Abstract
Objectives
The prevalence of chronic low back pain (CLBP) increases with age and several mechanisms are involved in the development of CLBP, including osteoporosis; however, no associations with sarcopenia have yet been identified.
Methods
In total, 100 patients with CLBP and 560 patients without CLBP (nCLBP) aged over 65 years were studied. Skeletal muscle mass index (SMI) and percentage of body fat were evaluated using whole-body dual-energy X-ray absorptiometry. Sarcopenia was diagnosed when the relative SMI was more than 2 standard deviations below the mean in young adults. Thus, the cutoff value for sarcopenia was defined according to Sanada's Japanese population data. Paraspinal muscle cross-sectional areas of the lumbar multifidus and the erector spinae muscles were calculated using magnetic resonance imaging.
Results
Forty patients (40.0%) from the CLBP group and 149 (26.6%) from the nCLBP group met the criteria of sarcopenia. SMI was significantly lower and the body fat ratio was significantly higher in the CLBP group compared with the nCLBP group. Sarcopenic obesity was significantly observed in the CLBP group. Lumbar multifidus and the erector spinae muscle cross sectional area were significantly lower in the CLBP group.
Conclusions
Elderly patients with CLBP have significantly lower skeletal muscle mass, and age-related mechanisms in sarcopenia are considered to be associated with chronic pain. Therapeutic procedures that are used to treat elderly aging muscle, including muscle strengthening and performance training, can possibly be a treatment for or used to prevent elderly CLBP.
Keywords: Sarcopenia, Chronic low back pain
1. Introduction
The neologisms sarcopenia, meaning “loss of flesh,” was first described in 1989 to refer to the age-related involuntary loss of skeletal muscle mass and function [1]. Since then, the concept of sarcopenia has become widely accepted and used in the field of gerontology to identify physical, metabolic, and functional impairment in elderly persons. Baumgartner et al. [2] summed the muscle masses of the 4 limbs from a dual-energy X-ray absorptiometry (DXA) scan to determine the appendicular skeletal muscle mass (ASM). They then defined a skeletal muscle mass index (SMI) as ASM/height2 (kg/m2), and proposed cutoff points of AMI by sex. The age- and sex-adjusted prevalence of sarcopenia increased from 13% to 24% in persons under 70 years of age to >50% in person over 80 years of age and was associated with physical disability in both men and women [2]. Sarcopenia is characterized by the atrophy of type II muscle fibers and a loss of satellite cell content in type II fibers [3]. Several mechanisms underlying the development of sarcopenia have been proposed, such as insulin-like growth factor-1 [4] mediated promotion of the proliferation of muscle satellite cells, inflammatory cytokine including tumor necrosis factor-alpha (TNF-α) [5], and interleukin (IL) [6], chronic low grade inflammation induced by oxidative stress [7], anabolic hormones [8], including testosterone, estrogen, and growth hormone. Thus, sarcopenia is a multidimensional phenomenon of ageing influenced by systemic molecular mechanisms rather than by local reactions. Given the expected rise in the number of elderly people, several researchers have sought to elucidate the mechanism of sarcopenia and develop effective targeted interventions.
The prevalence of chronic low back pain (CLBP) increases with age and more than 1 in 3 community-dwelling older adults experience low back pain (LBP) [9]. This demonstrates one of the most disabling and therapeutic challenges afflicting older adults [10]. Most elderly patients with CLBP are diagnosed with “nonspecific LBP,” often resulting in clinically heterogeneous and multifactorial complications. Several mechanisms may be involved in the development of LBP, including direct lesions of the lumbar vertebra, spinal mechanics, facet joints, and paravertebral muscles; however, correlation between radiographic findings and clinical symptoms is reportedly poor [11]. Conversely, mechanisms of LBP in patients with osteoporosis, which include an age-related reduction of bone mass and mineral density, have been investigated. Recently, acid-sensing ion channels and transient receptor potential channel vanilloid subfamily member 1 were found to be expressed in the sensory neuron innervating bone [12]. These receptors elicit pain signals upon activation by acid, causing bone pain [13]. Furthermore, osteoporosis modifies the 5-HT receptor to exhibit hyperalgesia due to the contribution of the serotonergic system [14]. Thus, osteoporosis medication, such as calcitonin [15], selective estrogen receptor modulator [16], and bisphosphonate [17], were reported to reduce osteoporotic pain. Recently, sarcopenia was also observed to occur with increasing age and may cause pain [18], [19]; therefore, systemic aging changes as causing chronic pain have clinical implications. The primary purpose of this study was to examine differences in the appearance of sarcopenia as an age-related systemic change in older adults with CLBP, and to evaluate the relationship between the age-associated loss of skeletal muscle mass and chronic pain.
2. Methods
This prospective study was conducted with a series of 100 patients with CLBP who had no symptoms in the lower extremities, were aged 65 years or older, and exhibit moderate to severe LBP persisting for a minimum of 3 months prior to treatment in the outpatient department of orthopedic surgery of our hospital between 2011 and 2015. All patients completed visual analogue scales (VAS; 0–10) for LBP and a validated Japanese version of the Roland Morris Disability Questionnaire (RDQ). As control subjects, 560 consecutive patients without CLBP (nCLBP) who visited our institute for the treatment of lumbar spinal stenosis with intermittent claudication, and/or numbness of lower extremities were recruited during the same period. Patients with motor weakness of the lower extremities and a history of spinal infection, spinal tumor, vertebral fractures or previous back surgery, were excluded. Ethical approval was granted by the Institutional Review Board and all patients provided written informed consent (approval number: 433).
2.1. Trunk muscle power measurement
Back and abdominal muscle strength was determined from the maximum isometric strength of the trunk muscles in a sitting posture with 30° lumbar extension (back muscle strength) or 30° lumbar flexion (abdominal muscle strength) using a digital muscle strength meter (Isoforce GT-300, 310; OG GIKEN Co., Ltd., Okayama, Japan).
2.2. X-ray image analysis
Antero-posterior and lateral X-ray images of the spine were obtained. The lumbar scoliosis and lordosis angle using Cobb method between the superior edge of L1 and S1, S1 inclination angle, the presence of spondylolisthesis (anterior slip > 4 mm), and the lumbar range of motion, which was defined as the difference in lumbar lordosis angle between flexion and extension, were measured.
2.3. Magnetic resonance image analysis
Axial T2-weighted slices at L1/2 and L4/5 were obtained to measure the cross-sectional area of the lumbar multifidus and the erector spinae muscles for each level. Paraspinal muscle cross-sectional areas for both the right and left side were added together for each subject. Vertebral endplate degeneration was evaluated according to Modic change [20]. Fat infiltration in the lumbar multifidus was evaluated according to the Kjaer classification [21].
2.4. Body composition study
Body composition was assessed using a whole-body DXA (Lunar iDXA, GE Healthcare, Tokyo, Japan). Whole body scans provided total lean body mass, total fat mass, and total body bone mineral content. Sarcopenia was defined as the loss of skeletal muscle mass of the arms and legs as appendicular lean mass, and SMI was obtained from ASM/height2 (kg/m2) [2]. Sarcopenia was considered to be present when a patients exhibit a relative SMI that was more than 2 standard deviations below the mean of young adults. Thus, the cutoff value for sarcopenia was defined according to Sanada's Japanese population data [22] (<6.87 kg/m2 for men and <5.46 kg/m2 for women). Sarcopenic obesity is the imbalance in muscle and fat mass, a combination of decreased SMI and increase fat in the elderly. The cutoff value was 28% body fat for men and 40% for women [23]. Based on the combination of sarcopenia and obesity cutoff values, subjects were classified into 4 groups: sarcopenic obesity, nonsarcopenic obesity, sarcopenic nonobesity, and nonsarcopenic nonobesity [23].
2.5. Statistics
The statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. A comparison between the 2 treatment groups was conducted using the t-test and chi-square test. A P-value less than 0.05 was assumed to indicate a statistically significant difference.
3. Results
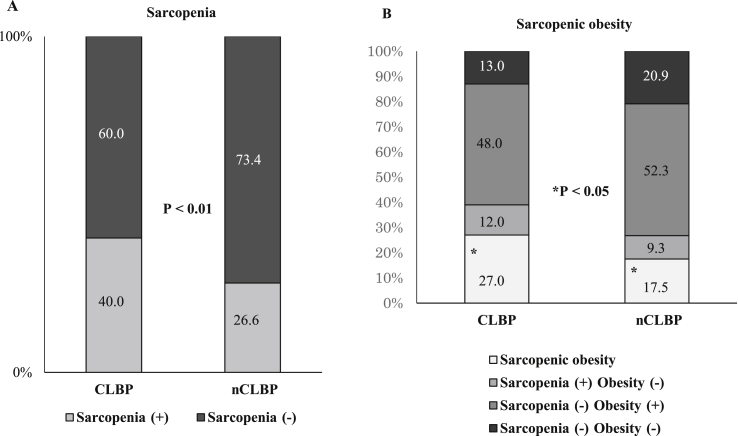
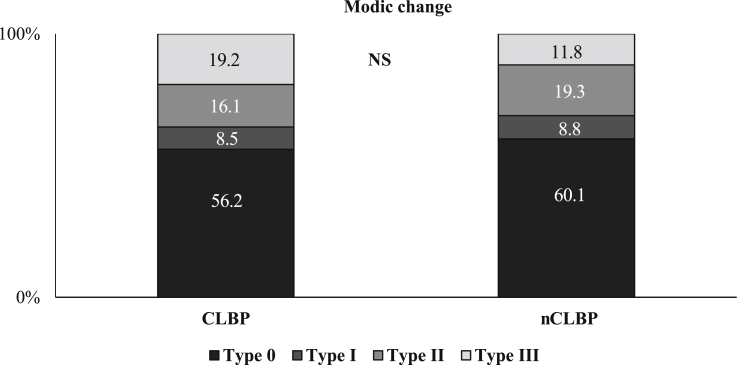
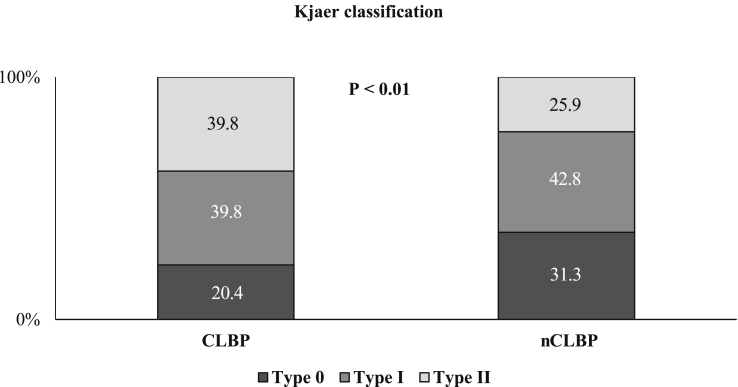
There were no substantial statistical differences in the baseline characteristics between the two study groups for age, sex, height. Body weight in the CLBP group was significantly lower, while body mass index was similar. There were no significant differences in trunk muscle strength, including lumbar extension and flexion, between the 2 groups. VAS and RDQ in the CLBP group were 6.5 and 16.9, respectively, with significant differences. There were no significant differences in radiographic findings, including lumbar lordosis, range of motion, sacral inclination angle, and presence of spondylolisthesis (Table 1). There were 189 cases (28.6%) in total that met the criteria of sarcopenia. Of these cases, 40 (40.0%) were from the CLBP group and 149 (26.6%) were from the nCLBP group. There were significant differences between the 2 groups (P < 0.01). Sarcopenic obesity was also significantly observed in the CLBP group (P < 0.05) (Fig. 1). SMI was significantly lower, while the body fat ratio was significantly higher in the CLBP group, compared with the nCLBP group both in male and female. Conversely, the bone mineral density (BMD) was similar in both groups. Paraspinal muscle cross sectional areas, including the lumbar multifidus and the erector spinae muscles at levels L1/2 and L4/5 were significantly lower in the CLBP group compared with the nCLBP group except for the L4/5 erector spinae muscle in male patient (Table 2). The distribution of Modic change was insignificant between the 2 groups (Fig. 2); however, multifidus degeneration using the Kjaer classification was significantly higher in the CLBP group compared with the nCLBP group (P < 0.01) (Fig. 3). A correlation between VAS and SMI was not identified.
Table 1.
Baseline subject characteristics.
| Characteristic | CLBP (n = 100) | nCLBP (n = 560) | P-value |
|---|---|---|---|
| Age, yr | 74.4 ± 6.0 | 73.2 ± 7.6 | 0.1306 |
| Sex, male:female | 45:55 | 304:256 | 0.0736 |
| Height, cm | 154.2 ± 8.9 | 155.9 ± 9.4 | 0.0866 |
| Weight, kg | 56.4 ± 10.3 | 59.2 ± 11.6 | <0.05 |
| BMI, kg/m2 | 23.6 ± 3.2 | 24.2 ± 3.5 | 0.1114 |
| Back muscle strength, N | 188.2 ± 73.5 | 157.9 ± 42.1 | 0.5775 |
| Abdominal muscle strength, N | 125.2 ± 68.4 | 110.6 ± 35.9 | 0.3241 |
| VAS | 6.5 ± 2.1 | 2.8 ± 1.8 | <0.01 |
| RDQ | 16.9 ± 7.9 | 12.9 ± 7.2 | 0.0425 |
| Lumbar lordosis, degree | 33.1 ± 11.8 | 32.5 ± 12.1 | 0.6370 |
| Lumbar range of motion, degree | 55.8 ± 23.2 | 51.2 ± 23.3 | 0.0726 |
| Sacral inclination angle, degree | 27.4 ± 8.8 | 27.0 ± 8.8 | 0.6673. |
| Presence of spondylolisthesis, % | 20.9 | 33.3 | 0.2880 |
Values are presented as mean ± standard deviation unless otherwise indicated.
CLBP, chronic low back pain; nCLBP, nonchronic low back pain; BMI, body mass index; VAS, visual analogue scale; RDQ, Roland Morris Disability Questionnaire.
Fig. 1.
Sarcopenia and sarcopenic obesity in CLBP. Prevalence of sarcopenia (A) and sarcopenic obesity (B) were shown. There were significantly more CLBP patients with sarcopenia and sarcopenic obesity. CLBP, chronic low back pain; nCLBP, nonchronic low back pain.
Table 2.
Comparison of muscle mass, fat mass, and bone mineral density.
| Variable | Male |
Female |
||||
|---|---|---|---|---|---|---|
| CLBP (n = 45) | nCLBP (n = 304) | P-value | CLBP (n = 55) | nCLBP (n = 254) | P-value | |
| Upper limb muscle mass, g | 4933.86 ± 865.98 | 5222.78 ± 857.53 | 0.0397 | 3073.13 ± 380.08 | 3366.62 ± 629.78 | 0.0009 |
| Lower limb muscle mass, g | 13,153.43 ± 2005.92 | 13,811.68 ± 2063.64 | 0.0481 | 9547.41 ± 1273.26 | 9935.256 ± 1552.39 | 0.0821 |
| SMI | 6.86 ± 1.07 | 7.21 ± 0.84 | 0.0133 | 5.78 ± 0.69 | 6.04 ± 0.85 | 0.0295 |
| Upper limb fat mass, g | 1781.18 ± 728.75 | 1655.43 ± 656.38 | 0.2428 | 1978.41 ± 553.97 | 2053.18 ± 998.24 | 0.5882 |
| Lower limb fat mass, g | 4509.52 ± 1530.68 | 4054.76 ± 1391.11 | 0.0464 | 4902.61 ± 1338.75 | 4861.08 ± 1826.70 | 0.8723 |
| Body fat ratio, % | 35.77 ± 6.71 | 27.69 ± 7.57 | <0.001 | 41.05 ± 4.09 | 34.25 ± 8,84 | <0.0001 |
| L2–4 BMD, g/cm2 | 1.36 ± 0.39 | 1.29 ± 0.25 | 0.0932 | 0.99 ± 0.26 | 1.02 ± 0.22 | 0.3399 |
| L2–4 YAM, % | 113.91 ± 32.23 | 107.62 ± 22.46 | 0.1039 | 86.53 ± 22.48 | 90.97 ± 19.03 | 0.1299 |
| L2–4 T score | 1.38 ± 3.19 | 0.82 ± 2.06 | 0.1165 | −1.25 ± 2.11 | −0.84 ± 1.78 | 0.1354 |
| CSA of multifidus (L1/2), mm2 | 327.23 ± 97.49 | 387.93 ± 131.59 | 0.0038 | 253.87 ± 83.01 | 282.85 ± 92.33 | 0.0312 |
| CSA of erector spinae (L1/2), mm2 | 2939.75 ± 790.76 | 3213.40 ± 699.08 | 0.0188 | 1913.81 ± 486.85 | 2242.10 ± 552.91 | <0.0001 |
| CSA of multifidus (L4/5), mm2 | 949.64 ± 280.31 | 1167.19 ± 342.75 | <0.0001 | 653.66 ± 281.80 | 836.23 ± 323.00 | 0.0001 |
| CSA of erector spinae (L4/5), mm2 | 2015.159 ± 595.16 | 2041.55 ± 546.10 | 0.7696 | 1493.69 ± 310.78 | 1694.61 ± 425.09 | 0.0009 |
Values are presented as mean ± standard deviation.
CLBP, chronic low back pain; nCLBP, nonchronic low back pain; SMI, skeletal muscle mass index; BMD, bone mineral density; YAM, young adult mean; CSA, cross sectional area.
Fig. 2.
Distribution of Modic change. The distribution of Modic change was insignificant between the 2 groups. CLBP, chronic low back pain; nCLBP, nonchronic low back pain; NS, not significant.
Fig. 3.
Degeneration of multifidus according to the distribution of Kjaer classification. Multifidus degeneration in the Kjaer classification was significantly higher in the CLBP group compared with the nCLBP group. (P < 0.01). CLBP, chronic low back pain; nCLBP, nonchronic low back pain.
4. Discussion
This study demonstrated that elderly patients with CLBP had a significantly lower skeletal muscle mass rather than BMD, and a higher percentage of body fat in whole-body DXA analysis. The prevalence of sarcopenia defined as SMI has been reported to be 19.0% in men and 14.3% in women [24], while sarcopenic obesity occurs in 5%–10% of patients [25], [26]. Thus, our observations that sarcopenia occurred in 40% of patients and sarcopenic obesity occurred in 27% of patients are considered to be large. Age-related muscle mass loss and fat degeneration suggested a possible influence on elderly CLBP. Since both muscle and bone decrease with age and a positive correlation between skeletal muscle and BMD has been reported [27], sarcopenia and osteoporosis may share etiological factors. There have been several studies regarding osteoporosis-associated skeletal pain. Our study observed that elderly CLBP correlated with skeletal muscle mass, but not with BMD, which supports our conclusion that age-related muscle loss (namely sarcopenia) is involved in elderly chronic pain without vertebral fracture. While trunk muscle atrophy (particularly in the lumbar multifidus) was previously identified as the cause of CLBP [28], evidence for fiber-type differentiation based on the presence of LBP is inconclusive [29]. Disuse, muscle denervation, and reflex inhibition have been proposed as possible mechanisms for muscle atrophy in patients with LBP [30]. As a cause of LBP, the evidence that morphological changes in trunk muscles may cause CLBP appears unsubstantiated. Local muscular changes seem to be a result of LBP rather than a cause, while decreased trunk muscle is likely implicated in recurrent LBP [31]. Since age-related muscle loss occurs in type II fibers [3], sarcopenic changes in trunk muscles, which contain abundant type I fibers compared with the extremities, are known to manifest more slowly compared with the limbs [32]. The present study demonstrated that the cross-sectional area of posterior trunk muscle was reduced in elderly patients with CLBP. However, no significant differences were seen in trunk muscle power. Thus, age-related trunk muscle atrophy may not be proposed as the cause of CLBP. Rather, CLBP occurs in older adults that have heterogeneous and multifactorial complications and that it should be treated as a systemic disorder rather than as a local dysfunction.
Sarcopenic pain has recently been shown to be correlated with fibromyalgia syndrome [18] and joint pain [19]; however, no previous study associated sarcopenia and CLBP. Although the relevance of sarcopenic mechanisms to age-related pain is unclear, chronic inflammation is potentially related to chronic pain. Not confined to sarcopenia, systemic low-grade inflammation, also called “inflamm-aging,” has been largely recognized as a feature of the aging process. Increased pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, act synergistically to promote the loss of muscle mass and function [33], and is referred to as sarcopenic obesity [34]. Inflammation signals are associated with muscle wasting, including sarcopenia and cachexia, and several studies have demonstrated an independent association of IL-6 with lower muscle mass. IL-6 can directly induce skeletal muscle atrophy, while treatment with an IL-6 receptor antibody was shown to inhibit muscle atrophy [6]. Additionally, the present study demonstrated an association between CLBP and fat mass. As is the case with sarcopenia, aging inflammation has been reported to be responsible for obesity in a manner that is similar to the relationship between sarcopenia obesity and inflammatory cytokine expression [35]. The vicious cycle that links inflammation and fat accumulation in aging is referred to as geriatric syndrome [36]. Similarly, inflammatory cytokines have been studied for their role in LBP. Several studies regarding increased plasma levels of TNF-α and IL-6 in patients with CLBP [37], [38] have reported that high serum IL-6 levels are associated with recovery in LBP [39]. Additionally, intradiscal administration of a TNF-α inhibitor has been used to treat discogenic LBP [40]. Thus, patients with age-related loss of muscle mass may have a higher perception of pain sensing.
The present study has several limitations, one of which is its cross-sectional nature. A longitudinal prospective study is necessary to establish sarcopenic pain, which is provoked and/or exacerbated chronic pain that is caused by an age-related loss of skeletal muscle mass. Furthermore, control subjects recruited in this study were not healthy individuals. While patients without CLBP were selected, they had degenerative lumbar spinal tissue that had the potential to be responsible for LBP. The lack of skeletal muscle evaluation, including grasping force and walking speed did not meet the criteria of sarcopenia.
5. Conclusions
Elderly patients with CLBP have significantly lower skeletal muscle mass, while age-related mechanisms in sarcopenia are considered to be associated with chronic pain.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors would like to thank Norio Sugimoto and Kazumasa Yamada for technical support.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Rosenberg I.W. Epidemiologic and methodologic problems in determining nutritional status of older persons. (Summary comments) Am J Clin Nutr. 1989;50:1231–1233. [PubMed] [Google Scholar]
- 2.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J., Taylor C.C., Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 4.Owino V., Yang S.Y., Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- 5.Visser M., Pahor M., Taaffe D.R., Goodpaster B.H., Simonsick E.M., Newman A.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 6.Haddad F., Zaldivar F., Cooper D.M., Adams G.R. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 7.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chahal H.S., Drake W.M. The endocrine system and ageing. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 9.Weiner D.K., Haggerty C.L., Kritchevsky S.B., Harris T., Simonsick E.M., Nevitt M. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4:311–320. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 10.Rudy T.E., Weiner D.K., Lieber S.J., Slaboda J., Boston J.R. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131:293–301. doi: 10.1016/j.pain.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., Modic M.T., Malkasian D., Ross J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 12.Mach D.B., Rogers S.D., Sabino M.C., Luger N.M., Schwei M.J., Pomonis J.D. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 13.Nagae M., Hiraga T., Wakabayashi H., Wang L., Iwata K., Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Ito A., Kumamoto E., Takeda M., Shibata K., Sagai H., Yoshimura M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J Neurosci. 2000;20:6302–6308. doi: 10.1523/JNEUROSCI.20-16-06302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata K., Takeda M., Ito A., Takeda M., Sagai H. Ovariectomy-induced hyperalgesia and antinociceptive effect of elcatonin, a synthetic eel calcitonin. Pharmacol Biochem Behav. 1998;60:371–376. doi: 10.1016/s0091-3057(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 16.Scharla S., Oertel H., Helsberg K., Kessler F., Langer F., Nickelsen T. Skeletal pain in postmenopausal women with osteoporosis: prevalence and course during raloxifene treatment in a prospective observational study of 6 months duration. Curr Med Res Opin. 2006;22:2393–2402. doi: 10.1185/030079906X154097. [DOI] [PubMed] [Google Scholar]
- 17.Ohtori S., Akazawa T., Murata Y., Kinoshita T., Yamashita M., Nakagawa K. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17:209–213. doi: 10.1016/j.jocn.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Koca I., Savas E., Ozturk Z.A., Boyaci A., Tutoglu A., Alkan S. The evaluation in terms of sarcopenia of patients with fibromyalgia syndrome. Wien Klin Wochenschr. 2016;128:816–821. doi: 10.1007/s00508-015-0821-8. [DOI] [PubMed] [Google Scholar]
- 19.Scott D., Blizzard L., Fell J., Jones G. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res Hob. 2012;64:30–37. doi: 10.1002/acr.20545. [DOI] [PubMed] [Google Scholar]
- 20.Modic M.T., Steinberg P.M., Ross J.S., Masaryk T.J., Carter J.R. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 21.Kjaer P., Bendix T., Sorensen J.S., Korsholm L., Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2. doi: 10.1186/1741-7015-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanada K., Miyachi M., Tanimoto M., Yamamoto K., Murakami H., Okumura S. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol. 2010;110:57–65. doi: 10.1007/s00421-010-1473-z. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner R.N., Wayne S.J., Waters D.L., Janssen I., Gallagher D., Morley J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 24.Newman A.B., Kupelian V., Visser M., Simonsick E., Goodpaster B., Nevitt M. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 25.Stenholm S., Harris T.B., Rantanen T., Visser M., Kritchevsky S.B., Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland Y., Lauwers-Cances V., Cristini C., Abellan van Kan G., Janssen I., Morley J.E. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study Am J Clin Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 27.Hida T., Ishiguro N., Shimokata H., Sakai Y., Matsui Y., Takemura M. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013;13:413–420. doi: 10.1111/j.1447-0594.2012.00918.x. [DOI] [PubMed] [Google Scholar]
- 28.Parkkola R., Rytökoski U., Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine (Phila Pa 1976) 1993;18:830–836. doi: 10.1097/00007632-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Cagnie B., Dhooge F., Schumacher C., De Meulemeester K., Petrovic M., van Oosterwijck J. Fiber typing of the erector spinae and multifidus muscles in healthy controls and back pain patients: a systematic literature review. J Manip Physiol Ther. 2015;38:653–663. doi: 10.1016/j.jmpt.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Hodges P., Holm A.K., Hansson T., Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31:2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 31.Freeman M.D., Woodham M.A., Woodham A.W. The role of the lumbar multifidus in chronic low back pain: a review. PM R. 2010;2:142–146. doi: 10.1016/j.pmrj.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Abe T., Loenneke J.P., Thiebaud R.S., Fukunaga T. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age (Dordr) 2014;36:813–821. doi: 10.1007/s11357-013-9600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvioli S., Capri M., Valensin S., Tieri P., Monti D., Ottaviani E. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni M., Mazzali G., Fantin F., Rossi A., Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Schrager M.A., Metter E.J., Simonsick E., Ble A., Bandinelli S., Lauretani F. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cesari M., Kritchevsky S.B., Baumgartner R.N., Atkinson H.H., Penninx B.W., Lenchik L. Sarcopenia, obesity, and inflammation–results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Schiltenwolf M., Buchner M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain. 2008;24:273–278. doi: 10.1097/AJP.0b013e31816111d3. [DOI] [PubMed] [Google Scholar]
- 38.Hasselhorn H.M., Theorell T., Vingård E. Musculoskeletal Intervention Center (MUSIC)-Norrtälje Study Group. Endocrine and immunologic parameters indicative of 6-month prognosis after the onset of low back pain or neck/shoulder pain. Spine (Phila Pa 1976) 2001;26:E24–E29. doi: 10.1097/00007632-200102010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Schistad E.I., Espeland A., Pedersen L.M., Sandvik L., Gjerstad J., Røe C. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. Eur J Pain. 2014;18:1394–1401. doi: 10.1002/j.1532-2149.2014.502.x. [DOI] [PubMed] [Google Scholar]
- 40.Sainoh T., Orita S., Miyagi M., Inoue G., Kamoda H., Ishikawa T. Single Intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med. 2016;17:40–45. doi: 10.1111/pme.12892. [DOI] [PubMed] [Google Scholar]