Abstract
Bisphosphonate (BP)-associated osteonecrosis of the jaw (ONJ) was first reported in oncology patients in 2003 and subsequently in osteoporosis patients in 2004. Since oral surgical procedures, such as tooth extraction, are also considered one of the major risk factors for ONJ, there is confusion among physicians, dentists, and patients—particularly osteoporosis patients currently taking BPs—regarding the safety of remaining on therapy surrounding these procedures. Many papers about BP-related ONJ (BRONJ) have been published to date. In addition to BRONJ, recent studies have reported an association between ONJ and the antiresorptive therapy denosumab (Dmab; a RANKL-inhibitor). BRONJ and Dmab-related ONJ are together referred to as antiresorptive agent-related ONJ (ARONJ). The pathogenesis of ARONJ still remains unknown. It is forecasted that there will be an increased incidence of patients with osteoporotic fractures and an increased number of prescriptions for antiresorptive agents in Asia in the future. However, prescriptions for antiresorptives for osteoporosis may be restricted in the Asian population as the occurrence of ARONJ may be higher as compared with those in other countries. In this review, we focused on the following topics as it pertains to the Asian osteoporotic population: the oral condition specific for osteoporosis patients; definition, staging, prevalence and incidence of ARONJ; imaging modalities for ARONJ; specific risk factors for ARONJ; prevention strategies for ARONJ, and; cooperation between physicians and dentists in the prevention of ARONJ. Ideally, the Asian Federation of Osteoporosis Societies would cooperate with one another and find more population-specific evidence for the prevention of ARONJ.
Keywords: Osteonecrosis, Jaw, Osteoporosis, Asia
1. Introduction
Since Marx [1] first described a new clinical entity, osteonecrosis of the jaw (ONJ), in oncology patients who had taken high dose intravenous (i.v.) bisphosphonates (BPs) in 2003, a large number of investigators have reported BP-related ONJ (BRONJ) in both basic and clinical studies (randomized controlled trials, cohort studies, observational studies, case-controlled studies, case series, case reports) and numerous reviews, guidelines, consensus papers, and position papers about its incidence and management have been authored. However, the precise pathogenesis of BRONJ remains unclear. BRONJ has also been reported in osteoporosis patients administered low-dose oral BPs [2], which caused confusion among physicians, dentists, and osteoporosis patients because there are many osteoporosis patients who have taken BPs worldwide but who have also had surgical dental procedures like tooth extraction which in itself is an important risk factor for BRONJ [3]. In addition to BPs, recent reports describe that another antiresorptive medication, denosumab (Dmab; a RANKL inhibitor), may also be associated with an increased incidence of ONJ [4]. ONJ associated with BPs or Dmab is termed antiresorptive agent-related ONJ (ARONJ).
Hip fracture rates are continuously rising in Asia, with the exception of Hong-Kong and Taiwan which possess fracture trends that more closely resemble those of western countries [5]. BPs are now the most frequently prescribed medication for preventing osteoporotic fractures. Although a Task Force of the American Society for Bone and Mineral Research (ASBMR) describes that the risk of BRONJ in Asia may be higher than that in other regions [6], access to BPs and Dmab should not be restricted for fear for very rare adverse events like ARONJ [7]. In Asia, accurate information regarding the risk of fracture if untreated versus the risk of ARONJ if treated should be shared among physicians, dentists and patients.
In this review, the incidence and management of ARONJ in Asia is discussed in patients treated for osteoporosis with an antiresorptive medication (BP or Dmab). All studies considered for inclusion in this review were published in English with humans as study subjects.
2. Oral health specific to patients treated for osteoporosis in Asia
2.1. What kind of oral conditions do osteoporosis patients have?
Before discussing the pathogenesis of ARONJ in patients with osteoporosis, it is imperative to understand the important role basic oral health plays in the risk of ONJ independent of antiresorptive therapies. The risk of osteoporosis and oral infectious diseases, like marginal and periapical periodontal diseases, both increase with aging. However, since Daniell [8] first demonstrated the association between progression of osteoporosis and increased risk of edentulous jaws in postmenopausal women, numerous other investigators have also demonstrated the association between osteoporosis and loss of teeth and periodontal disease in the elderly.
2.2. Association between osteoporosis, loss of teeth and periodontal disease
In a cross-sectional study of 1914 Japanese subjects aged 48–95 years recruited from the Adult Health Study, the self-reported number of teeth present was significantly associated with a greater femoral neck bone mineral density (BMD) in both men and women [9]. In the Korean National Health and Nutrition Examination Survey (KNHANES) 2008–2010 (3364 men over 50 years of age and 3951 postmenopausal women), Jang et al. [10] observed a significant association in postmenopausal women between the number of remaining teeth and presence of osteoporosis, most strongly when diagnosed at the femoral neck site. In a longitudinal study, Iwasaki et al. [11] observed a significant relationship between a change in BMD of the lumbar spine and femoral neck and number of lost teeth during a 5-year study period in 404 Japanese community-dwelling postmenopausal women. Although there is little data describing the association between BMD and the loss of teeth in Asia (except Japan and Korea), it is likely that Asian osteoporosis patients have a higher probability of having tooth extraction in comparison to individuals without osteoporosis.
In a cross-sectional study of 9977 participants aged ≥40 years from KNHANES, Kim et al. [12] reported a significant association between increased risk of periodontal disease and BMD of the lumbar spine, total hip, and femoral neck. Iwasaki et al. [13] demonstrated that a low BMD, measured at lumbar spine and proximal femur, was associated with severity of periodontal disease in 397 Japanese community-dwelling postmenopausal females. Similar findings have been observed in Hong Kong, Taiwan, India and Jordan [14], [15], [16], [17]. In a longitudinal study of a Taiwanese population-based database including data from 2527 patients with osteoporosis and 7575 matched non-osteoporosis individuals, Chang et al. [15] reported that the adjusted hazard ratio for periodontitis in patients with osteoporosis compared with individuals without osteoporosis during the 5-year follow-up was 1.14 (95% confidence interval [CI], 1.05–1.24; P < 0.01). These findings suggest that an Asian elderly population with osteoporosis may have an increased risk of periodontal disease, a major infectious disease of the oral cavity.
It has been previously shown that in 253 postmenopausal women with self-reported periodontal symptoms included gingival swelling, gingival bleeding, purulent discharge and tooth mobility that the odds of having low spine BMD was 2.01 (95% CI, 1.15–3.50) [18]. In 5127 osteoporosis patients and 50,498 nonosteoporotic controls from the Longitudinal Health Insurance Database of Taiwan, Huang et al. [19] found that in those participants with good oral hygiene maintenance, patients with periodontitis had 1.29-fold risk of osteoporosis as compared with those without periodontitis (95% CI, 1.12–1.49). On the other hand, in those with poor oral hygiene maintenance those patients with periodontitis had a 6.02-fold increased risk of osteoporosis as compared to those without periodontitis (95% CI, 4.65–7.81). This suggests that oral hygiene maintenance may also complicate the understanding of ONJ as it also appears to have an influence on the association between osteoporosis and periodontal disease.
2.3. Osteoporosis and delayed wound healing after tooth extraction
If osteoporosis patients have an increased risk of having periodontal disease and loss of teeth, then they may also have a greater chance of suffering from delayed wound healing after tooth extraction because it is likely that the infection due to severe periodontal disease may contribute to inhibiting the promotion of wound healing. Huang et al. [20] compared 19,399 osteoporosis patients who received dental extractions between 2000 and 2010 (osteoporosis cohort) with 38,669 age- and gender-matched controls selected from dental extraction patients without osteoporosis or osteonecrosis history (comparison cohort). They finally concluded that osteoporosis itself played a very important role for the development of ONJ in that those individuals with osteoporosis had prolonged wound healing after tooth extraction, while BPs had a synergistic effect. In a recent study designed to elucidate the association between osteoporosis and delayed wound healing after tooth extraction, self-reported kyphosis was used as a surrogate marker for spine fractures, owing to its significant association with spine fractures [21]. In this study, which included 518 subjects (134 men and 384 women) aged 55–97 years, those who self-reported mild-moderate kyphosis were significantly more likely to have delayed wound healing after tooth extraction as compared with those who reported no kyphosis (odds ratio [OR], 4.98; 95% CI, 1.86–13.38) [22].
The process of fracture healing by intramembranous ossification and/or endochondral ossification involves many well-orchestrated events including the signaling, recruitment and differentiation of mesenchymal stem cells during the early phase; formation of a hard callus and extracellular matrix, angiogenesis and revascularization during the midphase; and callus remodeling at the late phase of fracture healing. Cheung et al. [23] described that through clinical and animal research, many of these factors are shown to be impaired in osteoporotic bone. Impairment of the bone healing system also may contribute to prolonged wound healing after tooth extraction in patients with osteoporosis.
2.4. BPs and periodontal disease
If osteoporosis or low BMD is associated with an increased risk of periodontal disease and subsequent tooth loss, osteoporosis medications like BPs may then reduce the risk of periodontal disease. In fact, investigators in some countries have observed a protective effect of BPs against the progression of periodontal disease in randomized controlled trials [24], [25], [26] and in a clinical trial [27]. In the United States, Lane et al. [25] found that BP therapy group (n = 43) significantly improved periodontal measures, including clinical attachment level (CAL), probing depth (PD), and bleeding on probing (BOP), relative to the placebo group (n = 27) during the 6- to 12-month period (CAL, P = 0.0002; PD, P = 0.0156; BOP, P = 0.0079). Previous findings of up to 2 years of follow-up have indicated that BPs may improve periodontal disease, one of the main oral infections leading to osteomyelitis of the jaws. Using a mouse model, Tanoue et al. [28] recently demonstrated that bone formation was not impeded by short-term alendronate therapy, but was actually enhanced.
3. Definition, staging, prevalence, and incidence of ARONJ in Asia
In the year after Marx [1] first reported an increased risk of BRONJ in oncology patients who had taken high dose i.v. BPs (2003), Ruggiero et al. [2] reported BRONJ cases in osteoporosis patients who had taken low-dose oral BPs. They noted a growing number of patients referred for evaluation and management of “refractory osteomyelitis” of varying duration. The typical presentation of this “refractory osteomyelitis” was a “nonhealing” extraction socket or exposed jawbone with progression to sequestrum formation associated with localized swelling and purulent discharge. The lesions were refractory to conservative debridement procedures and antibiotic therapy. Subsequently, several definitions of BRONJ or ARONJ have been reported worldwide. The definition of ONJ used in Asian countries is shown in Table 1.
Table 1.
The definition of ONJ in Asia.
| Country | Definition (yr) | The content of definition |
|---|---|---|
| Japan | Original (2016) | (1) Patients have a history of treatment with BP or Dmab; (2) Patients have no history of radiation therapy to the jaw. Bone lesions of ARONJ must be differentiated from cancer metastasis to the jawbone by histological examination; (3) Exposure of alveolar bone in the oral cavity, jaw, and/or face is continuously observed for longer than 8 weeks after first detection by a medical or dental expert, or the bone is palpable in the intra- or extraoral fistula for longer than 8 weeks. These criteria do not apply to stage 0 ARONJ. |
| Korea | AAOMS position paper (2014) | (1) Current or previous treatment with antiresorptive or antiangiogenic agents; (2) Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than 8 weeks; (3) No history of radiation therapy to the jaws or obvious metastatic disease to the jaws. |
| Hong Kong | ASBMR (2007) and AAOMS position paper (2009) | ASBMR: BRONJ was defined as an area of exposed bone in the maxillofacial region that did not heal within 8 week after identification by a health care provider, in a patient who was receiving or had been exposed to a BPs and had not had radiation therapy to the craniofacial region. AAOMS: (1) Current or previous treatment with a BP; (2) Exposed bone in the maxillofacial region that has persisted for more than 8 weeks; (3) No history of radiation therapy to the jaws. |
| Taiwan Singapore India Jordan |
AAOMS position paper (2009) | (as defined above) |
ONJ, osteonecrosis of the jaw; BP, bisphosphonate; Dmab, denosumab; ARONJ, antiresorptive agent-related osteonecrosis of the jaw; AAOMS, American Association of Oral and Maxillofacial Surgeons; ASBMR, American Society for Bone and Mineral Research.
3.1. Definition and staging of BRONJ or ARONJ in Asian countries
Migliorati et al. [29] described BRONJ to be the unexpected development of necrotic bone in the oral cavity of a patient receiving BP treatment and who has not received radiotherapy to the head or neck. The BRONJ primarily developed spontaneously, but usually appeared after dental surgery procedures, with poor healing or absence of healing over a period of 6–8 weeks. A Task Force of the ASBMR made the following recommendation for a provisional case definition as follows [30]: a confirmed case of BRONJ was defined as an area of exposed bone in the maxillofacial region that did not heal within eight weeks after identification by a health care provider, in a patient who was receiving or had been exposed to a BP and had not had radiation therapy to the craniofacial region. The 8-week duration is consistent with a time frame where most trauma, extractions, and oral surgical procedures would have resulted in soft tissue closure, and exposed bone would no longer be present. In the event that the lesion was spontaneous or history was lacking regarding its duration, the 8-week duration would start at the time that exposed bone was first documented by a health care provider.
In 2009, the American Association of Oral and Maxillofacial Surgeons (AAMOS) position paper on BRONJ adopted a working definition to distinguish BRONJ from other delayed healing conditions [31]. According to the AAMOS working definition, patients are considered to have BRONJ if all of the following characteristics are present: (1) current or previous treatment with a BP; (2) exposed bone in the maxillofacial region that has persisted for more than eight weeks; (3) no history of radiation therapy to the jaws. In a revised position paper of AAOMS in 2014 [32], the diagnosis of BRONJ was changed to medication-related ONJ (MRONJ), which reflects a consideration of the fact that ONJ also occurs for denosumab and bevacizumab (an angiogenesis inhibitor) [33]. Patients may be considered to have MRONJ if all the following characteristics are present: (1) current or previous treatment with antiresorptive or antiangiogenic agents; (2) exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than eight weeks; (3) no history of radiation therapy to the jaws or obvious metastatic disease to the jaws. The International Task Force on ONJ similarly defined ARONJ as: (1) exposed bone in the maxillofacial region that does not heal within 8 weeks after identification by a health care provider; (2) exposure to an antiresorptive agent; and (3) no history of radiation therapy to the craniofacial region [34].
With consideration to the AAOMS 2014 position paper and international consensus, in 2016, the Japanese Allied Committee on ONJ determined the following definition for ARONJ [35]: (1) patients have a history of treatment with BP or Dmab; (2) patients have no history of radiation therapy to the jaw. Bone lesions of ARONJ must be differentiated from cancer metastasis to the jawbone by histological examination; (3) exposure of alveolar bone in the oral cavity, jaw, and/or face is continuously observed for longer than 8 weeks after first detection by a medical or dental expert, or the bone is palpable in the intra- or extraoral fistula for longer than eight weeks. These criteria do not apply to stage 0 ARONJ.
Countries within Asia do not have a consistent definition for what defines ARONJ. The 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons adopted the AAOMS 2014 position paper [36], Hong Kong has used both the ASBMR and AAOMS 2009 position papers [37], [38], and Taiwan, Singapore, India, and Jordan have used the AAOMS 2009 position paper [39], [40], [41], [42]. There is no published consensus or position for other Asian countries regarding the definition of ARONJ.
The differential diagnosis of BRONJ, or ARONJ, should specifically exclude other common intraoral conditions including periodontal disease, gingivitis or mucositis, infectious osteomyelitis, temporomandibular joint disease, sinusitis, periapical pathology caused by a carious infection, osteoradionecrosis, neuralgia-inducing cavitational osteonecrosis, and bone tumors or metastases. However, magnetic resonance imaging (MRI) findings imply the possibility that infectious osteomyelitis may precede BRONJ in patients who have taken BPs [43], [44]. In the absence of exposed bone, these conditions should not be considered as cases of BRONJ. However, the term “stage 0” ONJ that shows nonexposed bone is affirmed by AAOMS to refer to any or all symptoms or signs such as bone pain, fistula track formation, abscess formation, altered sensory function, or abnormal radiographic findings extending beyond the confines of the alveolar bone in patients on antiresorptive therapy [32]. International Task Force members, however, expressed concern that the use of such stage 0 terminology may lead to overdiagnosis of ONJ because these same presenting symptoms may ultimately lead to an alternative diagnosis [34]. Although this stage is reported to account for 25%–30% of ONJ, half of proposed stage 0 cases heal without progression to stage 1. Overdiagnosing patients with ONJ could lead to detrimental effects in their skeletal health, especially if modification or discontinuation of antiresorptive medication is entertained. Conversely, the Japanese Allied Committee on ONJ includes ONJ stage 0, which is consistent with the AAOMS proposal from a therapeutic point of view [35]. However, the Japanese Allied Committee on ONJ is concerned with the risk of overdiagnosis, so caution is strongly recommended in diagnosing stage 0 ONJ.
3.2. Prevalence and incidence of BRONJ or ARONJ in Asia
The International Task Force on ONJ systematically summarized prevalence and incidence of ARONJ for osteoporosis patients worldwide and reported that the prevalence of ONJ in patients prescribed oral BPs for the treatment of osteoporosis ranges from 0% to 0.04%, with the majority being below 0.001% [34]. The prevalence of ONJ in patients prescribed high dose i.v. BPs was significantly higher than those administered low-dose i.v. or oral BPs, with prevalence rates of 0%–0.348% and the majority being under 0.005%. The incidence of ONJ in patients prescribed oral BPs for the treatment of osteoporosis ranged from 1.04 to 69 per 100,000 patient-years and the incidence of ONJ in patients prescribed i.v. BPs for the treatment of osteoporosis ranged from 0 to 90 per 100,000 patient-years. The incidence of ONJ in patients who were prescribed Dmab ranged from 0 to 30.2 per 100,000 patient-years.
Prevalence and incidence of BRONJ in Asia are summarized in Table 2. In nationwide surveys, the Japanese Society of Oral and Maxillofacial Surgeons (JSOMS) found 263 patients with BRONJ between 2006 and 2008 [45]. Of these patients, 104 (39.5%) developed BRONJ following oral BP administration. Based on this data and assuming that approximately 600,000 Japanese osteoporosis patients had taken oral BPs, the estimated incidence for this population was calculated as 0.01%–0.02% [46]. However, given that analyses of the Japanese medical center-based claims data estimated that the number of patients prescribed oral BPs was 2,082,928 and 2,470,979 in 2007 and 2008, respectively [47], the revised estimate for BRONJ in Japan was 0.002%–0.003%. Of note, compliance to oral BPs is relatively low in Japan, so this may be an underestimate of the true BRONJ incidence. Recently, the JSOMS identified approximately 2400 BRONJ patients who were prescribed oral or i.v. BPs for the treatment of osteoporosis during 2011–2013 (not published in English). If it is estimated that 1.5 to 2 million osteoporosis patients used oral or low i.v. dose BPs in Japan during that period (Dmab became available, decreasing population on BPs from earlier years) the estimated incidence of BRONJ was calculated to be 0.04%–0.05% or 40 to 50/100,000 patient-years. This estimated incidence is similar to that of Korean osteoporosis patients [48], [49] and of osteoporosis patients in Europe, North America, and Australia [34]. In fact, the accurate incidence of BRONJ in Japan is unknown, since data regarding the precise total number of patients treated with BPs are not available at the present time. However, it is likely that BRONJ incidence in Japan is increased over time.
Table 2.
The prevalence and incidence of BRONJ in osteoporosis patients in Asia.
| Country | Study | Evaluated year | Prevalence | Estimated incidence rate |
|---|---|---|---|---|
| Japan | Yoneda et al. [46] | 2006–2008 | 0.01%–0.02% 0.85/100,000 patient-years |
|
| Japana | Yoneda et al. [35] | 2011–2013 | 40–50/100,000 patient-years | |
| Korea | Lee et al. [49] | 2008 | 0.04% | |
| Korea | Hong et al. [48] | 2008–2010 | 0.05%–0.07% | |
| Hong Kong | Kwok et al. [38] | 2002–2012 | 0.31% | 73.53/100,000 patient-years |
| Taiwan | Lin et al. [50] | 2003–2007 | 6.9–8.2/10,000 patient-years | |
| Taiwan | Yuh et al. [39] | 2006–2008 | 41.3/100,000 patient-years | |
| Taiwan | Chiu et al. [51] | 2000–2012 | 283/100,000 patient-years | |
| Taiwan | Huang et al. [20] | 2000–2010 | 11.72/10,000 patient-years |
BRONJ, bisphosphonate-related osteonecrosis of the jaw.
Incidence was estimated by author of the current paper.
On the other hand, the estimated incidence of BRONJ in Hong Kong and Taiwan is larger than that of Japan and Korea, as well as other countries [20], [38], [50], [51] (Table 2). Chiu et al. [51] reported the incidence of BRONJ as 283/100,000 patient-years, using a retrospective pharmacy database at their hospital between 2000 and 2012. The incidence in Taiwanese osteoporosis patients is approximately 4 times of that in Sweden [52]; however, the prevalence of BRONJ in a patient population at the University of Southern California was extremely high (4%) [53]. Prevalence and incidence rates calculated from the hospital-based investigations should be taken with caution due to a number of biases, including selection bias.
Chiu et al. [51] described that the overall frequencies of ONJ after oral BP use reported in Asian populations were inconsistent, with 0.46%–0.99% in Japanese patients [54] and 0.05%–0.07% in Korean patients [48]. However, this Japanese data was evaluated in a hospital-based cohort from BP-induced osteomyelitis of the jaw (OMJ), but not BRONJ, between 2000 and 2010, although the authors of this study described that OMJ had similar characteristics to those in BP-induced ONJ even if necrotic bone could not be clinically visualized. The Taiwanese data by Chiu et al. [51] cannot be easily compared with BP-induced OMJ data in Japan.
Additionally, the overall frequency of BP-induced OMJ among patients receiving other osteoporosis drugs, excluding Dmab, in Japan was estimated to be 0.071%–0.17% [54]. Similarly, Chiu et al. [51] reported that the incidence of BRONJ was 35/100,000 patient-years in osteoporosis patients who had been prescribed raloxifene. This implies that other osteoporosis medications aside from BP and Dmab may also induce OMJ. Further, osteoporosis itself might be associated with an increased risk of OMJ given osteoporosis patients have a higher risk of having oral infectious foci like severe periodontal diseases compared to individuals without osteoporosis. Lin et al. [50] observed no significant difference in the incidence of ONJ between osteoporosis patients who had taken either BPs or calcitonin/raloxifene using the National Health Insurance Research Database from 2003 to 2007; there were 25 potential ONJ cases in the alendronate group (n = 18,030) and 21 in the calcitonin/raloxifene group (n = 25,615).
To date, no BRONJ cases have been reported in a randomized controlled trial in Asian countries, although the number of participants was relatively smaller than those in other countries, especially in the United States or European countries [34]. Sugimoto et al. [55] observed one ONJ case in 810 participants in their Dmab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). DIRECT included a two-year randomized, double-blind, placebo-controlled phase and a 1-year open-label extension phase in which all subjects received Dmab. One ONJ case was found in subjects who received 2 years of placebo in the double-blind phase and 1 year of Dmab treatment (cross-over group), but not in subjects who received 3 years of Dmab (long-term group). This suggests that the incidence of Dmab-related ONJ may be about 62 per 100,000 patient-years. However, the number of possible Dmab-related ONJ cases in osteoporosis patients in Japan was only 5 between June, 2013 and December, 2015 [35]. The one ONJ case observed by Sugimoto et al. [55] might be an incidental finding. The International Task Force on ONJ reported that the incidence of ONJ in patients who are prescribed Dmab ranges from 0 to 30.2 per 100,000 patient-years [34]; however, we cannot estimate the incidence of Dmab-related ONJ because of a lack of data in Asia.
In Japan, an investigation found that in patients with ONJ approximately 40% between 2006 and 2008 and about half between 2011 and 2013 developed ONJ following oral BP administration [35], [46]. In Korea, 78.7% of ONJ occurred in patients who were receiving oral BPs and 21.3% were receiving BP through i.v. injection [49]. These results in Japan and Korea seem to be different from those obtained in other countries, which have shown a higher incidence of BRONJ in patients treated with high dose i.v. (95%) versus low dose oral BPs (5%) [34]. However, this is not specific to Asia because Rogers et al. [56] recently reported details of 368 BRONJ cases in the United Kingdom in which BP had been given orally in 207 (56%), intravenously in 125 (34%), both orally and intravenously in 27 (7%), and was unknown in 9 (2%). The number of patients with ONJ being more from oral BP group is likely a representation of the fact that oral BP are so widely used that the millions of patients on them collectively had more cases of ONJ than the smaller number of patients on i.v. BP.
4. Imaging for ARONJ
4.1. Role of imaging for ARONJ
The Japanese Society for Oral and Maxillofacial Radiology asked representatives from 23 oral radiology departments (25 responders), about the role of imaging modalities for BRONJ [57]. Sixteen responders (64%) selected panoramic radiographs as the best imaging modality for the screening of BRONJ and seventeen responders (68%) answered that intraoral radiographs are necessary for the diagnosis of BRONJ. Some reasons were given for the necessity of intraoral radiographs including the ability to easily observe the details of the teeth (lamina dura and periodontal ligament space) and changes in the surrounding trabecular bone structure (osteolysis or osteosclerosis). However, about half of the responders felt that the diagnosis of BRONJ based on imaging alone was difficult. No specific modality was selected for identifying the early signs of BRONJ, although 19 responders selected multidetector computed tomography (CT), followed by MRI, as the best modality for determining the extent of BRONJ. Almost all responders felt that the detection of early signs and determination of lesion extent on imaging were important roles of oral and maxillofacial radiologists.
Previous studies reported that lamina dura thickening and osteosclerotic changes may be early signs of BRONJ [46]. Hamada et al. [58] reported that measuring cancellous bone CT density has the potential to be a simple and quantitative method to detect the early stages of BRONJ. Taniguchi et al. [59] also described that the cancellous bone CT values were higher in the BP-treated group, including in patients with stage 0 BRONJ, and that CT may provide useful quantitative information. However, Suei [60] described that osteosclerotic changes indeed were frequent and remarkable, but not specific. He also mentioned that a relationship between lamina dura thickening and the lesion was doubtful.
4.2. What kind of imaging modalities should be used?
For patients undergoing treatment with low-dose antiresorptive agents, and who have no evident clinical manifestations of ARONJ, intraoral and panoramic radiographs in conjunction with clinical manifestations are sufficient for diagnosis [35]. Intraoral radiographs, which have high resolution, can reveal the site of infection in detail. For patients who are clinically suspected of having ONJ, CT and dental cone-beam CT are helpful for detecting early changes in trabecular and cortical bones of the jaw and in assessing sequestra, fistula formation, periosteal responses, and involved teeth. However, the use of dental cone-beam CT is limited to localized lesions and as a supplement to CT. CT must be combined with intraoral and panoramic radiographs. For cases in which differential diagnosis between ONJ and malignant tumors is required, the use of CT and MRI is recommended.
MRI may be useful for early assessment of changes in the bone marrow in osteoporosis patients with ONJ, as well as the assessment of the surrounding soft tissue [43], [44]. Ariji et al. [61] described that in the early stages of ONJ, the necrotic region displayed decreased signal intensity on T1-weighted images and normal signal intensity on T2-weighted images without fat saturation. In late stages of ONJ, the signal intensity of the bone marrow on T2-weighted images is more variable: the exposed diseased bone displayed decreased signal intensity and the unexposed diseased bone displayed increased signal intensity. For ONJ patients undergoing conservative and/or surgical treatment, the characteristics and extent of bone changes surrounding exposed bone can be assessed by CT and dental CT. Existing teeth that may be a cause of infection can be detected by intraoral radiographs.
Hong et al. [62] reported that 3-phase bone scintigraphy may provide a relatively sensitive means of detecting BRONJ and as such would be helpful for accurate BRONJ staging. Miyashita et al. [63] have proposed that the integrated single-photon emission CT and CT system (SPECT/CT) may allow oral and maxillofacial surgeons to identify an area of ONJ, especially when 3-dimensional SPECT and CT fusion images are offered. They have reported success in a patient where these imaging modalities were used together to determine the extent of the ONJ lesion, leading to a favourable patient prognosis. Simple positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) and PET/CT may also be useful for assessment of ONJ lesions. Yamazaki et al. [64] and Fatema et al. [65] demonstrated that FDG-PET may predict the effect of hyperbaric oxygen therapy in BRONJ. Despite there being many imaging modalities that can be employed, no specific differences in images between BRONJ and Dmab-related ONJ have been described.
5. Risk factors for ARONJ
5.1. Risk factors for ARONJ in Asian countries
Based on odds or hazard ratios obtained by systematic review, The International Task Force on ONJ described that significant risk factors for the development of ONJ in the osteoporosis patient population, in declining order of magnitude, included: suppuration; BP use; dental extraction; and anemia [34]. The Japanese Allied Committee on ONJ recently presented tooth extraction, dental implant, or apical/periodontal surgery as definitive local risk factors for ARONJ [35]. Additional local risk factors included ill-fitting dentures, excessive bite force, poor oral hygiene, periodontal disease, gingival abscess, inflammatory disease including apical periodontitis, mandibular torus, palatal torus, and mylohyoid line torus. Root canal and orthodontic treatments were not considered to be risk factors. However, this list of risk factors was not based on robust medical evidence, but rather was a summary of published reports investigated by the Allied Committee. Systemic factors included diabetes, rheumatoid arthritis (RA), hypocalcemia, hypoparathyroidism, osteomalacia, vitamin D deficiency, renal dialysis, anemia, and Paget disease of bone. Chiu et al. [51] presented that advanced age, drug duration, and coexisting diabetes and RA are contributing factors to the development of oral alendronate-related ONJ [51]. Paek et al. [66] reported that the effects of diabetes and hypertension were not statistically significant for the development of BRONJ. When not considering BP use, RA exhibited a high odds ratio of 3.23 (P = 0.094) for development of ONJ [66]. Furuya et al. [67] investigated participants of the Institute of Rheumatology, Rheumatoid Arthritis cohort study and found that the prevalence of ONJ was 0.094% among all RA patients and 0.26% among female RA patients ≥65 years of age, suggesting female gender and age to be a risk factor.
Lifestyle factors increasing the risk of ONJ included smoking, drinking, and obesity and coadministered agents related to ARONJ were corticosteroids, erythropoietin, angiogenic inhibitors and tyrosine kinase inhibitors [35]. Single-nucleotide polymorphisms in MMP-2 and cytochrome P450-2C genes were proposed as genetic risk factors [35]. With reference to genetic factors, Choi et al. [68] concluded that the CC homozygotes of rs2010963 and rs3025039 polymorphisms in the VEGF gene were associated with an increased risk of BRONJ in the Korean population. In Japan, many of the risk factors mentioned above for ARONJ has no published evidence to support their importance. Risk factors in other Asian countries are based on the AAOMS position paper [31], [32] and these include long duration of BPs use, use of corticosteroids, older age, diabetes, genetic factors, anemia, hyperthyroidism, dialysis, intraoral surgery such as tooth extraction, denture use, mandibular torus, mylohyoid ridge, palatine torus and dental or gingival abscesses.
5.2. Tooth extraction and long duration of BPs use for ONJ
In an investigation of a large Taiwanese cohort, Huang et al. [20] found that osteoporosis patients who had longer durations or higher dosages of BPs and higher frequency of dental extractions had a significantly higher risk for BRONJ than those without BPs treatment. In another Taiwanese cohort, Yuh et al. [39] found that tooth extraction was a major risk factor for the development of BRONJ. Additionally, in another large cohort study from Taiwan, Chiu et al. [51] described that advanced age, duration of BPs, and coexisting diabetes and RA were contributing factors for the development of BRONJ in osteoporosis patients who had used BPs. In Korea, Hong et al. [48] reported that long-term BPs therapy was a major risk factor for the development of ONJ. In an analysis of the National Cohort Study of Korea, Kwon et al. [69] adjusted for patient comorbidities and observed that patients with osteoporosis had increased odds of having BRONJ if they were continuous BP users (1–2 years; OR, 3.9; 95% CI, 2.4–6.2) as compared to nonusers, although there were no significant increases in recent short (0- to 1-year use) and past users.
As mentioned above, tooth extraction and longer duration of BPs use may be important risk factors for the development of ONJ. In a cohort study including all patients undergoing tooth extraction at a university hospital in Japan from 2006 to 2009, Yamazaki et al. [70] reported that BP administration was associated with the development of ONJ after tooth extraction, with an unadjusted risk ratio of 122.6 (95% CI, 14.4–1041.8). However, when stratified by age and route of BP administration, the risk ratio for ONJ patients aged 65 years or older with oral BPs administration compared to those without did not significantly increase. Matsumoto et al. [71] recently concluded that tooth extraction with proper wound closure to avoid secondary infection was effective for the prevention of ARONJ even in high-risk patients. Otto et al. [72] also concluded that risk factor may not be tooth extractions themselves, but rather the prevailing infectious conditions that may provide a key risk factor for the development of BRONJ.
Chiu et al. [51] reported that in the Taiwanese osteoporosis population the risk of ONJ began after 1 year of alendronate initiation and that the incidence of ONJ progressively increased with longer durations of alendronate treatment, ranging from 0.25% for 2 years of alendronate use to 0.92% for 10 years of alendronate use. In a large integrated health care delivery system in Northern California, Lo et al. [73] also found that the incidence of ONJ increased notably from 1 year of BP therapy to 4 years of therapy; however, they did not observe an occurrence of ONJ after 6 years of BPs use. Nakamura et al. [74] recently analyzed the Japanese Adverse Drug Event Report database and concluded that patients administered BPs for osteoporosis therapy should be closely monitored for the occurrence of BRONJ for at least 3 years following initiation. The results of the shape parameter of the Weibull distribution indicated that the risk of BRONJ with the oral BPs, minodronate and risedronate, was almost constant. However, the risk of BRONJ with i.v. BPs for oncology patients increased over time.
Sumi et al. [75] recently evaluated the impact of risk communication from pharmaceutical manufacturers and academic associations to the incidence of BRONJ by using previously constructed data from a retrospective cohort study. They described that the incidence of ONJ in the BP group increased in their cohort, although the rapid increase occurred 3 years after the risk communications began in 2006. They hypothesized that there might have been other causes for the rapid increase in the incidence of ONJ in their cohort in addition to risk communication; one possibility was the longer exposure to BPs in the cohort. However, since the incidence of ONJ was constant (about 0.2%) between 2000 and 2008 and the rapid increase in incidence occurred after 2009 (about 0.75%) in Japan, it is doubtful that longer exposure may be one of the risk factors explaining the rapid increase of ONJ in Japan.
Felsenberg et al. [76] did not observe a significant association between incidence of BRONJ and duration of BPs use in a prospective study of a German population. The Task Force of the ASBMR recently described that ONJ does not increase with BP therapy duration in the osteoporosis population [6]. Further investigations are needed to clarify whether longer duration of BPs and Dmab use is associated with an increased risk of ARONJ in osteoporosis populations in Asia.
6. How to prevent ARONJ
6.1. Case presentation
A 77-year-old Japanese woman visited our Hospital with complaints of spontaneous pain, gingival swelling and tooth mobility in the upper left jaw. She had taken alendronate for 7 years for osteoporosis. There were no other apparent risk factors for ONJ. In accordance with the first Japanese position paper on ONJ [46] (minor revision in 2012), the dentist discontinued alendronate for 3 months prior to extraction of the second molar because of a severe periodontal abscess in the upper left jaw. Medication was changed to vitamin D only. During the period of alendronate discontinuation, antibiotics were used for preventing the spread of infection. However, the symptoms worsened 1 month after discontinuation. Oral radiographs revealed the spread of dental infection to 1st and 2nd premolars in upper left jaw, indicating the occurrence of osteomyelitis of the left maxilla (Fig. 1). After waiting for three months following alendronate discontinuation, the dentist extracted the 2nd M as well as 1st and 2nd premolars. After the extraction of these teeth, ONJ occurred in the upper left jaw (Fig. 2, Fig. 3). Following surgical removal of the sequestrum, the ONJ resolved fully.
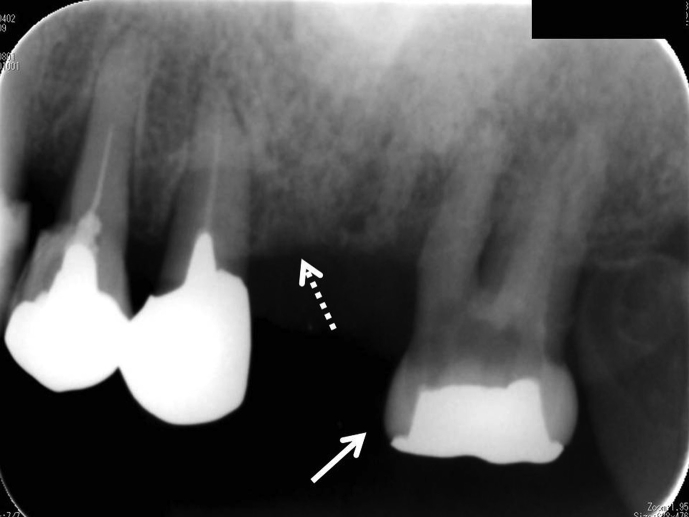
Fig. 1.
Oral radiograph of upper left jaw reveals 2nd M with periodontal abscess (white arrow) and periodontal involvement of (white dotted line) the 1st and 2nd premolars.
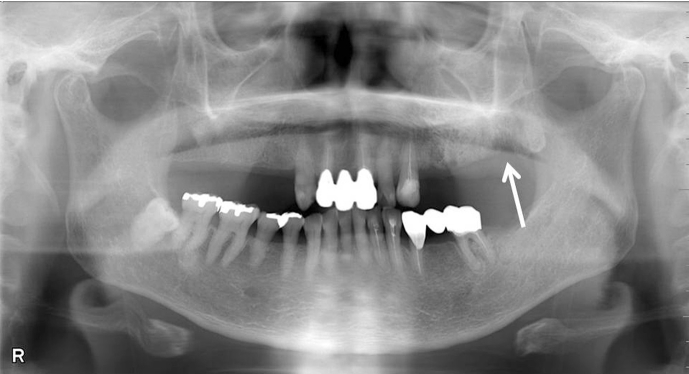
Fig. 2.
Dental panoramic radiograph after extraction of 2nd M (focus of infection) as well as 1st and 2nd premolars in upper left jaw reveals the occurrence of osteonecrosis of the jaw surrounded by osteosclerotic bone (white arrow).
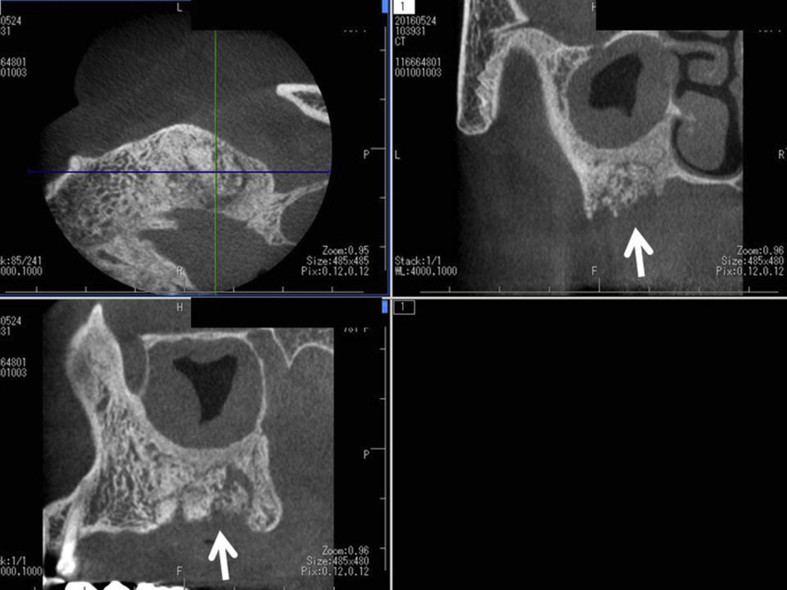
Fig. 3.
Dental cone beam computed tomography after extraction of the teeth shows the presence of sequestrum (white arrow) that seems to separate from surrounding bone in upper left jaw. Maxillary sinusitis is also found.
6.2. Dental emergency or temporary discontinuation in dental treatments
The AAOMS 2014 position paper described an increased incidence of BRONJ in patients with osteoporosis who were treated with BPs for longer than four years. This conclusion was based on retrospective studies with a small number of cases [73]. As a result, AAOMS recommended that patients who have received antiresorptive therapy for longer than 4 years and who have a low fracture risk but potentially high risk for ONJ that discontinuation of antiresorptive treatment for approximately 2 months prior to invasive dental treatment, such as tooth extraction, should be considered. Since the prior AAOMS recommendation was 3 months discontinuation prior to invasive dental treatment in those administered BPs for more than 3 years, the first Japanese position paper essentially mirrored this. Recently, the 2017 position paper of the Japanese Allied Committee on ONJ suggested that a BP temporary discontinuation prior to invasive dental treatment was not logically supported at the viewpoint of several pieces of background information collectively [35]. However, this did not deny the temporary discontinuation prescribed by the 2014 AAOMS position paper, resulting in further confusion among physicians, dentists, and osteoporosis patients.
When considering a temporary discontinuation of BPs before tooth extraction in osteoporosis patients, the larger benefit of preventing osteoporosis fractures in comparison with the smaller risk of ONJ and atypical fracture needs to be considered as a risk:benefit ratio. Brown et al. [77] described that ONJ and atypical fractures might be related to the use of BPs in osteoporosis, but they are exceedingly rare and they often occur with other comorbidities or concomitant medication use. Additionally, there is little clinical evidence that temporary discontinuation of BPs helps to prevent the occurrence of BRONJ resulting from invasive dental treatments [78]. Based on the physicochemical properties of BPs that are deposited and persist in the bone for a long period of time, it appears unlikely that a temporary BP discontinuation would prevent BRONJ.
On the other hand, it is suggested that dental infection may be a key event for BRONJ and that infection control before invasive dental treatment reduced the risk of BRONJ [35]. At the viewpoint of dental infection control, BPs should not be temporarily discontinued before tooth extraction even in osteoporosis patients with a low risk of fractures because the teeth that should be extracted may be a focus of spreading infection, resulting in the development of osteomyelitis and ONJ. In fact, a survey conducted by the Japan Osteoporosis Society (JOS) reported discontinuation of antiresorptive treatment before tooth extraction increased both fractures and ONJ, in contrast, there were no ONJ cases in osteoporosis patients who continued BPs before tooth extraction, suggesting that the timely dental procedures successfully prevented ONJ even under the usage of BPs [79]. The reason that the number of BRONJ cases rapidly increased between 2011 and 2013 in comparison with between 2006 and 2008 in osteoporosis patients in Japan may be more associated with dental emergencies related to extensive infection control. It is suggested that dental professionals first consider the dental emergencies rather than the recommendation from the AAOMS position paper regarding temporary discontinuation when confronted with teeth that should be extracted as soon as possible to preserve overall oral health. The case presented previously (6-1) may be a typical one of developing ONJ during the temporary discontinuation.
With respect to the prevention of ONJ, the International Task Force on ONJ in 2015 [34] recommended strategies to reduce the risk of ONJ that included completion of necessary oral surgery prior to the initiation of antiresorptive therapy, the use of antibiotics before and/or after the procedure, antimicrobial mouth rinsing, appropriate closure of the wound following tooth extraction, and maintenance of good oral hygiene. Additionally, the International Task Force presented the following concept for the prevention of ARONJ in 2016 [80]: (1) everyone should maintain good oral hygiene (stop smoking) and see their dentist every 6 months (or as recommended based on oral disease risk); (2) routine dental work, such as dental cleaning, fillings or root canals should be performed as usual and do not require stopping your osteoporosis treatment; (3) the recommendations for patients with osteoporosis receiving lower doses of BP or Dmab therapy are similar to those which apply to all individuals; (4) if oral surgery is needed, it is ideal to have the surgery completed before starting antiresorptive therapy, if possible. Individuals with risk factors for ONJ who are taking BP or Dmab therapy may be advised to stop treatment after dental surgery and to restart therapy after the surgical site has completely healed (mature soft tissue closure). However, recent progress in the treatment of osteoporosis is capable of prolonging the interval of drug administration. For instance, the interval of Dmab administration is 6-month and that of zoledronic acid is 1-yearly. If osteoporosis patients received them, the recommended temporary discontinuation itself seems to be nonsense. We have to propose the other rational strategy for the prevention of ONJ.
The above 2016 International Task Force recommendation is basically the same for ARONJ prevention in oncology patients [34]. In a prospective study of randomized patients with prostate cancer treated with zoledronic acid, Mücke et al. [81] recently demonstrated that the incidence rate per year (IR) of BRONJ in the control group was 0.073 cases/year while the IR in the prevention intervention group, even with a dental infection, was significantly decreased by 82% to 0.0131 (P < 0.001), although a significantly higher proportion of dental extractions was observed in the intervention group vs. control group (26.7% vs. 22.7%, P = 0.006). The incidence rate of ARONJ in osteoporosis patients is relatively low compared with that in oncology patients. It is reasonable that the recommendation mentioned by the International Task Force on ONJ may considerably reduce the incidence of ARONJ in osteoporosis patients.
7. Cooperation between physicians and dentists in the prevention of ARONJ
Both physicians and dentists need to be aware of ARONJ. Cooperation between physicians and dentists is necessary to prevent ARONJ, along with a common understanding that eliminating dental infections is a key factor for preventing it. Yoo et al. [82] randomly selected 264 dental practitioners (2%) from 13,405 dentists and reported that a total of 56.5% of the respondents had heard of BRONJ, but only 31.4% routinely recorded BP medication history. Most dentists were unaware of the 2009 AAOMS position paper. Dentists with <5 years of clinical experience were significantly more aware of ARONJ than those with >5 years of experience. Experience with treating patients with ONJ and recording medication histories was significantly greater in dental hospitals with >300 beds or university hospitals. Awareness of the severity of BRONJ was greatest among oral surgeons in this Korean Survey. This survey showed the lack of awareness and knowledge of BRONJ in Korean general dental practitioners, resulting in the possibility of less cooperation between physicians and dentists for preventing ONJ. In a survey of the Department of Dentistry, Riyadh Military Hospital, Kingdom of Saudi Arabia in 2010, Al-Mohaya et al. [83] found that physicians and dentists had low awareness and deficient knowledge regarding BRONJ, although most of the physicians did prescribe BPs to their patients. They concluded that intervention to raise awareness and knowledge among healthcare providers was needed. On the other hand, in a web-based survey in Ontario, Canada, where the rate of ARONJ was extremely low (1.04/100,000 patients-years [84]), 60% of general dentists and specialists, such as oral surgeons, had a good knowledge about BRONJ [85]. Most of them were not comfortable performing oral surgery in patients taking BPs; however, since the dentists who were comfortable had higher knowledge scores, they suggested greater educational efforts should be made to promote the knowledge of dentists regarding BP and BRONJ.
Recent surveys from both the Adequate Treatment of Osteoporosis Research Group (A-TOP mainly consisted of primary care doctors, 206 respondents) in Japan and JOS (mainly consisted of doctors belonging to academic institution, 629 respondents) indicated that almost all physicians (98%) had knowledge about BRONJ and the first Japanese position paper regarding BRONJ (75%–76%) [79], [86]. However, approximately 62% of respondents in the JOS group and 76% in A-TOP group did not request oral health care by a dentist before antiresorptive therapy, and 72% in both groups reported no cooperation between physicians and dentists in their region. We concluded that immediate development of a strategy for sharing information about ONJ among physicians, dentists, and patients should be required to reduce the incidence of both ONJ and skeletal events in osteoporosis treatment. Kim et al. [87] performed a survey in Korea and found that most of physicians except oncologist did not feel the necessity of dental examination before and during antiresorptive therapy. It is possible that poor cooperation between physicians and dentists may contribute to an increased incidence of ARONJ.
8. Conclusions
To date, there have been many reports about ONJ, BRONJ, ARONJ, and MRONJ in human studies worldwide; however, there is still a relatively smaller number of reports in Asian countries. References used in Asia are based on position papers or guidelines from other countries. However, since it is considered that the Asian population might have a higher incidence of BRONJ or ARONJ in comparison with the population of other countries, we at least need more information in Asia, such as the following: (1) What definitions for ARONJ should be used? (2) What is the incidence of ARONJ in Asian countries? (3) What are the specific risk factors for ARONJ? (4) How do Asians respond to oral surgical procedures such as tooth extraction with respect to the development of ONJ? (5) How knowledgeable is the Asian population about BRONJ or ARONJ.
The Asian Federation of Osteoporosis Societies (AFOS) consists of 10 Asian countries. Ideally, the countries in the AFOS should cooperate with each other and develop evidence specific for Asia for the prevention of ARONJ because both the number of osteoporotic fractures and the use of antiresorptive agents such as BPs and Dmab will increase in Asia compared with use in other countries in the future.
Conflict of interest
Akira Taguchi has received lecture fee from Asahi Kasei Pharma Co, MSD, Ono Pharmaceutical, Daiichi-Sankyo, Takeda Pharmaceutical, Chugai Pharmaceutical, and Teijin Pharma and consultant fee from Asahi Kasei Pharma Co. Masataka Shiraki has received consultant fee from Asahi-Kasei Pharama Co and MSD and lecture fee from Chugai Pharmaceutical, Teijin Pharma, Ono Pharmaceutical, Daiichi-Sankyo and Eisai Co. Aliya Khan has received research funds from Amgen, Merck, Shire and speaker honoraria from Amgen and Lilly. Archie Morrison has no conflicts of interest to declare.
Acknowledgments
We thank Dr. Shawn Davison in the Department of Education, University of Victoria, Canada, for his assistance in the literature review for this manuscript. This work was supported, in part, by JSPS KAKENHI (grant numbers JP15K11092, JP26463148).
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero S.L., Mehrotra B., Rosenberg T.J., Engroff S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Migliorati C.A., Siegel M.A., Elting L.S. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–514. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 4.Papapoulos S., Chapurlat R., Libanati C., Brandi M.L., Brown J.P., Czerwiński E. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Min Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballane G., Cauley J.A., Luckey M.M. Fuleihan Gel-H. Secular trends in hip fractures worldwide: opposing trends East versus West. J Bone Min Res. 2014;29:1745–1755. doi: 10.1002/jbmr.2218. [DOI] [PubMed] [Google Scholar]
- 6.Adler R.A., El-Hajj Fuleihan G., Bauer D.C., Camacho P.M., Clarke B.L., Clines G.A. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the american society for bone and mineral research. J Bone Min Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha S., Wang Z., Laucis N., Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996-2012: an ecological analysis. J Bone Min Res. 2015;30:2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniell H.W. Postmenopausal tooth loss. Contributions to edentulism by osteoporosis and cigarette smoking. Arch Intern Med. 1983;143:1678–1682. doi: 10.1001/archinte.143.9.1678. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A., Fujiwara S., Masunari N., Suzuki G. Self-reported number of remaining teeth is associated with bone mineral density of the femoral neck, but not of the spine, in Japanese men and women. Osteoporos Int. 2004;15:842–846. doi: 10.1007/s00198-004-1609-2. [DOI] [PubMed] [Google Scholar]
- 10.Jang K.M., Cho K.H., Lee S.H., Han S.B., Han K.D., Kim Y.H. Tooth loss and bone mineral density in postmenopausal South Korean women: the 2008-2010 Korea national health and Nutrition examination survey. Maturitas. 2015;82:360–364. doi: 10.1016/j.maturitas.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki M., Nakamura K., Yoshihara A., Miyazaki H. Change in bone mineral density and tooth loss in Japanese community-dwelling postmenopausal women: a 5-year cohort study. J Bone Min Metab. 2012;30:447–453. doi: 10.1007/s00774-011-0337-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.W., Kong K.A., Kim H.Y., Lee H.S., Kim S.J., Lee S.H. The association between bone mineral density and periodontitis in Korean adults (KNHANES 2008-2010) Oral Dis. 2014;20:609–615. doi: 10.1111/odi.12179. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki M., Taylor G.W., Nakamura K., Yoshihara A., Miyazaki H. Association between low bone mineral density and clinical attachment loss in Japanese postmenopausal females. J Periodontol. 2013;84:1708–1716. doi: 10.1902/jop.2013.120613. [DOI] [PubMed] [Google Scholar]
- 14.Shum I., Leung P.C., Kwok A., Corbet E.F., Orwoll E.S., Phipps K.R. Periodontal conditions in elderly men with and without osteoporosis or osteopenia. J Periodontol. 2010;81:1396–1402. doi: 10.1902/jop.2010.100052. [DOI] [PubMed] [Google Scholar]
- 15.Chang W.P., Chang W.C., Wu M.S., Pai J.T., Guo Y.C., Chen K.C. Population-based 5-year follow-up study in Taiwan of osteoporosis and risk of periodontitis. J Periodontol. 2014;85:e24–30. doi: 10.1902/jop.2013.130256. [DOI] [PubMed] [Google Scholar]
- 16.Juluri R., Prashanth E., Gopalakrishnan D., Kathariya R., Devanoorkar A., Viswanathan V. Association of postmenopausal osteoporosis and periodontal disease: a double-blind case-control study. J Int Oral Health. 2015;7:119–123. [PMC free article] [PubMed] [Google Scholar]
- 17.Al Habashneh R., Alchalabi H., Khader Y.S., Hazza'a A.M., Odat Z., Johnson G.K. Association between periodontal disease and osteoporosis in postmenopausal women in Jordan. J Periodontol. 2010;81:1613–1621. doi: 10.1902/jop.2010.100190. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi A., Suei Y., Ohtsuka M., Nakamoto T., Lee K., Sanada M. Relationship between self-reported periodontal status and skeletal bone mineral density in Japanese postmenopausal women. Menopause. 2005;12:144–148. doi: 10.1097/00042192-200512020-00007. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y.F., Chang C.T., Liu S.P., Muo C.H., Tsai C.H., Hong H.H. The impact of oral hygiene maintenance on the association between periodontitis and osteoporosis: a nationwide population-based cross sectional study. Med Baltim. 2016;95 doi: 10.1097/MD.0000000000002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y.F., Chang C.T., Muo C.H., Tsai C.H., Shen Y.F., Wu C.Z. Impact of bisphosphonate-related osteonecrosis of the jaw on osteoporotic patients after dental extraction: a population-based cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamimura M., Nakamura Y., Sugino N., Uchiyama S., Komatsu M., Ikegami S. Associations of self-reported height loss and kyphosis with vertebral fractures in Japanese women 60 years and older: a cross-sectional survey. Sci Rep. 2016;6:29199. doi: 10.1038/srep29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taguchi A., Kamimura M., Nakamura Y., Sugino N., Ichinose A., Maezumi H. Delayed wound healing after tooth extraction and self-reported kyphosis in Japanese men and women. Sci Rep. 2016;6:36309. doi: 10.1038/srep36309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung W.H., Miclau T., Chow S.K., Yang F.F., Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47(Suppl 2):S21–S26. doi: 10.1016/S0020-1383(16)47004-X. [DOI] [PubMed] [Google Scholar]
- 24.Rocha M.L., Malacara J.M., Sánchez-Marin F.J., Vazquez de la Torre C.J., Fajardo M.E. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol. 2004;75:1579–1585. doi: 10.1902/jop.2004.75.12.1579. [DOI] [PubMed] [Google Scholar]
- 25.Lane N., Armitage G.C., Loomer P., Hsieh S., Majumdar S., Wang H.Y. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005;76:1113–1122. doi: 10.1902/jop.2005.76.7.1113. [DOI] [PubMed] [Google Scholar]
- 26.Jeffcoat M.K., Cizza G., Shih W.J., Genco R., Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol. 2007;9:70–76. [PubMed] [Google Scholar]
- 27.Bhavsar N.V., Trivedi S.R., Dulani K., Brahmbhatt N., Shah S., Chaudhri D. Clinical and radiographic evaluation of effect of risedronate 5 mg as an adjunct to treatment of chronic periodontitis in postmenopausal women (12-month study) Osteoporos Int. 2016;27:2611–2619. doi: 10.1007/s00198-016-3577-8. [DOI] [PubMed] [Google Scholar]
- 28.Tanoue R., Koi K., Yamashita J. Effect of alendronate on bone formation during tooth extraction wound healing. J Dent Res. 2015;94:1251–1258. doi: 10.1177/0022034515592867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migliorati C.A., Casiglia J., Epstein J., Jacobsen P.L., Siegel M.A., Woo S.B. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American academy of oral medicine position paper. J Am Dent Assoc. 2005;136:1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 30.Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling P.R., Felsenberg D. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the american society for bone and mineral research. J Bone Min Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero S.L., Dodson T.B., Assael L.A., Landesberg R., Marx R.E., Mehrotra B. American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Greuter S., Schmid F., Ruhstaller T., Thuerlimann B. Bevacizumab-associated osteonecrosis of the jaw. Ann Oncol. 2008;19:2091–2092. doi: 10.1093/annonc/mdn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan A.A., Morrison A., Hanley D.A., Felsenberg D., McCauley L.K., O'Ryan F. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Min Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 35.Japanese Allied Committee on Osteonecrosis of the Jaw, Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S. Antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese allied committee on osteonecrosis of the jaw. J Bone Min Metab. 2017;35:6–19. doi: 10.1007/s00774-016-0810-7. [DOI] [PubMed] [Google Scholar]
- 36.Kim K.M., Rhee Y., Kwon Y.D., Kwon T.G., Lee J.K., Kim D.Y. Medication related osteonecrosis of the jaw: 2015 position statement of the Korean Society for bone and mineral research and the Korean association of oral and maxillofacial surgeons. J Bone Metab. 2015;22:151–165. doi: 10.11005/jbm.2015.22.4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong C.K., Ho S.T., Wong S.L. Osteonecrosis of the jaw after oral bisphosphonate for osteoporosis. Hong Kong Med J. 2010;16:145–148. [PubMed] [Google Scholar]
- 38.Kwok T., Choy T.K., Kwok W.L. Prevalence of bisphosphonate-related osteonecrosis of the jaw in Hong Kong. Hong Kong Med J. 2016;22(Suppl 2):S46–S47. [PubMed] [Google Scholar]
- 39.Yuh D.Y., Chang T.H., Huang R.Y., Chien W.C., Lin F.G., Fu E. The national-scale cohort study on bisphosphonate-related osteonecrosis of the jaw in Taiwan. J Dent. 2014;42:1343–1352. doi: 10.1016/j.jdent.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Suresh E., Pazianas M., Abrahamsen B. Safety issues with bisphosphonate therapy for osteoporosis. Rheumatol Oxf. 2014;53:19–31. doi: 10.1093/rheumatology/ket236. [DOI] [PubMed] [Google Scholar]
- 41.Kumar V., Sinha R.K. Bisphosphonate related osteonecrosis of the jaw: an update. J Maxillofac Oral Surg. 2014;13:386–393. doi: 10.1007/s12663-013-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baqain Z.H., Sawair F.A., Tamimi Z., Bsoul N., Al Edwan G., Almasad J.K. Osteonecrosis of jaws related to intravenous bisphosphonates: the experience of a Jordanian teaching hospital. Ann R Coll Surg Engl. 2010;92:489–494. doi: 10.1308/003588410X12699663903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Ferrer L., Bagán J.V., Martínez-Sanjuan V., Hernandez-Bazan S., García R., Jiménez-Soriano Y. MRI of mandibular osteonecrosis secondary to bisphosphonates. AJR Am J Roentgenol. 2008;190:949–955. doi: 10.2214/AJR.07.3045. [DOI] [PubMed] [Google Scholar]
- 44.Guggenberger R., Fischer D.R., Metzler P., Andreisek G., Nanz D., Jacobsen C. Bisphosphonate-induced osteonecrosis of the jaw: comparison of disease extent on contrast-enhanced MR imaging, [18F] fluoride PET/CT, and conebeam CT imaging. AJNR Am J Neuroradiol. 2013;34:1242–1247. doi: 10.3174/ajnr.A3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urade M., Tanaka N., Furusawa K., Shimada J., Shibata T., Kirita T. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;69:e364–e371. doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S. Bisphosphonate-related osteonecrosis of the jaw: position paper from the allied task force committee of Japanese society for bone and mineral research, Japan osteoporosis society, Japanese society of periodontology, Japanese society for oral and maxillofacial radiology, and Japanese society of oral and maxillofacial surgeons. J Bone Min Metab. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 47.Pharmaceuticals and medical devices agency safety information. Pharmaceuticals and Medical Devices Agency; Tokyo: 2010 September. http://www1.mhlw.go.jp/kinkyu/iyaku_j/iyaku_j/anzenseijyouhou/272.pdf [Internet] c2017 [cited 2017 Jan 7]. Available from: [Google Scholar]
- 48.Hong J.W., Nam W., Cha I.H., Chung S.W., Choi H.S., Kim K.M. Oral bisphosphonate-related osteonecrosis of the jaw: the first report in Asia. Osteoporos Int. 2010;21:847–853. doi: 10.1007/s00198-009-1024-9. [DOI] [PubMed] [Google Scholar]
- 49.Lee J.K., Kim K.W., Choi J.Y., Moon S.Y., Kim S.G., Kim C.H. Bisphosphonates-related osteonecrosis of the jaw in Korea: a preliminary report. J Korean Assoc Oral Maxillofac Surg. 2013;39:9–13. doi: 10.5125/jkaoms.2013.39.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin T.C., Yang C.Y., Kao Yang Y.H., Lin S.J. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population. Osteoporos Int. 2014;25:1503–1511. doi: 10.1007/s00198-014-2624-6. [DOI] [PubMed] [Google Scholar]
- 51.Chiu W.Y., Chien J.Y., Yang W.S., Juang J.M., Lee J.J., Tsai K.S. The risk of osteonecrosis of the jaws in Taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J Clin Endocrinol Metab. 2014;99:2729–2735. doi: 10.1210/jc.2013-4119. [DOI] [PubMed] [Google Scholar]
- 52.Ulmner M., Jarnbring F., Törring O. Osteonecrosis of the jaw in Sweden associated with the oral use of bisphosphonate. J Oral Maxillofac Surg. 2014;72:76–82. doi: 10.1016/j.joms.2013.06.221. [DOI] [PubMed] [Google Scholar]
- 53.Sedghizadeh P.P., Stanley K., Caligiuri M., Hofkes S., Lowry B., Shuler C.F. Oral bisphosphonate use and the prevalence of osteonecrosis of the jaw: an institutional inquiry. J Am Dent Assoc. 2009;140:61–66. doi: 10.14219/jada.archive.2009.0019. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki T., Yamori M., Yamamoto K., Saito K., Asai K., Sumi E. Risk of osteomyelitis of the jaw induced by oral bisphosphonates in patients taking medications for osteoporosis: a hospital-based cohort study in Japan. Bone. 2012;51:882–887. doi: 10.1016/j.bone.2012.08.115. [DOI] [PubMed] [Google Scholar]
- 55.Sugimoto T., Matsumoto T., Hosoi T., Miki T., Gorai I., Yoshikawa H. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT) Osteoporos Int. 2015;26:765–774. doi: 10.1007/s00198-014-2964-2. [DOI] [PubMed] [Google Scholar]
- 56.Rogers S.N., Palmer N.O., Lowe D., Randall C. United Kingdom nationwide study of avascular necrosis of the jaws including bisphosphonate-related necrosis. Br J Oral Maxillofac Surg. 2015;53:176–182. doi: 10.1016/j.bjoms.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi A., Akiyama H., Koseki T., Shimizutani K. Recognition of bisphosphonate-related osteonecrosis of the jaws among oral and maxillofacial radiologists: results from the questionnaire-based survey in Japan. Oral Radiol. 2013;29:98–104. [Google Scholar]
- 58.Hamada H., Matsuo A., Koizumi T., Satomi T., Chikazu D. A simple evaluation method for early detection of bisphosphonate-related osteonecrosis of the mandible using computed tomography. J Craniomaxillofac Surg. 2014;42:924–929. doi: 10.1016/j.jcms.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi T., Ariji Y., Nozawa M., Naitoh M., Kuroiwa Y., Kurita K. Computed tomographic assessment of early changes of the mandible in bisphosphonate-treated patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:362–372. doi: 10.1016/j.oooo.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Suei Y. Radiographic findings of bisphosphonate-related osteomyelitis of the jaw: investigation of the diagnostic points by comparison with radiation osteomyelitis, suppurative osteomyelitis, and diffuse sclerosing osteomyelitis. Oral Radiol. 2013;29:121–134. [Google Scholar]
- 61.Ariji Y., Ariji E. Role of magnetic resonance imaging in diagnosis of bisphosphonate-related osteonecrosis of the jaw. Oral Radiol. 2013;29:111–120. [Google Scholar]
- 62.Hong C.M., Ahn B.C., Choi S.Y., Kim D.H., Lee S.W., Kwon T.G. Implications of three-phase bone scintigraphy for the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Nucl Med Mol Imaging. 2012;46:162–168. doi: 10.1007/s13139-012-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyashita H., Shiba H., Kawana H., Nakahara T. Clinical utility of three-dimensional SPECT/CT imaging as a guide for the resection of medication-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2015;44:1106–1109. doi: 10.1016/j.ijom.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Yamazaki Y., Kitagawa Y., Hata H., Abe T., Murai C., Shiga T. Use of FDG PET to evaluate hyperbaric oxygen therapy for bisphosphonate-related osteonecrosis of the jaw. Clin Nucl Med. 2010;35:590–591. doi: 10.1097/RLU.0b013e3181e4df11. [DOI] [PubMed] [Google Scholar]
- 65.Fatema C.N., Sato J., Yamazaki Y., Hata H., Hattori N., Shiga T. FDG-PET may predict the effectiveness of hyperbaric oxygen therapy in a patient with bisphosphonate-related osteonecrosis of the jaw: report of a case. Odontology. 2015;103:105–108. doi: 10.1007/s10266-013-0129-y. [DOI] [PubMed] [Google Scholar]
- 66.Paek S.J., Park W.J., Shin H.S., Choi M.G., Kwon K.H., Choi E.J. Diseases having an influence on inhibition of angiogenesis as risk factors of osteonecrosis of the jaw. J Korean Assoc Oral Maxillofac Surg. 2016;42:271–277. doi: 10.5125/jkaoms.2016.42.5.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furuya T., Maeda S., Momohara S., Taniguchi A., Yamanaka H. Dental treatments, tooth extractions, and osteonecrosis of the jaw in Japanese patients with rheumatoid arthritis: results from the IORRA cohort study. J Bone Min Metab. 2017;35:344–350. doi: 10.1007/s00774-016-0763-x. [DOI] [PubMed] [Google Scholar]
- 68.Choi H., Lee J.H., Kim H.J., Park W., Lee J.H., Kim J.H. Genetic association between VEGF polymorphisms and BRONJ in the Korean population. Oral Dis. 2015;21:866–871. doi: 10.1111/odi.12355. [DOI] [PubMed] [Google Scholar]
- 69.Kwon J.W., Park E.J., Jung S.Y., Sohn H.S., Ryu H., Suh H.S. A large national cohort study of the association between bisphosphonates and osteonecrosis of the jaw in patients with osteoporosis: a nested case-control study. J Dent Res. 2015;94(9 Suppl) doi: 10.1177/0022034515587862. 212S-219S. [DOI] [PubMed] [Google Scholar]
- 70.Yamazaki T., Yamori M., Ishizaki T., Asai K., Goto K., Takahashi K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: a cohort study. Int J Oral Maxillofac Surg. 2012;41:1397–1403. doi: 10.1016/j.ijom.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 71.Matsumoto A., Sasaki M., Schmelzeisen R., Oyama Y., Mori Y., Voss P.J. Primary wound closure after tooth extraction for prevention of medication-related osteonecrosis of the jaw in patients under denosumab. Clin Oral Investig. 2017;21:127–134. doi: 10.1007/s00784-016-1762-y. [DOI] [PubMed] [Google Scholar]
- 72.Otto S., Tröltzsch M., Jambrovic V., Panya S., Probst F., Ristow O. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: a trigger for BRONJ development? J Craniomaxillofac Surg. 2015;43:847–854. doi: 10.1016/j.jcms.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 73.Lo J.C., O'Ryan F.S., Gordon N.P., Yang J., Hui R.L., Martin D. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura M., Umetsu R., Abe J., Matsui T., Ueda N., Kato Y. Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J Pharm Health Care Sci. 2015;1:34. doi: 10.1186/s40780-015-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumi E., Yamazaki T., Tanaka S., Yamamoto K., Nakayama T., Bessho K. The increase in prescriptions of bisphosphonates and the incidence proportion of osteonecrosis of the jaw after risk communication activities in Japan: a hospital-based cohort study. Pharmacoepidemiol Drug Saf. 2014;23:398–405. doi: 10.1002/pds.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Felsenberg D., López S., Gabbert T., Hoffmeister B. Osteonekrose des kiefers bei osteoporosepatienten: bisphosphonattherapie, risikofaktoren, klinische symptomatik, initiale interventionen und empfehlungen. Osteologie. 2012;21:207–212. [Google Scholar]
- 77.Brown J.P., Morin S., Leslie W., Papaioannou A., Cheung A.M., Davison K.S. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60:324–333. [PMC free article] [PubMed] [Google Scholar]
- 78.Hellstein J.W., Adler R.A., Edwards B., Jacobsen P.L., Kalmar J.R., Koka S. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 79.Taguchi A., Shiraki M., Sugimoto T., Ohta H., Soen S. Japan Osteoporosis Society. Lack of cooperation between physicians and dentists during osteoporosis treatment may increase fractures and osteonecrosis of the jaw. Curr Med Res Opin. 2016;32:1261–1268. doi: 10.1185/03007995.2016.1170005. [DOI] [PubMed] [Google Scholar]
- 80.Khan A.A., Morrison A., Kendler D.L., Rizzoli R., Hanley D.A., Felsenberg D. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J Clin Densitom. 2017;20:8–24. doi: 10.1016/j.jocd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 81.Mücke T., Deppe H., Hein J., Wolff K.D., Mitchell D.A., Kesting M.R. Prevention of bisphosphonate-related osteonecrosis of the jaws in patients with prostate cancer treated with zoledronic acid - a prospective study over 6 years. J Craniomaxillofac Surg. 2016;44:1689–1693. doi: 10.1016/j.jcms.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 82.Yoo J.Y., Park Y.D., Kwon Y.D., Kim D.Y., Ohe J.Y. Survey of Korean dentists on the awareness on bisphosphonate-related osteonecrosis of the jaws. J Investig Clin Dent. 2010;1:90–95. doi: 10.1111/j.2041-1626.2010.00024.x. [DOI] [PubMed] [Google Scholar]
- 83.Al-Mohaya M.A., Al-Khashan H.I., Mishriky A.M., Al-Otaibi L.M. Physicians' awareness of bisphosphonates-related osteonecrosis of the jaw. Saudi Med J. 2011;32:830–835. [PubMed] [Google Scholar]
- 84.Khan A.A., Rios L.P., Sándor G.K., Khan N., Peters E., Rahman M.O. Bisphosphonate-associated osteonecrosis of the jaw in Ontario: a survey of oral and maxillofacial surgeons. J Rheumatol. 2011;38:1396–1402. doi: 10.3899/jrheum.100221. [DOI] [PubMed] [Google Scholar]
- 85.Alhussain A., Peel S., Dempster L., Clokie C., Azarpazhooh A. Knowledge, practices, and opinions of ontario dentists when treating patients receiving bisphosphonates. J Oral Maxillofac Surg. 2015;73:1095–1105. doi: 10.1016/j.joms.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 86.Taguchi A., Shiraki M., Tsukiyama M., Miyazaki T., Soen S., Ohta H. Impact of osteonecrosis of the jaw on osteoporosis treatment in Japan: results of a questionnaire-based survey by the adequate treatment of osteoporosis (A-TOP) research group. Calcif Tissue Int. 2015;97:542–550. doi: 10.1007/s00223-015-0045-y. [DOI] [PubMed] [Google Scholar]
- 87.Kim J.W., Jeong S.R., Kim S.J., Kim Y. Perceptions of medical doctors on bisphosphonate-related osteonecrosis of the jaw. BMC Oral Health. 2016;16:92. doi: 10.1186/s12903-016-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]