBMD was 1.1%/yr in women which was significantly greater than that in men (0.2%/yr); the rate of loss in cortical vBMD was 0.3%/yr compared with 0.1%/yr in men. Similar trends were also observed at the tibia. As expected, polar SSI, SSI, and fracture load indices in men were greater than women, but the relative rate of decline in fracture load was not significantly different between men and women. Areal BMD has a better correlation with trabecular vBMD (r = 0.69) than with cortical vBMD (r = 0.35). Moreover, the correlation between areal BMD and bone polar strength strain index or fracture load index was model at either the radius (Fig. 1) or the tibia (Fig. 2).
Table 4.
Descriptive statistics on peripheral quantitative computer tomography bone parameters by sex.
| Variable | Women (n = 1383) | Men (n = 828) |
|---|---|---|
| Tibia 4% | ||
| Total bone area, mm2 | 906 ± 116 | 1150 ± 138 |
| Total BMC, mg/mm | 2.47 ± 0.42 | 3.50 ± 0.58 |
| Total BMD, mg/mm2 | 276 ± 48 | 306 ± 49 |
| Trabecular area, mm2 | 408 ± 52 | 517 ± 62 |
| Trabecular BMC, mg/mm | 198 ± 39 | 225 ± 42 |
| Total muscle area, mm2 | 5591 ± 1222 | 7396 ± 1503 |
| Tibia 38% | ||
| Total bone area, mm2 | 327 ± 51 | 417 ± 62 |
| Total BMC, mg/mm | 2.67 ± 0.43 | 3.53 ± 0.54 |
| Total BMD, mg/mm2 | 821 ± 106 | 851 ± 86 |
| Polar strength-strain index, mm3 | 1136 ± 227 | 1659 ± 332 |
| Strength-strain index X, mm4 | 707 ± 147 | 1030 ± 219 |
| Strength-strain index Y, mm3 | 627 ± 129 | 944 ± 201 |
| Cortical area, mm2 | 204 ± 34 | 273 ± 44 |
| Cortical density, mg/mm2 | 1179 ± 51 | 1183 ± 32 |
| Periosteal circumference, mm | 63.9 ± 5.3 | 72.1 ± 5.9 |
| Endosteal circumference, mm | 38.8 ± 6.4 | 42.1 ± 6.3 |
| Total muscle area, mm2 | 545 ± 84 | 704 ± 105 |
| Tibia 66% | ||
| Total bone area, mm2 | 456 ± 74 | 588 ± 94 |
| Total BMC, mg/mm | 2.88 ± 0.45 | 3.77 ± 0.58 |
| Total BMD, mg/mm2 | 640 ± 98 | 648 ± 82 |
| Polar strength-strain index, mm3 | 1958 ± 338 | 2332 ± 488 |
| Strength-strain index X, mm4 | 1084 ± 238 | 1577 ± 337 |
| Strength-strain index Y, mm3 | 820 ± 174 | 1236 ± 267 |
| Cortical area, mg/mm2 | 215 ± 36 | 285 ± 46 |
| Cortical bone density, mg/mm3 | 1140 ± 48 | 1142 ± 29 |
| Cortical thickness, cm | 3.34 ± 0.58 | 3.89 ± 0.60 |
| Periosteal circumference, mm | 75.4 ± 6.7 | 85.6 ± 7.8 |
| Endosteal circumference, mm | 54.4 ± 7.9 | 61.2 ± 8.3 |
| Total muscle area, mm2 | 8194 ± 1958 | 9018 ± 1878 |
| Fracture load X, mm4 | 3903 ± 856 | 5676 ± 1214 |
| Fracture load Y, mm4 | 2954 ± 625 | 4451 ± 962 |
| Radius 4% | ||
| Total BMC, mg/mm | 0.90 ± 0.17 | 01.38 ± 0.21 |
| Total bone area, mm2 | 287 ± 47 | 375 ± 63 |
| Total BMD, mg/mm3 | 321 ± 68 | 374 ± 61 |
| Trabecular area, mm2 | 129 ± 21 | 169 ± 28 |
| Trabecular BMD, mg/mm3 | 153 ± 43 | 209 ± 51 |
| Total muscle area, mm2 | 2455 ± 522 | 3924 ± 757 |
| Radius 38% | ||
| Total BMC, mg/mm | 0.84 ± 0.20 | 1.16 ± 0.22 |
| Polar strength strain index, mm3 | 197 ± 102 | 306 ± 115 |
| Strength-strain index X, mm3 | 112 ± 69 | 174 ± 78 |
| Strength-strain index Y, mm3 | 119 ± 59 | 185 ± 60 |
| Total bone area, mm2 | 124 ± 45 | 163 ± 40 |
| Total BMD, mg/mm3 | 701 ± 123 | 722 ± 94 |
| Periosteal circumference, mm | 39.0 ± 5.6 | 45.0 ± 4.9 |
| Endosteal circumference, mm | 27.8 ± 6.9 | 31.0 ± 6.0 |
| Total muscle area, mm2 | 274 ± 83 | 371 ± 95 |
| Radius 66% | ||
| Total BMC, mg/mm | 0.84 ± 0.20 | 1.16 ± 0.22 |
| Polar strength-strain index, mm3 | 196 ± 101 | 306 ± 115 |
| Strength-strain index X, mm3 | 112 ± 68 | 174 ± 78 |
| Strength-strain index Y, mm3 | 119 ± 58 | 185 ± 61 |
| Total bone area, mm2 | 123 ± 45 | 163 ± 40 |
| Total BMD, mg/mm3 | 701 ± 123 | 722 ± 94 |
| Periosteal circumference, mm | 39.0 ± 5.6 | 45.0 ± 4.9 |
| Endosteal circumference, mm | 27.8 ± 6.9 | 31.0 ± 6.0 |
| Fracture load X, mm4 | 404 ± 245 | 626 ± 281 |
| Fracture load Y, mm4 | 428 ± 210 | 667 ± 218 |
| Total muscle area, mm2 | 3818 ± 692 | 4797 ± 708 |
Values are presented as mean ± standard deviation.
BMC, bone mineral content; BMD, bone mineral density.
Fig. 1.
Correlation between dual-energy X-ray absorptiometry areal bone mineral density (BMD) and peripheral quantitative computer tomography volumetric BMD and bone strength indices at the radius. FNBMD, femoral neck BMD; LSBMD, lumbar spine BMD; Total.vBMD.4, total volumetric BMD at the 4% site; Total.vBMD.38, total volumetric BMD at the 38% site; Trab.vBMD4, trabecular vBMD at the 4% site; Cort.vBMD.38, cortical vBMD at the 38% site; Polar.SSI, polar strength-strain index; Fx.Load.X3, fracture load at the 66% site.
Fig. 2.
Correlation between dual-energy X-ray absorptiometry areal bone mineral density (BMD) and peripheral quantitative computer tomography volumetric BMD and bone strength indices at the tibia. FNBMD, femoral neck BMD; LSBMD, lumbar spine BMD; Total.vBMD.4, total volumetric BMD at the 4% site; Total.vBMD.38, total volumetric BMD at the 38% site; Trab.vBMD4, trabecular vBMD at the 4% site; Cort.vBMD.38, cortical vBMD at the 38% site; Polar.SSI, polar strength-strain index; Fx.Load.X3, fracture load at the 66% site.
We have also analyzed the TBS data from the VOS study [17]. On average, TBS were 4.5% higher in men than in women. Collectively, sex, age and height accounted for ∼28% of total variance in TBS. Relative importance analysis indicate that most of the explained variance was accounted by advancing age. TBS was strongly correlated with lumbar spine BMD (r = 0.73; P < 0.001). The age and height adjusted index of heritability of TBS was 0.46 [17], which was not much different from that of LSBMD (0.44). Furthermore, we found that the genetic correlation between TBS and LSBMD was 0.35, between TBS and femoral neck BMD was 0.21 [17].
4. Discussion
Although the prevalence and incidence of, and risk factors for, osteoporosis and its consequence of fracture are well documented in Caucasian populations, there are scarce data for Asian populations whose burden of hip fracture is expected to account for 30% of hip fractures worldwide [18]. The present VOS is designed to contribute substantially to the epidemiology and pathogenesis of osteoporosis in an Asian population. We consider that osteoporosis should be studied as part of a bigger network of chronic NCDs that are shared by common environmental and genetic factors. By dissecting the contributions of environmental and genetic factors to the covariation of diseases, we will have a better understanding of the linkage between osteoporosis and chronic diseases in the general population.
In this study, we found that the prevalence of osteoporosis among women and men aged 50 years and older was 14% and ∼5%, respectively. In women, our estimate of osteoporosis prevalence was lower than that in Koreans [19], but comparable to the National Health and Nutrition Examination Survey (NHANES) 2005–2006 estimate [20], and the discrepancy was probably due to the skeletal site used in the classification of osteoporosis. In the Korean study [19], osteoporosis was defined based on lumbar spine and/or femoral neck BMD, whereas in our study and NHANES 2005–2006, the classification of osteoporosis was based on femoral neck BMD only. In men, our estimate of osteoporosis prevalence was slightly lower than that in Korean men study which was 7.5% [19], but the difference was again likely due to the difference in BMD sites.
Among those aged 50 years and older, approximately 14% of women and 5.3% of men had osteoporosis (i.e., femoral neck BMD T-scores ≤ −2.5). The prevalence of osteoporosis and osteopenia increased with advancing age, reaching the highest among those aged 70 years and older (Table 3). Using the cutoff value of HbA1c ≥ 6.5% (48 mmol/mol), the prevalence of diabetes in women and men combined was 11%. There was no significant difference in the prevalence between women and men (P = 0.22). Among the 330 individuals with diabetes, only 125 (38%) were clinically known to have the disease and were on diabetic treatment.
Vietnam is an ideal setting for studying osteoporosis and NCDs, because the country is in a dynamic transition from developing to a more advanced status. The country's population is about 1.3% of the world population. The proportion of population living in urban areas is approximately 30%, and this figure is continuously increasing. During the past 20 years, the country has continued to be one of the world's fastest growing economies, with annual economic growth rate of ∼5% [21]. In parallel with the economic development, Vietnam has also undergone remarkable changes in disease burdens, and the existence of this trend presents Vietnam as an ideal setting for studying the dynamic of NCDs in transitional economies.
While communicable disease still remains a significant burden, the prevalence of chronic NCDs is rapidly increasing. Currently, major NCDs include obesity, diabetes, cardiovascular disease, cancer, musculoskeletal diseases, including osteoarthritis and osteoporosis. Our recent studies suggested that 10% of men and 29% of women have osteoporosis [8], 36% have knee osteoarthritis [22], and 12.3%% have type 2 diabetes [23]. The prevalence of diabetes, mostly undiagnosed, has increased almost 3 fold during the past two decades. The number of individuals with hypertension has doubled during the past 2 decades. The prevalence of osteoporosis and osteoarthritis is equivalent to those found in economically developed countries [8], [22]. Lung cancer alone accounted for more than 23,000 deaths in men and 4% of all deaths. According to a recent report, NCDs collectively account for 71% of total burden of disease in Vietnam, including 60% of all-cause deaths [11]. Compared with two decades ago, the disease burden attributable to NCDs represents a 30% increase.
Osteoporosis and many NCDs (e.g., cardiovascular disease, diabetes, cancer, and osteoarthritis) to a large extent, share common risk factors: smoking, excess alcohol intakes, poor nutrition, and insufficient physical activity. Vietnam is among the countries in the world that have high prevalence of smoking among men. Our recent study found that almost 50% of adult men are current smokers, and about 25% are regular alcohol drinkers. Moreover, more than 25% of adults are insufficiently active [24]. On the other hand, energy obtained from fats has doubled in 2 decades. With the urbanization that is being taken place in the country, the changes in lifestyle and nutrition are expected to be more pronounced in the coming years. The sharing of risk factors demands a new and more systematic approach to the study of osteoporosis, and it is our vision that VOS will make a contribution to the study of covariation of NCDs.
The scientific issues of this project are primarily concerned with environmental influences on osteoporosis. During the past 4 decades or so, the study of determinants of chronic diseases has been largely focused on the genetic component of disease susceptibility, and little attention has been on the environmental component. In reality, genetic effects account for between 15% and 40% of the variance of disease susceptibility in cancer, cardiovascular disease, obesity, diabetes and osteoarthritis. Through genomewide association studies (GWAS) several genetic variants have been identified to be associated with cancer, cardiovascular disease, obesity, diabetes and osteoarthritis. However, these genetic variants explain less than 10% of those diseases' variance [25].
The above fact, of course, suggests that the variability in osteoporosis susceptibility is mainly due to environmental factors. Environmental factors include, but not limited to, stress, lifestyle factors, nutritional factors, pollution and pollutants, and chemical exposures. The totality of environmental factors can be conceptualized as an “exposome” [26], analog of the genome that consists of genetic variants. By this conceptualization, study of environmental factors and chronic disease can be analytically based on the method of GWAS. With the current technology (i.e., high throughput Gas Chromatography - Time of Flight mass spectrometry), it is possible to quantify the total environmental exposure for an individual.
This project proposes a series of analyses and state-of-the-art technologies to address an important issue: how much variation in chronic NCDs is attributable to blood exposome and external environmental factors. The outcome of this analysis can potentially lead to the development of a new tool for early identification of high risk individuals and intervention on those components that are first affected, when reversal may be most possible, and hence contribute to the healthy aging. An innovative aspect of the project is the creation of the “diseasome” and exposome that will advance our understanding of the relationship between multiple comorbidities and environmental factors. Thus, the project's outcome also highlights the impact of environmental factors, including pollution, to public health in developing countries, and thus helps shape public health policy.
The findings of this study should of course be interpreted within the context of strengths and weaknesses. The study was based on a large, well characterized cohort who was recruited without any specific criteria that would affect the risk of chronic diseases. Therefore, our estimates of prevalence of chronic conditions are unlikely to be affected by selection bias. We have comprehensively collected a wide range of skeletal phenotypes and clinical conditions, that allows a network analysis of comorbidities for a future diseasome study. However, the study participants were from a highly urban population; therefore, the findings may not be generalizable to rural populations.
5. Conclusions
The Vietnam Osteoporosis Study is a major bone health research project in Vietnam and Asia. The Project will make use of advances in new scientific disciplines of network medicine, “exposome,” and genome to develop a unified predictive framework for annotating the human genome for genetic variants and network of environmental factors (e.g., chemical exposures) that likely contribute to the co-occurrence of osteoporosis and chronic diseases. We hope that the outcome of the Study will provide an insight into the pathogenesis of osteoporosis and its related conditions.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This research is funded by Foundation for Science and Technology Development of Ton Duc Thang University (FOSTECT, http://fostect.tdt.edu.vn; grant number: FOSTECT.2014.BR.09, and a grant from the Department of Science and Technology of Ho Chi Minh City; grant number: YT_2015_03. We sincerely thank Ms Tran Thi Ngoc Trang and Fr Pham Ba Lam for coordinating the recruitment of participants. We also thank doctors and medical students of the Pham Ngoc Thach University of Medicine for the data collection and clinical measurements.
Study team: Dr. Mai Duy Linh, Dr. Doan Cong Minh, Dr. Nguyen Da Thao Uyen, Dr. Lai Quoc Thai, Dr. Do Minh Tam, Dr. Do Thien An, Dr. Nguyen Cong Hoang, Dr. Chau Ngoc Minh Phuong, Dr. Nguyen Trung Thanh, Dr. Bui Dat Thinh, Dr. Nguyen Quynh Thu, Mr. Sam Vinh Loc, Dr. Bui Van Quoc, Dr. Nguyen Thi Dao Tien, Mr. Tran Xuan Truong, Ms. Thai Ngoc Tran, Ms Le Thi Phuong Thao, Mr. Nguyen Van Ngan, Ms. Nguyen Duong Thien Thanh, Mr. Nguyen Truong Trung Tin, Mr. Nguyen Tan Duc, Mr. Van Hai Long, Ms. Nguyen Le Phuong Hong, Ms Nguyen Thi Ngoc Yen, Ms. Nguyen Thi Thuy, Ms. Nguyen Duong Thao Quyen, Mr. Luu Minh Long, Mr. Do Tuan Kiet, Mr. Nguyen Tam Xuan Kiet, Ms. Nguyen Ngoc Nha Khanh, Mr. Vo Quang Hung, Mr. Duong Hai, Mr. Chau Quoc Khanh, Mr, Le Vinh Nghi, Ms Van Thi Ngoc Bich, Ms Tran Thi Nhung.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Nguyen N.D., Ahlborg H.G., Center J.R., Eisman J.A., Nguyen T.V. Residual lifetime risk of fractures in women and men. J Bone Min Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 2.Kanis J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 3.Cummings S.R., Black D.M., Rubin S.M. Lifetime risks of hip, Colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445–2448. [PubMed] [Google Scholar]
- 4.Center J.R., Bliuc D., Nguyen T.V., Eisman J.A. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297:387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 5.Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 6.Haentjens P., Magaziner J., Colón-Emeric C.S., Vanderschueren D., Milisen K., Velkeniers B. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost S.A., Nguyen N.D., Center J.R., Eisman J.A., Nguyen T.V. Excess mortality attributable to hip-fracture: a relative survival analysis. Bone. 2013;56:23–29. doi: 10.1016/j.bone.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Ho-Pham L.T., Nguyen U.D., Pham H.N., Nguyen N.D., Nguyen T.V. Reference ranges for bone mineral density and prevalence of osteoporosis in Vietnamese men and women. BMC Musculoskelet Disord. 2011;12:182. doi: 10.1186/1471-2474-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho-Pham L.T., Mai L.D., Pham H.N., Nguyen N.D., Nguyen T.V. Reference ranges for vertebral heights and prevalence of asymptomatic (undiagnosed) vertebral fracture in Vietnamese men and women. Arch Osteoporos. 2012;7:257–266. doi: 10.1007/s11657-012-0106-z. [DOI] [PubMed] [Google Scholar]
- 10.Nayak L., Tunga H., De R.K. Disease co-morbidity and the human Wnt signaling pathway: a network-wise study. OMICS. 2013;17:318–337. doi: 10.1089/omi.2012.0053. [DOI] [PubMed] [Google Scholar]
- 11.Harper C. World Health Organization; Geneva: 2011. Vietnam noncommunicable disease prevention and control programme 2002-2010. WHO Report 2011. [Google Scholar]
- 12.World Health Organization . World Health Organization; Geneva: 2011. Waist circumference and Waist–Hip ratio: report of a WHO expert consultation. WHO Tehnical Report 2011. [Google Scholar]
- 13.Bonita R., Winkelmann R., Douglas K.A., de Courten M., The W.H.O. Stepwise approach to surveillance (STEPS) of non-communicable disease risk factors. In: McQueen D.V., Puska P., editors. Global behavioral risk factor surveillance. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 9–22. [Google Scholar]
- 14.Silva B.C., Leslie W.D., Resch H., Lamy O., Lesnyak O., Binkley N. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Min Res. 2014;29:518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 15.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho-Pham L.T., Hans D., Doan M.C., Mai L.D., Nguyen T.V. Genetic determinant of trabecular bone score (TBS) and bone mineral density: a bivariate analysis. Bone. 2016;92:79–84. doi: 10.1016/j.bone.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C., Cole Z.A., Holroyd C.R., Earl S.C., Harvey N.C., Dennison E.M. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y.J., Oh H.J., Kim D.J., Lee Y., Chung Y.S. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008-2009. J Bone Min Res. 2012;27:1879–1886. doi: 10.1002/jbmr.1635. [DOI] [PubMed] [Google Scholar]
- 20.Looker A.C., Melton L.J., 3rd, Harris T.B., Borrud L.G., Shepherd J.A. Prevalence and trends in low femur bone density among older US adults: NHANES 2005-2006 compared with NHANES III. J Bone Min Res. 2010;25:64–71. doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The World Bank . The World Bank; Washington, DC: 2014. Science, technology and innovation in Vietnam. OECD reviews of innovation policy 2014. [Google Scholar]
- 22.Ho-Pham L.T., Lai T.Q., Mai L.D., Doan M.C., Pham H.N., Nguyen T.V. Prevalence of radiographic osteoarthritis of the knee and its relationship to self-reported pain. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094563. e94563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho-Pham L.T., Do T.T., Campbell L.V., Nguyen T.V. HbA1c-Based classification reveals epidemic of diabetes and prediabetes in Vietnam. Diabetes Care. 2016;39:e93–e94. doi: 10.2337/dc16-0654. [DOI] [PubMed] [Google Scholar]
- 24.The World Bank . The World Bank; Washington, DC: 2017. World development indicators [Internet]http://databank.worldbank.org/data/views/reports/tableview.aspx [cited 2017 Jan 13]. Available from: [Google Scholar]
- 25.Estrada K., Styrkarsdottir U., Evangelou E., Hsu Y.H., Duncan E.L., Ntzani E.E. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild C.P. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]