Abstract
Objectives
The calcemic and parathyroid hormone (PTH) responses to severe burn injury appear to differ between children and adults. In our limited studies children exhibited hypocalcemic hypoparathyroidism consistent with up-regulation of the parathyroid calcium-sensing receptor (CaSR) while adults did not, suggesting a developmental cutoff in cytokine-mediated up-regulation of the CaSR. This difference may be clinically important as published studies indicate that extracellular calcium (Ca) may stimulate the inflammatory response. The aim of this study was to examine the existing literature on burns to see if the differences between pediatric and adult calcemic and PTH responses to burn supported our findings providing stronger evidence to support this developmental difference.
Methods
We reviewed the National Library of Medicine database using the terms burns, PTH and ionized calcium and found 9 articles from 8 different medical centers; one was eliminated due to mixing of adults and children.
Results
There were 245 burn patients reported from the literature, 178 pediatric and 67 adults. The data are mostly consistent with our reported findings. Of the 10 pediatric patients with severe burns that we studied, mean ionized Ca concentration was below the lower limit of normal of 1.10 mM. The 67 adult burn patients reported in the literature had a mean blood ionized Ca concentration that was within the adult normal range or was lower than normal but with secondary hyperparathyroidism. Moreover, serum PTH concentrations were uniformly low in the 178 children in the burn literature but normal or mildly elevated in the 67 adults.
Conclusions
These results support the hypothesis that the difference between pediatric and adult victims is consistent with an age-related CaSR response to cytokine stimulation and may be consistent with a lower level of inflammation in children. Ionized Ca and PTH might serve as possible therapeutic targets to lower the inflammatory response in burn victims.
Keywords: Burns, Parathyroid hormone, Ionized calcium, Calcium-sensing receptor, Inflammation
1. Introduction
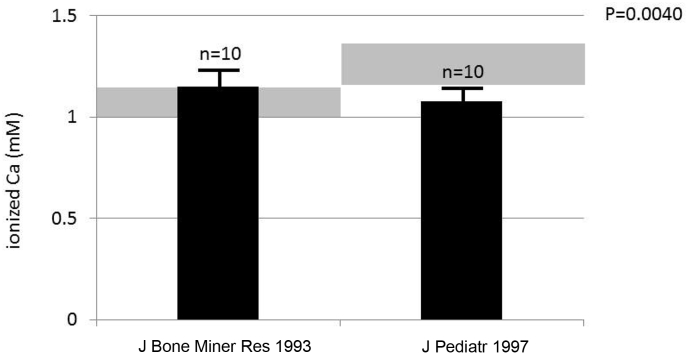
Severe burn injury incurs a hypermetabolic response including an acute inflammatory response that causes resorptive bone loss [1] and a stress response that involves increased endogenous glucocorticoid production, which contributes to hypodynamic or adynamic bone at approximately 2 weeks following severe burn injury in children [2], [3]. In the study of the metabolic response to burn injury we found that children and adults had a different calcemic response to burn injury (Fig. 1).
Fig. 1.
Mean and standard deviations of pediatric values from publication Klein et al. [4] (J Pediatr 1997) and Klein et al. [8] (J Bone Miner Res 1993) depicting blood ionized Ca 2 weeks following burn injury. The difference between pediatric and adult burn victims is significant (P = 0.0040). The grey areas surrounding each bar represent the normal range.
From our work, children present with sustained hypocalcemia, hypomagnesemia, and hypoparathyroidism [4] consistent with cytokine-mediated up-regulation of the parathyroid calcium-sensing receptor (CaSR) [5], [6], [7] while adults in general present with either normocalcemia or mild hypercalcemia [8]. The reason for this discrepancy is uncertain but one possibility could be that cytokine-mediated up-regulation of the CaSR is age-related.
This difference, if real, may be clinically important, inasmuch as we previously reported that in vitro calcium (Ca) affects chemokine production by normal human peripheral blood mononuclear cells [9]. This finding raises the possibility that cytokine-mediated bone resorption with consequent liberation of calcium into the blood could prolong or intensify the inflammatory response and up-regulation of the CaSR may blunt that effect. Moreover, Rossol et al. [10] had previously reported that extracellular calcium activates the nod-like receptor subtype P3 (NLRP3) inflammasome, eliciting the increased production of interleukin (IL)-1β both in vitro and in vivo. The NLRP3 is the largest of 4 molecular pattern recognition receptors or inflammasomes, which are caspase containing oligomers that are expressed in myeloid cells and are a component of the innate immune system. They aid in the production of IL-1 and IL-18.
The amount of data we have published is not adequate to answer whether or not there is evidence that cytokine-mediated up-regulation of the CaSR is developmentally regulated. Because other studies in the literature may be able to provide additional evidence to support our observation, the aim of this study was to undertake a literature review to see how much data there might be to support our hypothesis.
2. Methods
We accessed the National Library of Medicine database (PubMed) for all publications in English using the search terms of burns, parathyroid hormone, and ionized calcium levels in the blood of patients who were burned greater than 20% of total body surface area. We tried to avoid using multiple articles from the same medical center in order to diversify the patient population and avoid weighting the data in favor of one center over another. Similarly, we avoided combining data from various institutions given the differences in assays and normal ranges associated with them. The only statistics performed were unpaired t tests of ionized Ca (Fig. 1) and PTH (Fig. 2) between children and adults studied at our own institution.
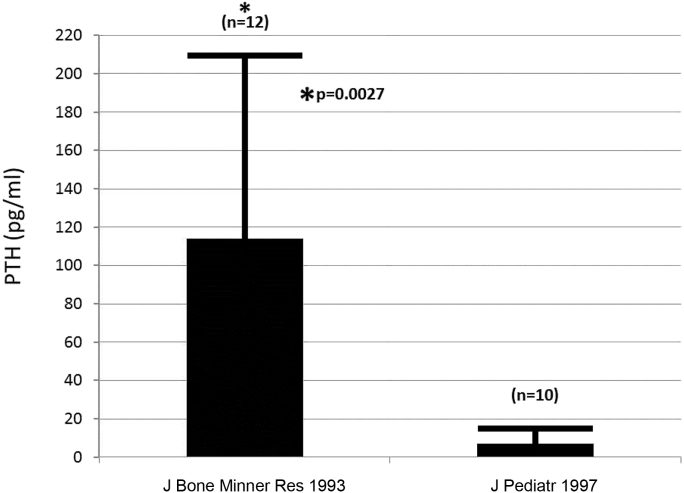
Fig. 2.
Mean and standard deviations of pediatric values from publication Klein et al. [4] (J Pediatr 1997) and Klein et al. [8] (J Bone Miner Res 1993) depicting serum parathyroid hormone (PTH) concentrations 2 weeks following burn injury. The difference between pediatric and adult burn victims is significant (*P = 0.0027 compared to pediatric values).
3. Results
Nine papers met the search criteria (Table 1) involving 178 children and 67 adults. Two of them were from the same institution because necessary data were reported separately [11], [12]. Inasmuch as the papers were limited to at most two per institution that covered the required data there was minimal opportunity for selection bias. A paper by Sobouti et al. [13] included 118 pediatric subjects but half of the study subjects had burns between 1% and 30% total body surface area. This was the only pediatric paper we encountered that published data on patients with minor burns. In this paper the ionized Ca values were at odds with our own findings although the PTH values were consistent with our report and that by Gottschlich et al. [11]. Moreover, while the ionized Ca data in the Sobouti paper were in the normal range for the assays we use, the normal range of the assay used in that paper was not given or referenced. The one paper eliminated was by Szyfelbein et al. [14], which included subjects ages 6–75 years without stratifying the results obtained in children and adults. That left 4 papers analyzing data from children: that by our group at Shriners Burns Hospital in Galveston, which studied 10 pediatric subjects [4], and those by Gottschlich et al. [11] and Mayes et al. [12] from Shriners Burns Hospital in Cincinnati, which studied 50 subjects, giving a total of 60 subjects in all and the one by Sobouti et al. [13] adding another 118 subjects. For the adults there were 4 studies, that from our institution [8] and that of Rousseau et al. [15] from Belgium and those by Lovén et al. [16] from Sweden and Dolecek et al. [17] from the Czech Republic. The total number of adult subjects from these four studies was 67.
Table 1.
Available references reporting ionized calcium and parathyroid hormone following burns.
| Study | Patient No. | Age (yr) | % TBSA | iPTH | iCa | Postburn day | Year published | Country |
|---|---|---|---|---|---|---|---|---|
| Klein et al. [8] | 12 | 25 ± 7 | 75 ± 15 | 12.1 ± 10.2 (1.0–7.0) pM | 1.15 ± 0.06 (1.00–1.15) mM | 24 ± 12 | 1993 | USA |
| Klein et al. [4] | 10 | 9.6 ± 4.7 | 57 ± 17 | 7 ± 3 (15–55) pg/mL | 1.08 ± 0.03 (1.12–1.37) mM | 20 ± 10 | 1997 | USA |
| Gottschlich et al. [11] | 50 | 0.7–18.4 | 56 ± 3 | 12.5 ± 7a (15–65) pg/mL | ND | 28 ± 3a | 2015 | USA |
| Rousseau et al. [15] | 20 | 18–78 | 23b | 10–114 (4–26) ng/L | 0.98–1.26 (1.14–1.3) mM | 7 | 2015 | Belgium |
| Lovén et al. [16] | 20 | 18–68 | >20 | 1.4–2.3a (1.1–2.5) μg/L | 1.03–1.12 (1.1–1.3) mM | 14 | 1984 | Sweden |
| Sobouti et al. [13] | 118 | 4.04 ± 3.04 | 1 to >50 | 11.1 ± 5.8 pg/mL (male) | 1.29 ± 0.06 mM | 0–7 | 2016 | Iran |
| 10.9 ± 7.6 pg/mL (female) | ||||||||
| Szyfelbein et al. [14] | 25 | 6–75 | 25–80 | ND | 1.03 ± 0.05 (0.79–0.90) mM | 1–35 | 1981 | USA |
| Dolecek et al. [17] | 15 | 18–74 | 8–58 | 17–42 pg/mL | 0.96–1.18 mM | 1–30 | 2003 | Czech Republic |
Values are presented as mean ± standard deviation (SD) or mean ± SD (range).
TBSA, total body surface area; iPTH, intact parathyroid hormone; iCa, ionized Ca; ND, not done or not disclosed.
Extrapolated.
Median.
In these subjects, ionized Ca remained in the normal range in the Galveston patients [8] and those of Rousseau et al. [15] but were either low or in the normal range in the subjects studied by Lovén et al. [16] and Dolecek et al. [17]. However, in the paper of Lovén et al. [16] urinary phosphate was elevated and urine calcium excretion was low suggesting that the patients in that study [16] were functionally hyperparathyroid. In the paper of Dolecek et al. [17] PTH was normal in the face of low ionized Ca concentration. Functional hyperparathyroidism is also consistent with data from the study of Rousseau et al. [15] which found blood concentration of fibroblast growth factor (FGF)-23 to be high, while in pediatric burn patients from our institution, FGF-23 was undetectable [18], suggesting functional hypoparathyroidism. When blood PTH concentrations were compared in the Galveston studies of children [4] and adults [8] the PTH in the children was significantly lower (Fig. 2).
Moreover, in the pediatric burn patients we studied [4] as well as in those studied by Gottschlich et al. [11], the mean serum intact PTH concentrations were below the lowest level of the normal range of each assay for age indicating that pediatric burn patients were hypoparathyroid. Surprisingly, even the PTH values in the paper of Sobouti et al. [13] were in the low range of the other assays using the same units of expression [4], [13] even though the normal range for those in the study of Sobouti et al. [13] was not provided. In our adult study [8], when PTH units were converted from pmol/L to pg/mL, the values of 114.1 ± 96 pg/mL were similar to those given by Rousseau et al. [15]. When these values were compared to our pediatric values of PTH [4] (Table 1), by unpaired t-test, the difference was significant, P = 0.0022 and by Welch t-test to accommodate unequal variances (P = 0.0027) (Fig. 2). While ionized Ca concentrations were not studied in the work of either Gottschlich et al. [11] or Mayes et al. [12], the latter study included 24-hour urinary Ca excretion, which ranged from low to high with the majority being normal or high [12], consistent with functional hypoparathyroidism as described in our study [4]. The Gottschlich paper did not convert urinary Ca excretion to mg/kg/d, which would have made the results comparable to ours and may have normalized for body size.
Data from studies with smaller burns were not excluded from the analysis mainly because of the similarity with data from larger burns. Thus in pediatric and adult burn patients not only was there a preponderance of data from smaller burns but patients could not be identified by burn size by only looking at the calcium and PTH data. While pediatric patients we studied with smaller burns, i.e., 20% total body surface area or less, did not appear to manifest the reduction in bone density seen in larger burns [19] we did not determine ionized Ca or PTH for purposes of this study.
Furthermore, a precise age of cutoff of the hypocalcemic-hypoparathyroid response to burns cannot be given. The pediatric studies involved patients up to age 18 years while 3 of the 4 adult studies covered patients age 18 and above. Given the discrepancies in calcemic responses to burn between the 2 groups, one can speculate that the age of transition might be somewhere around 18 years.
4. Discussion
The pediatric burns literature contains few papers looking at ionized Ca and PTH following severe burn injury. However, those that we could find were relatively uniform in demonstrating low serum concentration of PTH. Ours was the only paper that looked at ionized Ca sample and found uniformly low blood ionized Ca concentration in the 10 patients we studied. In contrast the 4 reliable adult studies all demonstrated normal to high PTH levels and low to high ionized Ca concentration but all but possibly one demonstrated a functional hyperparathyroidism. Thus, relative to given normal values for pediatric and adult populations, children with severe burns manifested hypocalcemia and hypoparathyroidism while adults even with small burns have normal ionized Ca levels with normal serum PTH concentration or slightly low ionized Ca levels with high serum PTH concentration.
Thus, the pediatric burns literature to date is consistent with the clinical presentation of up-regulation of the parathyroid CaSR that we have previously demonstrated in a sheep model of burn injury [5]. We believe that the experiments performed in the sheep model can be extrapolated to humans inasmuch as the clinical manifestation of up-regulation are demonstrated clearly in the pediatric burns patients. The up-regulation is manifested by hypocalcemic hypoparathyroidism and hypercalciuria [4]. Therefore, we speculate that the cytokine-mediated up-regulation of the CaSR that is seen in pediatric burn patients is turned off in adult burn victims, possibly by the hormones that are turned on at puberty. While no data are available on CaSR regulation in adults with burns, the hypocalcemia and hypoparathyroidism that are manifestations of up-regulation are absent in the published literature in adults with burns making the likelihood of CaSR up-regulation in burned adults very low. While vitamin D status is often associated with calcium metabolism, serum concentration of 25 hydroxyvitamin D is difficult to evaluate in acute burns because of the loss of serum constitutive proteins, such as vitamin D Binding Protein and albumin for at least 6 months postburn, as the available assays measure both free and bound 25-hydroxyvitamin D.
In trying to examine agreement among PTH assays we were handicapped in that not all papers listed the method used for assaying PTH. Furthermore, the literature has already noted major disagreements among PTH assays most notably in patients with chronic kidney disease [20] thus making it difficult to pool data and perform statistical analysis.
The potential importance of this observation is that present information suggests that the up-regulation of the parathyroid CaSR following pediatric burn injury may at least theoretically blunt either the intensity or the duration of the inflammatory response by lowering blood ionized Ca concentration and consequently reducing the amount of Ca stimulation of peripheral blood mononuclear cell chemokine production. Thus either the intensity or duration of the inflammatory response may be decreased [9], [10]. Finnerty et al. [21] and Jeschke et al. [22] have shown that inflammation in burned children is less intense than that in adults and that it takes a smaller percentage of total body surface area burn in adults than it does in children to produce morbidity and mortality. Thus ionized calcium reduction by up-regulation of the parathyroid CaSR in children may be a mechanism by which the systemic inflammatory response and burn morbidity are reduced in children compared to adults following burn injury and circulating calcium or the parathyroid CaSR may well serve as possible therapeutic targets to reduce inflammation in general.
5. Conclusions
Thus, the small number of studies of ionized calcium and PTH concentrations in adult and pediatric burn patients provide limited but largely consistent data suggesting a different set of responses of calcium, calcium sensing receptors, and parathyroid hormone to the inflammatory and stress-related stimuli following burn injury. These differences may point to differences in response to inflammation in younger and older patients with resultant differences in morbidity and mortality postburn.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
Data for this study were obtained in part by support from grants from Shriners Hospitals for Children and grant P50 protocol 4 GM60338 from the National Institutes of Health. We are grateful to Pamela Stevens, our data management coordinator, for the provision of the data set of children with minor burns.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Klein G.L., Xie Y., Qin Y.X., Lin L., Hu M., Enkhbaatar P. Preliminary evidence of early bone resorption in a sheep model of acute burn injury: an observational study. J Bone Miner Metab. 2014;32:136–141. doi: 10.1007/s00774-013-0483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein G.L., Herndon D.N., Goodman W.G., Langman C.B., Phillips W.A., Dickson I.R. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone. 1995;17:455–460. doi: 10.1016/8756-3282(95)00279-1. [DOI] [PubMed] [Google Scholar]
- 3.Klein G.L., Bi L.X., Sherrard D.J., Beavan S.R., Ireland D., Compston J.E. Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int. 2004;15:468–474. doi: 10.1007/s00198-003-1572-3. [DOI] [PubMed] [Google Scholar]
- 4.Klein G.L., Nicolai M., Langman C.B., Cuneo B.F., Sailer D.E., Herndon D.N. Dysregulation of calcium homeostasis after severe burn injury in children: possible role of magnesium depletion. J Pediatr. 1997;131:246–251. doi: 10.1016/s0022-3476(97)70161-6. [DOI] [PubMed] [Google Scholar]
- 5.Murphey E.D., Chattopadhyay N., Bai M., Kifor O., Harper D., Traber D.L. Up-regulation of the parathyroid calcium-sensing receptor after burn injury in sheep: a potential contributory factor to postburn hypocalcemia. Crit Care Med. 2000;28:3885–3890. doi: 10.1097/00003246-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen P.K., Rasmussen A.K., Butters R., Feldt-Rasmussen U., Bendtzen K., Diaz R. Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with an up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Commun. 1997;238:880–885. doi: 10.1006/bbrc.1997.7207. [DOI] [PubMed] [Google Scholar]
- 7.Canaff L., Zhou X., Hendy G.N. The proinflammatory cytokine, interleukin-6, up-regulates calcium-sensing receptor gene transcription via Stat1/3 and Sp1/3. J Biol Chem. 2008;283:13586–13600. doi: 10.1074/jbc.M708087200. [DOI] [PubMed] [Google Scholar]
- 8.Klein G.L., Herndon D.N., Rutan T.C., Sherrard D.J., Coburn J.W., Langman C.B. Bone disease in burn patients. J Bone Miner Res. 1993;8:337–345. doi: 10.1002/jbmr.5650080311. [DOI] [PubMed] [Google Scholar]
- 9.Klein G.L., Castro S.M., Garofalo R.P. The calcium-sensing receptor as a mediator of inflammation. Semin Cell Dev Biol. 2016;49:52–56. doi: 10.1016/j.semcdb.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossol M., Pierer M., Raulien N., Quandt D., Meusch U., Rothe K. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschlich M.M., Mayes T., Khoury J., Kagan R.J. Clinical trial of vitamin D(2) vs D(3) supplementation in critically Ill pediatric burn patients. JPEN J Parenter Enter Nutr. 2017;41:412–421. doi: 10.1177/0148607115587948. [DOI] [PubMed] [Google Scholar]
- 12.Mayes T., Gottschlich M.M., Khoury J., Kagan R.J. Investigation of bone health subsequent to vitamin D supplementation in children following burn injury. Nutr Clin Pract. 2015;30:830–837. doi: 10.1177/0884533615587720. [DOI] [PubMed] [Google Scholar]
- 13.Sobouti B., Riahi A., Fallah S., Ebrahimi M., Shafiee Sabet A., Ghavami Y. Serum 25-hydroxyvitamin D levels in pediatric burn patients. Trauma Mon. 2016;21 doi: 10.5812/traumamon.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szyfelbein S.K., Drop L.J., Martyn J.A. Persistent ionized hypocalcemia in patients during resuscitation and recovery phases of body burns. Crit Care Med. 1981;9:454–458. doi: 10.1097/00003246-198106000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau A.F., Damas P., Ledoux D., Lukas P., Carlisi A., Le Goff C. Vitamin D status after a high dose of cholecalciferol in healthy and burn subjects. Burns. 2015;41:1028–1034. doi: 10.1016/j.burns.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Lovén L., Nordström H., Lennquist S. Changes in calcium and phosphate and their regulating hormones in patients with severe burn injuries. Scand J Plast Reconstr Surg. 1984;18:49–53. doi: 10.3109/02844318409057402. [DOI] [PubMed] [Google Scholar]
- 17.Dolecek R., Tymonová J., Adámková M., Kadlcík M., Pohlídal A., Závodná R. Endocrine changes after burns: the bone involvement. Acta Chir Plast. 2003;45:95–103. [PubMed] [Google Scholar]
- 18.Klein G.L., Herndon D.N., Le P.T., Andersen C.R., Benjamin D., Rosen C.J. The effect of burn on serum concentrations of sclerostin and FGF23. Burns. 2015;41:1532–1535. doi: 10.1016/j.burns.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein G.L., Herndon D.N., Langman C.B., Rutan T.C., Young W.E., Pembleton G. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126:252–256. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]
- 20.Sturgeon C.M., Sprague S.M., Metcalfe W. Variation in parathyroid hormone immunoassay results–a critical governance issue in the management of chronic kidney disease. Nephrol Dial Transpl. 2011;26:3440–3445. doi: 10.1093/ndt/gfr614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnerty C.C., Jeschke M.G., Herndon D.N., Gamelli R., Gibran N., Klein M. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeschke M.G., Pinto R., Kraft R., Nathens A.B., Finnerty C.C., Gamelli R.L. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43:808–815. doi: 10.1097/CCM.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]